1. Introduction
Pesticides are used to protect plants, to control and destroy pests for plants. They could be classified from their spectrum of activity (ie. their target) (eg. Insecticides, acaricides, antiseptics, herbicides, etc), from their chemical hazards (from slightly to extremely hazardous) [
1] or from their chemical structure and composition. Regarding the latest classification, pesticides belong to three main groups: inorganics compounds (eg. Chlorates), biopesticides (eg. Bacteria, fungi) and organic compounds (eg. Organophosphate pesticides (OPP), carbamates). Among organic pesticides, there are several classes: organochlorine pesticides (OCP) (eg. DDT group), phenol derivatives, carbamates, aryloxycarboxylic acid derivatives, OPP (eg. phosphoric esters (dichlorvos)), organometallic, alcalaoids, quaternary ammonium salts (eg. Paraquat, diquat, chlormequat). OCPs and OPPs (malathion, parathion, diazinon, chlorpyrifos) and carbamates (carbaryl, carbofuran, aldicarb) are insecticides used to control pests in agricultural food commodities.
Foodstuffs of plant origin can be contaminated by pesticide residues. Moreover, pesticide residues can remain for years in soil and water [
2]. Food-producing animals can eat or drink contaminated products [
3,
4]. Therefore they can enter in the food chain.
The risks to human health from exposure to pesticides range from acute to chronic low-dose toxicity [
5,
6]. The toxic effects of pesticides can be different with risk factors depending on the exposure and the pesticide: local irritation (eg. Skin or ocular irritation), respiratory syndrome, allergic reactions, neurodevelopmental effects caused by enzyme inhibition (ie. Cholinesterase (OPP, carbamates) [
7], neurologic issues (eg. OCP), and oxidative damages.
Regulation 396/2005 established maximum residue limits (MRLs) for permitted pesticides in or on foodstuffs intended for human or animal consumption. These MRL were set on the basis of compliance with good agricultural practice and guaranted consumer safety [
8]. The control of pesticides is ensured through annual monitoring programs (NRMP) that requires Member States to sample, analyse and test an agreed range of products for pesticides to ensure compliance with pesticide MRLs and to assess consumer exposure. The most commonly used methods for the analysis of pesticides are chromatographic methods (gas chromatography and liquid chromatography coupled to mass spectrometry in tandem (GC-MS/MS, LC-MS/MS)) [
9,
10].
A pesticide detection method must have several characteristics, similar to those required for clinical point of care tests (POCT), which are very well defined by the acronym ASSURED. The ASSURED criteria defined by World Health Organization (WHO) means Affordable, Sensitive, Specific, User friendly, Rapid and Robust, Equipment-free and Deliverable to end users [
11,
12]. Biosensors for the detection of pesticides in food products are able to offer these performance characteristics [
13]. Therefore biosensors represent a promising avenue for pesticide detection [
14,
15,
16]. It has been explored since the 80’s [
17,
18,
19].
The detection of pesticide residues can be performed using different recognition elements: antibodies (immunosensors) [
20], molecularly imprinted polymers (MIPs) [
21,
22], aptamers [
23,
24], bacteria (Stoytcheva 2011) or yeast (Schofield 2007), enzymes [
25,
26], and more recently nanomaterials that have enzyme-like properties [
27,
28,
29]. Regarding the transduction elements, mainly electrochemical [
30,
31,
32,
33,
34,
35] and optical biosensors [
36,
37,
38] have been developed. In this review, we will focus on enzymatic colorimetric and dual-ratiometric biosensors.
This review will look at the different sensing principles of enzymatic colorimetric sensors, and will present illustrated examples of applications in water, plant and animal-derived food. Numerous colorimetric and dual ratiometric biosensors based on enzymatic inhibition have been developed for the detection of OPPs and carbamates in water and in various matrices of plant origin (ie. fruits, vegetables). Different types of substrates (ie. chemical, polymer and nanomaterials based) and/or fluorophores will be presented for colorimetric and fluorimetric detection. These biosensors could reach very low detection limits (down to ng/ml or ng/l). However, these methods are not necessarily specific to pesticides, since other chemical agents can inhibit acetylcholinesterase-type enzymes (eg Sarin, ....). This review will highlight that in recent years, nanomaterials have been increasingly used to improve detection sensitivity.
2. Principle of enzymatic biosensors for pesticide analysis
Enzymatic biosensors for pesticide detection were mostly based on the inhibition of the enzyme activity by the pesticide. The pesticide took the place of the enzyme substrate and thus inhibited the activity of the enzyme.
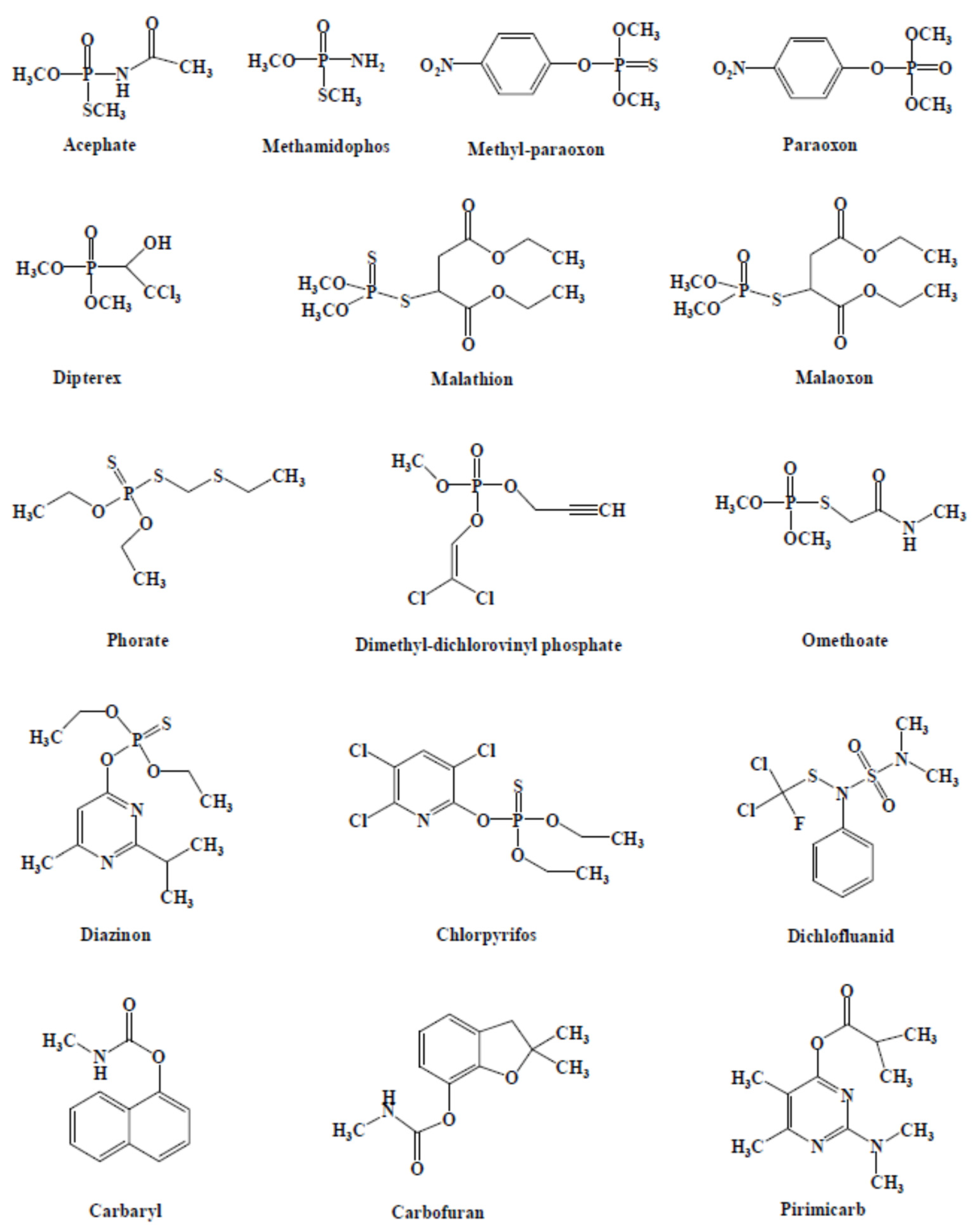
Figure 1 presented the structures of the main pesticides used as targets in AChE-based biosensors [
39].
The different enzymes used for the development of enzymatic biosensors for the detection of pesticides were: acetylcholinesterase (AChE) [
40], butyrylcholinesterase (BChE)[
41], choline oxidase (ChO) [
42], organophosphorus hydrolase (OPH) [
43], alkaline phosphatase (ALP) [
44], tyrosinase (TYR) [
45] and laccase (LAC) [
46].
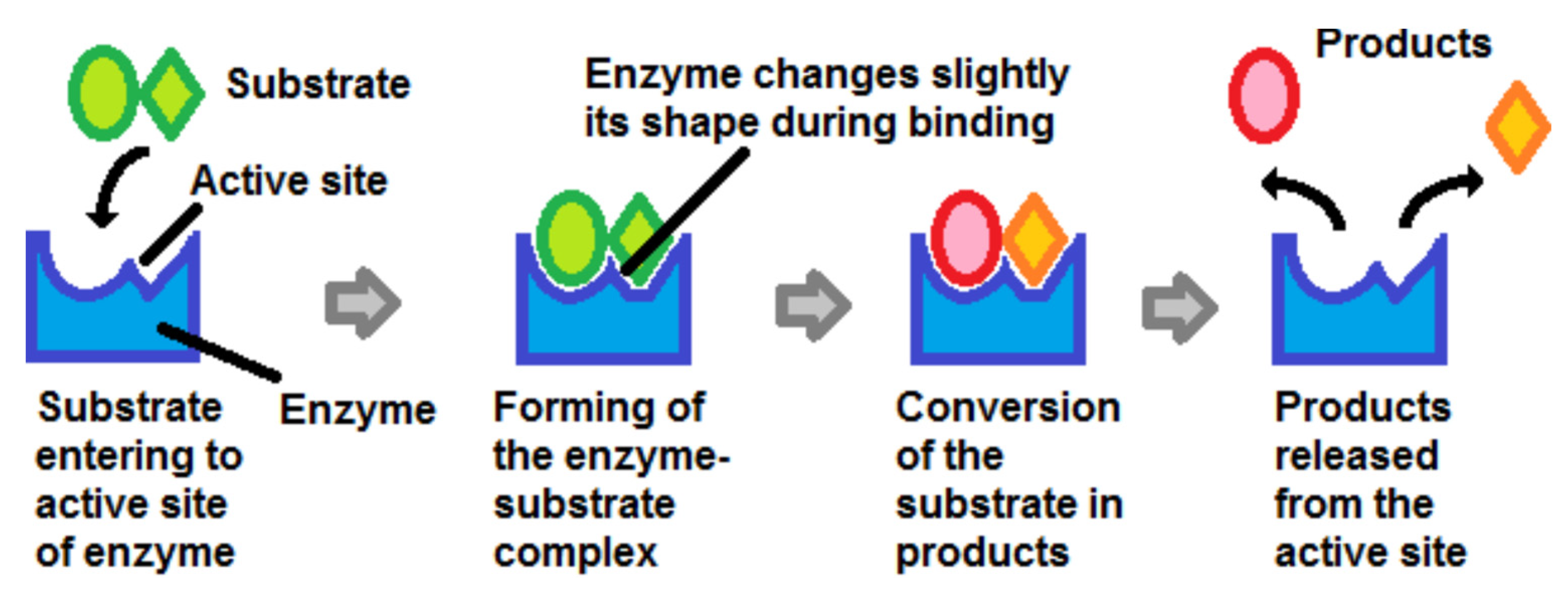
Whatever the enzyme, the substrate bound to the active site of the enzyme. The substrate was then converted into a product which was released into the environment and left room for a new substrate molecule to bind to the enzyme (
Figure 2).
This review will mainly focus on AChE because it was the mostly used enzyme. However, some examples of sensors based on ChO, and ALP will also be discussed.
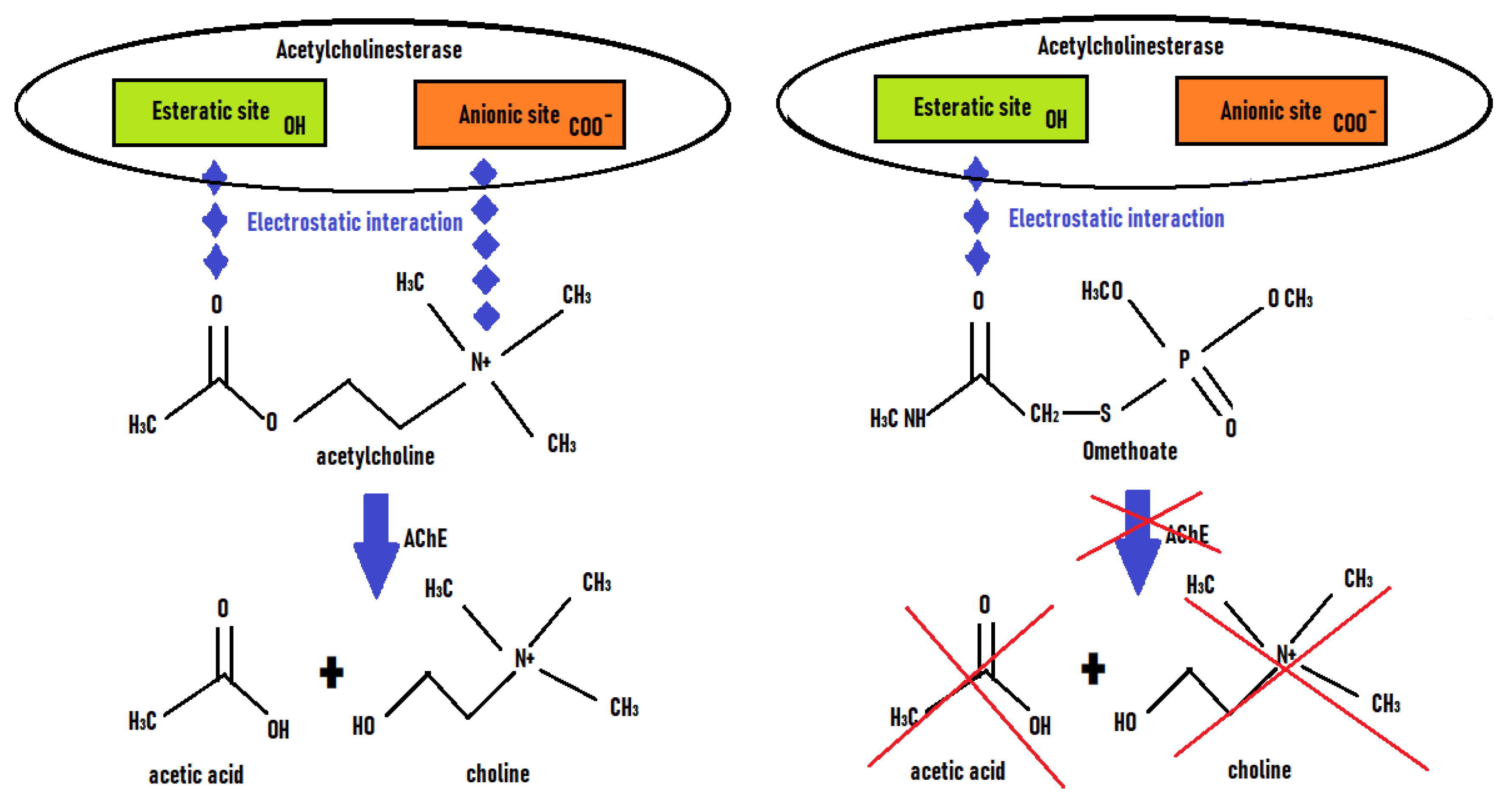
Acetylcholinesterase (AChE, EC 3.1.1.7) catalyzed the rapid hydrolysis of the neurotransmitter acetylcholine into acetate and choline (
Figure 3). Organophosphorus pesticides (OPPs) and carbamates had an inhibitory activity on enzymes such as AChE and BChE (EC 3.1.1.8) which made them potentially biocidal and toxic. This inhibition was due to the blocking of the esteratic site of the enzyme (phosphorylation or carbamylation of the OH of the serine of the active site) by the pesticide, which competed with the specific substrate of the enzyme (ie. Acetylcholine). Therefore the acetylcholine was no more transformed into choline. Generally the cation-binding site (ie. Anionic site) did not interact with OPP. Their inhibitory action led to an accumulation of acetylcholine at the level of synapses, resulting in over-activation of cholinergic receptors and cholinergic toxicity (e.g. involuntary movements, muscle paralysis, bradycardia, dyspnea). Therefore cholinesterases could be used as a recognition tool for the detection of pesticides.
The production of recombinant AChE could be a solution to develop more sensitive and selective biosensors for the detection of pesticides. This was due to a better knowledge and understanding of the interaction and binding of the inhibitor with the active site of the enzyme [
48,
49].
In the presence of ChOx (EC 1.1.3.17), following the catalytic activity of AChE, choline was oxydated to betaine and released hydrogen peroxide (H
2O
2). Then H
2O
2 could oxidise a chemical reagent (eg. Dye 2,20-azino-di-[3-ethylbenzthiazoline sulfonate (ABTS) [
42], 3,3’,5,5’-tetramethylbenzidine (TMB) [
50]) that produced a change of colour or fluorescence.
In the presence of the enzyme ALP (3.1.3.1), the substrate p-aminophenyl phosphate (p-APP) could be hydrolyzed to p-aminophenol (p-AP). The enzymatic sensor was usually based on the reduction of a chemical element by p-AP (ie. Reduction of manganese dioxide (MnO2) to Mn2+ [
51]). When the enzymatic activity was inhibited by a pesticide, the product p-AP was not formed so that the reduction did not occur.
The immobilisation of the enzyme was an essential step to maintain its structural integrity and therefore its function. It is necessary to guarantee the stability of the biosensor but also to allow access of the substrate and the inhibitor to the active site of the enzyme (ie. Relevant orientation of the enzyme) in order to obtain optimal sensitivity and specificity. Different immobilisation techniques were available: adsorption, covalent binding, entrapment, encapsulation, cross-linking [
52]. The cost of immobilisation partly related to the choice of the support materials should also be taken into account in the development of field biosensors.
3. Colorimetric enzymatic biosensors
The first colorimetric methods were developed to detect acetylcholinesterase activity [
53,
54]. Then development of colorimetric biosensors using AChE for the detection of inhibitors (ie. Pesticides, neurotoxic agents) started in the years 1990’s [
36,
55].
Basically the principle of the colorimetric detection of an enzymatic inhibitor was as follows. Without an inhibitor, enzymatic activity took place and so the product was synthesised by the catalytic activity of the enzyme. The formed product could react with a colorimetric substrate (eg. Dye precursor, nanoparticles) which produced a change of colour (by oxidation or reduction). In the presence of an inhibitor, the enzyme did not convert the substrate into the product. Thus the product could not perform the colorimetric detection mechanism.
There were different types of reagents used for the colorimetric detection of inhibition of the enzymatic activity of cholinesterases or related enzymes. They could be classified into three categories: chemical, polymer and nanomaterials based.
Table 1 shows examples of colorimetric enzyme-based biosensors developed for the detection of organophosphate pesticides and carbamates.
3.1. Chemical based
Chemical based enzymatic sensors use classical chemical reagents for the colorimetric detection of the enzymatic product.
3.1.1. Ellman
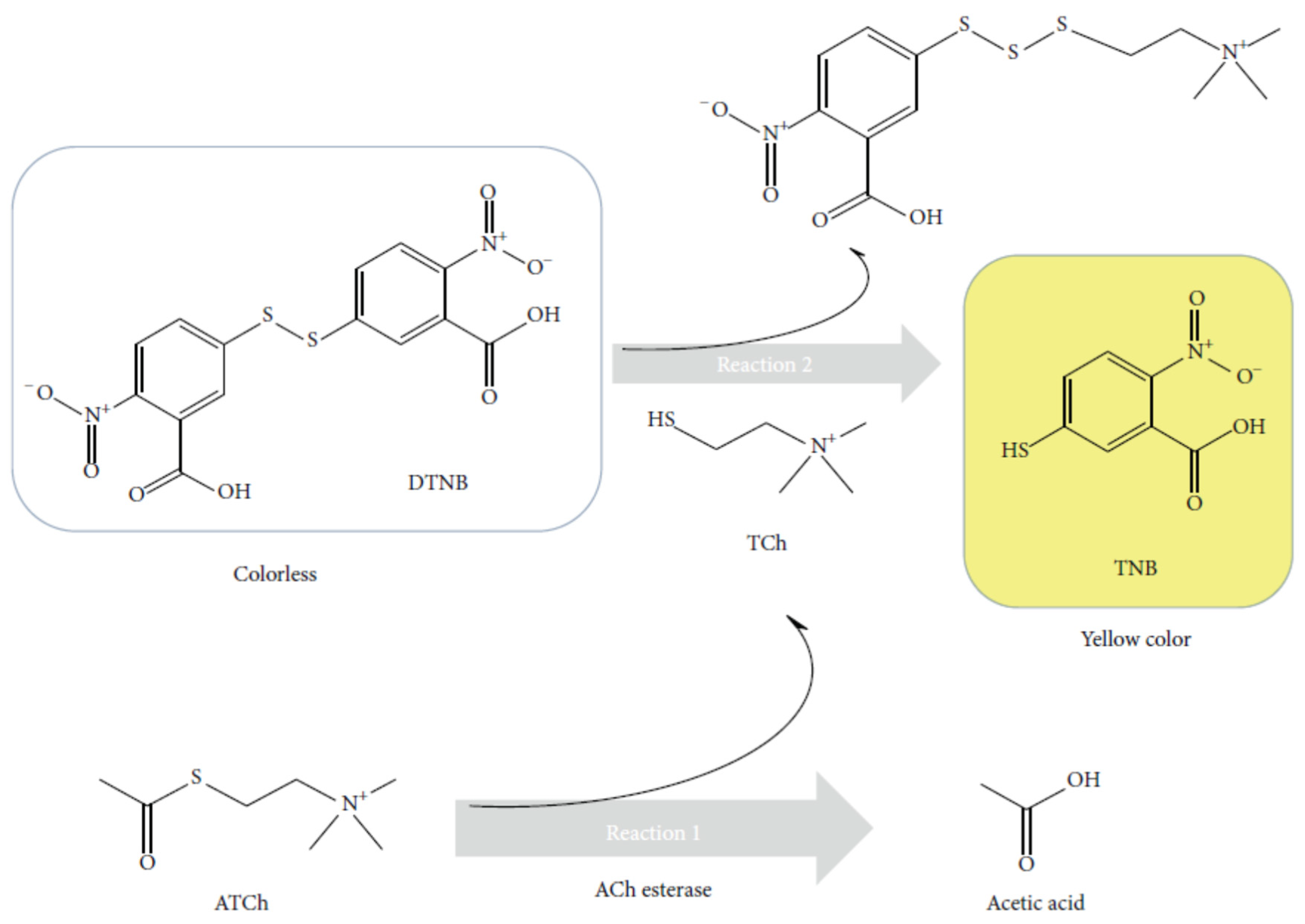
The Ellman’s method was a classical colorimetric method used for the detection of thiol groups [
54]. The mechanism of action of the Ellmann reagent was as follows [
88]. Thiols reacted with Ellmann's reagent, (5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) (faintly coloured) cleaving the disulphide bond to give 5-nitro-2-thiobenzoic acid (TNB) (yellow colour) in water at neutral and alkaline pH visible at 412 nm.
The Ellman method thus found application for the determination of cholinesterase (ChE) activity using thiocholine esters as substrates [
54,
89]. This method was applicable to acetylcholinesterase [
90] and butyrylcholinesterase detection [
91]. When thiocholine was produced by the catalytic activity of ChE, this product owing a thiol group could react with DTNB to form the yellow product TNB (
Figure 4). In the presence of an inhibitor, thiocholine was no more produced. Therefore DTNB was not transformed in TNB, the solution remained colourless. To measure cholinesterase activity accurately and precisely, the rate of hydrolysis of the substrate by the enzyme should be slower than the Ellman reaction between thiol groups and DTNB.
Caccioti et al. developed a multiplex-based 96-well system for the detection of malathion [
58]. Threee different biopolymers were tested for the immobilisation of AChE (i.e. polylactic acid (PLA), polycaprolactone (PCL), and poly-hydroxybutyrate-co-hydroxyvalerate (PHBV)). Biopolymeric electrospun fibrous materails are ecodesigned supports. Two methods of immobilisation were compared, physisorption and cross-linking with glutaraldehyde. Finally the biopolymer PHBV was selected as the best support to immobilise AChE by crosslinking method. The detection limit for paraoxon was 10 ng/ml. Kaur et al. developed an AChE biosensor for the detection of malathion [
59]. They also used the immobilisation of the enzyme onto the eggshell membvreane by cross-linking with glutraraldehyde. The detection limit was reported at 0.1 ng/ml.
One of the currently used support material to build colorimetric biosensors was paper (ie. Cellulose). Indeed it was a a low-cost and easy to use platform for the development of diagnostic tests [
92]. Paper support was biomolecules friendly. Immobilisation of enzymes was simple and non-selective adsorption was limited. The paper allowed the development of microfluidic systems, lateral flow assays that could allow the separation of different components. Examples of paper -based colorimetric sensors will be presented below. The colorimetric paper-based sensor sensor developed by Kavruk et al. was not enough sensitive, with a detection limit at 2.5 µg/ml [
62]. The enzyme was physically adsorbed on paper filter with the substrate and some stabilisers (sugar (eg. Glucose) or protein (bovine serum albumine (BSA)) to improve the biosensor shelf-life.
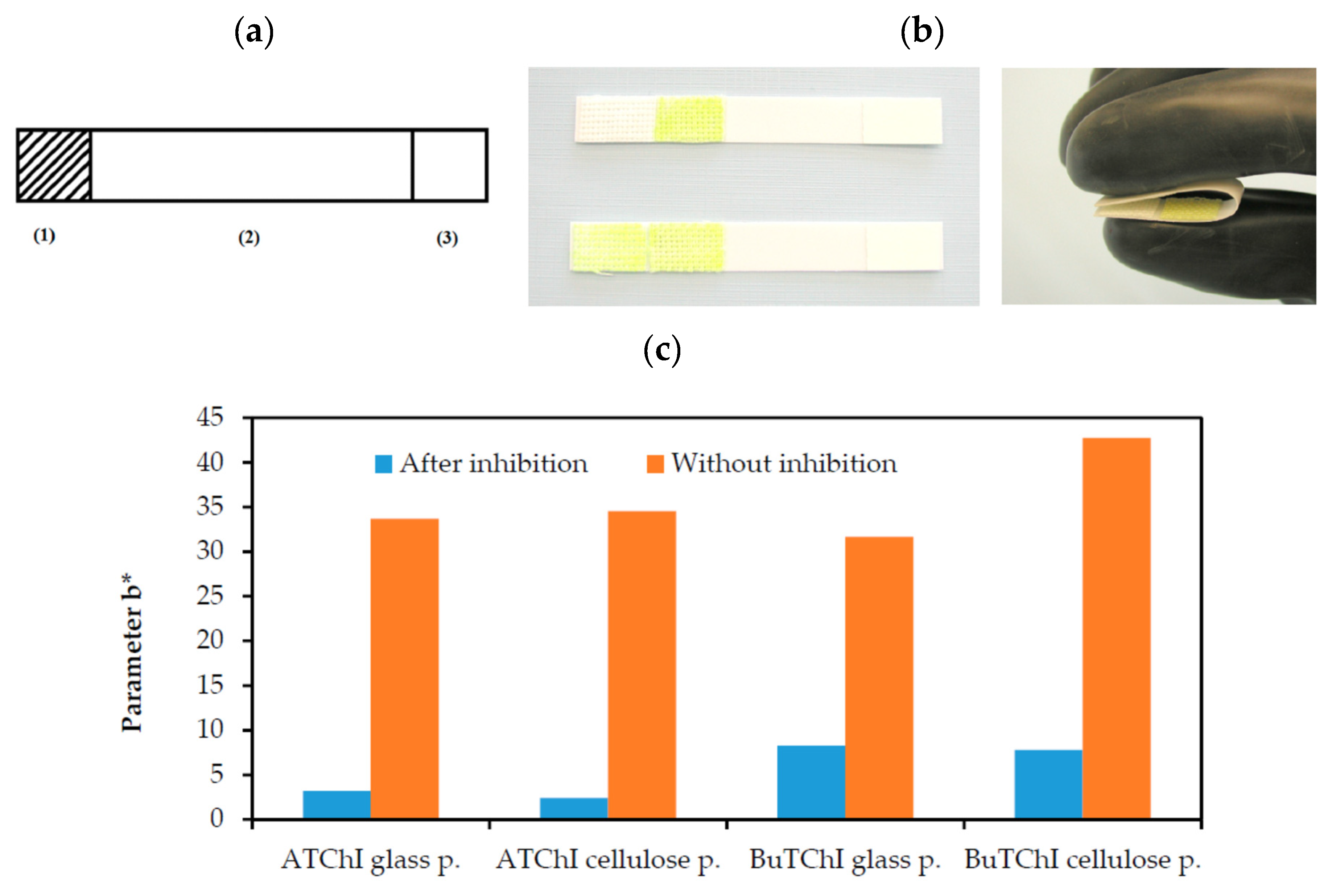
A team developed a paper-based sensor comparing different enzymes (AChE and BChE) and different support materials indicator (glass and cellulose) [
93]. This method used the Ellman’s reagent to detect the production of thiocholine. The fabrication of the sensor is presented in
Figure 5. All the reagents were deposited on the paper-based sensor on two different places (
Figure 5a). They were put in contact by folding the strip (
Figure 5b). The yellow colour was visible on the strip when the sample did not contain inhibitors. The comparative results between the two enzymes and the two different supports (glass and cellulose) were presented in
Figure 5c. The inhibition cause by the presence of phytostigmine was clearly measured in all cases. The best results were obtained with the enzyme AChE (LOD 0.0005 µg/ml).
Metal Organic Frameworks (MOFs) are crystalline porous nanomaterials constituted of self-assembly of inorganic metal centers (metal ions or metal clusters) (eg.iron, zinc, nickel, copper, zirconium, …) and bridging organic ligands (eg. imidazolate) [
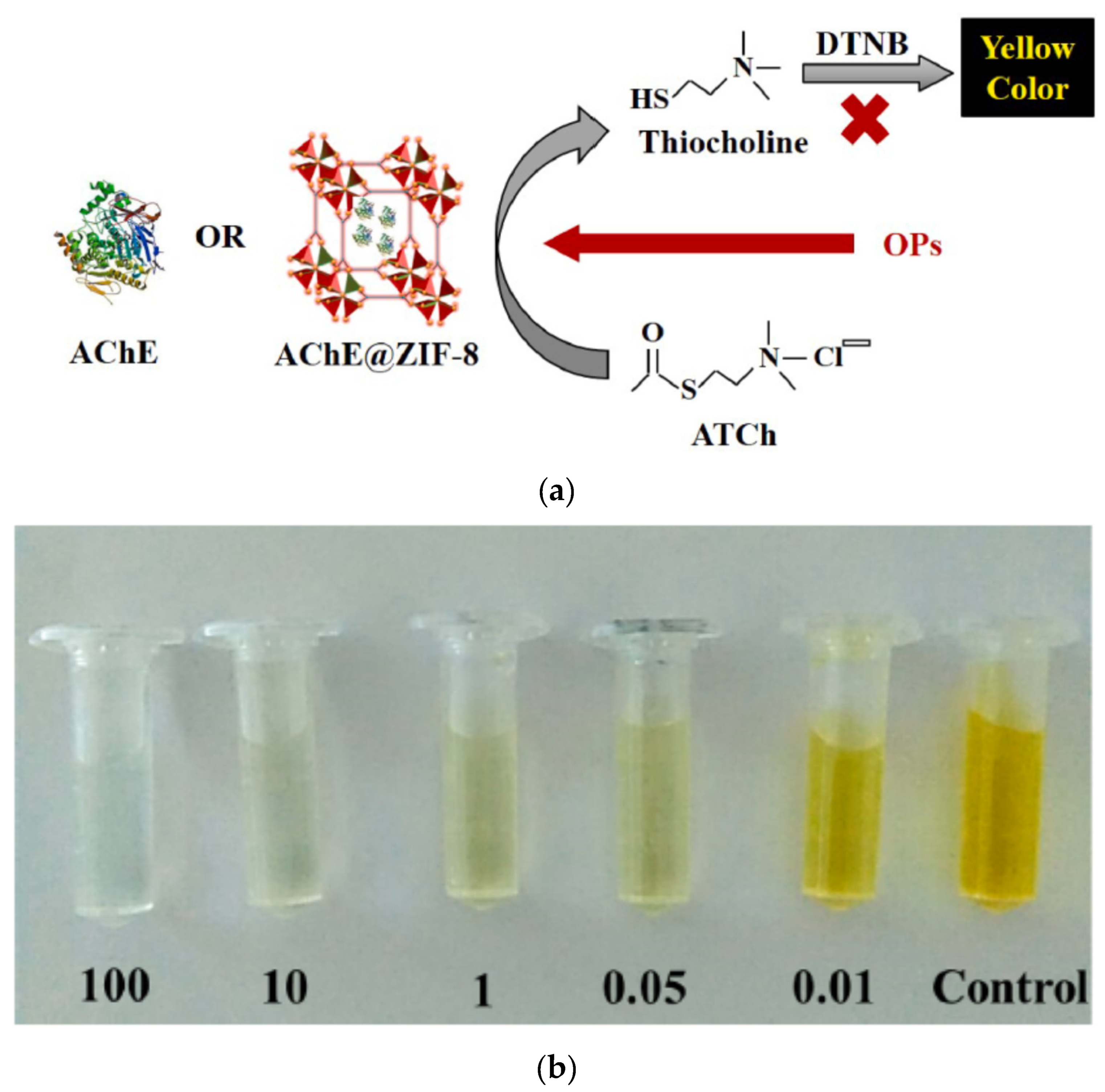
94]. The interesting properties of MOFs that could be exploited to develop sensitive enzymatic biosensors are their high surface area, porosity, and the reversible encapsulation/release of guest molecules like enzymes. The encapsulation of enzymes into MOFs could increase their stability, and enhance their catalytic activity. Kukkar et al. developed an acetylcholinesterase-based biosensor where the enzyme was encapsulated into zeolitic imidazolate framework (ZIF)-8 to produce a nanoreactor AChE@ZIF-8 NR (
Figure 6a) [
57]. They showed that the biosensor combining AChE and ZIF-8 showed a 10-fold increase in biocatalytic activities compared to the native AChE-based biosensor. The substrate DTNB was used for the colorimetric determination (λ = 412 nm) using UV–visible spectrophotometer. The change of colour in relation to the different glyphosate concentrations from 0.01 to 100 ng/ml could be visible by naked eyes (
Figure 6b). This biosensor was able to detect seven different OPPs. The LOD for three pesticides (glyphosate, glufosinate and malathion) were estimated almost identically as 10
-5 ng/ml. This colorimetric biosensor was developed in solution. Their perspective was to develop a paper-based biosensor strip based on the same principle.
The Ellman’s method faced some drawbacks such as the low stability of DTNB and the existence of interferences with substances containing thiol groups (eg. Amino acids (cysteine), reduced glutathione) [
95].
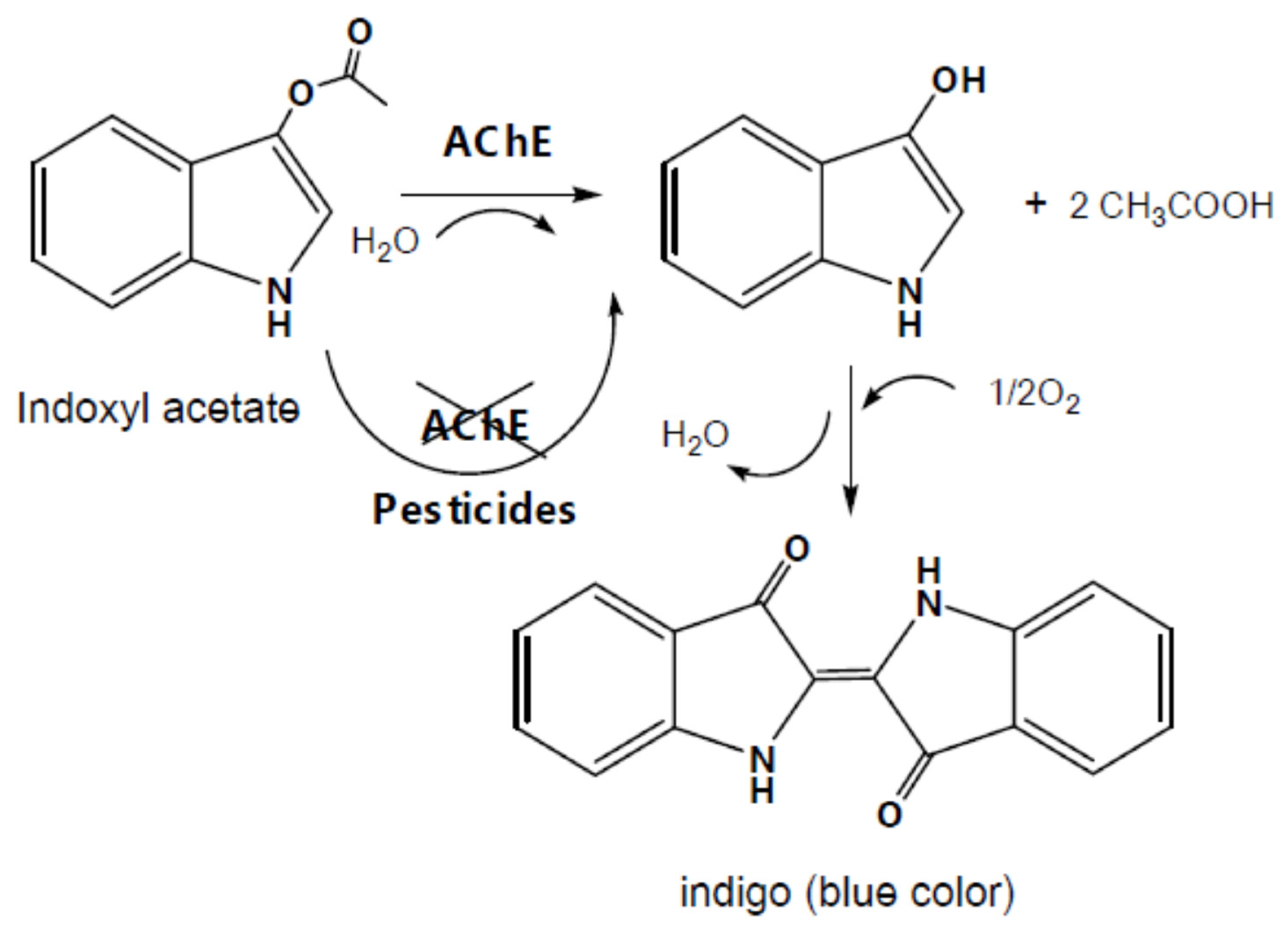
3.1.2. Indoxyl acetate (IDA), 2,6-dichloroindophenol acetate (DCIP) and indophenyl acetate (IPA)
The substrate indoxyl acetate (IDA) was a fluorogenic as well as chromogenic reagent acting also as a substrate of AChE and butyrylcholinesterase. Indeed IDA was hydrolysed in the presence of the enzyme AChE and H
2O to form a single indoxyl radical and acetic acid (
Figure 7). Then two single indoxyl radicals reacted together to form at the end (2,2ʹ-Biindoline)-3,3ʹ-dione (Indigo, blue). The change of colour from colourless to blue could be detected optically at 670 nm.
Pohanka et al. proposed an alternative chromogenic substrate to DTNB, the 2,6-dichloroindophenol acetate (DCIP), a blue redox dye (λ = 606 nm) [
95,
97]. In acidic medium, the blue DCIP (oxidised) was transformed into 2,6-dichloroindophenol that is colourless (reduced). They reported that no interferences with reduced glutathione and albumin were observed in the tested conditions. However ascorbic acid can interact with the detection because DCIP was initially used for the detection of ascorbic acid (ie. reducing agent). The highest drawback of this method was that the reaction is much longer than E”lman’s method because of a low turnover rate. The advantage of DCPI was that the change of colour is more intense than the one of Ellman’s reagent, so easier to measure.
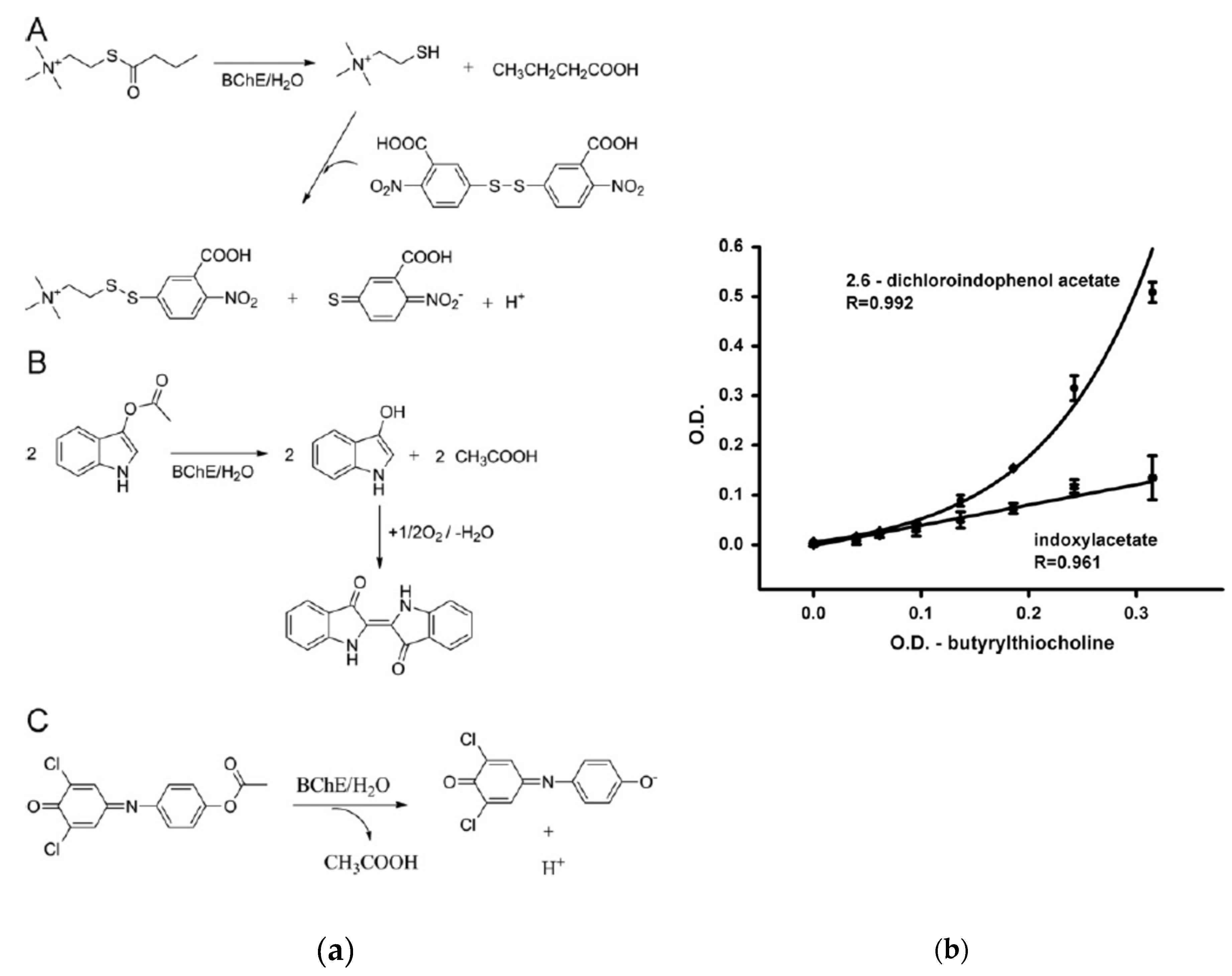
Pohanka et al compared three different substrates (DTNB, DCIP and IDA) to develop a biosensor for the detection of pesticides, based on BuChE (
Figure 8) [
97]. DCIP was reported as the best substrate because the lowest LOD was obtained and the biosensor was less sensitive to potential interferents.
The colorimetric reading could be performed visually, with a classical spectrophotometer or more recently with a smartphone. Reading with smartphone is more and more developed for field applications (ie. water quality monitoring [
98]). Smartphone reading could be based on smartphone applications [
74,
99] or softwares [
77] that could interpret the images taken by the smartphone.
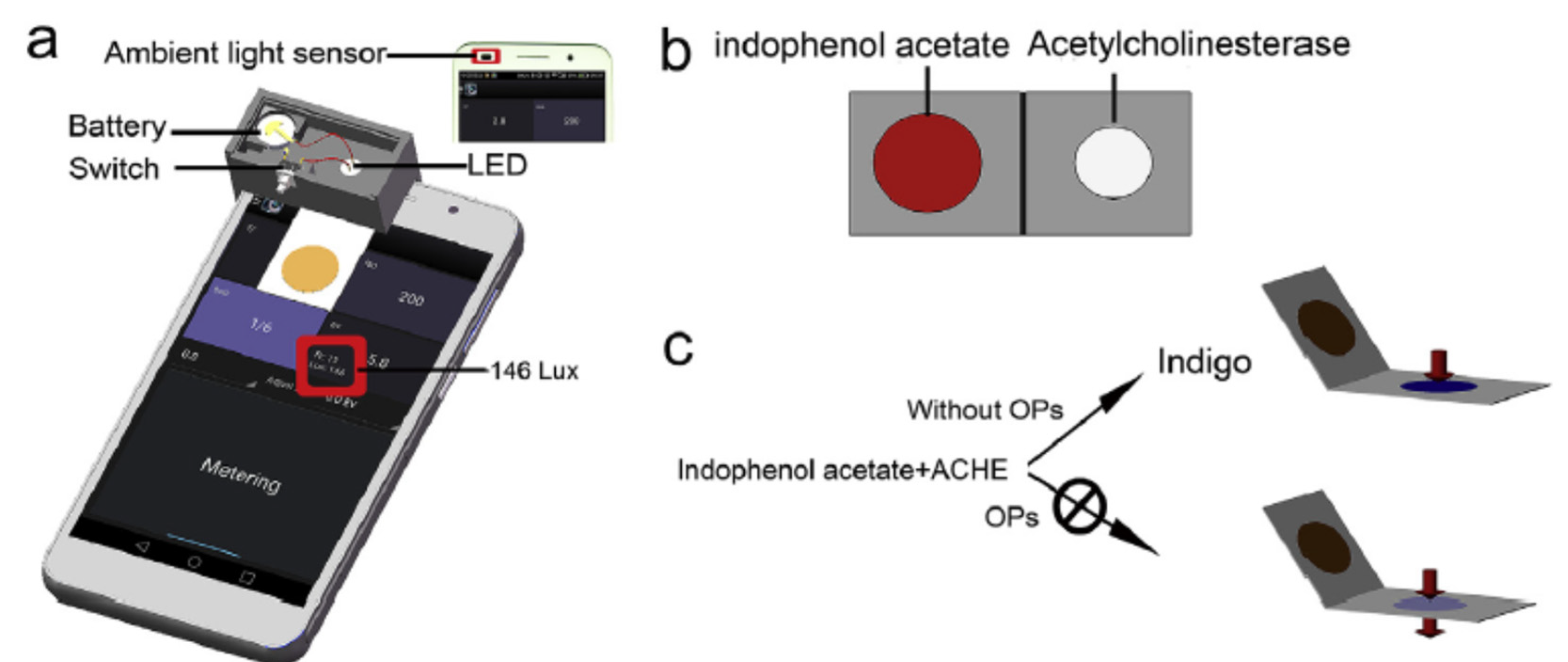
While classical spectrophotometer are benchtop equipment, the main advantages of smartphone reading are simplicity and portability. Digital camera based analytical devices like smartphones are widely used in everyday life, used and usable by everyone, at reasonable cost. Finally this technology is easily applicable to paper-based biosensors, lateral-flow immunoassays (LFIA) and other types of biosensors. Therefore smartphone reading are really a promising tool for hand-held assays, especially for monitoring pesticide residues in food products.
Indophenyl acetate (IPA) was used for the determination of AChE activity since the 1950’s. The substrate IPA was hydrolyzed by AChE at pH 8.0. The change of colour was from red-yellow to blue purple) [
53]. This simple method was optimised (eg. Substrate concentration, pH, time before absorbance measurement) and was reliable to measure between 25 to 150 units of acetylcholinesterase. More recently, some colorimetric biosensors based on AChE were developed based on IPA. For instance, Fu et al. developed a colorimetric biosensor based on a dipsticks and a smartphone reader for the detection of chlorpyrifos in cabbage [
63]. A colorimetric dipstick was produced, with two sides, one containing AChE and one the substrate IPA, that folded together to bring the reagents into contact, after the sample has been deposited (
Figure 9). The change of colour from red (indophenol acetate) to blue (indigo), was measured with a digital camera and a smartphone. The transmission light intensity was measured by the smartphone and the signal was interpreted by a free application. A LOD of 3.3 µg/ml was reported.
Nowadays, many smartphone readers used RGB principles for the colorimetric reading. RGB is an optical colour processing system, trichromy based on the three primary colours red, green and blue (RGB). A value between 0 and 1 is assigned to each of the 3 primary colours to define a colour that makes up an image.
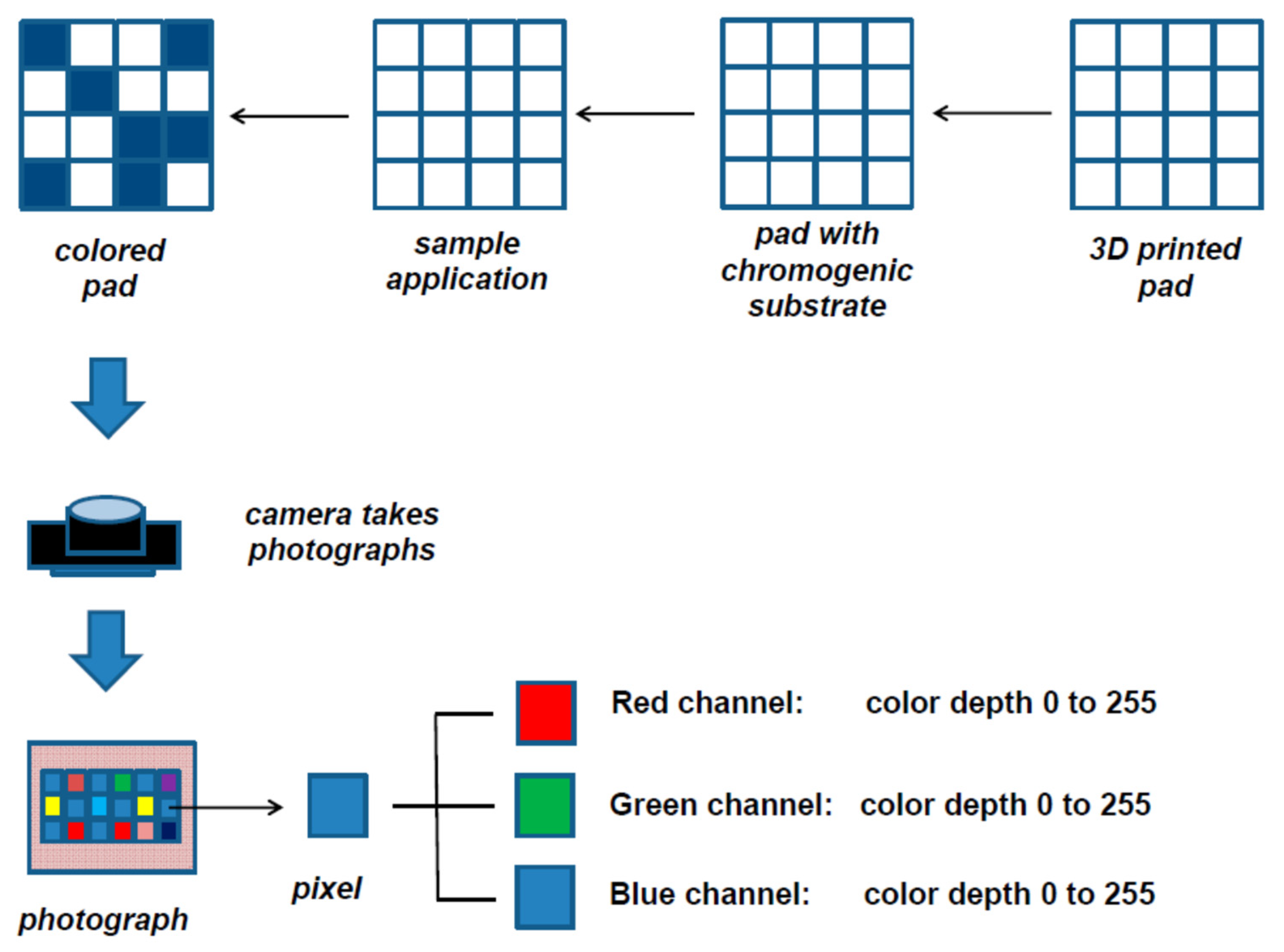
Pohanka et al. developed a biosensor for the detection of AChE and BuChE activity in plasma samples with a smartphone reading based on RGB principle [
100]. The substrate was deposited onto multiwell 3D-printed pad (
Figure 10). Then a smartphone (LED light included) was placed onto the pad, and a photograph in .jpg format was taken. A software was used to determine the colour depth of each well. The R value equal to 0 is the darkest red, while the R value 255 is the brightest red. They concluded that indoxylacetate provided a change in colour depth more than three times higher than that of 2,6-dichlorophenolindophenyl acetate. Amongst the three channels, the R channel appeared to be the most sensitive regarding the calibration curves, showing the lowest LOD. The readings were performed in parallel with a classical spectrophotometer to validate the smartphone readings. They observed no significant difference between BuChE activity determined by spectrophotometric method and smartphone reading.
3.1.3. Hestrin
The Hestrin method was an alternative to the Ellman method for visualising AChE inhibition [
101]. The main difference between the two methods was that the analytical signal depended on the concentration of the substrate (acetylcholine chloride) in the Hestrin method, whereas it depended on the concentration of the reaction product in the Ellman method. In the Hestrin method, acetylcholine was determined by its reaction with hydroxylamine at an alkaline pH, in the presence of ferrous ions. To our knowledge, this method was very little implemented to develop enzymatic biosensors for the detection of pesticides. This method was applied once to the monitoring of blood cholinesterase activity in workers exposed to organophosphate pesticides (nerve agents) [
102]. The conclusion was that the results obtained with this method were not very reproducible or valid.
3.1.4. TMB
The Horseradish peroxidase (HRP) enzyme catalysed the hydrolysis of substrates in the presence of hydrogen peroxide (H2O2). The substrate 3,3′,5,5′-tetramethylbenzidine (TMB) was the most currently used chromogenic substrate for the enzyme HRP. TMB was a colourless or pale blue-green substrate. After the action of HRP, TMB took on a blue colour whose absorbance could be measured between 620 and 655 nm.
A first alternative to HRP was based on the acetylcholine (ACh) itself mimicked peroxidase’s activity. A colorimetric system containing ACh, TMB and H
2O
2 was developed for the sensitive and selective determination of AChE activity and detection of its inhibitors. Therefore due to the catalysis of ACh by AChE, the peroxidase-like activity was affected and the signal decreased. This phenomenon was exploited for the detection detection of AChE activity with a LOD of 0.5 mU/ml for AChE activity and 4 ng/ml for OPP detection [
103].
A second alternative to HRP is to exploit the enzyme-like activity of some nanomaterials, called nanozymes, in combination with TMB. The catalytic properties of MnO
2 nanoparticles were frequently used for the development of colorimetric sensors. For instance, a colorimetric biosensor based on AChE was developed based on the oxidation power of manganese dioxide (MnO
2) nanosheets onto the substrate TMB (ie. mimic oxidase activity) [
104]. Indeed thiocholine (TCh) was able to reduce the oxidation power of MnO
2 nanosheets. Therefore less oxTMB were produced that resulted in decrease in the absorbance (λ = 652 nm) (ie. light blue to colourless). In the presence of an inhibitor, less TCh was generated. As a result, the blue colour was stronger and the absorbance increased. The combination of MnO
2 nanoflakes (NFs) and TMB was also used to develop a colorimetric bi-enzymatic sensor. Jin et al. developed a multienzyme-hydrogel sensor based [
50]. Firstly AChE catalyzed the hydrolysis of ACh and then choline is oxidised by choline oxidase (ChO) to produce H
2O
2. As a consequence, MnO
2 NFs lost their oxidation power and the oxidation of TMB was blocked. In the presence of an inhibitor, the production of H
2O
2 was reduced and MnO
2 nanoflakes recovered their oxdidation power and so oxTMB was produced (strong blue). The detection limit obtained for paraoxon was of 0.5 ng/ml. The sensor was able to detect 3 µg/ml of paraoxon in apple-banana juices by naked eyes reading. Jin et al. developed a multi-enzyme colorimetric biosensor for the detection of parathion-methyl based on the peroxidase-mimick activity of cobalt oxyhydroxide nanoflakes (CoOOH NFs) which catalyzed TMB into blue oxTMB with the help of H
2O
2 [
77]. Again the bi-enzymatic system was constituted of AChE and ChO. This biosensor was able to detect parathion methyl as low as 10 ng/ml.
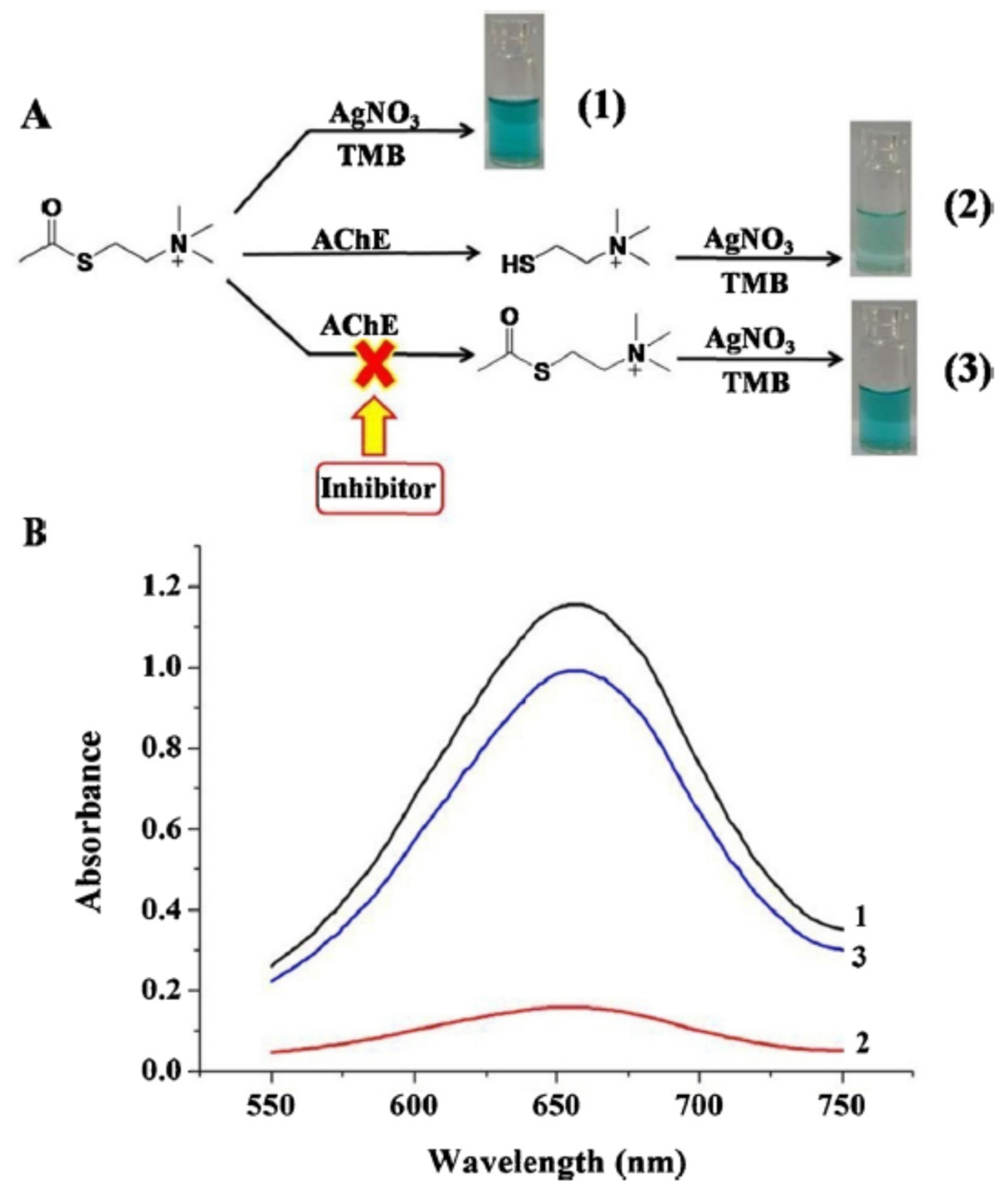
A colorimetric assay based on the combination Ag [I] ion–TMB was developed for the detection of inhibitors of AChE [
105]. Ag [I] ions were able to oxidise TMB (oxTMB (deep blue)). When AChe was introdiced, TCh produced by the enzymatic reaction reacted with Ag [I] ions. Therefore Ag [I] ions could not oxidise TMB and a pale blue colour was observed (reduced TMB (colourless)) (
Figure 11). In the presence of an inihibitor, the amount of TCh decreased and therefore the reaction with Ag [I] ions also decreased. Ag [I] ions could oxidize TMB and the colour was then be a deeper blue (oxTMB).
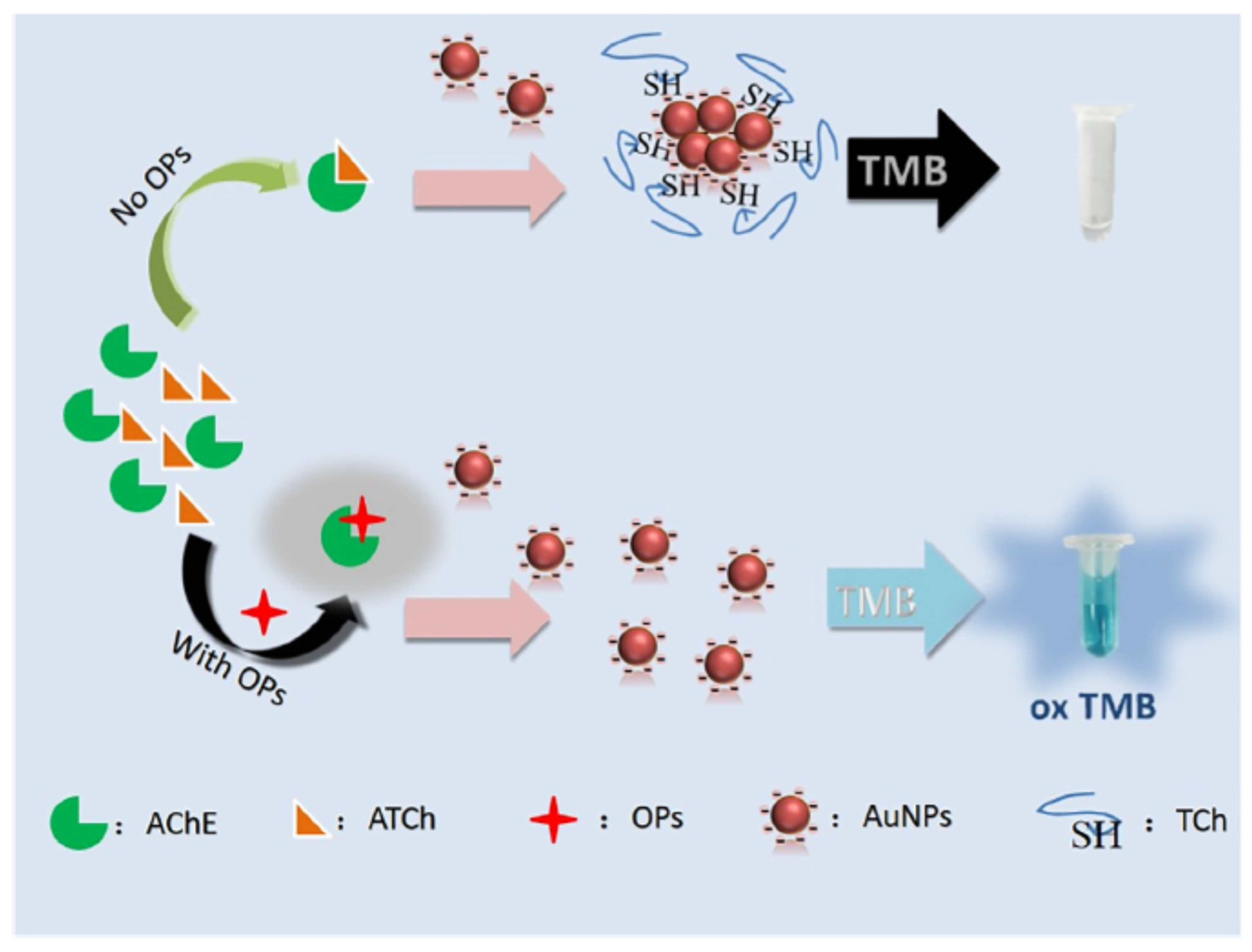
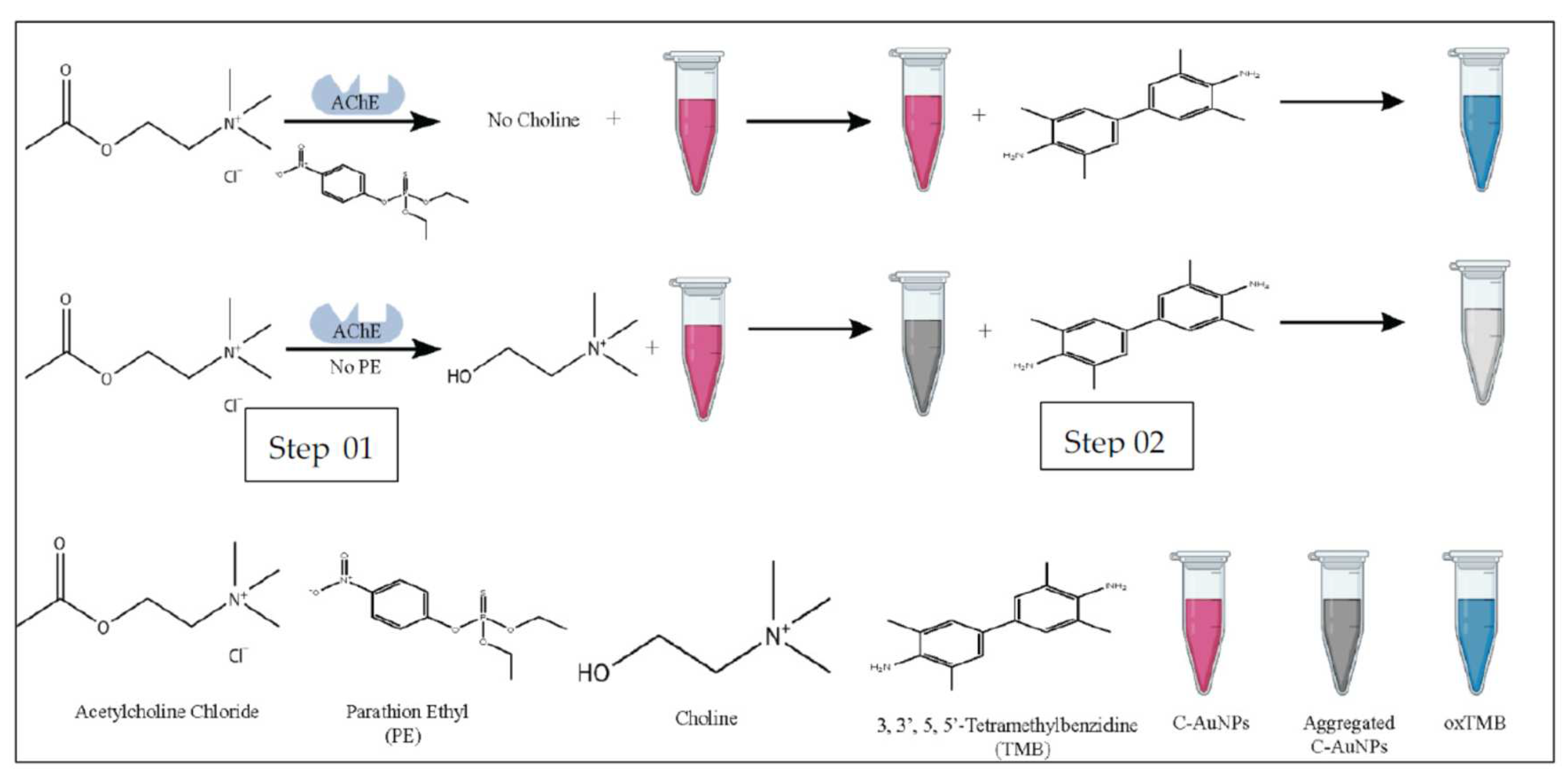
Gold nanoparticles (AuNP
S) were frequently used for the development of colorimetric sensors, especially in combination with TMB. Liu et al. developed an acetylcholinesterase assay for the detection of parathion methyl in water [
74]. When not aggregated, AuNPs could oxydise TMB. In the presence of TCh, the aggregation of AuNPs was induced and TMB remained not oxidised (colourless) (
Figure 12). When a pesticide inhibited the enzymatic activity, TMB could be oxidised by AuNPs because TCh is not produced. This biosensor showed a detection limit of 0.21 ng/ml.
Negatively charged cysteamine capped gold nanoparticles (C-AuNPs) were used to develop an AChE based colorimetric sensor for the detection of parathion ethyl [
76]. The peroxidase-like activity of C-AuNPs was exploited in combination with colorimetric detection with TMB. In the presence of AChE, choline reacted with C-AuNPs, which prevents the oxidation of TMB (colourless) (
Figure 13). In the presence of parathion ethyl, lesser choline was produced, the non aggregated C-AuNPs could oxidise TMB which gave a deep blue colour. The increase in the absorption at 652 nm was directly proportional to the concentration of pesticide. The LOD was reported at 5.8 ng/ml.
3.1.5. Others
The dye bromothymol blue was used to develop an AChE paper based colorimetric sensor for the detection of ofatox in tea [
72]. Test cards were prepared by spraying AChE directly onto paper filters, as well as enzyme substrate and bromothymol blue onto three different paper filters. Then the three papers were stacked to gether and pressed between two metallised PET film sheets. A hole is performed onto the test card to drop the sample. The LOD of the test card for Ofatox in tea was 0.1 ppm (µg/ml).
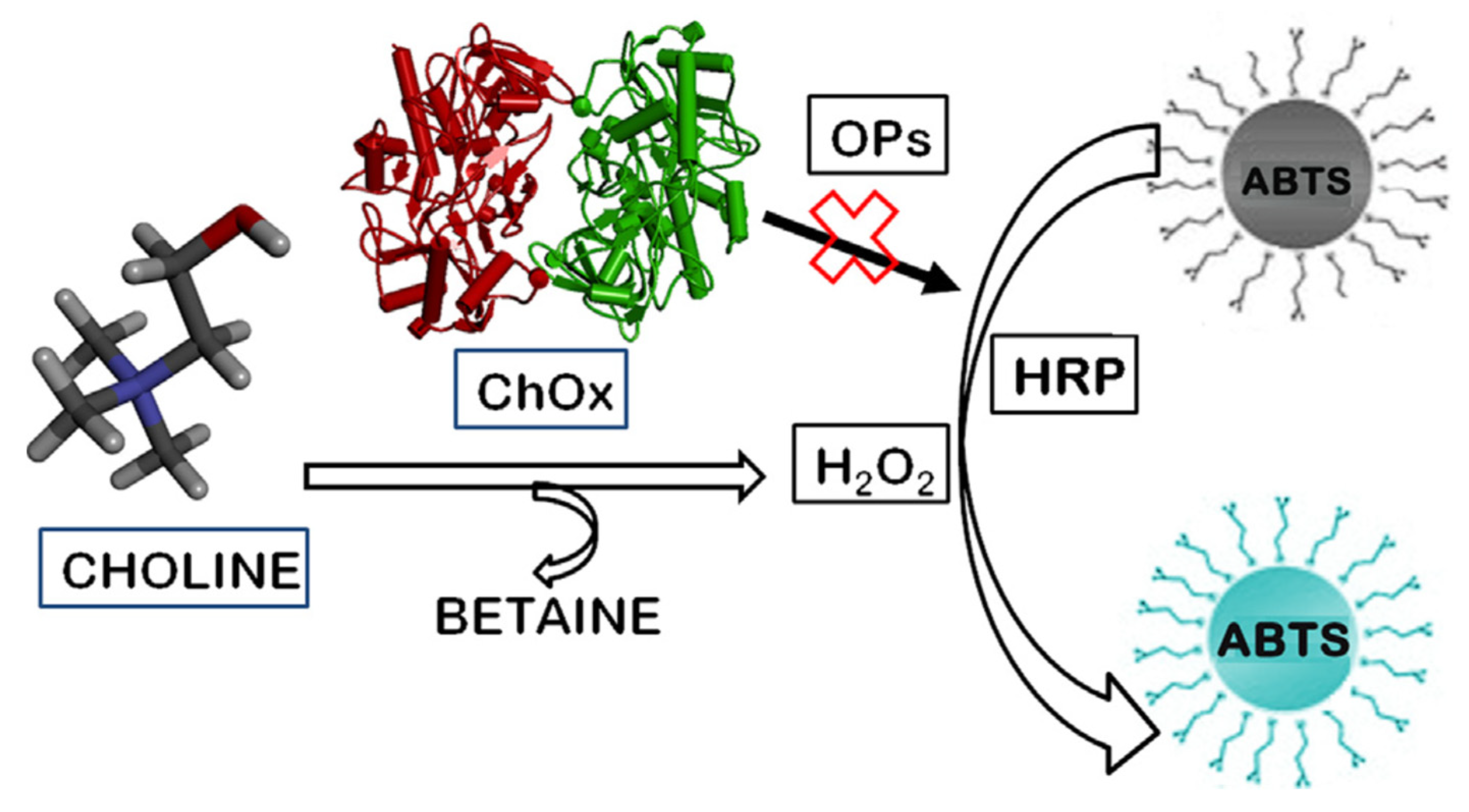
Another chromogenic substrate was used for the detection of pesticides, using the inhibition of another enzyme, ChOx (
Figure 14) [
42]. ChOx oxidised choline to betaine and H
2O
2. In the presence of the enzyme HRP and the generated H
2O
2, a dye, 2,20-azino-di-[3-ethylbenzthiazoline sulphonate] (ABTS) was oxidised. Then the absorbance increased at 734 nm and the dye changed from colourless to blue-green. In the presence of a pesticide, paraoxon methyl, the enzymatic activity of ChOx was inhibited which reduced the colour change and consequently the absorbance. The LOD was reported at 58 µM.
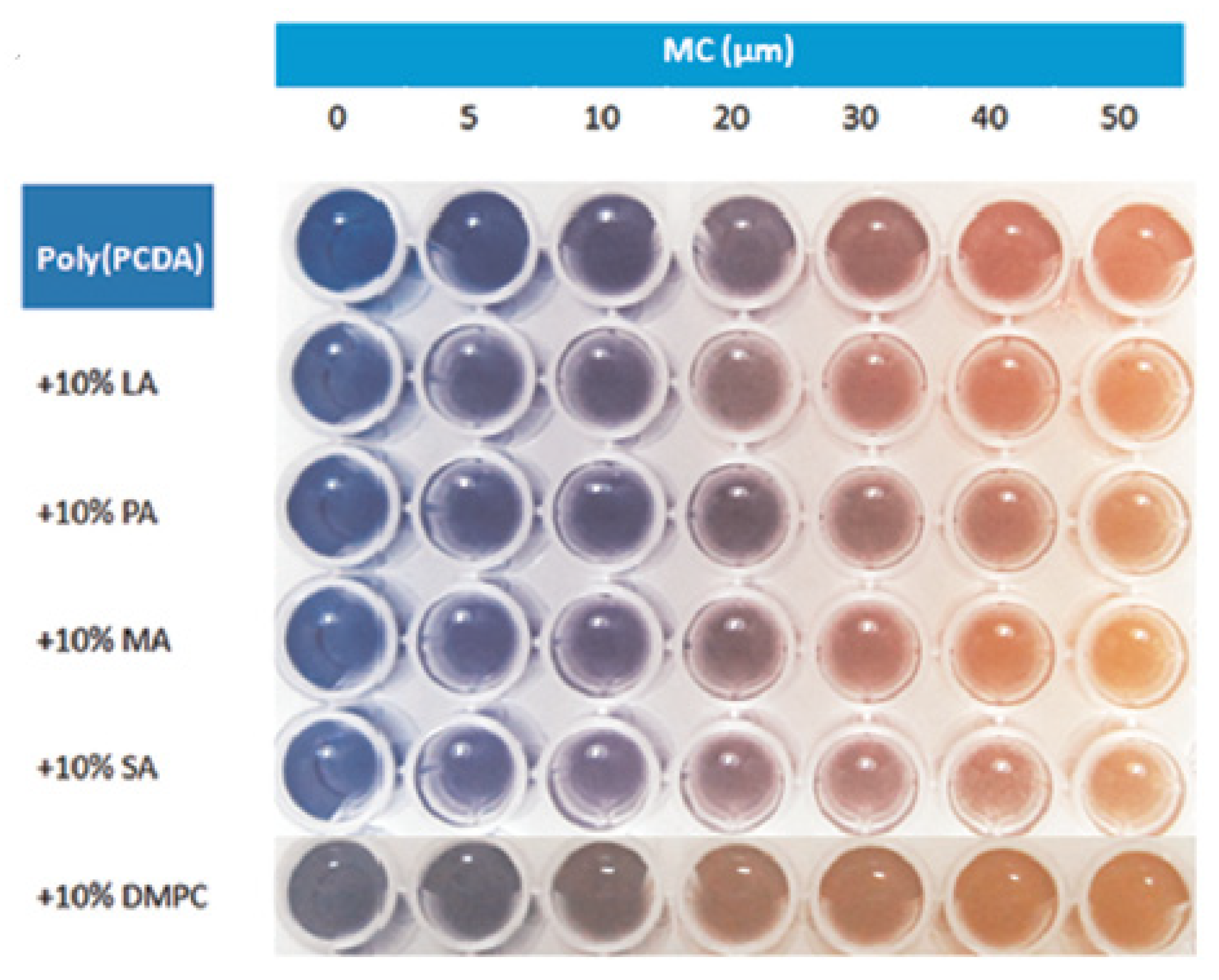
Polymers or nanofibres are often considered as promising alternative materials that are widely applied for colorimetric and fluorescence detection. Poly(10,12-pentacosadynoicacid) (poly-PCDA) vesicles were used to develop a colorimetric enzymatic biosensor based on the induction of the polymer by myristylcholine, which was one of the substrates of AChE [
78]. It was hypothetised that cationic surfactants like esters of choline could induce the colour transition of poly-PCDA. Indeed in the presence of myristylcholine, the colour transition of the polymer from blue to red induced by myristylcholine occurred (
Figure 15). Therefore when there is no pesticide, choline is produced, less myristylcholine is available; so the colour transition did not occur or decreased. In the presence of the pesticide, choline was not or less produced, more myristylcholine remained. Therefore the colour transition occurred. When poly-PCDA was used without lipids, the observation by naked eyes could only detect dichlorvos at 150 ppb (ng/ml) which is not enlough sensitive. Therefore they produced some poly-PCDA vesicles by mixing the polymer with different lipids. The lipid dimiristoylphosphatidyl-choline (DMPC) gave the best results with a LOD for dichlorvos of 6.7 ng/ml. Furthermore they were able to detect 50 ng/ml of dichlorvos by naked eyes with poly-PCDA vesicles which is three times more sensitive than with poly-PCDA alone.
3.2. Nanomaterials based
3.2.1. Gold and silver nanoparticles
The following optical properties of gold nanoparticles were very interesting for the development of colorimetric biosensors: good optical characteristics, high electron densities, good biocompatibility and catalytic performance [
106]. The change of colour between aggregated and disaggregated state of NPs was exploited to develop colorimetric biosensors, without the needs of other colorimetric substrates. In solution, dispersed AgNPs were yellow in colour, upon aggregation the nanoparticles appeared orange/red. Dispersed AuNPs in solution were red in colour, upon aggregation the nanoparticles appeared blue. AgNPs extinction coefficients were higher than those of AuNPs of the same average size [
107]. Yet AuNPs were more commonly used because the functionalisation of AgNPs often led to their degradation; AuNPs were more stable. Furthermore colour changes of AuNPs could be easily observed visually compared to AgNPs [
107].
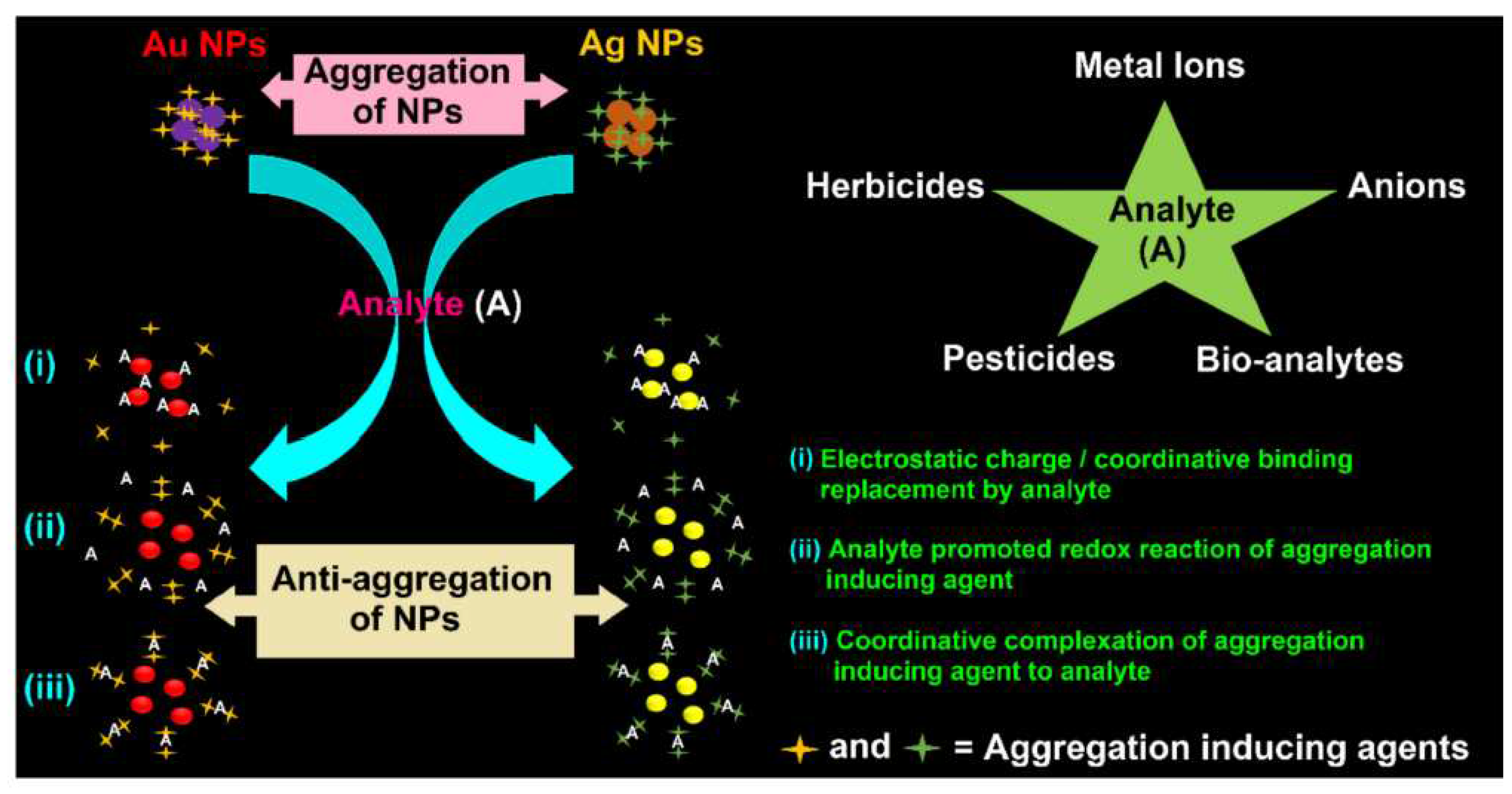
Some chemical substances (eg. Pesticides, herbicides, metallic ions) could prevent the aggregation of AgNPs and AuNPs.
Figure 16 shows how these analytes could interact with the gold and silver nanoparticles [
108]. The nanoparticles could aggregate in the presence of inducing agents. In the presence of the analyte, an anti-aggregation effect of the analyte occurred. Three different principles are illustrated: i) the anti-aggregation effect was caused by the replacing of the inducing agent by the analyte to the NPs, due to a repulsing effect of the inducing agent; (ii) the anti-aggregation effect occured because the analyte promoted the redox reaction of aggregation inducing agent and (iii) the anti-aggregation effect was due to the complexation of the analyte with the aggregation inducing agent.
For instance, thiocholine could induce the aggregation of AgNPs and AuNPs. A colorimetric sensor based on AChE and graphene quantum dots capped gold-nanocomposite particles (GQD-AuNPs) for the detection of chlorpyrifos was developed [
82]. The three-dimensional microfluidic paper-based analytical device (3D-μPAD) reached a very low LOD (0.0007 µg/ml). Another colorimetric sensor was based on the aggragation of AgNPs on graphene oxyde (GO) leaves in the presence of TCh [
80]. A LOD as low as 0.01 pM was reported for malathion in water and grapes.
Three different ways could be used to induce the aggregation of gold nanoparticles: addition of capping agent on the surface of AuNPs (e.g. thiols, amines, phosphines), surface functionalization (e.g. macromolecules, polymers) and addition of salt (e.g. NaCl) [
107]. High salt concentrations result in the aggregation of AuNPs. They can be used to enhance the stability of colloid AuNPs.
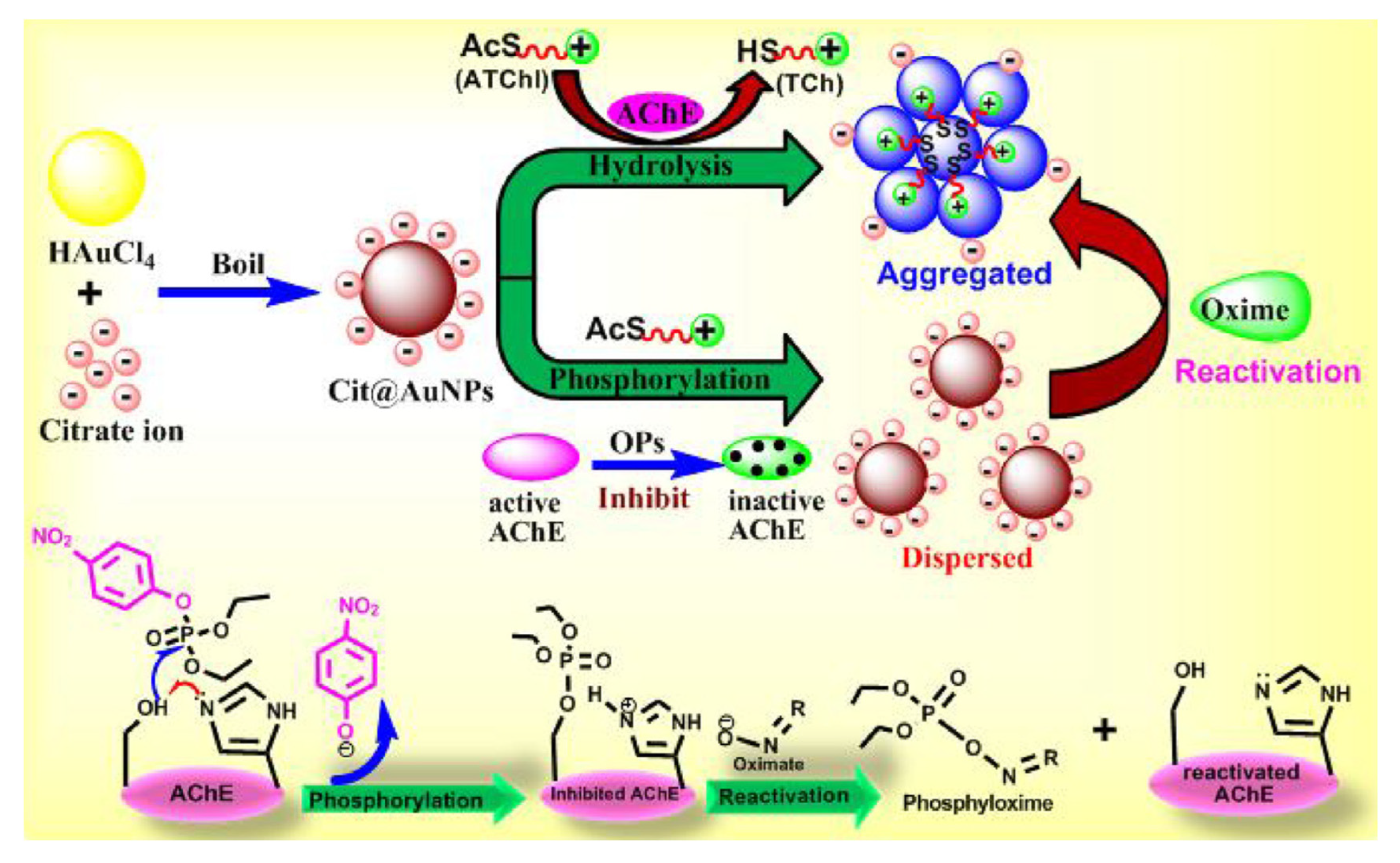
A colorimetric AChE-based biosensor was developed using citrate capped AuNPs for the detection of several OPP in water samples (
Figure 17) [
83]. Gold nanoparticles were aggregated when TCh was produced by the catalyctic activity of AChE. Indeed positively charged TCh was attracted by the negatively charged citrate groups located on the surface of AuNPs. On the contrary, AuNPs were dispersed in the presence of the pesticide because thicholine was not produced. The lowest LOD of 0.13 ng/ml was reported for paraoxon in water.
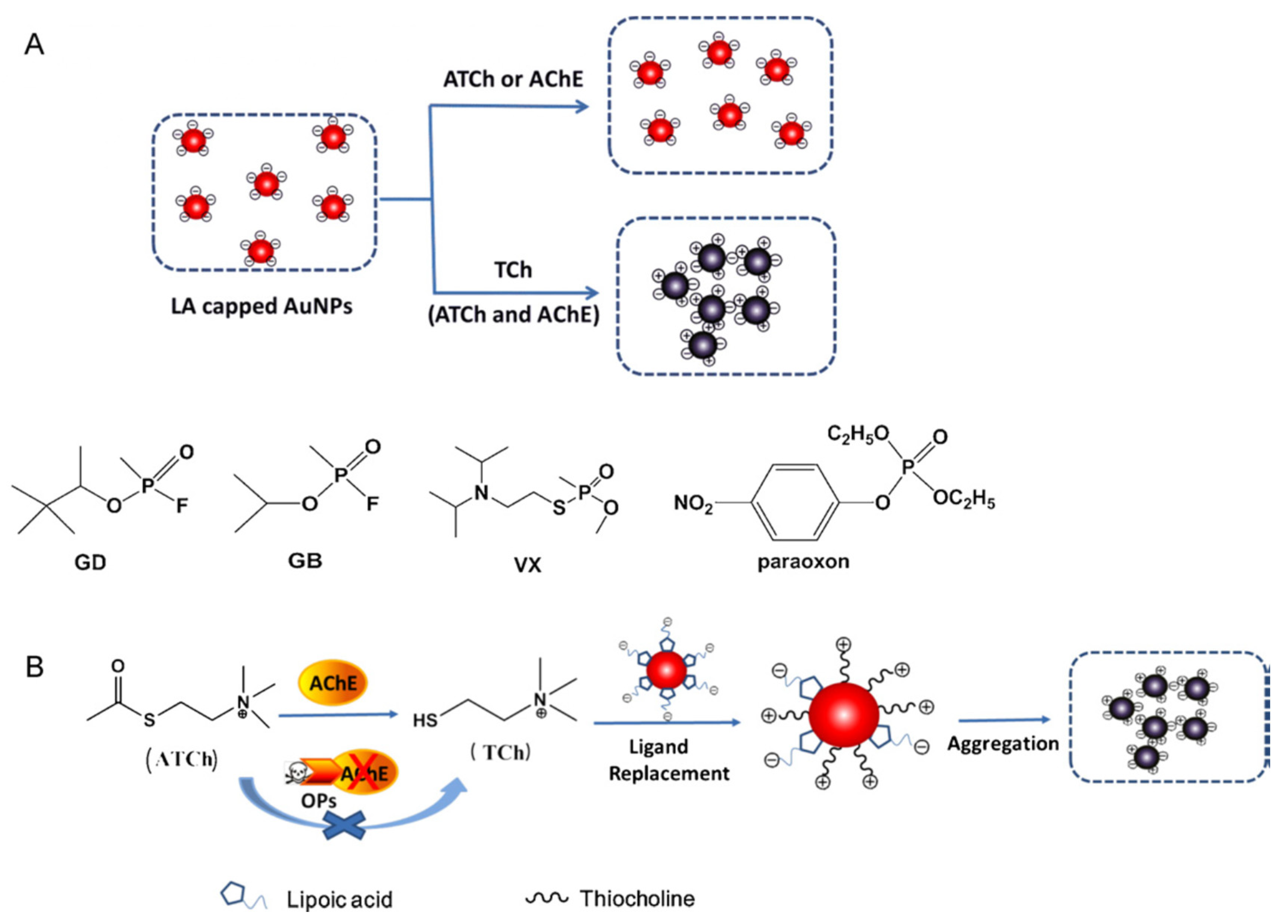
The aggregation potential of lipoic acid capped gold nanoparticles was used to develop a colorimetric sensor for the detection of paraoxon in apple juice (
Figure 18) [
87]. Thiocholine could induce the aggregation of LA capped AuNPs along with a distinct colour change from red to steel-blue. In the presence of a pesticide, TCh was no more produced, so that AuNPs could not aggregate anymore and the colour did not change. The reported LOD of 4.52.10
4 pM was very low.
Localized surface plasmon resonance (LSPR) is an optical phenomena generated by light when it interacts with conductive nanoparticles (NPs) [
109]. The adsorption of molecules (ie. pesticides) to the surface of nanoparticles caused surface plasmon resonance changes that could be monitored. It was observed that wavelength shifts of the LSPR were accompanied by colour changes thatcould be measured also.
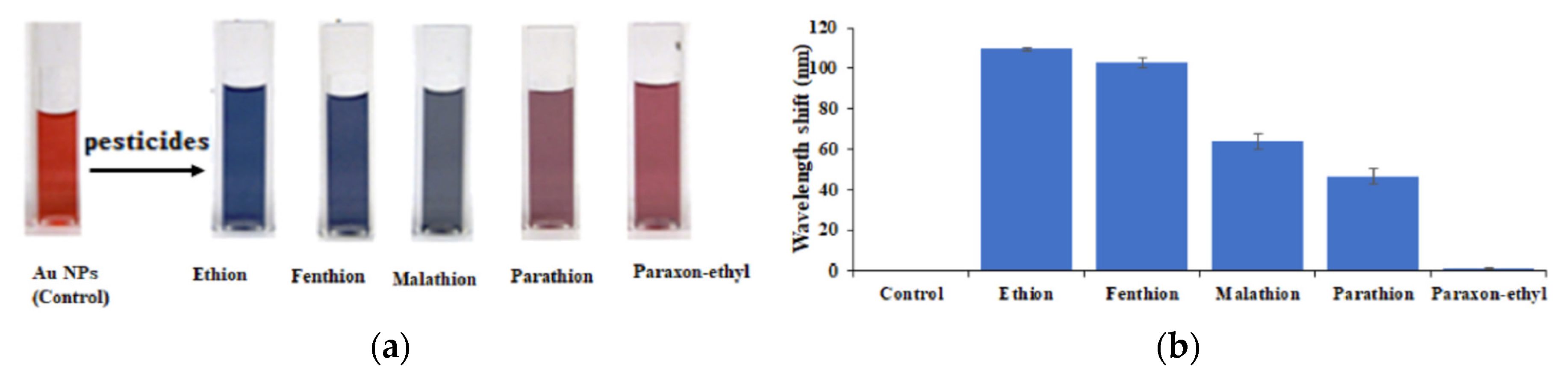
Figure 19 showed the colour changes in relation (a) to the different tested pesticides and (b) to the wavelenght shifts for the same pesticides [
110]. It was clear that the biosensor did not have the same detection sensitivity for all the pesticides tested. The best sensitivity was obtained for ethion and the worst for paraoxon ethyl.
Lv et al. developed an enzymatic biosensor based on a combination of ALP with gold and silver NPs [
85]. p-aminophenol (p-AP), the product of the catalytic activity of the ALP enzyme, was able to reduce Ag(I) ion to Ag metal that was spontaneously deposited on the surface of AuNPs to form Au@Ag NPs. The formation of Au@Ag NPs led to a change of colour, from red to yellow first, and then to gray. This change of colour could be detected visually or by spectrophotometry. The LOD were reported as 0.025 μg/l and 0.036 μg/l for methamidophos and malathion respectively.
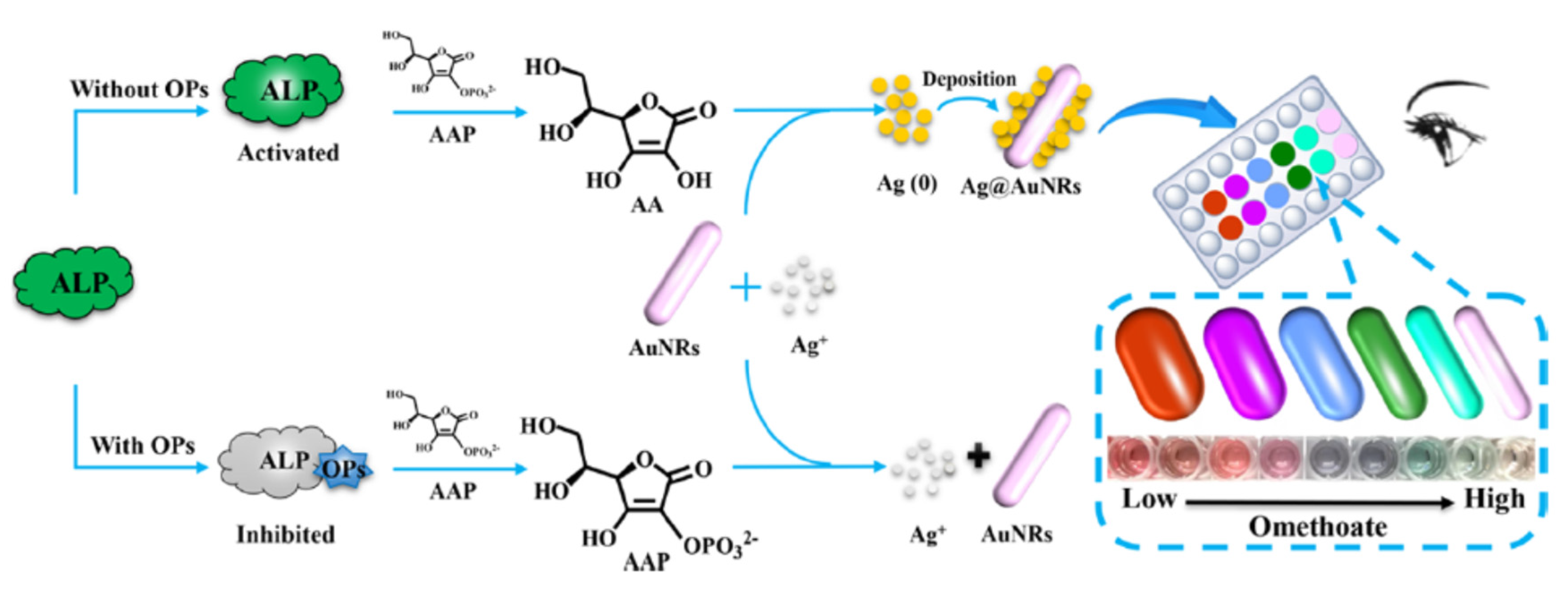
A colorimetric biosensor was developed based on the inhibition of the ALP-induced silver metallization on the surface of gold nanorods (Au NRs) in the presence of omethoate (
Figure 20) [
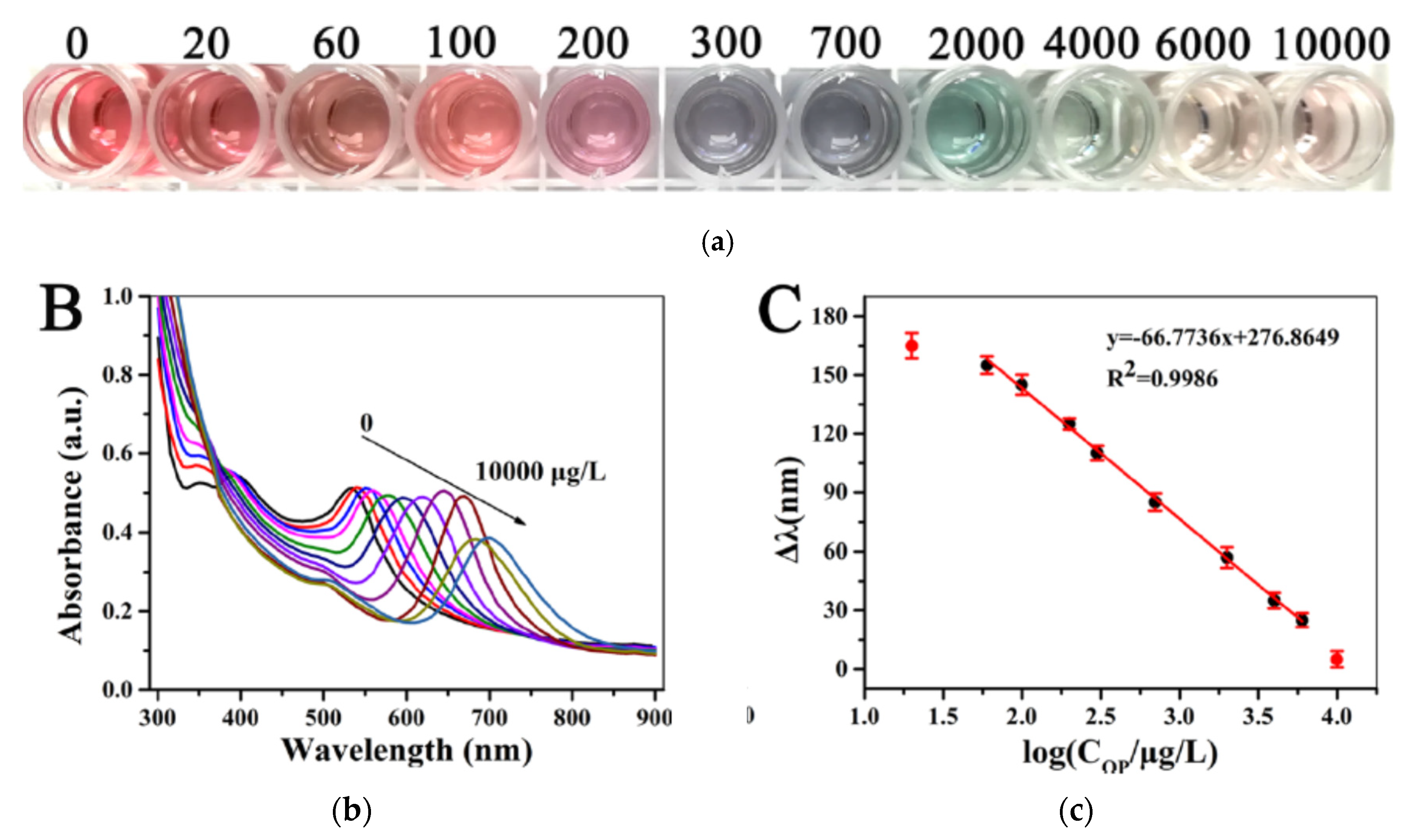
81]. The silver ions were reduced by ascorbic acid (AA), the product of the dephosphorylation of acid-2 phosphate (AAP) due to the catalytic activity of ALP. Then silver nanoshells were deposited onto the surface of AuNRs to form Ag@AuNRs. It resulted in a series of blue shifts on the LSPR peak. In the presence of omethoate, the catalytic activity of ALP was inhibited. Therefore silver ions were not reduced and so AuNRs were not modified. Rainbow-like multicolour changes from pink to green to cyan to violet to yellow-red could be observed that were correlated to the concentration of omethoate. Indeed the colour changes from the infrared to the visible range were related to the changes in the size, shape, and composition of Au NRs, in relation to the extent of Ag-shell deposition on the Au NRs. The actual changes of colour in the microwells in relation to the omethoate concentrations (from 20 to 10000 μg/l) could be visible by naked eyes (
Figure 21a). The wavelenght changes in the UV−vis spectra were visible in relation to the omethoate concentration (
Figure 21b). The wavelenghts difference were proportional to the concentration of omethoate and showed a good linear relationship (
Figure 21c). It was possible to semi-quantify the concentration of pesticide, even by naked eyes. The detection limit obtained with a microplate reader was low (83.2 ng/l).
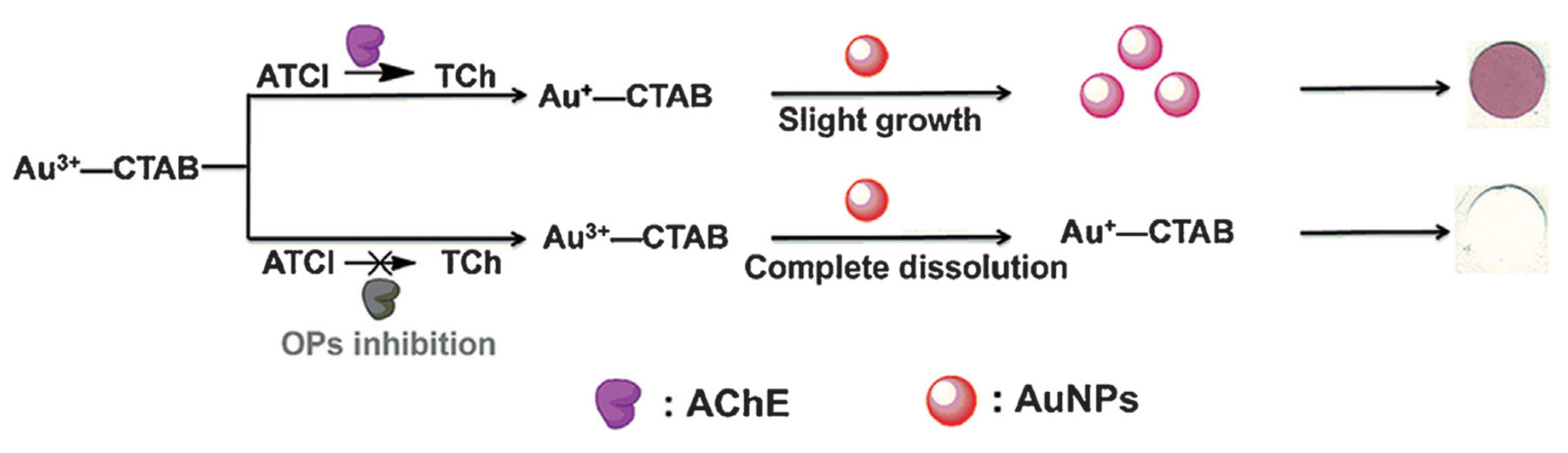
A gold nanoparticles-based paper colorimetric biosensor was developed for the detection of organophosphorous pesticides [
84]. This biosensor was based on the enzymatic hydrolysis reaction of acetylcholinesterase and gold nanoparticles (AuNPs) in Au
3+-cetyltrimethylammonium bromide (Au
3+-CTAB) solution-coated papers (
Figure 22). The property of Au3+ to dissolve the AuNPs was used, thus leading to a visible colour change. However when thiocholine was produced by AChE actibity, it reduced Au3+, preventing the dissolution of AuNPs and the change of colour. The role of CTAB was to stabilise the AuNPs and to prevent them from aggregation in high salinity conditions. The LOD obtained with the AuNPs-coated dipstick was 35 ng/ml for parathion.
3.2.2. Other nanoparticles
Manganese oxide nanoparticles (MnO
2) could be also used for the development of colorimetric biosensors. Qian et al. developed a biosensor based on MnO
2 NPs, UFO-shaped Au@MnO2 nanosheets and AChE inhibition by different OPP (chlorpyrifos, methyl, omethoate, bromophos-ethyl) [
79]. Thiocholine is a reducing agent for NPs. The colour of MnO
2 NPs changes from dark brown to light brown, while the colour of Au@MnO
2 nanosheets changes from green to pink. The LOD was 0.5 µg/l. The sensor was applied to the detection of OPP to grape juice and leaves (corn, rice, peanuts). Huang et al. developed an enzymatic biosensor based on alkaline phosphatase and a combination of gold and manganese oxide nanoparticles (ie. MnO
2-coated gold nanoparticles (GNP@MnO
2 NPs)) [
51]. p-aminophenol reduced the MnO
2 NPs to Mn
2+ ions., which broke the core-shell nanostructure GNP@MnO
2 NPs; thus the gold core is exposed to light. The colour changed from bright yellow (GNP@MnO2 NPs) to green (gold core). In the presence of malathion, the ALP activity was inhibited; thus the color change did not occur or more lightly A very low LOD of 0.82 pg/ml for malathion was reported. This method was applicable to actual samples of apple and lettuce.
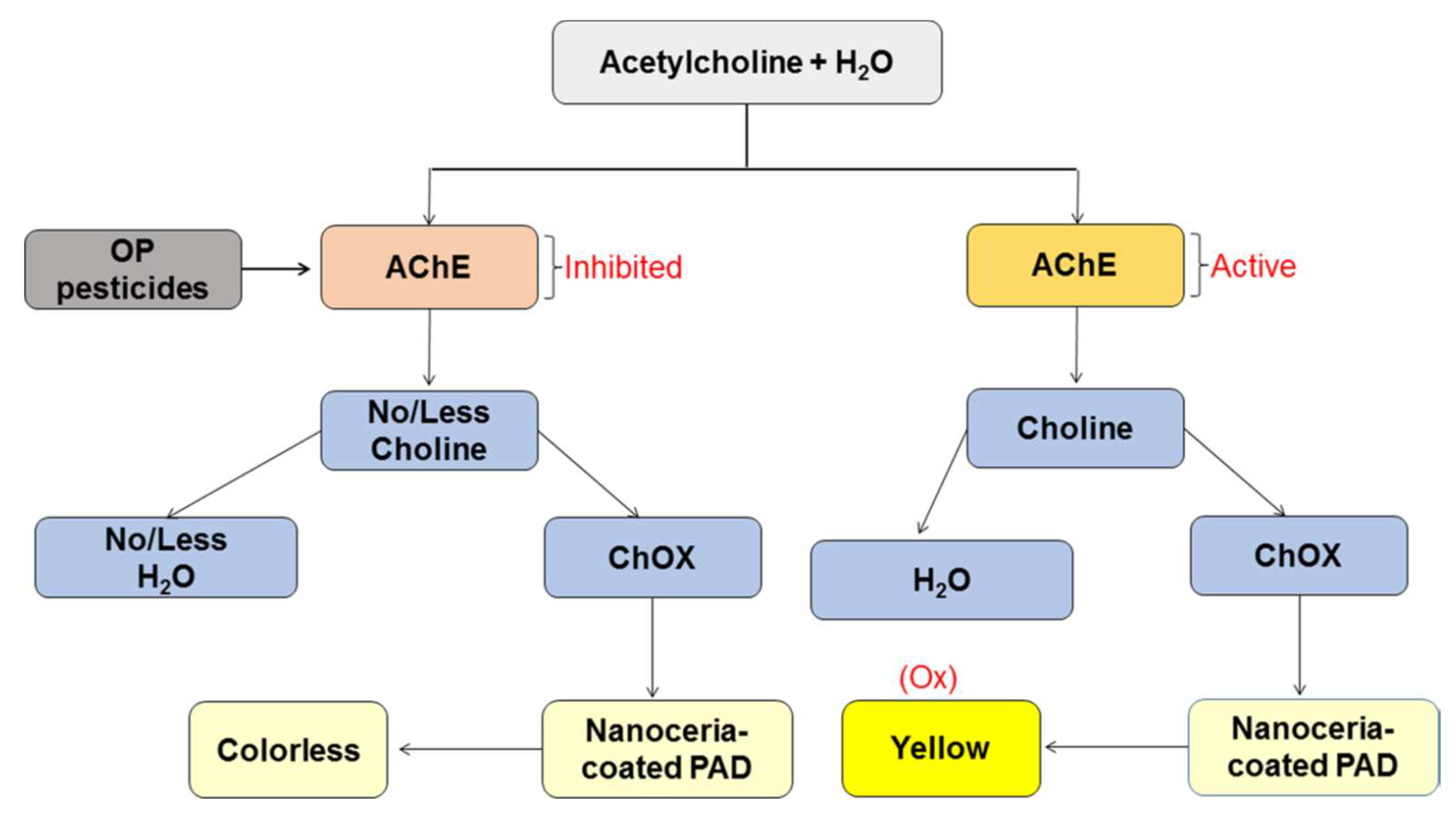
Cerium oxide nanoparticles (Nanoceria) are metal oxide nanoparticles, oxidase-like properties in aqueous environments [
111]. Nuanthavong et al. developed a nanoceria-based biosensor for the detection of methyl-paraoxon and chlorpyrifos-oxon [
86]. The principle of the paper-based analytical device (PAD) was summarised in
Figure 23 [
112]. In the presence of acetylcholine, H
2O
2 was produced by the bi-enzymatic system (AChE and ChO). Thus the colour changed from colorless to yellow when nanoceria were in the presence of H
2O
2. When AChE activity is inhibited by pesticides, less H
2O
2 was produced, then the PAD remained colorless or with less intense yellow colour. LOD of 18.3 ng/ml and 5.3 ng/ml were obtained for methyl-paraoxon and chlorpyrifos-oxon respectively.
To overcome some of the drawbacks of colorimetric biosensors, dual mode sensors gradually received extensive attention, especially dual colorimetric/fluorimetric sensors.
5. Dual enzymatic colorimetric/fluorimetric biosensors
The optical detection in dual enzymatic sensors is based on two modes of detection. Dual detection provides greater accuracy and reliability than single mode detection, because the two signals are independent of each other. One verifies and confirms the results of the other. Dual mode could eliminate matrix interference, reduce false positive and false negative results [
113].
Colorimetric and fluorimetric is the most popular dual paltform. Both signals could be readible by naked eyes, or by readers not so sophisticated. This dual mode combined the simplicity and rapidness of colorimetric methods and the sensitivity of fluorescent sensors.
Table 2 presents some examples of dual enzymatic colorimetric and fluorimetric sensors for the detection of pesticides. In most of the cases, nanomaterials were used as enhancers for the signal or even directly as transducers for dual colorimetric/fluorimetric biosensors.
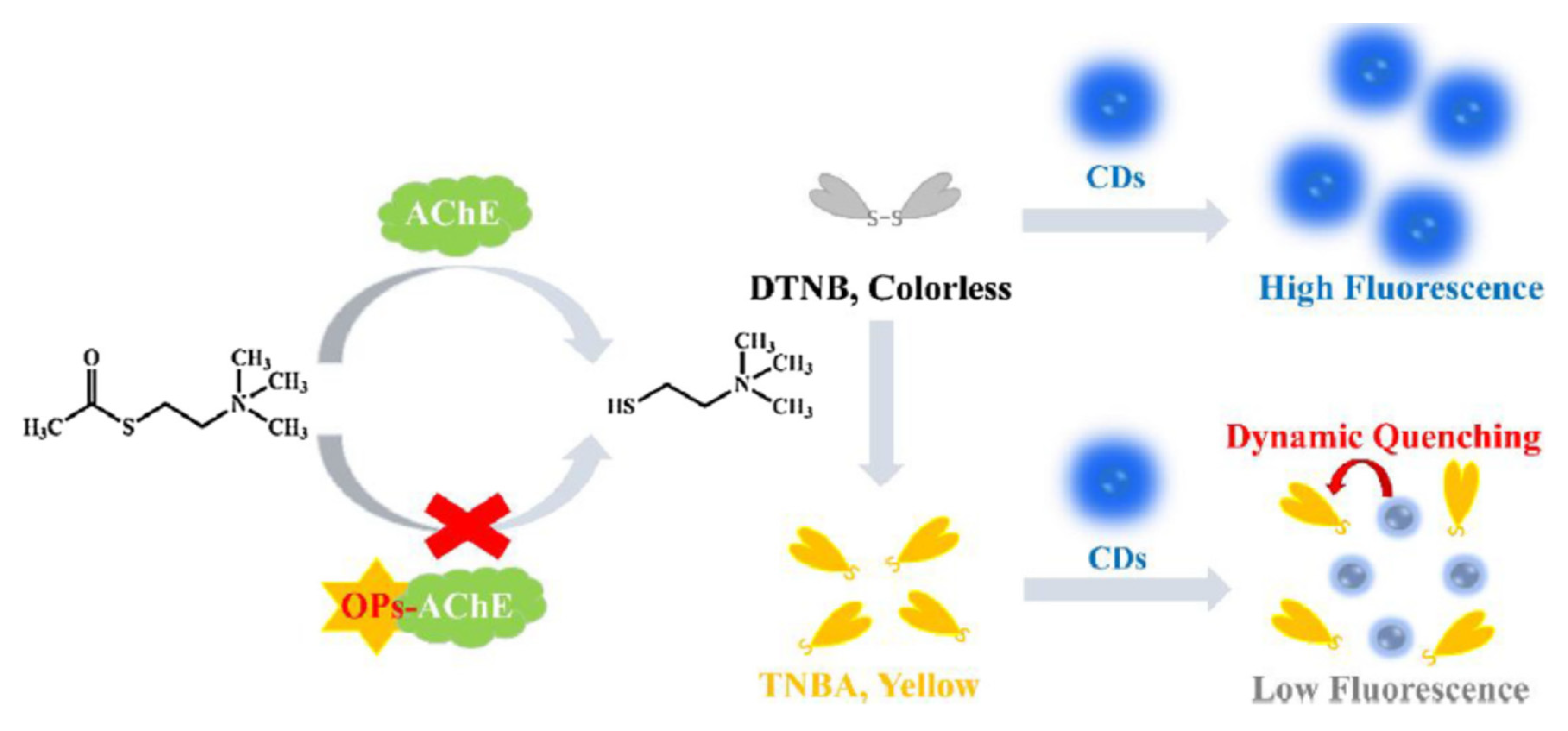
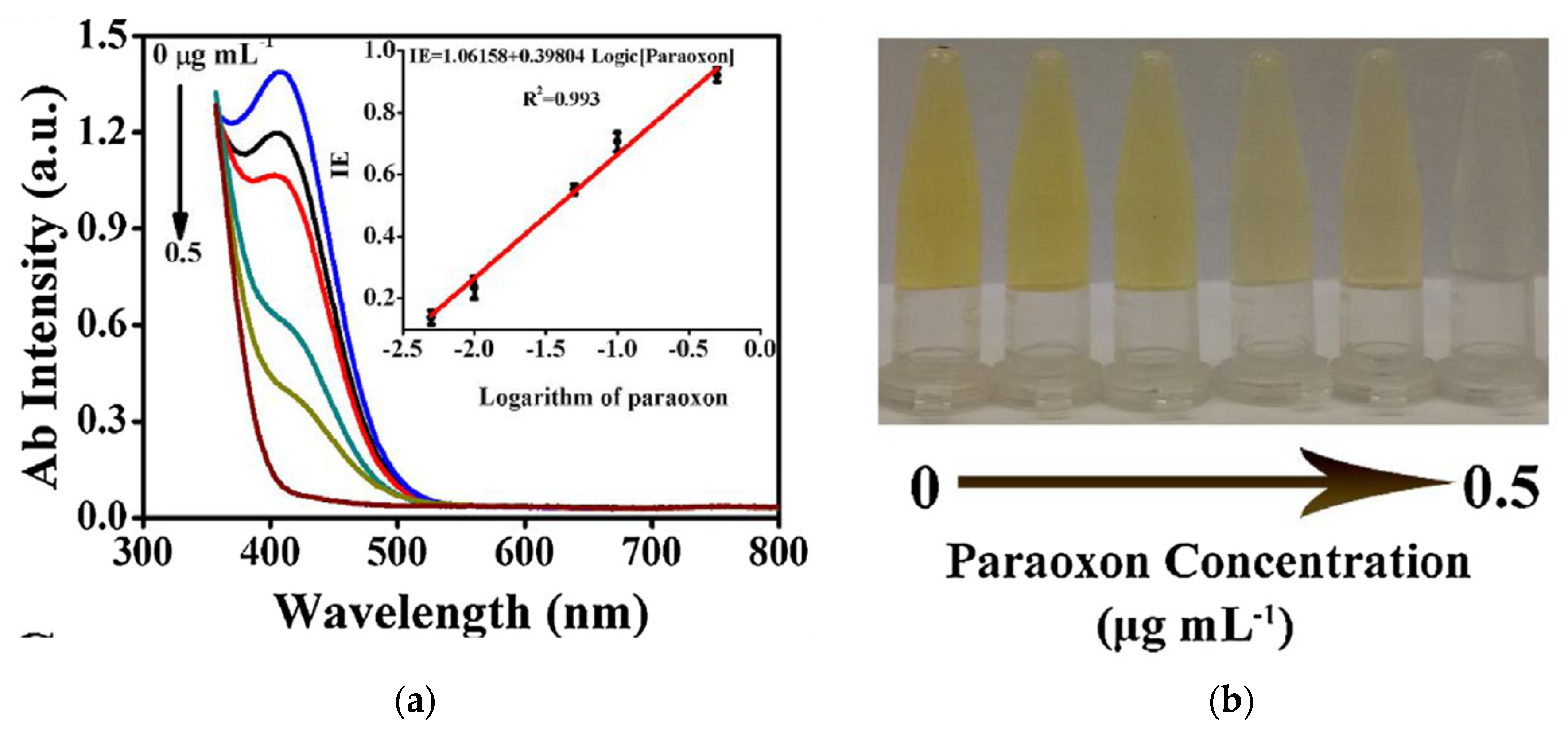
Li et al. developed a dual colorimetric/fluorimetric sensor for the detection of paraoxon based on acetylcholinesterase inhibition by pesticides [
120]. The substrate DTNB was decomposited by TCh to form a yellow coloured product, 5-thio-2-nitrobenzoic acid (TNBA) (
Figure 24). Thus TNBA quenched the fluorescence of carbon dots (CDs). In the presence of paraoxon, less TCh was produced and consequently less TNBA; the absorbance decreased and the fluorescence of CDs increased. The two signals fluorimetric and colorimetric moved in opposite directions as a function of paraoxon concentrations.
Figure 25a showed the UV/visible absorption spectra in relation to the concentrations of paraoxon. The colorimetric signal could be read by naked eyes and therefore could be used as a first screening step for field applications (
Figure 25b). Then the fluorescence signal could be used to confirm the first visual reading, with a greater sensitivity (
Figure 25c). A good linear relationship could be observed between inhibition efficiency (IE) and the concentration of paraoxon (
Figure 25d). The LOD was reported as 0.4 ng/ml. This biosensor was applied to actual samples, water, rice, and cabbage. The selectivity towards usual components of agricultural and biological samples (eg. Na+, K+, glucose, lactose, bovine serum albumine) was high.
A dual colorimetric and fluorimetric enzymatic sensor was developed for the detection of methyl parathion (MP) [
118]. The inner filtration effect (IFE) was observed when an absorber (ie. silver nanoparticles (AgNPs)) absorbed the fluorescence of the fluorophore (ie. graphene quantum dots (GQDs)). Furthermore the complex GQDs-AgNPs absorbed at 390 nm. Fluorescence decreased when the MP concentration increased (
Figure 26a,c), while the absorbance increased when the MP concentration increased (
Figure 26b,d). Indeed, TCh produced by AChE activity reacted with AgNPs which aggregated. Therefore the fluorescence of GQDs was recovered and the absorbance decreased. In the presence of MP, the AChE activity was inhibited. Therefore less TCh was produced, the fluorescence decreased and the aggregation of AgNPs led to an increase in the absorbance. The LOD of 0.017 µg/l reported for the fluorimetric assay was much better than the LOD of 0.2 µg/l for the colorimetric assay. The colorimetric assay is readible by naked eyes.
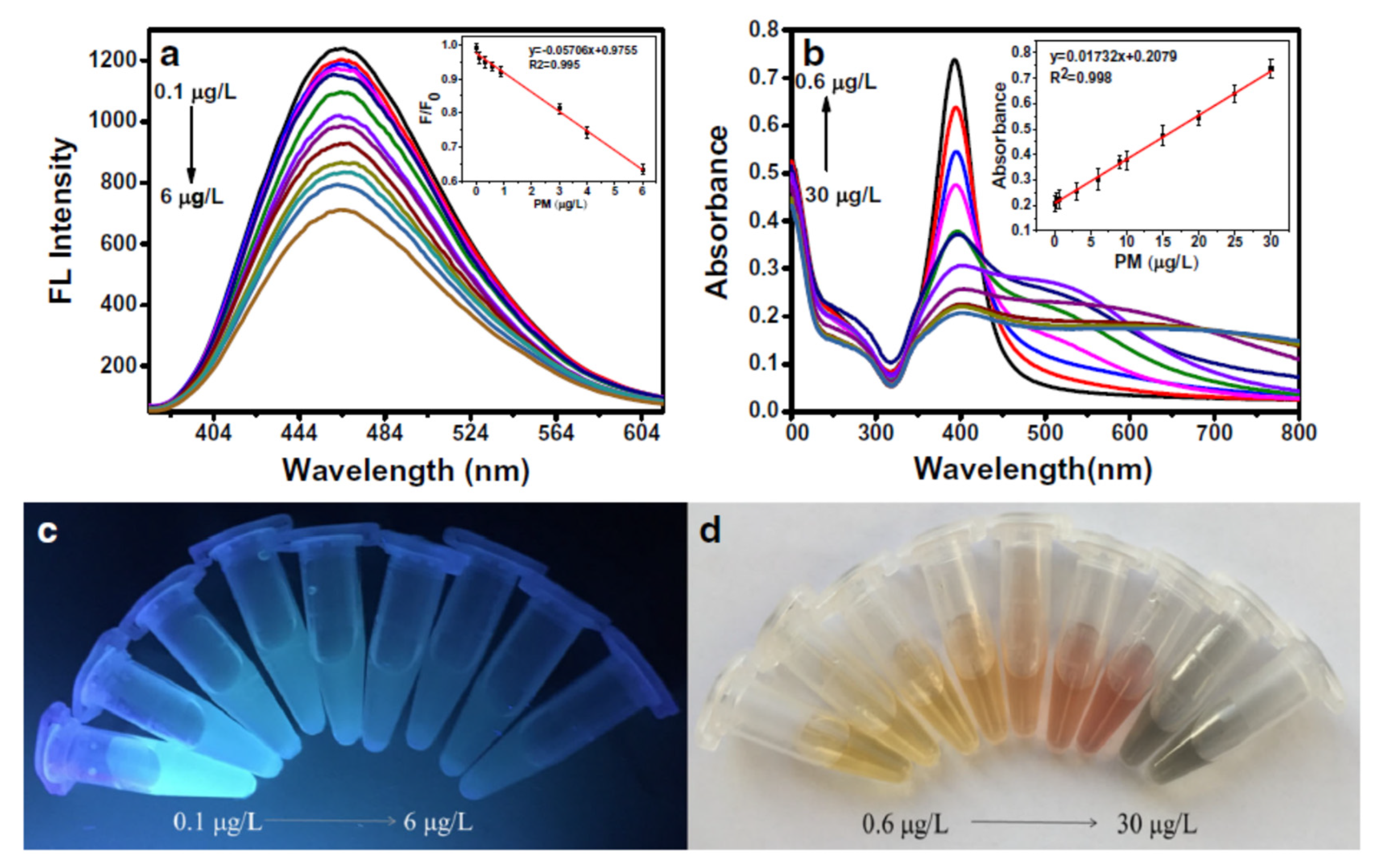
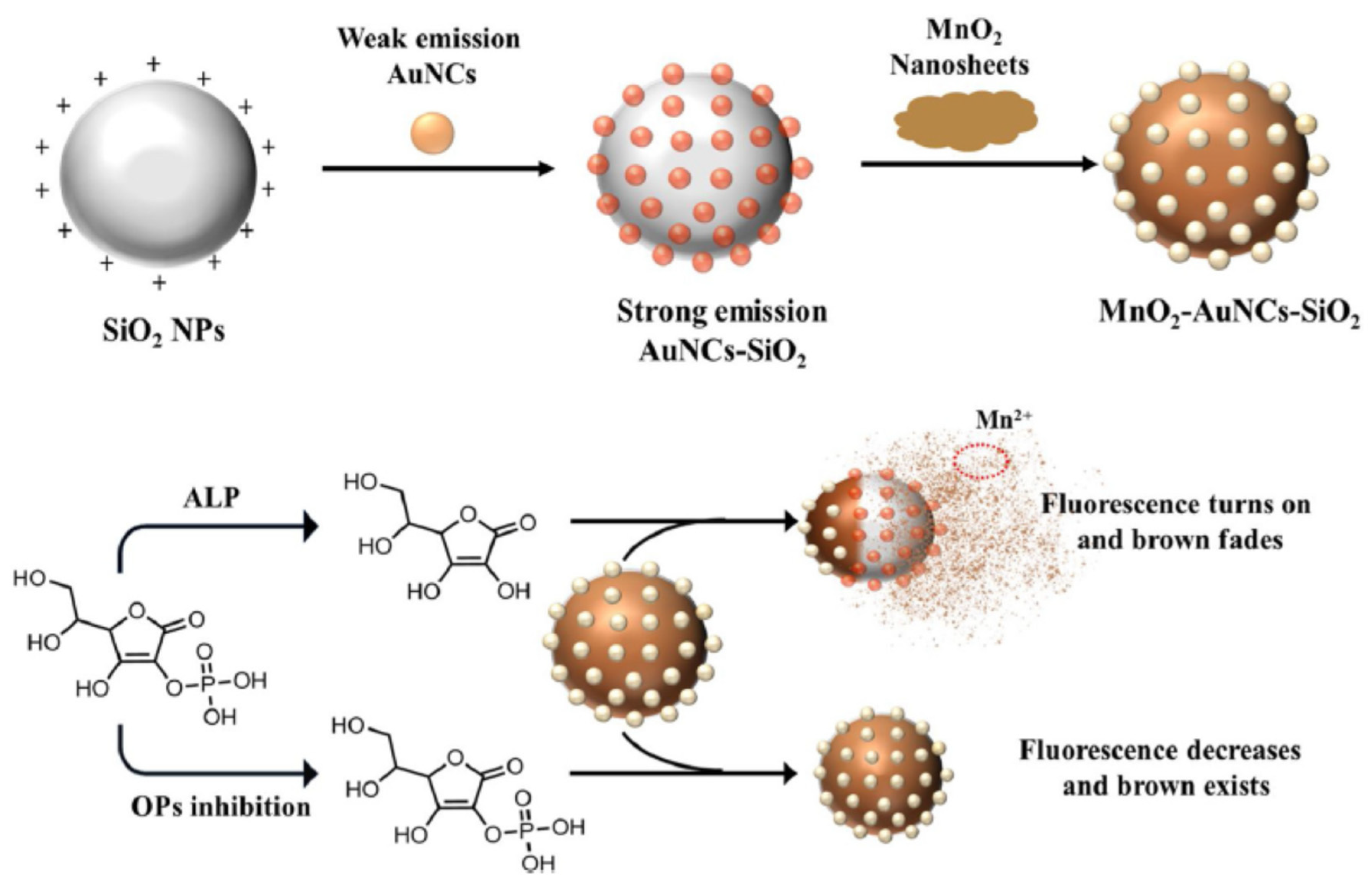
Cai et al. developed a dual ALP sensor based on MnO
2-AuNCs-SiO
2 nanocomposites for the detection of acephate (
Figure 27) [
119]. Pure gold nanoclusters (AuNCs) exhibited weak fluorescence, while strong fluorescence was emitted when AuNCs aggregated on the surface of silicium oxide nanoparticles (SiO
2 NPs). Due to IFE, manganese oxide (MnO
2) nanosheets quenched the fluorescence of AuNCs-SiO
2 NPs when MnO
2-AuNCs-SiO
2 composites were formed. Ascorbic acid, produced by ALP activity, was able to decompose MnO
2 nanosheets. In this case, the fluorescence of AuNCs-SiO
2 NPs was restored and the brown colour faded. In the presence of pesticides, less ascorbic acid was produced, less MnO
2 nanosheets were decomposed; thus the fluorescence was quenched by MnO
2 NPs and the colour was brown. The absorbance increased proportionally to the pesticide concentration while the fluorescence decreased. The LOD for fluorescence detection was reported as 0.4 μg/l and 0.09 μg/L for colorimetric analysis. Other pesticides, dimethoate, parathion-methyl, profenofos and isofenphos-methyl, were dtected on the dual-sensor, even it was with different sensitivities from acepahte because of different inhibitory effects.
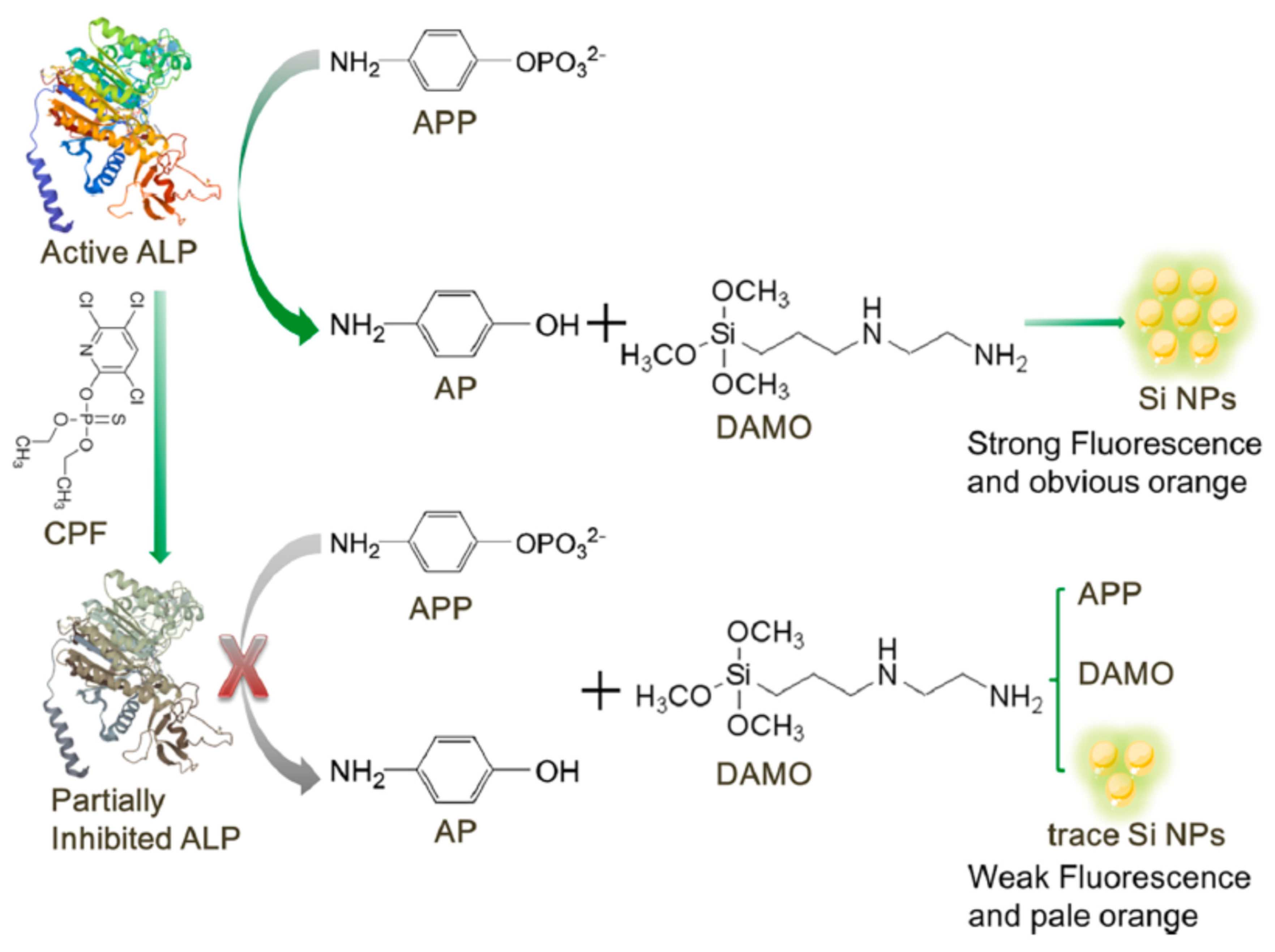
Jiang et al. developed a dual enzymatic sensor based on ALP inhibition for the detection of chlorpyrifos (
Figure 28) [
115]. p-aminophenol (p-AP) produced by ALP activity reacted with N-[3-(trimethoxysilyl) propyl]ethylenediamine (DAMO) to produce silicon based nanoparticles (Si BNPs). These Si BNPs could give colorimetric (orange color) and fluorimetric signals. In the presence of chlorpyrifos, the enzymatic activity is inhibited, thus less p-AP and Si BNPs were produced. The colorimetric (pale orange) and fluorimetric (weak fluorescence) signals decreased. Herein the two signals fluorimetric and colorimetric moved in the same direction as a function of chlorpyrifos concentrations. .The LOD were reported as 0.70 ng/ml and 1.90 ng/ml for fluorimetric and colorimetric sensor repsectively in lake water sample.
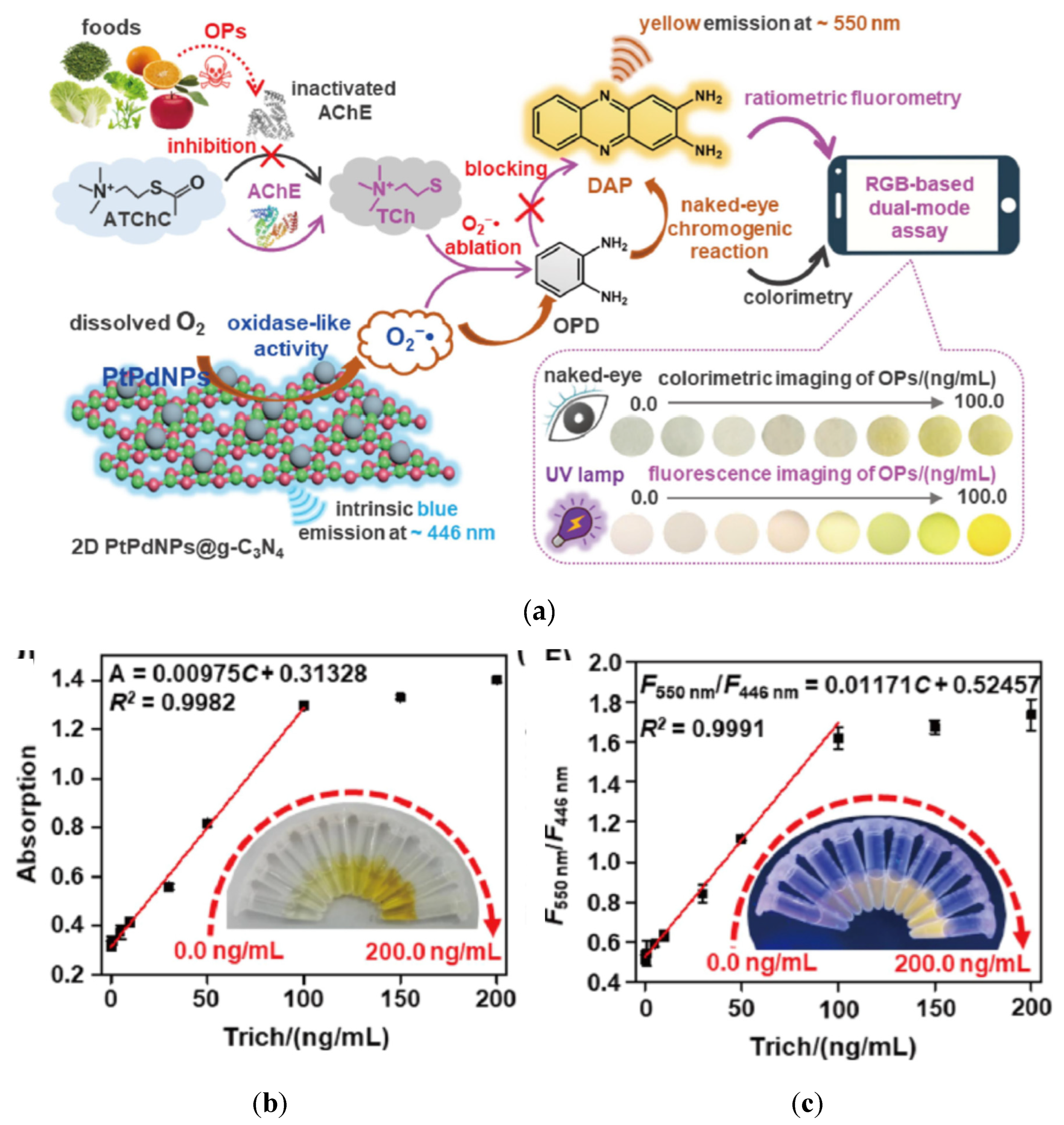
A dual colorimetric and fluorimetric AChE based sensor was developed for the detection of OPPs in fruits and vegetables [
114]. This sensor was based on the enzymatic activity of AChE, combined with an oxidase like activity of platinum palladium nanoparticles decorated graphite carbon nitride nanosheets (PtPdNPs@g-C
3N
4) (blue emission at 446 nm) (
Figure 29a). PtPdNPs converted dissolved O
2 into O
2- due to their oxidase like activity. Then the substrate (orthophenyldiamine (OPD)) which was not natively fluorescent, was oxidised by O
2- into a product 2,3-diaminophenothiazine (DAP) which was fluorescent (yellow emission at 550 nm). When ATCh was transformed into TCh by the AChE activity, TCh and OPD competed to react with O
2-. In the presence of an OPP, the AChE activity is inhibited. Therefore less TCh was produced, so more O
2- were available to react with OPD. Therefore more DAP was produced, which resulted in more strong fluorescence and increased absorbance (from light yellow to dark yellow). Again the two signals fluorimetric and colorimetric moved in the same direction as a function of OPP concentrations. The absorption and the fluorescence intensities were proportional to Trichlorfos (Trich) concentrations (
Figure 29b). For field applications, a portable filter paper smartphone-based sensor was developed. Images of colorimetric and fluorimetric signals were taken and analysed with an application to convert images into RGB values. Very low LOD were reported for colorimetric test 0.083 ng/ml and fluorimetric test 0.033 ng/ml.
6. Conclusions and future prospects
In summary, biosensors appeared to be relevant solutions for developing field-applicable, simple, fast and sensitive self-monitoring tools. Colorimetric enzymatic sensors were widely developed during the past ten years for the detection of pesticides in different matrices. This study discussed the different sensing principles of enzymatic colorimetric sensors. The main sensing principle involved the inhibition of enzymatic activity by the target analyte. The enzyme acetylcholinesterase was the most common used enzyme for the detection of pesticides. This review emphasised the increasing contribution of nanomaterials as a replacement for conventional colorimetric substrates (DTNB, TMB, IDA, ...). Furthermore nanomaterials could be used to increase the signal that allowed to develop very sensitive methods. Gold nanoparticles represented the most usual alternative to colorimetric traditional substrates. The limits of detection of gold nanoparticles based biosensors were very low, well below regulatory limits.
Dual-mode sensors have gradually received extensive attention and have been applied for the detection of pesticides. In this review, dual colorimetric/fluorimetric sensors for the detection of pesticides were presented. Dual detection provides greater accuracy and reliability than single mode detection, because the two signals are independent of each other. One verifies and confirms the results of the other. Dual mode could eliminate matrix interference, reduce false positive and false negative results.
The detection performance of colorimetric and dual colorimetric/fluorimetric sensors, and their applications in plant and animal derived food products, were discussed. The performance of colorimetric and dual enzymatic biosensors has improved considerably in recent years, thanks in particular to the development of nanomaterials and microfluidics.
However some challenges remain with enzymatic biosensors. A biosensor is constituted of two main elements, the bioreceptor and the transducer. Both of them participate to the performance of the developed biosensor. Acetylcholinesterase is not specific for pesticides; other chemical products (eg. neurotoxic agents (sarin)) could inhibit its activity and therefore be detected by AChE biosensors. The enzyme stability is also a critical point for the development of field sensors. More reliable immobilization protocols (polymers, nanomaterials) should be developed. The production of recombinant AChE could be a solution to develop more sensitive and selective biosensors for the detection of pesticides. Moreover, efficient immobilization method improves enzyme activity and stability, but also biosensor sensitivity. The enzyme activity is strongly correlated to the native structure of the enzyme that should not be degraded during immobilization.
Furthermore, the cost of nanomaterials could be very high. So even if nanomaterials could help to produce more sensitive biosensors, the cost of sensors for on site applications should be taken into account.
To reinforce the monitoring of pesticides on the field, the portability should be improved. It could be performed by the development of biosensors readable with smartphones. Indeed the colorimetric reading could be performed visually, with a classical spectrophotometer or more recently with a smartphone. Smartphones are emerging as potential replacements for the conventional laboratory instruments (ie. Spectrophotometer) [
123]. Smartphones are widely used in everyday life, easy to use and portable. These features enable to develop biosensors suitable for field use. Free applications and software are available to convert images into quantifiable, usable data (ie. RGB principle). Disposable test strips (eg. Paper-based analytical devices) could be assessed by naked eyes, with a portable spectrophotometer or even better with smartphone. These biosensors are cost-effective, easy to use, and portable. Therefore lateral flow assays are promising tools for self-monitoring of pesticides in the industry.
The second perspective is to replace the enzyme in the biosensor by nanomaterials due to their enzyme-like properties (nanozymes). Nanozymes could be used to develop biosensors in combination with natural enzymes (tandem catalytic performance) like acetylcholinesterase [50, 77] or could replace the native enzyme [
124]. Nanozymes became a promising alternative to natural enzymes in the past decade for the detection of pesticides, especially because of the better stability to environmental conditions [
29].
Finally the literature on the development of biosensors and especially enzymatic biosensors for the detection of pesticides is extensive. However, these methods are not currently used for routine monitoring [
125]. Many developed sensors remain at the step of proof of concept only. Sometimes the biosensor was not tested in actual conditions, with agricultural or biological samples. In some cases, one or two samples were tested to show the applicability. However during these research developments, no full validation were implemented. The limits of detection were determined mostly in buffer, which was a simpler matrix than biological or agricultural matrices. Therefore LOD were probably underestimated. Complex sample pre-treatment for some biological matrices could be an obstacle for the development of field biosensors.
Funding
This research received no external funding.
Data Availability Statement
Not applicable
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO, W.H.O. , The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification. 2019.
- Turdean, G., I. Popescu, and L. Oniciu, Biocapteurs ampérométriques à cholinestérases pour la détermination des pesticides organophosphorés. Canadian Journal of Chemistry, 2011, 80, 315–331. [Google Scholar]
- Do, Y.-S.; Kim, J.-B.; Kang, S.-H.; Kim, N.-Y.; Um, M.-N.; Park, Y.-B.; Oh, M.-S.; Yoon, M.-H. Risk assessment of pesticide residues in fruits collected in Gyeonggi-do, Korea from 2006 to 2010. Korean J. Pestic. Sci. 2012, 16, 85–97. [Google Scholar] [CrossRef]
- Mebdoua, S. , Pesticide Residues in Fruits and Vegetables, in Bioactive Molecules in Food, J.-M. Mérillon and K.G. Ramawat, Editors. 2018, Springer International Publishing: Cham. p. 1-39.
- Sanborn, M. , et al., Identifying and managing adverse environmental health effects: 4. Pesticides. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne, 2002. 166: 1431-6.
- Lockridge, O. and L.M. Schopfer, Chapter 59 - The cross-linking action of organophosphorus poisons; Implications for chronic neurotoxicity, in Handbook of Toxicology of Chemical Warfare Agents (Third Edition), R.C. Gupta, Editor. 2020, Academic Press: Boston. p. 1027-1033.
- Lim, L. and H.M. Bolstad, Organophosphate Insecticides: Neurodevelopmental Effects, in Encyclopedia of Environmental Health (Second Edition), J. Nriagu, Editor. 2019, Elsevier: Oxford. p. 785-791. [CrossRef]
- 8. REGULATION (EC) NO 396/2005 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC, O.J.o.t.E. Union, Editor. 2005. p. 1-16.
- Stoytcheva, M.; Zlatev, R. Organophosphorus Pesticides Analysis. 2011. [CrossRef]
- Zamora-Sequeira, R.; Starbird-Pérez, R.; Rojas-Carillo, O.; Vargas-Villalobos, S. What are the Main Sensor Methods for Quantifying Pesticides in Agricultural Activities? A Review. Molecules 2019, 24, 2659. [Google Scholar] [CrossRef]
- Das, B.; Franco, J.L.; Logan, N.; Balasubramanian, P.; Kim, M.I.; Cao, C. Nanozymes in Point-of-Care Diagnosis: An Emerging Futuristic Approach for Biosensing. Nano-Micro Lett. 2021, 13, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M. , et al., ASSURED-compliant point-of-care diagnostics for the detection of human viral infections. Reviews in Medical Virology, 2021. n/a(n/a): p. e2263.
- Gupta, S.; Kakkar, V. Development of Environmental Biosensors for Detection, Monitoring, and Assessment. 2020, 107–125. [CrossRef]
- Verma, N. and A. Bhardwaj, Biosensor Technology for Pesticides—A review. Applied Biochemistry and Biotechnology, 2015. 175, p. 3093-3119.
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Recent Progress in Biosensors for Environmental Monitoring: A Review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef]
- Issert, V., P. Grenier, and V. bellon maurel, Nouvelles méthodes d'analyse rapide pour le contrôle des résidus de pesticides : immunoessais et biocapteurs. 1997.
- Durand, P.; Nicaud, J.M.; Mallevialle, J. Detection of Organophosphorous Pesticides with an Immobilized Cholinesterase Electrode. J. Anal. Toxicol. 1984, 8, 112–117. [Google Scholar] [CrossRef]
- Campanella, L.; Cocco, R.; Sammartino, M.; Tomassetti, M. A new enzyme inhibition sensor for organophosphorus pesticides analysis. Sci. Total. Environ. 1992, 123-124, 1–16. [Google Scholar] [CrossRef]
- Palleschi, G.; Bernabei, M.; Cremisini, C.; Mascini, M. Determination of organophosphorus insecticides with a choline electrochemical biosensor. Sensors Actuators B: Chem. 1992, 7, 513–517. [Google Scholar] [CrossRef]
- González-Martínez, M. .; Brun, E.M.; Puchades, R.; Maquieira,.; Ramsey, K.; Rubio, F. Glyphosate Immunosensor. Application for Water and Soil Analysis. Anal. Chem. 2005, 77, 4219–4227. [Google Scholar] [CrossRef] [PubMed]
- Uygun, Z.O.; Dilgin, Y. A novel impedimetric sensor based on molecularly imprinted polypyrrole modified pencil graphite electrode for trace level determination of chlorpyrifos. Sensors Actuators B: Chem. 2013, 188, 78–84. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Yin, R.; Jensen, J.L. Molecularly imprinted polymer sensors for pesticide and insecticide detection in water. Anal. 2001, 126, 798–802. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Behera, B.K.; Parida, P.K.; Aralappanavar, V.K.; Mondal, S.; Dei, J.; Das, B.K.; Mukherjee, S.; Pal, S.; Weerathunge, P.; et al. Aptamer-based NanoBioSensors for seafood safety. Biosens. Bioelectron. 2023, 219, 114771. [Google Scholar] [CrossRef]
- Liu, B.; Tang, Y.; Yang, Y.; Wu, Y. Design an aptamer-based sensitive lateral flow biosensor for rapid determination of isocarbophos pesticide in foods. Food Control. 2021, 129, 108208. [Google Scholar] [CrossRef]
- Marty, J.-L.; Mionetto, N.; Noguer, T.; Ortega, F.; Roux, C. Enzyme sensors for the detection of pesticides. Biosens. Bioelectron. 1993, 8, 273–280. [Google Scholar] [CrossRef]
- Parellada, J.; Narváez, A.; López, M.; Domı́nguez, E.; Fernández, J.; Pavlov, V.; Katakis, I. Amperometric immunosensors and enzyme electrodes for environmental applications. Anal. Chim. Acta 1998, 362, 47–57. [Google Scholar] [CrossRef]
- Majdinasab, M.; Daneshi, M.; Marty, J.L. Recent developments in non-enzymatic (bio)sensors for detection of pesticide residues: Focusing on antibody, aptamer and molecularly imprinted polymer. Talanta 2021, 232, 122397. [Google Scholar] [CrossRef]
- Wu, H.; Wu, J.; Wang, H.; Liu, Y.; Han, G.; Zou, P. Sensitive and label-free chemiluminescence detection of malathion using exonuclease-assisted dual signal amplification and G-quadruplex/hemin DNAzyme. J. Hazard. Mater. 2021, 411, 124784. [Google Scholar] [CrossRef]
- Prasad, S.N.; Bansal, V.; Ramanathan, R. Detection of pesticides using nanozymes: Trends, challenges and outlook. TrAC Trends Anal. Chem. 2021, 144, 116429. [Google Scholar] [CrossRef]
- Imato, T.; Ishibashi, N. Potentiometric butyrylcholine sensor for organophosphate pesticides. Biosens. Bioelectron. 1995, 10, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Niunes, G.S. and D. Barcelo, Electrochemical biosensors for pesticide determination in food samples. Analusis magazine, 1998. 26, p. M156-M159.
- Everett, W.R.; Rechnitz, G.A. Enzyme-Based Electrochemical Biosensors for Determination of Organophosphorous and Carbamate Pesticides. Anal. Lett. 1999, 32, 1–10. [Google Scholar] [CrossRef]
- Trojanowicz, M. Determination of Pesticides Using Electrochemical Enzymatic Biosensors. Electroanalysis 2002, 14, 1311–1328. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Extraction and electrochemical sensing of pesticides in food and environmental samples by use of polydopamine-based materials. Chemosphere 2021, 266, 129222. [Google Scholar] [CrossRef]
- Andreescu, S.; Marty, J.-L. Twenty years research in cholinesterase biosensors: From basic research to practical applications. Biomol. Eng. 2006, 23, 1–15. [Google Scholar] [CrossRef]
- Roda, A.; Rauch, P.; Ferri, E.; Girotti, S.; Ghini, S.; Carrea, G.; Bovara, R. Chemiluminescent flow sensor for the determination of Paraoxon and Aldicarb pesticides. Anal. Chim. Acta 1994, 294, 35–42. [Google Scholar] [CrossRef]
- Barzen, C.; Brecht, A.; Gauglitz, G. Optical multiple-analyte immunosensor for water pollution control. Biosens. Bioelectron. 2002, 17, 289–295. [Google Scholar] [CrossRef]
- Su, D.; Li, H.; Yan, X.; Lin, Y.; Lu, G. Biosensors based on fluorescence carbon nanomaterials for detection of pesticides. TrAC Trends Anal. Chem. 2020, 134, 116126. [Google Scholar] [CrossRef]
- Xia, N.; Wang, Q.; Liu, L. Nanomaterials-Based Optical Techniques for the Detection of Acetylcholinesterase and Pesticides. Sensors 2015, 15, 499–514. [Google Scholar] [CrossRef]
- Pohanka, M. Biosensors containing acetylcholinesterase and butyrylcholinesterase as recognition tools for detection of various compounds. Chem. Pap. 2015, 69, 1–13. [Google Scholar] [CrossRef]
- Campanella, L.; Achilli, M.; Sammartino, M.; Tomassetti, M. Butyrylcholine enzyme sensor for determining organophosphorus inhibitors. Bioelectrochemistry Bioenerg. 1991, 26, 237–249. [Google Scholar] [CrossRef]
- Kaur, J.; Bandyopadhyay, D.; Singh, P.K. A simple and convenient choline oxidase inhibition based colorimetric biosensor for detection of organophosphorus class of pesticides. J. Mol. Liq. 2022, 347, 118258. [Google Scholar] [CrossRef]
- Jain, M.; Yadav, P.; Joshi, A.; Kodgire, P. Advances in detection of hazardous organophosphorus compounds using organophosphorus hydrolase based biosensors. Crit. Rev. Toxicol. 2019, 49, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhang, F.; Yang, L.; Yang, W.; Zhang, L.; Wang, Z. Paper-based multicolor sensor for on-site quantitative detection of 2,4-dichlorophenoxyacetic acid based on alkaline phosphatase-mediated gold nanobipyramids growth and colorimeter-assisted method for quantifying color. Talanta 2022, 245, 123489. [Google Scholar] [CrossRef]
- Fan, Y.-F.; Zhu, S.-X.; Hou, F.-B.; Zhao, D.-F.; Pan, Q.-S.; Xiang, Y.-W.; Qian, X.-K.; Ge, G.-B.; Wang, P. Spectrophotometric Assays for Sensing Tyrosinase Activity and Their Applications. Biosensors 2021, 11, 290. [Google Scholar] [CrossRef]
- Li, A.; Li, H.; Ma, Y.; Wang, T.; Liu, X.; Wang, C.; Liu, F.; Sun, P.; Yan, X.; Lu, G. Bioinspired laccase-mimicking catalyst for on-site monitoring of thiram in paper-based colorimetric platform. Biosens. Bioelectron. 2022, 207, 114199. [Google Scholar] [CrossRef]
- Vargas-Bernal, R., G. Herrera-Pérez, and E. Rodríguez-Miranda, Evolution and Expectations of Enzymatic Biosensors for Pesticides. Pesticides - Advances in Chemical and Botanical Pesticides. 2012.
- Schulze, H.; Vorlová, S.; Villatte, F.; Bachmann, T.T.; Schmid, R.D. Design of acetylcholinesterases for biosensor applications. Biosens. Bioelectron. 2003, 18, 201–209. [Google Scholar] [CrossRef]
- Xu, Y.-L.; Li, F.-Y.; Ndikuryayo, F.; Yang, W.-C.; Wang, H.-M. Cholinesterases and Engineered Mutants for the Detection of Organophosphorus Pesticide Residues. Sensors 2018, 18, 4281. [Google Scholar] [CrossRef]
- Jin, R.; Wang, F.; Li, Q.; Yan, X.; Liu, M.; Chen, Y.; Zhou, W.; Gao, H.; Sun, P.; Lu, G. Construction of multienzyme-hydrogel sensor with smartphone detector for on-site monitoring of organophosphorus pesticide. Sensors Actuators B: Chem. 2021, 327. [Google Scholar] [CrossRef]
- Huang, M.; Fan, Y.; Yuan, X.; Wei, L. Color-coded detection of malathion based on enzyme inhibition with dark-field optical microscopy. Sensors Actuators B: Chem. 2021, 353, 131135. [Google Scholar] [CrossRef]
- Hassan, M., T. Tamer, and A. Omer, Methods of Enzyme Immobilization. International Journal of Current Pharmaceutical Review and Research, 2016. 7: p. 385-392.
- Kramer, D.N.; Gamson, R.M. Colorimetric Determination of Acetylcholinesterase Activity. Anal. Chem. 1958, 30, 251–254. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Rogers, K.R. , et al., Acetylcholinesterase fiber-optic biosensor for detection of anticholinesterases. Fundamental and Applied Toxicology, 1991. 16, p. 810-820.
- Zhu, J.; Yin, X.; Zhang, W.; Chen, M.; Feng, D.; Zhao, Y.; Zhu, Y. Simultaneous and Sensitive Detection of Three Pesticides Using a Functional Poly(Sulfobetaine Methacrylate)-Coated Paper-Based Colorimetric Sensor. Biosensors 2023, 13, 309. [Google Scholar] [CrossRef]
- Kukkar, P.; Kukkar, D.; Younis, S.A.; Singh, G.; Singh, P.; Basu, S.; Kim, K.-H. Colorimetric biosensing of organophosphate pesticides using enzymatic nanoreactor built on zeolitic imdiazolate-8. Microchem. J. 2021, 166, 106242. [Google Scholar] [CrossRef]
- Cacciotti, I.; Pallotto, F.; Scognamiglio, V.; Moscone, D.; Arduini, F. Reusable optical multi-plate sensing system for pesticide detection by using electrospun membranes as smart support for acetylcholinesterase immobilisation. Mater. Sci. Eng. C 2020, 111, 110744. [Google Scholar] [CrossRef]
- Kaur, N.; Thakur, H.; Pathak, S.; Prabhakar, N. Acetylcholinesterase immobilised eggshell membrane-based optical biosensor for organophosphate detection. Int. J. Environ. Anal. Chem. 2015, 95, 1134–1147. [Google Scholar] [CrossRef]
- Ben Oujji, N.; Bakas, I.; Istamboulié, G.; Ait-Ichou, I.; Ait-Addi, E.; Rouillon, R.; Noguer, T. A Simple Colorimetric Enzymatic-Assay, based on immobilization of acetylcholinesterase by adsorption, for sensitive detection of organophosphorus insecticides in olive oil. Food Control. 2014, 46, 75–80. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Aswad, A.F. Bioactive Paper Sensor Based on the Acetylcholinesterase for the Rapid Detection of Organophosphate and Carbamate Pesticides. Int. J. Anal. Chem. 2014, 2014, 301410. [Google Scholar] [CrossRef] [PubMed]
- Kavruk, M.; Özalp, V.C.; Öktem, H.A. Portable Bioactive Paper-Based Sensor for Quantification of Pesticides. J. Anal. Methods Chem. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, C.; Xie, J.; Li, Z.; Qu, L.; Cai, X.; Ouyang, H.; Song, Y.; Du, D.; Lin, Y.; et al. Ambient light sensor based colorimetric dipstick reader for rapid monitoring organophosphate pesticides on a smart phone. Anal. Chim. Acta 2019, 1092, 126–131. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.; Park, S.J.; Kwon, C.; Noh, H. Development of Colorimetric Paper Sensor for Pesticide Detection Using Competitive-inhibiting Reaction. BioChip J. 2018, 12, 326–331. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Xiao, F.; Wu, Z.; Yu, R. Sensitive inkjet printing paper-based colormetric strips for acetylcholinesterase inhibitors with indoxyl acetate substrate. Talanta 2017, 162, 174–179. [Google Scholar] [CrossRef]
- Pohanka, M.; Vlcek, V. Preparation and performance of a colorimetric biosensor using acetylcholinesterase and indoxylacetate for assay of nerve agents and drugs. Interdiscip. Toxicol. 2015, 7, 215–218. [Google Scholar] [CrossRef]
- Pohanka, M. Acetylcholinesterase Based Dipsticks with Indoxylacetate as a Substrate for Assay of Organophosphates and Carbamates. Anal. Lett. 2012, 45, 367–374. [Google Scholar] [CrossRef]
- Yang, N.; Wang, P.; Xue, C.; Sun, J.; Mao, H.; Oppong, P.K. A portable detection method for organophosphorus and carbamates pesticide residues based on multilayer paper chip. J. Food Process. Eng. 2018, 41, e12867. [Google Scholar] [CrossRef]
- No, H.-Y.; Kim, Y.A.; Lee, Y.T.; Lee, H.-S. Cholinesterase-based dipstick assay for the detection of organophosphate and carbamate pesticides. Anal. Chim. Acta 2007, 594, 37–43. [Google Scholar] [CrossRef]
- Hossain, S.M.Z.; Luckham, R.E.; McFadden, M.J.; Brennan, J.D. Reagentless Bidirectional Lateral Flow Bioactive Paper Sensors for Detection of Pesticides in Beverage and Food Samples. Anal. Chem. 2009, 81, 9055–9064. [Google Scholar] [CrossRef]
- Ramos, F.; Ogunneye, A.; Barbarinde, N.; Erenas, M.; Capitán-Vallvey, L. Bioactive microfluidic paper device for pesticide determination in waters. Talanta 2020, 218, 121108. [Google Scholar] [CrossRef]
- Do, C. , CHOLINESTERASE IMMOBLIZATION FOR LARGE-SCALE PRODUCTION OF HIGH SENSITIVE TEST CARDS FOR RAPID DETECTION OF ORGANOPHOSPHORUS AND CARBAMATE PESTICIDES IN AGRICULTURAL PRODUCTS. Vietnam Journal of Science and Technology, 2018. 54: p. 189.
- Liang, L.; Jiang, Y.; Liu, F.; Wu, J.; Tian, L.; Zhao, S.; Ye, F. Smartphone flashlight-triggered covalent organic framework nanozyme activity: A universal scheme for visual point-of-care testing. Sensors Actuators B: Chem. 2023, 381. [Google Scholar] [CrossRef]
- Liu, B.; Wu, L.; Peng, Z.; Wu, S.; Qiu, P. Monitoring of parathion methyl using a colorimetric gold nanoparticle-based acetylcholinesterase assay. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2022, 268, 120665. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, J.; Yang, B.; Hao, H.; Lou, S. A green photocatalytic-biosensor for colorimetric detection of pesticide (carbaryl) based on inhibition of acetylcholinesterase. Talanta 2022, 246, 123525. [Google Scholar] [CrossRef]
- Shah, M.M.; Ren, W.; Irudayaraj, J.; Sajini, A.A.; Ali, M.I.; Ahmad, B. Colorimetric Detection of Organophosphate Pesticides Based on Acetylcholinesterase and Cysteamine Capped Gold Nanoparticles as Nanozyme. Sensors 2021, 21, 8050. [Google Scholar] [CrossRef]
- Jin, R.; Xing, Z.; Kong, D.; Yan, X.; Liu, F.; Gao, Y.; Sun, P.; Liang, X.; Lu, G. Sensitive colorimetric sensor for point-of-care detection of acetylcholinesterase using cobalt oxyhydroxide nanoflakes. J. Mater. Chem. B 2019, 7, 1230–1237. [Google Scholar] [CrossRef]
- Pimsen, R.; Khumsri, A.; Wacharasindhu, S.; Tumcharern, G.; Sukwattanasinitt, M. Colorimetric detection of dichlorvos using polydiacetylene vesicles with acetylcholinesterase and cationic surfactants. Biosens. Bioelectron. 2014, 62, 8–12. [Google Scholar] [CrossRef]
- Qian, Z.; Tan, R.; Zhang, X.; Leng, Y.; Chen, Z. MnO2 Nanosheet-Based colorimetric sensor Array: Toward identification of organophosphorus pesticides. Microchem. J. 2022, 181. [Google Scholar] [CrossRef]
- Alex A, V. , et al., An ultra-sensitive and selective AChE based colorimetric detection of malathion using silver nanoparticle-graphene oxide (Ag-GO) nanocomposite. Analytica Chimica Acta, 2021. 1142: p. 73-83.
- Zhang, Q.; Yu, Y.; Yun, X.; Luo, B.; Jiang, H.; Chen, C.; Wang, S.; Min, D. Multicolor Colorimetric Sensor for Detection of Omethoate Based on the Inhibition of the Enzyme-Induced Metallization of Gold Nanorods. ACS Appl. Nano Mater. 2020, 3, 5212–5219. [Google Scholar] [CrossRef]
- Chungchai, W. , et al., Development of a novel three-dimensional microfluidic paper-based analytical device (3D-μPAD) for chlorpyrifos detection using graphene quantum-dot capped gold nanocomposite for colorimetric assay. International Journal of Environmental Analytical Chemistry, 2019. 100: p. 1-19.
- Satnami, M.L.; Korram, J.; Nagwanshi, R.; Vaishanav, S.K.; Karbhal, I.; Dewangan, H.K.; Ghosh, K.K. Gold nanoprobe for inhibition and reactivation of acetylcholinesterase: An application to detection of organophosphorus pesticides. Sensors Actuators B: Chem. 2018, 267, 155–164. [Google Scholar] [CrossRef]
- Wu, S.; Li, D.; Wang, J.; Zhao, Y.; Dong, S.; Wang, X. Gold nanoparticles dissolution based colorimetric method for highly sensitive detection of organophosphate pesticides. Sensors Actuators B: Chem. 2017, 238, 427–433. [Google Scholar] [CrossRef]
- Lv, B.; Wei, M.; Liu, Y.; Liu, X.; Wei, W.; Liu, S. Ultrasensitive photometric and visual determination of organophosphorus pesticides based on the inhibition of enzyme-triggered formation of core-shell gold-silver nanoparticles. Microchim. Acta 2016, 183, 2941–2948. [Google Scholar] [CrossRef]
- Nouanthavong, S.; Nacapricha, D.; Henry, C.S.; Sameenoi, Y. Pesticide analysis using nanoceria-coated paper-based devices as a detection platform. Anal. 2016, 141, 1837–1846. [Google Scholar] [CrossRef]
- Sun, J. , et al., A simple, label-free AuNPs-based colorimetric ultrasensitive detection of nerve agents and highly toxic organophosphate pesticide. Biosensors and Bioelectronics, 2011. 28, p. 152-157.
- Luo, Y. , et al., 8 - Characterization and Analysis of Biopharmaceutical Proteins, in Separation Science and Technology, S. Ahuja and S. Scypinski, Editors. 2011, Academic Press. p. 283-359.
- inko, G. , et al., Limitation of the Ellman method: Cholinesterase activity measurement in the presence of oximes. Analytical Biochemistry, 2007. 370, p. 223-227.
- Pohanka, M.; Musilek, K.; Kuca, K. Progress of Biosensors Based on Cholinesterase Inhibition. Curr. Med. Chem. 2009, 16, 1790–1798. [Google Scholar] [CrossRef]
- Vymazalová, K., J. Kadlčák, and E. Halámek, Photocolorimetric Biosensor for Detection of Cholinergic Organophosphorus Compounds. Defence Science Journal, 2012. 62, p. pp.399-403.
- Pelton, R. Bioactive paper provides a low-cost platform for diagnostics. TrAC Trends Anal. Chem. 2009, 28, 925–942. [Google Scholar] [CrossRef]
- Matějovský, L.; Pitschmann, V. New Carrier Made from Glass Nanofibres for the Colorimetric Biosensor of Cholinesterase Inhibitors. Biosensors 2018, 8, 51. [Google Scholar] [CrossRef]
- Auer, B.; Telfer, S.G.; Gross, A.J. Metal Organic Frameworks for Bioelectrochemical Applications. Electroanalysis 2023, 35. [Google Scholar] [CrossRef]
- Pohanka, M.; Holas, O. Evaluation of 2,6-dichlorophenolindophenol acetate as a substrate for acetylcholinesterase activity assay. J. Enzym. Inhib. Med. Chem. 2015, 30, 796–799. [Google Scholar] [CrossRef]
- Apilux, A.; Isarankura-Na-Ayudhya, C.; Tantimongcolwat, T.; Prachayasittikul, V. Paper-based acetylcholinesterase inhibition assay combining a wet system for organophosphate and carbamate pesticides detection. EXCLI J. 2015, 14, 307–319. [Google Scholar] [CrossRef]
- Pohanka, M.; Drtinova, L. Spectrophotometric methods based on 2,6-dichloroindophenol acetate and indoxylacetate for butyrylcholinesterase activity assay in plasma. Talanta 2013, 106, 281–285. [Google Scholar] [CrossRef]
- Sicard, C.; Glen, C.; Aubie, B.; Wallace, D.; Jahanshahi-Anbuhi, S.; Pennings, K.; Daigger, G.T.; Pelton, R.; Brennan, J.D.; Filipe, C.D. Tools for water quality monitoring and mapping using paper-based sensors and cell phones. Water Res. 2015, 70, 360–369. [Google Scholar] [CrossRef]
- Balbach, S.; Jiang, N.; Moreddu, R.; Dong, X.; Kurz, W.; Wang, C.; Dong, J.; Yin, Y.; Butt, H.; Brischwein, M.; et al. Smartphone-based colorimetric detection system for portable health tracking. Anal. Methods 2021, 13, 4361–4369. [Google Scholar] [CrossRef]
- Pohanka, M.; Zakova, J. A Smartphone Camera Colorimetric Assay of Acetylcholinesterase and Butyrylcholinesterase Activity. Sensors 2021, 21, 1796. [Google Scholar] [CrossRef]
- Hestrin, S. , THE REACTION OF ACETYLCHOLINE AND OTHER CARBOXYLIC ACID DERIVATIVES WITH HYDROXYLAMINE, AND ITS ANALYTICAL APPLICATION. Journal of Biological Chemistry, 1949. 180, p. 249-261.
- Jun, D. , et al., Chapter 60 - Monitoring of blood cholinesterase activity in workers exposed to nerve agents, in Handbook of Toxicology of Chemical Warfare Agents (Third Edition), R.C. Gupta, Editor. 2020, Academic Press: Boston. p. 1035-1045.
- Han, T.; Wang, G. Peroxidase-like activity of acetylcholine-based colorimetric detection of acetylcholinesterase activity and an organophosphorus inhibitor. J. Mater. Chem. B 2019, 7, 2613–2618. [Google Scholar] [CrossRef]
- Sun, Y., H. Tan, and Y. Li, A colorimetric assay for acetylcholinesterase activity and inhibitor screening based on the thiocholine–induced inhibition of the oxidative power of MnO2 nanosheets on 3,3′,5,5′–tetramethylbenzidine. Microchimica Acta, 2018. 185, p. 446.
- Ni, P. , et al., Colorimetric assay for acetylcholinesterase and inhibitor screening based on the Ag [I] ion–3,3′,5,5′-tetramethylbenzidine (TMB). Sensors and Actuators B: Chemical, 2016. 226: p. 104-109.
- Liu, G.; Lu, M.; Huang, X.; Li, T.; Xu, D. Application of Gold-Nanoparticle Colorimetric Sensing to Rapid Food Safety Screening. Sensors 2018, 18, 4166. [Google Scholar] [CrossRef]
- Sulaiman, I.S.C.; Chieng, B.W.; Osman, M.J.; Ong, K.K.; Rashid, J.I.A.; Yunus, W.M.Z.W.; Noor, S.A.M.; Kasim, N.A.M.; Halim, N.A.; Mohamad, A. A review on colorimetric methods for determination of organophosphate pesticides using gold and silver nanoparticles. Microchim. Acta 2020, 187, 131. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.-W. Review on Anti-Aggregation-Enabled Colorimetric Sensing Applications of Gold and Silver Nanoparticles. Chemosensors 2022, 10, 536. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Dissanayake, N.M.; Arachchilage, J.S.; Samuels, T.A.; Obare, S.O. Highly sensitive plasmonic metal nanoparticle-based sensors for the detection of organophosphorus pesticides. Talanta 2019, 200, 218–227. [Google Scholar] [CrossRef]
- Thakur, N.; Manna, P.; Das, J. Synthesis and biomedical applications of nanoceria, a redox active nanoparticle. J. Nanobiotechnology 2019, 17, 1–27. [Google Scholar] [CrossRef]
- Ghosh, S.; AlKafaas, S.S.; Bornman, C.; Apollon, W.; Hussien, A.M.; Badawy, A.E.; Amer, M.H.; Kamel, M.B.; Mekawy, E.A.; Bedair, H. The application of rapid test paper technology for pesticide detection in horticulture crops: a comprehensive review. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 1–28. [Google Scholar] [CrossRef]
- Sun, R.; Li, Y.; Du, T.; Qi, Y. Recent advances in integrated dual-mode optical sensors for food safety detection. Trends Food Sci. Technol. 2023, 135, 14–31. [Google Scholar] [CrossRef]
- Shen, Y.; Gao, X.; Chen, H.; Wei, Y.; Yang, H.; Gu, Y. Ultrathin C3N4 nanosheets-based oxidase-like 2D fluorescence nanozyme for dual-mode detection of organophosphorus pesticides. J. Hazard. Mater. 2023, 451, 131171. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Miao, Y.; Ding, Z.; Lu, Y. In situ formed silicon-based nanoparticles enabled highly efficient dual-mode biosensing of chlorpyrifos. Food Chem. 2023, 403, 134243. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; He, Y.; Wang, Y.; Song, G. Fluorine-free synthesis of Ti3C2 MQDs for smartphone-based fluorescent and colorimetric determination of acetylcholinesterase and organophosphorus pesticides. Microchim. Acta 2021, 189, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, M.; Cao, J.; She, Y.; Zhu, Y.; Ye, J.; El-Aty, A.A.; Hacımüftüoğlu, A.; Wang, J.; Lao, S. Dual-mode detection of organophosphate pesticides in pear and Chinese cabbage based on fluorescence and AuNPs colorimetric assays. Food Chem. 2021, 364, 130326. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Lin, D.; Chen, Z.; Qiu, P. A dual-mode nanoprobe for the determination of parathion methyl based on graphene quantum dots modified silver nanoparticles. Anal. Bioanal. Chem. 2020, 412, 5583–5591. [Google Scholar] [CrossRef]
- Cai, Y.; Qiu, Z.; Lin, X.; Zeng, W.; Cao, Y.; Liu, W.; Liu, Y. Self-assembled nanomaterials based on aggregation-induced emission of AuNCs: Fluorescence and colorimetric dual-mode biosensing of organophosphorus pesticides. Sensors Actuators B: Chem. 2020, 321. [Google Scholar] [CrossRef]
- Li, H.; Yan, X.; Lu, G.; Su, X. Carbon dot-based bioplatform for dual colorimetric and fluorometric sensing of organophosphate pesticides. Sens. Actuators B Chem. 2018, 260, 563–570. [Google Scholar] [CrossRef]
- Luo, Q.-J.; Li, Y.-X.; Zhang, M.-Q.; Qiu, P.; Deng, Y.-H. A highly sensitive, dual-signal assay based on rhodamine B covered silver nanoparticles for carbamate pesticides. Chin. Chem. Lett. 2017, 28, 345–349. [Google Scholar] [CrossRef]
- Liu, D.; Chen, W.; Wei, J.; Li, X.; Wang, Z.; Jiang, X. A Highly Sensitive, Dual-Readout Assay Based on Gold Nanoparticles for Organophosphorus and Carbamate Pesticides. Anal. Chem. 2012, 84, 4185–4191. [Google Scholar] [CrossRef]
- Keçili, R. , et al., 2 - Smartphone-based optical and electrochemical sensing, in Smartphone-Based Detection Devices, C. Hussain, Editor. 2021, Elsevier. p. 19-36.
- Zhu, H.; Liu, P.; Xu, L.; Li, X.; Hu, P.; Liu, B.; Pan, J.; Yang, F.; Niu, X. Nanozyme-Participated Biosensing of Pesticides and Cholinesterases: A Critical Review. Biosensors 2021, 11, 382. [Google Scholar] [CrossRef]
- Garefalaki, V.; Manco, G.; Porzio, E. Use of biosensors for rapid and sensitive detection of pesticides in food samples for food safety chemical risk assessment. EFSA J. 2022, 20, e200922. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).