Submitted:
06 July 2023
Posted:
10 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Farmers
2.1.1. Farmers Demographic Characteristics and Farming Practices
2.1.2. Knowledge and Perceptions on Antimicrobials, Antibiotics, and Antibiotic Resistance among Farmers
2.1.3. Practices on Antimicrobial Use among Farmers
2.2. Animal Health Service Providers (AHSP)
2.2.1. Demographic Characteristics of the Veterinary Professionals and Paraprofessionals
2.2.2. Knowledge, Attitudes, and Practices towards Antimicrobials Use and AMR by the Animal Health Professionals.
2.3. One Health Practitioners
2.3.1. Demographic Characteristics
2.3.2. AMU/AMR Knowledge Practices and Perceptions among One Health Practitioners (OHPs)
3. Discussion
3.1. Farmers
3.2. Animal Health Service Providers (AHSP)
3.3. University, Research and One Health Partners
4. Materials and Methods
4.1. Survey Design
4.2. Sample Size Determinations
4.3. Survey Population
4.4. Survey Procedure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Joshi, M.P.; Hafner, T.; Twesigye, G.; Ndiaye, A.; Kiggundu, R.; Mekonnen, N.; et al. Strengthening multisectoral coordination on antimicrobial resistance: a landscape analysis of efforts in 11 countries. J Pharm Policy Pract 2021, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Wernli, D.; Harbarth, S.; Levrat, N.; Pittet, D. A ‘whole of United Nations approach’ to tackle antimicrobial resistance? A mapping of the mandate and activities of international organisations. BMJ Glob. Health 2022, 7, e008181. [Google Scholar] [CrossRef]
- Gajdács, M.; Urbán, E.; Stájer, A.; Baráth, Z. Antimicrobial Resistance in the Context of the Sustainable Development Goals: A Brief Review. Eur J Investig Health Psychol Educ 2021, 11, 71–82. [Google Scholar] [CrossRef]
- Jasovský, D.; Littmann, J.; Zorzet, A.; Cars, O. Antimicrobial resistance-a threat to the world’s sustainable development. Ups J Med Sci 2016, 121, 159–164. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect Drug Resist 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antimicrobial resistance in the farm-to-plate continuum: more than a food safety issue. Future Sci 2021, OA 7, FSO692. [Google Scholar] [CrossRef]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Hardcastle, T.C.; Haque, M.; et al. Surveillance of antimicrobial resistance in low- and middle-income countries: a scattered picture. Antimicrob Resist Infect Control 2021, 10, 63. [Google Scholar] [CrossRef]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob Health 2019, 4, e002104. [Google Scholar] [CrossRef]
- Sulis, G.; Sayood, S.; Gandra, S. Antimicrobial resistance in low- and middle-income countries: current status and future directions. Expert Rev Anti Infect Ther 2022, 20, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Bett, B. Situation analysis on antimicrobial resistance surveillance and control in Kenya Antimicrobial Resistance Research Hub, 2018, [WWW Document]. URL https://amr.cgiar.org/case-study/situation-analysis-antimicrobial-resistance-surveillance-and-control-kenya (accessed 07.02.2022).
- Maina, M.; Mwaniki, P.; Odira, E.; Kiko, N.; McKnight, J.; Schultsz, C.; et al. Antibiotic use in Kenyan public hospitals: Prevalence, appropriateness and link to guideline availability. Int J Infect Dis 2020, 99, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.A.; Vlieghe, E.; Mendelson, M.; Wertheim, H.; Ndegwa, L.; Villegas, M.V.; et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect 2017, 23, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Wesangula, E.N.; Githii, S.; Ndegwa, L. Implementing the national action plan on antimicrobial resistance in Kenya: Global expectations, national realities. Int J Infect Dis 2020, 101, 41. [Google Scholar] [CrossRef]
- Herrero, M.; Grace, D.; Njuki, J.; Johnson, N.; Enahoro, D.; Silvestri, S.; Rufino, M. The roles of livestock in developing countries. Anim 2012, 7, 1–16. [Google Scholar] [CrossRef]
- Thornton, P.K.; Rosenstock, T.; Förch, W.; Lamanna, C.; Bell, P.; Henderson, B.; Herrero, M. A Qualitative Evaluation of CSA Options in Mixed Crop-Livestock Systems in Developing Countries, in: Lipper, L.; McCarthy, N., Zilberman, D., Asfaw, S., Eds.; Branca, G. (Eds.), Climate Smart Agriculture : Building Resilience to Climate Change, Natural Resource Management and Policy. Springer International Publishing, Cham, 2018; pp. 385–423. [Google Scholar] [CrossRef]
- Salaheen, S.; Chowdhury, N.; Hanning, I.; Biswas, D. Zoonotic bacterial pathogens and mixed crop-livestock farming. Poult Sci 2015, 94, 1398–1410. [Google Scholar] [CrossRef]
- Ninh, L.K. Economic role of education in agriculture: evidence from rural Vietnam. J Econ Dev 2020, 23, 47–58. [Google Scholar] [CrossRef]
- Paltasingh, K.R.; Goyari, P. Impact of farmer education on farm productivity under varying technologies: case of paddy growers in India. Agric Food Econ 2018, 6, 7. [Google Scholar] [CrossRef]
- Phares, C.A.; Danquah, A.; Atiah, K.; Agyei, F.K.; Michael, O.-T. Antibiotics utilization and farmers’ knowledge of its effects on soil ecosystem in the coastal drylands of Ghana. PLoS ONE 2020, 15, e0228777. [Google Scholar] [CrossRef]
- Caudell, M.A.; Dorado-Garcia, A.; Eckford, S.; Creese, C.; Byarugaba, D.K.; Afakye, K.; et al. Towards a bottom-up understanding of antimicrobial use and resistance on the farm: A knowledge, attitudes, and practices survey across livestock systems in five African countries. PLoS ONE 2020, 15, e0220274. [Google Scholar] [CrossRef]
- Mouiche, M.M.M.; Moffo, F.; Betsama, J.D.B.; Mapiefou, N.P.; Mbah, C.K.; Mpouam, S.E.; et al. Challenges of antimicrobial consumption surveillance in food-producing animals in sub-Saharan African countries: Patterns of antimicrobials imported in Cameroon from 2014 to 2019. J Glob Antimicrob Resist 2020, 22, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Afakye, K.; Kiambi, S.; Koka, E.; Kabali, E.; Dorado-Garcia, A.; Amoah, A.; et al. The Impacts of Animal Health Service Providers on Antimicrobial Use Attitudes and Practices: An Examination of Poultry Layer Farmers in Ghana and Kenya. Antibiotics 2020, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.A.; Pinchbeck, G.L.; Fèvre, E.M.; Williams, N.J. A Cross-Sectional Survey of the Knowledge, Attitudes, and Practices of Antimicrobial Users and Providers in an Area of High-Density Livestock-Human Population in Western Kenya. Front Vet Sci 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Doidge, C.; Ruston, A.; Lovatt, F.; Hudson, C.; King, L.; Kaler, J. Farmers’ Perceptions of Preventing Antibiotic Resistance on Sheep and Beef Farms: Risk, Responsibility, and Action. Front Vet Sci 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Adekanye, U.O.; Ekiri, A.B.; Galipó, E.; Muhammad, A.B.; Mateus, A.; La Ragione, R.M.; et al. Knowledge, Attitudes and Practices of Veterinarians towards Antimicrobial Resistance and Stewardship in Nigeria. Antibiotics 2020, 9, E453. [Google Scholar] [CrossRef]
- Mangesho, P.E.; Caudell, M.A.; Mwakapeje, E.R.; Ole-Neselle, M.; Kimani, T.; Dorado-García, A.; et al. Knowing Is Not Enough: A Mixed-Methods Study of Antimicrobial Resistance Knowledge, Attitudes, and Practises among Maasai Pastoralists. Front Vet Sci 2021, 8. [Google Scholar] [CrossRef]
- Aziz, M.A.; Khan, A.H.; Adnan, M.; Ullah, H. Traditional uses of medicinal plants used by Indigenous communities for veterinary practices at Bajaur Agency, Pakistan. J Ethnobiol Ethnomedicine 2018, 14, 11. [Google Scholar] [CrossRef]
- Jayakumar, S.; Baskaran, N.; Arumugam, R.; Sathiskumar, S.; Pugazhenthi, M. Herbal medicine as a live practice for treating livestock ailments by indigenous people: A case study from the Konar community of Tamil Nadu. South Afr J Bot 2018, 118, 23–32. [Google Scholar] [CrossRef]
- Howland, O. Patterns of use, gathering, processing and administration of herbal and alternative medicines among people and livestock in Kenya: a study of local knowledge for One Health. J Glob Health Rep 2021, 5, e2021042. [Google Scholar] [CrossRef]
- Quynh, H.L.; Bich, T.N.T.; Hoang, L.T.; Erickson, V.I.; Padungtod, P. Quality testing of veterinary antimicrobial products used for livestock in Vietnam, 2018–2019. PLoS ONE 2021, 16, e0247337. [Google Scholar] [CrossRef]
- Alhaji, N.B.; Isola, T.O. Antimicrobial usage by pastoralists in food animals in North-central Nigeria: The associated socio-cultural drivers for antimicrobials misuse and public health implications. One Health 2018, 6, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Azabo, R.; Mshana, S.; Matee, M.; Kimera, S.I. Antimicrobial usage in cattle and poultry production in Dar es Salaam, Tanzania: pattern and quantity. BMC Vet Res 2022, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Gemeda, B.A.; Amenu, K.; Magnusson, U.; Dohoo, I.; Hallenberg, G.S.; Alemayehu, G.; et al. Antimicrobial Use in Extensive Smallholder Livestock Farming Systems in Ethiopia: Knowledge, Attitudes, and Practices of Livestock Keepers. Front Vet Sci 2020, 7. [Google Scholar] [CrossRef]
- Hoelzer, K.; Bielke, L.; Blake, D.P.; Cox, E.; Cutting, S.M.; Devriendt, B.; et al. Vaccines as alternatives to antibiotics for food producing animals. Part 1: challenges and needs. Vet Res 2018, 49, 64. [Google Scholar] [CrossRef] [PubMed]
- Raasch, S.; Postma, M.; Dewulf, J.; Stärk, K.D.C.; Grosse Beilage, E. Association between antimicrobial usage, biosecurity measures as well as farm performance in German farrow-to-finish farms. Porc Health Manag 2018, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Gichohi, P. The role of record keeping and maintenance in enhancing decision making among smallholder dairy farmers in Gitugi Ward in Murang’a County, Kenya. Inf Dev 2019, 36, 026666691987972. [Google Scholar] [CrossRef]
- Kuteesa, A.; Kyotalimye, M. Documentation and data handling: How can Africa promote record keeping and investment in data management? Afr J Food Agric Nutr Dev 2019, 19, 14171–14189. [Google Scholar] [CrossRef]
- Tham-Agyekum, E.; Appiah, P.; Nimoh, F. Assessing Farm Record Keeping Behaviour among Small-Scale Poultry Farmers in the Ga East Municipality. J Agric Sci 2010, 2. [Google Scholar] [CrossRef]
- Cuong, N.V.; Phu, D.H.; Van, N.T.B.; Dinh Truong, B.; Kiet, B.T.; Hien, B.V.; et al. High-Resolution Monitoring of Antimicrobial Consumption in Vietnamese Small-Scale Chicken Farms Highlights Discrepancies Between Study Metrics. Front Vet Sci 2019, 6, 174. [Google Scholar] [CrossRef]
- Schar, D.; Sommanustweechai, A.; Laxminarayan, R.; Tangcharoensathien, V. Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance. PLoS Med 2018, 15, e1002521. [Google Scholar] [CrossRef]
- Muloi, D.; Fèvre, E.M.; Bettridge, J.; Rono, R.; Ong’are, D.; Hassell, J.M.; et al. A cross-sectional survey of practices and knowledge among antibiotic retailers in Nairobi, Kenya. J Glob Health 2019, 9, 020412. [Google Scholar] [CrossRef] [PubMed]
- Fasina, F.O.; LeRoux-Pullen, L.; Smith, P.; Debusho, L.K.; Shittu, A.; Jajere, S.M.; et al. Knowledge, Attitudes, and Perceptions Associated With Antimicrobial Stewardship Among Veterinary Students: A Multi-Country Survey From Nigeria, South Africa, and Sudan. Front Public Health 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Md.S.; Rafa, N. Common Barriers, Attitudes and Practices of Veterinary Practitioners Regarding Antimicrobial Resistance and Stewardship in Bangladesh. Open Vet Sci 2021, 1, 72–80. [Google Scholar] [CrossRef]
- Odoi, A.; Samuels, R.; Carter, C.N.; Smith, J. Antibiotic prescription practices and opinions regarding antimicrobial resistance among veterinarians in Kentucky, USA. PLoS ONE 2021, 16, e0249653. [Google Scholar] [CrossRef]
- Tebug, S.F.; Mouiche, M.M.M.; Abia, W.A.; Teno, G.; Tiambo, C.K.; Moffo, F.; Awah-Ndukum, J. Antimicrobial use and practices by animal health professionals in 20 sub-Saharan African countries. Prev Vet Med 2021, 186, 105212. [Google Scholar] [CrossRef] [PubMed]
- Aworh, M.K.; Kwaga, J.K.P.; Okolocha, E.C. Assessing knowledge, attitude, and practices of veterinarians towards antimicrobial use and stewardship as drivers of inappropriate use in Abuja, Nigeria. One Health Outlook 2021, 3, 25. [Google Scholar] [CrossRef]
- Chema, S.; Gathuma, J.M. Kenya: The development of private services and the role of the Kenya Veterinary Association. Rev Sci Tech Int Off Epizoot 2004, 23, 331–40; discussion 391. [CrossRef]
- Heffernan, C.; Misturelli, F. The Delivery of Veterinary Services to the Poor: Preliminary findings from Kenya. 2002, available at: https://assets.publishing.service.gov.uk/media/57a08d2aed915d3cfd001868/R7359a.pdf, (16.04.2023). 2023.
- Jaime, G.; Hobeika, A.; Figuié, M. Access to Veterinary Drugs in Sub-Saharan Africa: Roadblocks and Current Solutions. Front Vet Sci 2022, 8, 558973. [Google Scholar] [CrossRef]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat Rev Microbiol 2021, 19, 287–302. [Google Scholar] [CrossRef]
- Vekemans, J.; Hasso-Agopsowicz, M.; Kang, G.; Hausdorff, W.P.; Fiore, A.; Tayler, E.; et al. Leveraging Vaccines to Reduce Antibiotic Use and Prevent Antimicrobial Resistance: A World Health Organization Action Framework. Clin Infect Dis 2021, 73, e1011–e1017. [Google Scholar] [CrossRef]
- ReAct. AMR stakeholder mapping From ReAct Europe – Action on Antibiotic Resistance, 2016. Available at: https://www.reactgroup.org/uploads/Stakeholder%20Analysis_ReActForWHO.pdf, (16.04.2023). 2023.
- Meseko, C.; Olabisi, M.; Ehizibolo, D.; Muraina, I. Veterinary Pharmaceuticals and Antimicrobial Resistance in Developing Countries. In: Veterinary Medicine and Pharmaceuticals, Bekoe, S.O.; Saravanan, M.; Adosraku, R.K.; Ramkumar, P.K. IntechOpen Publisher (https://www.intechopen.com/) 2019. [CrossRef]
- De Briyne, N.; Atkinson, J.; Pokludová, L.; Borriello, S.P.; Price, S. Factors influencing antibiotic prescribing habits and use of sensitivity testing amongst veterinarians in Europe. Vet Rec 2013, 173, 475. [Google Scholar] [CrossRef]
| Parameters | Farmers n (%) |
Animal health service providers n (%) |
One Health professionals/ training institutions n (%) |
|---|---|---|---|
| Gender | |||
| Male | 249 (65.3) | 36 (76.6) | 17 (53.1) |
| Female | 132 (34.7) | 11 (23.4) | 15 (46.9) |
| Age Groups | |||
| < 20 | 3 (0.8) | 21 (44.7) | 0 (0.0) |
| 20 – 29 | 76 (20.0) | 14 (36.2) | 14 (43.8) |
| 30 – 39 | 117 (30.7) | 7 (14.9) | 4 (12.5) |
| 40 – 49 | 117 (30.7) | 3 (6.4) | 9 (28.1) |
| 50 – 59 | 54 (14.3) | 2 (4.3) | 2 (6.3) |
| ≥ 60 | 14 (3.7) | 0 (0.0) | 3 (9.4) |
| Level of Education | |||
| None | 115 (30.2) | 0 (0.0) | 0 (0.0) |
| Primary | 65 (17.1) | 0 (0.0) | 0 (0.0) |
| Secondary | 121 (31.8) | 0 (0.0) | 0 (0.0) |
| Diploma | 32 (8.4) | 5 (10.6) | 1 (3.1) |
| Degree | 41 (10.8) | 24 (51.1) | 16 (50.0) |
| Masters/PhD | 7 (1.9) | 18 (38.3) | 15 (46.9) |
| Years of experience in the livestock value chain | |||
| < 1 | 20 (5.3) | 2 (4.3) | 6 (18.8) |
| 1 – 5 | 166 (43.6) | 23 (48.7) | 9 (28.1) |
| 6 – 10 | 60 (15.8) | 8 (17.0) | 3 (9.4) |
| 11 – 15 | 52 (13.7) | 7 (14.9) | 5 (15.6) |
| > 15 | 83 (21.8) | 7 (14.9) | 9 (28.1) |
| Characteristics | Level of education | Years of farming experience | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No formal (n = 115) (%) | Basic (n = 186) (%) | Tertiary (n = 80) (%) | < 1 (n = 20) (%) | 1 – 5 (n = 166) (%) | 6 – 10 (n = 60) (%) | 11 – 15 (n = 52) (%) | > 15 (n = 83) (%) | ||
| Prevention of diseases at the farm | |||||||||
| Practice farm hygiene | 86 (74.8) | 118 (63.4) | 49 (61.3) | 11 (55.0) | 115 (69.3) | 33 (55.0) | 33 (63.5) | 61 (73.5) | |
| Use commercial medicine | 11 (9.6) | 62 (33.3) | 18 (22.5) | 1 (5.0) | 34 (20.5) | 25 (41.7) | 14 (26.9) | 17 (20.5) | |
| Use feed supplements | 14 (12.2) | 93 (50.0) | 51 (63.8) | 9 (45.0) | 78 (47.0) | 26 (43.3) | 19 (36.5) | 26 (31.3) | |
| Action taken when livestock is unwell | |||||||||
| Call agrovet shop | 17 (15.3) | 28 (15.8) | 20 (27.0) | 8 (40.0) | 34 (21.9) | 7 (11.7) | 4 (7.8) | 12 (15.0) | |
| Call neighbour/ friend | 4 (3.6) | 2 (1.1) | 1 (1.4) | 1 (5.0) | 3 (1.9) | 1 (1.7) | 0 (0.0) | 2 (2.5) | |
| Call a veterinarian | 18 (16.2) | 106 (58.6) | 33 (44.6) | 9 (45.0) | 74 (47.7) | 29 (48.3) | 18 (35.3) | 27 (33.8) | |
| Implement treatment of own animals | 71 (64.0) | 43 (23.8) | 19 (25.7) | 2 (10.0) | 42 (27.1) | 23 (38.3) | 27 (53.0) | 39 (48.8) | |
| Take no action | 1 (0.9) | 2 (1.1) | 1 (1.4) | 0 (0.0) | 2 (1.3) | 0 (0.0) | 2 (3.9) | 0 (0.0) | |
| Variable | Parameter | Number (%) |
|---|---|---|
| Reason for using antimicrobials (n = 235) | Treatment | 187 (79.6) |
| Advice by veterinarian | 25 (10.6) | |
| Prevent animals from sickness | 22 (9.4) | |
| Increase productivity | 1 (0.4) | |
| Why use alternative to antimicrobials (n = 209) | Prevent resistance | 145 (86.8) |
| Available cheap option | 16 (9.6) | |
| Prefer traditional | 2 (1.2) | |
| Veterinarian’s advice | 4 (2.4) | |
| Source of antimicrobials (n = 349) | Agro-veterinary shops | 171 (49.0) |
| Veterinarians | 164 (47.0) | |
| Friends | 14 (4.0) | |
| Frequency of antimicrobial use (n = 322) | Whenever animal is sick | 249 (77.3) |
| Weekly | 32 (9.9) | |
| Monthly | 33 (10.3) | |
| Never | 4 (1.2) | |
| Twice a year | 4 (1.2) | |
| Usually get prescription (n = 355) | Yes | 228 (63.7) |
| Sometimes | 98 (24.6) | |
| No | 29 (8.3) | |
| Source of advice before buying antimicrobials (n = 163) | Agro-veterinary shops | 112 (63.7) |
| Local veterinarians | 39 (23.9) | |
| No advice | 12 (7.4) | |
| Antimicrobial Quality assurance (n = 355) | Seller recommendations | 131 (36.9) |
| Check expiry | 195 (54.9) | |
| Buy from agro-veterinary shops | 82 (23.1) | |
| Buy specific brands | 58 (16.3) | |
| Ask friends/neighbor | 29 (8.2) | |
| No quality assurance | 6 (1.7) | |
| Antimicrobial administration (n = 355) | Through injection | 243 (68.5) |
| Mix with water/other liquids | 151 (42.5) | |
| Mix with feeds | 66 (18.6) | |
| Duration of antimicrobial use (n = 355) | As prescribed | 220 (62.0) |
| As per manufacturer’s instruction | 84 (23.7) | |
| Before end of prescription | 25 (7.0) | |
| Longer than prescription | 15 (4.2) | |
| Record keeping (n = 355) | Livestock population | 158 (44.5) |
| Sales | 106 (29.9) | |
| Vaccination | 95 (26.8) | |
| Antimicrobials used | 49 (13.8) | |
| Disinfection | 23 (6.5) | |
| No record kept | 59 (16.6) | |
| Diseases treated by antimicrobials (n = 355) | Treat FMD (viral) | 121 (34.1) |
| Treat NCD (viral) | 111 (31.3) | |
| Treat mastitis (Bacterial) | 99 (27.9) | |
| Treat classical swine fever (viral) | 21 (5.9) | |
| Treat CCPP/Pox (Viral) | 14 (3.9) | |
| Disposal of excess/ expired antimicrobials (n = 355) | Keep for future use | 244 (68.7) |
| Give to neighbors | 42 (11.8) | |
| Throw in garbage | 35 (9.9) | |
| Bury | 27 (7.6) | |
| Burn | 16 (4.5) | |
| Veterinarians takes it away | 12 (3.4) |
| Variable | Parameter | Number (%) |
|---|---|---|
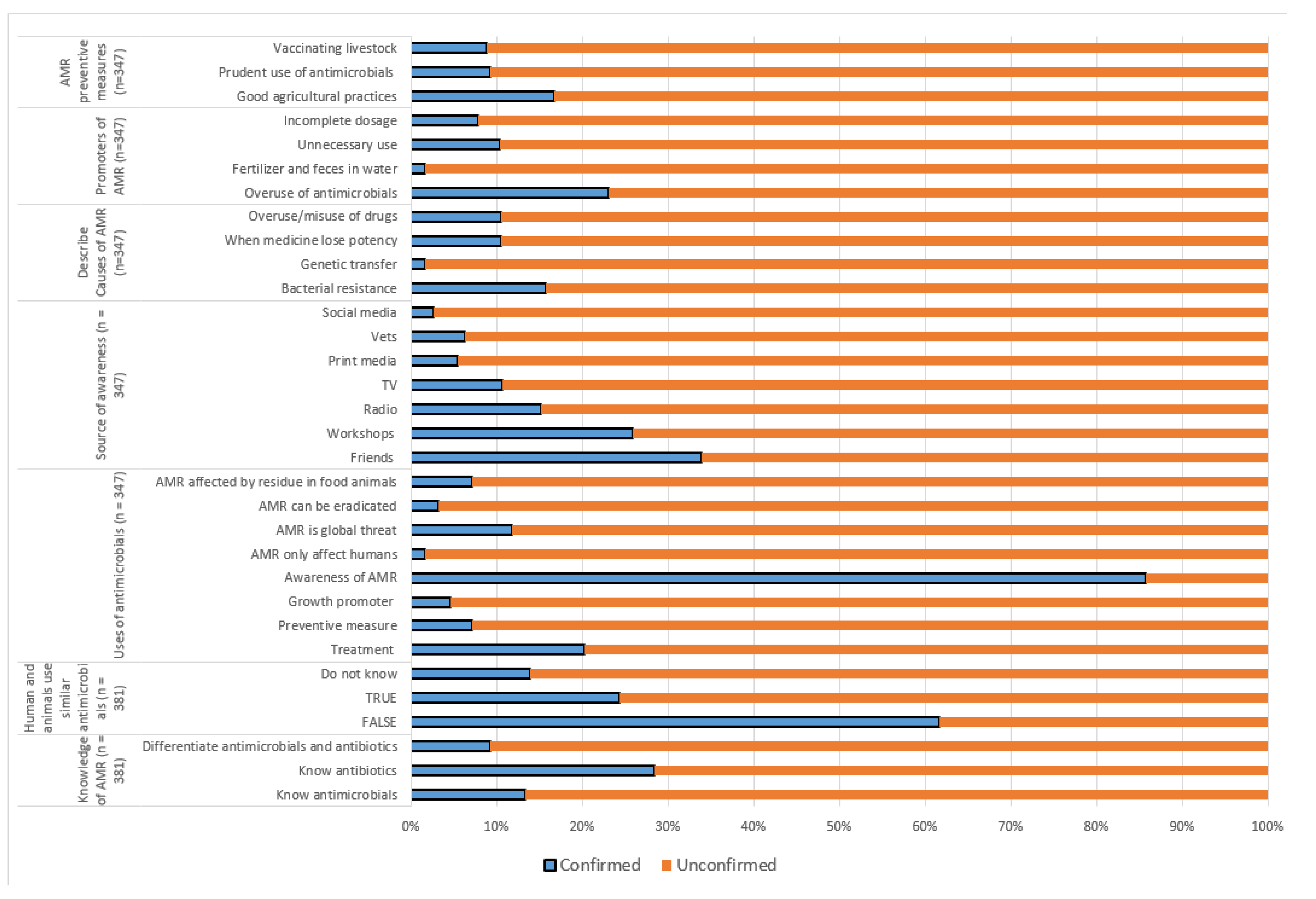
| Knowledge and awareness of antimicrobials | Know and described antibiotics correctly (n = 29) | 28 (96.6) |
| Know and described antimicrobials correctly (n = 30) | 28 (93.3) | |
| Know difference between antibiotic and antimicrobial (n = 20) | 20 (69.0) | |
| Have heard of antimicrobial resistance (n = 28) | 28 (100.0) | |
| Source of information about AMR (n = 25) | TV | 6 (23.1) |
| Workshop | 13 (50.0) | |
| Training program | 17 (65.4) | |
| Social media | 6 (23.1) | |
| Radio | 3 (11.5) | |
| Newspapers | 8 (30.8) | |
| Friends/relatives | 4 (15.4) | |
| What best describe antimicrobial resistance (n = 25) | No treatment due to ineffective medicine | 11 (42.3) |
| When microorganisms become irresponsive/less responsive to antimicrobial drugs | 21 (80.8) | |
| Lack of treatment because medicine lose potency and effectiveness | 10 (38.5) | |
| When microorganisms change | 19 (73.1) | |
| Understanding and perception on AMR (n = 25) | AMR in food animals is detected by number of antimicrobial residues in meat | 8 (30.8) |
| AMR is a global health threat | 22 (84.6) | |
| AMR can be eradicated | 12 (46.2) | |
| What promotes AMR (n = 25) | Incomplete dosage during antimicrobial treatment | 18 (69.2) |
| Appropriate use of antimicrobials in people and animals | 2 (7.7) | |
| Using water contaminated with fertilizer, feces, and antimicrobial residues | 9 (34.6) | |
| Unnecessary antimicrobial use in humans | 19 (73.1) | |
| Antimicrobial overuse in animals | 23 (88.5) | |
| Ways to prevent AMR (n = 20) | Good vaccination programs | 18 (69.2) |
| Good husbandry practices | 19 (73.1) | |
| Prudent antimicrobial use | 19 (73.1) | |
| Practicing hygiene, sanitation and biosecurity | 21 (80.8) |
| Variable | Parameter | Frequency (%) |
|---|---|---|
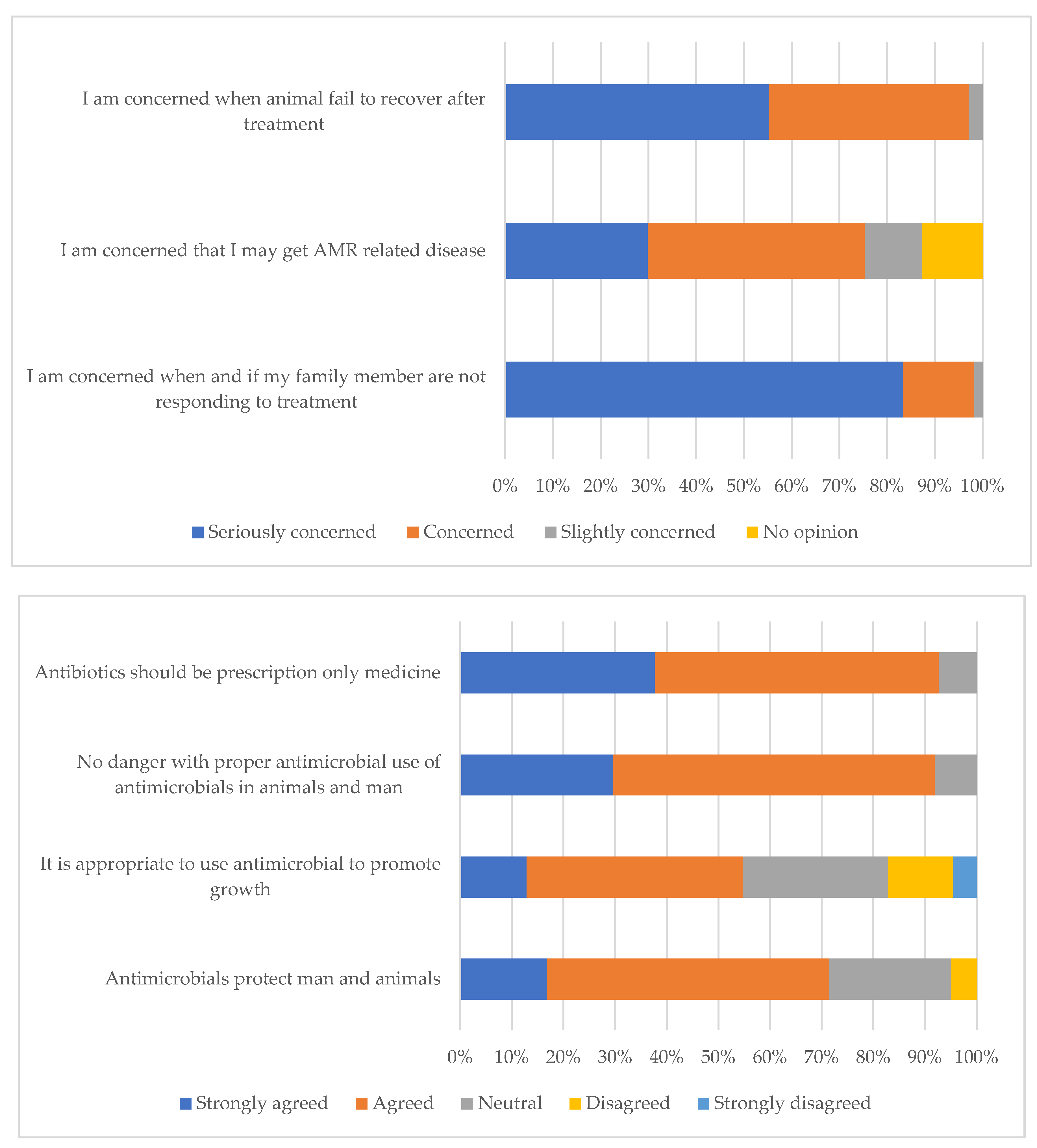
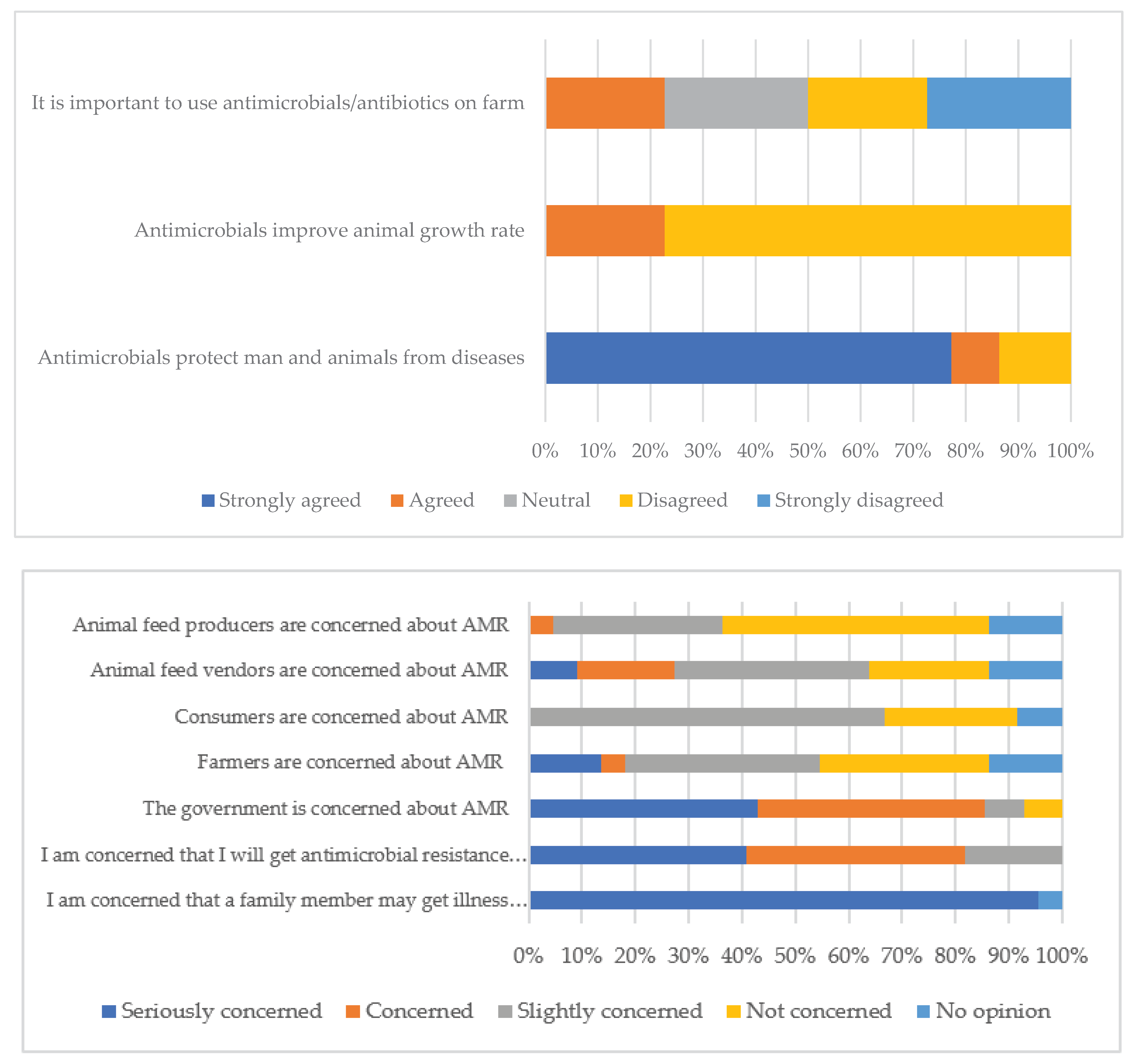
| Antimicrobials protect man and animals from diseases (n = 20) | Agreed | 9 (45.0) |
| Disagreed | 9 (45.0) | |
| Antimicrobials improve animal growth rate (n = 20) | Strongly disagreed | 18 (90.0) |
| Agreed | 1 (5.0) | |
| No danger if antimicrobials are properly used in animals (n = 20) | Strongly agreed | 14 (70.0) |
| Agreed | 5 (25.0) | |
| Antimicrobials should be given with prescriptions (n = 20) | Strongly agreed | 17 (85.0) |
| Agreed | 3 (15.0) | |
| Concerned that a family member may get illness not responding to medication (n = 20) | Seriously concerned | 17 (85.0) |
| Concerned | 2 (10.0) | |
| Worried of AMR related issues in future (n = 20) | Seriously concerned | 16 (80.0) |
| Concerned | 4 (20.0) | |
| Government concerned about antimicrobial resistance (n = 20) | Concerned | 17 (85.0) |
| Not concerned at all | 2 (10.0) | |
| No opinion | 1 (5.0) | |
| Farmers concerned about antimicrobial resistance (n = 20) | Concerned | 14 (70.0) |
| Not concerned at all | 5 (25.0) | |
| No opinion | 1 (5.0) | |
| Consumers concerned about antimicrobial resistance (n = 20) | Concerned | 16 (80.0) |
| Not concerned at all | 3 (15.0) | |
| No opinion | 1 (5.0) |
| Variable | Parameter | Frequency (%) |
|---|---|---|
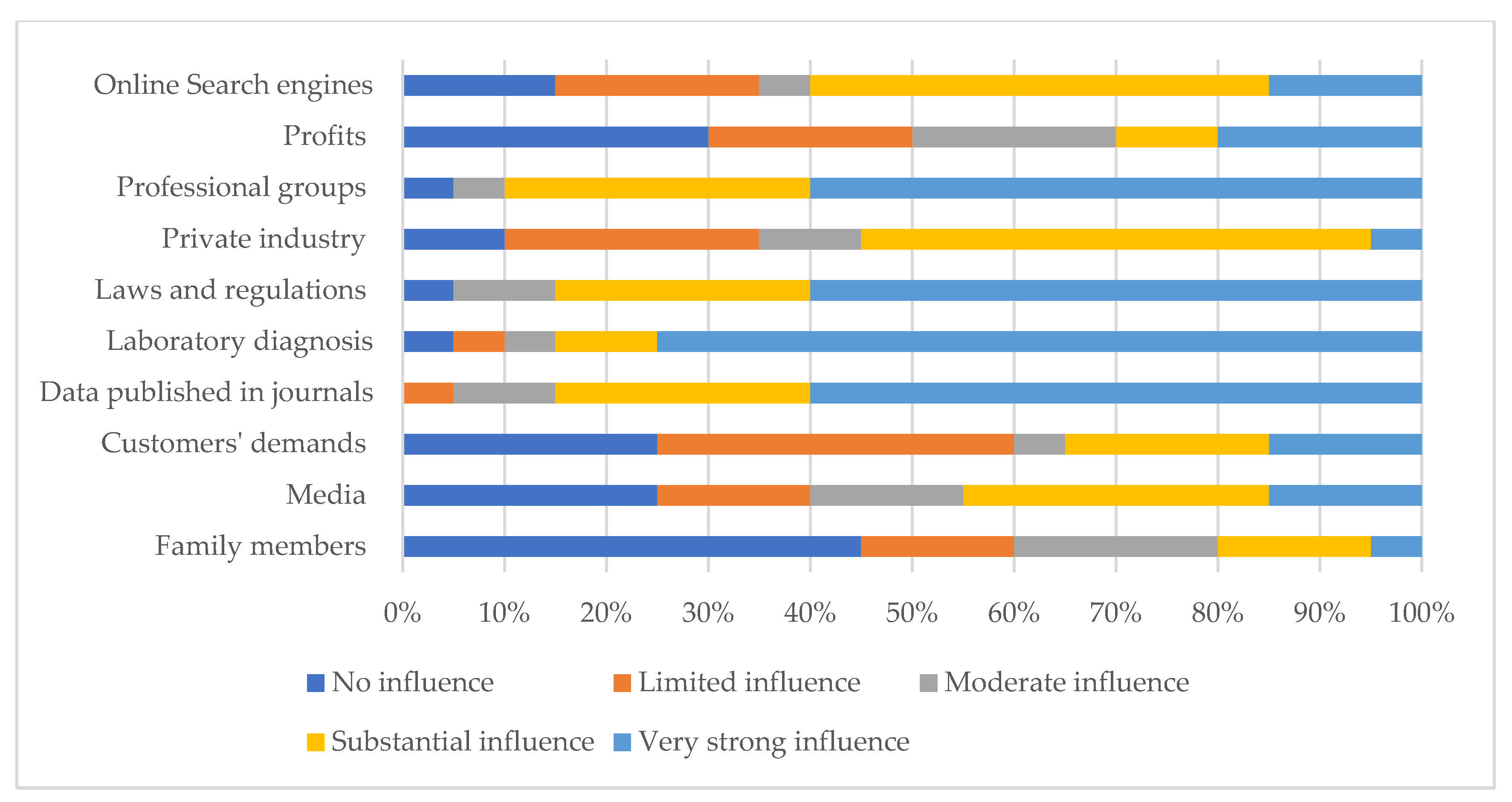
| Source of antimicrobial information (n = 18) | Another animal feed store/animal pharmaceutical store | 8 (44.4) |
| Facts on antimicrobial/antibiotic resistance | 3 (16.7) | |
| From suppliers/distributor/representative | 10 (55.6) | |
| Sales guidance for antimicrobials/antibiotics | 4 (22.2) | |
| The dosage and use in feed and water | 10 (55.6) | |
| Selling feed mixed with antibiotics (n = 19) | Never | 17 (89.5) |
| No (done previously but not at the moment) | 2 (10.5) | |
| Advice farmers on antimicrobial use (n = 18) | Sometimes | 1 (5.6) |
| Yes | 15 (83.3) | |
| During sale promotions | 2 (11.1) | |
| Occasion when farmers are advised on antimicrobial use (n = 18) | During sale promotions | 2 (11.1) |
| In possession of a prescription | 12 (77.8) | |
| Upon request by customer | 3 (16.7) | |
| Demand prescription before selling antimicrobials (n = 18) | No | 5 (27.8) |
| Sometimes | 5 (27.8) | |
| Yes | 8 (44.4) | |
| Record Keeping on antimicrobials used (n = 18) | Feel no need | 3 (16.7) |
| No records of clients with prescriptions kept | 11 (61.1) | |
| No records of prescriptions kept | 12 (66.7) | |
| Not a requirement by organization/business | 5 (27.8) | |
| Not required by law | 7 (38.9) | |
| Frequency of meeting farmer who use expired antimicrobial (n = 18) | At least once a month | 6 (33.3) |
| At least once a week | 2 (11.1) | |
| At least once every six months | 7 (38.9) | |
| At least once every two weeks | 2 (11.1) | |
| Everyday | 1 (5.6) | |
| Advice to farmers on antimicrobial disposal (n = 18) | Burn | 5 (27.8) |
| Bury in the ground | 3 (16.7) | |
| Keep for future use | 5 (27.8) | |
| Throw in the garbage | 3 (16.7) | |
| Return to the suppliers | 3 (16.7) | |
| Advice on use of antimicrobials (n = 18) | Advise use of antimicrobials to treat FMD | 10 (55.6) |
| Advise use of antimicrobials to treat NCD | 10 (55.6) |
| Variable | Parameter | Frequency (%) |
|---|---|---|
| Knowledge of AMR terminologies (n = 24) | Know and described antimicrobial correctly | 23 (95.8) |
| Know and described antibiotics correctly | 23 (95.8) | |
| Differentiated antimicrobials from antibiotics | 17 (70.8) | |
| Heard of AMR (n = 24) | Friends | 7 (29.2) |
| Workshops | 8 (33.3) | |
| Radio | 2 (8.3) | |
| TV | 6 (25.0) | |
| Print media | 5 (20.8) | |
| School training | 14 (58.3) | |
| Social media | 4 (16.7) | |
| Described aspects of AMR (n = 24) | Bacterial resistance | 18 (75.0) |
| Genetic transfer | 12 (50.0) | |
| When medicine lose potency | 12 (50.0) | |
| Overuse/misuse of drugs | 20 (83.3) | |
| Health issue affecting animal and plants | 18 (75.0) | |
| Promoters of AMR (n = 24) | Overuse of antimicrobials | 20 (83.3) |
| Fertilizer and feces in water | 13 (54.2) | |
| Unnecessary use | 22 (91.7) | |
| Incomplete dosage | 17 (70.8) | |
| AMR preventive measures (n = 24) | Good agricultural practices | 16 (66.7) |
| Prudent use of antimicrobials | 21 (87.5) | |
| Vaccinating livestock | 18 (75.0) |
| Variable | Parameter | Frequency (%) |
|---|---|---|
| Existing AMR curriculum (n = 11) | Yes | 8 (72.7) |
| No | 3 (27.3) | |
| AMR class duration (n = 11) | 1-3 hours (one class) | 7 (63.6) |
| I don’t know | 4 (36.4) | |
| AMR research type (n = 4) | Food safety | 1 (25) |
| Antimicrobial surveillance | 1 (25) | |
| Prevalence, mechanisms and outcomes | 1 (25) | |
| I do not know (no specific focus) | 1 (25) | |
| Specific subject of focus (n = 9) | Microbiology | 6 (66.7) |
| Public health/ One Health | 1 (11.1) | |
| AMR short course | 1 (11.1) | |
| I do not know (no specific focus) | 1 (11.1) | |
| Research results shared with stakeholders (n = 5) | Yes | 4 (80.0) |
| I am not sure/I do not know | 1 (20.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





