Submitted:
10 July 2023
Posted:
10 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. In Vitro Sun Protection Factor Evaluation
3. Materials and Methods
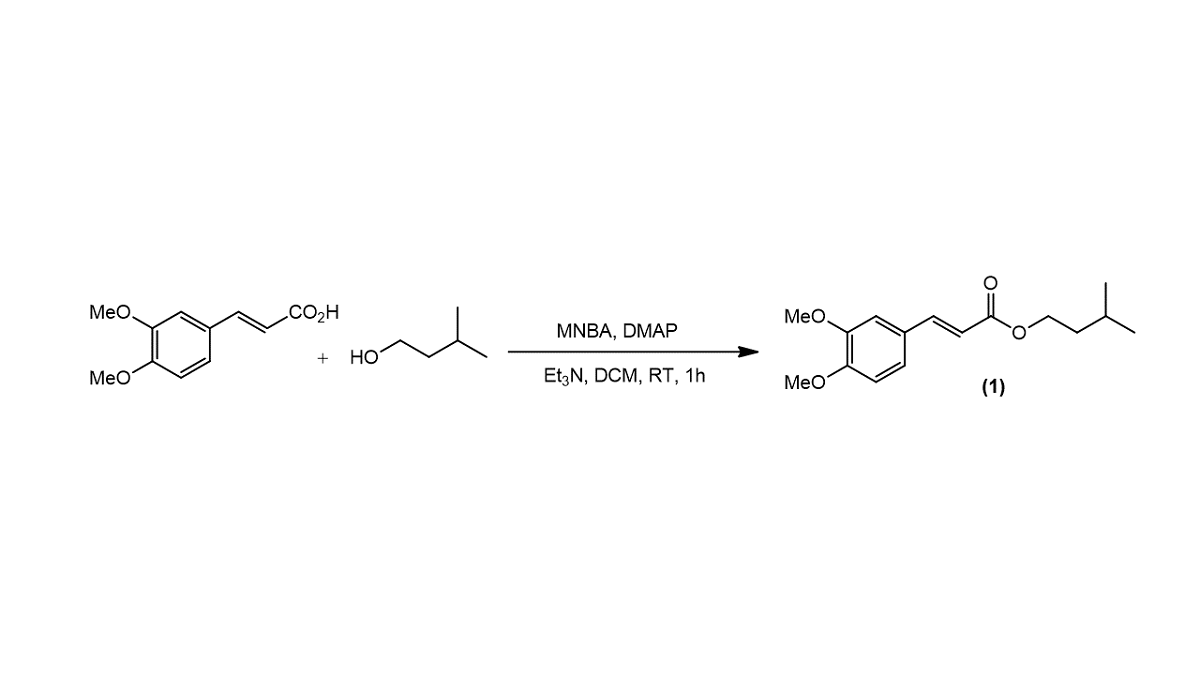
3.1. Synthesis of isoamyl (E)-3-(3,4-dimethoxyphenyl)acrylate (1)
3.3. In Vitro Sun Protection Factor Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steele, J.H.; Bozor, M.X.; Boyce, G.R. Transmutation of Scent: An Evaluation of the Synthesis of Methyl Cinnamate, a Commercial Fragrance, via a Fischer Esterification for the Second-Year Organic Laboratory. J. Chem. Educ. 2020, 97, 4127–4132. [Google Scholar] [CrossRef]
- Wong, N.G.K.; Dessent, C.E.H. Illuminating the Effect of the Local Environment on the Performance of Organic Sunscreens: Insights From Laser Spectroscopy of Isolated Molecules and Complexes. Front. Chem. 2022, 9, 812098. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Jiang, Y.; Zhang, H.; Huang, W.; Xie, Y.; Deng, C.; Xu, H.; Song, H.; Xu, H. Design, synthesis of Cinnamyl-paeonol derivatives with 1, 3-Dioxypropyl as link arm and screening of tyrosinase inhibition activity in vitro. Bioorg. Chem. 2021, 106, 104512. [Google Scholar] [CrossRef]
- Park, P.J. , & Cho, E.G. Kojyl Cinnamate Ester Derivatives Increase Adiponectin Expression and Stimulate Adiponectin-Induced Hair Growth Factors in Human Dermal Papilla Cells. Int. J. Mol. Sci. 2019, 20, 1859. [Google Scholar] [CrossRef]
- Ma, Y.; Yoo, J. History of sunscreen: An updated view. J. Cosmet. Dermatol. 2021, 20, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Martincigh, B.S.; Ollengo, M.A. The Photostabilizing Effect of Grape Seed Extract on Three Common Sunscreen Absorbers. J. Photochem. Photobiol. 2016, 92, 870–884. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed]
- Kuitunen, M.-L.; Cecilia Altamirano, J.; Siegenthaler, P.; Hannele Taure, T.; Antero Häkkinen, V.; Sinikka Vanninen, P. Derivatization and rapid GC-MS screening of chlorides relevant to the chemical weapons convention in organic liquid samples. Anal. Methods 2020, 12, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Amini, N.; Handayani, S.; Hudiyono, S. Toxicity test using brine shrimp lethality test (BSLT) and antioxidant assay of ricinoleic acid-based ester conjugate synthesized by Steglich esterification. AIP Conf. Proc. 2022, 2638, 070012. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, Y.A. Recent Development of Peptide Coupling Reagents in Organic Synthesis. Tetrahedron 2004, 60, 2447–2467. [Google Scholar] [CrossRef]
- Jordan, A.; Whymark, K.D.; Sydenham, J.; Sneddon, H.F. A Solvent-Reagent Selection Guide for Steglich-Type Esterification of Carboxylic Acids. Green. Chem. 2021, 23, 6405–6413. [Google Scholar] [CrossRef]
- Shiina, I.; Nakata, K. The first asymmetric esterification of free carboxylic acids with racemic alcohols using benzoic anhydrides and tetramisole derivatives: An application to the kinetic resolution of secondary benzylic alcohols. Tetrahedron Lett. 2007, 48, 8314–8317. [Google Scholar] [CrossRef]
- Manaia, E.B.; Kaminski, R.C. K.; Corrêa, M.A.; Chiavacci, L.A. Inorganic UV filters. Braz. J. Pharm. Sci. 2013, 49, 201–209. [Google Scholar] [CrossRef]
- Facts about Sunscreen. Available online: https://www.fda.gov/news-events/rumor-control/facts-about-sunscreen (accessed on 5 July 2023).
- Mansur, J.d.S.; Breder, M.N.R.; Mansur, M.C.d.A. Determinação do fator de proteção solar por espectrofotometria. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Koczurkiewicz, P.; Marona, H.; Pękala, E. Cinnamic acid derivatives in cosmetics: current use and future prospects. Int. J. Cosmet. Sci. 2018, 40, 356–366. [Google Scholar] [CrossRef]
- Antoniou, C.; Kosmadaki, M.G.; Stratigos, A.J.; Katsambas, A.D. Sunscreens – what’s important to know. J. Eur. Acad. Dermatol. Venereol 2008, 22, 1110–1118. [Google Scholar] [CrossRef]
- Kiss, B.; Bíró, T.; Czifra, G.; Tóth, B.; Kertész, Z.; Szikszai, Z.; Kiss, A.Z.; Juhászl, I.; Zouboulis, C.C.; Hunyadi, J. Investigation of micronized titanium dioxide penetration in human skin xenografts and its effect on cellular functions of human skin-derived cells. Exp. Dermatol. 2008, 17, 659–667. [Google Scholar] [CrossRef]
- Kinoshita, S.; Harabuchi, Y.; Inokuchi, Y.; Maeda, S.; Ehara, M.; Yamazaki, K.; Ebata, T. Substitution effect on the nonradiative decay and trans → cis photoisomerization route: a guideline to develop efficient cinnamate-based sunscreens. Phys. Chem. Chem. Phys. 2021, 23, 834–845. [Google Scholar] [CrossRef]
- Dalton, J.; Richings, G.W.; Woolley, J.M.; Abiola, T.T.; Habershon, S.; Stavros, V.G. Experimental and Computational Analysis of Para-Hydroxy Methylcinnamate following Photoexcitation. Molecules 2021, 26, 7621. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.P.; Li, C.X.; Xie, B.B.; Cui, G. Photoprotection Mechanism of p-Methoxy Methylcinnamate: A CASPT2 Study. J. Phys. Chem 2015, 119, 11488–11497. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




