1. Introduction
Musculoskeletal complications related to diabetes are recognized as being common and can lead to severe morbidity (1). More recent evidence highlights that limited joint mobility in patients with diabetes represents a population at risk for the development of early microvascular complications and hospitalisation (2,3). Besides, Gokcen et. al. have drawn our attention to musculoskeletal impairment in patients with prediabetes and indicated that the frequency of cheiroartropathy was 47.3% in prediabetes, and 28,8% in type 1 diabetes. They defined the musculoskeletal impairment of muscle, bone, and joint as an overlooked rheumatologic manifestation of diabetes (4). Fatemi et. al. reported that musculoskeletal manifestations in patients with prediabetes were as high as in patients with diabetes (5).
Children with diabetes may experience limited joint mobility, mainly due to the deteriorating effects of advanced glycation end-products (AGEs) on connective tissue (6). Temporomandibular joint, as a synovial joint, is subject to the same conditions involving other synovial joints, including type 1 diabetes. However, our knowledge on TMJ findings in type 1 diabetes patients is based on a single article. Collin et. al. has suggested that the prevalence of temporomandibular joint dysfunction (TMD) increased in patients with diabetes. Remarkably, peripheral neuropathy was identified to be a risk factor for severe TMD (7). In a type 2 spontaneous diabetes mellitus rat model study, thickness of the articular disc and diameter of retrodiscal capillaries of the TMJ were found to be significantly less (8). These findings suggest that TMD might be associated with diabetes, presumably due to the microvascular damage. Oles and Lexington reported that reactive hypoglycemia in children may be a significant factor associated with development of the TMD but they failed to evaluate mandibular movements (9). Despite the importance of musculoskeletal deterioration in type 1 diabetes, there remains a paucity of evidence on highly possible relation between TMD and type 1 diabetes.
Findings on the investigation of the incidence of TMD in children with type 1 diabetes and the assessment of the relation between TMD, type 1 diabetes and other influencing factors will provide us a comprehensive understanding of the epidemiological characteristics of TMD in children with type 1 diabetes and may encourage designing a program directed at the diagnosis, prevention, and treatment of that complication. The objectives of the present study were to evaluate the incidence of TMD in children with type 1 diabetes and to make a correlation between that incidence and clinical and laboratory parameters.
2. Materials and Methods
2.1. Study design and research population
The study design was cross-sectional. All consecutive patients with type 1 diabetes and aged 6-21 years treated at Istanbul University, Department of Pediatrics, Division of Endocrinology and Metabolism outpatient clinic, were asked to participate in the study between February 2019 and January 2020. Exclusion criteria were as follows: (i) presence of neurological disorders, (ii) presence of rheumatologic disorders, (iii) history of trauma which caused luxation or fracture or surgical operation to the mandible or TMJ.
All subjects had given written informed consent to their participation in the study, and the Institutional Review Board for Human Studies of Istanbul University, Istanbul Medicine Faculty had approved the study protocol (No: 2019/774).
2.2. Patients evaluation and data collection
The demographic details of patients including age, gender, body weights, heights, bone-age were recorded. The weights and heights were converted to standard deviation scores (SDS) according to the national standards (10, 11).
A detailed medical history was provided from all patients, which included duration of disease (defined as months since diagnosis) and complications i.e. ketoacidosis, gastroparesis, retinopathy, history of consanguineous marriage, as well as other medical diagnoses. Laboratory data included last four measurement of glycosylated hemoglobin (HbA1c (%)(mmol/mol)), vitamin B12, 25 OH Vitamin D, calcium (Ca), phosphorus (P), parathyroid hormone (PTH), alkaline phosphatase (ALP), microalbumin levels. Cheiroarthropathy was characterized according to physical examination. Positive prayer sign classified a subject as having cheiroartropathy. Prayer sign was referred to incomplete approximation of sides of opposite hands when the patient opposes palms and digits as if praying. The limitation of range of motion in other joints of the upper and lower extremity (shoulder, knee, elbow, ankle) and limitation of extension in the spine were also evaluated. These clinical examinations and data collection were conducted by a single examiner (A.P.O.).
2.3. Examination of TMJ
All patients were subjected to a standard dentofacial examination to identify signs or symptoms of TMJ involvement. The assessment included bilateral palpation of the masseter and temporal muscles along with the lateral parts of the TMJs. The interviewer asked the child to report whether his or her face hurt during palpation, whether the child heard a noise when opening the mouth wide. The answers marked as yes-no format.
The maximum mouth opening was recorded by measuring the vertical interincisal distance (the distance between the incisal edges of the upper and lower central incisors). The maximum lateral excursions were defined as right and left displacement of the lower incisors’ midline from the maxillary midline. The protrusive movement was calculated by measuring the horizontal distance between the upper and lower incisors during full closure and adding the distance the lower incisors traveled beyond the upper incisors. The Turkish version of the Short Fonseca Anamnestic Questionnaire was utilized and implemented (12, 13, 14). The participants were asked to respond to 10 questions. They were also asked to choose a single answer per question, with 10 for “yes,” 5 for “sometimes,” and 0 for “no.” After adding the scores together, respondents were classified as, No TMD (0–15), Mild TMD (20–40), Moderate TMD (45–65), or Severe TMD (70–100). The collection of TMJ data was performed by a single examiner (A.G.)
2.4. Statistical analysis
The sample size was calculated by expecting a statistically significant difference between the active maximum interincisal opening value measured in children with systemic disease with and without TMJ involvement. The sample size required for an α = 0.01 and 95% power meant 28 participants (15).
The data were analyzed using The IBM SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). The normality of the variables was checked using the Kolmogorov Smirnov test and the assumption of normality was provided. Categorical variables were compared using the χ2test. Student's t test and ANOVA were used for evaluation of continuous variables, and Tamhane's T2 tests was conducted for post-hoc evaluations after ANOVA. Patients were further subgrouped into with TMJ dysfunction (TMD+) and without dysfunction (TMD-) according to presence of at least a single TMJ symptom. Subgroup analyses were performed using the χ2 test to evaluate whether there were differences in demographic, clinical and laboratory parameters between patients with and without TMD. The correlations between continuous variables were carried out using Pearson’s correlation test. Results with p values less than 0.05 were considered as statistically significant.
3. Results
3.1. Patient characteristics
Descriptive statistics for the measurements obtained from the whole sample investigated were given in
Table 1. One hundred and one patients (51 female, 50 male) with type 1 diabetes were enrolled in this study. 50.49% of patients were female. Of the patient population, 23 (22.77%) were from consanguineous marriages. Almost half of the patients’ (48.51%) bone age assessment was recorded and the remaining records’ were not available. The mean bone age of these was 11.73 ± 3.87 years. The median HbA1c values of the patients was 8.95 ± 1.52% (74 ± 4 mmol/mol) with a mean duration of the type 1 diabetes as 71.2 ± 57.7 (range 1-360) months. Of the patients, just over half (54.45%) had a history of ketoacidosis. One of the patients had retinopathy and, none had a history of gastroparesis.
The baseline laboratory assays for the overall cohort are as follows: 1) 82.17%, 91.08%, 94.05%, and 82.17% of patients had serum Vitamin B12, Ca, PTH, and ALP levels within target levels, respectively; 2) The majority of patients (77.22%) had vitamin D deficiency; 3) From 101 subjects in the cohort, 17 (16.83%) had microalbuminuria; 4) 39.60% of the patients had higher P levels than target levels.
Of the patients, 26 (26.26%) had a chronic disease with type 1 diabetes. Of those, 50% had autoimmune thyroiditis, 23.07% had celiac disease, 3.84% had familial mediterranean fever, 3.84% had selective IgA deficiency, 3.84% had asthma, 3.84% had secondary hypothyroidism, 3.84% had hypertension, 3.84% had idiopathic aplastic anemia, 3.84% had Mauriac syndrome.
3.2. Physical examination findings
Cheiroarthropathy and limitation on the spine were determined only in four patients (3.96%). Of these patients, two were TMD+ presented as the pain in the masseter muscles and TMJ sounds. Since the sample size was very limited, the comparison of demographic and clinical variables could not be carried out. No limitation of range of motion in other joints of the body was detected in all patients. Subjects presented cheiroarthropathy were considered too few to analyze separately.
3.3. TMJ findings
In the patient group, 35 patients (34.65%) presented at least one clinical finding or symptoms defined as TMD+, 66 (65.34%) presented no dysfunction defined as TMD-. 13.9%, 12.9% and 8.9% of the children reported that their face hurt during palpation on temporal muscle, masseter muscle and condylar head area, respectively. 16.8% of the patients showed and reported that they heard a noise (clicking) when they opened their mouth wide. The most commonly reported symptom in patients was “TMJ sounds”, present in 14 (16.8%) children with type 1 diabetes overall, and in the 42.85% of those that reported an associated pain at masseter muscle, 28.57% reported temporal pain and 14.28% reported pain in the condylar area. Moreover, 11.88 % of the symptomatic patients reported more than one TMJ symptom, 6.93% reported two of these symptoms, 2.97% reported three symptoms and 1.98% reported all four.
Occlusal relationship distribution in the patient group was 62.4% Class I, 17.8% Class II, 19.8% Class III. Further post hoc analysis revealed that the patients who had Class II occlusion showed significantly lower maximum mouth opening than the patients with normal occlusion (95% CI: 0.82-9.57, p=0.02).
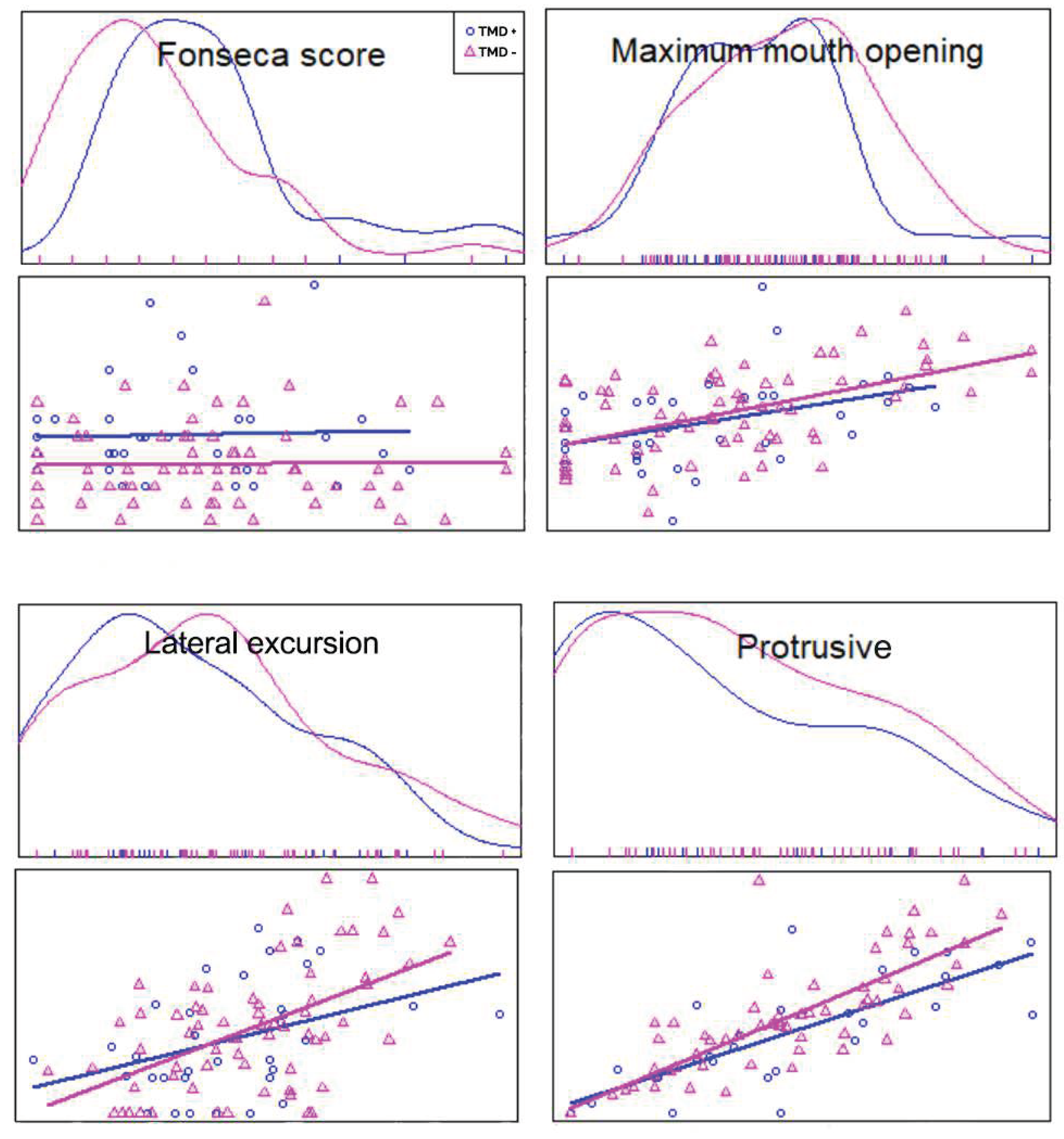
On average, the patients presented 20 ± 13.89 mean Fonseca score which was referred to mild TMD. Additionally, TMD+ group (25.71±14.71) and TMD- group (16.82±12.48) showed a statistically significant difference for the Fonseca scores (95% confidence interval [CI]: 3.38-14.4, p=0.000) (
Table 2,
Figure 1). The patients with TMD had greater Fonseca scores.
3.4. Relation of TMD to growth
Mean height SDS levels were -0.231.14 (
Table 1). In subjects with TMD (-0.421.16) compared to those without TMD (-0.131.12), height SDS was also lower (95% CI: -0.18-0.76, p<0.001) (
Table 2).
3.5. Relation of TMD to age and gender
The prevalence of TMD was not different in males compared to females. The mean age of patients with TMD did not show a significant difference from patients without TMD (
Table 1).
3.6. Relation of mandibular movements to type 1 diabetes
The mean maximal mouth opening in the TMD+ group was 34.51 ± 8.21 with respect to 36.32 ± 8.12 in the TMD- group. From the patients reported symptoms, there was statistically significant difference between the TMD+ and TMD- for maximal mouth opening (p = 0,000). However, no significant difference was found between TMD+ and TMD- groups in terms of lateral and protrusive excursions (
Table 2,
Figure 1).
3.7. Relation between TMD and HbA1c
The mean HbA1c was 9.15 ±1.65% (76 ± 4 mmol/mol) for TMD+ group, and 8.85 ±1.44% (73 ± 4 mmol/mol) for TMD- group. HbA1c levels of patients with TMD did not reveal any significant differences from patients without TMD (
Table 2). Also, no correlation was detected between Fonseca scores and mandibular movements and HbA1c.
3.8. Relation between TMD and diabetes age
Overall no difference existed in the mean duration of the type 1 diabetes disease between subjects with and without TMD (73.51±55.79 and 70.03±59.14, respectively) (
Table 2). There was no significant correlation between diabetes duration and Fonseca scores and mandibular range of motion.
3.9. Relation between concomitant diseases, HbA1c and TMJ movements
It was determined that the limitation in right and left lateral movements increased significantly in the presence of accompanying diseases (95% CI: 0.35-3.39, p=0.02; 95% CI: 0.04-2.84, p=0.04, respectively). No significant correlation exists between the TMJ movements, HbA1c levels and the presence of concomitant diseases.
3.10. Relation between TMD and serum levels
Mean serum phosphorus level was significantly lower in patients with TMD than patients without TMD (95% CI: 0.37-0.76, p=0.03) (
Table 2). Although mean serum microalbumin level in TMD+ group was slightly higher than normal range and values obtained in TMD- group, the difference was not statistically significant. Mean Vitamin B, 25 OH Vitamin D, Ca, PTH and ALP levels of patients with TMD did not show a significant difference from patients without TMD (
Table 2).
3.11. Relation between serum levels, HbA1c and TMJ movements
A significant correlation was found between the HbA1c and vitamin B12 levels, and between the HbA1c and Ca levels of the patients (95% CI: -0.4—0.03; coefficient= -.227; p = 0.026 and 95% CI: 0.1—0.46; coefficient= .295; p = 0.003, respectively). Furthermore, a nearly significant correlation was found between HbA1c and 25OH vitamin D levels (95% CI: -0.05—0.37; coefficient= -.191; p = 0.058). Regarding mandibular movements, the laboratory findings showed significant correlation only between left lateral movement and vitamin B levels (95% CI: -0.38—0.007; coefficient= -.202; p = 0.047).
4. Discussion
Granted that a great proportion of type 1 diabetes patients present with cheiroarthropathy, TMJ findings in type 1 diabetes are given far less attention than in other joints. This study analyzed the incidence of symptoms and signs of TMD in 101 children with type 1 diabetes. Overall incidence of TMD was 34.65% in this cohort. This result was further validated by a mean Fonseca score of 20 ± 13.89, which indicates mild TMD.
As expected, the patients with type 1 diabetes exhibited diminished inter-incisor distance, which is considered the most reliable clinical assessment in children (16). In a study evaluating the maximum mouth opening, right and left lateral and protrusive excursions, the average values of 6-year-old children were reported as 39.44 ± 4.42, 8.05±2.02, 7.77±1.65, 7.11±2.65, respectively (17). Another study reported that the mandibular range of motion of children aged 10-17 years with maximum opening of < 43 mm and laterotrusive movements < 8 mm or protrusive movements < 5 mm might be considered as being limited (18). According to the results of our study, all mandibular range of motion items were restricted in both TMD+ and TMD- children with type 1 diabetes. The limitation in the mandibular range of motion points crucial evidence that TMD in children with type 1 diabetes should not be overlooked.
Mouth opening capacity increases smoothly with age but shows a wide range within children of the same age group (17 - 19). Landtwing found a considerable range of maximum mouth opening starting with 34 mm to 55 mm for 5-year-old children and ending with 36 mm to 65 mm for 18-year-old subjects (20). In our study, we did not assign the children based on their age group, as this study included all the children that were investigated in the same center. The variability in the maximum amount of mouth opening of the children, even within the same age group, may have eliminated the potential bias that may have arisen in the present study due to not grouping based on age.
According to the literature, the prevalence of cheiroarthropathy has been found to range from 8% to 66% in type 1 diabetes (21, 22). Moreover, it was demonstrated that cheiroarthropathy is particularly common in the hands and shoulders and this effect possibly exceeds the prevalence of microvascular complications such as retinopathy, nephropathy, neuropathy, and cardiovascular disease (21). In the present study, objective measurement of the inter-incisor distance revealed that maximal mouth opening was decreased, and the mean Fonseca scores addressed mild TMJ dysfunction in children with type 1 diabetes. Interestingly, 35 patients (34.65%) presented TMJ dysfunction while cheiroarthorpathy and limitation on the spine were determined only in four patients (3.96%). This would appear to indicate that TMD is common in patients with type 1 diabetes and possibly exceeds the prevalence of cheiroarthropathy.
Previous data described an association between limited joint mobility and age and gender and short stature (2,6,23). On the contrary, Sousa et. al. supported that regarding the influences of the mandibular range of motion, age had a smaller influence than height and weight (17). The present study did not find a significant difference in age and gender between patients with TMD+ and TMD-. For this reason, we did not subgroup the participants based on their age and gender. However, in subjects with TMD compared to those without TMD, the only difference was found at height SDS. Thus, considering the significant decrease in height SDS values in TMD+ children, it is suggested that, for the diagnosis of TMD, specifically for clinical practice, height SDS may be considered.
In a large longitudinal study, lower height SDS in subjects with limited joint mobility presumed as the determinants of risk for the development of microalbuminuria (2). In a recent study, type 1 diabetes patients with microalbuminuria showed a more severe deterioration of joint mobility over a period of 15 years (24). Additional studies involving adults with type 1 diabetes have confirmed the association of limited joint mobility with microalbuminuria (6,25,26). In the current study, although not statistically significant, the mean level of serum microalbumin was slightly elevated in type 1 diabetes patients with TMD compared to patients without TMD. Future studies on the current topic are therefore needed to elucidate the relation between the development of TMD and microalbuminuria.
The studies evaluating the relation between the prevalence of limited joint mobility and poor glycemic control or diabetes duration present inconsistent data (2,4,21,23). Although several cross-sectional studies suggested that the most significant variables in the development of limited joint mobility are the duration of diabetes and glycemic control, it has been noted that if the time of appearance of joint changes could be determined, attained age was of greater importance (23). This finding further verified by a large longitudinal Oxford Regional Prospective Study (2,23). The present data showed that there is no relation between glycemic control and TMD. This substantiates previous findings in the literature. The studies that were unable to correlate the joint changes with diabetes control did conclude that age is a more important factor than diabetes control and duration. At that point, the risk for developing limited joint mobility was also found to be associated with pubertal onset (2,23). Rosenbloom et. al. reported that regardless of the duration of diabetes, joint stiffness usually appears during the second decade of life. Inconsistent with that, our cohort with TMD had a mean age of 13.93±3.86 years. It was also stated that the progression from mild to moderate or severe limitation averages two years (23). Thus, clinicians should consider that the second decade of life and regular follow-up of TMJ symptoms have great importance regardless of diabetes duration and glycemic control.
The most frequently recorded symptom in type 1 diabetes patients was “TMJ sounds”, presented in 16.8% of children, which is consistent with the literature, and in the 42.85% of those that reported an associated pain at masseter muscle, 28.57% reported temporal pain and 14.28% reported pain in the condylar area (24, 25). According to our experience, patients do not have considerably discomfort due to TMD and therefore tended to underrate the symptoms. However, TMJ sounds can be a symptom of an underlined pathological condition including disc displacement, deformity of articular disc, condylar subluxation, fibrous bands, or adhesions (26). An implication of these findings is the necessity of identifying TMD in type 1 diabetes patients.
The evidence from the current study demonstrated that patients with type 1 diabetes experience TMD in a fairly high percentage. Moreover, the present study indicated that type 1 diabetes children presented difficulty mostly in mouth opening. Other authors found percentages of TMD symptoms and signs between 10% and 25% among the children in their studies (27, 28). Our incidence data, which was higher than the range reported in the literature, raises the question whether a child’s diabetic condition might be an explanatory factor. However, since there is no study evaluated prevalence of TMD in children with type 1 diabetes, we cannot compare our results. It can be postulated that TMD may be a serious issue among children with type 1 diabetes. Temporomandibular joint dysfunction may clinically emerge as pain and functional limitations and may affect the ability to perform routine daily activities; however, patients and healthcare practitioners may not be aware of these avoidable complications (4, 29). For example, clinical care guidelines such as the American Diabetes Association’s Clinical Practice Recommendations recommend regular and frequent screening of retinopathy, neuropathy, nephropathy, and cardiovascular system, but there is no description of musculoskeletal disorders and no recommendations for routine examination of TMJ (30).
The results of our study have two major limitations. First, we did not use imaging techniques to establish TMD; instead, we classified children with type 1 diabetes with clinically established TMJ involvement based on the TMJ screening protocol. Further studies, which take magnetic resonance imaging into account, will need to be performed for differential diagnosis. The second limitation is, we assessed TMD by gathering information from both clinical examination and self-reported questions. Implementing a self-explanatory questionnaire in children for this research study may be a limitation due to the age group studied and may decrease the accuracy. However, if we didn`t collect such data, it would be difficult to compare these findings with the results in the existing literature regarding TMD. Also, it was noted that the questionnaires are valid and reliable for use in children with signs and symptoms of temporomandibular disorder (31, 32). A possible method to overcome the bias is to implement a longitudinal study and compare the patients with themselves. On the other hand, the strength of the present study lies in the sample size that was large enough to compare the findings between the groups. Although we acknowledge the TMJ is structurally a much different joint than other synovial joints we have referenced, evaluation of comprehensive set of variables that were assessed by clinical examination, laboratory assays and medical history enabled us to illustrate the effect of the type 1 diabetes on TMJ and provide some relevant criteria regarding the severity of disease. We believe that these findings will contribute to better understanding of the natural history of the disease and will contribute to advances in the implementation of more efficient diagnosis and control strategies.
5. Conclusions
In conclusion, using this well-phenotyped cohort of patients with type 1 diabetes, we have demonstrated that TMD is common, and its prevalence is likely to exceed the incidence of cheiroarthropathy. Therefore, patients suffering from type 1 diabetes should also be evaluated in terms of TMD seeing more frequently than the rheumatologic diseases on upper and lower extremity. The present study further suggests that the affection of TMJ could not be predicted based on the onset time of type 1 diabetes and glycemic control. The low height SDS may be of assistance to diagnose TMD. The importance of TMJ examinations in the overall clinical assessment of the pediatric patient should not be overlooked. Ultimately, understanding the characteristics of TMD in type 1 diabetes children should help to encourage the examination of TMJ in the diabetes clinics.
Author Contributions
A.G. collected the data and wrote the manuscript, A.P.O collected the data, M.G.G. and E.Y. contributed to statistical analysis, F.B. researched data and reviewed/edited the manuscript, Ö.D.O. researched data and reviewed/edited the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board for Human Studies of Istanbul University, Istanbul Medicine Faculty (No: 2019/774).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank to Duygu Kaya for her assistance with the collection of data and the subjects for their participation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gerrits, E.G. Limited joint mobility syndrome in diabetes mellitus: A minireview. World J Diabetes. 2015, 6, 1108. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Bahu, T.K.; Widmer, B.; Dalton, R.N.; Dunger, D.B. Longitudinal relation between limited joint mobility, height, insulin-like growth factor 1 levels, and risk of developing microalbuminuria: The Oxford Regional Prospective Study. Arch Dis Child. 2005, 90, 1039–1044. [Google Scholar] [CrossRef]
- Mineoka, Y.; Ishii, M.; Hashimoto, Y.; Hata, S.; Tominaga, H.; Nakamura, N.; et al. Limited joint mobility of the hand correlates incident hospitalisation with infection in patients with type 2 diabetes. Diabetes Res Clin Pract. 2020, 161, 108049. [Google Scholar] [CrossRef]
- Gokcen, N.; Cetinkaya Altuntas, S.; Coskun Benlidayi, I.; Sert, M.; Nazlican, E.; Sarpel, T. An overlooked rheumatologic manifestation of diabetes: Diabetic cheiroarthropathy. Clin Rheumatol. 2019, 38, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, A.; Iraj, B.; Barzanian, J.; Maracy, M.; Smiley, A. Musculoskeletal manifestations in diabetic versus prediabetic patients. Int J Rheum Dis. 2015, 18, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, A.L. Limited joint mobility in childhood diabetes: Discovery, description, and decline. J Clin Endocrinol Metab. 2013, 98, 466–473. [Google Scholar] [CrossRef]
- Collin, H.L.; Niskanen, L.; Uusitupa, M.; Töyry, J.; Collin, P.; Koivisto, A.M.; et al. Oral symptoms and signs in elderly patients with type 2 diabetes mellitus: A focus on diabetic neuropathy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000, 90, 299–305. [Google Scholar] [CrossRef]
- Uemura, M.; Toda, I.; Kawashima, W.; Yoshimoto, G.; Fang, Y.R.; Xu, Y.J.; et al. Morphological study of the articular disc and capillary of the retrodiscal tissue in a type 2 spontaneous diabetes mellitus rat model. Okajimas Folia Anat Jpn. 2015, 92, 53–59. [Google Scholar] [CrossRef]
- Oles, R.D. Glucose intolerance associated with temporomandibular joint pain-dysfunction syndrome. Oral Surgery, Oral Med Oral Pathol. 1977, 43, 546–553. [Google Scholar] [CrossRef]
- Neyzi, O.; Bundak, R.; Gokcay, G.; Gunoz, H.; Furman, A.; Darendeliler, F.; Bas, F. Reference Values for Weight, Height, Head Circumference, and Body Mass Index in Turkish Children. J Clin Res Pediatr Endocrinol. 2015, 7, 280–293. [Google Scholar] [CrossRef]
- Demir, K.; Konakci, E.; Ozkaya, G.; Kasap Demir, B.; Ozen, S.; Aydin, M.; Darendeliler, F. New Features for Child Metrics: Further Growth References and Blood Pressure Calculations. J Clin Res Pediatr Endocrinol. 2020, 12, 125–129. [Google Scholar] [CrossRef]
- Da Fonseca, D.M.; Gerson, B.; do Valle, A.L.; de Freitas, S.F.T. Diagnóstico pela anamnese da disfunção craniomandibular. RGO (Porto Alegre). 1994, 23–28.
- Stasiak, G.; Maracci, L.M.; de Oliveira Chami, V.; Pereira, D.D.; Tomazoni, F.; Bernardon Silva, T.; Ferrazzo, V.; Marquezan, M. TMD diagnosis: Sensitivity and specificity of the Fonseca Anamnestic Index. Cranio 2020, 27, 1–5. [Google Scholar] [CrossRef]
- Besime Ahu Kaynak, DDS, Serkan Taş, PhD, Yasemin Salkın, MSc, PT The accuracy and reliability of the Turkish version of the Fonseca anamnestic index in temporomandibular disorders Cranio. 2020.
- De Sonnaville WFC et., al. Mandibular range of motion in children with juvenile idiopathic arthritis with and without clinically established temporomandibular joint involvement and in healthy children; a cross-sectional study. Pediatr Rheumatol Online, J. 2021, 19, 106. [Google Scholar] [CrossRef]
- Stoustrup, P.; Twilt, M.; Spiegel, L.; Kristensen, K.D.; Koos, B.; Pedersen, T.K.; et al. Clinical orofacial examination in juvenile idiopathic arthritis: International consensus-based recommendations for monitoring patients in clinical practice and research studies. J Rheumatol. 2017, 44, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Sousa L M et., al. Evaluation of mandibular range of motion in Brazilian children and its correlation to age, height, weight, and gender. Braz Oral Res 2008, 22, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, C.; John, M.T.; Lautenschläger, C.; List, T. Mandibular jaw movement capacity in 10-17-yr-old children and adolescents: Normative values and the influence of gender, age, and temporomandibular disorders. European journal of oral sciences 2006, 114, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; van Waes, H.; Langerweger, C.; Molinari, L.; Saurenmann, R.K. Maximal mouth opening capacity: Percentiles for healthy children 4-17 years of age. Pediatr Rheumatol Online J. 2013, 11, 17. [Google Scholar] [CrossRef]
- Landtwing, K. Evaluation of the normal range of vertical mandibular opening in children and adolescents with special reference to age and stature. J Maxillofac Surg. 1978, 6, 157–162. [Google Scholar] [CrossRef]
- Larkin, M.E.; Barnie, A.; Braffett, B.H.; Cleary, P.A.; Diminick, L.; Harth, J.; et al. Musculoskeletal complications in type 1 diabetes. Diabetes Care. 2014, 37, 1863–1869. [Google Scholar] [CrossRef]
- Bañón, S.; Isenberg, D.A. Rheumatological manifestations occurring in patients with diabetes mellitus. Scand J Rheumatol. 2013, 42, 1–10. [Google Scholar] [CrossRef]
- Rosenbloom, A.L.; Silverstein, J.H.; Lezotte, D.C.; Riley, W.J.; Maclaren, N.K. Limited joint mobility in diabetes mellitus of childhood: Natural history and relationship to growth impairment. J Pediatr. 1982, 101, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Egermark-Eriksson, I.; Carlsson, G.E.; Ingervall, B. Prevalence of mandibular dysfunction and orofacial parafunction in 7-, 11- and 15-year-old Swedish children. Eur J Orthod. 1981, 3, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.M.; Fu, M.K.; Hägg, U. Prevalence of temporomandibular joint dysfunction (TMJD) in Chinese children and adolescents. A cross-sectional epidemiological study. Eur J Orthod. 1995, 17, 305–309. [Google Scholar] [PubMed]
- Tatlı, U.; Machon, V. Internal Derangements of the Temporomandibular Joint: Diagnosis and Management. In InTech Books; Intech, 2017; p. 48. [Google Scholar]
- Kobayashi et., al. Evaluation of oro-facial function in young subjects with temporomandibular disorders. J Oral Rehabil. 2014, 41, 496–506. [Google Scholar] [CrossRef]
- Inglehart, Marita, R. ; et al. "Self-reported temporomandibular joint disorder symptoms, oral health, and quality of life of children in kindergarten through grade 5: Do sex, race, and socioeconomic background matter?.". The Journal of the American Dental Association 2016, 147, 131–141. [CrossRef]
- Jedel, E.; Carlsson, J.; Stener-Victorin, E. Health-related quality of life in child patients with temporomandibular disorder pain. Eur J Pain. 2007, 11, 557–563. [Google Scholar] [CrossRef]
- Johnson, E.L.; Feldman, H.; Butts, A.; Chamberlain, J.; Collins, B.; Doyle-Delgado, K.; et al. Standards of medical care in diabetes—2020 abridged for primary care providers. Clin Diabetes. 2020.
- Barbosa, T.S.; Leme, M.S.; Castelo, P.M.; Gavião, M.B. Evaluating oral health-related quality of life measure for children and preadolescents with temporomandibular disorder. Health Qual Life Outcomes 2011, 9, 10–38. [Google Scholar] [CrossRef]
- De Santis, T.O.; Motta, L.J.; Biasotto-Gonzalez, D.A.; et al. Accuracy study of the main screening tools for temporomandibular disorder in children and adolescents. J Bodyw Mov Ther. 2014, 18, 87–91. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).