Submitted:
08 July 2023
Posted:
10 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- To confirm the efficiency of the proposed measurement system.
- To find out what are the qualities of the equipment.
- To look for aspects to improve and implement in the future.
2. Materials
2.1. NIRscan Nano Spectrometer
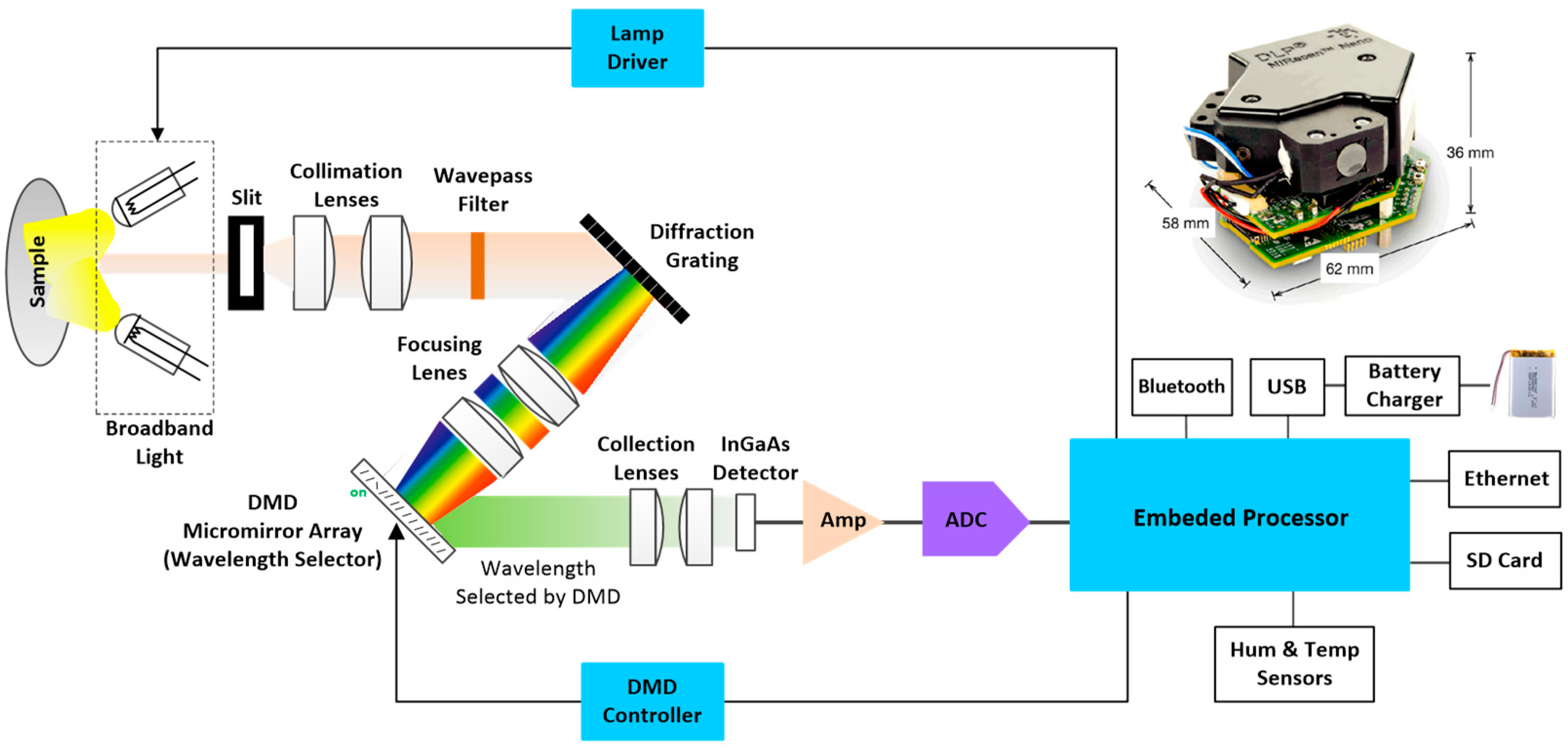
2.2 Measurement System
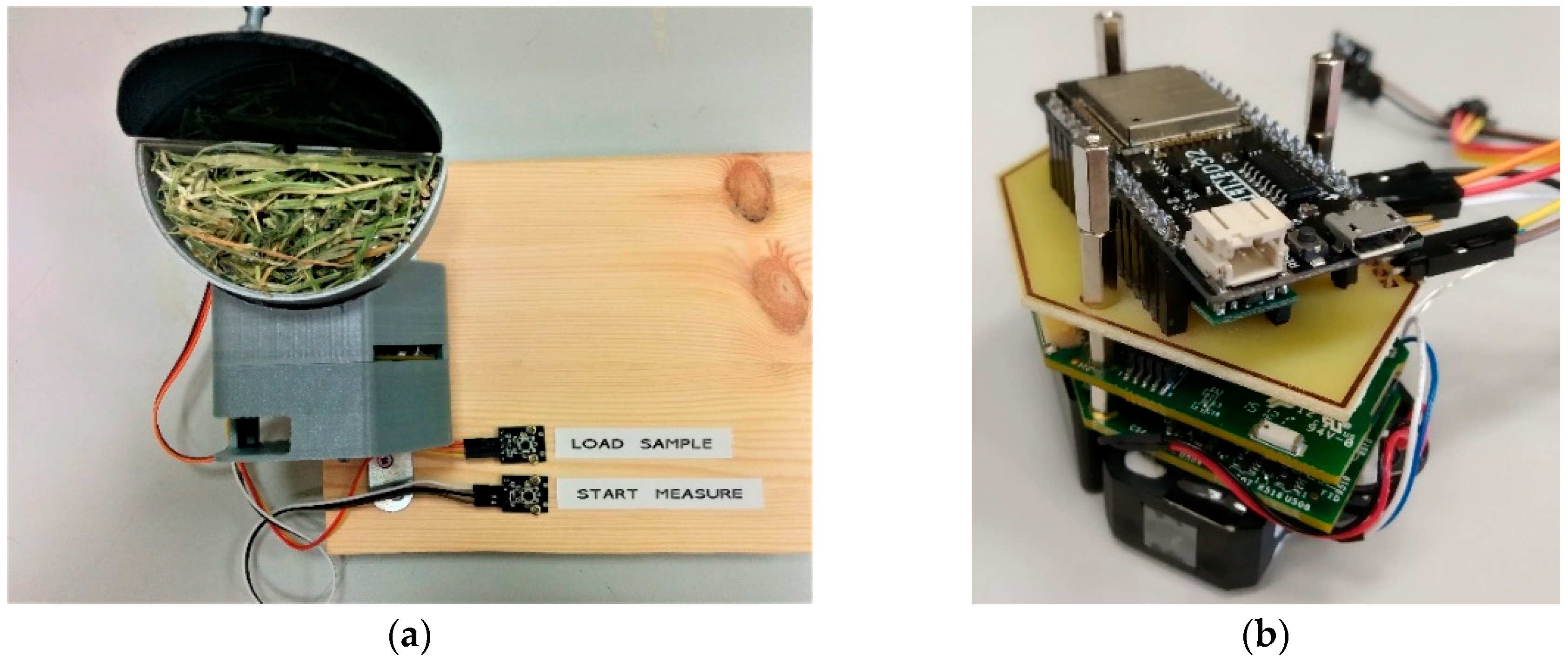
3. Methods
3.1. Forage Samples
| Parameter (%) | Mean | Min | Max | SD |
|---|---|---|---|---|
| NDF | 40.70 | 29.24 | 61.01 | 5.50 |
| MC | 10.64 | 8.47 | 13.71 | 0.91 |
| CP | 14.48 | 7.19 | 17.01 | 1.70 |
3.2. Spectral Acquisition
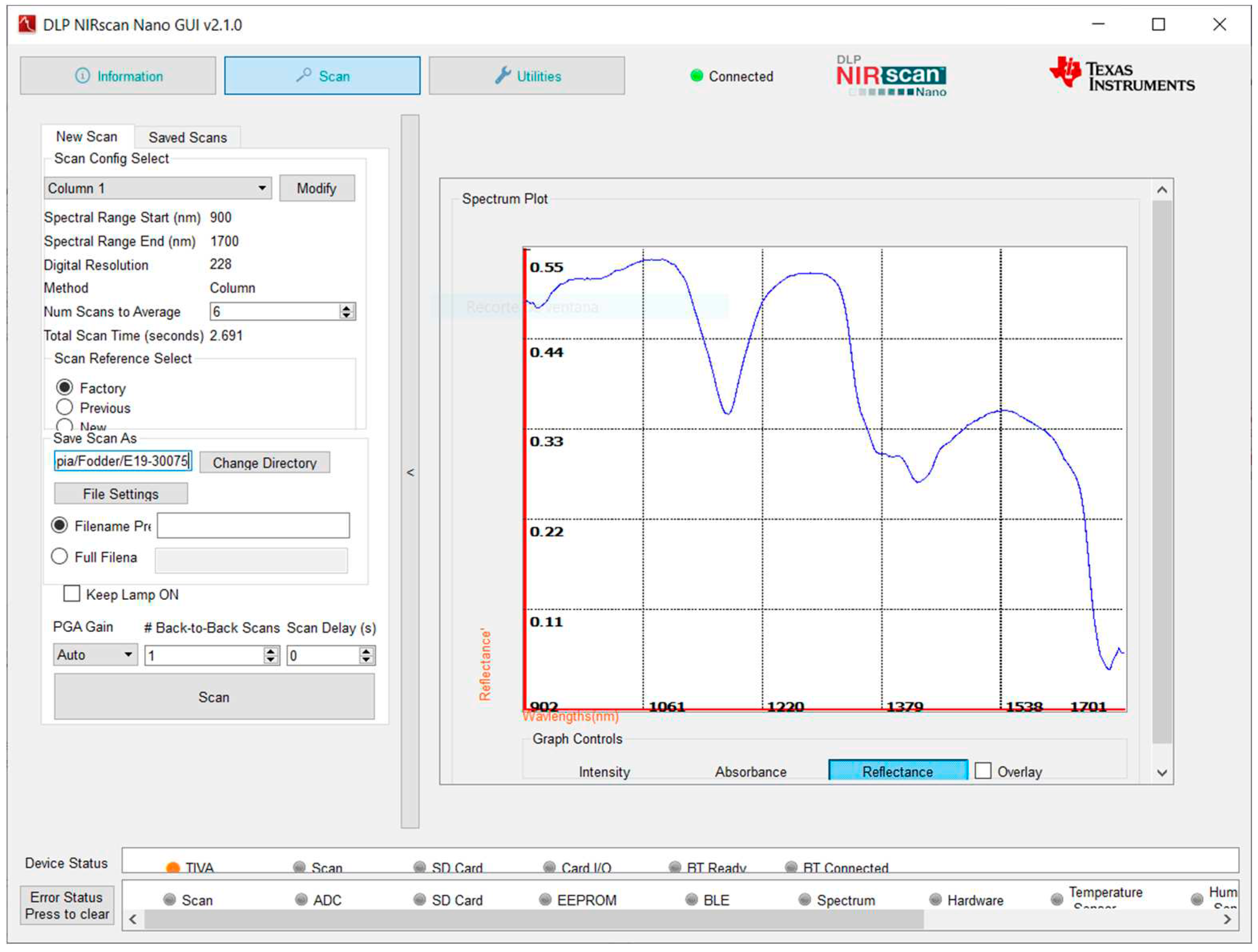
3.3. Spectra Data Processing
4. Results and Discussion
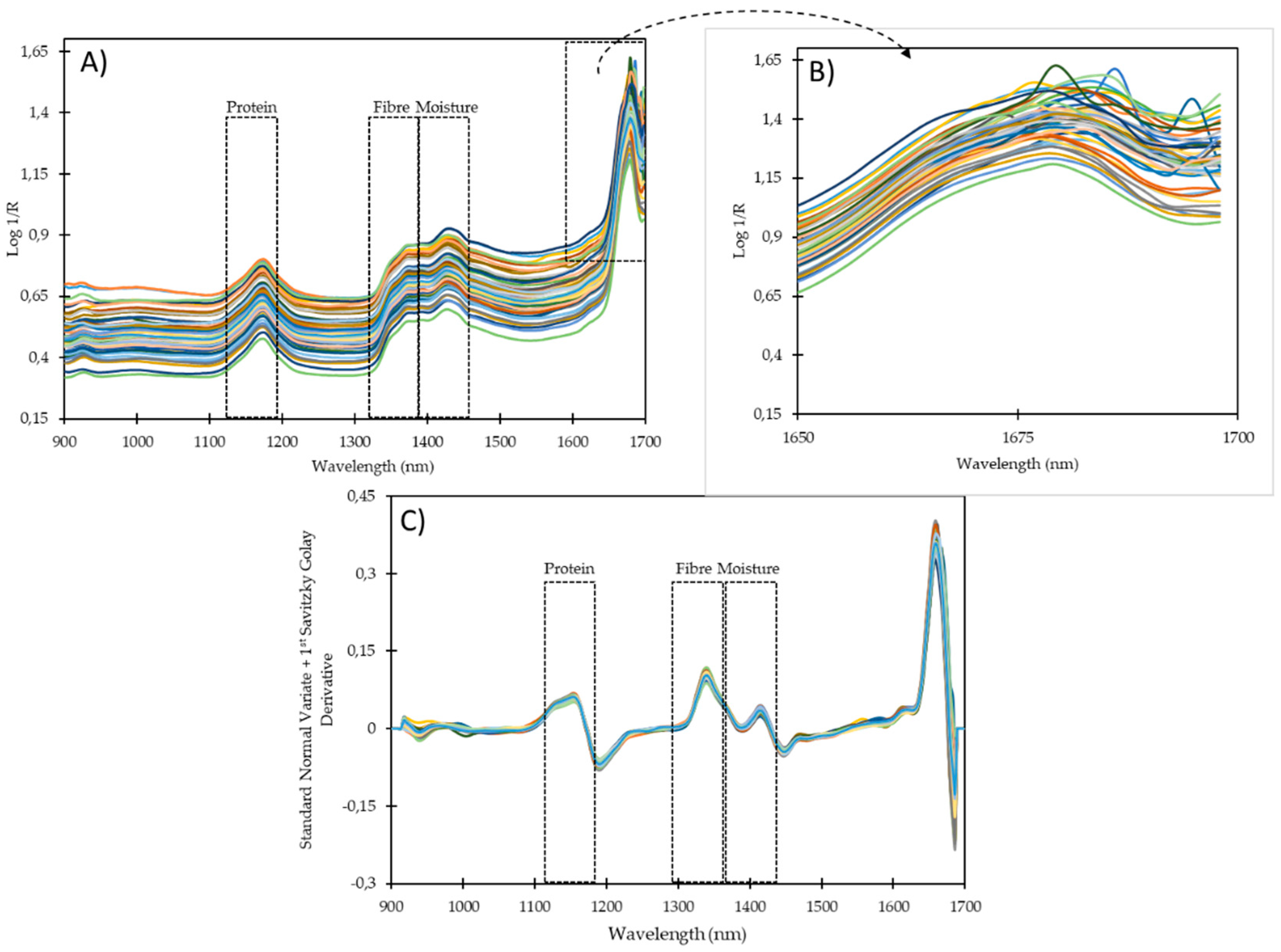
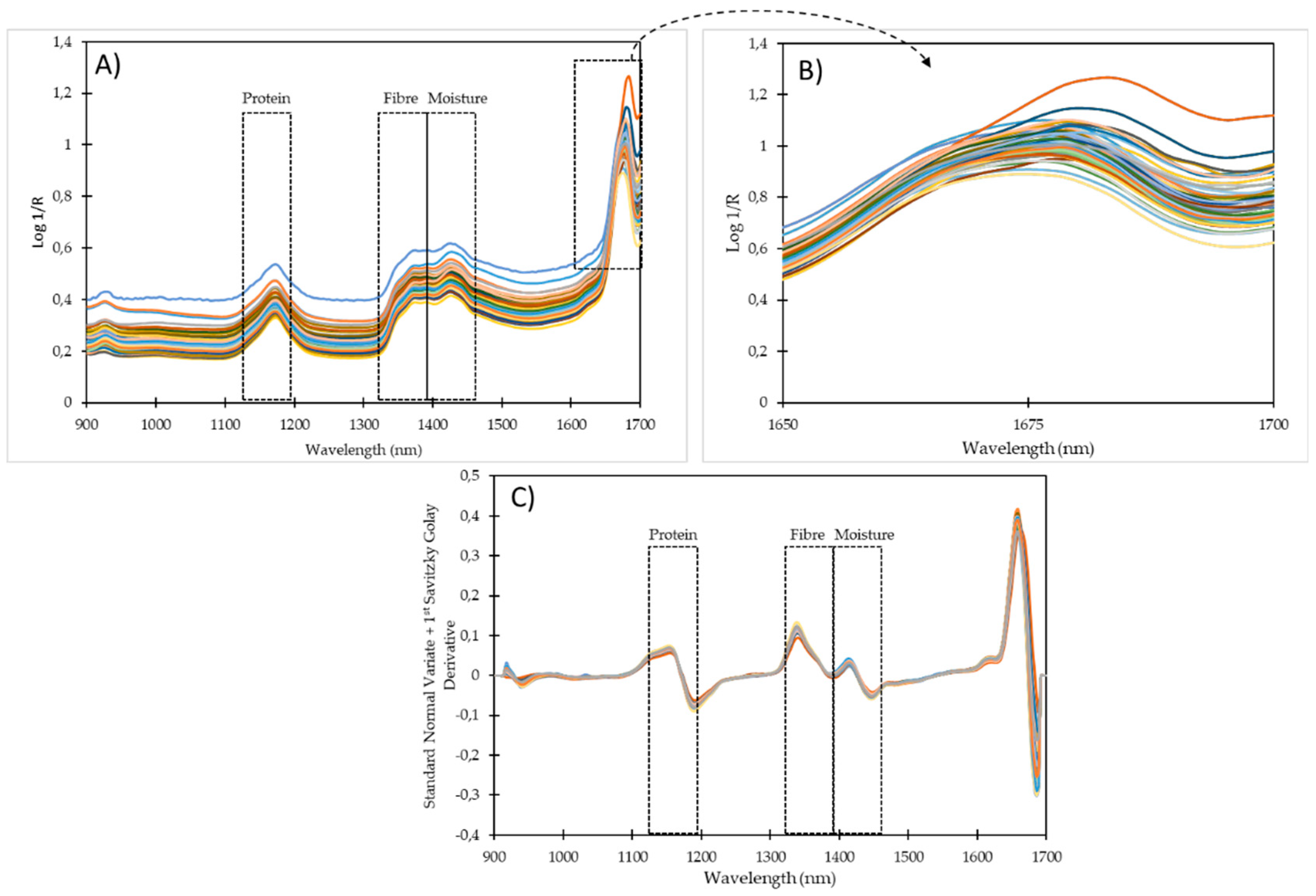
| 901−1700 nm | 901−1600 nm | |||
|---|---|---|---|---|
| Sample | Raw | Ground | Raw | Ground |
| 1 | 60529 | 27209 | 43283 | 14560 |
| 2 | 58378 | 23853 | 33386 | 10886 |
| 3 | 54190 | 20057 | 34085 | 7029 |
| 4 | 54854 | 30655 | 36262 | 17678 |
| 5 | 48472 | 33325 | 34239 | 21939 |
| Raw alfalfa | ||||
| Wavelength Range: | 901-1600 nm | 901-1700 nm | ||
| Math pretreatment | R2 | SEC | R2 | SEC |
| 1 4 4 SG | 0.898 | 1.554 | 0.883 | 1.670 |
| 2 4 4 SG | 0.784 | 2.184 | 0.786 | 2.289 |
| SNV 1 4 4SG | 0.911 | 1.392 | 0.791 | 2.187 |
| 1 4 4 SG SNV | 0.840 | 1.910 | 0.145 | 1.398 |
| SNV 2 4 4SG | 0.955 | 1.066 | 0.514 | 3.155 |
| 2 4 4 SG SNV | 0.726 | 2.558 | 0.540 | 3.238 |
| Ground Alfalfa | ||||
| Wavelength Range: | 901-1600 nm | 901-1700 nm | ||
| Math pretreatment | R2 | SEC | R2 | SEC |
| 1 4 4 SG | 0.756 | 2.749 | 0.598 | 2.830 |
| 2 4 4 SG | 0.842 | 2.258 | 0.623 | 3.371 |
| SNV 1 4 4SG | 0.761 | 2.694 | 0.043 | 5.383 |
| 1 4 4 SG SNV | 0.796 | 2.421 | 0.510 | 3.946 |
| SNV 2 4 4SG | 0.892 | 1.861 | 0.540 | 3.803 |
| 2 4 4 SG SNV | 0.730 | 2.860 | 0.524 | 3.321 |
| Raw alfalfa | ||||
| Wavelength Range: | 901-1600 nm | 901-1700 nm | ||
| Math pretreatment | R2 | SEC | R2 | SEC |
| 1 4 4 SG | 0.742 | 0.510 | 0.884 | 0.428 |
| 2 4 4 SG | 0.262 | 0.911 | 0.608 | 1.262 |
| SNV 1 4 4SG | 0.307 | 1.314 | 0.156 | 1.524 |
| 1 4 4 SG SNV | 0.678 | 0.842 | 0.257 | 1.378 |
| SNV 2 4 4SG | 0.885 | 0.377 | 0.345 | 0.855 |
| 2 4 4 SG SNV | 0.328 | 0.812 | 0.318 | 1.368 |
| Grounded Alfalfa | ||||
| Wavelength Range: | 901-1600 nm | 901-1700 nm | ||
| Math pretreatment | R2 | SEC | R2 | SEC |
| 1 4 4 SG | 0.671 | 0.986 | 0.706 | 0.927 |
| 2 4 4 SG | 0.906 | 0.530 | 0.290 | 1.014 |
| SNV 1 4 4SG | 0.773 | 0.816 | 0.790 | 0.650 |
| 1 4 4 SG SNV | 0.734 | 0.882 | 0.723 | 0.651 |
| SNV 2 4 4SG | 0.862 | 0.660 | 0.216 | 1.145 |
| 2 4 4 SG SNV | 0.820 | 0.746 | 0.179 | 1.433 |
| Raw alfalfa | ||||
| Wavelength Range: | 901-1600 nm | 901-1700 nm | ||
| Math pretreatment | R2 | SEC | R2 | SEC |
| 1 4 4 SG | 0.524 | 0.503 | 0.211 | 0.572 |
| 2 4 4 SG | 0.619 | 0.492 | 0.129 | 0.861 |
| SNV 1 4 4SG | 0.783 | 0.374 | 0.734 | 0.464 |
| 1 4 4 SG SNV | 0.502 | 0.579 | 0.312 | 0.491 |
| SNV 2 4 4SG | 0.675 | 0.409 | 0.679 | 0.434 |
| 2 4 4 SG SNV | 0.861 | 0.219 | 0.687 | 0.444 |
| Grounded Alfalfa | ||||
| Wavelength Range: | 901-1600 nm | 901-1700 nm | ||
| Math pretreatment | R2 | SEC | R2 | SEC |
| 1 4 4 SG | 0.650 | 0.530 | 0.652 | 0.506 |
| 2 4 4 SG | 0.770 | 0.435 | 0.243 | 0.819 |
| SNV 1 4 4SG | 0.570 | 0.586 | 0.670 | 0.519 |
| 1 4 4 SG SNV | 0.867 | 0.318 | 0.347 | 0.723 |
| SNV 2 4 4SG | 0.604 | 0.566 | 0.591 | 0.579 |
| 2 4 4 SG SNV | 0.781 | 0.424 | 0.301 | 0.625 |
| Parameter | Sampling | Math pretreatment | Range (nm) |
R2 | SEC |
|---|---|---|---|---|---|
| NDF | Raw | SNV 2 4 4 SG | 900-1600 | 0.955 | 1.066 |
| Ground | SNV 2 4 4 SG | 900-1600 | 0.892 | 1.861 | |
| CP | Raw | SNV 2 4 4 SG | 900-1600 | 0.885 | 0.377 |
| Ground | 2 4 4 SG | 900-1600 | 0.906 | 0.530 | |
| MC | Raw | 2 4 4 SG SNV | 900-1600 | 0.861 | 0.219 |
| Ground | 1 4 4 SG SNV | 900-1600 | 0.867 | 0.318 |
5. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baeten, V.; Manley, M.; Fernández-Pierna, J.A.; Downey, G.; Dardenne, P. Spectrometric technique: Fourier transform near-infrared (FT-NIR) spectroscopy. In Modern techniques for food authentication; Da-Wen, S., Ed. Elsevier, Dublin, UK, 2008, 117–147.
- De la Haba, M.J.; Fernández-Pierna, J.A.; Fumière, O.; Garrido-Varo, A.; Guerrero-Ginel, J.E.; Pérez-Marín D.C.; Dardenne, P.; Baeten, V. Discrimination of fish bones from other animal bones in the sedimented fraction of compound feeds by near infrared microscopy. J. Near Infrared Spectrosc. 2007, 15, 81–88.
- Fernández-Pierna, J.A.; Baeten, V.; Dardenne, P. Screening of compound feeds using NIR hyperspectral data. Chemometr. Intell. Lab. Syst. 2006, 84, 114–118. https://doi.org/10.1016/j.chemolab.2006.03.012. [CrossRef]
- Fernández-Ibáñez, M. del V.; Soldado, A.; Vicente, F.; Martínez-Fernández, A.; de la Roza-Delgado, B. Particle size optimisation in development of near infrared microscopy methodology to build spectral libraries of animal feeds. J. Near Infrared Spectrosc. 2008, 16, 243–248.
- Hermida, M.; Lois, A.; Rodriguez-Otero, J.L. Analysis of nitrogen fractions in silage by near-infrared spectroscopy. J. Agric. Food Chem. 2005, 53, 1374–1378.
- Volkers, K.C.; Wachendorf, M.; Loges, R.; Jovanovic, N.J.; Taube, F. Prediction of the quality of forage maize by near-infrared reflectance spectroscopy. Anim. Feed Sci. Technol. 2003, 109, 183–194. https://doi.org/10.1016/S0377-8401(03)00173-1. [CrossRef]
- Shenk, J.S.; Workman, J.J.; Westerhaus, M.O. Application of NIR spectroscopy to agricultural products. In Handbook of near infrared analysis; Burns, D.A., Ciurczak, E.W., Eds.; CRC Press, Boca Raton, FL, USA, 2008, 347–387.
- Bertrand, D.; Dufour, E. Infrared spectroscopy and its analytical applications. In Analytical techniques in the sciences; Editions Tec & Doc, Paris, France, 2000.
- Murray, I. Forage analysis by near infra-red spectroscopy. In Sward measurement handbook; Davies, A., Baker, R.D., Grant, S.A., Laidlaw, A.S., Eds.; NIR Publications, Chichester, UK, 1993, 155–177.
- Yan, H.; Siesler, H.W. Hand-held near-infrared spectrometers: State-of-the-art instrumentation and practical applications. NIR news 2018, 29, 8–12.
- Melendreras, C.; Soldado, A.; Costa-Fernández, J.M.; López, A.; Valledor, M.; Campo, J.C.; Ferrero, F.J. An affordable NIR spectroscopic system for fraud detection in olive oil. Sensors 2023, 23, 1728.
- Texas Instruments. DLP NIRscan™ Nano EVM User´s Guide. 2017. Available online: https://www.ti.com/lit/pdf/dlpu030 (Accessed on 2 January 2023).
- Van-Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2. [CrossRef]
- Dyer, S.A. Hadamard transform spectrometry. Chemom. Intell. Lab. Syst. 1991, 12, 101–115.
- Shenk, J.S.; Westerhaus, M.O. Routine Operation, Calibration, Development and Network System Management Manual. NIR Systems Inc.: Silver Spring, MD, USA, 1995.
- Shenk, J.S.; Westerhaus, M.O. Calibration the ISI way. Near infrared spectroscopy: The future waves. In NIR Publications; Chichester, UK, 1996, 198–202.
- Thompson, M.; Wood, R. Using uncertainty functions to predict and specify the performance of analytical methods. Accred. Qual. Assur. 2006, 10, 471–478. https://doi.org/10.1007/s00769-005-0040-5. [CrossRef]
- Rego G., Ferrero F., Valledor M., Campo J.C., Forcada S., Royo L.J., Soldado A. A portable IoT NIR spectroscopic system to analyze the quality of dairy farm forage. Computers and Electronics in Agriculture. 2020, 175, 1 – 8. https://doi.org/10.1016/j.compag.2020.105578. [CrossRef]
- Coelho, M.; Hembry, F.G. Laboratory methods of forage evaluation. Near infrared reflectance analysis. In Proceedings of the Program and Research Progress Reports, Baton Rouge, LA, USA, 195–199, 1982.
- Shenk, J.S.; Westerhaus, M.O.; Hoover, M.R. Infrared reflectance analysis of forages. Int. J. Dairy Sci. 1979, 62, 807–812.
- Martínez, M.L.; Garrido-Varo, A.; Pedro, E.D.; Sánchez, L. Effect of sample heterogeneity on near infrared meat analysis: The use of the RMS statistic. J. Near Infrared Spectrosc. 1998, 6, A313–A320.
- Berzaghi, P., Cherney, J.H., Casler, M.D. Prediction performance of portable near infrared reflectance instruments using preprocessed dried, ground forage samples,Comput. Electron. Agric. 2021, 182, 106013. https://doi.org/10.1016/j.compag.2021.106013. [CrossRef]
- Gorla, G., Taiana, A., Boqué, R., Bani, P., Gachiuta, O., Giussani, B. Unravelling error sources in miniaturized NIR spectroscopic measurements: The case study of forages, Anal. Chim. Acta,2022, 1211, 339900. https://doi.org/10.1016/j.aca.2022.339900. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





