Submitted:
29 June 2023
Posted:
10 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.2.1. Indirect Calorimetry
2.2.2. Accelerometery
2.2.3. Estimation of Energy Expenditure (EEACC)
2.3. Statistical Analysis
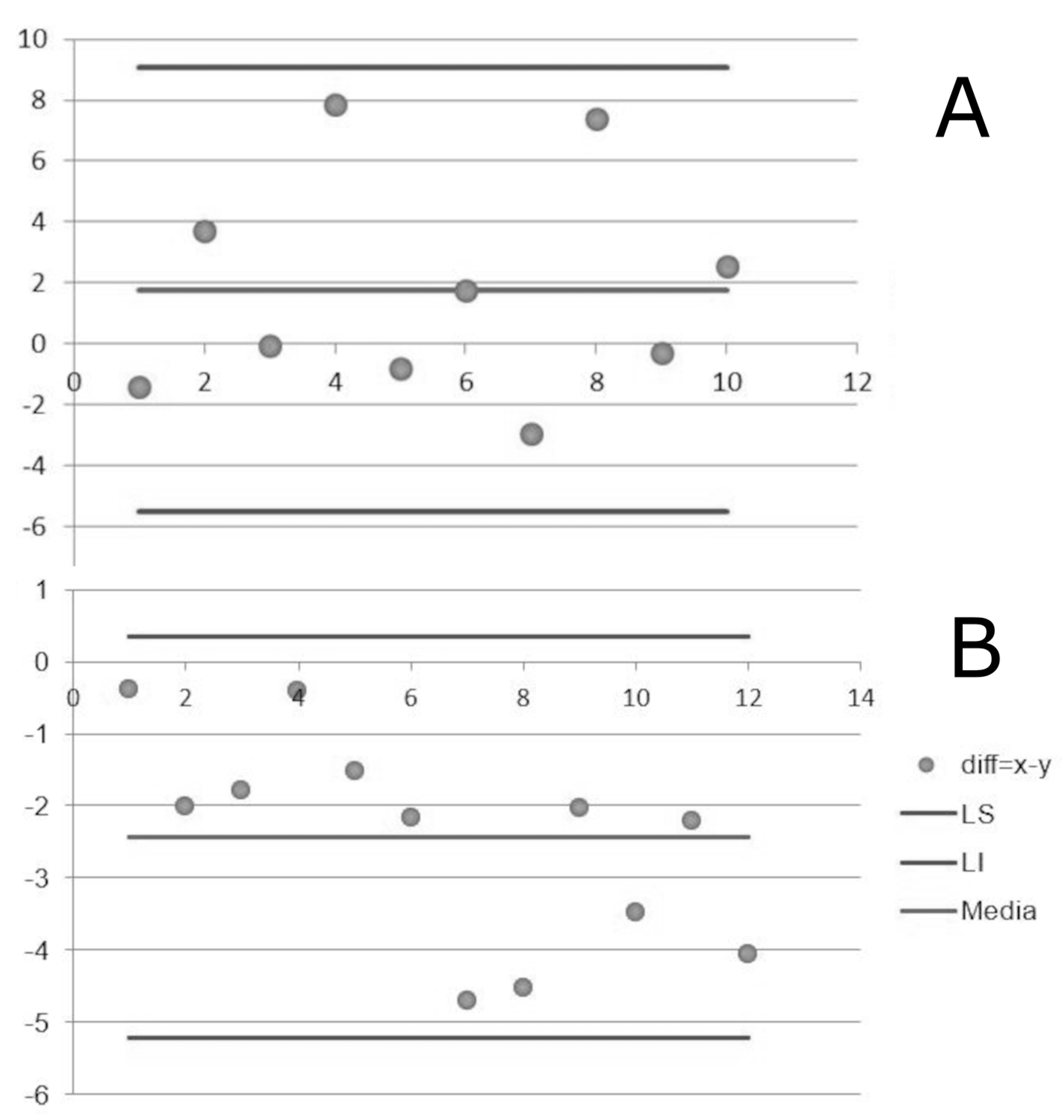
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ezeugwu, V.E.; Manns, P.J. Sleep Duration, Sedentary Behavior, Physical Activity, and Quality of Life after Inpatient Stroke Rehabilitation. J. Stroke Cerebrovasc. Dis. 2017, 26, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Sandroff, B.M.; Pilutti, L.A.; Klaren, R.E.; Baynard, T.; Fernhall, B. Physical Activity, Sedentary Behavior, and Aerobic Capacity in Persons with Multiple Sclerosis. J. Neurol. Sci. 2017, 372, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Dalgas, U.; Stenager, E.; Ingemann-Hansen, T. Review: Multiple Sclerosis and Physical Exercise: Recommendations for the Application of Resistance-, Endurance- and Combined Training. Mult Scler 2008, 14, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.H.; Kolle, E.; Dyrstad, S.M.; Holme, I.; Anderssen, S.A. Accelerometer-Determined Physical Activity in Adults and Older People. Med. Sci. Sports Exerc. 2012, 44, 266–272. [Google Scholar] [CrossRef]
- Jo, A.; Coronel, B.D.; Coakes, C.E.; Mainous, A.G. Is There a Benefit to Patients Using Wearable Devices Such as Fitbit or Health Apps on Mobiles? A Systematic Review. Am. J. Med. 2019, 132, 1394–1400.e1. [Google Scholar] [CrossRef]
- Stookey, A.D.; McCusker, M.G.; Sorkin, J.D.; Katzel, L.I.; Shaughnessy, M.; Macko, R.F.; Ivey, F.M. Test-Retest Reliability of Portable Metabolic Monitoring after Disabling Stroke. Neurorehabil. Neural Repair 2013, 27, 872–877. [Google Scholar] [CrossRef]
- Evenson, K.R.; Goto, M.M.; Furberg, R.D. Systematic Review of the Validity and Reliability of Consumer-Wearable Activity Trackers. Int. J. Behav. Nutr. Phys. Act. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Kuwashiro, T.; Sugimori, H.; Ago, T.; Kamouchi, M.; Kitazono, T. Risk Factors Predisposing to Stroke Recurrence within One Year of Non-Cardioembolic Stroke Onset: The Fukuoka Stroke Registry. Cerebrovasc. Dis. 2012, 33, 141–149. [Google Scholar] [CrossRef]
- Smith, A.C.; Saunders, D.H.; Mead, G. Cardiorespiratory Fitness after Stroke: A Systematic Review. Int. J. Stroke 2012, 7, 499–510. [Google Scholar] [CrossRef]
- Lynch, E.A.; Jones, T.M.; Simpson, D.B.; Fini, N.A.; Kuys, S.S.; Borschmann, K.; Kramer, S.; Johnson, L.; Callisaya, M.L.; Mahendran, N.; et al. Activity Monitors for Increasing Physical Activity in Adult Stroke Survivors. Cochrane Database Syst. Rev. 2018, 109, 103392. [Google Scholar] [CrossRef]
- English, C.; Healy, G.N.; Coates, A.; Lewis, L.K.; Olds, T.; Bernhardt, J. Sitting Time and Physical Activity after Stroke: Physical Ability Is Only Part of the Story. Top. Stroke Rehabil. 2016, 23, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.L.; Mulroy, S. The Energy Expenditure of Normal and Pathologic Gait. Gait Posture 1999, 9, 207–231. [Google Scholar] [CrossRef] [PubMed]
- Haute Autorité de Santé Prescription d’activité Physique et Sportive Accidents Vasculaires Cérébraux. 2018, 1–10.
- Dobkin, B.H. Wearable Motion Sensors to Continuously Measure Real-World Physical Activities. Curr. Opin. Neurol. 2013, 26, 602–608. [Google Scholar] [CrossRef]
- Lyden, K.; Kozey, S.L.; Staudenmeyer, J.W. Energy Expenditure and MET Prediction Equations. 2012, 111, 187–201. [CrossRef]
- Hall, K.S.; Howe, C.A.; Rana, S.R.; Martin, C.L.; Morey, M.C. METs and Accelerometry of Walking in Older Adults. Med. Sci. Sport. Exerc. 2013, 45, 574–582. [Google Scholar] [CrossRef]
- Bouten, C.V.C.; Sauren, A.A.H.J.; Verduin, M.; Janssen, J.D. Effects of Placement and Orientation of Body-Fixed Accelerometers on the Assessment of Energy Expenditure during Walking. Med. Biol. Eng. Comput. 1997, 35, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Caron, N.; Peyrot, N.; Caderby, T.; Verkindt, C.; Dalleau, G. Accelerometry-Based Method for Assessing Energy Expenditure in Patients with Diabetes during Walking. J. Hum. Nutr. Diet. 2019, 32, 531–534. [Google Scholar] [CrossRef]
- Levine, J.; Melanson, E.L.; Westerterp, K.R.; Hill, J.O. Tracmor System for Measuring Walking Energy Expenditure. Eur. J. Clin. Nutr. 2003, 57, 1176–1180. [Google Scholar] [CrossRef]
- Valenti, G.; Bonomi, A.G.; Westerterp, K.R. Body Acceleration as Indicator for Walking Economy in an Ageing Population. PLoS One 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bouten, C.V.C.; Westerterp, K.R.; Verduin, M.; Janssen, J.D. A Triaxial Accelerometer for the Assessment of Daily Physical Activity in Relation to Energy Expenditure. Eng. Med. Biol. Soc. 1993. Proc. 15th Annu. Int. Conf. IEEE 1993, 985–986. [Google Scholar]
- Bouten CV, Westerterp KR, Verduin M, J. J. Assessment of Energy Expenditure for Physical Activity Using a Triaxial Accelerometer. 1994.
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait Differences between Individuals with Post-Stroke Hemiparesis and Non-Disabled Controls at Matched Speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Slawinski, J.; Pradon, D.; Bensmail, D.; Roche, N.; Zory, R. Energy Cost of Obstacle Crossing in Stroke Patients. Am. J. Phys. Med. Rehabil. 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, D.C.; Bang, M.S.; Burke, D.T. An Algorithm to Assess Stiff-Legged Gait in Traumatic Brain Injury. J. Head Trauma Rehabil. 1999, 14, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Olney, S.J.; Griffin, M.P.; Monga, T.N.; McBride, I.D. Work and Power in Gait of Stroke Patients. Arch. Phys. Med. Rehabil. 1991, 72, 309–314. [Google Scholar]
- Olney, S.J.; Richards, C. Hemiparetic Gait Following Stroke. Part 1: Characteristics. Gait Posture 1996, 4, 136–148. [Google Scholar] [CrossRef]
- Waters, R L; Frazier, J; Garland, D E; Jordan, C; Perry, J. Electromyographic Gait Analysis before and after Operative Treatment for Hemiplegic Equinus and Equinovarus Deformity. J. Bone Jt. Surg. 1982, 64, 284–288. [Google Scholar] [CrossRef]
- Olney, S.J.; Monga, T.N.; Costigan, P.A. Mechanical Energy of Walking of Stroke Patients. Arch. Phys. Med. Rehabil. 1986, 67, 92–98. [Google Scholar] [CrossRef]
- Crapo, R.; Casaburi, R.; Coates, A.; Enright, P.; MacIntre, N.; McKay, R.; Johnson, D.; Wanger, J.; Zeballos, R.; Bittner, V.; et al. American Thoracic Society ATS Statement : Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Auvinet, B.; Gloria, E.; Renault, G.; Barrey, E. Runner’s Stride Analysis: Comparison of Kinematic and Kinetic Analyses under Field Conditions. Sci. Sports 2002, 17, 92–94. [Google Scholar] [CrossRef]
- Demonceau, M.; Donneau, A.F.; Croisier, J.L.; Skawiniak, E.; Boutaayamou, M.; Maquet, D.; Garraux, G. Contribution of a Trunk Accelerometer System to the Characterization of Gait in Patients with Mild-to-Moderate Parkinson’s Disease. IEEE J. Biomed. Heal. Informatics 2015, 19, 1803–1808. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Lancet (London, England) 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Robertson, J.V.G.; Pradon, D.; Bensmail, D.; Fermanian, C.; Bussel, B.; Roche, N. Relevance of Botulinum Toxin Injection and Nerve Block of Rectus Femoris to Kinematic and Functional Parameters of Stiff Knee Gait in Hemiplegic Adults. Gait Posture 2009, 29, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.; Delporte, L.; Arsenault, L.; Revol, P.; Lefevre, M.; Clevenot, D.; Boisson, D.; Mertens, P.; Rossetti, Y.; Luauté, J. Does the Rectus Femoris Nerve Block Improve Knee Recurvatum in Adult Stroke Patients? A Kinematic and Electromyographic Study. Gait Posture 2014, 39, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Price, K.; Bird, S.R.; Lythgo, N.; Raj, I.S.; Wong, J.Y.L.; Lynch, C. Validation of the Fitbit One, Garmin Vivofit and Jawbone UP Activity Tracker in Estimation of Energy Expenditure during Treadmill Walking and Running. J. Med. Eng. Technol. 2017, 41, 208–215. [Google Scholar] [CrossRef]
- Bai, Y.; Welk, G.J.; Nam, Y.H.; Lee, J.A.; Lee, J.M.; Kim, Y.; Meier, N.F.; Dixon, P.M. Comparison of Consumer and Research Monitors under Semistructured Settings. Med. Sci. Sports Exerc. 2016, 48, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Imboden, M.T.; Nelson, M.B.; Kaminsky, L.A.; Montoye, A.H. Comparison of Four Fitbit and Jawbone Activity Monitors with a Research-Grade ActiGraph Accelerometer for Estimating Physical Activity and Energy Expenditure. Br. J. Sports Med. 2018, 52, 844–850. [Google Scholar] [CrossRef]
- Crouter, S.E.; Churilla, J.R.; Bassett, D.R. Estimating Energy Expenditure Using Accelerometers. Eur. J. Appl. Physiol. 2006, 98, 601–612. [Google Scholar] [CrossRef]
- Compagnat, M.; Mandigout, S.; Chaparro, D.; Daviet, J.C.; Salle, J.Y. Validity of the Actigraph GT3x and Influence of the Sensor Positioning for the Assessment of Active Energy Expenditure during Four Activities of Daily Living in Stroke Subjects. Clin. Rehabil. 2018, 32, 1696–1704. [Google Scholar] [CrossRef]
- Compagnat, M.; Salle, J.Y.; Vinti, M.; Joste, R.; Daviet, J.C. The Best Choice of Oxygen Cost Prediction Equation for Computing Post-Stroke Walking Energy Expenditure Using an Accelerometer. Neurorehabil. Neural Repair 2022, 36, 298–305. [Google Scholar] [CrossRef]
| Control group | Patient with stroke | Mann-Whitney | |||||
|---|---|---|---|---|---|---|---|
| Médian | Q1 | Q3 | Médian | Q1 | Q3 | ||
| HR (bpm) | 140.0 | 98.1 | 143.7 | 116.0 | 90.1 | 126.5 | p=0.08 |
| (mL.min-1.kg-1) | 28.65 | 23.35 | 33.83 | 13.55 | 12.63 | 15.8 | p=0.0001 |
| Distance (m) | 686.5 | 660.0 | 729.7 | 341.0 | 310.0 | 442.0 | p=0.0001 |
| Median | Q1 | Q3 | Mann-Whitney | ||
|---|---|---|---|---|---|
| EEMETA(W·kg-1) | Control group | 9,85 | 8,18 | 11,89 | p<0,0001* |
| Patient with stroke | 5,0 | 4,56 | 5,46 | ||
| EEAcc(W·kg-1) | Control group | 8,57 | 7,86 | 11,24 | p=0,11 |
| Patient with stroke | 8,2 | 7,05 | 9,56 |
| Median | Q1 | Q3 | Correlation coefficient |
||
|---|---|---|---|---|---|
| Control group | EEMETA (W·kg-1) |
9.85 | 8.18 | 11.89 | r=0.09 ; p=0.79 |
| EEAcc(W·kg-1) | 8.57 | 7.86 | 11.24 | ||
| Patient with stroke | EEMETA (W·kg-1) |
5.0 | 4.56 | 5.46 | r=0.56 ; p=0.06 |
| EEAcc (W·kg-1) |
8.2 | 7.05 | 9.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





