Submitted:
06 July 2023
Posted:
10 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
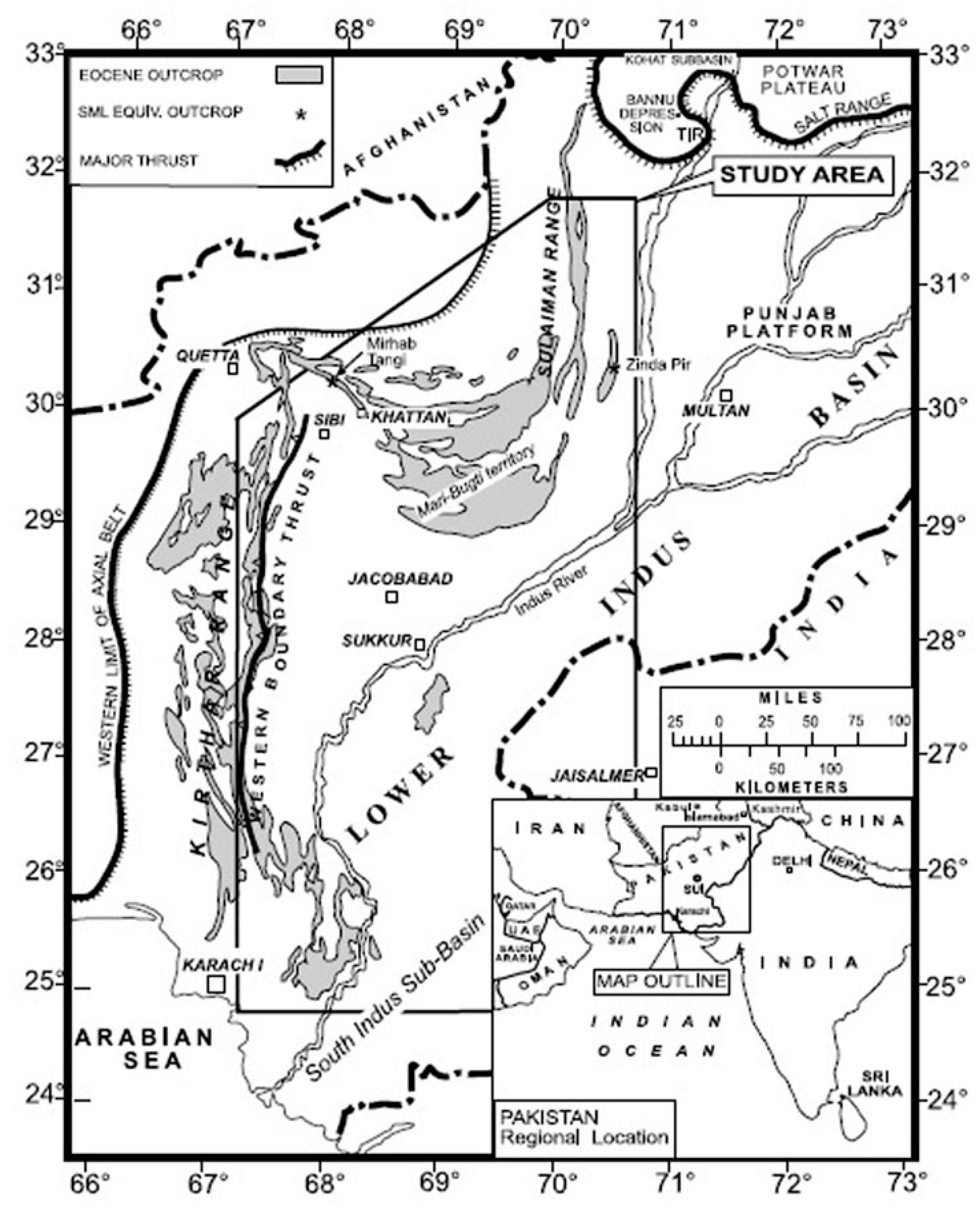
2. Geology and Tectonic Setting of Study Area
3. Materials and Methods
3.1. Materials

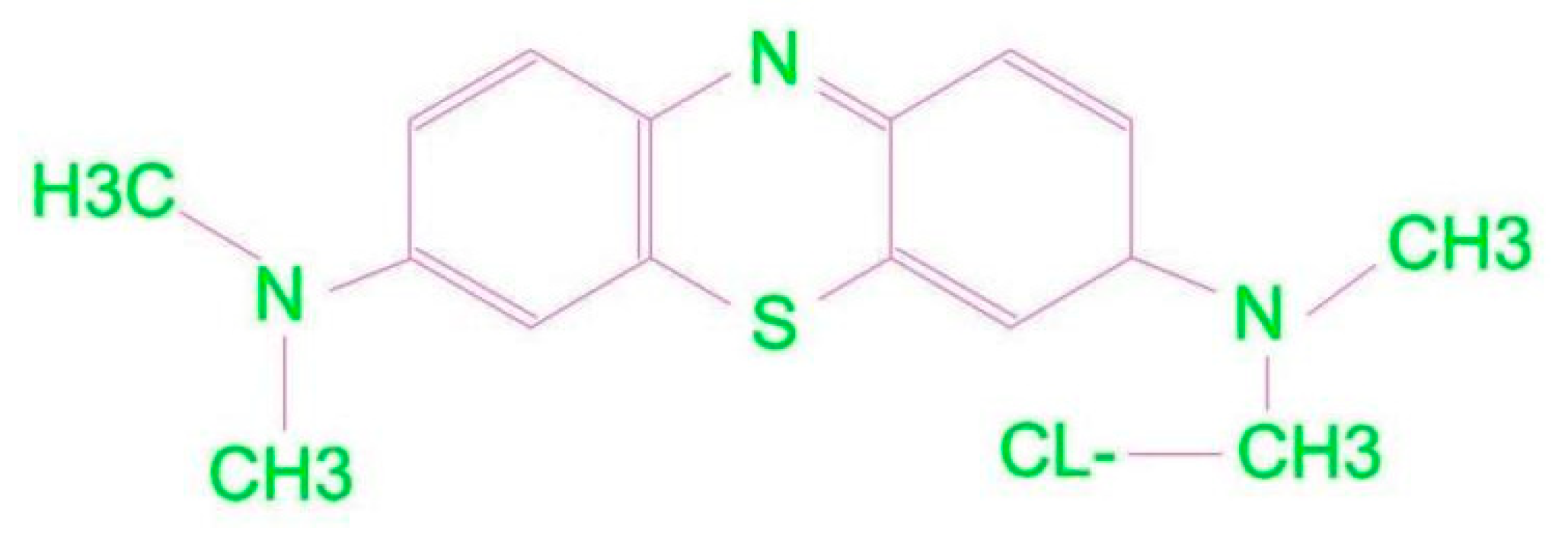
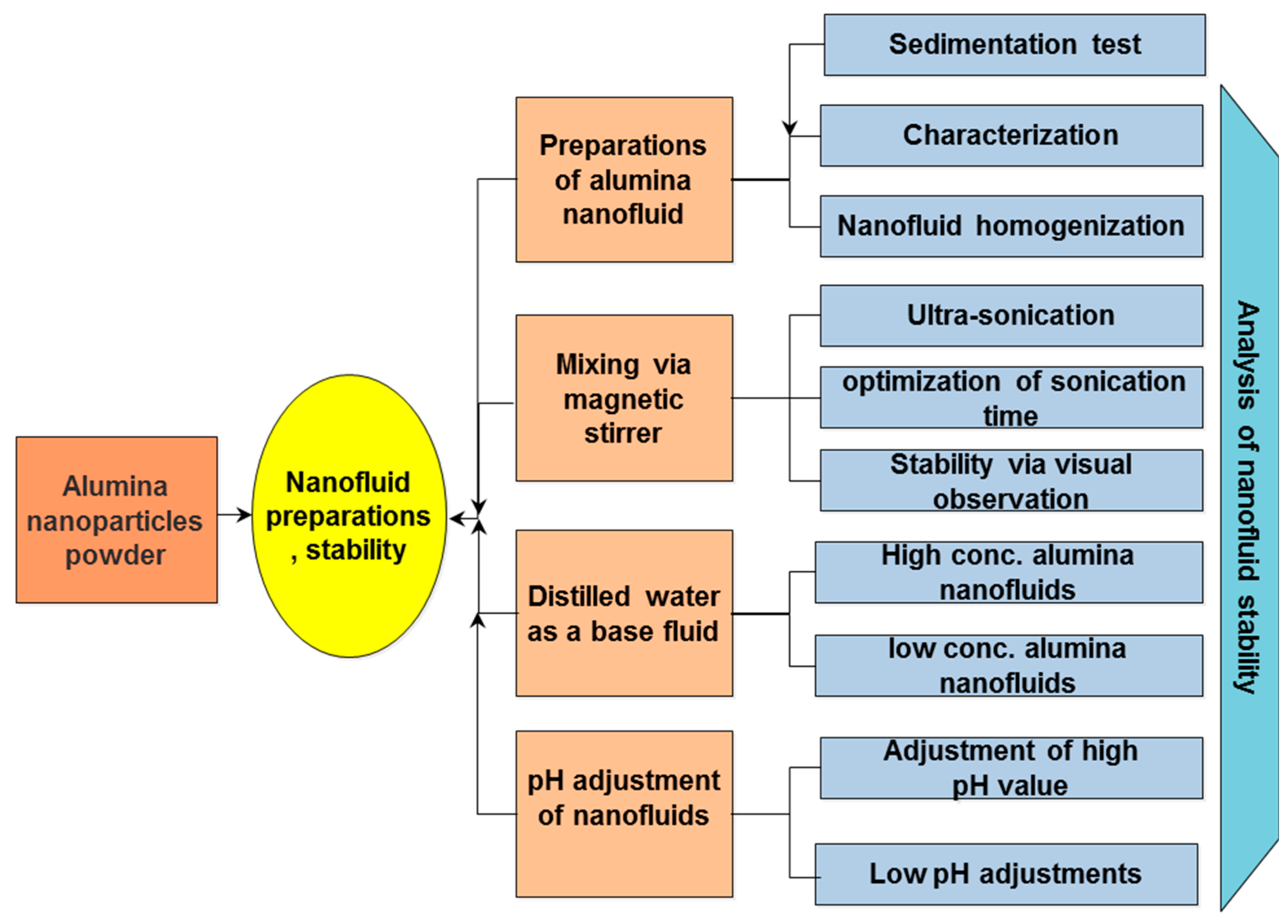
3.2. Fluids Formulations, Treatment and Stability
3.3. Sample Ageing Procedure
4. Experimental Methods
4.1. X-ray Diffraction Analysis XRD
4.2. Fourier Transforms Infrared (FTIR) Spectroscopy
4.3. Surface Features Characterization via Atomic Force Microscopy (AFM)
4.4. Surface Morphology via SEM
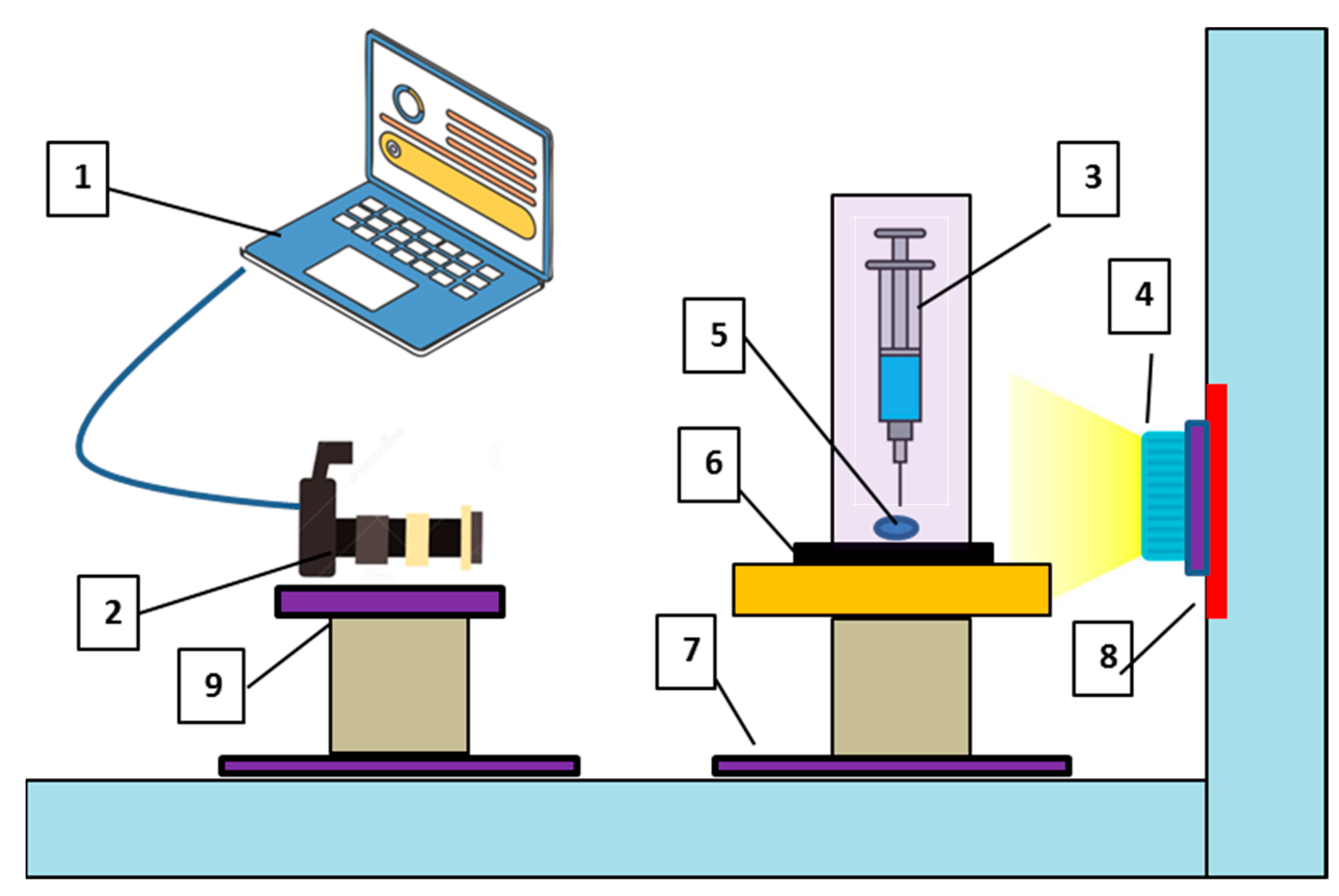
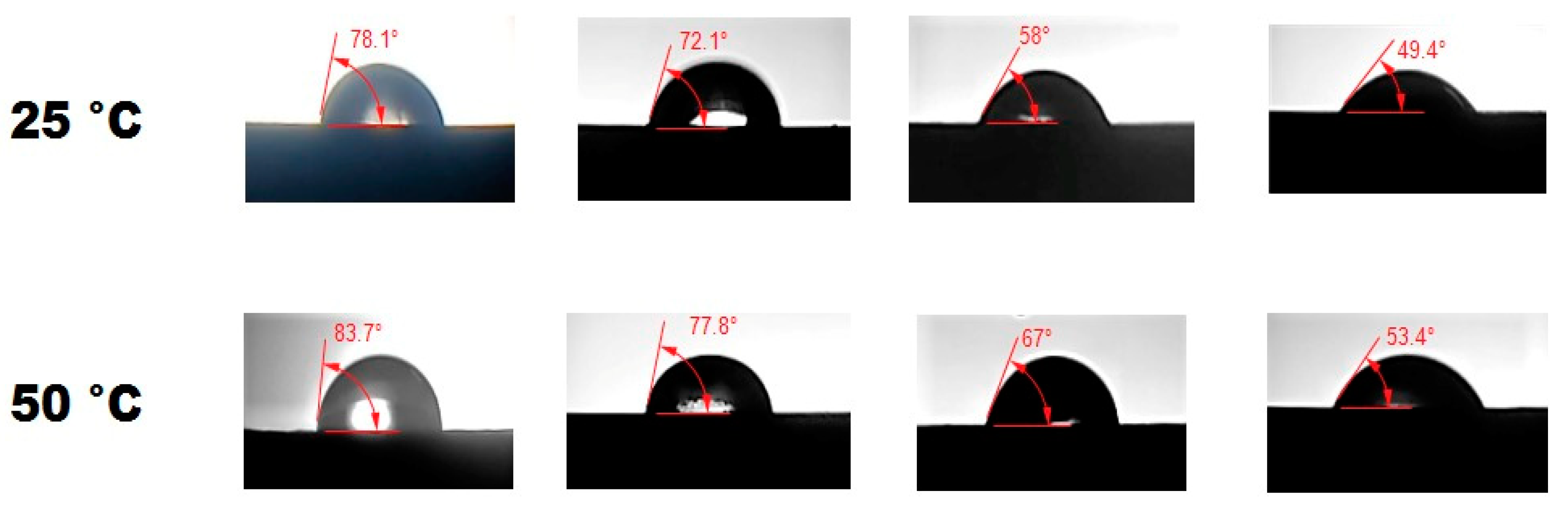
4.5. Wettability Determination via CA Measurement
- The sample stage is carefully flattened so that the dispensed liquid droplets remain aligned on the sample surface.
- Fill the syringe with designated amount of fluid via pumping chamber.
- Dispense the brine drop which stays on the sample surface after deposition. The light is illuminated behind the dispensed drop so that camera can capture the magnified image via optical lens appropriately.
- The captured images via HD camera wereanalysed precisely using the image-J software and the CA was determined with accuracy.
5. Results and Discussions
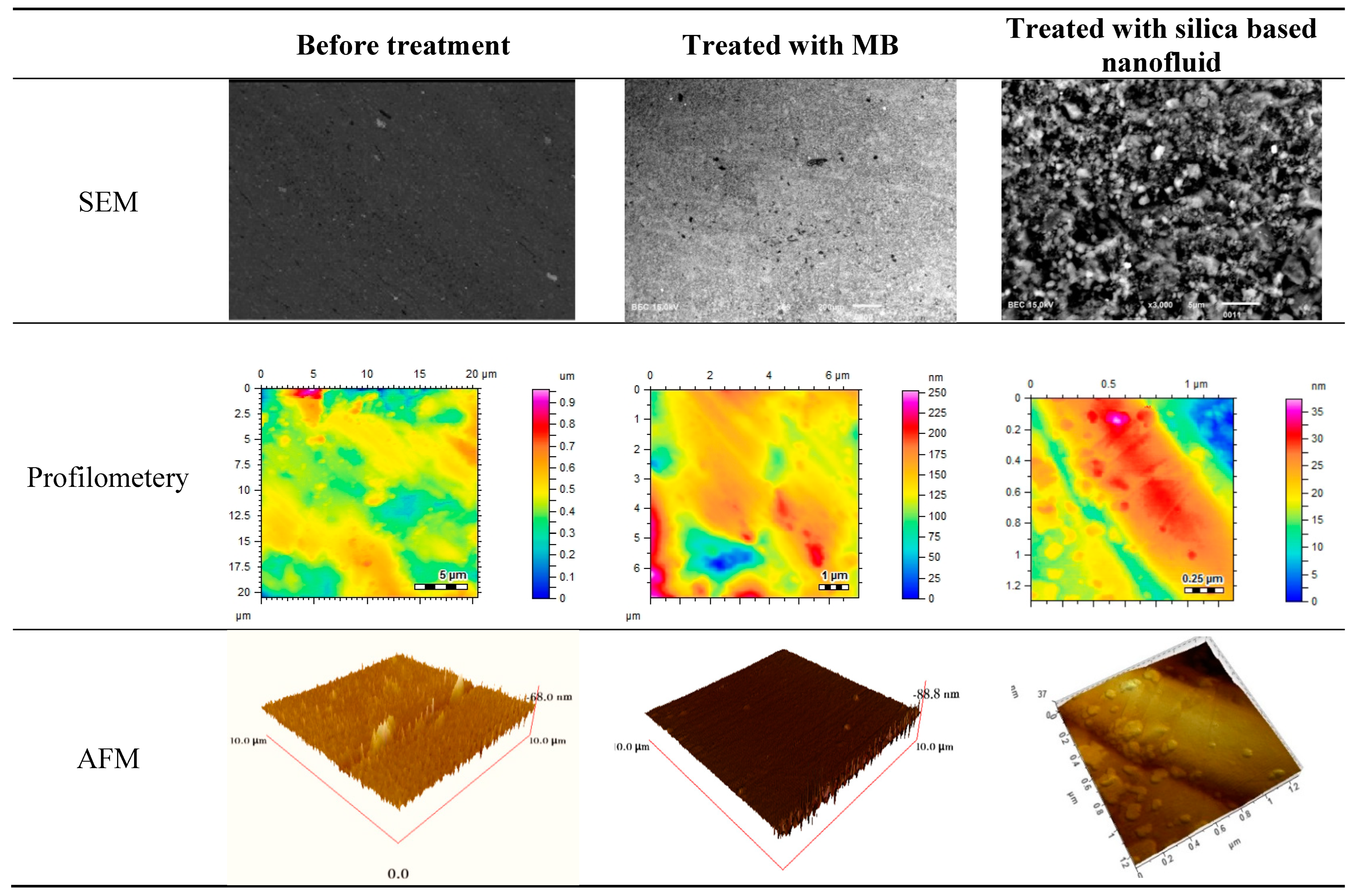
5.1. Surface Features Evaluation via SEM, Profilometry and AFM
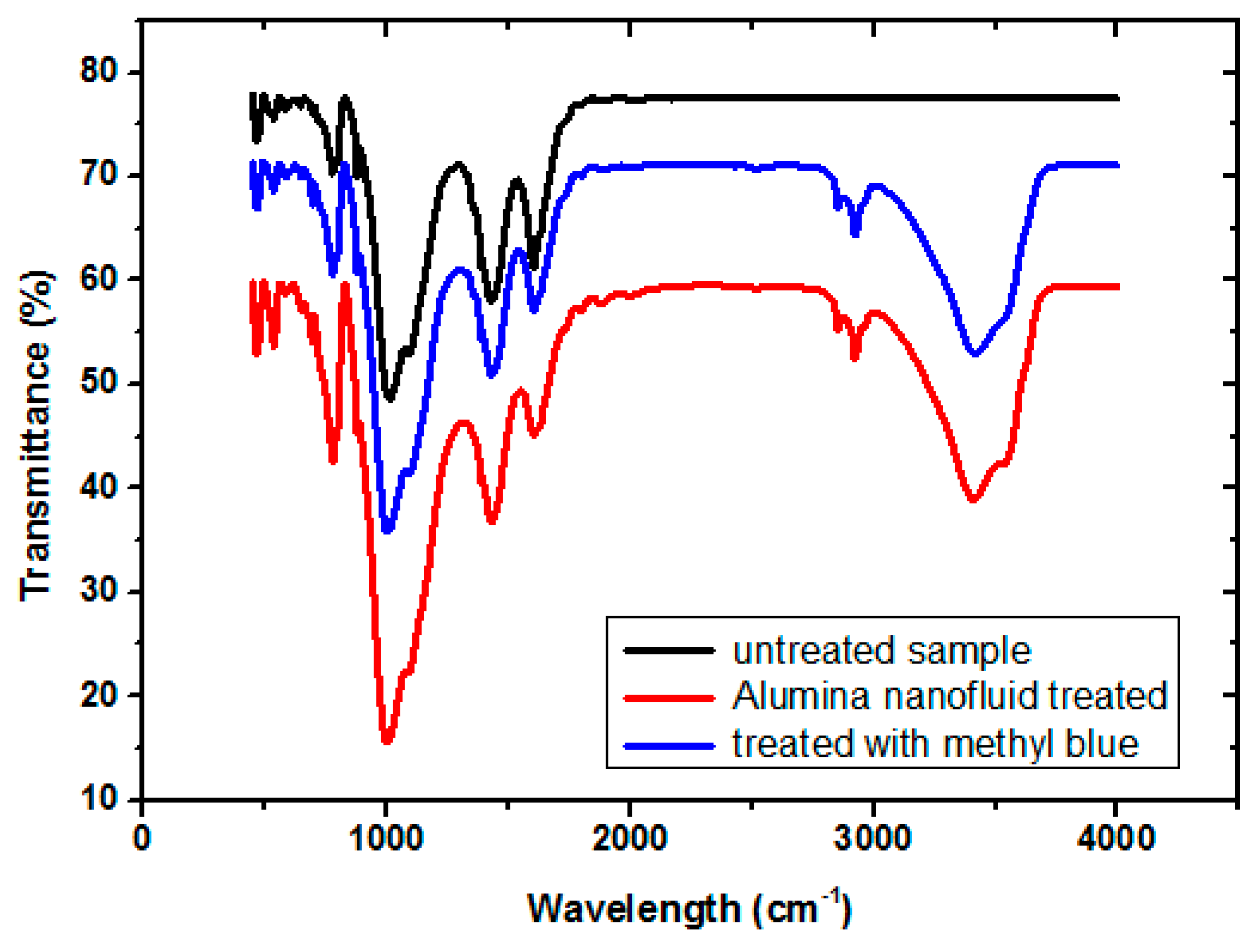
5.2. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
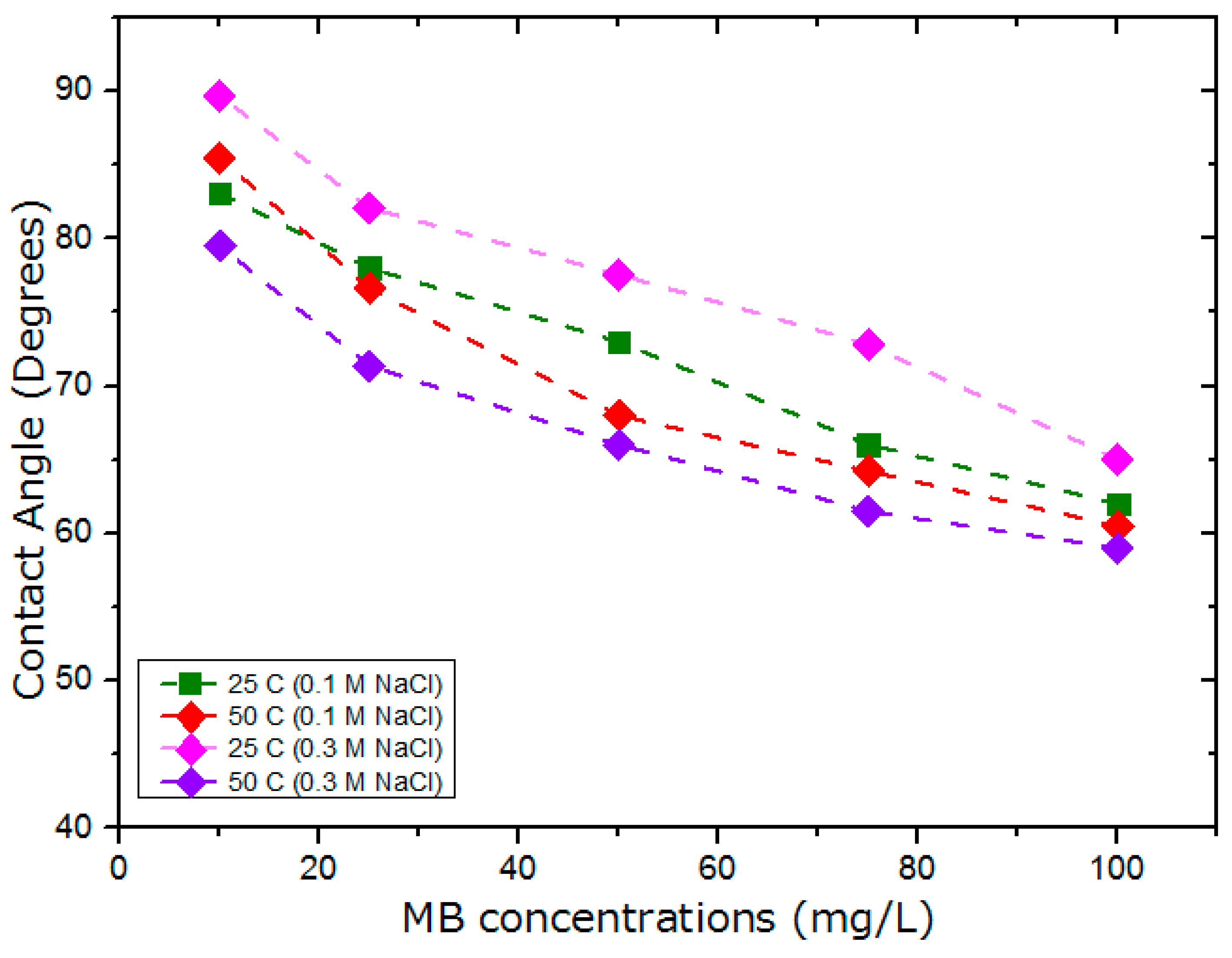
5.3. Effect of MB on Wettability
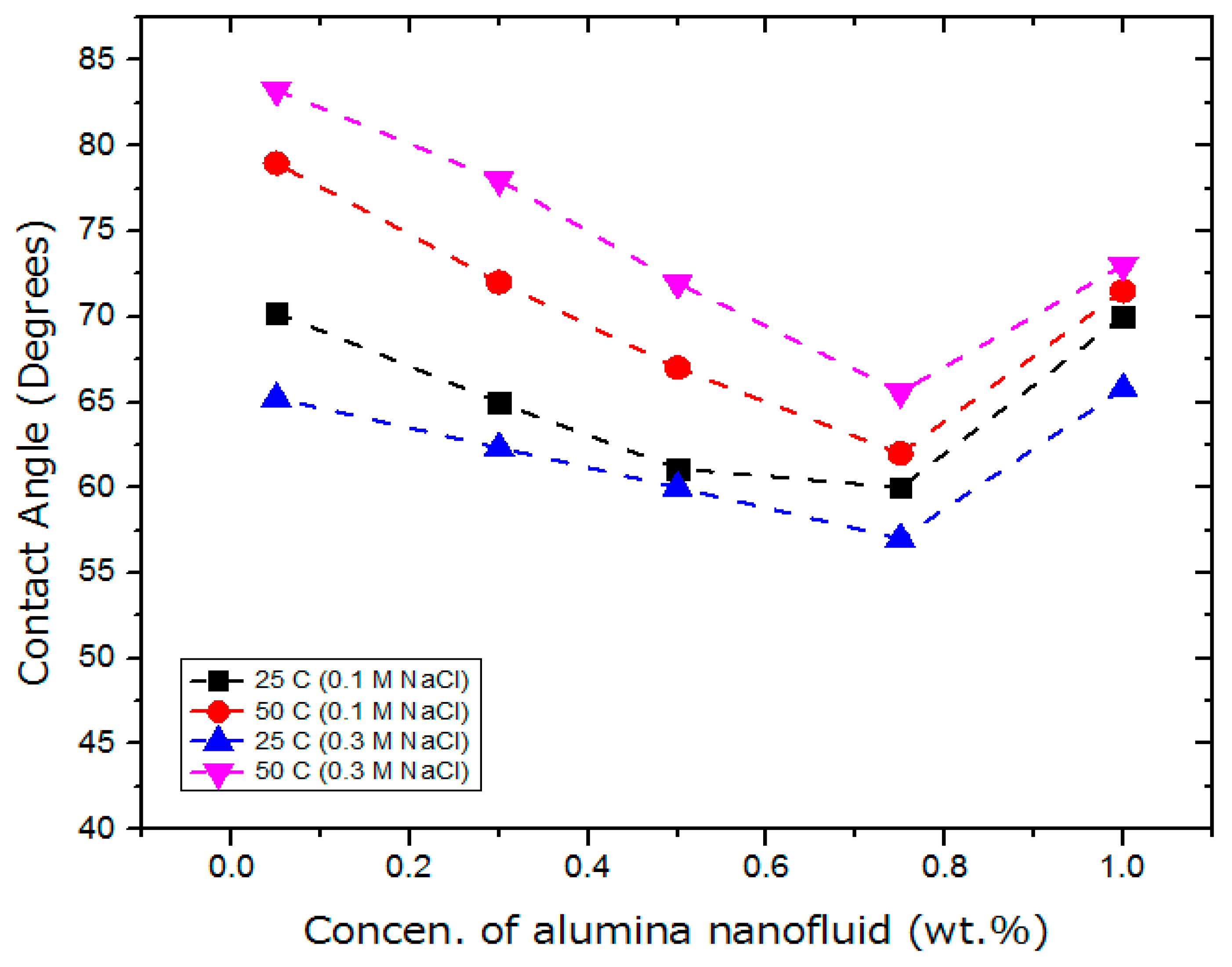
5.4. Effect of Alumina Based Nanofluid on Wettability
6. Conclusions
Funding
Acknowledgments
References
- Davies, A.; Simmons, M.D. Demand for ‘advantaged’hydrocarbons during the 21st century energy transition. Energy Reports 2021, 7, 4483–4497. [Google Scholar] [CrossRef]
- Shar, A.M.; Ali, M.; Bhutto, D.K.; Alanazi, A.; Akhondzadeh, H.; Keshavarz, A.; Iglauer, S.; Hoteit, H. Cryogenic Liquid Nitrogen Fracking Effects on Petro-Physical and Morphological Characteristics of the Sembar Shale Gas Formation in the Lower Indus Basin, Pakistan. Energy & Fuels 2022, 36, 13743–13752. [Google Scholar] [CrossRef]
- Bhutto, D.K.; Shar, A.M.; Abbasi, G.R.; Ansari, U. Shale Wettability Characteristics via Air/Brines and Air/Oil Contact Angles and Influence of Controlling Factors: A Case Study of Lower Indus Basin, Pakistan. ACS omega 2022. [Google Scholar] [CrossRef] [PubMed]
- Shar, A.M.; Mahesar, A.A.; Abbasi, G.R.; Narejo, A.A.; Hakro, A.A.A.D. Influence of diagenetic features on petrophysical properties of fine-grained rocks of Oligocene strata in the Lower Indus Basin, Pakistan. Open Geosciences 2021, 13, 517–531. [Google Scholar] [CrossRef]
- Ali Abro, W.; Majeed Shar, A.; Sang Lee, K.; Ali Narejo, A. An integrated analysis of mineralogical and microstructural characteristics and petrophysical properties of carbonate rocks in the lower Indus Basin, Pakistan. Open Geosciences 2019, 11, 1151–1167. [Google Scholar] [CrossRef]
- Mahesar, A.A.; Shar, A.M.; Ali, M.; Tunio, A.H.; Uqailli, M.A.; Mohanty, U.S.; Akhondzadeh, H.; Iglauer, S.; Keshavarz, A. Morphological and petro physical estimation of eocene tight carbonate formation cracking by cryogenic liquid nitrogen; a case study of Lower Indus basin, Pakistan. Journal of Petroleum Science and Engineering 2020, 192, 107318. [Google Scholar] [CrossRef]
- Alhamad, F.; Ali, M.; Ali, M.; Abid, H.; Hoteit, H.; Iglauer, S.; Keshavarz, A. Effect of methyl orange on wettability of sandstone formations: Implications for enhanced oil recovery. Energy Reports 2022, 8, 12357–12365. [Google Scholar] [CrossRef]
- Morad, S.; Al-Aasm, I.; Sirat, M.; Sattar, M. Vein calcite in cretaceous carbonate reservoirs of Abu Dhabi: Record of origin of fluids and diagenetic conditions. Journal of Geochemical Exploration 2010, 106, 156–170. [Google Scholar] [CrossRef]
- Paganoni, M.; Al Harthi, A.; Morad, D.; Morad, S.; Ceriani, A.; Mansurbeg, H.; Al Suwaidi, A.; Al-Aasm, I.S.; Ehrenberg, S.N.; Sirat, M. Impact of stylolitization on diagenesis of a Lower Cretaceous carbonate reservoir from a giant oilfield, Abu Dhabi, United Arab Emirates. Sedimentary Geology 2016, 335, 70–92. [Google Scholar] [CrossRef]
- Eslahati, M.; Mehrabianfar, P.; Isari, A.A.; Bahraminejad, H.; Manshad, A.K.; Keshavarz, A. Experimental investigation of Alfalfa natural surfactant and synergistic effects of Ca2+, Mg2+, and SO42− ions for EOR applications: Interfacial tension optimization, wettability alteration and imbibition studies. Journal of Molecular Liquids 2020, 310, 113123. [Google Scholar] [CrossRef]
- Souayeh, M.; Al-Maamari, R.S.; Karimi, M.; Aoudia, M. Wettability alteration and oil recovery by surfactant assisted low salinity water in carbonate rock: The impact of nonionic/anionic surfactants. Journal of Petroleum Science and Engineering 2021, 197, 108108. [Google Scholar] [CrossRef]
- Al-Anssari, S.; Arif, M.; Wang, S.; Barifcani, A.; Lebedev, M.; Iglauer, S. Wettability of nanofluid-modified oil-wet calcite at reservoir conditions. Fuel 2018, 211, 405–414. [Google Scholar] [CrossRef]
- Naik, S.; You, Z.; Bedrikovetsky, P. Rate enhancement in unconventional gas reservoirs by wettability alteration. Journal of Natural Gas Science and Engineering 2015, 26, 1573–1584. [Google Scholar] [CrossRef]
- Safari, M.; Jye, J.W.J.; Rahimi, A.; Gholami, R.; Yisong, L.; Khur, W.S. Salinity adjustment to improve the efficiency of nano glass flakes (NGFs) in interfacial tension reduction. Journal of Petroleum Science and Engineering 2022, 212, 109874. [Google Scholar] [CrossRef]
- Iglauer, S.; Wu, Y.; Shuler, P.; Tang, Y.; Goddard III, W.A. New surfactant classes for enhanced oil recovery and their tertiary oil recovery potential. Journal of Petroleum science and Engineering 2010, 71, 23–29. [Google Scholar] [CrossRef]
- Iglauer, S.; Wu, Y.; Shuler, P.; Tang, Y.; Goddard III, W.A. Alkyl polyglycoside surfactant–alcohol cosolvent formulations for improved oil recovery. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2009, 339, 48–59. [Google Scholar] [CrossRef]
- Golabi, E.; SEYEDIN, A.F.; AYAT, E.S. Chemical induced wettability alteration of carbonate reservoir rocks. 2009.
- Austad, T.; Standnes, D.C. Spontaneous imbibition of water into oil-wet carbonates. Journal of Petroleum Science and Engineering 2003, 39, 363–376. [Google Scholar] [CrossRef]
- Al-Anssari, S.; Barifcani, A.; Wang, S.; Maxim, L.; Iglauer, S. Wettability alteration of oil-wet carbonate by silica nanofluid. Journal of colloid and interface science 2016, 461, 435–442. [Google Scholar] [CrossRef]
- Ekechukwu, G.K.; Khishvand, M.; Kuang, W.; Piri, M.; Masalmeh, S. The effect of wettability on waterflood oil recovery in carbonate rock samples: A systematic multi-scale experimental investigation. Transport in Porous Media 2021, 138, 369–400. [Google Scholar] [CrossRef]
- Iglauer, S.; Pentland, C.; Busch, A. CO2 wettability of seal and reservoir rocks and the implications for carbon geo-sequestration. Water Resources Research 2015, 51, 729–774. [Google Scholar] [CrossRef]
- Wang, S.; Edwards, I.M.; Clarens, A.F. Wettability phenomena at the CO2–brine–mineral interface: implications for geologic carbon sequestration. Environmental science & technology 2013, 47, 234–241. [Google Scholar] [CrossRef]
- Al-Hadhrami, H.S.; Blunt, M.J. Thermally induced wettability alteration to improve oil recovery in fractured reservoirs. SPE Reservoir Evaluation & Engineering 2001, 4, 179–186. [Google Scholar] [CrossRef]
- Deng, X.; Kamal, M.S.; Patil, S.; Hussain, S.M.S.; Zhou, X. A review on wettability alteration in carbonate rocks: Wettability modifiers. Energy & Fuels 2019, 34, 31–54. [Google Scholar] [CrossRef]
- Mohan, K.; Gupta, R.; Mohanty, K. Wettability altering secondary oil recovery in carbonate rocks. Energy & Fuels 2011, 25, 3966–3973. [Google Scholar] [CrossRef]
- Lager, A.; Webb, K.; Black, C. Impact of brine chemistry on oil recovery. In Proceedings of the IOR 2007-14th European symposium on improved oil recovery, 2007; pp. cp-24-00020. [Google Scholar] [CrossRef]
- Saboori, R.; Azin, R.; Osfouri, S.; Sabbaghi, S.; Bahramian, A. Wettability alteration of carbonate cores by alumina-nanofluid in different base fluids and temperature. Journal of Sustainable Energy Engineering 2018, 6, 84–98. [Google Scholar] [CrossRef]
- Nazari Moghaddam, R.; Bahramian, A.; Fakhroueian, Z.; Karimi, A.; Arya, S. Comparative study of using nanoparticles for enhanced oil recovery: wettability alteration of carbonate rocks. Energy & Fuels 2015, 29, 2111–2119. [Google Scholar] [CrossRef]
- Izadi, N.; Nasernejad, B. Newly engineered alumina quantum dot-based nanofluid in enhanced oil recovery at reservoir conditions. Scientific Reports 2022, 12, 9505. [Google Scholar] [CrossRef]
- Murshed, S.S.; Tan, S.-H.; Nguyen, N.-T. Temperature dependence of interfacial properties and viscosity of nanofluids for droplet-based microfluidics. Journal of Physics D: Applied Physics 2008, 41, 085502. [Google Scholar] [CrossRef]
- Lu, T.; Li, Z.; Zhou, Y.; Zhang, C. Enhanced oil recovery of low-permeability cores by SiO2 nanofluid. Energy & fuels 2017, 31, 5612–5621. [Google Scholar] [CrossRef]
- Ali, M.; Shar, A.M.; Mahesar, A.A.; Al-Yaseri, A.; Yekeen, N.; Memon, K.R.; Keshavarz, A.; Hoteit, H. Experimental evaluation of liquid nitrogen fracturing on the development of tight gas carbonate rocks in the Lower Indus Basin, Pakistan. Fuel 2022, 309, 122192. [Google Scholar] [CrossRef]
- Rezvani, H.; Panahpoori, D.; Riazi, M.; Parsaei, R.; Tabaei, M.; Cortés, F.B. A novel foam formulation by Al2O3/SiO2 nanoparticles for EOR applications: A mechanistic study. Journal of Molecular Liquids 2020, 304, 112730. [Google Scholar] [CrossRef]
- Alnarabiji, M.S.; Yahya, N.; Nadeem, S.; Adil, M.; Baig, M.K.; Ghanem, O.B.; Azizi, K.; Ahmed, S.; Maulianda, B.; Klemeš, J.J. Nanofluid enhanced oil recovery using induced ZnO nanocrystals by electromagnetic energy: Viscosity increment. Fuel 2018, 233, 632–643. [Google Scholar] [CrossRef]
- AfzaliTabar, M.; Alaei, M.; Bazmi, M.; Khojasteh, R.R.; Koolivand-Salooki, M.; Motiee, F.; Rashidi, A. Facile and economical preparation method of nanoporous graphene/silica nanohybrid and evaluation of its Pickering emulsion properties for Chemical Enhanced oil Recovery (C-EOR). Fuel 2017, 206, 453–466. [Google Scholar] [CrossRef]
- Patel, A.; Xue, Y.; Hartley, R.; Sant, V.; Eles, J.R.; Cui, X.T.; Stolz, D.B.; Sant, S. Hierarchically aligned fibrous hydrogel films through microfluidic self-assembly of graphene and polysaccharides. Biotechnology and bioengineering 2018, 115, 2654–2667. [Google Scholar] [CrossRef]
- Hill, D.; Barron, A.R.; Alexander, S. Controlling the wettability of plastic by thermally embedding coated aluminium oxide nanoparticles into the surface. Journal of colloid and interface science 2020, 567, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kadri, I.B. Petroleum geology of Pakistan: Pakistan Petroleum Limited. Karachi, Pakistan 1995.
- Siddiqui, N.K. Sui Main Limestone: Regional geology and the analysis of original pressures of a closed-system reservoir in central Pakistan. AAPG bulletin 2004, 88, 1007–1035. [Google Scholar] [CrossRef]
- Khalid, P.; Ehsan, M.I.; Khurram, S.; Ullah, I.; Ahmad, Q.A. Reservoir quality and facies modeling of the early Eocene carbonate stratigraphic unit of the Middle Indus Basin, Pakistan. Frontiers in Earth Science 2022, 10, 1063877. [Google Scholar] [CrossRef]
- Alhamad, F.; Sedev, R.; Ali, M.; Ali, M.; Hoteit, H.; Iglauer, S.; Keshavarz, A. Effect of methyl orange on the hydrogen wettability of sandstone formation for enhancing the potential of underground hydrogen storage. Energy & Fuels 2023, 37, 6149–6157. [Google Scholar] [CrossRef]
- Pentland, C.H.; Itsekiri, E.; Al Mansoori, S.K.; Iglauer, S.; Bijeljic, B.; Blunt, M.J. Measurement of nonwetting-phase trapping in sandpacks. Spe Journal 2010, 15, 274–281. [Google Scholar] [CrossRef]
- Haghighi, O.M.; Zargar, G.; Khaksar Manshad, A.; Ali, M.; Takassi, M.A.; Ali, J.A.; Keshavarz, A. Effect of environment-friendly non-ionic surfactant on interfacial tension reduction and wettability alteration; implications for enhanced oil recovery. Energies 2020, 13, 3988. [Google Scholar] [CrossRef]
- Nazarahari, M.J.; Manshad, A.K.; Ali, M.; Ali, J.A.; Shafiei, A.; Sajadi, S.M.; Moradi, S.; Iglauer, S.; Keshavarz, A. Impact of a novel biosynthesized nanocomposite (SiO2@ Montmorilant@ Xanthan) on wettability shift and interfacial tension: Applications for enhanced oil recovery. Fuel 2021, 298, 120773. [Google Scholar] [CrossRef]
- Iglauer, S.; Ali, M.; Keshavarz, A. Hydrogen wettability of sandstone reservoirs: implications for hydrogen geo-storage. Geophysical Research Letters 2021, 48, e2020GL090814. [Google Scholar] [CrossRef]
- Arif, M.; Abu-Khamsin, S.A.; Zhang, Y.; Iglauer, S. Experimental investigation of carbonate wettability as a function of mineralogical and thermo-physical conditions. Fuel 2020, 264, 116846. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; Issa, A.A.; Khraisheh, M.A.; Walker, G.M. Sorption of Zn (II), Pb (II), and Co (II) using natural sorbents: equilibrium and kinetic studies. Water research 2006, 40, 2645–2658. [Google Scholar] [CrossRef]
- Nayak, P.S.; Singh, B. Instrumental characterization of clay by XRF, XRD and FTIR. Bulletin of materials science 2007, 30, 235–238. [Google Scholar] [CrossRef]
- Hosseini, M.; Sedev, R.; Ali, M.; Ali, M.; Fahimpour, J.; Keshavarz, A.; Iglauer, S. Hydrogen-wettability alteration of Indiana limestone in the presence of organic acids and nanofluid. International Journal of Hydrogen Energy 2023. [Google Scholar] [CrossRef]
| S.No. | Properties | Values (Al2O3) |
|---|---|---|
| 1 | Molecular weight | 101.96 |
| 2 | Form | Solid |
| 3 | Diameter | 25-30nm |
| 4 | Specific surface area | 30-42 m2/g |
| 5 | Purity | 99.96 |
| 6 | Supplier | Sigma-Aldrich |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





