1. Introduction
Fermentation of foods is an ancient science used to process and preserve foods. The products of fermentation have been found to possess great nutritional and therapeutic benefits for humans. This accrue from the breakdown and conversion of food components resulting in improved food digestibility and nutrient availability, detoxification and degradation of anti-nutrients, synthesis and increased availability of Vitamin B12, niacin, provision of prebiotic and probiotic components [
1].
Increasingly, fermented foods and beverages are being researched for their health benefits in preventing, treating or managing chronic diseases. Fermented foods and beverages produced by the metabolic activity of microorganisms such as bacteria, yeast, and fungi have gained attention for their potential health benefits, including for individuals with diabetes [
2]. Several studies have reported the health benefits of fermented foods and beverages, which include enhancement of gut microbiota, reducing cardiovascular disease risks, antioxidant, antimicrobial, anti-inflammation, anti-obesity, anti-cancer, anti-hypertensive, enhancing insulin secretion and immune-modulatory activities [
2,
3,
4,
5,
6].
Fermented beverages remarkably, are increasingly gaining recognition as functional foods, especially with therapeutic benefits against chronic diseases. Many fermented beverages such as “kunu” (fermented millet) “kefir” (fermented milk), “kombucha” (fermented tea), beer, wine, etc, exist around the world [
6,
7,
8] and have been reported to mitigate the incidence of several chronic conditions [
4,
9,
10].
Diabetes is a chronic metabolic disorder characterized by high blood glucose levels due to insulin resistance or insufficient insulin secretion. According to IDF [
11] reports, over 537 million people currently live with diabetes globally, with a projection that this number will increase to 700 million by 2045.
Studies have shown that fermented foods can improve gut microbiota composition and function, potentially reducing diabetes risk. For example, a study by Naruszewicz et al. [
12] found that daily consumption of fermented milk containing Lactobacillus fermentum LF15 and Lactobacillus plantarum 299v significantly reduced LDL cholesterol and triglycerides in patients with type 2 diabetes. Another study by Kim and Shin [
13] found that water-soluble chicory extracts in fermented beverages reduced glucose uptake in rats.
Fermented beverages may also have anti-inflammatory and antioxidant properties, capacity to improve insulin sensitivity and reduce diabetes risk. Xiao et al. [
14] found that rats fed a cinnamon extract diet had improved insulin sensitivity and reduced inflammation. However, inter-individual variability in gut microbiota composition and response to dietary interventions may limit the generalizability of these findings.
The effects of fermented foods and beverages on glycemic control and other diabetes markers have been reported. For instance, Nilsson et al. [
15] found that a fermented oatmeal drink resulted in a significantly lower glycemic response in healthy subjects. Additionally, a fermented milk drink improved insulin sensitivity and reduced fasting blood glucose levels in individuals with type 2 diabetes [
16]. These studies suggest the benefits of fermented beverages for glycemic control and insulin sensitivity in individuals with diabetes.
However, the potential mechanisms underlying the beneficial effects of fermented beverages on diabetes are not fully understood. Fermentation may increase the bioavailability and bioactivity of certain nutrients like peptides and polyphenols, which might have antidiabetic effects [
14]. These bioactivities are attributed to the influence of the fermenting microorganisms on the gut microbiota, which then mediate the effects of fermented foods and beverages on glycaemic control, regulate appetite and food intake, reduce inflammation and inhibit carbohydrate metabolizing enzymes [
12,
16,
17,
18].
Rice (Oryza sativa) in various forms (polished, brown, white, etc) is a staple cereal grain food in many diets all over the world [
19,
20]. Rice is not often recommended for diabetics because of its high glycemic index which tends to increase the risk of diabetes [
21,
22]. However, fermentation is known to increase nutrient content and remove/decrease anti-nutritional factors of many cereal grains [
23].
In view of the increasing consumer demand for functional foods, a non-conventional, non- alcoholic beverage was prepared from polished rice grains, spices and groundnuts, simulating a traditional, Nigerian millet-based beverage (“Kunu”).
The aim of this study was to assess the potential of fermented rice beverages to inhibit alpha-glucosidase, alpha-amylase and pancreatic lipase in vitro. This is to the end that consuming rice in a different form may have functional anti-diabetic benefits through inhibition of the above mentioned enzymes.
2. Materials and Methods
This was an in vitro study invovling fermented rice beverages: fermented rice alone (FKR) and fermented rice plus roasted peanuts/groundnuts (FKRG) produced using spontaneous (native flora) fermentation. Inhibitory capacity of the freeze-dried extracts against alpha-glucosidase, alpha-amylase and pancreatic lipase were then evaluated and compared against standards (positive controls). The standards used were- Epigallocatechin gallate (EGCG 200 µM) for alpha-glucosidase, Acarbose [500 µM] for alpha-amylase, and Orlistat (50 µM) for pancreatic lipase respectively.
All reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) and freshly prepared for the assays. Buffer: 67 mM potassium monobasic anhydrous phosphate pH 6.8, 3 mM reduced glutathione, p-NP-Gluc: 10 mM p-Nitrophenyl α-D-glucopyranoside, Na2CO3: 100 mM sodium carbonate, Enzyme: 50 μg/mL α–glucosidase from Saccharomyces cerevisiae, Positive control: Epigallocatechin gallate (EGCG), Dulbecco’s Phosphate Buffered Saline (PBS), porcine pancreatin (1 mg/mL) in PBS, Starch solution: 2 mg/mL in distilled water, Stop solution: 1 M HCl in distilled water, Iodine reagent: 0.127 g iodine; 0.083 g potassium iodide in 100 mL distilled water, Positive control: Acarbose.
The fermented rice beverage was prepared as for Nigerian fermented millet beverage (Kunu) [
24] with modifications. Briefly, rice (250 g in 1000 mL tap water), was soaked and allowed to spontaneously ferment (native fermentation) for 48 h after which 5 g of ginger, 3 g cloves and 1 g cayenne pepper were added. It was ground into a smooth paste using a laboratory blender (Waring Commercial, Torrington, CT 06790), then sieved using a muslin cloth. The sieved slurry was allowed to settle for 3 h after which the clear liquid was decanted. The slurry was divided into two equal halves, half of the thick slurry was put in a medium sized bowl and boiling hot water (about 500 mL), was added to it and mixed to give a porridge/pap-like product. The remaining half was then added to this and mixed thoroughly to produce the rice ‘kunu’-this is the fermented rice kunu (FKR). The fermented rice plus groundnut beverage was prepared following the same procedure as described, except that 50 g of roasted peeled groundnuts, soaked for 1 h was added to the fermented rice just before grinding. This is the fermented rice kunu with groundnuts (FKRG).
The fermented rice beverages were stored in glass bottles at 4°C in the refrigerator. One hundred (100 mL) of each beverage was taken, filtered under pressure using a Buckner funnel and filter paper (Whatman No. 1) and freeze-dried to obtain samples for analyses.
Test samples (fermented rice beverages) were reconstituted in dimethyl sulfoxide (DMSO) to a final concentration of 100 mg/mL. Samples were diluted serially in assay buffer to concentrations of 500, 250, 125, 62.5 and 31.25 μg/mL.
Into a 96 well micro-titer plate, 10 μL of sample and 70 μL enzyme were added and incubated at 37°C for 10 minutes. After this, 20 μL p-NP-Gluc was added and incubated at 37°C for 20 minutes. Finally, 25 μL Na
2CO
3 was added to the mixture, then Absorbance was measured at 410 nm using a BioTek
® PowerWave XS spectrophotometer (Winooski, VT, USA). No enzyme and no substrate controls were included and the percentage α-glucosidase inhibition was calculated as:
The α-amylase inhibition assay was also performed in a 96-well micro-titer plate in quadruplicate. To the 96-well plate, 15 μL sample and 5 μL of enzyme were incubated for 10 min at 37°C. Then 20 μL starch solution was added, incubated for 30 min at 37°C, after which the reaction was quenched with 10 μL stop solution and 75 μL iodine reagent was added. Absorbance was measured at 580 nm using a BioTek
® PowerWave XS spectrophotometer (Winooski, VT, USA). No enzyme and no substrate controls were included, and the percentage α-amylase inhibition was calculated as:
where
amylase activity = A580nm without enzyme − A580nm with enzyme.
The pancreatic lipase inhibition assay was performed as described by Pringle
et al. [
26]. Briefly, 10 μL sample plus 5 μL enzyme were incubated at 37°C for 15 minutes. Then 170 μL of substrate with reaction buffer were incubated at 37°C for 25 minutes. The absorbance was measured at 405 nm using a BioTek
® PowerWave XS spectrophotometer (Winooski, VT, USA). No enzyme and no substrate controls were included. Orlistat (50 µM) was used as a positive control and the percentage lipase inhibition was calculated as:
All assays were performed in triplicates and data were expressed as mean ± standard deviation (SD), using one way analysis of variance (ANOVA) and Fischer’s Least Significant Difference on the MINITAB 17 statistical software package. Mean values were regarded as significantly different at P < 0.05.
3. Results
3.1. Alpha-glucosidase inhibition
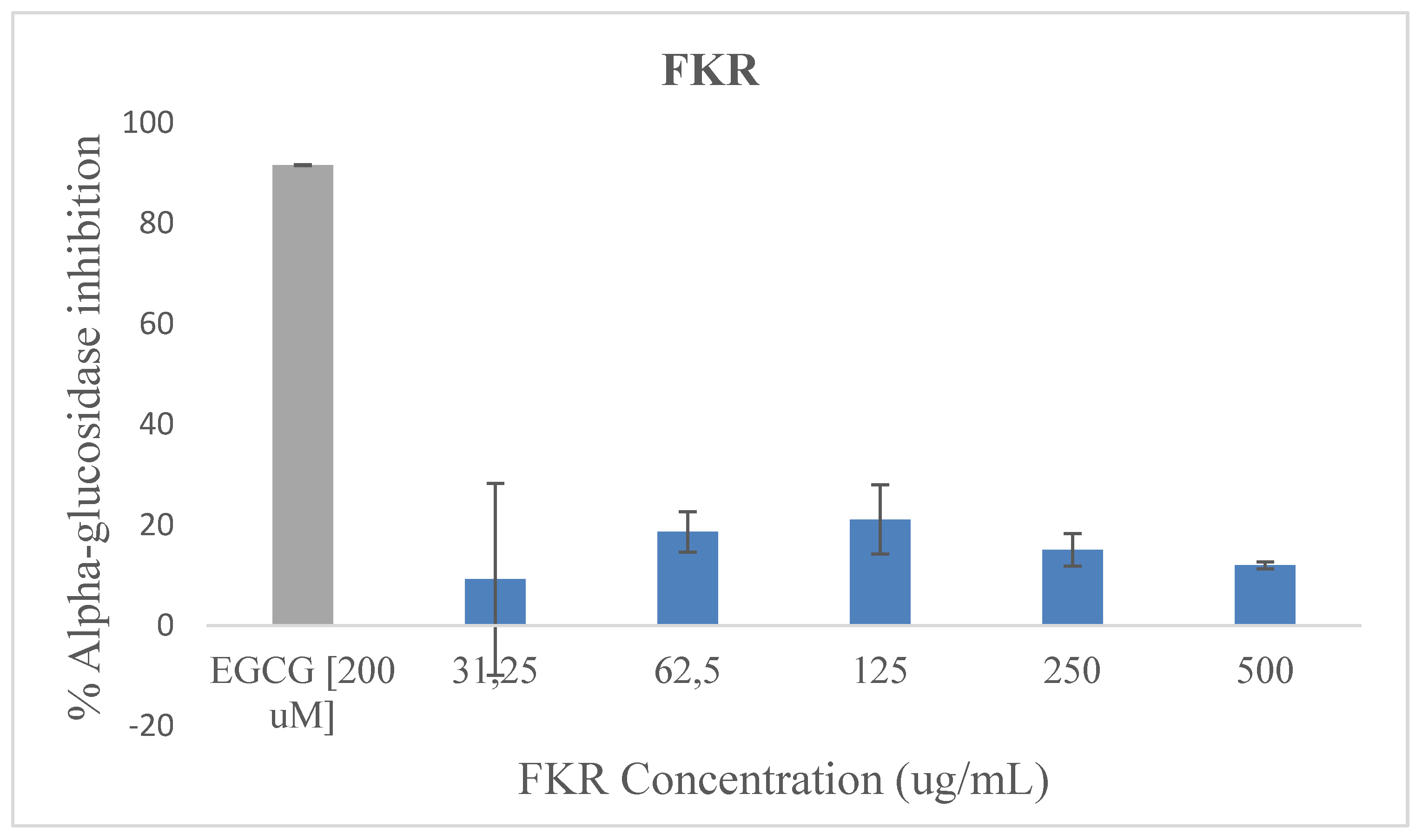
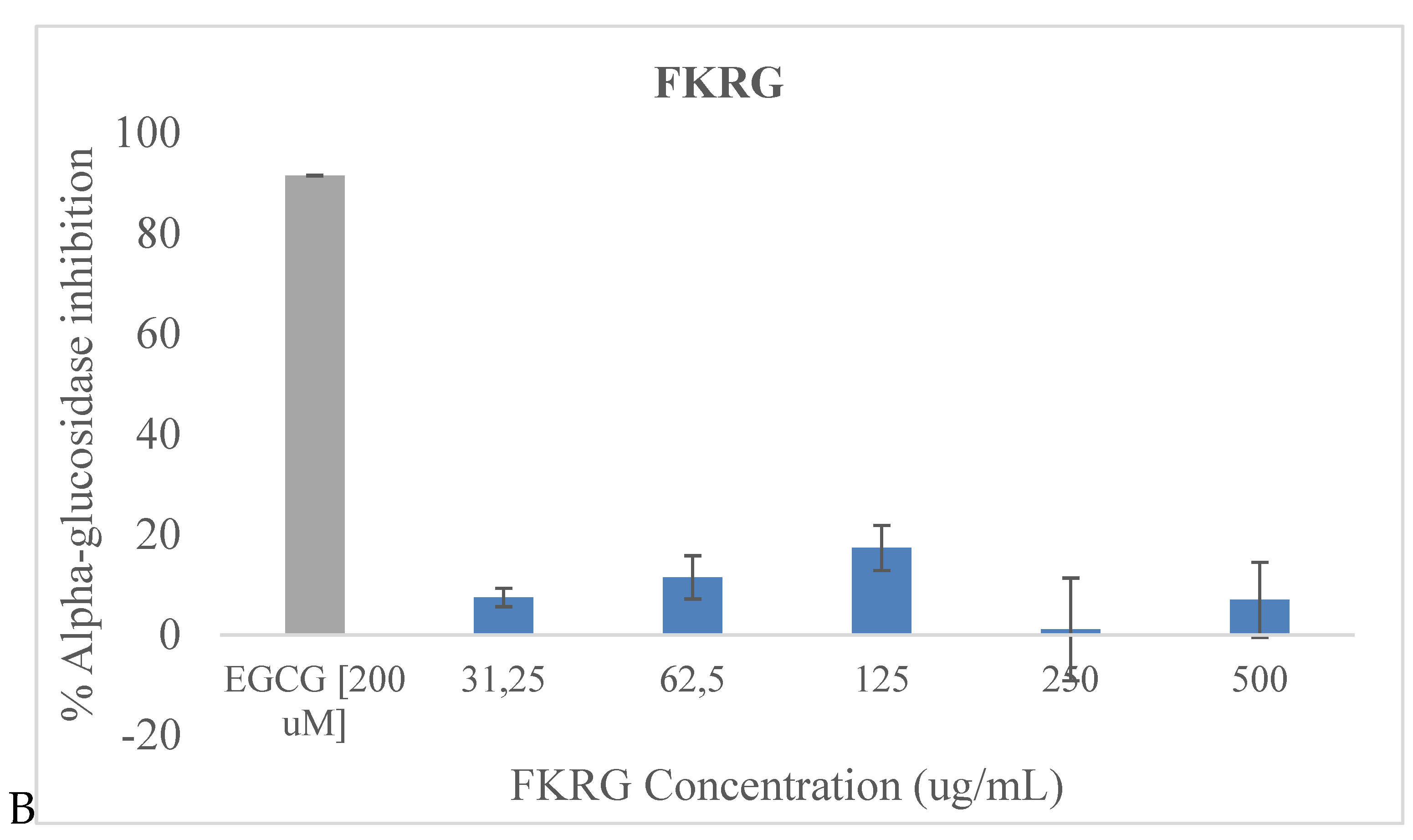
Alpha-glucosidase inhibition capacity of the samples is shown in
Figure 1. The two fermented rice beverages (FKR and FKRG) exhibited modest alpha-glucosidase inhibition activity, though these were considerably low compared to EGCG which was used as the standard/positive control. FKR exerted inhibition of α-glucosidase at a range of 9.23-21.11% and FKRG 1.11-17.36% at the various concentrations respectively. For both samples, the most significant inhibition approaching 20% occurred at the 125 μg/mL concentration. It is noteworthy however, that the inhibitory effect of these beverage samples were not significantly (P > 0.05) concentration dependent.
ECGC [200 µM] was used as positive control. Values are means ± SD (n = 3); P < 0.05.
3.2. Alpha-amylase inhibition
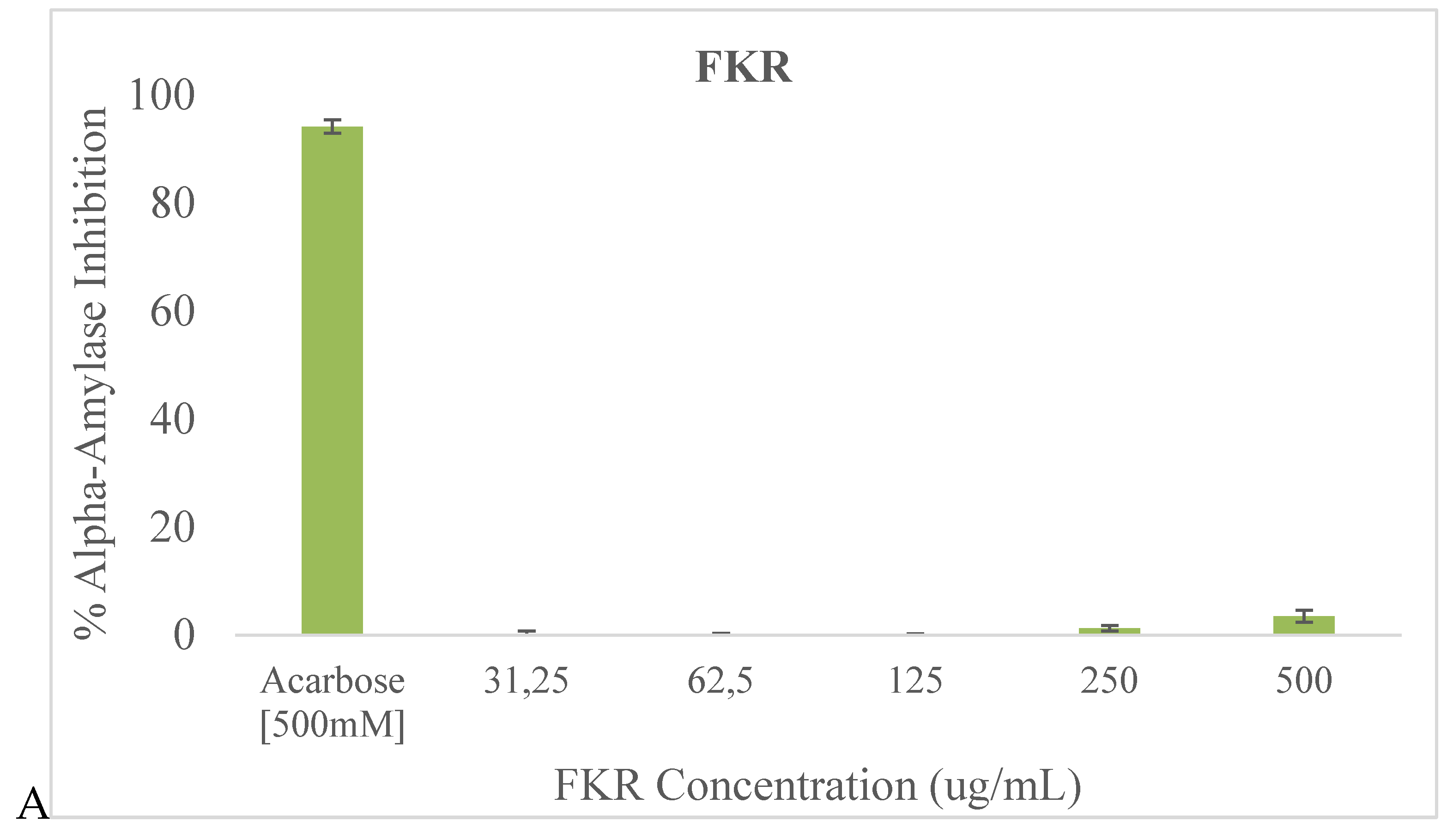
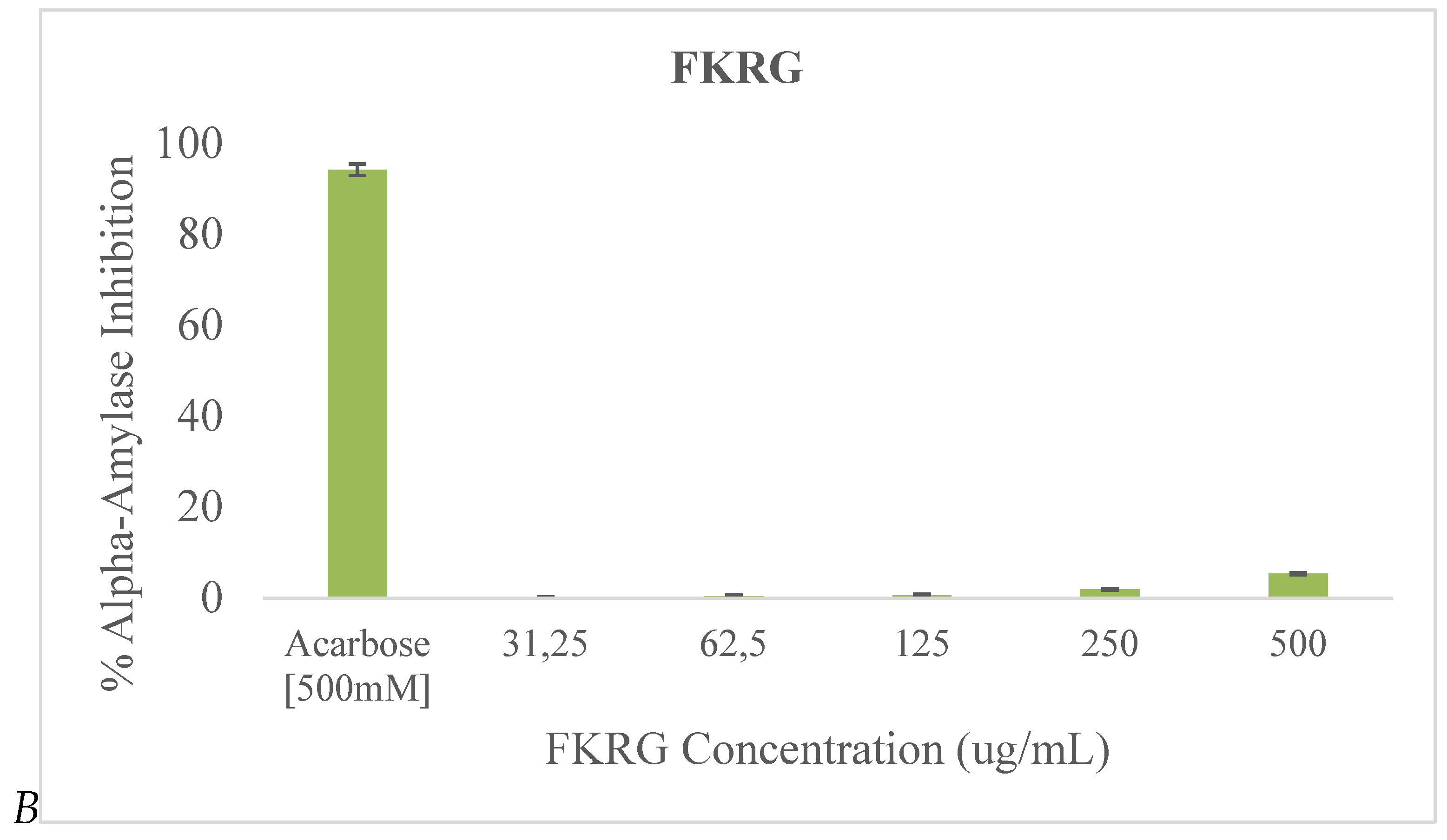
Both FKR and FKRG exhibited moderate alpha-amylase inhibition activity at the tested concentrations, but very low inhibitory activity compared to acarbose (
Figure 2). FKRG was found to have the higher alpha-amylase inhibition activity. For both samples, the most significant inhibition occurred at the 500 μg/mL concentration.
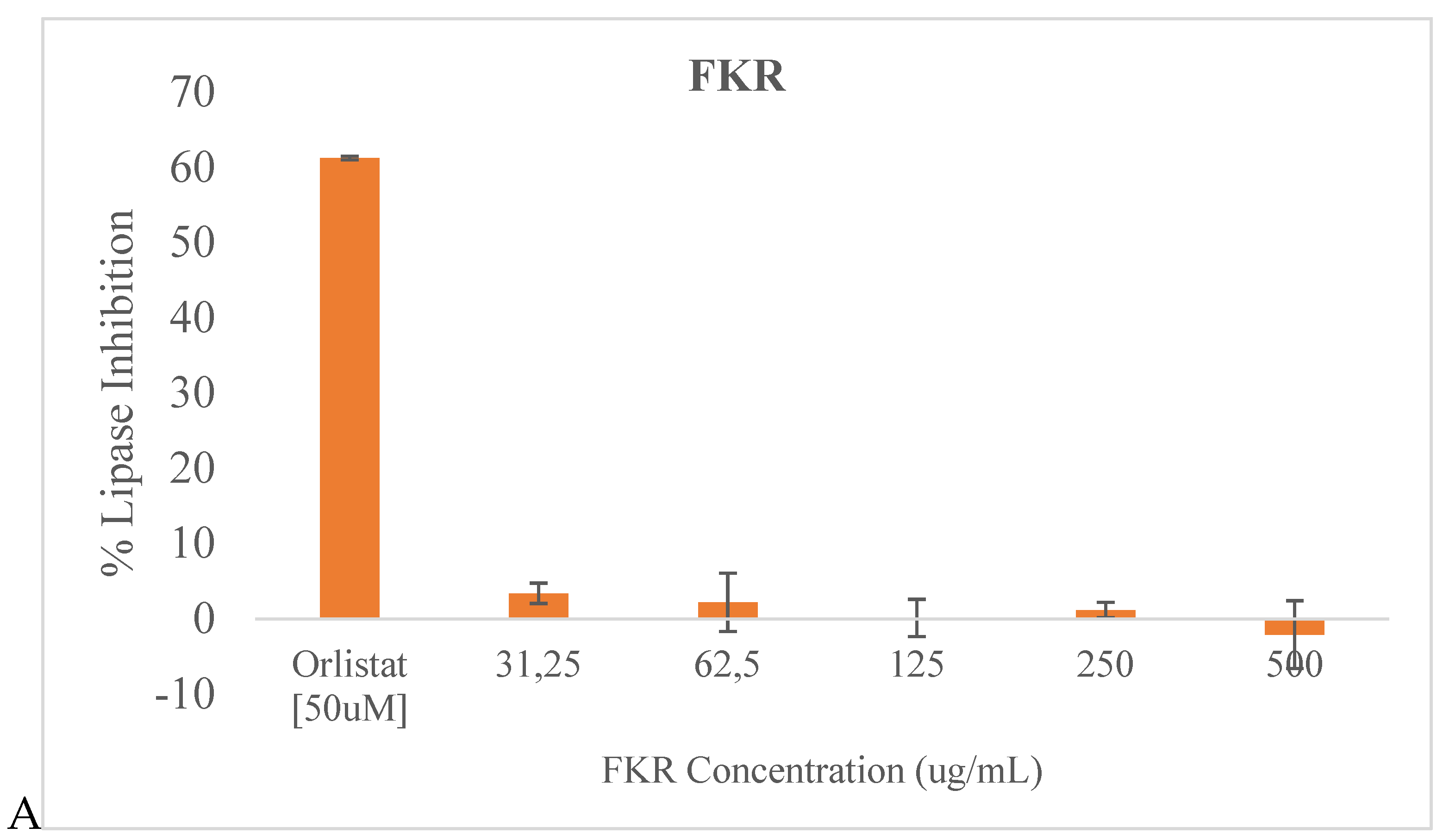
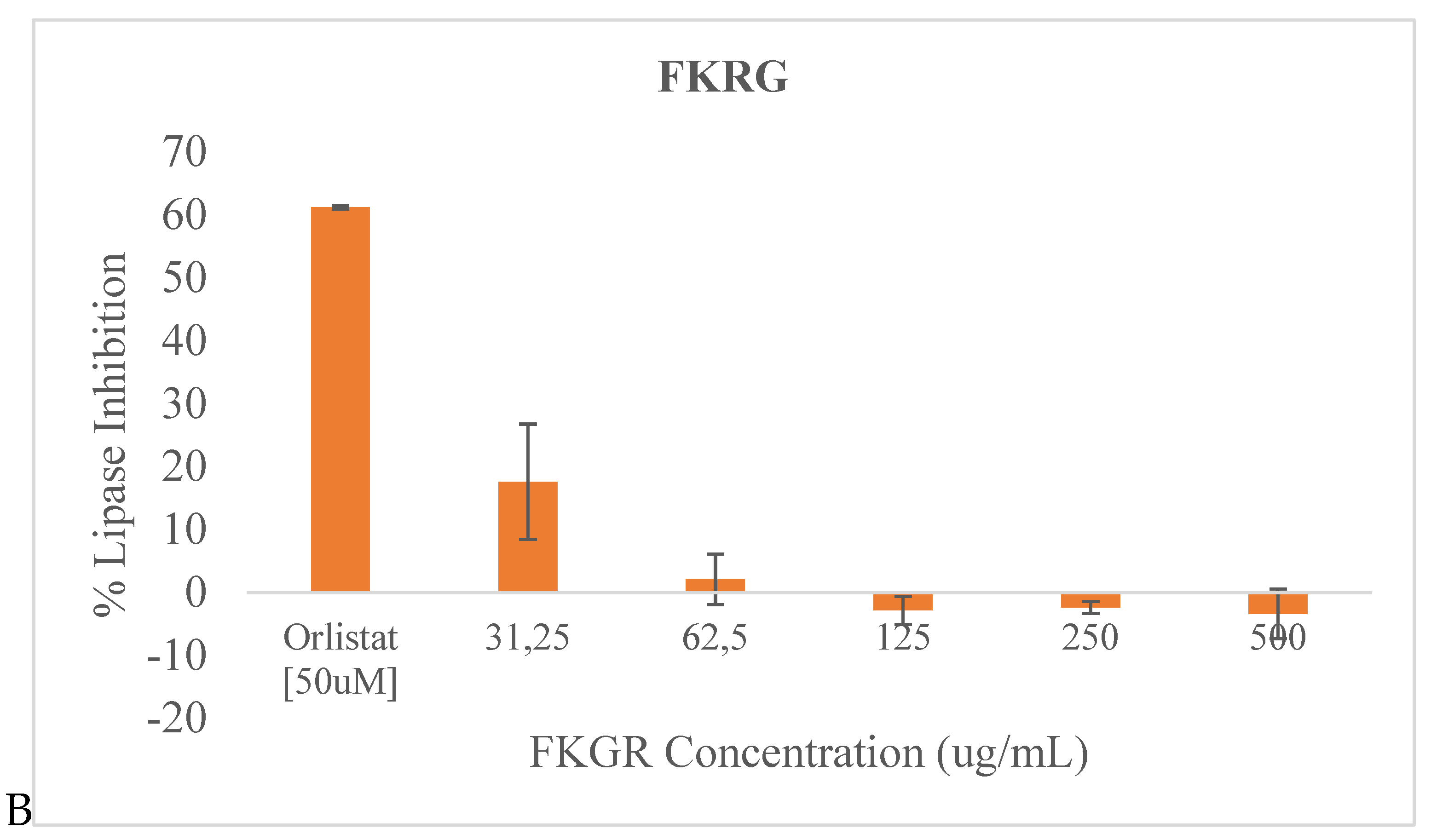
3.3. Lipase inhibition
The potential pancreatic lipase inhibition activity of the two fermented rice beverages FKR and FKRG are illustrated in
Figure 3.
No significant pancreatic lipase inhibitory activity was observed for sample FKR at the tested concentrations compared to Orlistat (a standard anti-obesity drug) used as control in this study. Although no significant inhibitory activity was observed in most of the tested concentrations for FKRG also, at 31.25 ug/mL there was an inhibitory effect of approximately 15%, which was not seen at the higher concentrations.
4. Discussion
Postprandial hyperglycaemia is a major contributing factor in the development of diabetes and represents an important therapeutic target. Anti-diabetic drugs that retard glucose absorption after a meal through the inhibition of carbohydrate hydrolysing enzymes are known to be effective in decreasing postprandial hyperglycaemia [
26,
27,
28]. Therefore, retarding the absorption of glucose by inhibition of α-amylase and α-glucosidases (carbohydrate hydrolyzing enzymes), is a major therapeutic approach for decreasing postprandial hyperglycemia.
Alpha-Glucosidase is a membrane-bound enzyme located at the brush-border of epithelial cells in the small intestine, which catalyses the final stage of starch digestion by hydrolysing terminal glucose molecules from the non-reducing ends of oligosaccharides [
29]. Thus, α-glucosidase inhibitors are frequently used as oral antidiabetic drugs in the early stages of type 2 diabetes to combat postprandial hyperglycaemia and obesity [
30]. These inhibitors prevent postprandial hyperglycaemia by slowing down the digestion of carbohydrates and consequently the rate at which glucose can be absorbed into the general circulation. Acarbose and other α-glucosidase inhibitors act via a competitive inhibition mechanism and ideally bind to all four catalytic domains of the enzyme to effectively inhibit the hydrolysis of oligosaccharides [
31].
Starch digestion is strongly associated with glycemia-related problems such as diabetes and metabolic syndrome including obesity and atherosclerosis. Pancreatic and salivary alpha-amylase and four small intestine mucosal alpha-glucosidase subunits are required to digest starch into glucose [
32]. When given orally, acarbose inhibits alpha-glucosidase in the brush border of the small intestines and reduces the rate of digestion of complex carbohydrates. Inhibitors of alpha-glucosidase exhibit the same mechanism to retard glycation of proteins, thereby reducing glycated hemoglobin and glycation end products in collagen [
33].
Alpha-amylase hydrolyses starch at inner alpha-1,4 glycosidic linkages to give linear and branched malto-oligosaccharides and disaccharides which then serve as substrates for intestinal alpha-glucosidases that hydrolyse the disaccharides to monosaccharides such as glucose [
32,
34]. Alpha-amylase is the limiting enzyme and therefore determines digestion and consequent rate of glucose release, thus alpha-amylase inhibitors decrease the high glucose levels that can occur after a meal by slowing the speed with which alpha-amylase can convert starch to simple sugars [
35].
Starch is hydrolyzed by pancreatic α-amylase into oligosaccharides, which is further hydrolyzed by α-glucosidase into glucose in the jejunum [
36]. Therefore, glucose absorption could be delayed by impeding these two enzymes, thus suppressing the postprandial blood glucose levels. The moderate inhibition of α-glucosidase exhibited by the two fermented beverages FKR and FKRG indicates that both beverages have the potential to prevent postprandial hyperglycemia. The most significant inhibition (IC50) for both samples occurred at the 125 μg/mL, with inhibition approaching 20%.
The significant (P < 0.05) alpha-amylase inhibition exhibited by both samples, occurred at the 500 μg/mL testing concentration. This could be attributed to increased nutrient and polyphenolic content as a result of fermentation, as well as increase in antioxidant activity. This is so because during fermentation, plant cell walls are broken down liberating antioxidants, phytochemicals and nutrients present in foods are metabolized into simpler and unique health-beneficial components by the fermenting microorganisms [
37]. In addition, several studies have reported that fermentation improves nutrient content and availability, polyphenolic content and enhances antioxidant content and capacity of functional foods through reduction or neutralization of tannins and phytates, resulting in greater bioavailability of nutrients, phytochemicals, vitamins and minerals amongst others [
37,
38,
39,
40].
The inhibitory activities of FRK and FRKG against α-amylase and α-glucosidase enzymes could be attributed to the additive polyphenolic contents resulting from the ingredients. According to Noviasari et al. [
41], polyphenol and flavonoid content found in many foods have great capacity of inhibiting α-amylase and α-glucosidase. Moreover, the phenolic content of rice could be responsible for inhibition of the two enzymes, also legumes (peanuts in this case) and spices are known to be rich in protein, phytochemicals, dietary fibre, vitamins, and other micronutrients beneficial for human health [
42,
43]. These results agree with Ramakrishna et al. [
44] who also reported low to moderate alpha-glucosidase inhibition for fermented barley flour extracts. When enzymes involved in starch degradation are inhibited, the release of glucose is slowed down and its level in the blood is reduced [
45].
Dietary lipid digestion occurs through the hydrolysis of triacylglycerols within the intestinal lumen to monoacyl glycerols by pancreatic lipase. This inhibition is one of the most promising methods for combating obesity and hypertriglyceridemia in type 2 diabetes [
46]. Although the inhibitory action against pancreatic lipase was very low for FKR and FKRG compared to orlistat the standard drug, at 31.25 ug/mL, there was a significant (P < 0.05) inhibitory effect of approximately 15%, by FKRG which was absent at higher concentrations. Insulin resistance has been associated with dyslipidemia (hypertriglyceridemia and low HDL-cholesterol) and could lead to increased synthesis and secretion of very low-density lipoprotein, resulting in increased plasma triglyceride levels and decreased plasma HDL-cholesterol levels.
According to Ignat et al. [
42], non-alcoholic cereal-based beverages are healthy drinks that impact positively on human health and their consumption improves liver function, gut microbiota and reduces the incidence of nosocomial infections. Although, fermented foods and beverages may not replace medical treatment, they could serve as adjuvants or alternative therapies in treating and managing the condition through reducing oxidative stress, glycemic control, recovery or maintenance of body weight [
43,
47].
The use of spontaneous fermentation (native flora) implies that different populations of microorganisms are involved.
Use of the freeze-dried filterate from the rice beverage could have been responsible for the low/moderate inhibitions observed, since the beverage is supposed to be consumed as a slurry.
Also, no study on the fermenting organisms of the beverages were performed.
5. Conclusions
The present results reveal the potential of fermented rice beverage to moderately inhibit carbohydrate metabolizing enzymes (α-amylase, α-glucosidase) and pancreatic lipase in vitro and could modulate postprandial hyperglycemia in type 2 diabetes. Again, better inhibitory action of the fermented beverages against α-amylase, α-glucosidase and pancreatic lipase may be possible in vivo, which thus calls for further studies.
Author Contributions
Conceptualization, G.A.O.; Methodology, G.A.O.; O.T.T.; C.O-Y.; J.N.; Formal Analysis, G.A.O.; and O.T.T.; Data Curation, G.A.O.; Writing–Original Draft Preparation, G.A.O.; and O.T.T.; Writing–Review & Editing, G.A.O.; OT.T.; C.O-Y.; J.N.; O.O.; and A.J.A.; Supervision, G.A.O.; Funding Acquisition, G.A.O.
Funding
This study was supported by the South Africa National Research Foundation, Research and Innovation Support and Advancement (NRF: RISA) CSURG: Grant No: 121264.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article.
Acknowledgments
The authors acknowledge the support of Govan Mbeki Research and Development Centre, University of Fort Hare, Eastern Cape, South Africa; Prof Maryna de Venter: Department of Biochemistry and Microbiology, Nelson Mandela University, PE 6031, South Africa, and her laboratory staff, are appreciated for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lian, J. Health Benefit of Plant-base Fermented Food and Beverage on Type 2 Diabetes Mellitus. Highlights Sci. Eng. Technol. 2022, 11, 229–238. [Google Scholar] [CrossRef]
- Tamang, J.P.; Shin, D.-H.; Jung, S.-J.; Chae, S.-W. Functional Properties of Microorganisms in Fermented Foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Mota de Carvalho, N.; Costa, E.M.; Silva, S.; Pimentel, L.; Fernandes, T.H.; Pintado, M.E. Fermented Foods and Beverages in Human Diet and Their Influence on Gut Microbiota and Health. Fermentation 2018, 4, 90. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Cuamatzin-García, L.; Rodríguez-Rugarcía, P.; El-Kassis, E.G.; Galicia, G.; Meza-Jiménez, M.d.L.; Baños-Lara, M.d.R.; Zaragoza-Maldonado, D.S.; Pérez-Armendáriz, B. Traditional Fermented Foods and Beverages from around the World and Their Health Benefits. Microorganisms 2022, 10, 1151. [Google Scholar] [CrossRef]
- Coton, M.; Pawtowski, A.; Taminiau, B.; Burgaud, G.; Deniel, F.; Coulloumme-Labarthe, L.; Fall, A.; Daube, G.; Coton, E. Unraveling microbial ecology of industrial-scale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Makwa, *!!! REPLACE !!!*. 9. Makwana M, Hati S. Fermented beverages and their health benefits. InFermented beverages 2019 Jan 1 (pp. 1-29). Woodhead Publishing.
- Keșa, A.-L.; Pop, C.R.; Mudura, E.; Salanță, L.C.; Pasqualone, A.; Dărab, C.; Burja-Udrea, C.; Zhao, H.; Coldea, T.E. Strategies to Improve the Potential Functionality of Fruit-Based Fermented Beverages. Plants 2021, 10, 2263. [Google Scholar] [CrossRef]
- IDF Diabetes Atlas 9th Edition. 2019. International Diabetes Federation. IDF Diabetes Atlas. 10th edition, 2023. Available online: https://www.diabetesatlas.org/en/.
- Naruszewicz M, Johansson ML, Zapolska-Downar D, et al. Effect of Lactobacillus fermentum LF 15 and Lactobacillus plantarum 299v on blood lipids and other biomarkers of cardiovascular disease in patients with type 2 diabetes: a randomised controlled trial. Atherosclerosis 2018, 268, 72–80.
- Kim M, Shin HK. The water-soluble chicory extract reduces glucose uptake from the perfused jejunum in rats. J Nutr. 1998, 128, 1734–1737.
- Xiao J, Zhang R, Wu X, et al. Metabolic profiles and pharmacokinetics of procyanidins in rats fed a cinnamon extract diet. Food Funct. 2018, 9, 4725–4736.
- Nilsson, A.C.; Östman, E.M.; Knudsen, K.E.B.; Holst, J.J.; Björck, I.M.E. A Cereal-Based Evening Meal Rich in Indigestible Carbohydrates Increases Plasma Butyrate the Next Morning, J. Nutr. 2010, 140, 1932–1936. [Google Scholar] [CrossRef] [PubMed]
- Healey, G.R.; Murphy, R.; Brough, L.; A Butts, C.; Coad, J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr. Rev. 2017, 75, 1059–1080. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Fei, X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina 2015, 52, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, F.; Wu, W. Effects of rice bran rancidity on the oxidation and structural characteristics of rice bran protein. LWT 2019, 120, 108943. [Google Scholar] [CrossRef]
- Ai, X.; Wu, C.; Yin, T.; Zhur, O.; Liu, C.; Yan, X.; Yi, C.; Liu, D.; Xiao, L.; Li, W.; et al. Antidiabetic Function of Lactobacillus fermentum MF423-Fermented Rice Bran and Its Effect on Gut Microbiota Structure in Type 2 Diabetic Mice. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ 2012, 15, 344.
- Bhavadharini, B.; Mohan, V.; Dehghan, M.; Rangarajan, S.; Swaminathan, S.; Rosengren, A.; Wielgosz, A.; Avezum, A.; Lopez-Jaramillo, P.; Lanas, F.; et al. White Rice Intake and Incident Diabetes: A Study of 132,373 Participants in 21 Countries. Diabetes Care 2020, 43, 2643–2650. [Google Scholar] [CrossRef]
- Negrete-Romero, B.; Valencia-Olivares, C.; Baños-Dossetti, G.A.; Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Nutritional Contributions and Health Associations of Traditional Fermented Foods. Fermentation 2021, 7, 289. [Google Scholar] [CrossRef]
- Adebayo GB, Otunola GA, Ajao TA. Physicochemical, microbiological and sensory characteristics of kunu prepared from millet, maize and guinea corn and stored at selected temperatures. Advance Journal of Food Science and Technology 2010, 2, 41–46.
- Pringle NA, van de Venter M, Koekemoer TC. Comprehensive in vitro antidiabetic screening of Aspalathus linearis using a target-directed screening platform and cellomics. Food & Function 2021, 12, 1020–1038.
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738–111738. [Google Scholar] [CrossRef]
- Mitiku H, Kim TY, Kang H, Apostolidis E, Lee JY, Kwon YI. Selected coffee (Coffea arabica L.) extracts inhibit intestinal α-glucosidases activities in-vitro and postprandial hyperglycemia in SD Rats. BMC Complementary Medicine and Therapies 2022, 22, 249. [CrossRef] [PubMed]
- Narumalla J, Sheela D, Dixit R. In-vitro α-amylase and α-glucosidase inhibitory activity of coccinia grandis fruits and hyptis suaveolens seeds extracts. Journal of Pharmaceutical Negative Results 2022, 31, 4015–4022.
- Elferink, H.; Bruekers, J.P.J.; Veeneman, G.H.; Boltje, T.J. A comprehensive overview of substrate specificity of glycoside hydrolases and transporters in the small intestine. Cell. Mol. Life Sci. 2020, 77, 4799–4826. [Google Scholar] [CrossRef]
- Min, C.Y.; Yoon, J.-H.; Hahn, S.; Cho, Y.M. Efficacy and safety of combination therapy with an α-glucosidase inhibitor and a dipeptidyl peptidase-4 inhibitor in patients with type 2 diabetes mellitus: A systematic review with meta-analysis. J. Diabetes Investig. 2017, 9, 893–902. [Google Scholar] [CrossRef]
- Roskar I, Molek P, Vodnik M, Stempelj M, Strukelj B, Lunder M. Peptide modulators of alpha-glucosidase. Journal of Diabetes Investigation 2015, 6, 625–631.
- Lin, A.H.-M.; Ao, Z.; Quezada-Calvillo, R.; Nichols, B.L.; Lin, C.-T.; Hamaker, B.R. Branch pattern of starch internal structure influences the glucogenesis by mucosal Nt-maltase-glucoamylase. Carbohydr. Polym. 2014, 111, 33–40. [Google Scholar] [CrossRef]
- Alam, F.; Shafique, Z.; Amjad, S.T.; Bin Asad, M.H.H. Enzymes inhibitors from natural sources with antidiabetic activity: A review. Phytotherapy Res. 2018, 33, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Gangoiti, J.; Corwin, S.F.; Lamothe, L.M.; Vafiadi, C.; Hamaker, B.R.; Dijkhuizen, L. Synthesis of novel α-glucans with potential health benefits through controlled glucose release in the human gastrointestinal tract. Crit. Rev. Food Sci. Nutr. 2018, 60, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Nair SS, Kavrekar V, Mishra A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. European Journal of Experimental Biology 2013, 3, 128–132.
- Koh, W.; Uthumporn, U.; Rosma, A.; Yuen, K. Fermented pumpkin-based beverage inhibits key enzymes of carbohydrate digesting and extenuates postprandial hyperglycemia in type-2 diabetic rats. Acta Aliment. 2018, 47, 495–503. [Google Scholar] [CrossRef]
- Sharma, R.; Diwan, B.; Singh, B.P.; Kulshrestha, S. Probiotic fermentation of polyphenols: potential sources of novel functional foods. Food Prod. Process. Nutr. 2022, 4, 1–16. [Google Scholar] [CrossRef]
- Adebo, O.A.; Medina-Meza, I.G. Impact of Fermentation on the Phenolic Compounds and Antioxidant Activity of Whole Cereal Grains: A Mini Review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef]
- Zhao, Y.-S.; Eweys, A.S.; Zhang, J.-Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.-B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Effect of Fermentation on the Nutritional Quality of the Selected Vegetables and Legumes and Their Health Effects. Life 2023, 13, 655. [Google Scholar] [CrossRef]
- Noviasari, S.; Kusnandar, F.; Setiyono, A.; Budijanto, S. Antioxidant activity and inhibition of α-amylase and α-glucosidase in fermented black rice bran-based analog rice. AIMS Agric. Food 2022, 7, 61–72. [Google Scholar] [CrossRef]
- Ignat, M.V.; Salanță, L.C.; Pop, O.L.; Pop, C.R.; Tofană, M.; Mudura, E.; Coldea, T.E.; Borșa, A.; Pasqualone, A. Current Functionality and Potential Improvements of Non-Alcoholic Fermented Cereal Beverages. Foods 2020, 9, 1031. [Google Scholar] [CrossRef]
- Otunola, G.A.; Afolayan, A.J. In vitro Alpha-amylase inhibition, antioxidant, nutritional and sensory properties of functional spice-blend fortified cookies. Funct. Foods Heal. Dis. 2022, 12, 56. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Sarkar, D.; Dogramaci, M.; Shetty, K. Kefir Culture-Mediated Fermentation to Improve Phenolic-Linked Antioxidant, Anti-Hyperglycemic and Human Gut Health Benefits in Sprouted Food Barley. Appl. Microbiol. 2021, 1, 377–407. [Google Scholar] [CrossRef]
- Diowksz, A.; Malik, A.; Jaśniewska, A.; Leszczyńska, J. The Inhibition of Amylase and ACE Enzyme and the Reduction of Immunoreactivity of Sourdough Bread. Foods 2020, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Kim, J.-I.; Ryu, C.-H.; Kang, M.-J. Effects of Fermented Beverage in Subjects with Metabolic Syndrome. Prev. Nutr. Food Sci. 2021, 26, 12–20. [Google Scholar] [CrossRef]
- Souza, C.d.A.; de Oliveira. A.C.L.; Rolim, V.A.d.O.; Bogsan, C.S.B. Traditional Fermented Foods as an Adjuvant Treatment to Diabetes. Curr. Geriatr. Rep. 2020, 9, 242–250. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).