Submitted:
07 July 2023
Posted:
10 July 2023
You are already at the latest version
Abstract
Keywords:
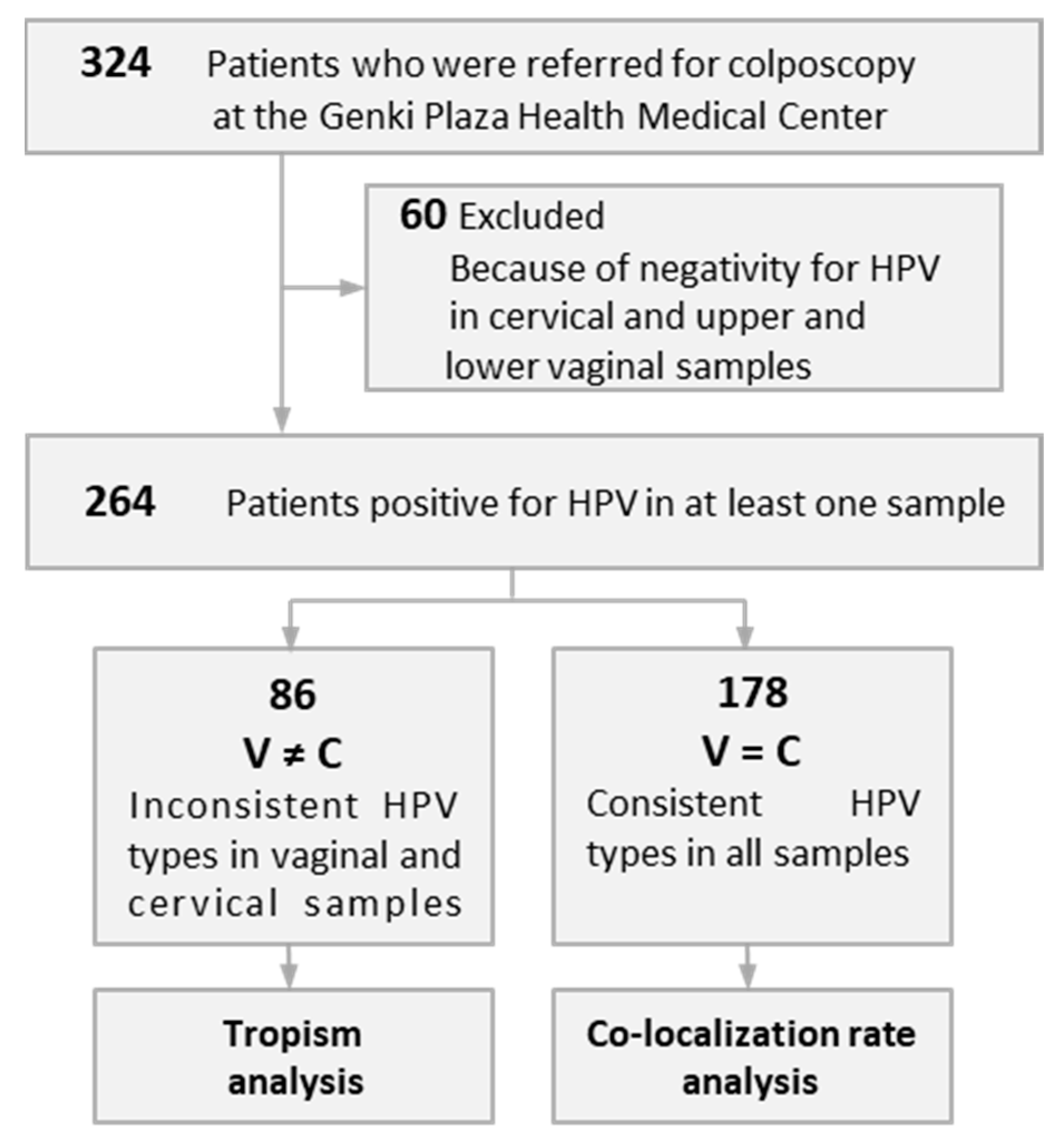
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients and Specimen Collection
4.2. HPV Genotyping Using LBC Samples
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Doorslaer, K.; Li, Z.; Xirasagar, S.; Maes, P.; Kaminsky, D.; Liou, D.; Sun, Q.; Kaur, R.; Huyen, Y.; McBride, A.A. The Papillomavirus Episteme: a major update to the papillomavirus sequence database. Nucleic Acids Res. 2017, 45, D499–D506. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human papillomaviruses. IARC Monogr. Eval. Carcinog. Risks Hum. 2007, 90, 1–636. [Google Scholar]
- de Sanjosé, S.; Brotons, M.; Pavón, M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Mirkovic, J.; Howitt, B.E.; Roncarati, P.; Demoulin, S.; Suarez-Carmona, M.; Hubert, P.; McKeon, F.D.; Xian, W.; Li, A.; Delvenne, P.; Crum, C.P.; Michael, H. Carcinogenic HPV infection in the cervical squamo-columnar junction. J. Pathol. 2015, 236, 265–271. [Google Scholar] [CrossRef]
- de Sanjosé, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.-R.; Vallejos, C.S.; Ruiz, P.A.d.; Lima, M.A.; Guimera, N.; Clavero, O.; Alejo, M.; Llombart-Bosch, A.; Cheng-Yang, C.; Tatti, S.A.; Kasamatsu, E.; Iljazovic, E.; Odida, M.; Prado, R.; Seoud, M.; Grce, M.; Usubutun, A.; Jain, A.; Suarez, G.A.H.; Lombardi, L.E.; Banjo, A.; Menéndez, C.; Domingo, E.J.; Velasco, J.; Nessa, A.; Chichareon, S.C.B.; Qiao, Y.L.; Lerma, E.; Garland, S.M.; Sasagawa, T.; Ferrera, A.; Hammouda, D.; Mariani, L.; Pelayo, A.; Steiner, I.; Oliva, E.; Meijer, C.J.; Al-Jassar, W.F.; Cruz, E.; Wright, T.C.; Puras, A.; Llave, C.L.; Tzardi, M.; Agorastos, T.; Garcia-Barriola, V.; Clavel, C.; Ordi, J.; Andújar, M.; Castellsagué, X.; Sánchez, G.I.; Nowakowski, A.M.; Bornstein, J.; Muñoz, N.; Bosch, F.X. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Bzhalava, D.; Guan, P.; Franceschi, S.; Dillner, J.; Clifford, G. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 2013, 445, 224–231. [Google Scholar] [CrossRef]
- Halec, G.; Alemany, L.; Lloveras, B.; Schmitt, M.; Alejo, M.; Bosch, F.X.; Tous, S.; Klaustermeier, J.E.; Guimerà, N.; Grabe, N.; Lahrmann, B.; Gissmann, L.; Quint, W.; Bosch, F.X.; de Sanjosé, S.; Pawlita, M. Pathogenic role of the eight probably/possibly carcinogenic HPV types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J. Pathol. 2014, 234, 441–451. [Google Scholar] [CrossRef]
- Münger, K.; Howley, P.M. Human papillomavirus immortalization and transformation functions. Virus Res. 2002, 89, 213–228. [Google Scholar] [CrossRef]
- Fehrmann, F.; Laimins, L.A. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene 2003, 22, 5201–5207. [Google Scholar] [CrossRef]
- De Villiers, E.-M.; Fauquet, C.; Broker, T.R.; Bernard, H.-U.; Zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef]
- Baldwin, S.; Santos, C.; Brown, E.M.; Nuño, T.; Giuliano, A.; Davis, J.; Garcia, F. Comparison of type-specific human papillomavirus data from self and clinician directed sampling. Gynecol. Oncol. 2005, 97, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Castle, P.E.; Jeronimo, J.; Schiffman, M.; Herrero, R.; Rodríguez, A.C.; Bratti, M.C.; Hildesheim, A.; Wacholder, S.; Long, L.R.; Neve, L.; Pfeiffer, R.; Burk, R.D. Age-related changes of the cervix influence human papillomavirus type distribution. Cancer Res. 2006, 66, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- D'Souza, G.; Burk, R.D.; Zhong, Y.; Minkoff, H.; Stewart Massad, L.; Xue, X.; Heather Watts, D.; Anastos, K.; Palefsky, J.M.; Levine, A.M.; Colie, C.; Castle, P.E.; Strickler, H.D. Cervicovaginal human papillomavirus (HPV)-infection before and after hysterectomy: evidence of different tissue tropism for oncogenic and nononcogenic HPV types in a cohort of HIV-positive and HIV-negative women. Int. J. Cancer 2012, 131, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Castle, P.E.; Schiffman, M.; Bratti, M.C.; Hildesheim, A.; Herrero, R.; Hutchinson, M.L.; Rodriguez, A.C.; Wacholder, S.; Sherman, M.E.; Kendall, H.; Viscidi, R.P.; Jeronimo, J.; Schussler, J.E.; Burk, R.D. A population-based study of vaginal human papillomavirus infection in hysterectomized women. J. Infect. Dis. 2004, 190, 458–467. [Google Scholar] [CrossRef]
- Castle, P.E.; Rodriguez, A.C.; Porras, C.; Herrero, R.; Schiffman, M.; Gonzalez, P.; Hildesheim, A.; Burk, R.D. A comparison of cervical and vaginal human papillomavirus. Sex. Transm. Dis. 2007, 34, 849–855. [Google Scholar] [CrossRef]
- Winer, R.L.; Hughes, J.P.; Feng, Q.; O’Reilly, S.; Kiviat, N.B.; Koutsky, L.A. Comparison of incident cervical and vulvar/vaginal human papillomavirus infections in newly sexually active young women. J. Infect. Dis. 2009, 199, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Tsimplaki, E.; Argyri, E.; Michala, L.; Kouvousi, M.; Apostolaki, A.; Magiakos, G.; Papassideri, I.; Panotopoulou, E. Human papillomavirus genotyping and e6/e7 mRNA expression in Greek women with intraepithelial neoplasia and squamous cell carcinoma of the vagina and vulva. J. Oncol. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Zhang, S.; Saito, M.; Okayama, K.; Okodo, M.; Kurose, N.; Sakamoto, J.; Sasagawa, T. HPV Genotyping by molecular mapping of tissue samples in vaginal squamous intraepithelial neoplasia (VaIN) and vaginal squamous cell carcinoma (VaSCC). Cancers (Basel) 2021, 13, 3260. [Google Scholar] [CrossRef]
- Halec, G.; Schmitt, M.; Dondog, B.; Sharkhuu, E.; Wentzensen, N.; Gheit, T.; Tommasino, M.; Kommoss, F.; Bosch, F.X.; Franceschi, S.; Clifford, G.; Gissmann, L.; Pawlita, M. Biological activity of probable/possible high-risk human papillomavirus types in cervical cancer. Int. J. Cancer 2013, 132, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Okayama, K.; Kimura, H.; Teruya, K.; Ishii, Y.; Fujita, K.; Fujii, M.; Oda, M.; Sasagawa, T.; Okodo, M. Correlation between human papillomavirus codetection profiles and cervical intraepithelial neoplasia in Japanese women. Microorganisms 2020, 8, 1863. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Bosch, F.X.; De Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M.; Group, I.A.f.R.o.C.M.C.C.S. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Saslow, D.; Solomon, D.; Lawson, H.W.; Killackey, M.; Kulasingam, S.L.; Cain, J.; Garcia, F.A.; Moriarty, A.T.; Waxman, A.G.; Wilbur, D.C.; Wentzensen, N.; Downs, L.S.; Spitzer, M.; Moscicki, A.-B.; Franco, E.L.; Stoler, M.H.; Schiffman, M.; Castle, P.E.; Myers, E.R.; Chelmow, D.; Herzig, A.; Kim, J.J.; Kinney, W.; Herschel, W.L.; Waldman, J. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J. Clin. 2012, 62, 147–172. [Google Scholar] [CrossRef]
- Arbyn, M.; Ronco, G.; Anttila, A.; Meijer, C.J.; Poljak, M.; Ogilvie, G.; Koliopoulos, G.; Naucler, P.; Sankaranarayanan, R.; Peto, J. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012, 30, F88–F99. [Google Scholar] [CrossRef]
- Castle, P.E.; Schiffman, M.; Burk, R.D.; Wacholder, S.; Hildesheim, A.; Herrero, R.; Bratti, M.C.; Sherman, M.E.; Lorincz, A. Restricted cross-reactivity of hybrid capture 2 with nononcogenic human papillomavirus types. Cancer Epidemiol. Biomarkers Prev 2002, 11, 1394–1399. [Google Scholar]
- Okodo, M.; Okayama, K.; Teruya, K.; Tanabe, K.; Ito, C.; Ishii, Y.; Fujii, M.; Kimura, H.; Oda, M. Effects of menstrual cycle on the accumulation of human papillomavirus-infected cells exfoliated from the cervix that drift into the vagina. Microorganisms 2022, 10, 693. [Google Scholar] [CrossRef]
- Khan, M.J.; Massad, L.S.; Kinney, W.; Gold, M.A.; Mayeaux Jr, E.; Darragh, T.M.; Castle, P.E.; Chelmow, D.; Lawson, H.W.; Huh, W.K. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol. Oncol. 2016, 141, 364–370. [Google Scholar] [CrossRef]
- Murta, E.F.; Neves Junior, M.A.; Sempionato, L.R.F.; Costa, M.C.; Maluf, P.J. Vaginal intraepithelial neoplasia: clinical-therapeutic analysis of 33 cases. Arch. Gynecol. Obstet. 2005, 272, 261–264. [Google Scholar] [CrossRef]
- Frega, A.; Sopracordevole, F.; Assorgi, C.; Lombardi, D.; De Sanctis, V.; Catalano, A.; Matteucci, E.; Milazzo, G.N.; Ricciardi, E.; Moscarini, M. Vaginal intraepithelial neoplasia: a therapeutical dilemma. Anticancer Res. 2013, 33, 29–38. [Google Scholar]
- Kohaar, I.; Thakur, N.; Salhan, S.; Batra, S.; Singh, V.; Sharma, A.; Sodhani, P.; Das, B.C.; Sarkar, D.P.; Bharadwaj, M. TNF α–308G/A polymorphism as a risk factor for HPV associated Cervical Cancer in Indian population. Anal. Cell. Pathol. 2007, 29, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kohaar, I.; Hussain, S.; Thakur, N.; Tiwari, P.; Nasare, V.; Batra, S.; Singh, V.; Bhambani, S.; Das, B.C.; Sarkar, D.P.; Bharadwaj, M. Association between human leukocyte antigen class II alleles and human papillomavirus-mediated cervical cancer in Indian women. Hum. Immunol. 2009, 70, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Truett, G.E.; Heeger, P.; Mynatt, R.L.; Truett, A.A.; Walker, J.A.; Warman, M.L. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 2000, 29, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Okodo, M.; Okayama, K.; Teruya, K.; Sasagawa, T. Uniplex E6/E7 PCR method detecting E6 or E7 genes in 39 human papillomavirus types. J. Med. Virol. 2018, 90, 981–988. [Google Scholar] [CrossRef]
| Prevalence (95% CI) % | |||
|---|---|---|---|
| Cervical samples | Vaginal samples | ||
| Upper | Lower | ||
| HPV types | |||
| HPV16 | 12.9 (8.8–16.9) | 10.6 (6.9–14.3) | 4.5 (2.0–7.1) |
| HPV18 | 1.9 (0.2–3.5) | 1.1 (0.0–2.4) | 0 |
| HPV31 | 9.5 (5.9–13) | 7.6 (4.4–10.8) | 2.7 (0.7–4.6) |
| HPV33 | 2.7 (0.7–4.6) | 2.3 (0.5–4.1) | 1.1 (0.0–2.4) |
| HPV35 | 0.4 (0.1–1.1) | 0.4 (0.0–1.1) | 0.4 (0.0–1.1) |
| HPV39 | 3.8 (1.5–6.1) | 3.4 (1.2–5.6) | 1.9 (0.2–3.5) |
| HPV45 | 1.1 (0.0–2.4) | 1.1 (0.0–2.4) | 0.8 (0.0–1.8) |
| HPV51 | 13.6 (9.5–17.8) | 10.2 (6.6–13.9) | 4.5 (2.0–7.1) |
| HPV52 | 25.0 (19.8–30.2) | 19.3 (14.6–24.1) | 11.4 (7.5–15.2) |
| HPV56 | 9.5 (5.9–13.0) | 8.0 (4.7–11.2) | 5.3 (2.6–8.0) |
| HPV58 | 12.9 (8.8–16.9) | 12.1 (8.2–16.1) | 5.3 (2.6–8.0) |
| HPV59 | 4.2 (1.8–6.6) | 4.5 (2.0–7.1) | 3.4 (1.2–5.6) |
| HPV26 | 0.4 (0.0–1.1) | 0 | 0 |
| HPV30 | 0.8 (0.0–1.8) | 0.8 (0.0–1.8) | 0.4 (0.0–1.1) |
| HPV34 | 1.9 (0.2–3.5) | 2.3 (0.5–4.1) | 1.1 (0.0–2.4) |
| HPV53 | 8.0 (4.7–11.2) | 8.3 (5.0–11.7) | 4.2 (1.8–6.6) |
| HPV66 | 8.3 (5.0–11.7) | 8.3 (5.0–11.7) | 5.7 (2.9–8.5) |
| HPV67 | 3.8 (1.5–6.1) | 2.7 (0.7–4.6) | 1.5 (0.0–3.0) |
| HPV69 | 1.1 (0.0–2.4) | 1.1 (0.0–2.4) | 0.8 (0.0–1.8) |
| HPV68 | 3.0 (1.0–5.1) | 3.0 (1.0–5.1) | 1.5 (0.0–3.0) |
| HPV70 | 1.5 (0.0–3.0) | 0 | 0.8 (0.0–1.8) |
| HPV73 | 0 | 0.4 (0.0–1.1) | 0 |
| HPV82 | 7.6 (4.4–10.8) | 6.8 (3.8–9.9) | 0 |
| HPV6 | 1.5 (0.0–3.0) | 1.5 (0.0–3.0) | 1.5 (0.0–3.0) |
| HPV11 | 0.4 (0.0–1.1) | 0.4 (0.0–1.1) | 0 |
| HPV40 | 2.7 (0.7–4.6) | 3.0 (1.0–5.1) | 0 |
| HPV42 | 6.4 (3.5–9.4) | 7.2 (4.1–10.3) | 4.5 (2.0–7.1) |
| HPV44 | 0.4 (0.0–1.1) | 0.4 (0.0–1.1) | 0.4 (0.0–1.1) |
| HPV54 | 3.4 (1.2–5.6) | 3.8 (1.5–6.1) | 1.9 (0.2–3.5) |
| HPV55 | 1.9 (0.3–3.5) | 2.3 (0.5–4.1) | 1.9 (0.2–3.5) |
| HPV61 | 3.4 (1.2–5.6) | 3.4 (1.2–5.6) | 1.1 (0.0–2.4) |
| HPV62 | 4.9 (2.3–7.5) | 5.7 (2.9–8.5) | 2.7 (0.7–4.6) |
| HPV71 | 2.7 (0.7–4.6) | 2.7 (0.7–4.6) | 0.4 (0.0–1.1) |
| HPV74 | 8.7 (5.3–12.1) | 9.8 (6.3–13.4) | 5.7 (2.9–8.5) |
| HPV81 | 3.4 (1.2–5.6) | 4.2 (1.8–6.6) | 1.9 (0.2–3.5) |
| HPV84 | 0.8 (0.0–1.8) | 1.5 (0.0–3.0) | 1.1 (0.0–2.4) |
| HPV89 | 0.8 (0.0–1.8) | 0.8 (0.0–1.8) | 0.4 (0.0–1.1) |
| HPV90 | 4.5 (2.0–7.1) | 5.3 (2.6–8.0) | 3.4 (1.2–5.6) |
| Risk groups | |||
| High-risk type | 95.1 (92.5–97.7) | 80.7 (75.9–85.4) | 41.3 (35.3–47.2) |
| Possible high-risk type | 34.5 (29.1–40.6) | 35.2 (29.5–41.0) | 17.4 (12.8–22.0) |
| Low-risk type | 39.0 (33.1–44.9) | 50.4 (44.3–56.4) | 26.9 (21.5–32.2) |
| α phylogenetic groups | |||
| α-5 | 21.6 (16.6–26.6) | 18.2 (13.5–22.8) | 6.8 (3.8–9.9) |
| α-7 | 15.2 (10.8–19.5) | 14.8 (10.5–19.1) | 8.3 (5.0–11.7) |
| α-9 | 65.2 (59.4–70.9) | 54.9 (48.9–60.9) | 26.9 (21.5–32.2) |
| α-6 | 25.8 (20.8–31.4) | 25.4 (20.1–30.6) | 15.5 (11.2–19.9) |
| α-11/α-1/α-8/α-10/α-13/α-3/α-15 | 40.9 (35.0–46.8) | 53.0 (47.0–59.1) | 28.0 (22.6–33.4) |
| Cases | Age | HPV types | All HPV types detected | |||
|---|---|---|---|---|---|---|
| Preferentially infected the cervix | Preferentially infected the vagina | Cervical samples | Vaginal samples | |||
| Upper | Lower | |||||
| 1 | 51 | 56 (HR, α-6) | − | 56, 62 | 62 | Negative |
| 2 | 37 | − | 81 (LR, α-3) | Negative | 81 | 81 |
| 3 | 31 | 31 (HR, α-9) | − | 31, 81, 82 | 81, 82 | Negative |
| 4 | 34 | − | 16 (HR, α-9) | 39, 56, 71 | 16, 39, 56, 71 | 39, 56 |
| 5 | 24 | − | 90 (LR, α-15) | 59 | 59, 90 | 59, 90 |
| 6 | 41 | 16 (HR, α-9) | − | 16 | Negative | Negative |
| 7 | 50 | − | 84 (LR, α-3) | Negative | 84 | 84 |
| 8 | 31 | 52 (HR, α-9) | − | 16, 45, 52, 40 | 16, 45, 40 | 16, 45, 40 |
| 9 | 40 | − | 56 (HR, α-6) | Negative | 56 | 56 |
| 10 | 50 | − | 66 (pHR, α-6) | 51, 56, 58 | 51, 56, 58 | 51, 56, 66 |
| 11 | 35 | 52 (HR, α-9) | − | 52, 53, 74 | 53, 74 | 53, 74 |
| 12 | 26 | − | 59 (HR, α-7) | 56, 66, 74, 90 | 56, 59, 66, 74, 90 | 56, 59, 66, 74, 90 |
| 13 | 40 | 56 (HR, α-6) | − | 56 | Negative | Negative |
| 14 | 50 | 51 (HR, α-5) | − | 51, 58 | 58 | 58 |
| 15 | 45 | − | 34 (pHR, α-11) | Negative | Negative | 34 |
| 16 | 26 | 58 (HR, α-9) | − | 52, 58 | 52 | 52 |
| 17 | 58 | − | 52 (HR, α-9) | 34 | 52, 34 | 34 |
| 18 | 45 | − | 34 (pHR, α-11) | 31,74 | 31, 34, 74 | Negative |
| 19 | 26 | − | 54 (LR, α-13), 66 (pHR, α-5), 74 (LR, α-10), 90 (LR, α-15) | 56 | 56, 54, 66 | 56, 54, 66, 74, 90 |
| 20 | 42 | 39 (HR, α-7) | − | 39, 58 | 58 | 58 |
| 21 | 43 | 51 (HR, α-5) | − | 51 | Negative | Negative |
| 22 | 31 | 52 (HR, α-9) | − | 52 | Negative | Negative |
| 23 | 47 | 16 (HR, α-9) | − | 16 | Negative | Negative |
| 24 | 42 | 51 (HR, α-5) | − | 51 | Negative | Negative |
| 25 | 30 | − | 40 (LR, α-8) | 39 | 39, 40 | 39, 40 |
| 26 | 45 | 31 (HR, α-9) | − | 31,74 | 74 | 74 |
| 27 | 28 | 16 (HR, α-9) | − | 16, 52, 56, 55, 74, 81 | 52, 56, 55, 74, 81 | 52, 56, 55, 74, 81 |
| 28 | 43 | 58 (HR, α-9) | − | 52, 58 | 52 | 52 |
| 29 | 56 | 16 (HR, α-9) | − | 16, 42 | 42 | 42 |
| 30 | 53 | − | 58 (HR, α-9), 82 (pHR, α-5) | 45, 51, 90 | 45, 51, 58, 82, 90 | 90 |
| 31 | 25 | 56 (HR, α-6) | − | 56, 34 | 34 | 34 |
| 32 | 32 | 16 (HR, α-9) | − | 16 | Negative | Negative |
| 33 | 46 | 52 (HR, α-9) | − | 52 | Negative | Negative |
| 34 | 53 | 51 (HR, α-5), 82 (pHR, α-5) | − | 51, 82 | Negative | Negative |
| 35 | 34 | 82 (pHR, α-5) | − | 51, 82 | 51 | 51 |
| 36 | 53 | 52 (HR, α-9) | − | 52 | Negative | Negative |
| 37 | 29 | 52 (HR, α-9) | − | 33, 52 | 33 | Negative |
| 38 | 48 | − | 62 (LR, α-3) | 52, 53, 81 | 52, 53, 62, 81 | 53, 62 |
| 39 | 51 | 58 (HR, α-9) | − | 51, 58 | 51 | 51 |
| 40 | 32 | − | 81 (LR, α-3) | 31 | 31, 81 | 31, 81 |
| 41 | 31 | − | 42 (LR, α-1) | 58 | 58 | 58, 42 |
| 42 | 50 | 66 (pHR, α-6) | − | 40, 66 | 40 | Negative |
| 43 | 51 | 51 (HR, α-5) | − | 51, 56, 58 | 56, 58 | 56 |
| 44 | 40 | 56 (HR, α-6) | − | 56 | Negative | Negative |
| 45 | 49 | 67 (pHR, α-9) | − | 67 | Negative | Negative |
| 46 | 48 | 52 (HR, α-9) | − | 52 | Negative | Negative |
| 47 | 59 | − | 74 (LR, α-10) | 44 | 44, 74 | 44 |
| 48 | 51 | − | 11 (LR, α-10) | 35, 67 | 35, 11, 67 | 35 |
| 49 | 33 | 31 (HR, α-9) | − | 31, 58, 74 | 58, 74 | 58, 74 |
| 50 | 26 | − | 55 (LR, α-10) | 52, 56, 59, 30, 54, 74 | 52, 56, 59, 30, 54, 55, 74 | 52, 56, 59, 30, 54, 55, 74 |
| 51 | 37 | 52 (HR, α-9) | − | 52 | Negative | Negative |
| 52 | 37 | − | 42 (LR, α-1), 62 (LR, α-3) | 51, 53, 82 | 51, 42, 53, 62, 82 | 51, 42, 53, 62, 82 |
| 53 | 54 | 31 (HR, α-9) | 58 (HR, α-9), 66 (pHR, α-6) | 31, 51, 82, 6b | 51, 58, 6b, 82 | 6b, 66, 82 |
| 54 | 57 | 16 (HR, α-9) | − | 16, 42 | 42 | 42 |
| 55 | 31 | 52 (HR, α-9) | − | 31, 52, 58, 70 | 31, 58, 70 | 31, 58, 70 |
| 56 | 40 | 52 (HR, α-9) | − | 52 | Negative | Negative |
| 57 | 51 | 82 (pHR, α-5) | − | 51, 58, 82 | 51, 58 | 51, 58 |
| 58 | 65 | 53 (pHR, α-6) | − | 53 | Negative | Negative |
| 59 | 28 | − | 53 (pHR, α-6), 74 (LR, α-10) | 16, 52 | 16, 52, 53, 74 | 16, 52, 53, 74 |
| 60 | 46 | − | 74 (LR, α-10) | 31 | 31, 74 | 74 |
| 61 | 30 | 66 (pHR, α-6) | − | 53, 66 | 53 | 53 |
| 62 | 37 | 52 (HR, α-9) | − | 52 | Negative | Negative |
| 63 | 32 | 31 (HR, α-9) | − | 31 | Negative | Negative |
| 64 | 25 | − | 84 (LR, α-3) | Negative | 84 | 84 |
| 65 | 38 | 18 (HR, α-7) | − | 18 | Negative | Negative |
| 66 | 47 | − | 66 (pHR, α-6), 90 (LR, α-15) | 16 | 16, 66, 90 | Negative |
| 67 | 54 | 52 (HR, α-9) | − | 52 | Negative | Negative |
| 68 | 28 | − | 90 (LR, α-15) | 42 | 42 | 42, 90 |
| 69 | 40 | 16 (HR, α-9) | 59 (HR, α-7) | 16, 74 | 59, 74 | 74 |
| 70 | 43 | − | 53 (pHR, α-6) | 18 | 18, 53 | 18, 53 |
| 71 | 33 | 51 (HR, α-5) | − | 51, 74 | 74 | 74 |
| 72 | 42 | − | 67 (pHR, α-9) | Negative | Negative | 67 |
| 73 | 30 | − | 74 (LR, α-10) | 16, 39 | 16, 39 | 16, 39, 74 |
| 74 | 49 | 67 (pHR, α-9) | − | 67 | Negative | Negative |
| 75 | 26 | 56 (HR, α-6) | − | 56 | Negative | Negative |
| 76 | 34 | 51 (HR, α-5) | − | 51 | Negative | Negative |
| 77 | 27 | − | 54 (LR, α-13) | 34 | 34, 54 | 34, 54 |
| 78 | 50 | 66 (pHR, α-6) | − | 40, 66 | 40 | 40 |
| 79 | 46 | − | 73 (pHR, α-11) | 54, 61 | 61, 73 | 54,61, 73 |
| 80 | 51 | 58 (HR, α-9) | − | 51, 56, 58 | 51, 56 | 51, 56 |
| 81 | 35 | 26 (pHR, α-5) | − | 26, 90 | 90 | Negative |
| 82 | 56 | − | 89 (LR, α-3) | Negative | Negative | 89 |
| 83 | 26 | 52 (HR, α-9) | − | 52, 59 | 59 | Negative |
| 84 | 29 | 33(HR, α-9), 52 (HR, α-9) | − | 33, 52 | Negative | Negative |
| 85 | 28 | − | 42 (LR, α-1) | 16, 54, 82 | 16, 42, 54, 82 | 16, 54, 82 |
| 86 | 27 | 18 (HR, α-7), 59 (HR, α-7) | − | 18, 31, 52, 59, 55, 67, 74, 90 | 31, 52, 55, 67, 74, 90 | 31, 55, 67, 74, 90 |
| Preferentially infected | Ratio(cervix / vagina) | |||
|---|---|---|---|---|
| N | Cervix | Vagina | ||
| Risk groups | ||||
| High-risk type | 54 | 87.0%** | 13.0% | 6.7 |
| Possible high-risk type | 21 | 47.6% | 52.4% | 0.9 |
| Low-risk type | 24 | 0% | 100.0%** | 0.0 |
| α phylogenetic groups | ||||
| α-5 | 12 | 91.7%* | 8.3% | 11.0 |
| α-7 | 6 | 66.7% | 33.3% | 2.0 |
| α-9 | 38 | 86.8%** | 13.2% | 6.6 |
| α-6 | 16 | 56.3% | 43.8% | 1.3 |
| α-11/α-1/α-8/α-10/α-13/α-3/α-15 | 27 | 0% | 100.0%** | 0.0 |
| Combination of infectingHPV types | Samples (n) | Colocalization rate by LR typesin cervical samples | Colocalization rate by HR typesin vaginal samples | ||
|---|---|---|---|---|---|
| LR | 18 | 34.8% | − | ||
| LR | + pHR | 8 | |||
| LR | + HR | 24 | 73.6% | ||
| LR | + HR | + pHR | 12 | ||
| HR | 79 | − | |||
| HR | + pHR | 16 | |||
| pHR | 21 | − | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





