Submitted:
09 July 2023
Posted:
11 July 2023
You are already at the latest version
Abstract
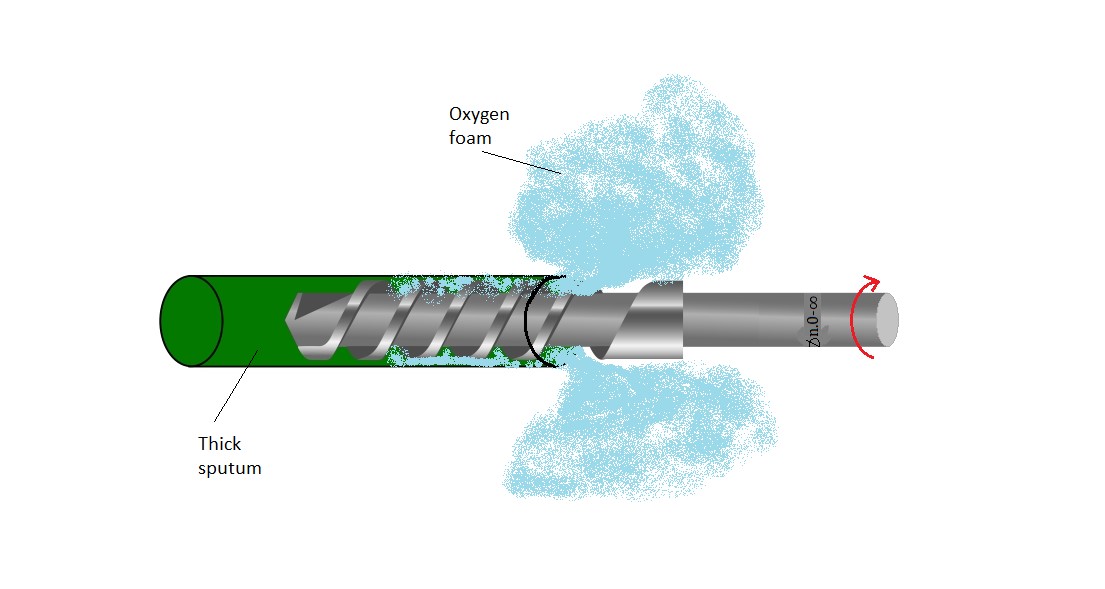
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Sahebnasagh, A.; Mojtahedzadeh, M.; Najmeddin, F.; Najafi, A.; Safdari, M.; Rezai Ghaleno, H.; et al. A perspective on erythropoietin as a potential adjuvant therapy for acute lung injury/acute respiratory distress syndrome in patients with COVID-19. Arch. med. res. 2020, 51, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Elezkurtaj, S.; Greuel, S.; Ihlow, J.; Michaelis, E.G.; Bischoff, P.; Kunze, C.A.; et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci. Rep. 2021, 11, 4263. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Duke, T.; Chisti, M.J.; Kepreotes, E.; Kalinowski, V.; Li, J. Efficacy of high-flow nasal cannula vs standard oxygen therapy or nasal continuous positive airway pressure in children with respiratory distress: a meta-analysis. J. Pediatr. 2019, 215, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Cinesi Gómez, C.; Peñuelas Rodríguez, Ó.; Luján Torné, M.; Egea Santaolalla, C.; Masa Jiménez J., F.; García Fernández, J.; et al. Clinical consensus recommendations regarding non-invasive respiratory support in the adult patient with acute respiratory failure secondary to SARS-CoV-2 infection. Recomendaciones de consenso respecto al soporte respiratorio no invasivo en el paciente adulto con insuficiencia respiratoria aguda secundaria a infección por SARS-CoV-2. Med. Intensiva (Engl Ed). 2020, 44, 429–438. [Google Scholar] [CrossRef]
- Marini J., J. Advances in the support of respiratory failure: putting all the evidence together. Crit. Care. 2015, 19, S4. [Google Scholar] [CrossRef]
- Mendes, N.F.; Jara, C.P.; Mansour, E.; Araújo, E.P.; Velloso, L.A. Asthma and COVID-19: a systematic review. Allergy Asthma Clin. Immunol. 2021, 17, 5. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Urakov, A.; Muhutdinov, N.; Yagudin, I.; Suntsova, D.; Svetova, M. Brain hypoxia caused by respiratory obstruction wich should not be forgotten in COVID-19 disease. Turk. J. Med. Sci. 2022, 52, 1504–1505. [Google Scholar] [CrossRef]
- Dhont, S.; Derom, E.; Van Braeckel, E.; Depuydt, P.; Lambrecht, B.N. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir. Res. 2020, 21, 198. [Google Scholar] [CrossRef]
- Cronin J., N.; Camporota, L.; Formenti, F. Mechanical ventilation in COVID-19: A physiological perspective. Exp. Physiol. 2022, 107, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Yaroshetskiy, A.I.; Avdeev, S.N.; Politov, M.E.; Nogtev, P.V.; Beresneva, V.G.; Sorokin, Y.D.; et al. Potential for the lung recruitment and the risk of lung overdistension during 21 days of mechanical ventilation in patients with COVID-19 after noninvasive ventilation failure: the COVID-VENT observational trial. BMC anesthesiol. 2022, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Cattaneo, E.; Florio, G.; Ippolito, M.; Zanella, A.; Cortegiani, A.; et al. Mechanical ventilation parameters in critically ill COVID-19 patients: a scoping review. Crit. care (London, England). 2021, 25, 115. [Google Scholar] [CrossRef] [PubMed]
- Lentz, S.; Roginski M., A.; Montrief, T.; Ramzy, M.; Gottlieb, M.; Long, B. Initial emergency department mechanical ventilation strategies for COVID-19 hypoxemic respiratory failure and ARDS. Am. J. Emerg. Med. 2020, 38, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Xiong, J.; Feng, Z.; Shi, Y. Extracorporeal membrane oxygenation (ECMO): does it have a role in the treatment of severe COVID-19? Int. J. Infect. Dis. 2020, 94, 78–80. [Google Scholar] [CrossRef]
- Maqsood, U.; Patel, N. Extracorporeal membrane oxygenation (ECMO) for near-fatal asthma refractory to conventional ventilation. BMJ Case Rep. 2018, 2018, bcr2017223276. [Google Scholar] [CrossRef]
- Steinack, C.; Lenherr, R.; Hendra, H.; Franzen, D. The use of life-saving extracorporeal membrane oxygenation (ECMO) for pregnant woman with status asthmaticus. J. Asthma. 2017, 54, 84–88. [Google Scholar] [CrossRef]
- Kronibus, N.; Seiler, F.; Danziger, G.; Muellenbach R., M.; Reyher, C.; Becker, A.P.; et al. Respiratory physiology of COVID-19 and influenza associated acute respiratory distress syndrome. J. Clin. Med. 2022, 11, 6237. [Google Scholar] [CrossRef]
- Jäckel, M.; Rilinger, J.; Lang C., N.; Zotzmann, V.; Kaier, K.; Stachon, P.; et al. Outcome of acute respiratory distress syndrome requiring extracorporeal membrane oxygenation in Covid-19 or influenza: A single-center registry study. Artif. Organs. 2021, 45, 593–601. [Google Scholar] [CrossRef]
- Odigwe, C.; Krieg, J.; Owens, W.; Lopez, C.; Arya, R.R. Usefulness of extracorporeal membrane oxygenation in status asthmaticus with severe tracheal stenosis. Proc. (Bayl. Univ. Med. Cent.). 2020, 33, 404–406. [Google Scholar] [CrossRef]
- Lazar H., L. Commentary: Extracorporeal membrane oxygenation: Is it life-saving and cost effective for all patients? JTCVS open. 2020, 1, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Cavarocchi, N.C.; Wallace, S.; Hong, E.Y.; Tropea, A.; Byrne, J.; Pitcher, H.T.; et al. A cost-reducing extracorporeal membrane oxygenation (ECMO) program model: a single institution experience. Perfusion. 2015, 30, 148–153. [Google Scholar] [CrossRef]
- Barrot, L.; Asfar, P.; Mauny, F.; Winiszewski, H.; Montini, F.; Badie, J.; et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N. Engl. J. Med. 2020, 382, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Semler, M.W.; Casey, J.D.; Lloyd, B.D.; Hastings, P.G.; Hays, M.A.; Stollings, J.L.; et al. Oxygen-saturation targets for critically Ill adults receiving mechanical ventilation. N. Engl. J. Med. 2022, 387, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- James A., L.; Paré P., D.; Hogg J., C. The mechanics of airway narrowing in asthma. Am. Rev. Respir. Dis. 1989, 139, 242–246. [Google Scholar] [CrossRef]
- O'Sullivan, M.J.; Jang, J.H.; Panariti, A.; Bedrat, A.; Ijpma, G.; Lemos, B.; et al. Airway epithelial cells drive airway smooth muscle cell phenotype switching to the proliferative and pro-inflammatory phenotype. Front. Physiol. 2021, 12, 687654. [Google Scholar] [CrossRef]
- Charriot, J.; Volpato, M.; Petit, A.; Vachier, I.; Bourdin, A. Methods of sputum and mucus assessment for muco-bsotructive lung diseases in 2022: Time to "unplug" from our daily routine! Cells. 2022, 11, 812. [Google Scholar] [CrossRef]
- Ehre, C.; Rushton, Z.L.; Wang, B.; Hothem, L.N.; Morrison, C.B.; Fontana N., C.; et al. An improved inhaled mucolytic to treat airway muco-obstructive diseases. Am. J. Respir. Crit. Care Med. 2019, 199, 171–180. [Google Scholar] [CrossRef]
- Djukanović, R.; Roche, W.R.; Wilson, J.W.; Beasley, C.R.; Twentyman, O.P.; Howarth, R.H.; et al. Mucosal inflammation in asthma. Am. Rev. Respir. Dis. 1990, 142, 434–457. [Google Scholar] [CrossRef]
- Suissa, S.; Ernst, P.; Benayoun, S.; Baltzan, M.; Cai, B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N. Engl. J. Med. 2000, 343, 332–336. [Google Scholar] [CrossRef]
- Krings, J.G.; Wojcik, K.M.; Chen, V.; Sekhar T., C.; Harris, K.; Zulich, A.; et al. Symptom-driven inhaled corticosteroid/long-acting beta-agonist therapy for adult patients with asthma who are non-adherent to daily maintenance inhalers: a study protocol for a pragmatic randomized controlled trial. Trials. 2022, 23, 975. [Google Scholar] [CrossRef] [PubMed]
- Romão, M.; Bulhosa, C.; Mendes, Z.; Sousa, C.; Silva, G.; Pereira, M.; et al. Characteristics of oral corticosteroid users among persons with asthma on GINA step 3 therapy and above: A cross-sectional study in portuguese community pharmacies. J. Asthma Allergy. 2022, 15, 1579–1592. [Google Scholar] [CrossRef]
- Bengtson, L.G.S.; Yu, Y.; Wang, W.; Cao, F.; Hulbert, E.M.; Wolbeck, R.; et al. Inhaled corticosteroid-containing treatment escalation and outcomes for patients with asthma in a U.S. health care organization. J. Manag. Care Spec. Pharm. 2017, 23, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Crossingham, I.; Turner, S.; Ramakrishnan, S.; Fries, A.; Gowell, M.; Yasmin, F.; et al. Combination fixed-dose beta agonist and steroid inhaler as required for adults or children with mild asthma. Cochrane Database Syst. Rev. 2021, 5, CD013518. [Google Scholar] [CrossRef] [PubMed]
- O'Shea, O.; Stovold, E.; Cates, C.J. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events. Cochrane Database Syst. Rev. 2021, 4, CD007694. [Google Scholar] [CrossRef]
- Priya, S.P.; Sunil, P.M.; Varma, S.; Brigi, C.; Isnadi M.F.A., R.; Jayalal, J.A.; et al. Direct, indirect, post-infection damages induced by coronavirus in the human body: an overview. Virusdisease. 2022, 33, 429–444. [Google Scholar] [CrossRef]
- Kirtipal, N.; Kumar, S.; Dubey, S.K.; Dwivedi, V.D.; Gireesh Babu, K.; Malý, P.; et al. Understanding on the possible routes for SARS CoV-2 invasion via ACE2 in the host linked with multiple organs damage. Infect. Genet. Evol. 2022, 99, 105254. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Jaber, V.R.; Pogue, A.I.; Zhao, Y. SARS-CoV-2 invasion and pathological links to prion disease. Biomolecules. 2022, 12, 1253. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber V., R.; Lukiw, W.J. SARS-CoV-2, long COVID, prion disease and neurodegeneration. Front. Neurosci. 2022, 16, 1002770. [Google Scholar] [CrossRef]
- Duzgun, S.A.; Durhan, G.; Demirkazik, F.B.; Akpinar, M.G.; Ariyurek, O.M. COVID-19 pneumonia: the great radiological mimicker. Insights into imaging. 2020, 11, 118. [Google Scholar] [CrossRef]
- Su, W.L.; Lu, K.C.; Chan, C.Y.; Chao, Y.C. COVID-19 and the lungs: A review. J. Infect. Public Health. 2021, 14, 1708–1714. [Google Scholar] [CrossRef]
- Aguiar, D.; Lobrinus, J.A.; Schibler, M.; Fracasso, T.; Lardi, C. Inside the lungs of COVID-19 disease. Int. J. Legal Med. 2020, 134, 1271–1274. [Google Scholar] [CrossRef]
- Cui X, Chen W, Zhou H, Gong Y. ; Zhu B.; Lv X.; et al. Pulmonary edema in COVID-19 patients: Mechanisms and treatment potential. Front. Pharmacol. 2021, 12, 664349. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet. 2021, 398, 622–637. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, M.; Jiang, L.; Chen, N.; Tu, S.; Wei, Y.; et al. Effects of the lower airway secretions on airway opening pressures and suction pressures in critically Ill COVID-19 patients: A computational simulation. Ann. Biomed. Eng. 2020, 48, 3003–3013. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, F.I.; Morgan, R.C.; Dhawan, N.; Dinh, J.; Yatzkan, G.; Michel, G. Airway hygiene in COVID-19 pneumonia: Treatment responses of 3 critically Ill cruise ship employees. Am. J. Case. Rep. 2020, 21, e926596. [Google Scholar] [CrossRef]
- Coelho Dos Reis, J.G.A.; Ferreira, G.M.; Lourenço, A.A.; Ribeiro Á., L. , da Mata C.P.D.S. M., de Melo Oliveira P.; et al. Ex-vivo mucolytic and anti-inflammatory activity of BromAc in tracheal aspirates from COVID-19. Biomed. Pharmacother. 2022, 148, 112753. [Google Scholar] [CrossRef]
- Kumar, S.S.; Binu, A.; Devan, A.R.; Nath, L.R. Mucus targeting as a plausible approach to improve lung function in COVID-19 patients. Med. Hypotheses. 2021, 156, 110680. [Google Scholar] [CrossRef]
- Voynow J., A.; Rubin, B.K. Mucins, mucus, and sputum. Chest. 2009, 135, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Urakov, A.L.; Urakova, N.A.; Yagudin, I.I.; Svetova, M.D.; Suntsova, D.O. COVID-19: Artificial sputum, respiratory obstruction method and screening of pyolitic and antihypoxic drugs. BioImpacts. 2022, 12, 393–394. [Google Scholar] [CrossRef]
- Evans, C.M.; Koo, J.S. Airway mucus: the good, the bad, the sticky. Pharmacol. Ther. 2009, 121, 332–348. [Google Scholar] [CrossRef] [PubMed]
- Altiner, A.; Wilm, S.; Däubener, W.; Bormann, C.; Pentzek, M.; Abholz H., H.; et al. Sputum colour for diagnosis of a bacterial infection in patients with acute cough. Scand. J. Prim. Health Care. 2009, 27, 70–73. [Google Scholar] [CrossRef]
- Keshishyan, S.; DeLorenzo, L.; Hammoud, K.; Avagyan, A.; Assallum, H.; Harris, K. Infections causing central airway obstruction: role of bronchoscopy in diagnosis and management. J. Thorac. Dis. 2017, 9, 1707–1724. [Google Scholar] [CrossRef]
- Sick-Samuels, A.C.; Linz, M.; Bergmann, J.; Fackler, J.C.; Berenholtz, S.M.; Ralston, S.L.; et al. Diagnostic stewardship of endotracheal aspirate cultures in a PICU. Pediatrics. 2021, 147, e20201634. [Google Scholar] [CrossRef]
- Prinzi A., M.; Wattier, R.L.; Curtis, D.J.; Ziniel, S.I.; Fitzgerald, A.; Pearce, K.; et al. Impact of organism reporting from endotracheal aspirate cultures on antimicrobial prescribing practices in mechanically ventilated pediatric patients. J. Clin. Microbiol. 2022, 60, e0093022. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Jiang, Z.; Li, H.; Guo, C.; Lu, H.; Luo, W.; et al. Small airway dysfunction in cough variant asthma: Prevalence, clinical, and pathophysiological features. Front. Physiol. 2022, 12, 761622. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Yang, A.X.; Sieve, A.; Kuo, S.H.; Mudalagiriyappa, S.; Vieson, M.; et al. Pulmonary mycosis drives forkhead box protein A2 degradation and mucus hypersecretion through activation of the spleen tyrosine kinase-epidermal growth factor receptor-AKT/extracellular signal-regulated kinase 1/2 signaling. Am. J Pathol. 2021, 191, 108–130. [Google Scholar] [CrossRef] [PubMed]
- Urakov, A.L.; Shabanov, P.D. Acute respiratory syndrome-2 (SARS-CoV-2): A solution of hydrogen peroxide and sodium bicarbonate as an expectorant for recanalization of the respiratory tract and blood oxygenation in respiratory obstruction (review). Rev. Clin. Pharmacol. Drug. Ther. 2021, 19, 383–393. [Google Scholar] [CrossRef]
- Ramos, F.L.; Krahnke, J.S.; Kim, V. Clinical issues of mucus accumulation in COPD. Int. J Chron. Obstruct. Pulmon. Dis. 2014, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Aliberti, S.; Blasi, F. Management of bronchiectasis in adults. Eur. Respir. J. 2015, 45, 1446–1462. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.A.; Chen, A.C.H.; Radicioni G, Lourie R. ; Martin M.; Broomfield A.; et al. Airway mucus hyperconcentration in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2020, 201, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Esther, C.R.Jr. , Muhlebach M.S.; Ehre C.; Hill D.B.; Wolfgang M.C.; Kesimer M.; et al. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci. Transl. Med. 2019, 11, eaav3488. [Google Scholar] [CrossRef]
- Urakov, A.; Urakova, N. COVID-19: Cause of death and medications. IP Int. J Compr. Adv. Pharmacol, 2020, 5, 45–48. [Google Scholar] [CrossRef]
- Kato, T.; Asakura, T.; Edwards, C.E.; Dang, H.; Mikami, Y.; Okuda, K.; et al. Prevalence and mechanisms of mucus accumulation in COVID-19 lung disease. Am. J. Respir. Crit. Care Med. 2022, 206, 1336–1352. [Google Scholar] [CrossRef] [PubMed]
- Meyerholz D., K.; Reznikov, L.R. Influence of SARS-CoV-2 on airway mucus production: A review and proposed model. Vet. Pathol. 2022, 59, 578–585. [Google Scholar] [CrossRef]
- Maskin, L.P.; Olarte, G.L.; Palizas F., Jr. , Velo A.E.; Lurbet M.F.; Bonelli I.; et al. High dose dexamethasone treatment for Acute Respiratory Distress Syndrome secondary to COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020, 21, 743. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Griesel, M.; Mikolajewska, A.; Metzendorf, M.I.; Fischer, A.L.; Stegemann, M.; et al. Systemic corticosteroids for the treatment of COVID-19: Equity-related analyses and update on evidence. Cochrane Database Syst. Rev. 2022, 11, CD014963. [Google Scholar] [CrossRef]
- Cochrane, M.G.; Bala, M.V.; Downs, K.E.; Mauskopf, J.; Ben-Joseph, R.H. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 2000, 117, 542–550. [Google Scholar] [CrossRef]
- Shea, B.J.; Grimshaw, J.M.; Wells, G.A. Boers M.; Andersson N.; Hamel C.; et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol, 2007, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Forrest, J. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J Evid-Based Dent. Pr. 2001, 1, 136–141. [Google Scholar] [CrossRef]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Boehm B.; Hansen J.W. Spiral development: Experience, principles, and refinements. SEI Joint Program Office: Pittsburgh, US, 9 February 2000. 9 February. [CrossRef]
- Alshamrani, A.; Bahattab, A. A comparison between three SDLC models waterfall model, spiral model, and incremental/iterative model. Int. J. Comput. Sci. 2015, 12, 106–111. [Google Scholar]
- Soriano, J.B. Planetary respiratory health for asthma, rhinoconjunctivitis and eczema. Eur. Respir. J. 2022, 60, 2200440. [Google Scholar] [CrossRef] [PubMed]
- Pijnenburg, M.W.; Nantanda, R. Rising and falling prevalence of asthma symptoms. Lancet. 2021, 398, 1542–1543. [Google Scholar] [CrossRef]
- Asher, M.I.; Rutter, C.E.; Bissell, K.; Chiang, C.Y.; El Sony, A.; Ellwood, E.; et al. Worldwide trends in the burden of asthma symptoms in school-aged children: Global Asthma Network Phase I cross-sectional study. Lancet. 2021, 398, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Dastoorpoor, M.; Khodadadi, N.; Madadizadeh, F.; Raji, H.; Shahidizadeh, E.; Idani, E.; et al. Assessing the prevalence and severity of asthma, rhinitis, and eczema among schoolchildren (6-7 and 13-14 years old) in Khuzestan, Iran: a cross-sectional survey. BMC Pediatr. 2022, 22, 463. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, K.; Salvi, S.S.; Reddel, H.K. Closing gaps in asthma care in India – World asthma day 2022. Indian J. Med. Res. 2022, 156, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Vogtel, M.; Michels, A. Role of intermittent hypoxia in the treatment of bronchial asthma and chronic obstructive pulmonary disease. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 206–213. [Google Scholar] [CrossRef]
- Haider, T.; Casucci, G.; Linser, T.; Faulhaber, M.; Gatterer, H.; Ott, G.; et al. Interval hypoxic training improves autonomic cardiovascular and respiratory control in patients with mild chronic obstructive pulmonary disease. J. Hypertens. 2009, 27, 1648–1654. [Google Scholar] [CrossRef]
- Serebrovskaya, T.V.; Swanson, R.J.; Kolesnikova, E.E. Intermittent hypoxia: mechanisms of action and some applications to bronchial asthma treatment. J. Physiol. Pharmacol. 2003, 54, 35–41. [Google Scholar] [PubMed]
- Chen, P.S.; Chiu, W.T.; Hsu, P.L.; Lin, S.C.; Peng, I.C.; Wang, C.Y.; et al. Pathophysiological implications of hypoxia in human diseases. J. Biomed. Sci. 2020, 27, 63. [Google Scholar] [CrossRef] [PubMed]
- Quirt, J.; Hildebrand, K.J.; Mazza, J.; Noya, F.; Kim, H. Asthma. Allergy Asthma Clin. Immunol. 2018, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Creticos P., S. (2003). Treatment options for initial maintenance therapy of persistent asthma: a review of inhaled corticosteroids and leukotriene receptor antagonists. Drugs. 2003, 63, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Moamary, M.S.; Alhaider, S.A.; Alangari, A.A.; Idrees, M.M.; Zeitouni, M.O.; Al Ghobain, M.O.; et al. The saudi initiative for asthma - 2021 update: Guidelines for the diagnosis and management of asthma in adults and children. Ann. Thorac. Med. 2021, 16, 4–56. [Google Scholar] [CrossRef] [PubMed]
- Al-Moamary, M.S.; Al-Lehebi, R.; Idrees, M.M.; Zeitouni, M.O. When single-inhaler triple therapy is a preferred option in asthma management? Ann. Thorac. Med. 2022, 17, 185–188. [Google Scholar] [CrossRef]
- Marín-Cassinello, A.; Vega-Hernández, M.C.; Lumbreras-Lacarra, B.; De Arriba-Méndez, S.; Pellegrini-Belinchón, J. Prevalence of symptoms, severity and diagnosis of asthma in adolescents in the province of Salamanca, Spain: Global asthma network (GAN) Phase I. Allergol. Immunopathol. (Madr). 2021, 49, 106–112. [Google Scholar] [CrossRef]
- Lai, C.K.; Beasley, R.; Crane, J.; Foliaki, S.; Shah, J.; Weiland, S.; et al. Global variation in the prevalence and severity of asthma symptoms: phase three of the international study of asthma and allergies in childhood (ISAAC). Thorax. 2009, 64, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.; Douwes, J. The global epidemiology of asthma in children. Int. J. Tuberc. Lung. Dis. 2006, 10, 125–132. [Google Scholar]
- Pearce, N.; Douwes, J. Lifestyle changes and childhood asthma. Indian J. Pediatr. 2013, 80, S95–S99. [Google Scholar] [CrossRef]
- Holzer, S.S.; Engelhart, L.; Crown, W.H.; L'Herrou, T.A.; Kennedy, S.T. Asthma treatment costs using inhaled corticosteroids. Am. J. Manag. Care. 1997, 3, 891–897. [Google Scholar] [PubMed]
- Lage, M.J.; Gross, G.N.; Brewster, C.; Spalitto, A. Outcomes and costs of patients with persistent asthma treated with beclomethasone dipropionate hydrofluoroalkane or fluticasone propionate. Adv. Ther. 2009, 26, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Spangler, D.L. The role of inhaled corticosteroids in asthma treatment: a health economic perspective. Am. J. Manag. Care. 2012, 18, S35–S39. [Google Scholar] [PubMed]
- Lavorini, F.; Janson, C.; Braido, F.; Stratelis, G.; Løkke, A. What to consider before prescribing inhaled medications: a pragmatic approach for evaluating the current inhaler landscape. Ther. Adv. Respir. Dis. 2019, 13, 1753466619884532. [Google Scholar] [CrossRef]
- Martonen, T.; Fleming, J.; Schroeter, J.; Conway, J.; Hwang, D. In silico modeling of asthma. Adv. Drug Deliv. Rev. 2003, 55, 829–849. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, C.E.; Sossa-Briceño, M.P.; Castro-Rodriguez, J.A. Dexamethasone or prednisolone for asthma exacerbations in children: A cost-effectiveness analysis. Pediatr. Pulmonol. 2020, 55, 1617–1623. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, C.E.; Sossa-Briceño, M.P.; Castro-Rodriguez, J.A. Advantage of inhaled corticosteroids as additional therapy to systemic corticosteroids for pediatric acute asthma exacerbations: a cost-effectiveness analysis. J Asthma. 2020, 57, 949–958. [Google Scholar] [CrossRef]
- Urakov, A.; Urakova, N. Recent Insights into the Management of Inflammation in Asthma [Letter]. J. Inflamm. Res. 2021, 14, 4603–4604. [Google Scholar] [CrossRef]
- Rubin B., K. Mucolytics, expectorants, and mucokinetic medications. Respir. Care. 2007, 52, 859–865. [Google Scholar]
- Fuloria, M.; Rubin, B.K. Evaluating the efficacy of mucoactive aerosol therapy. Respir. Care. 2000, 45, 868–873. [Google Scholar]
- Kurukulaaratchy, R.J.; Rupani, H.; Fong, W.C.G.; Kyyaly, A. (2021). A role for mucolytics and xpeectorants in aiding inhaled therapies in asthma? [Response to Letter]. J. Inflamm. Res. 2021, 14, 5183–5185. [Google Scholar] [CrossRef]
- Kratochvil, M.J.; Kaber, G.; Demirdjian, S.; Cai, P.C.; Burgener, E.B.; Nagy, N.; et al. Biochemical, biophysical, and immunological characterization of respiratory secretions in severe SARS-CoV-2 infections. JCI insight. 2022, 7, e152629. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Duvall, M.G. Invasive and noninvasive ventilation strategies for acute respiratory failure in children with coronavirus disease 2019. Curr. Opin. Pediatr. 2021, 33, 311–318. [Google Scholar] [CrossRef]
- Pham, T.; Brochard, L.J.; Slutsky, A.S. Mechanical ventilation: State of the art. Mayo. Clin. Proc. 2017, 92, 1382–1400. [Google Scholar] [CrossRef] [PubMed]
- Urakov, A.L.; Urakova, N.A. COVID-19: What drug can be used to treat a new coronavirus disease and why. J. Bio. Innov. 2020, 9, 241–251. [Google Scholar] [CrossRef]
- Parrilla, F.J.; Morán, I.; Roche-Campo, F.; Mancebo, J. Ventilatory strategies in obstructive lung disease. Semin. Resp. Crit. Care. 2014, 35, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guo, S.; He, Y.; Zuo, Q.; Liu, D.; Xiao, M.; et al. COVID-19 is distinct from SARS-CoV-2-negative community-acquired pneumonia. Front. Cell. Infect. Mi. 2020, 10, 322. [Google Scholar] [CrossRef]
- Kim, KH. Timing of musculoskeletal steroid injections in pain practice during Coronavirus disease 2019 (COVID-19) vaccine administration. Korean J. Pain. 2022, 35, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.; Poh, C.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Harcourt, J.; Tamin, A.; Lu, X.; Kamili, S.; Sakthivel, S.K.; Murray, J.; et al. Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States. Emerg. Infect. Dis. 2020, 26, 1266–1273. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.M.; Wang, Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412. [Google Scholar] [CrossRef]
- Winters, M.E.; Hu, K.; Martinez, J.P.; Mallemat, H.; Brady, W.J. The critical care literature 2020. Am J Emerg Med. 2021, 50, 683–692. [Google Scholar] [CrossRef]
- Lu, Y.; Cui, Y.; Shi, J.Y.; Zhou Y., P.; Wang, C.X.; Zhang Y., C. Efficacy of high flow nasal oxygen therapy in children with acute respiratory failure. Chin. J. Pediatr, 2021, 59, 20–26. [Google Scholar] [CrossRef]
- Petersen, L.; Friend, J.; Merritt, S. Single ventilator for multiple patients during COVID19 surge: matching and balancing patients. Crit. Care. 2020, 24, 357. [Google Scholar] [CrossRef] [PubMed]
- Holanda, M.; Pinheiro, B. COVID-19 pandemic and mechanical ventilation: facing the present, designing the future. J. Bras. Pneumol. 2020, 46, e20200282. [Google Scholar] [CrossRef]
- Urakov, A.L.; Urakova, N.; Fisher, E.L.; Yagudin, I.I.; Suntsova, D.O.; Svetova, M.D.; Shubina, Z.V.; Muhutdinov, N.M. Inhalation of an aerosol solution of hydrogen peroxide and sodium bicarbonate for the urgent recanalization of the respiratory tract after blockage by mucus and pus. JMBDD. 2022, 1, 2. [Google Scholar] [CrossRef]
- Shabanov, P.D.; Fisher, E.L.; Urakov, A.L. Hydrogen peroxide formulations and methods of their use for blood oxygen saturation. J. Med. Pharm. Allied Sci. 2022, 11, 5489–5493. [Google Scholar] [CrossRef]
- Karam, O.; Nellis, M.E. Transfusion management for children supported by extracorporeal membrane oxygenation. Transfusion. 2021, 61, 660–664. [Google Scholar] [CrossRef]
- Fan, E.; Sorbo, D.; Goligher, E.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; et al. An official american thoracic/european society of intensive care medicine/society of critical care medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef]
- Wunsch, H. Mechanical ventilation in COVID-19: Interpreting the current epidemiology. Am. J. Respir. Crit. Care Med. 2020, 202, 1–4. [Google Scholar] [CrossRef]
- Urakov, A.L. COVID-19: Original simple and cheap extrapulmonary oxygenation as an alternative to ECMO. J. Bio. Innov. 2020, 9, 648–654. [Google Scholar] [CrossRef]
- Dhamija, A.; Kakuturu, J.; Schauble, D.; Hayanga, H.K.; Jacobs, J.P.; Badhwar, V.; et al. Outcome and cost of nurse-led vs perfusionist-led extracorporeal membrane oxygenation. Ann. Thorac. Surg. 2021, 25, S0003–4975. [Google Scholar] [CrossRef]
- Fisher, E.; Urakov, A.; Svetova, M.; Suntsova, D.; Yagudin, I. COVID-19: intrapulmonary alkaline hydrogen peroxide can immediately increase blood oxygenation. Med. Cas. 2021, 55, 135–138. [Google Scholar] [CrossRef]
- Urakov, A.; Urakova, N.; Nikolenko, V.; Belkharoeva, R.; Achkasov, E.; Kochurova, E.; Gavryushova, L.; Sinelnikov, M. Current and emerging methods for treatment of hemoglobin related cutaneous discoloration: a literature review. Heliyon. 2021, 7, e059542. [Google Scholar] [CrossRef] [PubMed]
- Urakov, A.L.; Urakova, N.A. COVID-19: Optimization of respiratory biomechanics by aerosol pus solvent. Russ. J. Biomech. 2021, 25, 86–90. [Google Scholar] [CrossRef]
- Urakov, А.L.; Urakova, N.A. COVID-19: intrapulmonary injection of hydrogen peroxide solution eliminates hypoxia and normalizes respiratory biomechanics. Russ. J. Biomech. 2021, 25, 350–356. [Google Scholar] [CrossRef]
- Urakov, A.L.; Stolyarenko, A.P.; Kopitov, M.V.; Bashirov, I.I. Dynamics of the local temperature of blood, pus, mucus and catalase solution when they interact with a solution of hydrogen peroxide in vitro. Thermol. Int. 2021, 31, 150–152. [Google Scholar]
- Kasatkin, A.; Urakov, A. Effect of hydrogen peroxide on erythrocyte temperature in vitro. Chem. Biol. Interact. 2022, 109837. [Google Scholar] [CrossRef]
- Urakov, A.L. Pus solvents as new drugs with unique physical and chemical property. Rev. Clin. Pharmacol. Drug Ther. 2019, 17, 89–95. [Google Scholar] [CrossRef]
- Lombardi, C.; Gani, F.; Berti, A.; Comberiati, P.; Peroni, D.; Cottini, M. Asthma and COVID-19: a dangerous liaison? . Asthma Res Pract. 2021, 7, 9. [Google Scholar] [CrossRef]
- Schultze, A.; Walker A., J.; MacKenna, B.; Morton C., E.; Bhaskaran, K.; Brown J., P.; et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020, 8, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Adir, Y.; Saliba, W.; Beurnier, A. , & Humbert, M. Asthma and COVID-19: an update. Eur Respir Rev. 2021, 30, 210152. [Google Scholar] [CrossRef] [PubMed]
- Adir, Y.; Humbert, M.; Saliba, W. COVID-19 risk and outcomes in adult asthmatic patients treated with biologics or systemic corticosteroids: Nationwide real-world evidence. J Allergy Clin Immunol. 2021, 148, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Beurnier, A.; Yordanov, Y.; Dechartres, A.; Dinh, A.; Debuc, E.; Lescure F., X.; et al. Characteristics and outcomes of asthmatic outpatients with COVID-19 who receive home telesurveillance. ERJ Open Res. 2022, 8, 00012–2022. [Google Scholar] [CrossRef]
- Bafadhel, M.; Faner, R.; Taillé, C.; Russell R. E., K.; Welte, T.; Barnes P., J.; et al. Inhaled corticosteroids for the treatment of COVID-19. Eur Respir Rev. 2022, 31, 220099. [Google Scholar] [CrossRef]
- An T., J.; Kim, Y.; Park Y., B.; Kim, K.; Cho D., Y.; Yoo K., H.; et al. Inhaled corticosteroid is not associated with a poor prognosis in COVID-19. Respirology. 2021, 26, 812–815. [Google Scholar] [CrossRef]
- Lai C., C. The impact of inhaled corticosteroid on SARS-CoV2 infection. J Allergy Clin Immunol Pract. 2021, 9, 2939–2940. [Google Scholar] [CrossRef]
- Chen, Y. , & Li, L. Influence of corticosteroid dose on viral shedding duration in patients with COVID-19. Clin Infect Dis. 2021, 72, 1298–1300. [Google Scholar] [CrossRef]
- Griesel, M.; Wagner, C.; Mikolajewska, A.; Stegemann, M.; Fichtner, F.; Metzendorf M., I.; et al. Inhaled corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. 2022, 3, CD015125. [Google Scholar] [CrossRef]
- Paltiel A., D.; Fuhlbrigge A., L.; Kitch B., T.; Liljas, B.; Weiss S., T.; Neumann P., J.; et al. Cost-effectiveness of inhaled corticosteroids in adults with mild-to-moderate asthma: results from the asthma policy model. J Allergy Clin Immunol. 2001, 108, 39–46. [Google Scholar] [CrossRef]
- Mortimer, K.; Reddel H., K.; Pitrez P., M.; Bateman E., D. Asthma management in low and middle income countries: case for change. Eur Respir J. 2022, 60, 2103179. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, K.; Lesosky, M.; García-Marcos, L.; Asher M., I.; Pearce, N.; Ellwood, E. , et al.The burden of asthma, hay fever and eczema in adults in 17 countries: GAN phase I study. Eur Respir J. 2022, 60, 2102865. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, M.; Pati, I.; Masiello, F.; Pupella, S.; De Angelis, V. Corticosteroids use for COVID-19: an overview of systematic reviews. Infez Med. 2022, 30, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Diab, N.; Patel, M.; O'Byrne, P.; Satia, I. Narrative review of the mechanisms and treatment of cough in asthma, cough variant asthma, and non-asthmatic eosinophilic bronchitis. Lung. 2022, 200, 707–716. [Google Scholar] [CrossRef]
- Chaudhuri, R.; McMahon A., D.; Thomson L., J.; MacLeod K., J.; McSharry C., P.; Livingston, E.; et al. Effect of inhaled corticosteroids on symptom severity and sputum mediator levels in chronic persistent cough. J Allergy Clin Immunol. 2004, 113, 1063–1070. [Google Scholar] [CrossRef]
- De Vincentis, A.; Baldi, F.; Calderazzo, M.; Caliceti, U.; Guarnieri, G.; Lombardi, F.; et al. Chronic cough in adults: recommendations from an Italian intersociety consensus. Aging Clin Exp Res. 2022, 34, 1529–1550. [Google Scholar] [CrossRef]
- Riccioni, G.; Di Ilio, C.; D'Orazio, N. Review: Pharmacological treatment of airway remodeling: inhaled corticosteroids or antileukotrienes? Ann Clin Lab Sci. 2004, 34, 138–142. [Google Scholar] [PubMed]
- Ye, Z.; Wang, Y.; Colunga-Lozano L., E.; Prasad, M.; Tangamornsuksan, W.; Rochwerg, B.; Yao, L.; et al. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2020, 192, E756–E767. [Google Scholar] [CrossRef]
- Johns, M.; George, S.; Taburyanskaya, M.; Poon Y., K. A Review of the evidence for corticosteroids in COVID-19. J Pharm Pract. 2020, 35, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Tran V., T.; Mahévas, M.; Bani-Sadr, F.; Robineau, O.; Perpoint, T.; Perrodeau, E.; et al. Corticosteroids in patients hospitalized for COVID-19 pneumonia who require oxygen: observational comparative study using routine care data. Clin Microbiol Infect. 2021, 27, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Gallay, L.; Tran V., T.; Perrodeau, E.; Vignier, N.; Mahevas, M.; Bisio, F.; et al. Fourteen-day survival among older adults with severe infection with severe acute respiratory syndrome coronavirus 2 treated with corticosteroid: a cohort study. Clin Microbiol Infect. 2021, 27, 1145–1150. [Google Scholar] [CrossRef]
- Kratochvil M., J.; Kaber, G.; Demirdjian, S.; Cai P., C.; Burgener E., B.; Nagy, N.; et al. Biochemical, biophysical, and immunological characterization of respiratory secretions in severe SARS-CoV-2 infections. JCI insight, 2022, 7, e152629. [Google Scholar] [CrossRef] [PubMed]
- Walenga R., L.; Longest P., W. (2016). Current inhalers deliver very small doses to the lower tracheobronchial airways: Assessment of healthy and constricted lungs. J Pharm Sci. 2016, 105, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Hayden, R.; Hills, T.; Al-Samkari, H.; Casey, J.; Del Sorbo, L.; et al. Therapeutic advances in COVID-19. Nat Rev Nephrol. 2023, 19, 38–52. [Google Scholar] [CrossRef]
- Shaefi, S.; Brenner S., K.; Gupta, S.; O'Gara B., P.; Krajewski M., L.; Charytan D., M.; et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021, 47, 208–221. [Google Scholar] [CrossRef]
- Tran A.; Fernando S. M.; Rochwerg B.; Barbaro R. P.; Hodgson C. L.; Munshi L.; et al. Prognostic factors associated with mortality among patients receiving venovenous extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Lancet Respir Med. 2022, S2213-2600(22)00296-X. Advance online publication. [CrossRef]
- Mishra, V.; Svennevig J., L.; Bugge J., F.; Andresen, S.; Mathisen, A.; Karlsen, H.; et al. Cost of extracorporeal membrane oxygenation: evidence from the Rikshospitalet University Hospital, Oslo, Norway. Eur J Cardiothorac Surg. 2010, 37, 339–342. [Google Scholar] [CrossRef]
- Hayanga J. W., A.; Aboagye, J.; Bush, E.; Canner, J.; Hayanga H., K.; Klingbeil, A.; et al. Contemporary analysis of charges and mortality in the use of extracorporeal membrane oxygenation: A cautionary tale. JTCVS Open. 2020, 1, 61–70. [Google Scholar] [CrossRef]
- Abdollahi M.; Hosseini A. Hydrogen peroxide. Encyclopedia of Toxicology (Third Edition). 2014, 967-970. [CrossRef]
- Cervantes Trejo, A.; Castañeda I., D.; Rodríguez A., C.; Andrade Carmona V., R.; Mercado M. D. P., C.; Vale L., S.; et al. Hydrogen peroxide as an adjuvant therapy for COVID-19: A case series of patients and caregivers in the Mexico city metropolitan area. Evid Based Complement Alternat Med. 2021, 5592042. [Google Scholar] [CrossRef]
- Urakov, A.; Urakova, N.; Reshetnikov, A. Oxygen alkaline dental's cleaners from tooth plaque, food debris, stains of blood and pus: A narrative review of the history of inventions. J Int Soc Prev Community Dent. 2019, 9, 427–433. [Google Scholar] [CrossRef]
- BiCARB study group. Clinical and cost-effectiveness of oral sodium bicarbonate therapy for older patients with chronic kidney disease and low-grade acidosis (BiCARB): a pragmatic randomised, double-blind, placebo-controlled trial. BMC Med. 2020, 18, 91. [Google Scholar] [CrossRef]
- Hazard P., B.; Griffin J., P. Calculation of sodium bicarbonate requirement in metabolic acidosis. Am J Med Sci. 1982, 283, 18–22. [Google Scholar] [CrossRef] [PubMed]
| The title and number of the patent for the invention | Ingredient concentration indicators | Method of application |
|---|---|---|
| Lympho-substitute for local maintaining viability of organs and tissues in hypoxia and ischemia (RU Patent No. 2586292) | 0.01-0.02% Hydrogen peroxide 0.88% Sodium chloride 0.06-0.1% Glucose |
Intratissue injection at the site of ischemia and/or hypoxia |
| Bruise bleacher (RU Patent No. 2539380) | 0.03-0.01% Hydrogen peroxide 1.8% Sodium bicarbonate 0.25% EDTA |
Intradermal injection into the bruise area |
| Bleaching agent (RU Patent No. 2589682) | 0.01-0.03% Hydrogen peroxide 1.7% Sodium bicarbonate 0.125-0.25% Lidocaine hydrochloride 0.25% EDTA |
|
| Аgent for intradermal bruise whitening (RU Patent No. 2573382) | 0.01-0.03% Hydrogen peroxide 1.8% Sodium bicarbonate 0.001-0.05% Polysorbate |
|
| Method for skin discoloration in bruising area (RU Patent No. 2582215) | 0.03% Hydrogen peroxide 1.8% Sodium bicarbonate 0.25% EDTA Local temperature +37 - +42 °C |
|
| Method for whitening of sore under nail (RU Patent No.2641386) | 0.03% Hydrogen peroxide 1.8% Sodium bicarbonate Local temperature +37 - +42 °C |
Injection into the blood at hematoma cavity under the nail |
| Method for whitening of bruise under eye (RU Patent No. 2639283) | 0,03% Hydrogen peroxide 1,8% Sodium bicarbonate Temperature +42 °C |
Skin application |
| E.M.Soikher's hyperoxygenated agent for saturation of venous blood with oxygen (RU Patent No. 2538662) | 0.29% Hydrogen peroxide 0.85% Sodium chloride 0.10% Sodium bicarbonate |
Injection into venous donor blood |
| Aerated mouthwash (RU Patent No. 2635992) | 0.1-0.3 % Hydrogen peroxide 0.6% Sodium chloride 0.15% Sodium hydrophosphate 0.05% Sodium dihydrophosphate |
Irrigate the mouth |
| Agent for increasing resistance to hypoxia (RU Patent No. 2604129) | 0.3-0.5% Hydrogen peroxide Gas oxygen at an overpressure of 0.2 ATM |
Enteral administration |
| Energy drink (RU Patent No. 2639493) | 0.3-0.5% Hydrogen peroxide 7% Glucose 0.7% Ethyl alcohol Gas Oxygen at an overpressure of 0.2 ATM |
|
| Glass washing liquid (RU Patent No. 2763882) | 0.06-0.5% Hydrogen peroxide 0.1% Colorless detergent 0.08-0.1% Ammonia |
Spray on the dirty glass of the vehicle |
| Multipurpose solution for epibulbar instillations (RU Patent No. 2452478) | 0.55-1.0% Hydrogen peroxide 1.0-1.5% Sodium bicarbonate 0.5-1.0% Lidocaine hydrochloride |
Instillation into the conjunctival cavity |
| Methods of diagnostics and treatment of clotted hemothorax by A.Y. Malchikov (RU Patent No. 2368333) | 1.5% Hydrogen peroxide 5% Sodium bicarbonate Temperature +37 °С |
Injection inside a blood clot |
| Bleaching opener of dried blood for wrapping bandages adhered to a wound (RU Patent No. 2653465) | 0.75-1.0% Hydrogen peroxide 1.2% Sodium bicarbonate 0.5% Lidocaine hydrochloride |
Wetting bandages stuck to the wound |
| Frictional toothpaste (RU Patent No. 2626669) | 0.5-1.5% Hydrogen peroxide 9.5-10% Sodium bicarbonate |
Wiping the surface of teeth in the mouth |
| Method of extrapulmonary blood oxygenation (RU Application No. 2020120367, 15.12.2021) | 3% Hydrogen peroxide Gas Oxygen under excess pressure 0.2-0.8 ATM Temperature +36-+42 °С |
Enteral administration |
| Method of newborn resuscitation in asphyxia (RU Application No. 2021103789, 15.08.2022) | 3% Hydrogen peroxide 1.8% Sodium bicarbonate Temperature +37-+42 °С |
Intrapulmonary injection |
| Method of emergency intrapulmonary blood oxygenation for COVID-19 (RU Application No. 2021114105 from 18.05.2021) | 3% Hydrogen peroxide 1.8% Sodium bicarbonate |
Intrapulmonary injection |
| Lung oxygenation method for COVID-19 (RU Application No. 2021102618, 04.08.2022) | 3% Hydrogen peroxide 1.8% Sodium bicarbonate Temperature +37-+42 °С |
Intrapulmonary injection |
| Softening agent for thick and viscous pus (RU Patent No. 2360685) | 2.7-3.3% Hydrogen peroxide 5.0-10.0% Sodium bicarbonate |
Injection into a mass of thick pus |
| Hyper-gassed and hyper-osmotic antiseptic mixture (RU Patent No. 2331441) | 2.7-3.3% Hydrogen peroxide 2.0-10.0% Sodium chloride Gas Carbon dioxide an overpressure of 0.2 ATM |
|
| Method and means for removing sulfur plug (RU Patent No. 2468776) | 2.7-3.3% Hydrogen peroxide 2.0-10.0% Sodium chloride Gas Carbon dioxide an overpressure of 0.2 ATM |
|
| Method of using plaque removal solution with irrigation agent (RU Patent No. 2723138) | 2.7-3.3 % Hydrogen peroxide 2.0-10.0 % Sodium bicarbonate Gas Argon at equilibrium pressure of 3-4 ATM Temperature +43 - +65 °C |
Pour into the container of a dental irrigator, and from it into the oral cavity |
| Means for intravital skin whitening near blue eyes (RU Patent No. 2639485) | 3±0.3% Hydrogen peroxide Sodium bicarbonate in an amount that ensures precipitation at a temperature of +45 °C 2.0% Lidocaine Hydrochloride |
Application to the skin in the bruise area |
| A way of treating long-term non-healing wounds (RU Patent No. 2187287) | 3% Hydrogen peroxide Temperature +37 - +42 °C |
Irrigation of the wound surface |
| Bleaching cleanser of dentures (RU Patent No. 2659952) | 3.0±0.3% Hydrogen peroxide 2.0-10.0% Sodium bicarbonate Gas Oxygen under excess pressure 0.2 ATM Local temperature +37 - +42 °C |
Baths for whitening dentures |
| Descolorant of blood (RU Patent No. 2647371) | 3±0.3% Hydrogen peroxide ≥10% Sodium bicarbonate Local temperature +42 °C |
Rehabilitation of wounds and cavities |
| The method of bleaching a bruise under the nail (RU Patent No.2631592) | 3% Hydrogen peroxide 10% Sodium bicarbonate Local temperature +37 - +42 °C |
Injection into the hematoma cavity under the nail |
| Means for physical endurance increase (RU Patent No. 2634271) | 3% Hydrogen peroxide 7% Glucose Gas oxygen at an overpressure of 0.2 ATM |
Enteral administration |
| Method of emergency bleaching of skin hematoma under eye (RU Patent No. 2679334) | 3% Hydrogen peroxide 10% Sodium bicarbonate Local temperature +37 - +42 °C |
Injection into the cavity of a hematoma under the eye |
| Method for emergency bleaching and blood crust removal from skin in place of squeezed out acne (RU Patent No. 2631593) | 3% Hydrogen peroxide 10% Sodium bicarbonate |
Application to the skin in the acne area |
| Method of express cleaning of blood stains off clothes (RU Patent No. 2371532) | 3% Hydrogen peroxide 4% Sodium bicarbonate |
Impregnation of textile fabrics |
| Uterine lavage technique (RU Patent No. 2327471) | 3% Hydrogen peroxide 0.9% Sodium chloride Local temperature +42 - +45 °C |
Injection into the blood inside the uterus for uterine bleeding |
| Uterine lavage technique (RU Patent No. 2327471) | 3% Hydrogen peroxide 0.9% Sodium chloride Local temperature +42 - +45 °C |
Injection into the blood inside the uterus for uterine bleeding |
| Method of maintenance of live fish during transportation and storage (RU Patent No. 2563151) | 6% Hydrogen peroxide | Injection into water with fish in a single dose of 0.2mL/kg of fish |
| Peeling agent for foot hyperkeratosis (RU Patent No. 2730451) | 0.5-20% Hydrogen peroxide 3.0-5.0% Potassium hydroxide Gas Oxygen an overpressure of 0.2 ATM pH 13.0-14.0 Osmotic activity 350-560 mosmol/L of water Local temperature +38 - +42 °C |
Irrigation of the feet with baths |
| Aerosol for inhalations in obstructive bronchitis (RU Patent No.2735502) | 0.3-0.5% hydrogen peroxide 1.2% sodium bicarbonate 0.5% lidocaine hydrochloride pH 8.5 Osmotic activity 280–300 mosmol/L of water Local temperature +41 – +55 °C |
Aerosol for inhalation |
| Aerosol for invasive mechanical ventilation in COVID-19 (RU Patent No.2742505) | 0.3-0.5% hydrogen peroxide 2-10% sodium bicarbonate 0.5% lidocaine hydrochloride and the rest water for injection pH 8.5, Osmotic activity 370-1990 mosmol/L of water Local temperature +37 - +55 °С |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





