Submitted:
07 July 2023
Posted:
10 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The molecular basis of FXPAC
2.1. Molecular basis of the FMR1 locus
2.2. Molecular mechanisms leading to FXTAS pathology: RNA toxicity and RAN translation at CGG repeats: mechanistic insights and their contribution to disease pathology
2.3. Therapeutic perspectives to FXTAS from a RAN translation perspective
2.4. Genetic Modifiers in Fragile X-Associated Tremor Ataxia Syndrome (FXTAS)
2.5. The use of human pluripotent stem cell-based neurodevelopmental models for FXTAS
2.6. Shared molecular mechanism with other repeat expansion disorders
2.7. Mitochondrial dysfunction in PM carriers
2.8. Omics studies (metabolomics and proteomics) in PM carriers
2.9. CGG Short Tandem Repeat (STR) expansions
3. Clinical involvement in children who have a PM
- Increasing efforts to prepare support organizations, genetic counselors, and healthcare practitioners to be able to respond to and treat children who have a PM and who are symptomatic.
- Detailed characterization of the pediatric phenotype – both at clinically actionable and sub-threshold levels.
- Efforts to study outcomes at a population scale through newborn screening that may provide evidence-base around developmental trajectories and risks.
- Clarified testing indications and potentially, modified diagnostic testing workflows to ensure that symptomatic children with PMs do not miss out on comprehensive genetic testing with microarray and potentially other methodology (WES or WGS).
4. FXPAC and relationships with genetic markers
4.1. FXTAS: neurological/cognitive phenotypes
4.2. FXTAS spectrum: non-syndromic neurological, cognitive, and psychiatric involvements
4.3. Do PM cognitive and motor deficits represent a distinct form of neural involvement, or are they prodromal to FXTAS?
4.4. Major psychiatric issues (FXAND)
4.4.1. Anxiety
4.4.2. Depression
4.4.3. Substance abuse
4.4.4. Autism spectrum disorder (ASD) and the broad autism phenotype (BAP)
4.5. Other FXPAC-related symptoms and conditions
4.5.1. Hypertension.
4.5.2. Metabolic Syndrome
4.5.3. Chronic fatigue
4.5.4. Chronic pain and fibromyalgia
4.5.5. Sleep problems
5. FXTAS clinical and protective mechanisms
6. Reproductive and health implications in women who carry the PM
6.1. Fragile X- associated primary ovarian insufficiency (FXPOI)
6.2. Medications to treat FXAND in FXPOI
6.3. Psychotherapy to treat FXAND in FXPOI
6.4. Early diagnosis and Carrier Screening
6.5. Future Directions
7. Neuroimaging findings in FXTAS
7.1. Structural brain differences associated with FXTAS
7.2. Functional brain differences associated with FXTAS
8. The neuropathology of FXTAS
9. FXTAS treatment
9.1. Treatment Trials Specific to FXTAS
9.2. Management of Neurologic symptoms in FXTAS
9.3. Lifestyle changes in FXTAS
- Vegetables of all types—dark green; red and orange; beans, peas, and lentils; starchy; and other vegetables,
- Fruits, especially whole fruits,
- Grains, at least half of which are whole grain,
- Dairy, including fat-free or low-fat milk, yogurt, and cheese, and/ or lactose-free versions and fortified soy beverages and yogurt as alternatives,
- Protein foods, including lean meats, poultry, and eggs; seafood; beans, peas, and lentils; and nuts, seeds, and soy products,
- Oils, including vegetable oils and oils in food, such as seafood and nuts.
9.4. Future directions to advance treatment in FXTAS
10. Screening for fragile X and FXPAC
10.1. Diagnosis via cascade testing
10.2. Newborn screening
10.3. Carrier and prenatal screening
10.4. Genetic testing pathways
11. Shining a light on the FMR1 PM: what we know, what we think we know and what we need to know
- Fertility related issues – the need for increased knowledge and better pathways for fertility related issues associated with the PM gene, particularly for younger women.
- Implications for early diagnosis and intervention in children with the PM - Studies suggest a small group of children with the PM may have developmental issues.
- CGG repeat number recognized as only part of the evolving picture - research indicating activation ratio, FMR1 mRNA, FMRP levels, AGG interruptions and allelic instability as also important factors to consider.
- Lifestyle measures - Multiple presenters mentioned the importance of healthy lifestyle as a protective measure against risk factors associated with the PM including an emphasis on limiting alcohol, not smoking, importance of exercise and good diet, and avoiding excess environmental toxins and high stress.
- It was noted that many PM have high levels of functioning and achievements.
- Many PM also face the challenges of children with developmental issues and FXS.
12. NZ Fragile X Community Response to PM Research (Fragile X New Zealand)
13. What’s in a name? (National Fragile X Foundation (NFXF) and Fragile X Association of Australia (FXAA))
13.1. Terminology
13.2. The importance of appropriate terminology
14. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verkerk, A.J.; Pieretti, M.; Sutcliffe, J.S.; Fu, Y.H.; Kuhl, D.P.; Pizzuti, A.; Reiner, O.; Richards, S.; Victoria, M.F.; Zhang, F.P.; et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991, 65, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Cronister, A.; Schreiner, R.; Wittenberger, M.; Amiri, K.; Harris, K.; Hagerman, R.J. Heterozygous fragile X female: historical, physical, cognitive, and cytogenetic features. Am J Med Genet 1991, 38, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.L. Premature ovarian failure in the fragile X syndrome. Am J Med Genet 2000, 97, 189–194. [Google Scholar] [CrossRef]
- Mailick, M.R.; Hong, J.; Greenberg, J.; Smith, L.; Sherman, S. Curvilinear association of CGG repeats and age at menopause in women with FMR1 premutation expansions. Am J Med Genet B Neuropsychiatr Genet 2014, 165b, 705–711. [Google Scholar] [CrossRef]
- Sullivan, A.K.; Marcus, M.; Epstein, M.P.; Allen, E.G.; Anido, A.E.; Paquin, J.J.; Yadav-Shah, M.; Sherman, S.L. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod 2005, 20, 402–412. [Google Scholar] [CrossRef]
- Tassone, F.; Hagerman, R.J.; Taylor, A.K.; Gane, L.W.; Godfrey, T.E.; Hagerman, P.J. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet 2000, 66, 6–15. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Leehey, M.; Heinrichs, W.; Tassone, F.; Wilson, R.; Hills, J.; Grigsby, J.; Gage, B.; Hagerman, P.J. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 2001, 57, 127–130. [Google Scholar] [CrossRef]
- Jacquemont, S.; Hagerman, R.J.; Leehey, M.; Grigsby, J.; Zhang, L.; Brunberg, J.A.; Greco, C.; Des Portes, V.; Jardini, T.; Levine, R.; et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet 2003, 72, 869–878. [Google Scholar] [CrossRef]
- Jacquemont, S.; Hagerman, R.J.; Leehey, M.A.; Hall, D.A.; Levine, R.A.; Brunberg, J.A.; Zhang, L.; Jardini, T.; Gane, L.W.; Harris, S.W.; et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA 2004, 291, 460–469. [Google Scholar] [CrossRef]
- Hall, D.A.; Birch, R.C.; Anheim, M.; Jønch, A.E.; Pintado, E.; O'Keefe, J.; Trollor, J.N.; Stebbins, G.T.; Hagerman, R.J.; Fahn, S.; et al. Emerging topics in FXTAS. J Neurodev Disord 2014, 6, 31. [Google Scholar] [CrossRef]
- Greco, C.M.; Berman, R.F.; Martin, R.M.; Tassone, F.; Schwartz, P.H.; Chang, A.; Trapp, B.D.; Iwahashi, C.; Brunberg, J.; Grigsby, J.; et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain 2006, 129, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.J.; Hagerman, P. Fragile X-associated tremor/ataxia syndrome — features, mechanisms and management. Nature Reviews Neurology 2016, 12, 403–412. [Google Scholar] [CrossRef]
- Aydin, E.Y.; Schneider, A.; Protic, D.; Wang, J.Y.; Martínez-Cerdeño, V.; Tassone, F.; Tang, H.T.; Perlman, S.; Hagerman, R.J. Rapidly Progressing Neurocognitive Disorder in a Male with FXTAS and Alzheimer's Disease. Clin Interv Aging 2020, 15, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cerdeño, V.; Wang, J.Y.; Grigsby, J.; Hall, D.; J., H.R. FXTAS New Advances and Treatments. In Fragile X Syndrome and Premutation Disorders; Hagerman, R., Hagerman, P., Eds.; Mac Keith Press: London, UK, 2020; pp. 83–96. [Google Scholar]
- Famula, J.; Ferrer, E.; Hagerman, R.J.; Tassone, F.; Schneider, A.; Rivera, S.M.; Hessl, D. Neuropsychological changes in FMR1 premutation carriers and onset of fragile X-associated tremor/ataxia syndrome. J Neurodev Disord 2022, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Hall, D.A. FXTAS, FXPOI, and Other Premutation Disorders; Flora Tassone, Hall, D.A., Ed.; Springer International Publishing: Switzerland, 2016. [Google Scholar]
- Wang, J.; Napoli, E.; Kim, K.; McLennan, Y.A.; Hagerman, R.J.; Giulivi, C. Brain Atrophy and White Matter Damage Linked to Peripheral Bioenergetic Deficits in the Neurodegenerative Disease FXTAS. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Greco, C.M.; Hagerman, R.J.; Tassone, F.; Chudley, A.E.; Del Bigio, M.R.; Jacquemont, S.; Leehey, M.; Hagerman, P.J. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain 2002, 125, 1760–1771. [Google Scholar] [CrossRef]
- Ariza, J.; Steward, C.; Rueckert, F.; Widdison, M.; Coffman, R.; Afjei, A.; Noctor, S.C.; Hagerman, R.; Hagerman, P.; Martínez-Cerdeño, V. Dysregulated iron metabolism in the choroid plexus in fragile X-associated tremor/ataxia syndrome. Brain Res 2015, 1598, 88–96. [Google Scholar] [CrossRef]
- Salcedo-Arellano, M.J.; Wang, J.Y.; McLennan, Y.A.; Doan, M.; Cabal-Herrera, A.M.; Jimenez, S.; Wolf-Ochoa, M.W.; Sanchez, D.; Juarez, P.; Tassone, F.; et al. Cerebral Microbleeds in Fragile X-Associated Tremor/Ataxia Syndrome. Mov Disord 2021, 36, 1935–1943. [Google Scholar] [CrossRef]
- Salcedo-Arellano, M.J.; Wolf-Ochoa, M.W.; Hong, T.; Amina, S.; Tassone, F.; Lechpammer, M.; Hagerman, R.; Martínez-Cerdeño, V. Parkinsonism Versus Concomitant Parkinson's Disease in Fragile X-Associated Tremor/Ataxia Syndrome. Mov Disord Clin Pract 2020, 7, 413–418. [Google Scholar] [CrossRef]
- Seritan, A.L.; Kim, K.; Benjamin, I.; Seritan, I.; Hagerman, R.J. Risk Factors for Cognitive Impairment in Fragile X-Associated Tremor/Ataxia Syndrome. J Geriatr Psychiatry Neurol 2016, 29, 328–337. [Google Scholar] [CrossRef]
- Schneider, A.; Summers, S.; Tassone, F.; Seritan, A.; Hessl, D.; Hagerman, P.; Hagerman, R. Women with Fragile X-associated Tremor/Ataxia Syndrome. Mov Disord Clin Pract 2020, 7, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Sellier, C.; Freyermuth, F.; Tabet, R.; Tran, T.; He, F.; Ruffenach, F.; Alunni, V.; Moine, H.; Thibault, C.; Page, A.; et al. Sequestration of DROSHA and DGCR8 by expanded CGG RNA repeats alters microRNA processing in fragile X-associated tremor/ataxia syndrome. Cell Rep 2013, 3, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Duan, R.; Qurashi, A.; Qin, Y.; Tian, D.; Rosser, T.C.; Liu, H.; Feng, Y.; Warren, S.T. Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome. Neuron 2007, 55, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Sofola, O.A.; Jin, P.; Qin, Y.; Duan, R.; Liu, H.; de Haro, M.; Nelson, D.L.; Botas, J. RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a Drosophila model of FXTAS. Neuron 2007, 55, 565–571. [Google Scholar] [CrossRef]
- Holm, K.N.; Herren, A.W.; Taylor, S.L.; Randol, J.L.; Kim, K.; Espinal, G.; Martiínez-Cerdeño, V.; Pessah, I.N.; Hagerman, R.J.; Hagerman, P.J. Human Cerebral Cortex Proteome of Fragile X-Associated Tremor/Ataxia Syndrome. Front Mol Biosci 2020, 7, 600840. [Google Scholar] [CrossRef]
- Todd, P.K.; Oh, S.Y.; Krans, A.; He, F.; Sellier, C.; Frazer, M.; Renoux, A.J.; Chen, K.C.; Scaglione, K.M.; Basrur, V.; et al. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron 2013, 78, 440–455. [Google Scholar] [CrossRef]
- Rosario, R.; Stewart, H.L.; Choudhury, N.R.; Michlewski, G.; Charlet-Berguerand, N.; Anderson, R.A. Evidence for a fragile X messenger ribonucleoprotein 1 (FMR1) mRNA gain-of-function toxicity mechanism contributing to the pathogenesis of fragile X-associated premature ovarian insufficiency. Faseb j 2022, 36, e22612. [Google Scholar] [CrossRef]
- Napoli, E.; Ross-Inta, C.; Wong, S.; Omanska-Klusek, A.; Barrow, C.; Iwahashi, C.; Garcia-Arocena, D.; Sakaguchi, D.; Berry-Kravis, E.; Hagerman, R.; et al. Altered zinc transport disrupts mitochondrial protein processing/import in fragile X-associated tremor/ataxia syndrome. Hum Mol Genet 2011, 20, 3079–3092. [Google Scholar] [CrossRef]
- Napoli, E.; Song, G.; Wong, S.; Hagerman, R.; Giulivi, C. Altered Bioenergetics in Primary Dermal Fibroblasts from Adult Carriers of the FMR1 Premutation Before the Onset of the Neurodegenerative Disease Fragile X-Associated Tremor/Ataxia Syndrome. Cerebellum 2016, 15, 552–564. [Google Scholar] [CrossRef]
- Giulivi, C.; Napoli, E.; Tassone, F.; Halmai, J.; Hagerman, R. Plasma metabolic profile delineates roles for neurodegeneration, pro-inflammatory damage and mitochondrial dysfunction in the FMR1 premutation. Biochem J 2016, 473, 3871–3888. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Duffy, D.L.; Martin, N.G.; Tassone, F.; Atkinson, A.; Storey, E. 'Essential Tremor' Phenotype in FMR1 Premutation/Gray Zone Sibling Series: Exploring Possible Genetic Modifiers. Twin Res Hum Genet 2021, 24, 95–102. [Google Scholar] [CrossRef]
- Johnson, D.; Santos, E.; Kim, K.; Ponzini, M.D.; McLennan, Y.A.; Schneider, A.; Tassone, F.; Hagerman, R.J. Increased Pain Symptomatology Among Females vs. Males With Fragile X-Associated Tremor/Ataxia Syndrome. Front Psychiatry 2021, 12, 762915. [Google Scholar] [CrossRef]
- Coffey, S.M.; Cook, K.; Tartaglia, N.; Tassone, F.; Nguyen, D.V.; Pan, R.; Bronsky, H.E.; Yuhas, J.; Borodyanskaya, M.; Grigsby, J.; et al. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A 2008, 146a, 1009–1016. [Google Scholar] [CrossRef]
- Cordeiro, L.; Abucayan, F.; Hagerman, R.; Tassone, F.; Hessl, D. Anxiety disorders in fragile X premutation carriers: Preliminary characterization of probands and non-probands. Intractable Rare Dis Res 2015, 4, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Farzin, F.; Perry, H.; Hessl, D.; Loesch, D.; Cohen, J.; Bacalman, S.; Gane, L.; Tassone, F.; Hagerman, P.; Hagerman, R. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr 2006, 27, S137–S144. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E.; Leslie, M.; Novak, G.; Hamilton, D.; Shubeck, L.; Charen, K.; Abramowitz, A.; Epstein, M.P.; Lori, A.; Binder, E.; et al. Depression and anxiety symptoms among women who carry the FMR1 premutation: impact of raising a child with fragile X syndrome is moderated by CRHR1 polymorphisms. Am J Med Genet B Neuropsychiatr Genet 2012, 159b, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Aishworiya, R.; Protic, D.; Tang, S.J.; Schneider, A.; Tassone, F.; Hagerman, R. Fragile X-Associated Neuropsychiatric Disorders (FXAND) in Young Fragile X Premutation Carriers. Genes (Basel) 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Clifford, S.; Dissanayake, C.; Bui, Q.M.; Huggins, R.; Taylor, A.K.; Loesch, D.Z. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. J Autism Dev Disord 2007, 37, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.B., Jr.; Raspa, M.; Olmsted, M.; Holiday, D.B. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A 2008, 146a, 2060–2069. [Google Scholar] [CrossRef]
- Aziz, M.; Stathopulu, E.; Callias, M.; Taylor, C.; Turk, J.; Oostra, B.; Willemsen, R.; Patton, M. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet B Neuropsychiatr Genet 2003, 121b, 119–127. [Google Scholar] [CrossRef]
- Chonchaiya, W.; Au, J.; Schneider, A.; Hessl, D.; Harris, S.W.; Laird, M.; Mu, Y.; Tassone, F.; Nguyen, D.V.; Hagerman, R.J. Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum Genet 2012, 131, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Hagerman, R.J.; Duyzend, M.; Budimirovic, D.B.; Eichler, E.E.; Tassone, F. Genomic studies in fragile X premutation carriers. J Neurodev Disord 2014, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chan, D.C. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet 2009, 18, R169–R176. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Napoli, E.; Wong, S.; Hagerman, R.; Liu, S.; Tassone, F.; Giulivi, C. Altered redox mitochondrial biology in the neurodegenerative disorder fragile X-tremor/ataxia syndrome: use of antioxidants in precision medicine. Mol Med 2016, 22, 548–559. [Google Scholar] [CrossRef]
- Ligsay, A.; El-Deeb, M.; Salcedo-Arellano, M.J.; Schloemerkemper, N.; Grayson, J.S.; Hagerman, R. General Anesthetic Use in Fragile X Spectrum Disorders. J Neurosurg Anesthesiol 2019, 31, 285–290. [Google Scholar] [CrossRef]
- Muzar, Z.; Adams, P.E.; Schneider, A.; Hagerman, R.J.; Lozano, R. Addictive substances may induce a rapid neurological deterioration in fragile X-associated tremor ataxia syndrome: A report of two cases. Intractable Rare Dis Res 2014, 3, 162–165. [Google Scholar] [CrossRef]
- Muzar, Z.; Lozano, R.; Schneider, A.; Adams, P.E.; Faradz, S.M.; Tassone, F.; Hagerman, R.J. Methadone use in a male with the FMRI premutation and FXTAS. Am J Med Genet A 2015, 167, 1354–1359. [Google Scholar] [CrossRef]
- Sodhi, D.K.; Hagerman, R. Fragile X Premutation: Medications, Therapy and Lifestyle Advice. Pharmgenomics Pers Med 2021, 14, 1689–1699. [Google Scholar] [CrossRef]
- Kaplan, E.S.; Cao, Z.; Hulsizer, S.; Tassone, F.; Berman, R.F.; Hagerman, P.J.; Pessah, I.N. Early mitochondrial abnormalities in hippocampal neurons cultured from Fmr1 pre-mutation mouse model. J Neurochem 2012, 123, 613–621. [Google Scholar] [CrossRef]
- Cao, Z.; Hulsizer, S.; Tassone, F.; Tang, H.T.; Hagerman, R.J.; Rogawski, M.A.; Hagerman, P.J.; Pessah, I.N. Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Hum Mol Genet 2012, 21, 2923–2935. [Google Scholar] [CrossRef]
- Aishworiya, R.; Protic, D.; Hagerman, R. Autism spectrum disorder in the fragile X premutation state: possible mechanisms and implications. J Neurol 2022, 269, 4676–4683. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.M.; Cogswell, J.; Goodrich, J.E.; Mu, Y.; Nguyen, D.V.; Brass, S.D.; Hagerman, R.J. Fatigue and body mass index in the Fragile X premutation carrier. Fatigue: Biomedicine, Health & Behavior 2014, 2, 64–72. [Google Scholar] [CrossRef]
- Summers, S.M.; Cogswell, J.; Goodrich, J.E.; Mu, Y.; Nguyen, D.V.; Brass, S.D.; Hagerman, R.J. Prevalence of restless legs syndrome and sleep quality in carriers of the fragile X premutation. Clin Genet 2014, 86, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E.W., A.C.; Allen, E.G.; Wald, K.; Rajkovic, A.; Hagerman, R.J.; Sherman, S.L. Fragile X Syndrome and Premutation Disorders: New Developments and Treatments; Hagerman, R., Hagerman, P., Eds.; Mac Keith Press: London, 2020; pp. 75–82. [Google Scholar]
- Hagerman, R.J.; Protic, D.; Rajaratnam, A.; Salcedo-Arellano, M.J.; Aydin, E.Y.; Schneider, A. Fragile X-Associated Neuropsychiatric Disorders (FXAND). Front Psychiatry 2018, 9, 564. [Google Scholar] [CrossRef]
- Winarni, T.I.; Chonchaiya, W.; Sumekar, T.A.; Ashwood, P.; Morales, G.M.; Tassone, F.; Nguyen, D.V.; Faradz, S.M.; Van de Water, J.; Cook, K.; et al. Immune-mediated disorders among women carriers of fragile X premutation alleles. Am J Med Genet A 2012, 158a, 2473–2481. [Google Scholar] [CrossRef]
- Hamlin, A.A.; Sukharev, D.; Campos, L.; Mu, Y.; Tassone, F.; Hessl, D.; Nguyen, D.V.; Loesch, D.; Hagerman, R.J. Hypertension in FMR1 premutation males with and without fragile X-associated tremor/ataxia syndrome (FXTAS). Am J Med Genet A 2012, 158a, 1304–1309. [Google Scholar] [CrossRef]
- Au, J.; Akins, R.S.; Berkowitz-Sutherland, L.; Tang, H.T.; Chen, Y.; Boyd, A.; Tassone, F.; Nguyen, D.V.; Hagerman, R. Prevalence and risk of migraine headaches in adult fragile X premutation carriers. Clin Genet 2013, 84, 546–551. [Google Scholar] [CrossRef]
- Tassanakijpanich, N.; McKenzie, F.J.; McLennan, Y.A.; Makhoul, E.; Tassone, F.; Jasoliya, M.J.; Romney, C.; Petrasic, I.C.; Napalinga, K.; Buchanan, C.B.; et al. Hypermobile Ehlers-Danlos syndrome (hEDS) phenotype in fragile X premutation carriers: case series. J Med Genet 2022, 59, 687–690. [Google Scholar] [CrossRef]
- McKenzie, F.J.; Tassankijpanich, N.; Epps, K.C.; March, S.K.; Hagerman, R.J. Spontaneous Coronary Artery Dissection in Females With the Fragile X FMR1 Premutation. JACC Case Rep 2020, 2, 40–44. [Google Scholar] [CrossRef]
- Hunsaker, M.R.; Greco, C.M.; Spath, M.A.; Smits, A.P.; Navarro, C.S.; Tassone, F.; Kros, J.M.; Severijnen, L.A.; Berry-Kravis, E.M.; Berman, R.F.; et al. Widespread non-central nervous system organ pathology in fragile X premutation carriers with fragile X-associated tremor/ataxia syndrome and CGG knock-in mice. Acta Neuropathol 2011, 122, 467–479. [Google Scholar] [CrossRef]
- Bourgeois, J.A.; Coffey, S.M.; Rivera, S.M.; Hessl, D.; Gane, L.W.; Tassone, F.; Greco, C.; Finucane, B.; Nelson, L.; Berry-Kravis, E.; et al. A review of fragile X premutation disorders: Expanding the psychiatric perspective. The Journal of Clinical Psychiatry 2009, 70, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, J.A.; Seritan, A.L.; Casillas, E.M.; Hessl, D.; Schneider, A.; Yang, Y.; Kaur, I.; Cogswell, J.B.; Nguyen, D.V.; Hagerman, R.J. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry 2011, 72, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Losh, M.; Klusek, J.; Martin, G.E.; Sideris, J.; Parlier, M.; Piven, J. Defining genetically meaningful language and personality traits in relatives of individuals with fragile X syndrome and relatives of individuals with autism. Am J Med Genet B Neuropsychiatr Genet 2012, 159b, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Tonnsen, B.L.; McCary, L.M.; Ford, A.L.; Golden, R.N.; Bailey, D.B., Jr. Trajectory and Predictors of Depression and Anxiety Disorders in Mothers With the FMR1 Premutation. Biol Psychiatry 2016, 79, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Gossett, A.; Sansone, S.; Schneider, A.; Johnston, C.; Hagerman, R.; Tassone, F.; Rivera, S.M.; Seritan, A.L.; Hessl, D. Psychiatric disorders among women with the fragile X premutation without children affected by fragile X syndrome. Am J Med Genet B Neuropsychiatr Genet 2016, 171, 1139–1147. [Google Scholar] [CrossRef]
- Kraan, C.M.; Hocking, D.R.; Georgiou-Karistianis, N.; Metcalfe, S.A.; Archibald, A.D.; Fielding, J.; Trollor, J.; Bradshaw, J.L.; Cohen, J.; Cornish, K.M. Impaired response inhibition is associated with self-reported symptoms of depression, anxiety, and ADHD in female FMR1 premutation carriers. Am J Med Genet B Neuropsychiatr Genet 2014, 165b, 41–51. [Google Scholar] [CrossRef]
- Movaghar, A.; Page, D.; Brilliant, M.; Baker, M.W.; Greenberg, J.; Hong, J.; DaWalt, L.S.; Saha, K.; Kuusisto, F.; Stewart, R.; et al. Data-driven phenotype discovery of FMR1 premutation carriers in a population-based sample. Sci Adv 2019, 5, eaaw7195. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Bui, M.Q.; Hammersley, E.; Schneider, A.; Storey, E.; Stimpson, P.; Burgess, T.; Francis, D.; Slater, H.; Tassone, F.; et al. Psychological status in female carriers of premutation FMR1 allele showing a complex relationship with the size of CGG expansion. Clin Genet 2015, 87, 173–178. [Google Scholar] [CrossRef]
- Johnson, K.; Herring, J.; Richstein, J. Fragile X Premutation Associated Conditions (FXPAC). Front Pediatr 2020, 8, 266. [Google Scholar] [CrossRef]
- Kenneson, A.; Zhang, F.; Hagedorn, C.H.; Warren, S.T. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet 2001, 10, 1449–1454. [Google Scholar] [CrossRef]
- Allen, E.G.; He, W.; Yadav-Shah, M.; Sherman, S.L. A study of the distributional characteristics of FMR1 transcript levels in 238 individuals. Hum Genet 2004, 114, 439–447. [Google Scholar] [CrossRef]
- Tassone, F.; Beilina, A.; Carosi, C.; Albertosi, S.; Bagni, C.; Li, L.; Glover, K.; Bentley, D.; Hagerman, P.J. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. Rna 2007, 13, 555–562. [Google Scholar] [CrossRef]
- Primerano, B.; Tassone, F.; Hagerman, R.J.; Hagerman, P.; Amaldi, F.; Bagni, C. Reduced FMR1 mRNA translation efficiency in fragile X patients with premutations. Rna 2002, 8, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Peprah, E.; He, W.; Allen, E.; Oliver, T.; Boyne, A.; Sherman, S.L. Examination of FMR1 transcript and protein levels among 74 premutation carriers. J Hum Genet 2010, 55, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Yrigollen, C.M.; Martorell, L.; Durbin-Johnson, B.; Naudo, M.; Genoves, J.; Murgia, A.; Polli, R.; Zhou, L.; Barbouth, D.; Rupchock, A.; et al. AGG interruptions and maternal age affect FMR1 CGG repeat allele stability during transmission. J Neurodev Disord 2014, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Nolin, S.L.; Glicksman, A.; Ersalesi, N.; Dobkin, C.; Brown, W.T.; Cao, R.; Blatt, E.; Sah, S.; Latham, G.J.; Hadd, A.G. Fragile X full mutation expansions are inhibited by one or more AGG interruptions in premutation carriers. Genet Med 2015, 17, 358–364. [Google Scholar] [CrossRef]
- Yrigollen, C.M.; Tassone, F.; Durbin-Johnson, B.; Tassone, F. The role of AGG interruptions in the transcription of FMR1 premutation alleles. PLoS ONE 2011, 6, e21728. [Google Scholar] [CrossRef]
- Ludwig, A.L.; Raske, C.; Tassone, F.; Garcia-Arocena, D.; Hershey, J.W.; Hagerman, P.J. Translation of the FMR1 mRNA is not influenced by AGG interruptions. Nucleic Acids Res 2009, 37, 6896–6904. [Google Scholar] [CrossRef]
- Ladd, P.D.; Smith, L.E.; Rabaia, N.A.; Moore, J.M.; Georges, S.A.; Hansen, R.S.; Hagerman, R.J.; Tassone, F.; Tapscott, S.J.; Filippova, G.N. An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum Mol Genet 2007, 16, 3174–3187. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Hayward, B.E.; Zafarullah, M.; Kumar, J.; Durbin Johnson, B.; Holmans, P.; Usdin, K.; Tassone, F. Both cis and trans-acting genetic factors drive somatic instability in female carriers of the FMR1 premutation. Sci Rep 2022, 12, 10419. [Google Scholar] [CrossRef]
- Pretto, D.I.; Mendoza-Morales, G.; Lo, J.; Cao, R.; Hadd, A.; Latham, G.J.; Durbin-Johnson, B.; Hagerman, R.; Tassone, F. CGG allele size somatic mosaicism and methylation in FMR1 premutation alleles. J Med Genet 2014, 51, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Aishworiya, R.; Hwang, Y.H.; Santos, E.; Hayward, B.; Usdin, K.; Durbin-Johnson, B.; Hagerman, R.; Tassone, F. Clinical implications of somatic allele expansion in female FMR1 premutation carriers. Sci Rep 2023, 13, 7050. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, C.S.; Nolin, S.L.; Cohen, I.; Sudhalter, V.; Bialer, M.G.; Ding, X.H.; Jenkins, E.C.; Zhong, N.; Brown, W.T. Tissue differences in fragile X mosaics: mosaicism in blood cells may differ greatly from skin. Am J Med Genet 1996, 64, 296–301. [Google Scholar] [CrossRef]
- Maddalena, A.; Yadvish, K.N.; Spence, W.C.; Howard-Peebles, P.N. A fragile X mosaic male with a cryptic full mutation detected in epithelium but not in blood. Am J Med Genet 1996, 64, 309–312. [Google Scholar] [CrossRef]
- Taylor, A.K.; Tassone, F.; Dyer, P.N.; Hersch, S.M.; Harris, J.B.; Greenough, W.T.; Hagerman, R.J. Tissue heterogeneity of the FMR1 mutation in a high-functioning male with fragile X syndrome. Am J Med Genet 1999, 84, 233–239. [Google Scholar] [CrossRef]
- Fernández, E.; Gennaro, E.; Pirozzi, F.; Baldo, C.; Forzano, F.; Turolla, L.; Faravelli, F.; Gastaldo, D.; Coviello, D.; Grasso, M.; et al. FXS-Like Phenotype in Two Unrelated Patients Carrying a Methylated Premutation of the FMR1 Gene. Front Genet 2018, 9, 442. [Google Scholar] [CrossRef]
- Jiraanont, P.; Sweha, S.R.; AlOlaby, R.R.; Silva, M.; Tang, H.T.; Durbin-Johnson, B.; Schneider, A.; Espinal, G.M.; Hagerman, P.J.; Rivera, S.M.; et al. Clinical and molecular correlates in fragile X premutation females. eNeurologicalSci 2017, 7, 49–56. [Google Scholar] [CrossRef]
- Del Hoyo Soriano, L.; Thurman, A.J.; Harvey, D.J.; Ted Brown, W.; Abbeduto, L. Genetic and maternal predictors of cognitive and behavioral trajectories in females with fragile X syndrome. J Neurodev Disord 2018, 10, 22. [Google Scholar] [CrossRef]
- Lyon, M.F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 1961, 190, 372–373. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, J.; Wang, Y. X-Chromosome Inactivation and Related Diseases. Genet Res (Camb) 2022, 2022, 1391807. [Google Scholar] [CrossRef]
- Devys, D.; Lutz, Y.; Rouyer, N.; Bellocq, J.P.; Mandel, J.L. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet 1993, 4, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Franke, P.; Leboyer, M.; Hardt, J.; Sohne, E.; Weiffenbach, O.; Biancalana, V.; Cornillet-Lefebre, P.; Delobel, B.; Froster, U.; Schwab, S.G.; et al. Neuropsychological profiles of FMR-1 premutation and full-mutation carrier females. Psychiatry Research 1999, 87, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Loesch, D.Z.; Huggins, R.M.; Hagerman, R.J. Phenotypic variation and FMRP levels in fragile X. Ment Retard Dev Disabil Res Rev 2004, 10, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Godler, D.E.; Slater, H.R.; Bui, Q.M.; Ono, M.; Gehling, F.; Francis, D.; Amor, D.J.; Hopper, J.L.; Hagerman, R.; Loesch, D.Z. FMR1 intron 1 methylation predicts FMRP expression in blood of female carriers of expanded FMR1 alleles. J Mol Diagn 2011, 13, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Potanos, K.; Weinberg, D.; Zhou, L.; Goetz, C.G. Fragile X-associated tremor/ataxia syndrome in sisters related to X-inactivation. Ann Neurol 2005, 57, 144–147. [Google Scholar] [CrossRef]
- Hall, D.A.; Robertson-Dick, E.E.; O'Keefe, J.A.; Hadd, A.G.; Zhou, L.; Berry-Kravis, E. X-inactivation in the clinical phenotype of fragile X premutation carrier sisters. Neurol Genet 2016, 2, e45. [Google Scholar] [CrossRef]
- Abrams, M.T.; Reiss, A.L.; Freund, L.S.; Baumgardner, T.L.; Chase, G.A.; Denckla, M.B. Molecular-neurobehavioral associations in females with the fragile X full mutation. Am J Med Genet 1994, 51, 317–327. [Google Scholar] [CrossRef]
- Hessl, D.; Dyer-Friedman, J.; Glaser, B.; Wisbeck, J.; Barajas, R.G.; Taylor, A.; Reiss, A.L. The influence of environmental and genetic factors on behavior problems and autistic symptoms in boys and girls with fragile X syndrome. Pediatrics 2001, 108, E88. [Google Scholar] [CrossRef]
- Heine-Suñer, D.; Torres-Juan, L.; Morlà, M.; Busquets, X.; Barceló, F.; Picó, G.; Bonilla, L.; Govea, N.; Bernués, M.; Rosell, J. Fragile-X syndrome and skewed X-chromosome inactivation within a family: A female member with complete inactivation of the functional X chromosome. American Journal of Medical Genetics Part A 2003, 122A, 108–114. [Google Scholar] [CrossRef]
- Talebizadeh, Z.; Bittel, D.C.; Veatch, O.J.; Kibiryeva, N.; Butler, M.G. Brief report: non-random X chromosome inactivation in females with autism. J Autism Dev Disord 2005, 35, 675–681. [Google Scholar] [CrossRef]
- Stembalska, A.; Łaczmańska, I.; Gil, J.; Pesz, K.A. Fragile X syndrome in females - a familial case report and review of the literature. Dev Period Med 2016, 20, 99–104. [Google Scholar]
- Sobesky, W.E.; Taylor, A.K.; Pennington, B.F.; Bennetto, L.; Porter, D.; Riddle, J.; Hagerman, R.J. Molecular/clinical correlations in females with fragile X. Am J Med Genet 1996, 64, 340–345. [Google Scholar] [CrossRef]
- Tassone, F.; Pan, R.; Amiri, K.; Taylor, A.K.; Hagerman, P.J. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn 2008, 10, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Godler, D.E.; Tassone, F.; Loesch, D.Z.; Taylor, A.K.; Gehling, F.; Hagerman, R.J.; Burgess, T.; Ganesamoorthy, D.; Hennerich, D.; Gordon, L.; et al. Methylation of novel markers of fragile X alleles is inversely correlated with FMRP expression and FMR1 activation ratio. Hum Mol Genet 2010, 19, 1618–1632. [Google Scholar] [CrossRef] [PubMed]
- Hadd, A.G.; Filipovic-Sadic, S.; Zhou, L.; Williams, A.; Latham, G.J.; Berry-Kravis, E.; Hall, D.A. A methylation PCR method determines FMR1 activation ratios and differentiates premutation allele mosaicism in carrier siblings. Clin Epigenetics 2016, 8, 130. [Google Scholar] [CrossRef]
- Protic, D.; Polli, R.; Hwang, Y.H.; Mendoza, G.; Hagerman, R.; Durbin-Johnson, B.; Hayward, B.E.; Usdin, K.; Murgia, A.; Tassone, F. Activation Ratio Correlates with IQ in Female Carriers of the FMR1 Premutation. Cells 2023, 12, 1711. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Hagerman, P. Fragile X-associated tremor/ataxia syndrome - features, mechanisms and management. Nat Rev Neurol 2016, 12, 403–412. [Google Scholar] [CrossRef]
- Loomis, E.W.; Sanz, L.A.; Chédin, F.; Hagerman, P.J. Transcription-associated R-loop formation across the human FMR1 CGG-repeat region. PLoS Genet 2014, 10, e1004294. [Google Scholar] [CrossRef]
- Malik, I.; Kelley, C.P.; Wang, E.T.; Todd, P.K. Author Correction: Molecular mechanisms underlying nucleotide repeat expansion disorders. Nat Rev Mol Cell Biol 2021, 22, 644. [Google Scholar] [CrossRef]
- Iwahashi, C.K.; Yasui, D.H.; An, H.J.; Greco, C.M.; Tassone, F.; Nannen, K.; Babineau, B.; Lebrilla, C.B.; Hagerman, R.J.; Hagerman, P.J. Protein composition of the intranuclear inclusions of FXTAS. Brain 2006, 129, 256–271. [Google Scholar] [CrossRef]
- Sellier, C.; Rau, F.; Liu, Y.; Tassone, F.; Hukema, R.K.; Gattoni, R.; Schneider, A.; Richard, S.; Willemsen, R.; Elliott, D.J.; et al. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. Embo j 2010, 29, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Qurashi, A.; Liu, H.; Ray, L.; Nelson, D.L.; Duan, R.; Jin, P. Chemical screen reveals small molecules suppressing fragile X premutation rCGG repeat-mediated neurodegeneration in Drosophila. Hum Mol Genet 2012, 21, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Poidevin, M.; Li, H.; Chen, D.; Jin, P. MicroRNA-277 modulates the neurodegeneration caused by Fragile X premutation rCGG repeats. PLoS Genet 2012, 8, e1002681. [Google Scholar] [CrossRef]
- Khalili, K.; Del Valle, L.; Muralidharan, V.; Gault, W.J.; Darbinian, N.; Otte, J.; Meier, E.; Johnson, E.M.; Daniel, D.C.; Kinoshita, Y.; et al. Puralpha is essential for postnatal brain development and developmentally coupled cellular proliferation as revealed by genetic inactivation in the mouse. Mol Cell Biol 2003, 23, 6857–6875. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, S.; Feldmann, H.M.; Ding, H.; Jung, C.K.; Bojarski, L.; Renner-Müller, I.; Schüller, U.; Kretzschmar, H.; Wolf, E.; Herms, J. Lack of Pur-alpha alters postnatal brain development and causes megalencephaly. Hum Mol Genet 2012, 21, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Shaw, C.; Yu, P.; Parghi, D.; Poidevin, M.; Jin, P.; Nelson, D.L. CGG repeats in RNA modulate expression of TDP-43 in mouse and fly models of fragile X tremor ataxia syndrome. Hum Mol Genet 2014, 23, 5906–5915. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Krans, A.; Freibaum, B.D.; Taylor, J.P.; Todd, P.K. TDP-43 suppresses CGG repeat-induced neurotoxicity through interactions with HnRNP A2/B1. Hum Mol Genet 2014, 23, 5036–5051. [Google Scholar] [CrossRef]
- Zu, T.; Gibbens, B.; Doty, N.S.; Gomes-Pereira, M.; Huguet, A.; Stone, M.D.; Margolis, J.; Peterson, M.; Markowski, T.W.; Ingram, M.A.; et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci U S A 2011, 108, 260–265. [Google Scholar] [CrossRef]
- Banez-Coronel, M.; Ranum, L.P.W. Repeat-associated non-AUG (RAN) translation: insights from pathology. Lab Invest 2019, 99, 929–942. [Google Scholar] [CrossRef]
- Kearse, M.G.; Green, K.M.; Krans, A.; Rodriguez, C.M.; Linsalata, A.E.; Goldstrohm, A.C.; Todd, P.K. CGG Repeat-Associated Non-AUG Translation Utilizes a Cap-Dependent Scanning Mechanism of Initiation to Produce Toxic Proteins. Mol Cell 2016, 62, 314–322. [Google Scholar] [CrossRef]
- Krans, A.; Skariah, G.; Zhang, Y.; Bayly, B.; Todd, P.K. Neuropathology of RAN translation proteins in fragile X-associated tremor/ataxia syndrome. Acta Neuropathologica Communications 2019, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.E.; Rodriguez, C.M.; Monroe, J.; Xing, J.; Krans, A.; Flores, B.N.; Barsur, V.; Ivanova, M.I.; Koutmou, K.S.; Barmada, S.J.; et al. CGG repeats trigger translational frameshifts that generate aggregation-prone chimeric proteins. Nucleic Acids Res 2022, 50, 8674–8689. [Google Scholar] [CrossRef] [PubMed]
- Sellier, C.; Buijsen, R.A.M.; He, F.; Natla, S.; Jung, L.; Tropel, P.; Gaucherot, A.; Jacobs, H.; Meziane, H.; Vincent, A.; et al. Translation of Expanded CGG Repeats into FMRpolyG Is Pathogenic and May Contribute to Fragile X Tremor Ataxia Syndrome. Neuron 2017, 93, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Buijsen, R.A.; Visser, J.A.; Kramer, P.; Severijnen, E.A.; Gearing, M.; Charlet-Berguerand, N.; Sherman, S.L.; Berman, R.F.; Willemsen, R.; Hukema, R.K. Presence of inclusions positive for polyglycine containing protein, FMRpolyG, indicates that repeat-associated non-AUG translation plays a role in fragile X-associated primary ovarian insufficiency. Hum Reprod 2016, 31, 158–168. [Google Scholar] [CrossRef]
- Ma, L.; Herren, A.W.; Espinal, G.; Randol, J.; McLaughlin, B.; Martinez-Cerdeño, V.; Pessah, I.N.; Hagerman, R.J.; Hagerman, P.J. Composition of the Intranuclear Inclusions of Fragile X-associated Tremor/Ataxia Syndrome. Acta Neuropathol Commun 2019, 7, 143. [Google Scholar] [CrossRef]
- Zhang, Y.; Glineburg, M.R.; Basrur, V.; Conlon, K.; Wright, S.E.; Krans, A.; Hall, D.A.; Todd, P.K. Mechanistic convergence across initiation sites for RAN translation in fragile X associated tremor ataxia syndrome. Hum Mol Genet 2022, 31, 2317–2332. [Google Scholar] [CrossRef]
- Asamitsu, S.; Yabuki, Y.; Ikenoshita, S.; Kawakubo, K.; Kawasaki, M.; Usuki, S.; Nakayama, Y.; Adachi, K.; Kugoh, H.; Ishii, K.; et al. CGG repeat RNA G-quadruplexes interact with FMRpolyG to cause neuronal dysfunction in fragile X-related tremor/ataxia syndrome. Sci Adv 2021, 7. [Google Scholar] [CrossRef]
- Linsalata, A.E.; He, F.; Malik, A.M.; Glineburg, M.R.; Green, K.M.; Natla, S.; Flores, B.N.; Krans, A.; Archbold, H.C.; Fedak, S.J.; et al. DDX3X and specific initiation factors modulate FMR1 repeat-associated non-AUG-initiated translation. EMBO Rep 2019, 20, e47498. [Google Scholar] [CrossRef]
- Rodriguez, C.M.; Wright, S.E.; Kearse, M.G.; Haenfler, J.M.; Flores, B.N.; Liu, Y.; Ifrim, M.F.; Glineburg, M.R.; Krans, A.; Jafar-Nejad, P.; et al. A native function for RAN translation and CGG repeats in regulating fragile X protein synthesis. Nat Neurosci 2020, 23, 386–397. [Google Scholar] [CrossRef]
- Haify, S.N.; Mankoe, R.S.D.; Boumeester, V.; van der Toorn, E.C.; Verhagen, R.F.M.; Willemsen, R.; Hukema, R.K.; Bosman, L.W.J. Lack of a Clear Behavioral Phenotype in an Inducible FXTAS Mouse Model Despite the Presence of Neuronal FMRpolyG-Positive Aggregates. Front Mol Biosci 2020, 7, 599101. [Google Scholar] [CrossRef]
- Abstract_book. Conference Proceedings. In Proceedings of The 5th International Conference on FMR1 Premutation: Molecular Mechanism, Clinical Involvments and Target Treatments, Bay of Islands, New Zealand.
- Tseng, Y.J.; Sandwith, S.N.; Green, K.M.; Chambers, A.E.; Krans, A.; Raimer, H.M.; Sharlow, M.E.; Reisinger, M.A.; Richardson, A.E.; Routh, E.D.; et al. The RNA helicase DHX36-G4R1 modulates C9orf72 GGGGCC hexanucleotide repeat-associated translation. J Biol Chem 2021, 297, 100914. [Google Scholar] [CrossRef] [PubMed]
- Green, K.M.; Glineburg, M.R.; Kearse, M.G.; Flores, B.N.; Linsalata, A.E.; Fedak, S.J.; Goldstrohm, A.C.; Barmada, S.J.; Todd, P.K. RAN translation at C9orf72-associated repeat expansions is selectively enhanced by the integrated stress response. Nat Commun 2017, 8, 2005. [Google Scholar] [CrossRef] [PubMed]
- Zafarullah, M.; Durbin-Johnson, B.; Fourie, E.S.; Hessl, D.R.; Rivera, S.M.; Tassone, F. Metabolomic Biomarkers Are Associated With Area of the Pons in Fragile X Premutation Carriers at Risk for Developing FXTAS. Front Psychiatry 2021, 12, 691717. [Google Scholar] [CrossRef]
- Zafarullah, M.; Li, J.; Salemi, M.; Phinney, B.; Durbin-Johnson, B.P.; Hagerman, R.; Hessl, D.; Rivera, S.M.; Tassone, F. Blood proteome profiling reveals biomarkers and pathways alterations in Fragile X premutation carriers at risk for developing FXTAS. International Journal of Molecular Biology 2023. in review. [Google Scholar]
- Derbis, M.; Kul, E.; Niewiadomska, D.; Sekrecki, M.; Piasecka, A.; Taylor, K.; Hukema, R.K.; Stork, O.; Sobczak, K. Short antisense oligonucleotides alleviate the pleiotropic toxicity of RNA harboring expanded CGG repeats. Nat Commun 2021, 12, 1265. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.E.; Lim, J.; Linsalata, A.; Kang, Y.; Malik, I.; Allen, E.G.; Cao, Y.; Shubeck, L.; Johnston, R.; Huang, Y.; et al. Identification of PSMB5 as a genetic modifier of fragile X-associated tremor/ataxia syndrome. Proc Natl Acad Sci U S A 2022, 119, e2118124119. [Google Scholar] [CrossRef]
- Konieczny, P.; Mukherjee, S.; Stepniak-Konieczna, E.; Taylor, K.; Niewiadomska, D.; Piasecka, A.; Walczak, A.; Baud, A.; Dohno, C.; Nakatani, K.; et al. Cyclic mismatch binding ligands interact with disease-associated CGG trinucleotide repeats in RNA and suppress their translation. Nucleic Acids Res 2021, 49, 9479–9495. [Google Scholar] [CrossRef]
- Filley, C.M.; Brown, M.S.; Onderko, K.; Ray, M.; Bennett, R.E.; Berry-Kravis, E.; Grigsby, J. White matter disease and cognitive impairment in FMR1 premutation carriers. Neurology 2015, 84, 2146–2152. [Google Scholar] [CrossRef]
- Kong, H.E.; Lim, J.; Zhang, F.; Huang, L.; Gu, Y.; Nelson, D.L.; Allen, E.G.; Jin, P. Metabolic pathways modulate the neuronal toxicity associated with fragile X-associated tremor/ataxia syndrome. Hum Mol Genet 2019, 28, 980–991. [Google Scholar] [CrossRef]
- Fan, J.; Tao, W.; Li, X.; Li, H.; Zhang, J.; Wei, D.; Chen, Y.; Zhang, Z. The Contribution of Genetic Factors to Cognitive Impairment and Dementia: Apolipoprotein E Gene, Gene Interactions, and Polygenic Risk. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013, 9, 106–118. [Google Scholar] [CrossRef]
- Raulin, A.C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.C. ApoE in Alzheimer's disease: pathophysiology and therapeutic strategies. Mol Neurodegener 2022, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.W.; Akay, L.A.; Davila-Velderrain, J.; von Maydell, D.; Mathys, H.; Davidson, S.M.; Effenberger, A.; Chen, C.Y.; Maner-Smith, K.; Hajjar, I.; et al. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature 2022, 611, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Greco, C.M.; Hunsaker, M.R.; Seritan, A.L.; Berman, R.F.; Gane, L.W.; Jacquemont, S.; Basuta, K.; Jin, L.W.; Hagerman, P.J.; et al. Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav 2012, 11, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Rodriguez-Revenga, L.; Madrigal, I.; Alvarez-Mora, M.I.; Oliva, R.; Milà, M. High apolipoprotein E4 allele frequency in FXTAS patients. Genet Med 2013, 15, 639–642. [Google Scholar] [CrossRef]
- Avitzour, M.; Mor-Shaked, H.; Yanovsky-Dagan, S.; Aharoni, S.; Altarescu, G.; Renbaum, P.; Eldar-Geva, T.; Schonberger, O.; Levy-Lahad, E.; Epsztejn-Litman, S.; et al. FMR1 epigenetic silencing commonly occurs in undifferentiated fragile X-affected embryonic stem cells. Stem Cell Reports 2014, 3, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Koscielska, K.A.; Cao, Z.; Hulsizer, S.; Grace, N.; Mitchell, G.; Nacey, C.; Githinji, J.; McGee, J.; Garcia-Arocena, D.; et al. Signaling defects in iPSC-derived fragile X premutation neurons. Hum Mol Genet 2012, 21, 3795–3805. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Kraff, J.; Tang, H.T.; Cilia, R.; Canesi, M.; Pezzoli, G.; Goldwurm, S.; Hagerman, P.J.; Tassone, F. Screen for excess FMR1 premutation alleles among males with parkinsonism. Arch Neurol 2007, 64, 1002–1006. [Google Scholar] [CrossRef]
- Popovic, D.; Vucic, D.; Dikic, I. Ubiquitination in disease pathogenesis and treatment. Nat Med 2014, 20, 1242–1253. [Google Scholar] [CrossRef]
- Wenzel, H.J.; Hunsaker, M.R.; Greco, C.M.; Willemsen, R.; Berman, R.F. Ubiquitin-positive intranuclear inclusions in neuronal and glial cells in a mouse model of the fragile X premutation. Brain Res 2010, 1318, 155–166. [Google Scholar] [CrossRef]
- Vardinon, N.; Spirer, Z.; Goldhar, J.; Kacevman, B.; Eylan, E. Human milk anti-E. coli antibodies: relationship to maternal parity. Eur J Pediatr 1979, 130, 173–180. [Google Scholar] [CrossRef]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Gutekunst, C.A.; Li, S.H.; Yi, H.; Mulroy, J.S.; Kuemmerle, S.; Jones, R.; Rye, D.; Ferrante, R.J.; Hersch, S.M.; Li, X.J. Nuclear and neuropil aggregates in Huntington's disease: relationship to neuropathology. J Neurosci 1999, 19, 2522–2534. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.S.L.; Xu, Z.; Chen, Z.; Tan, Y.J.; Lim, W.K.; Ting, S.K.S.; Yu, W.Y.; Cheng, Q.H.; Foo, J.N.; Tan, E.K.; et al. NOTCH2NLC-linked neuronal intranuclear inclusion body disease and fragile X-associated tremor/ataxia syndrome. Brain 2020, 143, e69. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.; Kelley, C.P.; Wang, E.T.; Todd, P.K. Molecular mechanisms underlying nucleotide repeat expansion disorders. Nat Rev Mol Cell Biol 2021, 22, 589–607. [Google Scholar] [CrossRef]
- Todd, P.K.; Paulson, H.L. RNA-mediated neurodegeneration in repeat expansion disorders. Ann Neurol 2010, 67, 291–300. [Google Scholar] [CrossRef]
- Mankodi, A.; Logigian, E.; Callahan, L.; McClain, C.; White, R.; Henderson, D.; Krym, M.; Thornton, C.A. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 2000, 289, 1769–1773. [Google Scholar] [CrossRef]
- Brook, J.D.; McCurrach, M.E.; Harley, H.G.; Buckler, A.J.; Church, D.; Aburatani, H.; Hunter, K.; Stanton, V.P.; Thirion, J.P.; Hudson, T.; et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell 1992, 69, 385. [Google Scholar] [CrossRef]
- Mahadevan, M.S.; Yadava, R.S.; Yu, Q.; Balijepalli, S.; Frenzel-McCardell, C.D.; Bourne, T.D.; Phillips, L.H. Reversible model of RNA toxicity and cardiac conduction defects in myotonic dystrophy. Nat Genet 2006, 38, 1066–1070. [Google Scholar] [CrossRef]
- Oh, S.Y.; He, F.; Krans, A.; Frazer, M.; Taylor, J.P.; Paulson, H.L.; Todd, P.K. RAN translation at CGG repeats induces ubiquitin proteasome system impairment in models of fragile X-associated tremor ataxia syndrome. Hum Mol Genet 2015, 24, 4317–4326. [Google Scholar] [CrossRef]
- Koehorst, E.; Núñez-Manchón, J.; Ballester-López, A.; Almendrote, M.; Lucente, G.; Arbex, A.; Chojnacki, J.; Vázquez-Manrique, R.P.; Gómez-Escribano, A.P.; Pintos-Morell, G.; et al. Characterization of RAN Translation and Antisense Transcription in Primary Cell Cultures of Patients with Myotonic Dystrophy Type 1. J Clin Med 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Furling, D.; Lam le, T.; Agbulut, O.; Butler-Browne, G.S.; Morris, G.E. Changes in myotonic dystrophy protein kinase levels and muscle development in congenital myotonic dystrophy. Am J Pathol 2003, 162, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Pizza, F.; Scaglione, C.; Tonon, C.; Lodi, R.; Barbiroli, B.; Ambrosetto, P.; Martinelli, P. A case of fragile X premutation tremor/ataxia syndrome with evidence of mitochondrial dysfunction. Mov Disord 2006, 21, 1541–1542. [Google Scholar] [CrossRef]
- Ross-Inta, C.; Omanska-Klusek, A.; Wong, S.; Barrow, C.; Garcia-Arocena, D.; Iwahashi, C.; Berry-Kravis, E.; Hagerman, R.J.; Hagerman, P.J.; Giulivi, C. Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem J 2010, 429, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tassone, F.; Berman, R.F.; Hagerman, P.J.; Hagerman, R.J.; Willemsen, R.; Pessah, I.N. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet 2010, 19, 196–208. [Google Scholar] [CrossRef]
- Alvarez-Mora, M.I.; Podlesniy, P.; Gelpi, E.; Hukema, R.; Madrigal, I.; Pagonabarraga, J.; Trullas, R.; Mila, M.; Rodriguez-Revenga, L. Fragile X-associated tremor/ataxia syndrome: Regional decrease of mitochondrial DNA copy number relates to clinical manifestations. Genes Brain Behav 2019, 18, e12565. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Annesley, S.J.; Trost, N.; Bui, M.Q.; Lay, S.T.; Storey, E.; De Piazza, S.W.; Sanislav, O.; Francione, L.M.; Hammersley, E.M.; et al. Novel Blood Biomarkers Are Associated with White Matter Lesions in Fragile X- Associated Tremor/Ataxia Syndrome. Neurodegener Dis 2017, 17, 22–30. [Google Scholar] [CrossRef]
- Fisher, P.R.; Allan, C.Y.; Sanislav, O.; Atkinson, A.; Ngoei, K.R.W.; Kemp, B.E.; Storey, E.; Loesch, D.Z.; Annesley, S.J. Relationships between Mitochondrial Function, AMPK, and TORC1 Signaling in Lymphoblasts with Premutation Alleles of the FMR1 Gene. IJMS 2021, 22, 10393. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Kemp, B.E.; Bui, M.Q.; Fisher, P.R.; Allan, C.Y.; Sanislav, O.; Ngoei, K.R.W.; Atkinson, A.; Tassone, F.; Annesley, S.J.; et al. Cellular Bioenergetics and AMPK and TORC1 Signalling in Blood Lymphoblasts Are Biomarkers of Clinical Status in FMR1 Premutation Carriers. Front Psychiatry 2021, 12, 747268. [Google Scholar] [CrossRef]
- Cid-Samper, F.; Gelabert-Baldrich, M.; Lang, B.; Lorenzo-Gotor, N.; Ponti, R.D.; Severijnen, L.; Bolognesi, B.; Gelpi, E.; Hukema, R.K.; Botta-Orfila, T.; et al. An Integrative Study of Protein-RNA Condensates Identifies Scaffolding RNAs and Reveals Players in Fragile X-Associated Tremor/Ataxia Syndrome. Cell Rep 2018, 25, 3422–3434.e3427. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Deng, H.; Xu, S.; Zhang, J. MicroRNAs Regulate Mitochondrial Function in Cerebral Ischemia-Reperfusion Injury. Int J Mol Sci 2015, 16, 24895–24917. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lee, Y.; Yeom, K.H.; Kim, Y.K.; Jin, H.; Kim, V.N. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev 2004, 18, 3016–3027. [Google Scholar] [CrossRef]
- Tafuri, F.; Ronchi, D.; Magri, F.; Comi, G.P.; Corti, S. SOD1 misplacing and mitochondrial dysfunction in amyotrophic lateral sclerosis pathogenesis. Front Cell Neurosci 2015, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Gohel, D.; Sripada, L.; Prajapati, P.; Singh, K.; Roy, M.; Kotadia, D.; Tassone, F.; Charlet-Berguerand, N.; Singh, R. FMRpolyG alters mitochondrial transcripts level and respiratory chain complex assembly in Fragile X associated tremor/ataxia syndrome [FXTAS]. Biochim Biophys Acta Mol Basis Dis 2019, 1865, 1379–1388. [Google Scholar] [CrossRef]
- Jové, M.; Portero-Otín, M.; Naudí, A.; Ferrer, I.; Pamplona, R. Metabolomics of human brain aging and age-related neurodegenerative diseases. J Neuropathol Exp Neurol 2014, 73, 640–657. [Google Scholar] [CrossRef]
- Peng, B.; Li, H.; Peng, X.X. Functional metabolomics: from biomarker discovery to metabolome reprogramming. Protein Cell 2015, 6, 628–637. [Google Scholar] [CrossRef]
- Napoli, E.; Song, G.; Schneider, A.; Hagerman, R.; Eldeeb, M.A.; Azarang, A.; Tassone, F.; Giulivi, C. Warburg effect linked to cognitive-executive deficits in FMR1 premutation. Faseb j 2016, 30, 3334–3351. [Google Scholar] [CrossRef]
- Napoli, E.; Schneider, A.; Wang, J.Y.; Trivedi, A.; Carrillo, N.R.; Tassone, F.; Rogawski, M.; Hagerman, R.J.; Giulivi, C. Allopregnanolone Treatment Improves Plasma Metabolomic Profile Associated with GABA Metabolism in Fragile X-Associated Tremor/Ataxia Syndrome: a Pilot Study. Mol Neurobiol 2019, 56, 3702–3713. [Google Scholar] [CrossRef]
- Zafarullah, M.; Palczewski, G.; Rivera, S.M.; Hessl, D.R.; Tassone, F. Metabolic profiling reveals dysregulated lipid metabolism and potential biomarkers associated with the development and progression of Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS). Faseb j 2020, 34, 16676–16692. [Google Scholar] [CrossRef]
- Abbasi, D.A.; Nguyen, T.T.A.; Hall, D.A.; Robertson-Dick, E.; Berry-Kravis, E.; Cologna, S.M. Characterization of the Cerebrospinal Fluid Proteome in Patients with Fragile X-Associated Tremor/Ataxia Syndrome. Cerebellum 2022, 21, 86–98. [Google Scholar] [CrossRef]
- Deng, J.; Yu, J.; Li, P.; Luan, X.; Cao, L.; Zhao, J.; Yu, M.; Zhang, W.; Lv, H.; Xie, Z.; et al. Expansion of GGC Repeat in GIPC1 Is Associated with Oculopharyngodistal Myopathy. Am J Hum Genet 2020, 106, 793–804. [Google Scholar] [CrossRef]
- Ishiura, H.; Shibata, S.; Yoshimura, J.; Suzuki, Y.; Qu, W.; Doi, K.; Almansour, M.A.; Kikuchi, J.K.; Taira, M.; Mitsui, J.; et al. Noncoding CGG repeat expansions in neuronal intranuclear inclusion disease, oculopharyngodistal myopathy and an overlapping disease. Nat Genet 2019, 51, 1222–1232. [Google Scholar] [CrossRef]
- Sone, J.; Mitsuhashi, S.; Fujita, A.; Mizuguchi, T.; Hamanaka, K.; Mori, K.; Koike, H.; Hashiguchi, A.; Takashima, H.; Sugiyama, H.; et al. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat Genet 2019, 51, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, Y. Tonotopic differentiation of presynaptic neurotransmitter-releasing machinery in the auditory brainstem during the prehearing period and its selective deficits in Fmr1 knockout mice. J Comp Neurol 2022, 530, 3248–3269. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.H.; Yang, K.; Du, G.Q.; Chen, Y.K.; Cao, C.Y.; Qiu, Y.S.; He, J.; Lv, H.D.; Qu, Q.Q.; Chen, J.N.; et al. GGC Repeat Expansion of RILPL1 is Associated with Oculopharyngodistal Myopathy. Ann Neurol 2022, 92, 512–526. [Google Scholar] [CrossRef]
- Annear, D.J.; Vandeweyer, G.; Elinck, E.; Sanchis-Juan, A.; French, C.E.; Raymond, L.; Kooy, R.F. Abundancy of polymorphic CGG repeats in the human genome suggest a broad involvement in neurological disease. Sci Rep 2021, 11, 2515. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.E.; Nichol Edamura, K.; Cleary, J.D. Repeat instability: mechanisms of dynamic mutations. Nat Rev Genet 2005, 6, 729–742. [Google Scholar] [CrossRef]
- Essop, F.B.; Krause, A. Diagnostic, carrier and prenatal genetic testing for fragile X syndrome and other FMR-1-related disorders in Johannesburg, South Africa: a 20-year review. S Afr Med J 2013, 103, 994–998. [Google Scholar] [CrossRef]
- Kraan, C.M.; Bui, Q.M.; Field, M.; Archibald, A.D.; Metcalfe, S.A.; Christie, L.M.; Bennetts, B.H.; Oertel, R.; Smith, M.J.; du Sart, D.; et al. FMR1 allele size distribution in 35,000 males and females: a comparison of developmental delay and general population cohorts. Genet Med 2018, 20, 1627–1634. [Google Scholar] [CrossRef]
- Madrigal, I.; Xunclà, M.; Tejada, M.I.; Martínez, F.; Fernández-Carvajal, I.; Pérez-Jurado, L.A.; Rodriguez-Revenga, L.; Milà, M. Intermediate FMR1 alleles and cognitive and/or behavioural phenotypes. Eur J Hum Genet 2011, 19, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Choudhary, N.S.; Tassone, F.; Durbin-Johnson, B.; Hansen, R.; Hertz-Picciotto, I.; Pessah, I. Identification of expanded alleles of the FMR1 Gene in the CHildhood Autism Risks from Genes and Environment (CHARGE) study. J Autism Dev Disord 2013, 43, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Raspa, M.; Wylie, A.; Wheeler, A.C.; Kolacz, J.; Edwards, A.; Heilman, K.; Porges, S.W. Sensory Difficulties in Children With an FMR1 Premutation. Front Genet 2018, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.B., Jr.; Sideris, J.; Roberts, J.; Hatton, D. Child and genetic variables associated with maternal adaptation to fragile X syndrome: a multidimensional analysis. Am J Med Genet A 2008, 146a, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Kraan, C.M.; Hocking, D.R.; Bradshaw, J.L.; Fielding, J.; Cohen, J.; Georgiou-Karistianis, N.; Cornish, K.M. Neurobehavioural evidence for the involvement of the FMR1 gene in female carriers of fragile X syndrome. Neurosci Biobehav Rev 2013, 37, 522–547. [Google Scholar] [CrossRef]
- Lachiewicz, A.M.; Dawson, D.V.; Spiridigliozzi, G.A.; McConkie-Rosell, A. Arithmetic difficulties in females with the fragile X premutation. Am J Med Genet A 2006, 140, 665–672. [Google Scholar] [CrossRef]
- Wheeler, A.C.; Bailey, D.B., Jr.; Berry-Kravis, E.; Greenberg, J.; Losh, M.; Mailick, M.; Milà, M.; Olichney, J.M.; Rodriguez-Revenga, L.; Sherman, S.; et al. Associated features in females with an FMR1 premutation. J Neurodev Disord 2014, 6, 30. [Google Scholar] [CrossRef]
- Cornish, K.M.; Kraan, C.M.; Bui, Q.M.; Bellgrove, M.A.; Metcalfe, S.A.; Trollor, J.N.; Hocking, D.R.; Slater, H.R.; Inaba, Y.; Li, X.; et al. Novel methylation markers of the dysexecutive-psychiatric phenotype in FMR1 premutation women. Neurology 2015, 84, 1631–1638. [Google Scholar] [CrossRef]
- Brooker, R.J.; Buss, K.A.; Lemery-Chalfant, K.; Aksan, N.; Davidson, R.J.; Goldsmith, H.H. The development of stranger fear in infancy and toddlerhood: normative development, individual differences, antecedents, and outcomes. Dev Sci 2013, 16, 864–878. [Google Scholar] [CrossRef]
- Brooker, R.J.; Kiel, E.J.; Buss, K.A. Early social fear predicts kindergarteners' socially anxious behaviors: Direct associations, moderation by inhibitory control, and differences from nonsocial fear. Emotion 2016, 16, 997–1010. [Google Scholar] [CrossRef]
- Klusek, J.; Thurman, A.J.; Abbeduto, L. Maternal Pragmatic Language Difficulties in the FMR1 Premutation and the Broad Autism Phenotype: Associations with Individual and Family Outcomes. J Autism Dev Disord 2022, 52, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Maltman, N.; Guilfoyle, J.; Nayar, K.; Martin, G.E.; Winston, M.; Lau, J.C.Y.; Bush, L.; Patel, S.; Lee, M.; Sideris, J.; et al. The Phenotypic Profile Associated With the FMR1 Premutation in Women: An Investigation of Clinical-Behavioral, Social-Cognitive, and Executive Abilities. Front Psychiatry 2021, 12, 718485. [Google Scholar] [CrossRef] [PubMed]
- Strawn, J.R.; Lu, L.; Peris, T.S.; Levine, A.; Walkup, J.T. Research Review: Pediatric anxiety disorders - what have we learnt in the last 10 years? J Child Psychol Psychiatry 2021, 62, 114–139. [Google Scholar] [CrossRef] [PubMed]
- Tolan, P.H.; Dodge, K.A. Children's mental health as a primary care and concern: a system for comprehensive support and service. Am Psychol 2005, 60, 601–614. [Google Scholar] [CrossRef]
- Walter, H.J.; Bukstein, O.G.; Abright, A.R.; Keable, H.; Ramtekkar, U.; Ripperger-Suhler, J.; Rockhill, C. Clinical Practice Guideline for the Assessment and Treatment of Children and Adolescents With Anxiety Disorders. J Am Acad Child Adolesc Psychiatry 2020, 59, 1107–1124. [Google Scholar] [CrossRef]
- Leehey, M.A.; Berry-Kravis, E.; Min, S.J.; Hall, D.A.; Rice, C.D.; Zhang, L.; Grigsby, J.; Greco, C.M.; Reynolds, A.; Lara, R.; et al. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord 2007, 22, 203–206. [Google Scholar] [CrossRef]
- O'Keefe, J.A.; Robertson-Dick, E.; Dunn, E.J.; Li, Y.; Deng, Y.; Fiutko, A.N.; Berry-Kravis, E.; Hall, D.A. Characterization and Early Detection of Balance Deficits in Fragile X Premutation Carriers With and Without Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS). Cerebellum 2015, 14, 650–662. [Google Scholar] [CrossRef]
- Fraint, A.; Vittal, P.; Szewka, A.; Bernard, B.; Berry-Kravis, E.; Hall, D.A. New observations in the fragile X-associated tremor/ataxia syndrome (FXTAS) phenotype. Front Genet 2014, 5, 365. [Google Scholar] [CrossRef]
- Hall, D.A.; Leehey, M.A.; Hagerman, R.J.; Pelak, V.S. Eye Movements in Fragile X-Associated Tremor/Ataxia Syndrome. J Neuroophthalmol 2021, 41, e661–e664. [Google Scholar] [CrossRef]
- Wong, L.M.; Goodrich-Hunsaker, N.J.; McLennan, Y.; Tassone, F.; Zhang, M.; Rivera, S.M.; Simon, T.J. Eye movements reveal impaired inhibitory control in adult male fragile X premutation carriers asymptomatic for FXTAS. Neuropsychology 2014, 28, 571–584. [Google Scholar] [CrossRef]
- Moser, C.; Schmitt, L.; Schmidt, J.; Fairchild, A.; Klusek, J. Response Inhibition Deficits in Women with the FMR1 Premutation are Associated with Age and Fall Risk. Brain Cogn 2021, 148, 105675. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, J.; Brega, A.G.; Jacquemont, S.; Loesch, D.Z.; Leehey, M.A.; Goodrich, G.K.; Hagerman, R.J.; Epstein, J.; Wilson, R.; Cogswell, J.B.; et al. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS). J Neurol Sci 2006, 248, 227–233. [Google Scholar] [CrossRef]
- Grigsby, J.; Brega, A.G.; Engle, K.; Leehey, M.A.; Hagerman, R.J.; Tassone, F.; Hessl, D.; Hagerman, P.J.; Cogswell, J.B.; Bennett, R.E.; et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology 2008, 22, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, J.; Brega, A.G.; Leehey, M.A.; Goodrich, G.K.; Jacquemont, S.; Loesch, D.Z.; Cogswell, J.B.; Epstein, J.; Wilson, R.; Jardini, T.; et al. Impairment of executive cognitive functioning in males with fragile X-associated tremor/ataxia syndrome. Mov Disord 2007, 22, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D.; Sherman, J.C. The cerebellar cognitive affective syndrome. Brain 1998, 121 Pt 4, 561–579. [Google Scholar] [CrossRef]
- Hocking, D.R.; Loesch, D.Z.; Stimpson, P.; Tassone, F.; Atkinson, A.; Storey, E. Relationships of Motor Changes with Cognitive and Neuropsychiatric Features in FMR1 Male Carriers Affected with Fragile X-Associated Tremor/Ataxia Syndrome. Brain Sci 2022, 12. [Google Scholar] [CrossRef]
- Storey, E.; Bui, M.Q.; Stimpson, P.; Tassone, F.; Atkinson, A.; Loesch, D.Z. Relationships between motor scores and cognitive functioning in FMR1 female premutation X carriers indicate early involvement of cerebello-cerebral pathways. Cerebellum Ataxias 2021, 8, 15. [Google Scholar] [CrossRef]
- Hocking, D.R.; Loesch, D.Z.; Stimpson, P.; Tassone, F.; Atkinson, A.; Storey, E. Delineating the Relationships Between Motor, Cognitive-Executive and Psychiatric Symptoms in Female FMR1 Premutation Carriers. Front Psychiatry 2021, 12, 742929. [Google Scholar] [CrossRef]
- Fay-Karmon, T.; Hassin-Baer, S. The spectrum of tremor among carriers of the FMR1 premutation with or without the fragile X-associated tremor/ataxia syndrome (FXTAS). Parkinsonism Relat Disord 2019, 65, 32–38. [Google Scholar] [CrossRef]
- Apartis, E.; Blancher, A.; Meissner, W.G.; Guyant-Maréchal, L.; Maltête, D.; De Broucker, T.; Legrand, A.P.; Bouzenada, H.; Thanh, H.T.; Sallansonnet-Froment, M.; et al. FXTAS: new insights and the need for revised diagnostic criteria. Neurology 2012, 79, 1898–1907. [Google Scholar] [CrossRef]
- Juncos, J.L.; Lazarus, J.T.; Graves-Allen, E.; Shubeck, L.; Rusin, M.; Novak, G.; Hamilton, D.; Rohr, J.; Sherman, S.L. New clinical findings in the fragile X-associated tremor ataxia syndrome (FXTAS). Neurogenetics 2011, 12, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; Jennings, D.; Seibyl, J.; Tassone, F.; Marek, K. FMR1 gene expansion and scans without evidence of dopaminergic deficits in parkinsonism patients. Parkinsonism Relat Disord 2010, 16, 608–611. [Google Scholar] [CrossRef]
- Ceravolo, R.; Antonini, A.; Volterrani, D.; Rossi, C.; Goldwurm, S.; Di Maria, E.; Kiferle, L.; Bonuccelli, U.; Murri, L. Dopamine transporter imaging study in parkinsonism occurring in fragile X premutation carriers. Neurology 2005, 65, 1971–1973. [Google Scholar] [CrossRef] [PubMed]
- Wojtala, J.; Heber, I.A.; Neuser, P.; Heller, J.; Kalbe, E.; Rehberg, S.P.; Storch, A.; Linse, K.; Schneider, C.; Gräber, S.; et al. Cognitive decline in Parkinson's disease: the impact of the motor phenotype on cognition. J Neurol Neurosurg Psychiatry 2019, 90, 171–179. [Google Scholar] [CrossRef]
- Jacquemont, S.; Leehey, M.A.; Hagerman, R.J.; Beckett, L.A.; Hagerman, P.J. Size bias of fragile X premutation alleles in late-onset movement disorders. J Med Genet 2006, 43, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Leehey, M.A.; Berry-Kravis, E.; Goetz, C.G.; Zhang, L.; Hall, D.A.; Li, L.; Rice, C.D.; Lara, R.; Cogswell, J.; Reynolds, A.; et al. FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology 2008, 70, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Loesch, D.Z.; Tassone, F.; Atkinson, A.; Stimpson, P.; Trost, N.; Pountney, D.L.; Storey, E. Differential Progression of Motor Dysfunction Between Male and Female Fragile X Premutation Carriers Reveals Novel Aspects of Sex-Specific Neural Involvement. Front Mol Biosci 2020, 7, 577246. [Google Scholar] [CrossRef]
- Cornish, K.M.; Li, L.; Kogan, C.S.; Jacquemont, S.; Turk, J.; Dalton, A.; Hagerman, R.J.; Hagerman, P.J. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex 2008, 44, 628–636. [Google Scholar] [CrossRef]
- Cornish, K.M.; Hocking, D.R.; Moss, S.A.; Kogan, C.S. Selective executive markers of at-risk profiles associated with the fragile X premutation. Neurology 2011, 77, 618–622. [Google Scholar] [CrossRef]
- Kogan, C.S.; Cornish, K.M. Mapping self-reports of working memory deficits to executive dysfunction in Fragile X Mental Retardation 1 (FMR1) gene premutation carriers asymptomatic for FXTAS. Brain Cogn 2010, 73, 236–243. [Google Scholar] [CrossRef]
- Kogan, C.S.; Turk, J.; Hagerman, R.J.; Cornish, K.M. Impact of the Fragile X mental retardation 1 (FMR1) gene premutation on neuropsychiatric functioning in adult males without fragile X-associated Tremor/Ataxia syndrome: a controlled study. Am J Med Genet B Neuropsychiatr Genet 2008, 147b, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.S.G.; Whalley, H.C.; Kind, P.C.; Stanfield, A.C. Decreased functional brain response to emotional arousal and increased psychiatric symptomology in FMR1 premutation carriers. Psychiatry Res Neuroimaging 2019, 285, 9–17. [Google Scholar] [CrossRef]
- Hashimoto, R.; Backer, K.C.; Tassone, F.; Hagerman, R.J.; Rivera, S.M. An fMRI study of the prefrontal activity during the performance of a working memory task in premutation carriers of the fragile X mental retardation 1 gene with and without fragile X-associated tremor/ataxia syndrome (FXTAS). J Psychiatr Res 2011, 45, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hashimoto, R.; Tassone, F.; Simon, T.J.; Rivera, S.M. Altered neural activity of magnitude estimation processing in adults with the fragile X premutation. J Psychiatr Res 2013, 47, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Koldewyn, K.; Hessl, D.; Adams, J.; Tassone, F.; Hagerman, P.J.; Hagerman, R.J.; Rivera, S.M. Reduced Hippocampal Activation During Recall is Associated with Elevated FMR1 mRNA and Psychiatric Symptoms in Men with the Fragile X Premutation. Brain Imaging Behav 2008, 2, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Gabis, L.V.; Shaham, M.; Attia, O.L.; Kowal, T.; David, S.; Banet-Levi, Y.; Shefer, S.; Gabis, D.; Mula-Topf, D.; Avrech Bar, M.; et al. An escalating continuum of learning and attention difficulties from premutation to full mutation in female carriers of FMR1 expansion. Front Neurol 2023, 14, 1135630. [Google Scholar] [CrossRef] [PubMed]
- Shelton, A.L.; Cornish, K.; Fielding, J. Long term verbal memory recall deficits in fragile X premutation females. Neurobiol Learn Mem 2017, 144, 131–135. [Google Scholar] [CrossRef]
- Shelton, A.L.; Cornish, K.; Kraan, C.; Georgiou-Karistianis, N.; Metcalfe, S.A.; Bradshaw, J.L.; Hocking, D.R.; Archibald, A.D.; Cohen, J.; Trollor, J.N.; et al. Exploring inhibitory deficits in female premutation carriers of fragile X syndrome: through eye movements. Brain Cogn 2014, 85, 201–208. [Google Scholar] [CrossRef]
- Shelton, A.L.; Cornish, K.M.; Godler, D.E.; Clough, M.; Kraan, C.; Bui, M.; Fielding, J. Delineation of the working memory profile in female FMR1 premutation carriers: the effect of cognitive load on ocular motor responses. Behav Brain Res 2015, 282, 194–200. [Google Scholar] [CrossRef]
- Shelton, A.L.; Cornish, K.M.; Kraan, C.M.; Lozano, R.; Bui, M.; Fielding, J. Executive Dysfunction in Female FMR1 Premutation Carriers. Cerebellum 2016, 15, 565–569. [Google Scholar] [CrossRef]
- Sterling, A.M.; Mailick, M.; Greenberg, J.; Warren, S.F.; Brady, N. Language dysfluencies in females with the FMR1 premutation. Brain Cogn 2013, 82, 84–89. [Google Scholar] [CrossRef]
- Yang, J.C.; Simon, C.; Niu, Y.Q.; Bogost, M.; Schneider, A.; Tassone, F.; Seritan, A.; Grigsby, J.; Hagerman, P.J.; Hagerman, R.J.; et al. Phenotypes of hypofrontality in older female fragile X premutation carriers. Ann Neurol 2013, 74, 275–283. [Google Scholar] [CrossRef]
- Klusek, J.; Hong, J.; Sterling, A.; Berry-Kravis, E.; Mailick, M.R. Inhibition deficits are modulated by age and CGG repeat length in carriers of the FMR1 premutation allele who are mothers of children with fragile X syndrome. Brain Cogn 2020, 139, 105511. [Google Scholar] [CrossRef]
- Klusek, J.; Porter, A.; Abbeduto, L.; Adayev, T.; Tassone, F.; Mailick, M.R.; Glicksman, A.; Tonnsen, B.L.; Roberts, J.E. Curvilinear Association Between Language Disfluency and FMR1 CGG Repeat Size Across the Normal, Intermediate, and Premutation Range. Front Genet 2018, 9, 344. [Google Scholar] [CrossRef]
- Maltman, N.; DaWalt, L.S.; Hong, J.; Baker, M.W.; Berry-Kravis, E.M.; Brilliant, M.H.; Mailick, M. FMR1 CGG Repeats and Stress Influence Self-Reported Cognitive Functioning in Mothers. Am J Intellect Dev Disabil 2023, 128, 1–20. [Google Scholar] [CrossRef]
- Goodrich-Hunsaker, N.J.; Wong, L.M.; McLennan, Y.; Tassone, F.; Harvey, D.; Rivera, S.M.; Simon, T.J. Adult Female Fragile X Premutation Carriers Exhibit Age- and CGG Repeat Length-Related Impairments on an Attentionally Based Enumeration Task. Front Hum Neurosci 2011, 5, 63. [Google Scholar] [CrossRef]
- Goodrich-Hunsaker, N.J.; Wong, L.M.; McLennan, Y.; Srivastava, S.; Tassone, F.; Harvey, D.; Rivera, S.M.; Simon, T.J. Young adult female fragile X premutation carriers show age- and genetically-modulated cognitive impairments. Brain Cogn 2011, 75, 255–260. [Google Scholar] [CrossRef]
- Bredin-Oja, S.L.; Warren, S.F.; Swinburne Romine, R.E.; Fleming, K.K.; Brady, N.; Berry-Kravis, E. Word retrieval difficulty in adult females with the FMR1 premutation: Changes over time and across contexts. Brain Cogn 2021, 148, 105694. [Google Scholar] [CrossRef]
- Klusek, J.; Fairchild, A.; Moser, C.; Mailick, M.R.; Thurman, A.J.; Abbeduto, L. Family history of FXTAS is associated with age-related cognitive-linguistic decline among mothers with the FMR1 premutation. J Neurodev Disord 2022, 14, 7. [Google Scholar] [CrossRef]
- Maltman, N.; Klusek, J.; DaWalt, L.; Hong, J.; Sterling, A.; Berry-Kravis, E.; Mailick, M.R. Verbal inhibition declines among older women with high FMR1 premutation expansions: A prospective study. Brain Cogn 2022, 159, 105851. [Google Scholar] [CrossRef]
- Grigsby, J.; Brega, A.G.; Bennett, R.E.; Bourgeois, J.A.; Seritan, A.L.; Goodrich, G.K.; Hagerman, R.J. Clinically significant psychiatric symptoms among male carriers of the fragile X premutation, with and without FXTAS, and the mediating influence of executive functioning. Clin Neuropsychol 2016, 30, 944–959. [Google Scholar] [CrossRef]
- Hippolyte, L.; Battistella, G.; Perrin, A.G.; Fornari, E.; Cornish, K.M.; Beckmann, J.S.; Niederhauser, J.; Vingerhoets, F.J.; Draganski, B.; Maeder, P.; et al. Investigation of memory, executive functions, and anatomic correlates in asymptomatic FMR1 premutation carriers. Neurobiol Aging 2014, 35, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Birch, R.C.; Hocking, D.R.; Cornish, K.M.; Menant, J.C.; Georgiou-Karistianis, N.; Godler, D.E.; Wen, W.; Hackett, A.; Rogers, C.; Trollor, J.N. Preliminary evidence of an effect of cerebellar volume on postural sway in FMR1 premutation males. Genes Brain Behav 2015, 14, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E.; Epstein, M.P.; Tinker, S.W.; Abramowitz, A.; Sherman, S.L. The FMR1 premutation and attention-deficit hyperactivity disorder (ADHD): evidence for a complex inheritance. Behav Genet 2012, 42, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Klusek, J.; Schmidt, J.; Fairchild, A.J.; Porter, A.; Roberts, J.E. Altered sensitivity to social gaze in the FMR1 premutation and pragmatic language competence. J Neurodev Disord 2017, 9, 31. [Google Scholar] [CrossRef]
- Dembo, R.S.; Hong, J.; DaWalt, L.S.; Berry-Kravis, E.M.; Mailick, M.R. Health Effects of Sleep Quality in Premutation Carrier Mothers of Individuals With Fragile X Syndrome. Am J Intellect Dev Disabil 2023, 128, 254–268. [Google Scholar] [CrossRef]
- Kraan, C.M.; Hocking, D.R.; Georgiou-Karistianis, N.; Metcalfe, S.A.; Archibald, A.D.; Fielding, J.; Trollor, J.; Bradshaw, J.L.; Cohen, J.; Cornish, K.M. Age and CGG-repeat length are associated with neuromotor impairments in at-risk females with the FMR1 premutation. Neurobiol Aging 2014, 35, 2179.e2177-2113. [Google Scholar] [CrossRef]
- Wang, J.Y.; Hessl, D.; Hagerman, R.J.; Simon, T.J.; Tassone, F.; Ferrer, E.; Rivera, S.M. Abnormal trajectories in cerebellum and brainstem volumes in carriers of the fragile X premutation. Neurobiol Aging 2017, 55, 11–19. [Google Scholar] [CrossRef]
- Hashimoto, R.; Srivastava, S.; Tassone, F.; Hagerman, R.J.; Rivera, S.M. Diffusion tensor imaging in male premutation carriers of the fragile X mental retardation gene. Mov Disord 2011, 26, 1329–1336. [Google Scholar] [CrossRef]
- Mailick, M.; Hong, J.; Greenberg, J.; Dawalt, L.S.; Baker, M.W.; Rathouz, P.J. FMR1 genotype interacts with parenting stress to shape health and functional abilities in older age. Am J Med Genet B Neuropsychiatr Genet 2017, 174, 399–412. [Google Scholar] [CrossRef]
- Cornish, K.M.; Kogan, C.S.; Li, L.; Turk, J.; Jacquemont, S.; Hagerman, R.J. Lifespan changes in working memory in fragile X premutation males. Brain Cogn 2009, 69, 551–558. [Google Scholar] [CrossRef]
- Sévin, M.; Kutalik, Z.; Bergman, S.; Vercelletto, M.; Renou, P.; Lamy, E.; Vingerhoets, F.J.; Di Virgilio, G.; Boisseau, P.; Bezieau, S.; et al. Penetrance of marked cognitive impairment in older male carriers of the FMR1 gene premutation. J Med Genet 2009, 46, 818–824. [Google Scholar] [CrossRef]
- Hartley, S.L.; DaWalt, L.S.; Hong, J.; Greenberg, J.S.; Mailick, M.R. Positive Emotional Support in Premutation Carrier Mothers of Adolescents and Adults With Fragile X Syndrome: Gene by Environment Interactions. Am J Intellect Dev Disabil 2019, 124, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, M.M.; Barker, E.T.; Greenberg, J.S.; Hong, J.; Coe, C.; Almeida, D. Differential sensitivity to life stress in FMR1 premutation carrier mothers of children with fragile X syndrome. Health Psychol 2012, 31, 612–622. [Google Scholar] [CrossRef] [PubMed]
- O'Keefe, J.A.; Robertson, E.E.; Ouyang, B.; Carns, D.; McAsey, A.; Liu, Y.; Swanson, M.; Bernard, B.; Berry-Kravis, E.; Hall, D.A. Cognitive function impacts gait, functional mobility and falls in fragile X-associated tremor/ataxia syndrome. Gait Posture 2018, 66, 288–293. [Google Scholar] [CrossRef] [PubMed]
- O'Keefe, J.A.; Guan, J.; Robertson, E.; Biskis, A.; Joyce, J.; Ouyang, B.; Liu, Y.; Carnes, D.; Purcell, N.; Berry-Kravis, E.; et al. The Effects of Dual Task Cognitive Interference and Fast-Paced Walking on Gait, Turns, and Falls in Men and Women with FXTAS. Cerebellum 2021, 20, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Koldewyn, K.; Hashimoto, R.; Schneider, A.; Le, L.; Tassone, F.; Cheung, K.; Hagerman, P.; Hessl, D.; Rivera, S.M. Male carriers of the FMR1 premutation show altered hippocampal-prefrontal function during memory encoding. Front Hum Neurosci 2012, 6, 297. [Google Scholar] [CrossRef] [PubMed]
- Cvejic, R.C.; Hocking, D.R.; Wen, W.; Georgiou-Karistianis, N.; Cornish, K.M.; Godler, D.E.; Rogers, C.; Trollor, J.N. Reduced caudate volume and cognitive slowing in men at risk of fragile X-associated tremor ataxia syndrome. Brain Imaging Behav 2019, 13, 1128–1134. [Google Scholar] [CrossRef]
- Filley, C.M. Fragile X tremor ataxia syndrome and white matter dementia. Clin Neuropsychol 2016, 30, 901–912. [Google Scholar] [CrossRef]
- Shelton, A.L.; Cornish, K.M.; Godler, D.; Bui, Q.M.; Kolbe, S.; Fielding, J. White matter microstructure, cognition, and molecular markers in fragile X premutation females. Neurology 2017, 88, 2080–2088. [Google Scholar] [CrossRef]
- Wang, J.Y.; Grigsby, J.; Placido, D.; Wei, H.; Tassone, F.; Kim, K.; Hessl, D.; Rivera, S.M.; Hagerman, R.J. Clinical and Molecular Correlates of Abnormal Changes in the Cerebellum and Globus Pallidus in Fragile X Premutation. Front Neurol 2022, 13, 797649. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Chan, S.H.; Khan, S.; Schneider, A.; Nanakul, R.; Teichholtz, S.; Niu, Y.Q.; Seritan, A.; Tassone, F.; Grigsby, J.; et al. Neural substrates of executive dysfunction in fragile X-associated tremor/ataxia syndrome (FXTAS): a brain potential study. Cereb Cortex 2013, 23, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Flavell, J.; Franklin, C.; Nestor, P.J. A Systematic Review of Fragile X-Associated Neuropsychiatric Disorders. J Neuropsychiatry Clin Neurosci 2023, 35, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P. Diagnostic and statistical manual of mental disorders, 5th ed.; American Psychiatric Association Publishing, 2013. [Google Scholar]
- Baxter, A.J.; Scott, K.M.; Vos, T.; Whiteford, H.A. Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol Med 2013, 43, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Franke, P.; Leboyer, M.; Gänsicke, M.; Weiffenbach, O.; Biancalana, V.; Cornillet-Lefebre, P.; Croquette, M.F.; Froster, U.; Schwab, S.G.; Poustka, F.; et al. Genotype-phenotype relationship in female carriers of the premutation and full mutation of FMR-1. Psychiatry Res 1998, 80, 113–127. [Google Scholar] [CrossRef]
- Johnston, C.; Eliez, S.; Dyer-Friedman, J.; Hessl, D.; Glaser, B.; Blasey, C.; Taylor, A.; Reiss, A. Neurobehavioral phenotype in carriers of the fragile X premutation. Am J Med Genet 2001, 103, 314–319. [Google Scholar] [CrossRef]
- Hessl, D.; Tassone, F.; Loesch, D.Z.; Berry-Kravis, E.; Leehey, M.A.; Gane, L.W.; Barbato, I.; Rice, C.; Gould, E.; Hall, D.A.; et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet 2005, 139b, 115–121. [Google Scholar] [CrossRef]
- Hunter, J.E.; Rohr, J.K.; Sherman, S.L. Co-occurring diagnoses among FMR1 premutation allele carriers. Clin Genet 2010, 77, 374–381. [Google Scholar] [CrossRef]
- Schneider, A.; Johnston, C.; Tassone, F.; Sansone, S.; Hagerman, R.J.; Ferrer, E.; Rivera, S.M.; Hessl, D. Broad autism spectrum and obsessive-compulsive symptoms in adults with the fragile X premutation. Clin Neuropsychol 2016, 30, 929–943. [Google Scholar] [CrossRef]
- Kenna, H.A.; Tartter, M.; Hall, S.S.; Lightbody, A.A.; Nguyen, Q.; de los Angeles, C.P.; Reiss, A.L.; Rasgon, N.L. High rates of comorbid depressive and anxiety disorders among women with premutation of the FMR1 gene. Am J Med Genet B Neuropsychiatr Genet 2013, 162b, 872–878. [Google Scholar] [CrossRef]
- Santos, E.; Emeka-Nwonovo, C.; Wang, J.Y.; Schneider, A.; Tassone, F.; Hagerman, P.; Hagerman, R. Developmental aspects of FXAND in a man with the FMR1 premutation. Mol Genet Genomic Med 2020, 8, e1050. [Google Scholar] [CrossRef] [PubMed]
- Polussa, J.; Schneider, A.; Hagerman, R. Molecular Advances Leading to Treatment Implications for Fragile X Premutation Carriers. Brain Disord Ther 2014, 3. [Google Scholar] [CrossRef]
- Zhang, A.; Borhneimer, L.A.; Weaver, A.; Franklin, C.; Hai, A.H.; Guz, S.; Shen, L. Cognitive behavioral therapy for primary care depression and anxiety: a secondary meta-analytic review using robust variance estimation in meta-regression. J Behav Med 2019, 42, 1117–1141. [Google Scholar] [CrossRef]
- Wheeler, A.; Raspa, M.; Hagerman, R.; Mailick, M.; Riley, C. Implications of the FMR1 Premutation for Children, Adolescents, Adults, and Their Families. Pediatrics 2017, 139, S172–s182. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E.; Allen, E.G.; Abramowitz, A.; Rusin, M.; Leslie, M.; Novak, G.; Hamilton, D.; Shubeck, L.; Charen, K.; Sherman, S.L. Investigation of phenotypes associated with mood and anxiety among male and female fragile X premutation carriers. Behav Genet 2008, 38, 493–502. [Google Scholar] [CrossRef]
- Roberts, J.E.; Bailey, D.B., Jr.; Mankowski, J.; Ford, A.; Sideris, J.; Weisenfeld, L.A.; Heath, T.M.; Golden, R.N. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet 2009, 150b, 130–139. [Google Scholar] [CrossRef]
- Seritan, A.L.; Bourgeois, J.A.; Schneider, A.; Mu, Y.; Hagerman, R.J.; Nguyen, D.V. Ages of Onset of Mood and Anxiety Disorders in Fragile X Premutation Carriers. Curr Psychiatry Rev 2013, 9, 65–71. [Google Scholar] [CrossRef]
- Dorn, M.B.; Mazzocco, M.M.; Hagerman, R.J. Behavioral and psychiatric disorders in adult male carriers of fragile X. J Am Acad Child Adolesc Psychiatry 1994, 33, 256–264. [Google Scholar] [CrossRef]
- Bush, L.; Scott, M.N. Neuropsychological and ASD phenotypes in rare genetic syndromes: A critical review of the literature. Clin Neuropsychol 2022, 36, 993–1027. [Google Scholar] [CrossRef]
- Klusek, J.; Martin, G.E.; Losh, M. Consistency between research and clinical diagnoses of autism among boys and girls with fragile X syndrome. J Intellect Disabil Res 2014, 58, 940–952. [Google Scholar] [CrossRef]
- Lee, M.; Martin, G.E.; Berry-Kravis, E.; Losh, M. A developmental, longitudinal investigation of autism phenotypic profiles in fragile X syndrome. J Neurodev Disord 2016, 8, 47. [Google Scholar] [CrossRef]
- Martin, G.E.; Bush, L.; Klusek, J.; Patel, S.; Losh, M. A Multimethod Analysis of Pragmatic Skills in Children and Adolescents With Fragile X Syndrome, Autism Spectrum Disorder, and Down Syndrome. J Speech Lang Hear Res 2018, 61, 3023–3037. [Google Scholar] [CrossRef]
- Bolton, P.; Macdonald, H.; Pickles, A.; Rios, P.; Goode, S.; Crowson, M.; Bailey, A.; Rutter, M. A case-control family history study of autism. J Child Psychol Psychiatry 1994, 35, 877–900. [Google Scholar] [CrossRef] [PubMed]
- Piven, J.; Palmer, P.; Jacobi, D.; Childress, D.; Arndt, S. Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. Am J Psychiatry 1997, 154, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Cornish, K.; Burack, J.A.; Rahman, A.; Munir, F.; Russo, N.; Grant, C. Theory of mind deficits in children with fragile X syndrome. J Intellect Disabil Res 2005, 49, 372–378. [Google Scholar] [CrossRef] [PubMed]
- White, S.J.; Gerber, D.; Sanchez Hernandez, R.D.; Efiannayi, A.; Chowdhury, I.; Partington, H.; Moss, J.F. Autistic traits and mental health in women with the fragile-X premutation: maternal status versus genetic risk. Br J Psychiatry 2021, 218, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.C.; Van Driesche, S.J.; Zhang, C.; Hung, K.Y.; Mele, A.; Fraser, C.E.; Stone, E.F.; Chen, C.; Fak, J.J.; Chi, S.W.; et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 2011, 146, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Klusek, J.; Fairchild, A.J.; Roberts, J.E. Vagal Tone as a Putative Mechanism for Pragmatic Competence: An Investigation of Carriers of the FMR1 Premutation. J Autism Dev Disord 2019, 49, 197–208. [Google Scholar] [CrossRef]
- Winston, M.; Nayar, K.; Hogan, A.L.; Barstein, J.; La Valle, C.; Sharp, K.; Berry-Kravis, E.; Losh, M. Physiological regulation and social-emotional processing in female carriers of the FMR1 premutation. Physiol Behav 2020, 214, 112746. [Google Scholar] [CrossRef]
- Hessl, D.; Wang, J.M.; Schneider, A.; Koldewyn, K.; Le, L.; Iwahashi, C.; Cheung, K.; Tassone, F.; Hagerman, P.J.; Rivera, S.M. Decreased fragile X mental retardation protein expression underlies amygdala dysfunction in carriers of the fragile X premutation. Biol Psychiatry 2011, 70, 859–865. [Google Scholar] [CrossRef]
- Klusek, J.; McGrath, S.E.; Abbeduto, L.; Roberts, J.E. Pragmatic Language Features of Mothers With the FMR1 Premutation Are Associated With the Language Outcomes of Adolescents and Young Adults With Fragile X Syndrome. J Speech Lang Hear Res 2016, 59, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Coplan, R.J.; Weeks, M. Shy and soft-spoken: shyness, pragmatic language, and socio-emotional adjustment in early childhood. Infant and Child Development 2009, 18, 238–254. [Google Scholar] [CrossRef]
- Rodas, N.V.; Eisenhower, A.; Blacher, J. Structural and Pragmatic Language in Children with ASD: Longitudinal Impact on Anxiety and Externalizing Behaviors. J Autism Dev Disord 2017, 47, 3479–3488. [Google Scholar] [CrossRef] [PubMed]
- Ostchega, Y.; Fryar, C.D.; Nwankwo, T.; Nguyen, D.T. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017-2018. NCHS Data Brief 2020, 1–8. [Google Scholar]
- Tassanakijpanich, N.; Cohen, J.; Cohen, R.; Srivatsa, U.N.; Hagerman, R.J. Cardiovascular Problems in the Fragile X Premutation. Front Genet 2020, 11, 586910. [Google Scholar] [CrossRef]
- Gruber, N.; Haham, L.M.; Raanani, H.; Cohen, Y.; Gabis, L.; Berkenstadt, M.; Ries-Levavi, L.; Elizur, S.; Pinhas-Hamiel, O. Female fragile X premutation carriers are at increased risk for metabolic syndrome from early adulthood. Nutr Metab Cardiovasc Dis 2022, 32, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.P.; Zarrouf, F.A. A systematic review of chronic fatigue syndrome: don't assume it's depression. Prim Care Companion J Clin Psychiatry 2008, 10, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Hamlin, A.; Liu, Y.; Nguyen, D.V.; Tassone, F.; Zhang, L.; Hagerman, R.J. Sleep apnea in fragile X premutation carriers with and without FXTAS. Am J Med Genet B Neuropsychiatr Genet 2011, 156b, 923–928. [Google Scholar] [CrossRef]
- El-Deeb, M.; Adams, P.; Schneider, A.; Salcedo-Arellano, M.J.; Tassone, F.; Hagerman, R. Fentanyl overdose in a female with the FMR1 premutation and FXTAS. J Mol Genet (Isleworth) 2018, 1. [Google Scholar] [CrossRef]
- Leehey, M.A.; Legg, W.; Tassone, F.; Hagerman, R. Fibromyalgia in fragile X mental retardation 1 gene premutation carriers. Rheumatology 2011, 50, 2233–2236. [Google Scholar] [CrossRef]
- Hall, D.A.; Hermanson, M.; Dunn, E.; Stebbins, G.; Merkitch, D.; Ouyang, B.; Berry-Kravis, E.; Jhaveri, M. The Corpus Callosum Splenium Sign in Fragile X-Associated Tremor Ataxia Syndrome. Mov Disord Clin Pract 2017, 4, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Martorell, L.; Tondo, M.; Garcia-Fructuoso, F.; Naudo, M.; Alegre, C.; Gamez, J.; Genovés, J.; Poo, P. Screening for the presence of FMR1 premutation alleles in a Spanish population with fibromyalgia. Clin Rheumatol 2012, 31, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Revenga, L.; Madrigal, I.; Blanch-Rubió, J.; Elurbe, D.M.; Docampo, E.; Collado, A.; Vidal, J.; Carbonell, J.; Estivill, X.; Mila, M. Screening for the presence of FMR1 premutation alleles in women with fibromyalgia. Gene 2013, 512, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Chonchaiya, W.; Nguyen, D.V.; Au, J.; Campos, L.; Berry-Kravis, E.M.; Lohse, K.; Mu, Y.; Utari, A.; Hervey, C.; Wang, L.; et al. Clinical involvement in daughters of men with fragile X-associated tremor ataxia syndrome. Clin Genet 2010, 78, 38–46. [Google Scholar] [CrossRef]
- Tentindo, G.S.; Fishman, S.M.; Li, C.S.; Wang, Q.; Brass, S.D. The prevalence and awareness of sleep apnea in patients suffering chronic pain: an assessment using the STOP-Bang sleep apnea questionnaire. Nat Sci Sleep 2018, 10, 217–224. [Google Scholar] [CrossRef]
- Napoli, E.; Flores, A.; Mansuri, Y.; Hagerman, R.J.; Giulivi, C. Sulforaphane improves mitochondrial metabolism in fibroblasts from patients with fragile X-associated tremor and ataxia syndrome. Neurobiol Dis 2021, 157, 105427. [Google Scholar] [CrossRef]
- Muzar, Z.; Lozano, R. Current research, diagnosis, and treatment of fragile X-associated tremor/ataxia syndrome. Intractable Rare Dis Res 2014, 3, 101–109. [Google Scholar] [CrossRef]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: common mechanisms and strategies for new treatments. Mol Neurodegener 2022, 17, 23. [Google Scholar] [CrossRef]
- Aiello Bowles, E.J.; Crane, P.K.; Walker, R.L.; Chubak, J.; LaCroix, A.Z.; Anderson, M.L.; Rosenberg, D.; Keene, C.D.; Larson, E.B. Cognitive Resilience to Alzheimer's Disease Pathology in the Human Brain. J Alzheimers Dis 2019, 68, 1071–1083. [Google Scholar] [CrossRef]
- Kotagal, V.; Bohnen, N.I.; Müller, M.L.; Koeppe, R.A.; Frey, K.A.; Langa, K.M.; Albin, R.L. Educational attainment and motor burden in Parkinson's disease. Mov Disord 2015, 30, 1143–1147. [Google Scholar] [CrossRef]
- Brega, A.G.; Reynolds, A.; Bennett, R.E.; Leehey, M.A.; Bounds, L.S.; Cogswell, J.B.; Hagerman, R.J.; Hagerman, P.J.; Grigsby, J. Functional status of men with the fragile X premutation, with and without the tremor/ataxia syndrome (FXTAS). Int J Geriatr Psychiatry 2009, 24, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Saito, N.; Reed, D.; Eldeeb, M.; Schneider, A.; Hessl, D.; Tassone, F.; Beckett, L.; Hagerman, R. Aging in Fragile X Premutation Carriers. Cerebellum 2016, 15, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Dembo, R.S.; DaWalt, L.S.; Brilliant, M.; Berry-Kravis, E.M.; Mailick, M. The effect of college degree attainment on neurodegenerative symptoms in genetically at-risk women. SSM Popul Health 2022, 19, 101262. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.M. Clinical practice. Primary ovarian insufficiency. N Engl J Med 2009, 360, 606–614. [Google Scholar] [CrossRef]
- Hipp, H.S.; Charen, K.H.; Spencer, J.B.; Allen, E.G.; Sherman, S.L. Reproductive and gynecologic care of women with fragile X primary ovarian insufficiency (FXPOI). Menopause 2016, 23, 993–999. [Google Scholar] [CrossRef]
- Pan, M.L.; Chen, L.R.; Tsao, H.M.; Chen, K.H. Polycystic ovarian syndrome and the risk of subsequent primary ovarian insufficiency: a nationwide population-based study. Menopause 2017, 24, 803–809. [Google Scholar] [CrossRef]
- Shuster, L.T.; Rhodes, D.J.; Gostout, B.S.; Grossardt, B.R.; Rocca, W.A. Premature menopause or early menopause: long-term health consequences. Maturitas 2010, 65, 161–166. [Google Scholar] [CrossRef]
- Cedars, M.I. Biomarkers of ovarian reserve--do they predict somatic aging? Semin Reprod Med 2013, 31, 443–451. [Google Scholar] [CrossRef]
- Hundscheid, R.D.; Smits, A.P.; Thomas, C.M.; Kiemeney, L.A.; Braat, D.D. Female carriers of fragile X premutations have no increased risk for additional diseases other than premature ovarian failure. Am J Med Genet A 2003, 117a, 6–9. [Google Scholar] [CrossRef]
- Allen, E.G.; Sullivan, A.K.; Marcus, M.; Small, C.; Dominguez, C.; Epstein, M.P.; Charen, K.; He, W.; Taylor, K.C.; Sherman, S.L. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod 2007, 22, 2142–2152. [Google Scholar] [CrossRef]
- Roeters van Lennep, J.E.; Heida, K.Y.; Bots, M.L.; Hoek, A. Cardiovascular disease risk in women with premature ovarian insufficiency: A systematic review and meta-analysis. Eur J Prev Cardiol 2016, 23, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Reiss, S.; Zalles, L.; Gbekie, C.; Lozano, R. Identity and Reproductive Aspects in Females with Fragile X Syndrome. Womens Health Rep (New Rochelle) 2021, 2, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.G.; Charen, K.; Hipp, H.S.; Shubeck, L.; Amin, A.; He, W.; Hunter, J.E.; Sherman, S.L. Clustering of comorbid conditions among women who carry an FMR1 premutation. Genet Med 2020, 22, 758–766. [Google Scholar] [CrossRef]
- Besterman, A.D.; Wilke, S.A.; Mulligan, T.E.; Allison, S.C.; Hagerman, R.; Seritan, A.L.; Bourgeois, J.A. Towards an Understanding of Neuropsychiatric Manifestations in Fragile X Premutation Carriers. Future Neurol 2014, 9, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, J.A.; Coffey, S.M.; Rivera, S.M.; Hessl, D.; Gane, L.W.; Tassone, F.; Greco, C.; Finucane, B.; Nelson, L.; Berry-Kravis, E.; et al. A review of fragile X premutation disorders: Expanding the psychiatric perspective. The Journal of Clinical Psychiatry 2009, 70, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Kirwin, J.L.; Gören, J.L. Duloxetine: a dual serotonin-norepinephrine reuptake inhibitor for treatment of major depressive disorder. Pharmacotherapy 2005, 25, 396–410. [Google Scholar] [CrossRef]
- Buoli, M.; Serati, M.; Cahn, W. Alternative pharmacological strategies for adult ADHD treatment: a systematic review. Expert Rev Neurother 2016, 16, 131–144. [Google Scholar] [CrossRef]
- Mazza, M.; Harnic, D.; Catalano, V.; Janiri, L.; Bria, P. Duloxetine for premenstrual dysphoric disorder: a pilot study. Expert Opin Pharmacother 2008, 9, 517–521. [Google Scholar] [CrossRef]
- Ramos, M.G.; Hara, C.; Rocha, F.L. Duloxetine treatment for women with premenstrual dysphoric disorder: a single-blind trial. Int J Neuropsychopharmacol 2009, 12, 1081–1088. [Google Scholar] [CrossRef]
- Shah, N.R.; Jones, J.B.; Aperi, J.; Shemtov, R.; Karne, A.; Borenstein, J. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder: a meta-analysis. Obstet Gynecol 2008, 111, 1175–1182. [Google Scholar] [CrossRef]
- Hofmann, S.G.; Asnaani, A.; Vonk, I.J.; Sawyer, A.T.; Fang, A. The Efficacy of Cognitive Behavioral Therapy: A Review of Meta-analyses. Cognit Ther Res 2012, 36, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Twohig, M.P.; Levin, M.E. Acceptance and Commitment Therapy as a Treatment for Anxiety and Depression: A Review. Psychiatr Clin North Am 2017, 40, 751–770. [Google Scholar] [CrossRef] [PubMed]
- Michael W. Otto; Jasper A. J. Smits, and; Hannah E. Reese. Combined Psychotherapy and Pharmacotherapy for Mood and Anxiety Disorders in Adults: Review and Analysis. FOCUS 2006, 4, 204–214. [CrossRef]
- Maina, G.; Mauri, M.; Rossi, A. Anxiety and depression. The Medical clinics of North America 2016, 72 4, 745–977. [Google Scholar]
- Curtiss, J.E.; Levine, D.S.; Ander, I.; Baker, A.W. Cognitive-Behavioral Treatments for Anxiety and Stress-Related Disorders. Focus (Am Psychiatr Publ) 2021, 19, 184–189. [Google Scholar] [CrossRef]
- Sockol, L.E.; Epperson, C.N.; Barber, J.P. A meta-analysis of treatments for perinatal depression. Clin Psychol Rev 2011, 31, 839–849. [Google Scholar] [CrossRef]
- Busse, J.W.; Montori, V.M.; Krasnik, C.; Patelis-Siotis, I.; Guyatt, G.H. Psychological intervention for premenstrual syndrome: a meta-analysis of randomized controlled trials. Psychother Psychosom 2009, 78, 6–15. [Google Scholar] [CrossRef]
- Pan, J.X.; Xia, J.J.; Deng, F.L.; Liang, W.W.; Wu, J.; Yin, B.M.; Dong, M.X.; Chen, J.J.; Ye, F.; Wang, H.Y.; et al. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: a targeted metabolomics study. Transl Psychiatry 2018, 8, 130. [Google Scholar] [CrossRef]
- Thoma, N.; Pilecki, B.; McKay, D. Contemporary Cognitive Behavior Therapy: A Review of Theory, History, and Evidence. Psychodyn Psychiatry 2015, 43, 423–461. [Google Scholar] [CrossRef]
- AlHadi, A.N.; AlGhofili, H.H.; Almujaiwel, N.A.; Alsweirky, H.M.; Albeshr, M.F.; Almogbel, G.T. Perception and barriers to the use of cognitive-behavioral therapy in the treatment of depression in primary healthcare centers and family medicine clinics in Saudi Arabia. J Family Community Med 2021, 28, 77–84. [Google Scholar] [CrossRef]
- Steel, Z.; McDonald, R.; Silove, D.; Bauman, A.; Sandford, P.; Herron, J.; Minas, I.H. Pathways to the first contact with specialist mental health care. Aust N Z J Psychiatry 2006, 40, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Juang, B.-T.; Ludwig, A.L.; Benedetti, K.L.; Gu, C.; Collins, K.; Morales, C.; Asundi, A.; Wittmann, T.; L'Etoile, N.; Hagerman, P.J. Expression of an expanded CGG-repeat RNA in a single pair of primary sensory neurons impairs olfactory adaptation in Caenorhabditis elegans. Human Molecular Genetics 2014, 23, 4945–4959. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, E.W. Physical activity in the prevention and treatment of anxiety and depression. Nord J Psychiatry 2008, 62 Suppl 47, 25–29. [Google Scholar] [CrossRef]
- Shelly, K.E.; Candelaria, N.R.; Li, Z.; Allen, E.G.; Jin, P.; Nelson, D.L. Ectopic expression of CGG-repeats alters ovarian response to gonadotropins and leads to infertility in a murine FMR1 premutation model. Hum Mol Genet 2021, 30, 923–938. [Google Scholar] [CrossRef]
- Ennis, S.; Ward, D.; Murray, A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet 2006, 14, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Tejada, M.-I.; García-Alegría, E.; Bilbao, A.; Martínez-Bouzas, C.; Beristain, E.; Poch, M.; Ramos-Arroyo, M.A.; López, B.; Fernandez Carvajal, I.; Ribate, M.-P.; et al. Analysis of the molecular parameters that could predict the risk of manifesting premature ovarian failure in female premutation carriers of fragile X syndrome. Menopause (New York, N.Y.) 2008, 15, 945–949. [Google Scholar] [CrossRef]
- Spath, M.A.; Feuth, T.B.; Smits, A.P.; Yntema, H.G.; Braat, D.D.; Thomas, C.M.; van Kessel, A.G.; Sherman, S.L.; Allen, E.G. Predictors and risk model development for menopausal age in fragile X premutation carriers. Genet Med 2011, 13, 643–650. [Google Scholar] [CrossRef]
- Allen, E.G.; Charen, K.; Hipp, H.S.; Shubeck, L.; Amin, A.; He, W.; Nolin, S.L.; Glicksman, A.; Tortora, N.; McKinnon, B.; et al. Refining the risk for fragile X-associated primary ovarian insufficiency (FXPOI) by FMR1 CGG repeat size. Genet Med 2021, 23, 1648–1655. [Google Scholar] [CrossRef]
- Brunberg, J.A.; Jacquemont, S.; Hagerman, R.J.; Berry-Kravis, E.M.; Grigsby, J.; Leehey, M.A.; Tassone, F.; Brown, W.T.; Greco, C.M.; Hagerman, P.J. Fragile X premutation carriers: characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. AJNR Am J Neuroradiol 2002, 23, 1757–1766. [Google Scholar]
- Cohen, S.; Masyn, K.; Adams, J.; Hessl, D.; Rivera, S.; Tassone, F.; Brunberg, J.; DeCarli, C.; Zhang, L.; Cogswell, J.; et al. Molecular and imaging correlates of the fragile X-associated tremor/ataxia syndrome. Neurology 2006, 67, 1426–1431. [Google Scholar] [CrossRef]
- Hashimoto, R.; Javan, A.K.; Tassone, F.; Hagerman, R.J.; Rivera, S.M. A voxel-based morphometry study of grey matter loss in fragile X-associated tremor/ataxia syndrome. Brain 2011, 134, 863–878. [Google Scholar] [CrossRef] [PubMed]
- Battistella, G.; Niederhauser, J.; Fornari, E.; Hippolyte, L.; Gronchi Perrin, A.; Lesca, G.; Forzano, F.; Hagmann, P.; Vingerhoets, F.J.; Draganski, B.; et al. Brain structure in asymptomatic FMR1 premutation carriers at risk for fragile X-associated tremor/ataxia syndrome. Neurobiol Aging 2013, 34, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Shelton, A.L.; Wang, J.Y.; Fourie, E.; Tassone, F.; Chen, A.; Frizzi, L.; Hagerman, R.J.; Ferrer, E.; Hessl, D.; Rivera, S.M. Middle Cerebellar Peduncle Width-A Novel MRI Biomarker for FXTAS? Front Neurosci 2018, 12, 379. [Google Scholar] [CrossRef]
- Birch, R.C.; Hocking, D.R.; Cornish, K.M.; Menant, J.C.; Lord, S.R.; Georgiou-Karistianis, N.; Godler, D.E.; Wen, W.; Rogers, C.; Trollor, J.N. Selective subcortical contributions to gait impairments in males with the FMR1 premutation. J Neurol Neurosurg Psychiatry 2017, 88, 188–190. [Google Scholar] [CrossRef]
- Hocking, D.R.; Birch, R.C.; Bui, Q.M.; Menant, J.C.; Lord, S.R.; Georgiou-Karistianis, N.; Godler, D.E.; Wen, W.; Hackett, A.; Rogers, C.; et al. Cerebellar volume mediates the relationship between FMR1 mRNA levels and voluntary step initiation in males with the premutation. Neurobiol Aging 2017, 50, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Hagerman, R.J.; Rivera, S.M. A multimodal imaging analysis of subcortical gray matter in fragile X premutation carriers. Mov Disord 2013, 28, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Bostan, A.C.; Strick, P.L. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 2018, 19, 338–350. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Pandya, D.N. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex 2008, 44, 1037–1066. [Google Scholar] [CrossRef]
- Adams, J.S.; Adams, P.E.; Nguyen, D.; Brunberg, J.A.; Tassone, F.; Zhang, W.; Koldewyn, K.; Rivera, S.M.; Grigsby, J.; Zhang, L.; et al. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS). Neurology 2007, 69, 851–859. [Google Scholar] [CrossRef]
- Renaud, M.; Perriard, J.; Coudray, S.; Sévin-Allouet, M.; Marcel, C.; Meissner, W.G.; Chanson, J.B.; Collongues, N.; Philippi, N.; Gebus, O.; et al. Relevance of corpus callosum splenium versus middle cerebellar peduncle hyperintensity for FXTAS diagnosis in clinical practice. J Neurol 2015, 262, 435–442. [Google Scholar] [CrossRef]
- Wang, J.Y.; Hessl, D.H.; Hagerman, R.J.; Tassone, F.; Rivera, S.M. Age-dependent structural connectivity effects in fragile x premutation. Arch Neurol 2012, 69, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.E.; Adams, J.S.; Nguyen, D.V.; Hessl, D.; Brunberg, J.A.; Tassone, F.; Zhang, W.; Koldewyn, K.; Rivera, S.M.; Grigsby, J.; et al. Psychological symptoms correlate with reduced hippocampal volume in fragile X premutation carriers. Am J Med Genet B Neuropsychiatr Genet 2010, 153b, 775–785. [Google Scholar] [CrossRef]
- Jäkälä, P.; Hänninen, T.; Ryynänen, M.; Laakso, M.; Partanen, K.; Mannermaa, A.; Soininen, H. Fragile-X: neuropsychological test performance, CGG triplet repeat lengths, and hippocampal volumes. J Clin Invest 1997, 100, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Hessl, D.; Schneider, A.; Tassone, F.; Hagerman, R.J.; Rivera, S.M. Fragile X-associated tremor/ataxia syndrome: influence of the FMR1 gene on motor fiber tracts in males with normal and premutation alleles. JAMA Neurol 2013, 70, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Hocking, D.R.; Loesch, D.Z.; Trost, N.; Bui, M.Q.; Hammersley, E.; Francis, D.; Tassone, F.; Storey, E. Total and Regional White Matter Lesions Are Correlated With Motor and Cognitive Impairments in Carriers of the FMR1 Premutation. Front Neurol 2019, 10, 832. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Trost, N.; Bui, M.Q.; Hammersley, E.; Lay, S.T.; Annesley, S.J.; Sanislav, O.; Allan, C.Y.; Tassone, F.; Chen, Z.P.; et al. The Spectrum of Neurological and White Matter Changes and Premutation Status Categories of Older Male Carriers of the FMR1 Alleles Are Linked to Genetic (CGG and FMR1 mRNA) and Cellular Stress (AMPK) Markers. Front Genet 2018, 9, 531. [Google Scholar] [CrossRef]
- Giulivi, C.; Wang, J.Y.; Hagerman, R.J. Artificial neural network applied to fragile X-associated tremor/ataxia syndrome stage diagnosis based on peripheral mitochondrial bioenergetics and brain imaging outcomes. Sci Rep 2022, 12, 21382. [Google Scholar] [CrossRef]
- Wang, J.Y.; Hessl, D.; Tassone, F.; Kim, K.; Hagerman, R.J.; Rivera, S.M. Interaction between ventricular expansion and structural changes in the corpus callosum and putamen in males with FMR1 normal and premutation alleles. Neurobiol Aging 2020, 86, 27–38. [Google Scholar] [CrossRef]
- Arpin, D.J.; Mitchell, T.; Archer, D.B.; Burciu, R.G.; Chu, W.T.; Gao, H.; Guttuso, T.; Hess, C.W.; Lai, S.; Malaty, I.A.; et al. Diffusion Magnetic Resonance Imaging Detects Progression in Parkinson's Disease: A Placebo-Controlled Trial of Rasagiline. Mov Disord 2022, 37, 325–333. [Google Scholar] [CrossRef]
- Burciu, R.G.; Chung, J.W.; Shukla, P.; Ofori, E.; Li, H.; McFarland, N.R.; Okun, M.S.; Vaillancourt, D.E. Functional MRI of disease progression in Parkinson disease and atypical parkinsonian syndromes. Neurology 2016, 87, 709–717. [Google Scholar] [CrossRef]
- Yang, J.C.; Rodriguez, A.; Royston, A.; Niu, Y.Q.; Avar, M.; Brill, R.; Simon, C.; Grigsby, J.; Hagerman, R.J.; Olichney, J.M. Memantine Improves Attentional Processes in Fragile X-Associated Tremor/Ataxia Syndrome: Electrophysiological Evidence from a Randomized Controlled Trial. Sci Rep 2016, 6, 21719. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Chi, L.; Teichholtz, S.; Schneider, A.; Nanakul, R.; Nowacki, R.; Seritan, A.; Reed, B.; DeCarli, C.; Iragui, V.J.; et al. ERP abnormalities elicited by word repetition in fragile X-associated tremor/ataxia syndrome (FXTAS) and amnestic MCI. Neuropsychologia 2014, 63, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Niu, Y.Q.; Simon, C.; Seritan, A.L.; Chen, L.; Schneider, A.; Moghaddam, S.T.; Hagerman, P.J.; Hagerman, R.J.; Olichney, J.M. Memantine effects on verbal memory in fragile X-associated tremor/ataxia syndrome (FXTAS): a double-blind brain potential study. Neuropsychopharmacology 2014, 39, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
- Seritan, A.L.; Nguyen, D.V.; Mu, Y.; Tassone, F.; Bourgeois, J.A.; Schneider, A.; Cogswell, J.B.; Cook, K.R.; Leehey, M.A.; Grigsby, J.; et al. Memantine for fragile X-associated tremor/ataxia syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2014, 75, 264–271. [Google Scholar] [CrossRef] [PubMed]
- McCormick, E.M.; Arnemann, K.L.; Ito, T.; Hanson, S.J.; Cole, M.W. Latent functional connectivity underlying multiple brain states. Netw Neurosci 2022, 6, 570–590. [Google Scholar] [CrossRef]
- Brown, S.S.G.; Basu, S.; Whalley, H.C.; Kind, P.C.; Stanfield, A.C. Age-related functional brain changes in FMR1 premutation carriers. Neuroimage Clin 2018, 17, 761–767. [Google Scholar] [CrossRef] [PubMed]
- McKinney, W.S.; Bartolotti, J.; Khemani, P.; Wang, J.Y.; Hagerman, R.J.; Mosconi, M.W. Cerebellar-cortical function and connectivity during sensorimotor behavior in aging FMR1 gene premutation carriers. Neuroimage Clin 2020, 27, 102332. [Google Scholar] [CrossRef]
- McKinney, W.S.; Wang, Z.; Kelly, S.; Khemani, P.; Lui, S.; White, S.P.; Mosconi, M.W. Precision Sensorimotor Control in Aging FMR1 Gene Premutation Carriers. Front Integr Neurosci 2019, 13, 56. [Google Scholar] [CrossRef]
- Park, S.H.; Wang, Z.; McKinney, W.; Khemani, P.; Lui, S.; Christou, E.A.; Mosconi, M.W. Functional motor control deficits in older FMR1 premutation carriers. Exp Brain Res 2019, 237, 2269–2278. [Google Scholar] [CrossRef]
- Hessl, D.; Rivera, S.; Koldewyn, K.; Cordeiro, L.; Adams, J.; Tassone, F.; Hagerman, P.J.; Hagerman, R.J. Amygdala dysfunction in men with the fragile X premutation. Brain 2007, 130, 404–416. [Google Scholar] [CrossRef]
- Tassone, F.; Hagerman, R.J.; Garcia-Arocena, D.; Khandjian, E.W.; Greco, C.M.; Hagerman, P.J. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. J Med Genet 2004, 41, e43. [Google Scholar] [CrossRef] [PubMed]
- Ariza, J.; Rogers, H.; Monterrubio, A.; Reyes-Miranda, A.; Hagerman, P.J.; Martínez-Cerdeño, V. A Majority of FXTAS Cases Present with Intranuclear Inclusions Within Purkinje Cells. Cerebellum 2016, 15, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Buijsen, R.A.; Sellier, C.; Severijnen, L.A.; Oulad-Abdelghani, M.; Verhagen, R.F.; Berman, R.F.; Charlet-Berguerand, N.; Willemsen, R.; Hukema, R.K. FMRpolyG-positive inclusions in CNS and non-CNS organs of a fragile X premutation carrier with fragile X-associated tremor/ataxia syndrome. Acta Neuropathol Commun 2014, 2, 162. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.; Ariza, J.; Monterrubio, A.; Hagerman, P.; Martínez-Cerdeño, V. Cerebellar Mild Iron Accumulation in a Subset of FMR1 Premutation Carriers with FXTAS. Cerebellum 2016, 15, 641–644. [Google Scholar] [CrossRef]
- Ariza, J.; Rogers, H.; Hartvigsen, A.; Snell, M.; Dill, M.; Judd, D.; Hagerman, P.; Martínez-Cerdeño, V. Iron accumulation and dysregulation in the putamen in fragile X-associated tremor/ataxia syndrome. Mov Disord 2017, 32, 585–591. [Google Scholar] [CrossRef]
- Wang, J.Y.; Sonico, G.J.; Salcedo-Arellano, M.J.; Hagerman, R.J.; Martínez-Cerdeño, V. A postmortem MRI study of cerebrovascular disease and iron content at end-stage of fragile X-associated tremor/ataxia syndrome. Res Sq 2023. [Google Scholar] [CrossRef]
- Dufour, B.D.; Amina, S.; Martinez-Cerdeno, V. FXTAS presents with upregulation of the cytokines IL12 and TNFα. Parkinsonism Relat Disord 2021, 82, 117–120. [Google Scholar] [CrossRef]
- Niu, Y.Q.; Yang, J.C.; Hall, D.A.; Leehey, M.A.; Tassone, F.; Olichney, J.M.; Hagerman, R.J.; Zhang, L. Parkinsonism in fragile X-associated tremor/ataxia syndrome (FXTAS): revisited. Parkinsonism Relat Disord 2014, 20, 456–459. [Google Scholar] [CrossRef]
- Martínez-Cerdeño, V.; Lechpammer, M.; Hagerman, P.J.; Hagerman, R. Two FMR1 premutation cases without nuclear inclusions. Mov Disord 2017, 32, 1328–1329. [Google Scholar] [CrossRef]
- Paucar, M.; Nennesmo, I.; Svenningsson, P. Pathological Study of a FMR1 Premutation Carrier With Progressive Supranuclear Palsy. Front Genet 2018, 9, 317. [Google Scholar] [CrossRef]
- Sacino, A.N.; Prokop, S.; Walsh, M.A.; Adamson, J.; Subramony, S.H.; Krans, A.; Todd, P.K.; Giasson, B.I.; Yachnis, A.T. Fragile X-associated tremor ataxia syndrome with co-occurrent progressive supranuclear palsy-like neuropathology. Acta Neuropathol Commun 2019, 7, 158. [Google Scholar] [CrossRef]
- Salcedo-Arellano, M.J.; Hagerman, R.J. Recent research in fragile X-associated tremor/ataxia syndrome. Curr Opin Neurobiol 2022, 72, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.; Hagerman, P. Fragile X-associated tremor/ataxia syndrome: pathophysiology and management. Curr Opin Neurol 2021, 34, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; Berry-Kravis, E.; Hagerman, R.J.; Hagerman, P.J.; Rice, C.D.; Leehey, M.A. Symptomatic treatment in the fragile X-associated tremor/ataxia syndrome. Mov Disord 2006, 21, 1741–1744. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; O'Keefe J, A. Fragile x-associated tremor ataxia syndrome: the expanding clinical picture, pathophysiology, epidemiology, and update on treatment. Tremor Other Hyperkinet Mov (N Y) 2012, 2. [Google Scholar] [CrossRef]
- Hall, D.; Todorova-Koteva, K.; Pandya, S.; Bernard, B.; Ouyang, B.; Walsh, M.; Pounardjian, T.; Deburghraeve, C.; Zhou, L.; Losh, M.; et al. Neurological and endocrine phenotypes of fragile X carrier women. Clin Genet 2016, 89, 60–67. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Hall, D.A.; Coffey, S.; Leehey, M.; Bourgeois, J.; Gould, J.; Zhang, L.; Seritan, A.; Berry-Kravis, E.; Olichney, J.; et al. Treatment of fragile X-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Clin Interv Aging 2008, 3, 251–262. [Google Scholar] [CrossRef]
- Wang, J.Y.; Trivedi, A.M.; Carrillo, N.R.; Yang, J.; Schneider, A.; Giulivi, C.; Adams, P.; Tassone, F.; Kim, K.; Rivera, S.M.; et al. Open-Label Allopregnanolone Treatment of Men with Fragile X-Associated Tremor/Ataxia Syndrome. Neurotherapeutics 2017, 14, 1073–1083. [Google Scholar] [CrossRef]
- Budimirovic, D.B. Can a Neurosteroid Ameliorate Fragile X-Associated Tremor/Ataxia Syndrome? Neurotherapeutics 2017, 14, 1070–1072. [Google Scholar] [CrossRef]
- Hall, D.A.; Robertson, E.E.; Leehey, M.; McAsey, A.; Ouyang, B.; Berry-Kravis, E.; O'Keefe, J.A. Open-label pilot clinical trial of citicoline for fragile X-associated tremor/ataxia syndrome (FXTAS). PLoS ONE 2020, 15, e0225191. [Google Scholar] [CrossRef]
- Hall, D.A.; Berry-Kravis, E. Fragile X syndrome and fragile X-associated tremor ataxia syndrome. Handb Clin Neurol 2018, 147, 377–391. [Google Scholar] [CrossRef]
- Artusi, C.A.; Farooqi, A.; Romagnolo, A.; Marsili, L.; Balestrino, R.; Sokol, L.L.; Wang, L.L.; Zibetti, M.; Duker, A.P.; Mandybur, G.T.; et al. Deep brain stimulation in uncommon tremor disorders: indications, targets, and programming. J Neurol 2018, 265, 2473–2493. [Google Scholar] [CrossRef] [PubMed]
- Zesiewicz, T.A.; Wilmot, G.; Kuo, S.H.; Perlman, S.; Greenstein, P.E.; Ying, S.H.; Ashizawa, T.; Subramony, S.H.; Schmahmann, J.D.; Figueroa, K.P.; et al. Comprehensive systematic review summary: Treatment of cerebellar motor dysfunction and ataxia: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med 2003, 348, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Stamatakis, E.; Lee, I.M.; Bennie, J.; Freeston, J.; Hamer, M.; O'Donovan, G.; Ding, D.; Bauman, A.; Mavros, Y. Does Strength-Promoting Exercise Confer Unique Health Benefits? A Pooled Analysis of Data on 11 Population Cohorts With All-Cause, Cancer, and Cardiovascular Mortality Endpoints. Am J Epidemiol 2018, 187, 1102–1112. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med 2018, 52, 154–160. [Google Scholar] [CrossRef]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol 2009, 94, 1062–1069. [Google Scholar] [CrossRef]
- Herold, F.; Törpel, A.; Schega, L.; Müller, N.G. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements - a systematic review. Eur Rev Aging Phys Act 2019, 16, 10. [Google Scholar] [CrossRef]
- Wang, P.; Song, M.; Eliassen, A.H.; Wang, M.; Fung, T.T.; Clinton, S.K.; Rimm, E.B.; Hu, F.B.; Willett, W.C.; Tabung, F.K.; et al. Optimal dietary patterns for prevention of chronic disease. Nat Med 2023, 29, 719–728. [Google Scholar] [CrossRef]
- Snetselaar, L.G.; de Jesus, J.M.; DeSilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020-2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr Today 2021, 56, 287–295. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Rapamycin for longevity: opinion article. Aging (Albany NY) 2019, 11, 8048–8067. [Google Scholar] [CrossRef] [PubMed]
- Mannick, J.B.; Lamming, D.W. Targeting the biology of aging with mTOR inhibitors. Nat Aging 2023, 3, 642–660. [Google Scholar] [CrossRef] [PubMed]
- Moses, J.W.; Leon, M.B.; Popma, J.J.; Fitzgerald, P.J.; Holmes, D.R.; O'Shaughnessy, C.; Caputo, R.P.; Kereiakes, D.J.; Williams, D.O.; Teirstein, P.S.; et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003, 349, 1315–1323. [Google Scholar] [CrossRef]
- Sasongko, T.H.; Ismail, N.F.; Zabidi-Hussin, Z. Rapamycin and rapalogs for tuberous sclerosis complex. Cochrane Database Syst Rev 2016, 7, Cd011272. [Google Scholar] [CrossRef]
- Arriola Apelo, S.I.; Neuman, J.C.; Baar, E.L.; Syed, F.A.; Cummings, N.E.; Brar, H.K.; Pumper, C.P.; Kimple, M.E.; Lamming, D.W. Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell 2016, 15, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Tang, C.; He, H.; Duan, R. Activation of mTOR ameliorates fragile X premutation rCGG repeat-mediated neurodegeneration. PLoS ONE 2013, 8, e62572. [Google Scholar] [CrossRef]
- Lamming, D.W.; Ye, L.; Sabatini, D.M.; Baur, J.A. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest 2013, 123, 980–989. [Google Scholar] [CrossRef]
- Kaeberlein, M.; Galvan, V. Rapamycin and Alzheimer's disease: Time for a clinical trial? Sci Transl Med 2019, 11. [Google Scholar] [CrossRef]
- Urfer, S.R.; Kaeberlein, T.L.; Mailheau, S.; Bergman, P.J.; Creevy, K.E.; Promislow, D.E.L.; Kaeberlein, M. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience 2017, 39, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Li, Y.; Allen, E.G.; Jin, P. Therapeutic Development for CGG Repeat Expansion-Associated Neurodegeneration. Front Cell Neurosci 2021, 15, 655568. [Google Scholar] [CrossRef]
- Jin, P.; Zarnescu, D.C.; Zhang, F.; Pearson, C.E.; Lucchesi, J.C.; Moses, K.; Warren, S.T. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron 2003, 39, 739–747. [Google Scholar] [CrossRef]
- Alvarado, C.X.; Makarious, M.B.; Vitale, D.; Koretsky, M.J.; Bandres-Ciga, S.; Iwaki, H.; Levine, K.; Singleton, A.; Faghri, F.; Nalls, M.A.; et al. omicSynth: an Open Multi-omic Community Resource for Identifying Druggable Targets across Neurodegenerative Diseases. medRxiv 2023. [Google Scholar] [CrossRef]
- Gabis, L.V.; Hochberg, O.; Leon Attia, O.; Banet-Levi, Y.; Topf, D.; Shefer, S. Prolonged Time Lag to Final Diagnosis of Fragile X Syndrome. J Pediatr 2018, 193, 217–221.e211. [Google Scholar] [CrossRef]
- Bailey, D.B., Jr.; Raspa, M.; Bishop, E.; Holiday, D. No change in the age of diagnosis for fragile x syndrome: findings from a national parent survey. Pediatrics 2009, 124, 527–533. [Google Scholar] [CrossRef] [PubMed]
- McConkie-Rosell, A.; Abrams, L.; Finucane, B.; Cronister, A.; Gane, L.W.; Coffey, S.M.; Sherman, S.; Nelson, L.M.; Berry-Kravis, E.; Hessl, D.; et al. Recommendations from multi-disciplinary focus groups on cascade testing and genetic counseling for fragile X-associated disorders. J Genet Couns 2007, 16, 593–606. [Google Scholar] [CrossRef]
- Raspa, M.; Edwards, A.; Wheeler, A.C.; Bishop, E.; Bailey, D.B., Jr. Family Communication and Cascade Testing for Fragile X Syndrome. J Genet Couns 2016, 25, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Abrams, L.; Coffey, S.M.; Hall, D.A.; Greco, C.; Gane, L.W.; Grigsby, J.; Bourgeois, J.A.; Finucane, B.; Jacquemont, S.; et al. Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines. Mov Disord 2007, 22, 2018–2030, quiz 2140. [Google Scholar] [CrossRef]
- Acharya, K.; Schindler, A. Developmental and behavioral pediatricians' attitudes toward screening for fragile X. Am J Intellect Dev Disabil 2013, 118, 284–293. [Google Scholar] [CrossRef]
- Andermann, A.; Blancquaert, I.; Beauchamp, S.; Déry, V. Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Bull World Health Organ 2008, 86, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.B., Jr.; Berry-Kravis, E.; Gane, L.W.; Guarda, S.; Hagerman, R.; Powell, C.M.; Tassone, F.; Wheeler, A. Fragile X Newborn Screening: Lessons Learned From a Multisite Screening Study. Pediatrics 2017, 139, S216–s225. [Google Scholar] [CrossRef]
- Christie, L.; Wotton, T.; Bennetts, B.; Wiley, V.; Wilcken, B.; Rogers, C.; Boyle, J.; Turner, C.; Hansen, J.; Hunter, M.; et al. Maternal attitudes to newborn screening for fragile X syndrome. Am J Med Genet A 2013, 161a, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.C.; Gwaltney, A.; Raspa, M.; Okoniewski, K.C.; Berry-Kravis, E.; Botteron, K.N.; Budimirovic, D.; Hazlett, H.C.; Hessl, D.; Losh, M.; et al. Emergence of Developmental Delay in Infants and Toddlers With an FMR1 Mutation. Pediatrics 2021, 147. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F. Newborn screening for fragile X syndrome. JAMA Neurol 2014, 71, 355–359. [Google Scholar] [CrossRef]
- Lee, S.; Taylor, J.L.; Redmond, C.; Hadd, A.G.; Kemppainen, J.A.; Haynes, B.C.; Shone, S.; Bailey, D.B., Jr.; Latham, G.J. Validation of Fragile X Screening in the Newborn Population Using a Fit-for-Purpose FMR1 PCR Assay System. J Mol Diagn 2020, 22, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F. Advanced technologies for the molecular diagnosis of fragile X syndrome. Expert Rev Mol Diagn 2015, 15, 1465–1473. [Google Scholar] [CrossRef]
- Bailey, D.B., Jr.; Wheeler, A.; Berry-Kravis, E.; Hagerman, R.; Tassone, F.; Powell, C.M.; Roche, M.; Gane, L.W.; Sideris, J. Maternal Consequences of the Detection of Fragile X Carriers in Newborn Screening. Pediatrics 2015, 136, e433–e440. [Google Scholar] [CrossRef]
- Bailey, D.B., Jr.; Gehtland, L.M.; Lewis, M.A.; Peay, H.; Raspa, M.; Shone, S.M.; Taylor, J.L.; Wheeler, A.C.; Cotten, M.; King, N.M.P.; et al. Early Check: translational science at the intersection of public health and newborn screening. BMC Pediatr 2019, 19, 238. [Google Scholar] [CrossRef]
- Gallego, P.K.; Burris, J.L.; Rivera, S.M. Visual motion processing deficits in infants with the fragile X premutation. J Neurodev Disord 2014, 6, 29. [Google Scholar] [CrossRef]
- Wheeler, A.C.; Sideris, J.; Hagerman, R.; Berry-Kravis, E.; Tassone, F.; Bailey, D.B., Jr. Developmental profiles of infants with an FMR1 premutation. J Neurodev Disord 2016, 8, 40. [Google Scholar] [CrossRef]
- Bailey, D.B., Jr.; Bishop, E.; Raspa, M.; Skinner, D. Caregiver opinions about fragile X population screening. Genet Med 2012, 14, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Archibald, A.D.; Hickerton, C.L.; Wake, S.A.; Jaques, A.M.; Cohen, J.; Metcalfe, S.A. "It gives them more options": preferences for preconception genetic carrier screening for fragile X syndrome in primary healthcare. J Community Genet 2016, 7, 159–171. [Google Scholar] [CrossRef]
- Metcalfe, S.A.; Martyn, M.; Ames, A.; Anderson, V.; Archibald, A.D.; Couns, G.D.G.; Carter, R.; Cohen, J.; Cotter, M.; GenCouns, M.; et al. Informed decision making and psychosocial outcomes in pregnant and nonpregnant women offered population fragile X carrier screening. Genet Med 2017, 19, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Xie, W.; Chen, J.; Tang, W.; Deng, X.; Li, H.; Peng, Y.; Wang, D.; Yang, S.; Zhang, Y.; et al. Implementation of fragile X syndrome carrier screening during prenatal diagnosis: A pilot study at a single center. Mol Genet Genomic Med 2021, 9, e1711. [Google Scholar] [CrossRef] [PubMed]
- Finucane, B.; Abrams, L.; Cronister, A.; Archibald, A.D.; Bennett, R.L.; McConkie-Rosell, A. Genetic counseling and testing for FMR1 gene mutations: practice guidelines of the national society of genetic counselors. J Genet Couns 2012, 21, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Martyn, M.; Anderson, V.; Archibald, A.; Carter, R.; Cohen, J.; Delatycki, M.; Donath, S.; Emery, J.; Halliday, J.; Hill, M.; et al. Offering fragile X syndrome carrier screening: a prospective mixed-methods observational study comparing carrier screening of pregnant and non-pregnant women in the general population. BMJ Open 2013, 3, e003660. [Google Scholar] [CrossRef]
- Miller, D.T.; Adam, M.P.; Aradhya, S.; Biesecker, L.G.; Brothman, A.R.; Carter, N.P.; Church, D.M.; Crolla, J.A.; Eichler, E.E.; Epstein, C.J.; et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet 2010, 86, 749–764. [Google Scholar] [CrossRef]
- Spector, E.; Behlmann, A.; Kronquist, K.; Rose, N.C.; Lyon, E.; Reddi, H.V. Laboratory testing for fragile X, 2021 revision: a technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2021, 23, 799–812. [Google Scholar] [CrossRef]
- Biancalana, V.; Glaeser, D.; McQuaid, S.; Steinbach, P. EMQN best practice guidelines for the molecular genetic testing and reporting of fragile X syndrome and other fragile X-associated disorders. Eur J Hum Genet 2015, 23, 417–425. [Google Scholar] [CrossRef]
- Hayward, B.; Loutaev, I.; Ding, X.; Nolin, S.L.; Thurm, A.; Usdin, K.; Smith, C.B. Fragile X syndrome in a male with methylated premutation alleles and no detectable methylated full mutation alleles. Am J Med Genet A 2019, 179, 2132–2137. [Google Scholar] [CrossRef] [PubMed]
- Tabolacci, E.; Pomponi, M.G.; Remondini, L.; Pietrobono, R.; Nobile, V.; Pennacchio, G.; Gurrieri, F.; Neri, G.; Genuardi, M.; Chiurazzi, P. Methylated premutation of the FMR1 gene in three sisters: correlating CGG expansion and epigenetic inactivation. Eur J Hum Genet 2020, 28, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Jiraanont, P.; Kumar, M.; Tang, H.T.; Espinal, G.; Hagerman, P.J.; Hagerman, R.J.; Chutabhakdikul, N.; Tassone, F. Size and methylation mosaicism in males with Fragile X syndrome. Expert Rev Mol Diagn 2017, 17, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Archibald, A.D.; Smith, M.J.; Burgess, T.; Scarff, K.L.; Elliott, J.; Hunt, C.E.; McDonald, Z.; Barns-Jenkins, C.; Holt, C.; Sandoval, K.; et al. Reproductive genetic carrier screening for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy in Australia: outcomes of 12,000 tests. Genet Med 2018, 20, 513–523. [Google Scholar] [CrossRef]

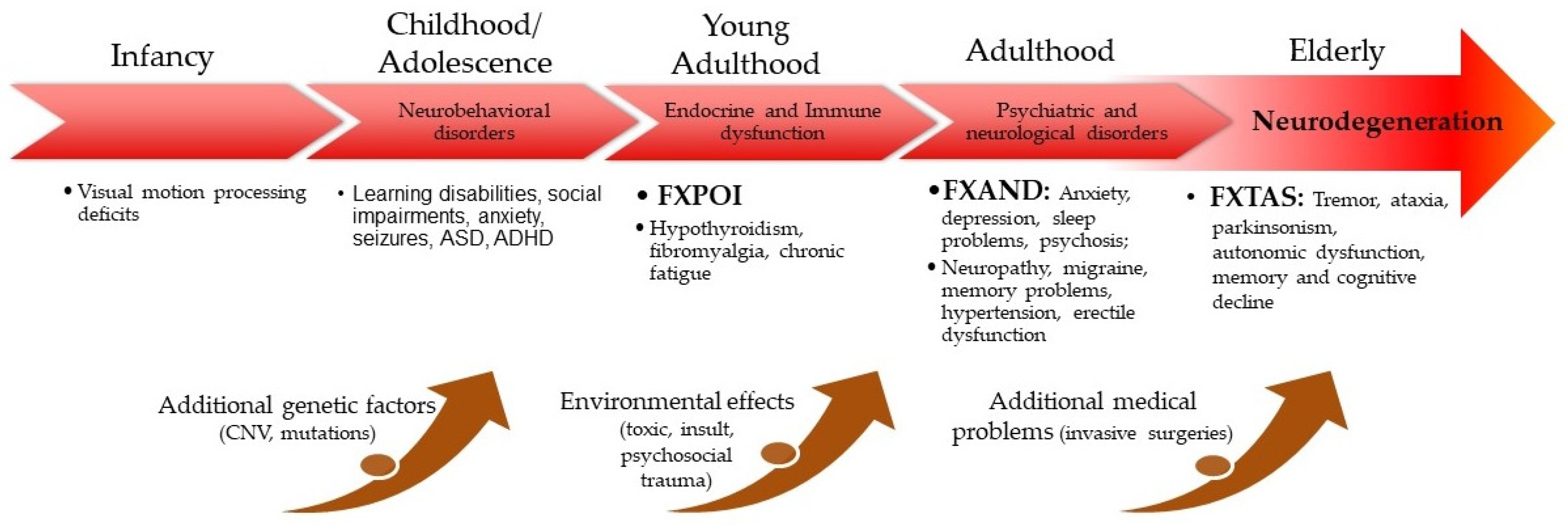
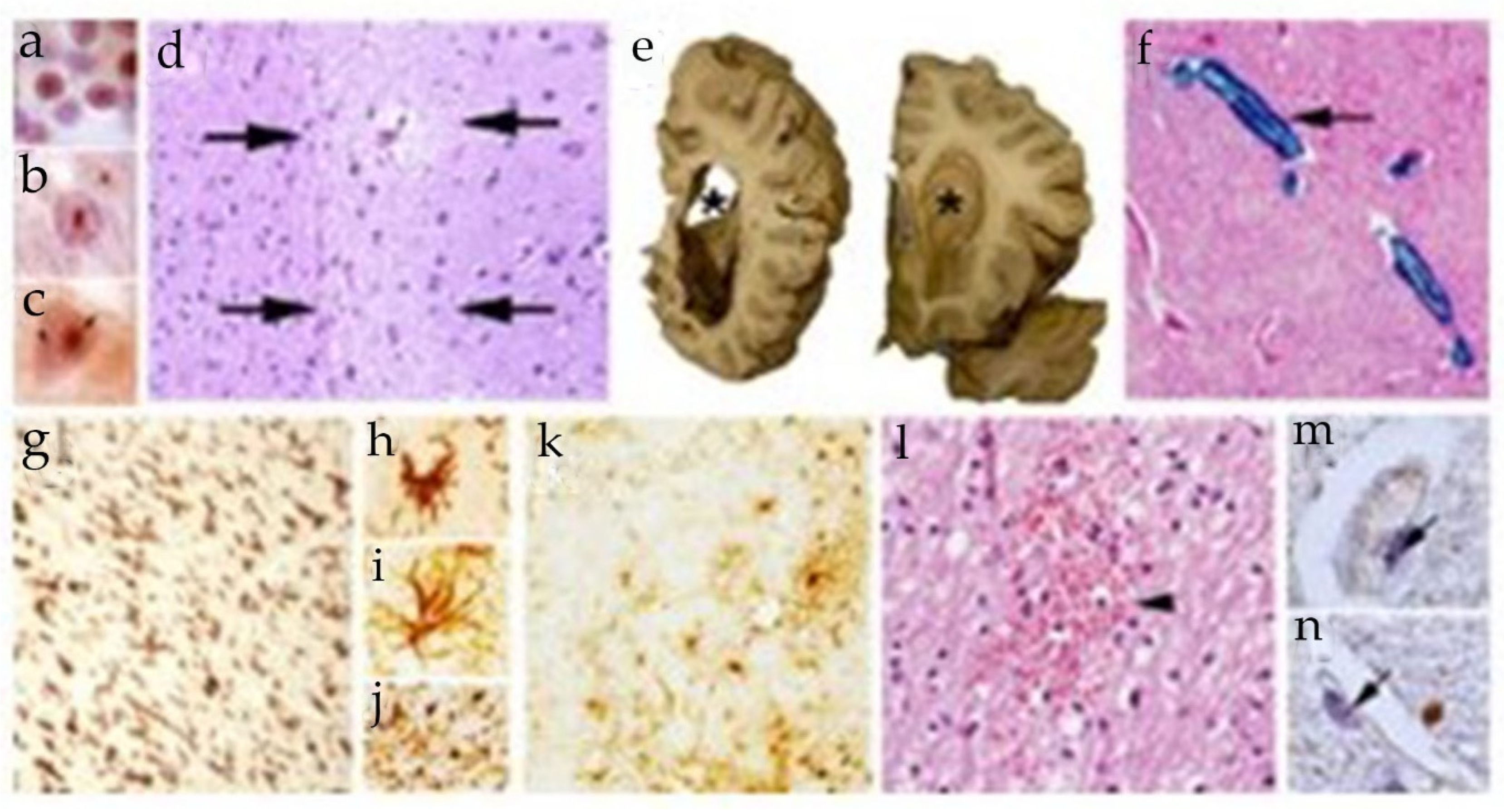
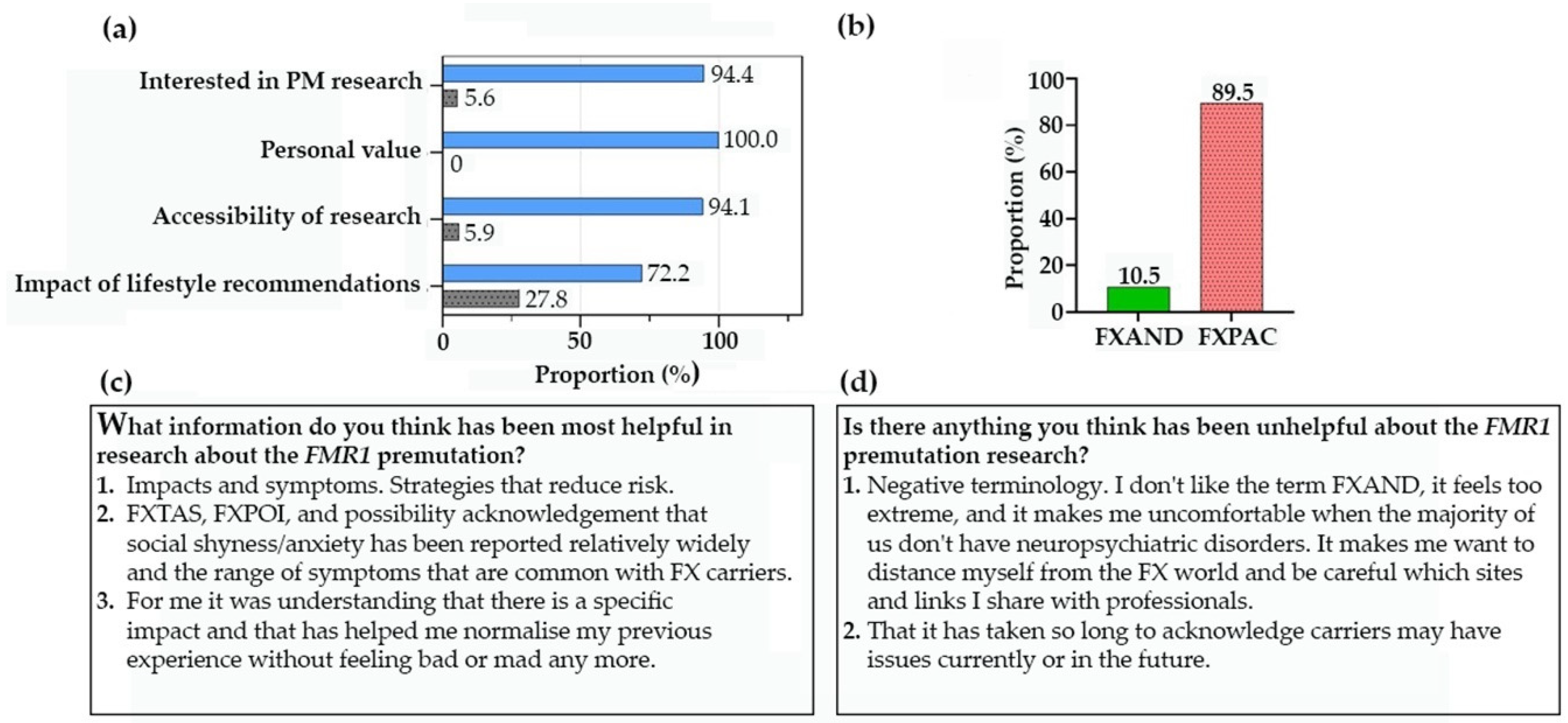
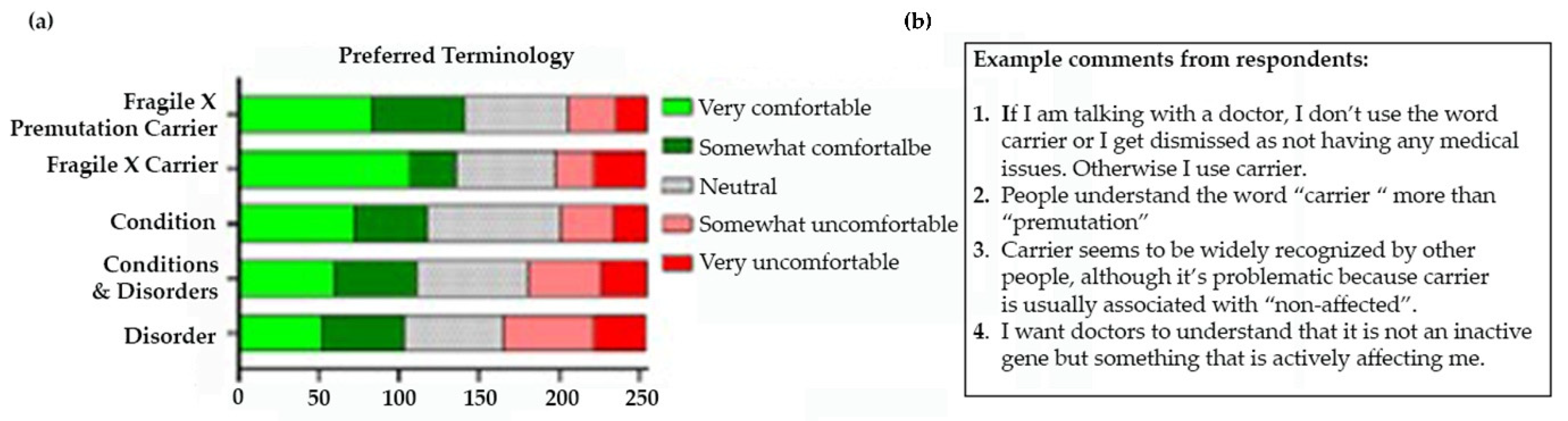

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




