1. Introduction
Decellularized pericardium is a type of tissue that has been stripped of its cellular components, leaving only the extracellular matrix [
1]. This process is typically performed using chemical or mechanical methods. Decellularized pericardium is utilized as a scaffold for tissue engineering and regenerative medicine applications, such as repairing or replacing damaged heart tissue [
2]. The extracellular matrix acts as a template for the growth and differentiation of new cells, which can be seeded onto the decellularized tissue before implantation [
3].
Decellularized pericardium is considered a promising option for tissue engineering and regenerative medicine, but like any new technology, there are potential complications that must be taken into account [
4]. Some of these potential complications include rejection, tissue remodeling, and infection [
4].
The use of antibiotics in decellularized tissues, like decellularized pericardium, is a mean to reduce the risk of infection and promote healing after implantation [
5]. The decellularization process removes all cells and DNA, leaving behind extracellular matrix scaffold that can be impregnated with antibiotics [
6]. The antibiotics can be incorporated into the tissue before, during, or after decellularization [
7]. There are several advantages to using antibiotic-impregnated decellularized tissues. Antibiotics can help prevent bacterial growth and lower the risk of infection following implantation. They can also assist in reducing inflammation and promoting tissue repair, leading to improved healing outcomes [
8]. Some antibiotics, like gentamicin, can remain active in the tissue for an extended period, providing prolonged protection against infection. Different antibiotics can be used depending on the specific needs of the patient, and the concentration can be tailored to achieve the desired effect [
9]. It's important to note that while antibiotics can be effective in preventing infection, they can also cause side effects and contribute to antibiotic resistance if overused.
Silver nanoparticles (AgNPs) have been researched for its antimicrobial properties and potential use in decellularized tissues. AgNPs have a high surface area to volume ratio, which makes them highly effective in killing bacteria, viruses, and other microorganisms [
10]. The use of AgNPs in decellularized tissues, such as decellularized pericardium, can reduce infection risk. The objective of this study was to develop a bovine pericardium impregnated with vancomycin (VAN) or AgNPs, evaluating their
in vitro antimicrobial activity as well as prophylaxis in a mouse model of
S. aureus infection.
2. Results
2.1. Decellularization and histological and morphometric analysis
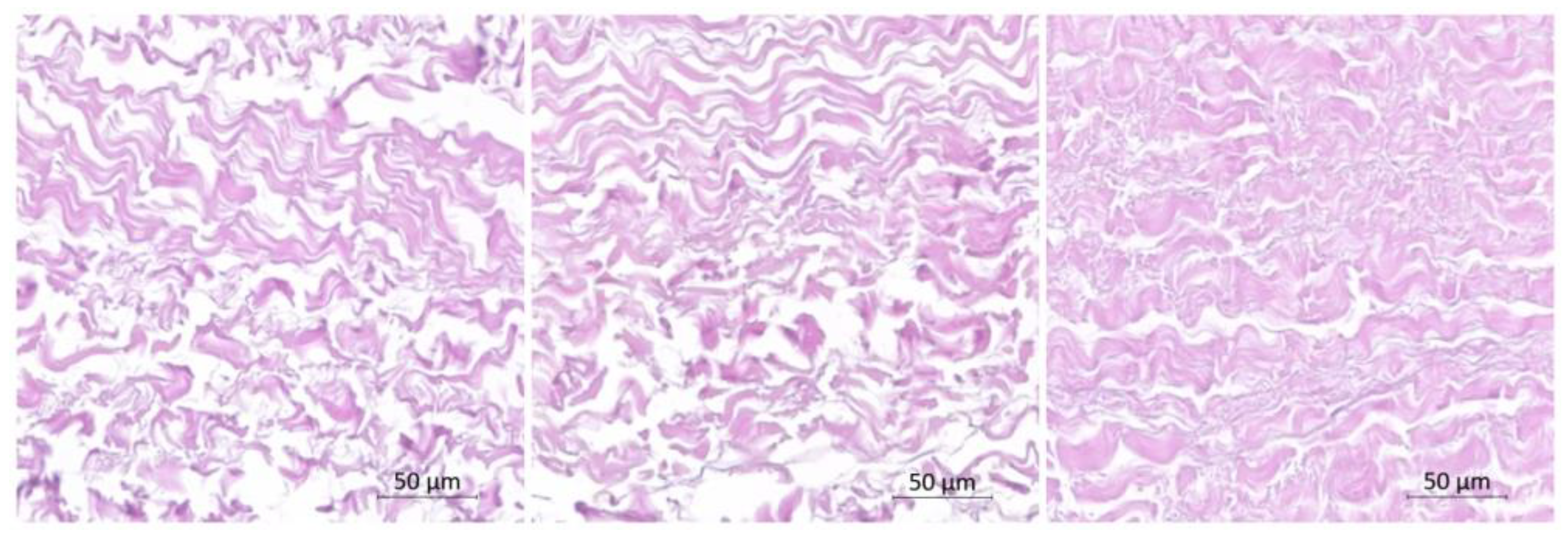
The H&E staining revealed the absence of nuclei, confirming the success of the decellularization process. Importantly, the decellularization process did not result in significant alterations to the structure of collagen and elastic fibers within the pericardium.
When comparing the decellularized bovine pericardium with the VAN group and the AgNP group, slight reductions in collagen fiber content were observed. Additionally, there was an increase in the corrugation amplitude of the collagen fibers in these groups, indicating some structural changes compared to the decellularized pericardium (
Figure 1). These findings suggest that the incorporation of VAN and AgNPs may have influenced the collagen fiber arrangement within the pericardium samples.
2.2. Impregnation of pericardium with AgNPs or vancomycin
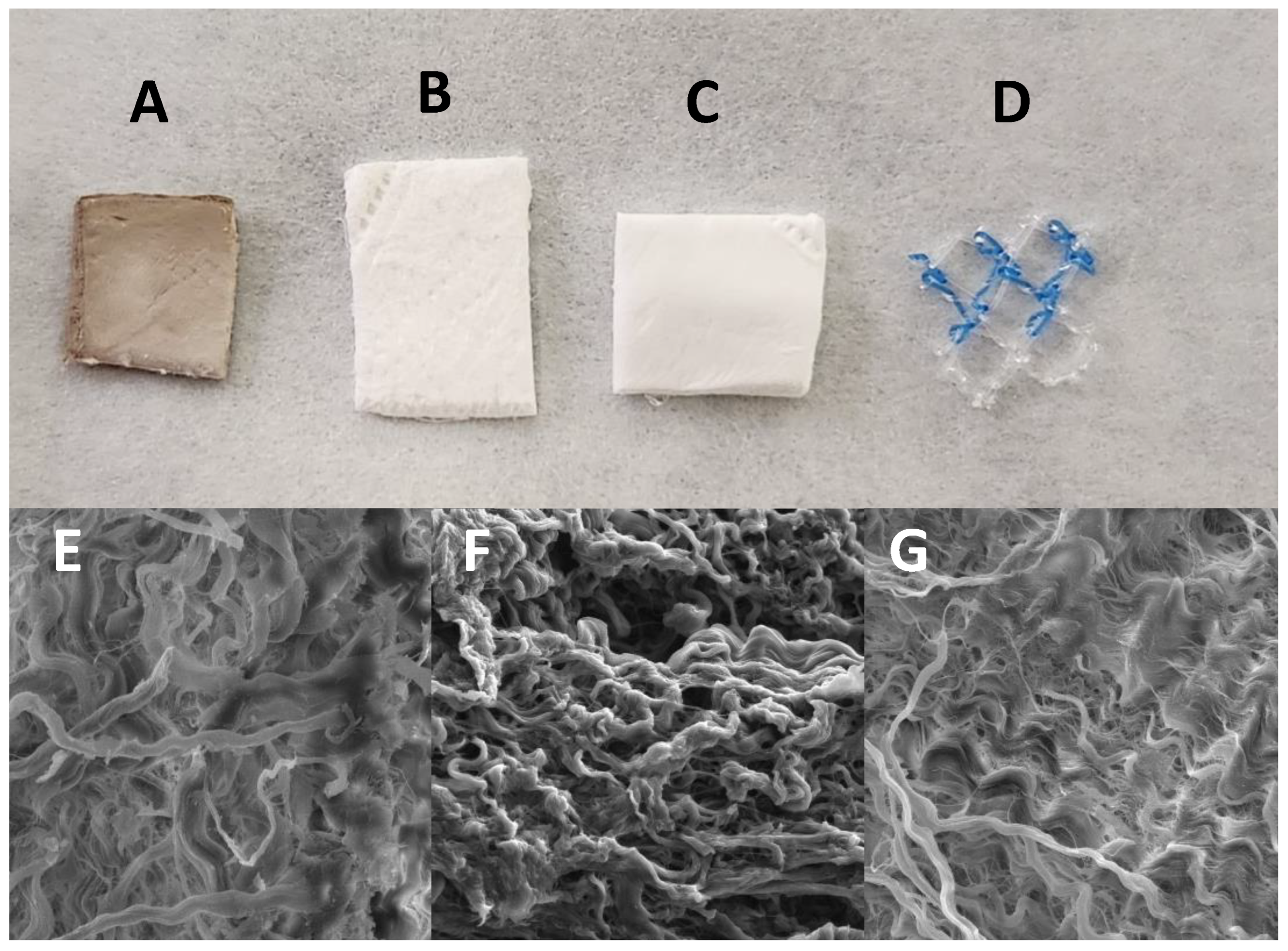
Macroscopic characteristics of the pericardium are presented in
Figure 2. The pericardium impregnated with AgNPs exhibits a darker color, attributed to the presence of oxidized silver. In contrast, the membrane impregnated with VAN tends to have a slightly yellowish hue compared to the non-impregnated membrane. Scanning electron microscopy analysis reveals no significant changes in collagen fibers between the different impregnated membranes (
Figure 2E,F) when compared to the non-impregnated membrane (
Figure 2G).
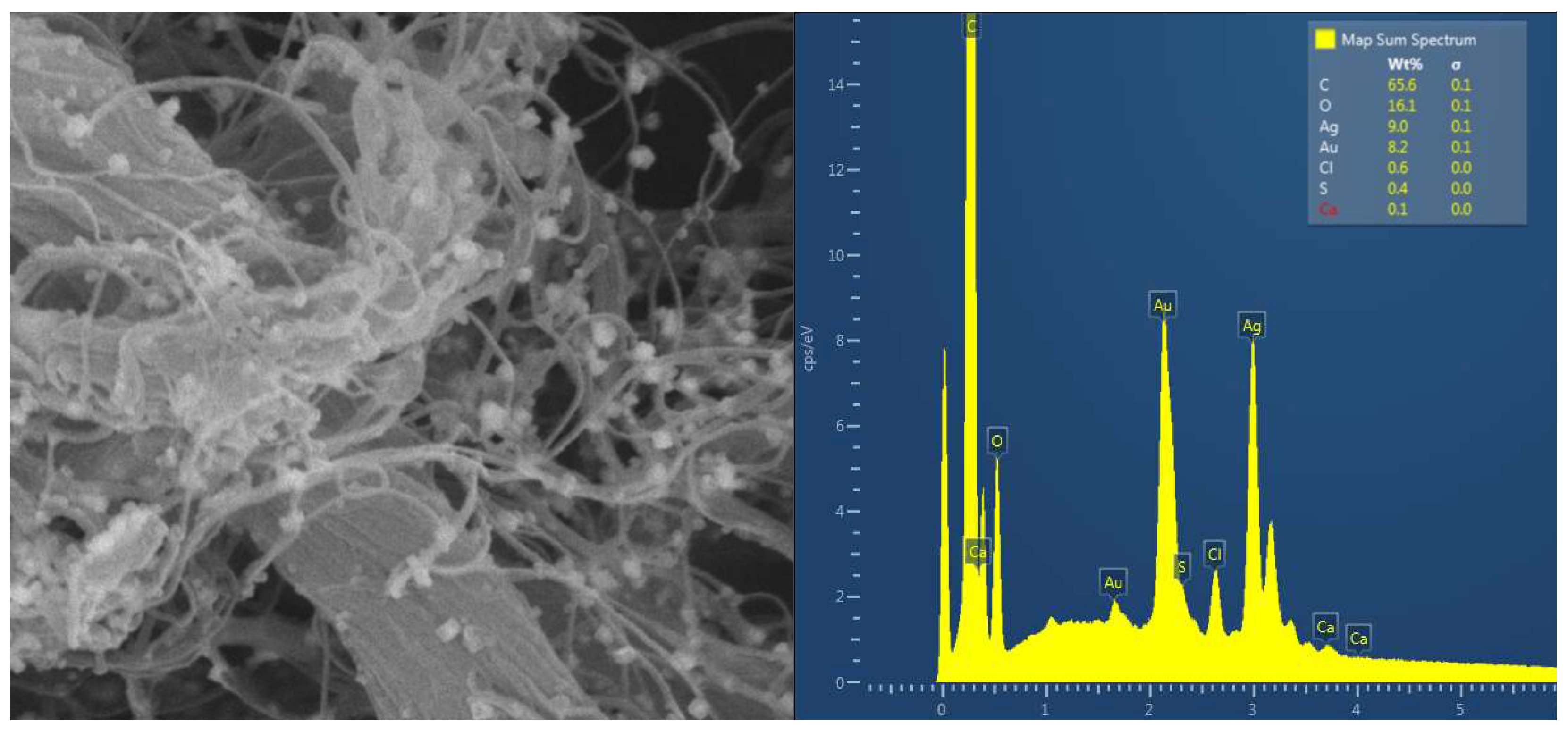
The impregnation of AgNPs was evaluated through microbiological tests and EDS analysis. EDS analysis of the AgNP-impregnated samples confirms the presence of silver peaks, indicating successful silver deposition in the tissue (
Figure 3). Additionally, SEM analysis at a magnification of 50,000x allows for visualizing the nanoparticles within the tissue (
Figure 3).
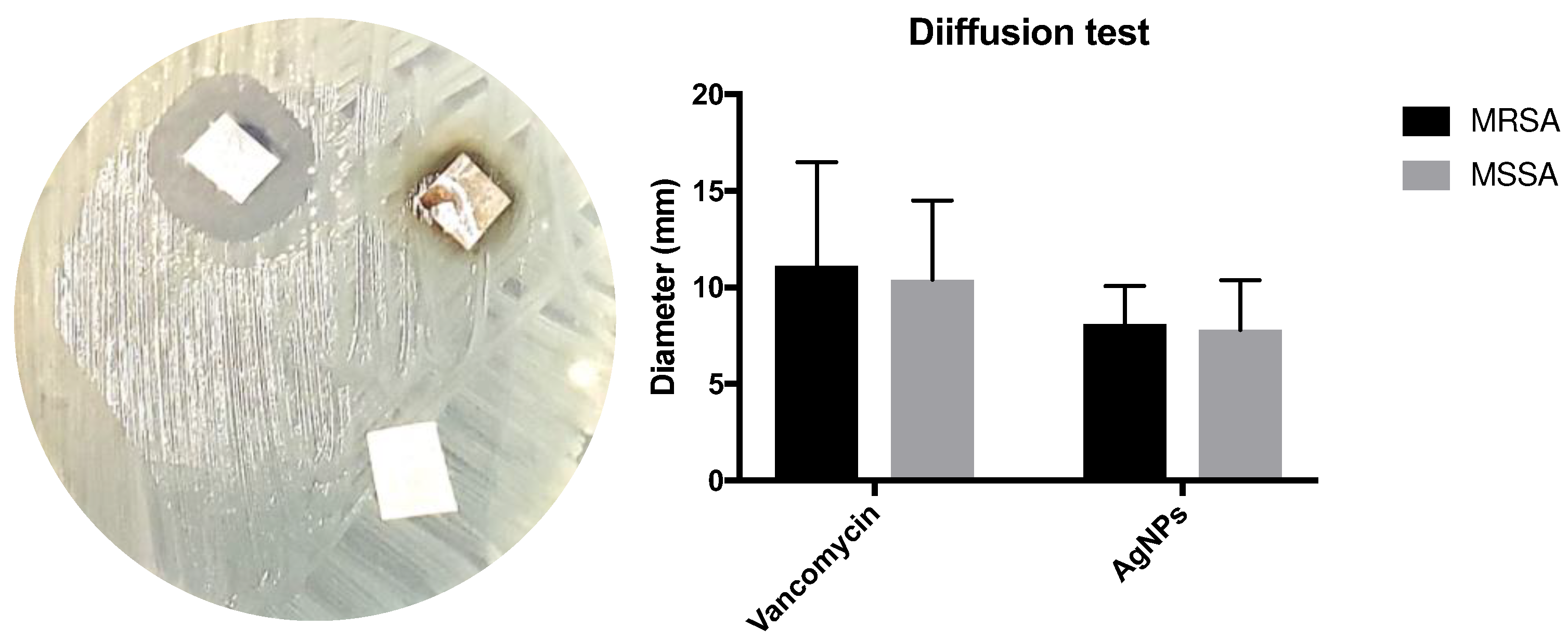
In the qualitative microbiological evaluation, the pericardium models exhibited inhibition halos on the agar, which ranged from 7 to 21 mm for the VAN group and from 6 to 15 mm for the AgNP group. No inhibition halo was observed in the control group (
Figure 4). The average diameter of the inhibition halos was similar between the VAN and AgNP groups.
2.3. Biofilm evaluation
The biofilm “in well” was not presented in the VAN group, but was presented in all tests with other groups, including AgNP group. The quantitative analysis of biofilm “in tissue”, the control group presented 2.41 x10
8 cfu/g (IQR 25-75% 1.51-3.19), the polypropylene was 3.06 x10
7 cfu/g (IQR 25-75% 2.20-4.58), the AgNPs was 4.81 x10
7 cfu/g (IQR 25-75% 3.75-5.27), and VAN was 3.50 x10
4 cfu/g (IQR 25-75% 2.01-4.39). The only tissue with significant difference was VAN against control (P = 0.002) (
Figure 5).
2.4. Animal model
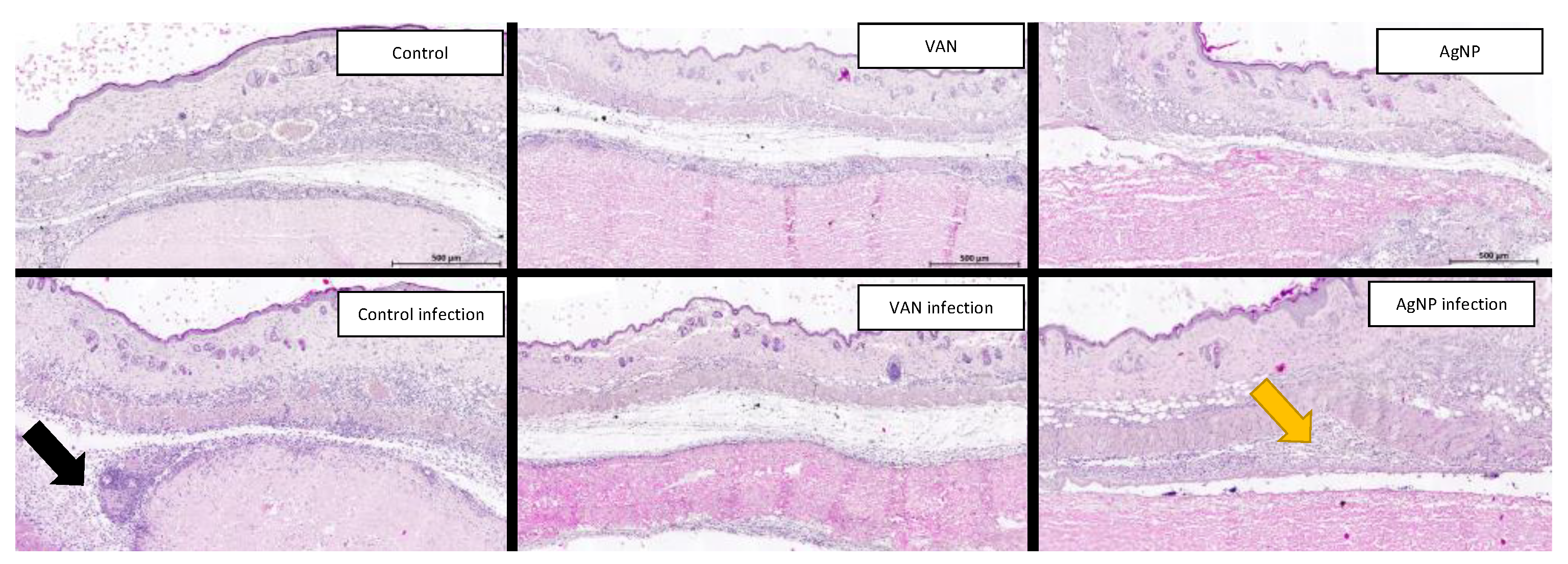
In the group without infection, the histological observations revealed a similar pattern characterized by a typical fibrotic process around the implant, accompanied by minimal to no inflammatory response. Inflammatory process was absent in all animals with AgNP membrane. Conversely, in the group with infection, histological analysis of the control group exhibited a pronounced inflammatory response surrounding the implant, primarily characterized by the presence of neutrophils (
Figure 6). Furthermore, clusters of gram-positive cocci were identified not only around the pericardium but also within the pericardial tissue, suggesting bacterial invasion into the collagen structure. In the VAN and AgNP groups, the inflammatory response was minimal, and only a few bacteria were identified. However, it is worth noting that the inflammatory aspect was more pronounced in the AgNP group compared to the VAN group.
2.5. Quantitative microbiological analysis
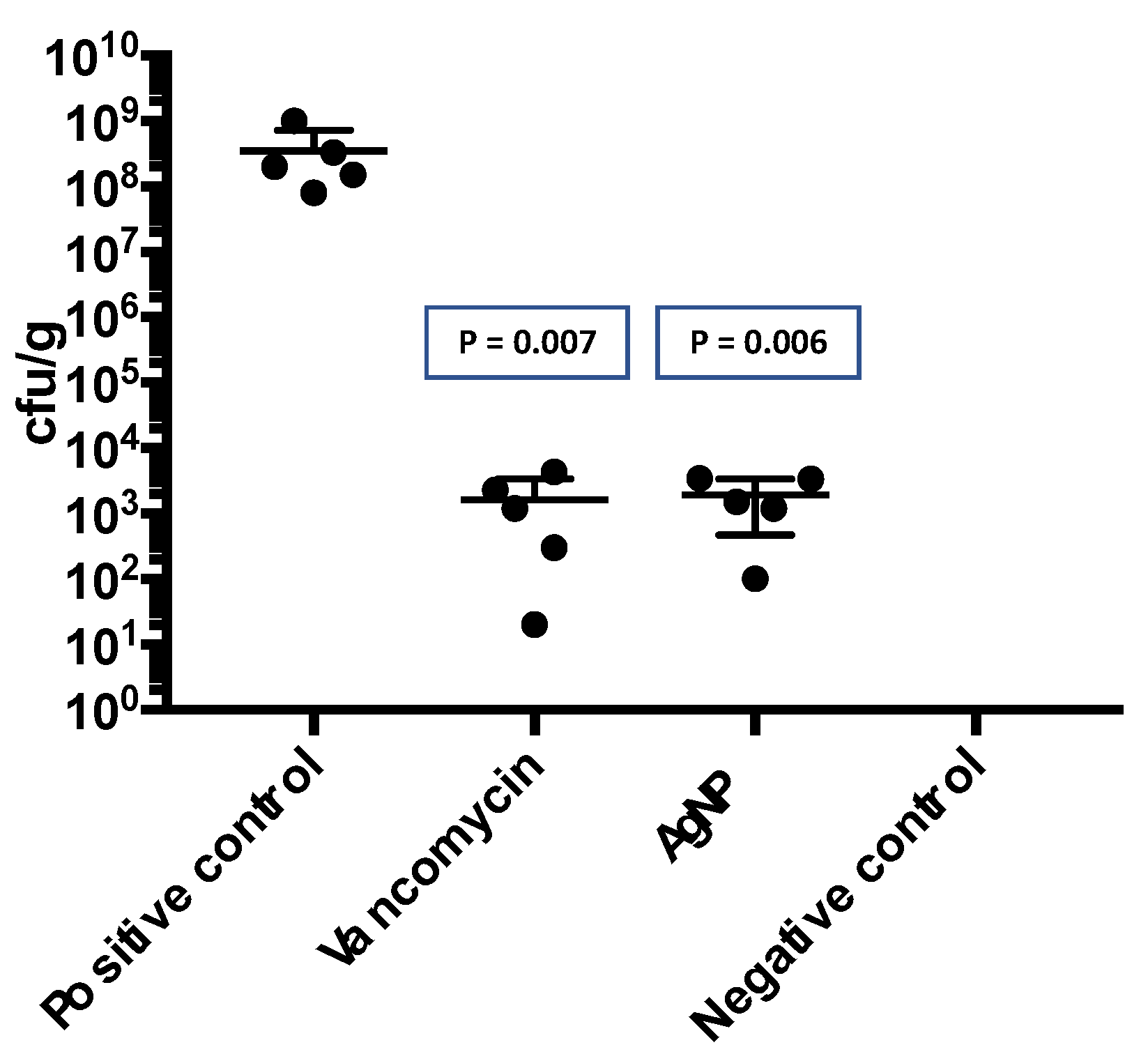
In the quantitative microbiological analysis of the membranes removed from the animals after infection, a significant presence of bacteria was observed in the control group. However, there was a substantial reduction in the number of microorganisms in both the VAN and AgNP groups. The control group had a median of 3.52 x 10
8 cfu/g (interquartile range [IQR] 1.15-6.65), while the VAN group had a median of 1.64 x 10
3 cfu/g (IQR 0.61-3.30), and the AgNP group had a median of 1.92 x 10
3 cfu/g (IQR 0.65-3.42) (
Figure 7). These results indicate that both VAN and AgNP impregnation were effective in reducing the bacterial load in the pericardial membranes.
2.6. Biomechanical Assay
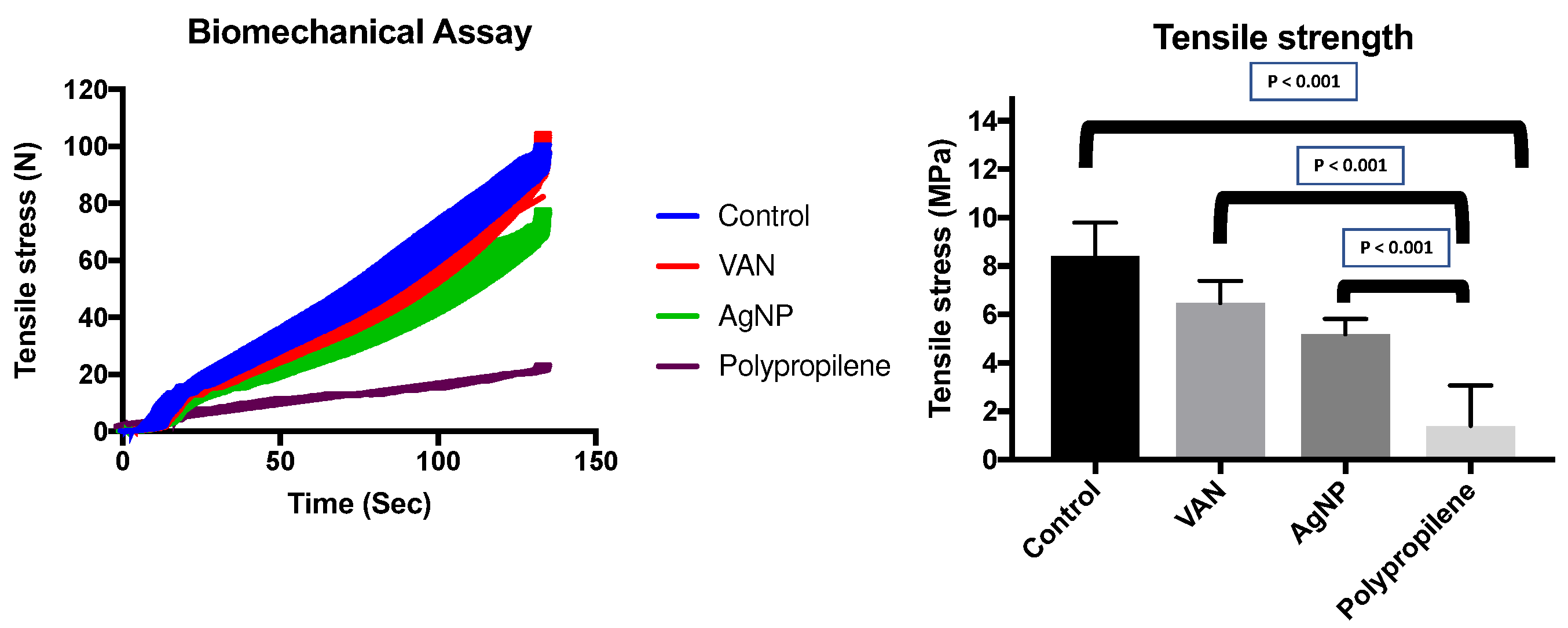
Significant differences in tensile strength were observed among the different pericardial membranes and the polypropylene mesh, with the polypropylene mesh showing the lowest resistance. When comparing the pericardial membranes, the AgNP-impregnated pericardium exhibited lower tensile strength compared to the VAN-impregnated pericardium, and the VAN-impregnated pericardium showed slightly lower strength than the control pericardium (
Figure 8).
The tensile strength values were as follows: Control = 8.74 MPa (interquartile range [IQR] 6.87-9.63), VAN = 7.01 MPa (IQR 6.17-8.34), AgNP = 6.22 MPa (IQR 4.95-8.43). In contrast, the polypropylene group had a significantly lower tensile strength of 0.39 MPa (IQR 0.24-3.55, P < 0.001) (
Figure 8). These results indicate that the pericardial membranes had comparable tensile strength, while the polypropylene mesh exhibited substantially weaker mechanical properties.
2.7. Ethylene oxide toxic residues
The test of toxic residues of ethylene oxide was negative.
3. Discussion
In this study, we developed a lyophilized and decellularized bovine pericardium impregnated with vancomycin or silver nanoparticles to identify potential in vitro antimicrobial activity as well as prophylaxis in a mice model of S. aureus infection.
Bovine pericardium is an important natural biomaterial for treatment of multiple pathological conditions in the regenerative medicine. Their application range covers cardiac valve reconstruction [
11], ophthalmic surgery [
12,
13], bony and periodontal defects [
14,
15], gingival recessions [
16], reconstruction and closure of blood vessels [
17], abdominal wall repair [
18]. All scaffolds designed for clinical use that utilize tissues derived from biologic sources must undergo a series of processing steps designed to rid the material of diseases causing agents and provide the sterility required for surgical use [
19]. To overcome the limitations of current scaffold materials in terms of antimicrobial properties, we have developed lyophilized and decellularized bovine pericardium impregnated with vancomycin or silver nanoparticles. Several studies have been published in recent years covering the possible effects of the use of nanoparticles as an antibiotic agent [
20].
We developed two biological membranes with antimicrobial activity. The vancomycin impregnation presented a potent antimicrobial activity against MRSA and MSSA, which was identified in vitro (agar plate) and in vivo (animal model). It was interesting the anti-biofilm activity avoiding the biofilm formation in the pericardium and plastic around due to elution of antibiotic through culture media, which was not found in the AgNP group. Some studies have shown the weak activity of vancomycin on biofilms, but the rapid diffusion in the broth allowed a faster antimicrobial activity before biofilm formation [
9,
21,
22,
23,
24].
The advantage of vancomycin is the well know activity and safety of this antibiotic used by intravenous route, impregnation of orthopedic devices, including topic use in spine surgery [
25]. However, the use of AgNPs is still a challenge because potential cytotoxicity found in some studies [
26,
27,
28]. Furthermore, there is no standard of AgNPs commercially available for clinical use. Nevertheless, silver has a broader spectrum of action, including Gram-negative bacilli, which is not found with vancomycin [
29].
Lyophilization is a well-established method used to stabilize the structure, bioactivity and composition of proteins, so freeze-drying alone was not expected to alter the growth factor, glycosaminoglycan, or fibronectin content of the group VAN and group AgNP [
19]. However, lyophilization process may cause fragmentation of the collagen, which was suggested in the preliminary analysis in this study. However, when we performed the SEM and biomechanical assay, this microstructural finding was not confirmed.
The three-dimensional architecture of this biomaterial following decellularization, vancomycin or silver nanoparticles, drying by lyophilization, and terminal sterilization with ethylene oxide gas showed similar characteristics, and the tensile stress of all groups of pericardium was similar. We have included a polypropylene group to demonstrate the properties of this material in comparison with biological membrane. The synthetic material has replaced the biological products because of safety and costs [
30]. However, it is important to report that in some cases, the impregnated biological material represent a better choice in infected areas, because the pericardium is easy to remove, instead of synthetic material which mixes with the fibrous tissue, needing extensive cleaning [
31].
We evaluated the capacity of decellularized bovine pericardium impregnated with vancomycin or silver nanoparticles against S. aureus infections in vivo. Our study demonstrated that group VAN and group AgNP presented antibacterial effects after subcutaneous implantation in the spine. There was significantly less inflammatory aspect in the group AgNP when compared to the group VAN.
4. Materials and Methods
4.1. Tissue preparation and decontamination
Bovine pericardium was obtained from a local slaughterhouse and then prepared in physiological saline solution. Adherent adipose tissue was removed using scissors and forceps. The pericardia were decontaminated in RPMI 1640 medium (Sigma-Aldrich, St. Louis, USA) containing antibiotics (240 µg/mL cefoxitin, 50 µg/mL vancomycin, 120 µg/mL lincomycin and 100 µg/mL polymyxin B) and were kept at 2-8°C for 24 hours. Persistent positive cultures after decontamination were excluded [
32]. It has been observed that there is not enough antibiotic present in the processed pericardium to inhibit bacterial growth [
6]. Each step of the process is depicted in the
Figure 9.
4.2. Decellularization
The pericardia were subjected to a decellularization process involving shaking conditions with a solution containing 0.1% sodium dodecyl sulfate (SDS) (w/v) (Sigma-Aldrich, St. Louis, USA) and 7 mM ethylenediaminetetraacetic acid (Sigma-Aldrich, St. Louis, USA) for 24 hours at room temperature. Subsequently, they underwent a washing step using 70% ethanol (v/v) for 24 hours at room temperature. This was followed by a 10-day washing period with a 0.9% sodium chloride (NaCl) solution (Baxter International, Deerfield, USA) to eliminate any remaining substances and cellular debris [
32].
4.3. Impregnation of pericardium with AgNPs or vancomycin
The procedure used to prepare 50 nm AgNPs was adapted from Dong et al. [
33]. To incorporate the AgNPs into the pericardium, physical adsorption was employed, based on a modified version of the model described by Becerril-Juárez et al. (2012) [
34]. In this process, pericardium samples measuring 1 x 1 cm were added to the AgNPs solution at room temperature and kept for 60 minutes, protected from light. After the incorporation of AgNPs, the samples were maintained at room temperature for an additional 60 minutes and then subjected to lyophilization (AgNP group). In contrast, the VAN group followed a different procedure utilizing VAN (patented process). Following a similar protocol, the samples were kept at room temperature for 60 minutes after the VAN impregnation and subsequently underwent lyophilization. The control group underwent the same procedure described above but without the impregnation of AgNPs or VAN (control group). To enhance resistance, all samples were fixed in 0.5% glutaraldehyde for 1 hour prior to impregnation. Terminal sterilization was achieved using ethylene oxide.
4.4. Qualitative microbiological analysis
In each group, five samples were punched into 6 x 6 mm squares. These samples were then plated on Muller-Hinton agar plates with S. aureus, with a turbidity equivalent to 0.5 McFarland, which corresponds to a concentration of 3 x 108 cfu/mL. The diameter of the inhibition zone surrounding the samples was measured after 24 hours of incubation at 37°C, indicating the antibacterial activity. For these tests, we included 10 clinical isolates of Meticillin-Susceptible S. aureus (MSSA) and 10 Meticillin-Resistant S. aureus (MRSA).
4.5. Biofilm production on pericardium
A 1:10 dilution was prepared in TSB broth using a microbe 0.5 McFarland suspension of a clinical isolate of MRSA. This dilution process was carried out until a bacterial concentration of 10
7 CFU/mL was achieved [
35]. Next, 10 mL of TSB broth was added to sterile 24-well plates, completely covering the membranes (0.5 x 0.5 mm, n=4 for each group, including a polypropylene group), and the plates were agitated at 120 rpm for 2 hours. This allowed the specimens to be exposed to the broth and facilitate bacterial adhesion.
After the incubation period, the specimens were transferred to a new sterile 24-well plate containing 0.9% NaCl. This step was performed to remove any planktonic (free-floating) cells from the material. Following the removal of planktonic cells, the specimens were transferred to another sterile 24-well plate and submerged in 10 mL of TSB broth at 37°C for 24 hours without agitation. This allowed the cells adhering to the device surface to form a biofilm. After the biofilm formation, the specimens were submerged in 1.5 mL conical tubes filled with 1 mL of sterile 0.9% NaCl to remove any residual and unadhered/planktonic cells (step I). This washing step (step I) was repeated three times.
Following the washing steps, the specimens were placed into 1.5 mL conical tubes filled with 1 mL of 0.9% NaCl for further processing. They were subjected to sonication for 5 minutes using an ultrasonic bath with a Soniclean 15 device (Sanders Medical, Santa Rita do Sapucaí, Brazil) operating at a frequency of approximately 40 kHz and a temperature of 35°C. [
36]. After the sonication step (step II), the supernatant (100 μL) was collected and inoculated onto TSA agar plates for growth evaluation and cell count (cfu/mL). This allowed for the assessment of bacterial colonies and determination of the viable bacterial count in the samples.
4.6. Scanning Electronic Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
For the SEM and EDS analysis, three pericardial samples of each group were transferred to sterile Petri dishes filled with the primary fixative agent (0.68 g sucrose, 0.42 g sodium cacodylate, 0.6 mL 30% glutaraldehyde) (Merck, Darmstadt, Germany) and 19.4 mL of deionized water in sufficient quantity to cover the specimen for 45 minutes [
37]. After that, the specimens were transferred to a buffer (composed of sucrose and sodium cacodylate at the above concentrations) for 10 minutes. Then, the samples were dehydrated in ethanol in a series of increasing concentrations (35%, 50%, 70%, 100%) for 10 minutes in each concentration. Then, the samples were transferred to another Petri dish and covered with 100% hexamethyldisilane (Merck, Darmstadt, Germany) for 10 minutes. After the fixation step, the samples were metalized with gold particles in metalizing equipment with a Q150R ES rotary pump (Quorum Technologies, Lewes, UK), and later fixed in a metal base for observation under Mira 3 (Tescan, Czech Republic) in different magnifications. The EDS analysis was carried concomitantly with SEM for AgNPs detection and confirm the impregnation.
4.7. Biomechanical Assay
The biomechanical assay of bovine pericardium was performed with tensile test to compare the effects of impregnation on tissue properties of tensile strength. An EMIC DL 500 universal testing machine was used to perform the tests. Five samples of each group were included in the assay. A control group of polypropylene mesh was included, once this type of membrane is commonly used for inguinal hernia correction. The collagen fibers traction direction was random. The dumbbell-shaped sample was based on the ASTM D1708-13 standard because of the small-size tissue available to the tests. To obtain the samples, it was necessary to manufacture a steel rule die according to standard specifications, which was used to cut the pericardium. Before each test, sample thickness, width and initial length values were provided to the equipment. Thickness and width are necessary to calculate the cross-sectional area and consequently the stress (equation 1). Thickness was measured with a thickness gauge at three points of the tissue. Pre-load of 0.1 N and 5 mm/min velocity were applied to the test.
Equation 1. Stress calculation.
where:
σ is the stress in megapascal [MPa];
F is the force in newton [N] and
A is the cross-sectional area in square millimeter [mm2].
4.8. Ethylene oxide toxic residues
The determination of the concentration of toxic residues of ethylene oxide used in sterilization was carried out as required by ISO10993-7 [
38]
.
4.9. Animal model
The study was granted approval by the Ethical Committee of the PUCPR (Curitiba, Brazil). All procedures adhered to the regulations outlined in the Brazilian animal protection law. A total of thirty male C57BL/6N mice (FIOCRUZ, Curitiba, PR, Brazil) were included in the study, distributed across six study groups as follows: VAN and infection (n=5), VAN without infection (n=5), AgNPs and infection (n=5), AgNPs without infection (n=5), control and infection (n=5), and control without infection (n=5).
Each mouse received a subcutaneous implant of a 1 x 1 cm pericardium on both sides of the spine, with one implant designated for histological analysis and the other for tissue culture. In the group with bacterial contamination, a suspension containing 0.1 mL of bacteria was pipetted onto the implant after it was placed in the tissue pocket. The inoculum used was Staphylococcus aureus ATCC 25923TM with a medium concentration of 1 × 105 cfu/mL.
The surgeon conducting the procedure was unaware of the group assignments, except for the presence of bacterial contamination. Following the surgery, the animals were monitored for a period of five days before scheduled euthanasia. The surgical and euthanasia procedures were previously published, providing detailed information about the methods employed [
39]. Following euthanasia, the pericardium and the surrounding soft tissue (on the right side) were immersed in 10% formalin for subsequent histological analysis. This involved preserving the tissues in a formalin solution to maintain their structural integrity and prepare them for microscopic examination. On the other hand, the pericardium on the left side was designated for quantitative microbiological analysis. This involved analyzing the pericardium tissue to quantify the bacterial load present, allowing for the assessment of microbial colonization and infection.
4.10. Histological analysis
Histological analyses were conducted on the pericardium groups both before implantation to assess morphology and after implantation to evaluate bacterial and inflammatory responses. Longitudinal cuts measuring 3.0 mm in length were made, and the tissue samples were fixed in neutral buffered formalin 10%. Subsequently, the samples were embedded in paraffin, and 4-µm thick sections were obtained.
To assess the morphological integrity of the pericardium, hematoxylin and eosin (H&E) staining was performed. An optical microscope BX51 (Olympus Tokyo, Japan) was used for visual examination of the stained sections. Fresh pericardium tissue was used as a control to compare and evaluate any changes caused by the decellularization process.
The prepared slides were scanned using an Axio Scan.Z1 slide scanner (Carl Zeiss Microscopy GmbH, Jena, Germany), and subsequent image processing and analysis were performed using Zen lite software (Carl Zeiss, Jena, Germany). This allowed for detailed examination and documentation of the histological features and changes observed in the pericardium samples.
4.11. Quantitative microbiological analysis of animal model
The left pericardium inserted in the animals was disrupted in 10 mL of NaCl with a tissue handheld rotor-stator homogenizer (Qiagen®). Serial dilution 1:10 with plating was performed for quantitative bacterial count in cfu/g of tissue.
4.12. Statistical analysis
The SEM, EDS and histological data were qualitative and descriptive. For quantitative microbiological analysis, the median mg/mL obtained with quantitative results was analyzed by the Mann-Whitney test and presented with a median with interquartile range (25-75%). The difference was significant when p <0.05. The data was calculated, analyzed, and plotted using Prism 7.0 (Graphpad, San Diego, CA).
5. Conclusions
In conclusion, impregnated biological membrane based on bovine pericardium can be a promising product with antimicrobial activity which can be used with prophylactic or therapeutic purposes. The study confirmed the antimicrobial activity of vancomycin and AgNP was well as the safety and preservation of biomechanical properties of the tissue.
6. Patents
We have sent this product for patent registration.
Author Contributions
Conceptualization, Felipe Tuon and Daniele Colatusso; methodology, Paula Hansen Suss and Leticia Ramos Dantas; formal analysis, Felipe Tuon and Jaime Lopes Rocha; resources, Felipe Tuon; data curation, Felipe Tuon and Marcelo Loureiro; writing—original draft preparation, Felipe Tuon and Jaime Rocha; writing—review and editing, Felipe Tuon and Marcelo Loureiro. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of PUCPR (protocol code 01/2021 approved 18/Jan/2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on demand to corresponding author.
Acknowledgments
We thank Paulo Soares (PPGEM PUCPR) by SEM and EDS tests.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed Eng Online 2019, 18, 24. [CrossRef]
- Murin, P.; Weixler, V.H.M.; Kuschnerus, K.; Romanchenko, O.; Lorenzen, V.; Nordmeyer, J.; Cho, M.Y.; Sigler, M.; Photiadis, J. Pulmonary artery augmentation using decellularized equine pericardium (Matrix Patch): initial single-centre experience. Eur J Cardiothorac Surg 2021, 60, 1094-1101. [CrossRef]
- Suzuki, M.; Kimura, T.; Yoshida, Y.; Kobayashi, M.; Hashimoto, Y.; Takahashi, H.; Shimizu, T.; Anzai, S.; Nakamura, N.; Kishida, A. In Vitro Tissue Reconstruction Using Decellularized Pericardium Cultured with Cells for Ligament Regeneration. Polymers (Basel) 2022, 14, 2351-2356. [CrossRef]
- da Costa, F.D.; Santos, L.R.; Collatusso, C.; Matsuda, C.N.; Lopes, S.A.; Cauduro, S.; Roderjan, J.G.; Ingham, E. Thirteen years' experience with the Ross Operation. J Heart Valve Dis 2009, 18, 84-94.
- Ostdiek, A.M.; Ivey, J.R.; Hansen, S.A.; Gopaldas, R.; Grant, S.A. Feasibility of a nanomaterial-tissue patch for vascular and cardiac reconstruction. J Biomed Mater Res B Appl Biomater 2016, 104, 449-457. [CrossRef]
- Kraft, L.; Ribeiro, V.S.T.; de Nazareno Wollmann, L.C.F.; Suss, P.H.; Tuon, F.F. Determination of antibiotics and detergent residues in decellularized tissue-engineered heart valves using LC-MS/MS. Cell Tissue Bank 2020, 21, 573-584. [CrossRef]
- Dantas, L.R.; Ribeiro, V.S.T.; Kraft, L.; Pinho, R.A.; Suss, P.H.; Vasconcellos, F.T.F.; de Noronha, L.; Tuon, F.F. Collagen matrices are preserved following decellularization of a bovine bone scaffold. Cell Tissue Bank 2022, 23, 531-540. [CrossRef]
- Wali, N.; Shabbir, A.; Wajid, N.; Abbas, N.; Naqvi, S.Z.H. Synergistic efficacy of colistin and silver nanoparticles impregnated human amniotic membrane in a burn wound infected rat model. Sci Rep 2022, 12, 6414. [CrossRef]
- Pedroni, M.A.; Ribeiro, V.S.T.; Cieslinski, J.; Lopes, A.P.A.; Kraft, L.; Suss, P.H.; Tuon, F.F. Different concentrations of vancomycin with gentamicin loaded PMMA to inhibit biofilm formation of Staphylococcus aureus and their implications. J Orthop Sci 2022, 14, S0949-2658(0922)00336-00330. [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl Mater Interfaces 2019, 11, 37381-37396. [CrossRef]
- Ionescu, M.I. Mitral valvar grafts. Br Heart J 1971, 33, Suppl:56-59. [CrossRef]
- Quaranta, L.; Riva, I.; Floriani, I.C. Outcomes of using a sutureless bovine pericardial patch graft for Ahmed glaucoma valve implantation. Eur J Ophthalmol 2013, 23, 738-742. [CrossRef]
- Gupta, M.; Puri, P.; Rennie, I.G. Use of bovine pericardium as a wrapping material for hydroxyapatite orbital implants. Br J Ophthalmol 2002, 86, 288-289. [CrossRef]
- Rothamel, D.; Schwarz, F.; Fienitz, T.; Smeets, R.; Dreiseidler, T.; Ritter, L.; Happe, A.; Zoller, J. Biocompatibility and biodegradation of a native porcine pericardium membrane: results of in vitro and in vivo examinations. Int J Oral Maxillofac Implants 2012, 27, 146-154.
- Stavropoulos, A.; Chiantella, G.; Costa, D.; Steigmann, M.; Windisch, P.; Sculean, A. Clinical and histologic evaluation of a granular bovine bone biomaterial used as an adjunct to GTR with a bioresorbable bovine pericardium collagen membrane in the treatment of intrabony defects. J Periodontol 2011, 82, 462-470. [CrossRef]
- Schlee, M.; Ghanaati, S.; Willershausen, I.; Stimmlmayr, M.; Sculean, A.; Sader, R.A. Bovine pericardium based non-cross linked collagen matrix for successful root coverage, a clinical study in human. Head Face Med 2012, 8, 6. [CrossRef]
- D'Andrilli, A.; Ibrahim, M.; Venuta, F.; De Giacomo, T.; Coloni, G.F.; Rendina, E.A. Glutaraldehyde preserved autologous pericardium for patch reconstruction of the pulmonary artery and superior vena cava. Ann Thorac Surg 2005, 80, 357-358. [CrossRef]
- Limpert, J.N.; Desai, A.R.; Kumpf, A.L.; Fallucco, M.A.; Aridge, D.L. Repair of abdominal wall defects with bovine pericardium. Am J Surg 2009, 198, e60-65. [CrossRef]
- Hodde, J.; Janis, A.; Ernst, D.; Zopf, D.; Sherman, D.; Johnson, C. Effects of sterilization on an extracellular matrix scaffold: part I. Composition and matrix architecture. J Mater Sci Mater Med 2007, 18, 537-543. [CrossRef]
- Allaker, R.P. The use of nanoparticles to control oral biofilm formation. J Dent Res 2010, 89, 1175-1186. [CrossRef]
- Chen, X.; Thomsen, T.R.; Winkler, H.; Xu, Y. Influence of biofilm growth age, media, antibiotic concentration and exposure time on Staphylococcus aureus and Pseudomonas aeruginosa biofilm removal in vitro. BMC Microbiol 2020, 20, 264. [CrossRef]
- Douthit, C.; Gudenkauf, B.; Hamood, A.; Mudaliar, N.; Caroom, C.; Jenkins, M. Effects of powdered rifampin and vancomycin solutions on biofilm production of staphylococcus aureus on orthopedic implants. J Clin Orthop Trauma 2020, 11, S113-S117. [CrossRef]
- Kaneko, H.; Nakaminami, H.; Ozawa, K.; Wajima, T.; Noguchi, N. In vitro anti-biofilm effect of anti-methicillin-resistant Staphylococcus aureus (anti-MRSA) agents against the USA300 clone. J Glob Antimicrob Resist 2021, 24, 63-71. [CrossRef]
- Okae, Y.; Nishitani, K.; Sakamoto, A.; Kawai, T.; Tomizawa, T.; Saito, M.; Kuroda, Y.; Matsuda, S. Estimation of Minimum Biofilm Eradication Concentration (MBEC) on In Vivo Biofilm on Orthopedic Implants in a Rodent Femoral Infection Model. Front Cell Infect Microbiol 2022, 12, 896978. [CrossRef]
- Tuon, F.F.; Romero, R.; Gasparetto, J.; Cieslinski, J. Vancomycin trough level and loading dose. Infect Drug Resist 2018, 11, 2393-2396. [CrossRef]
- Baker, C.; Pradhan, A.; Pakstis, L.; Pochan, D.J.; Shah, S.I. Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol 2005, 5, 244-249. [CrossRef]
- Correa, J.M.; Mori, M.; Sanches, H.L.; da Cruz, A.D.; Poiate, E., Jr.; Poiate, I.A. Silver nanoparticles in dental biomaterials. Int J Biomater 2015, 2015, 485275. [CrossRef]
- Mathur, P.; Jha, S.; Ramteke, S.; Jain, N.K. Pharmaceutical aspects of silver nanoparticles. Artif Cells Nanomed Biotechnol 2018, 46, 115-126. [CrossRef]
- Anuj, S.A.; Gajera, H.P.; Hirpara, D.G.; Golakiya, B.A. Bacterial membrane destabilization with cationic particles of nano-silver to combat efflux-mediated antibiotic resistance in Gram-negative bacteria. Life Sci 2019, 230, 178-187. [CrossRef]
- Gonzalez, E.A.; Nandy, P.; Lucas, A.D.; Hitchins, V.M. Designing for cleanability: The effects of material, surface roughness, and the presence of blood test soil and bacteria on devices. Am J Infect Control 2017, 45, 194-196. [CrossRef]
- Tuon, F.F.; Cieslinski, J.; Ono, A.F.M.; Goto, F.L.; Machinski, J.M.; Mantovani, L.K.; Kosop, L.R.; Namba, M.S.; Rocha, J.L. Microbiological profile and susceptibility pattern of surgical site infections related to orthopaedic trauma. Int Orthop 2019, 43, 1309-1313. [CrossRef]
- Wollmann, L.; Suss, P.; Mendonca, J.; Luzia, C.; Schittini, A.; Rosa, G.; Costa, F.; Tuon, F.F. Characterization of Decellularized Human Pericardium for Tissue Engineering and Regenerative Medicine Applications. Arq Bras Cardiol 2019, 113, 11-17. [CrossRef]
- Dong, H.W., D.; Sun, G.; Hinestroza, J. Assembly of Metal Nanoparticles on Electrospun Nylon 6 Nanofibers by Control of Interfacial Hydrogen-Bonding Interactions. Chem. Mater. 2015, 20, 5.
- Becerril-Juáres, I.G.M.-L., R.A.; Ureña-Nuñez, F.; Arenas-Alatorre, J.A.; Hinestroza, J.P.; Sánchez-Mendieta, V. . Silver micro-, submicro- and nano-crystals using bovine bone as template. Formation of a silver/bovine bone composite. Mat. Lett. 2012, 85, 4.
- Franklin, M.J.; Chang, C.; Akiyama, T.; Bothner, B. New Technologies for Studying Biofilms. Microbiol Spectr 2015, 3, 10.1128. [CrossRef]
- Trampuz, A.; Piper, K.E.; Jacobson, M.J.; Hanssen, A.D.; Unni, K.K.; Osmon, D.R.; Mandrekar, J.N.; Cockerill, F.R.; Steckelberg, J.M.; Greenleaf, J.F.; et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 2007, 357, 654-663. [CrossRef]
- da Rocha, L.; Ribeiro, V.S.T.; de Andrade, A.P.; Goncalves, G.A.; Kraft, L.; Cieslinski, J.; Suss, P.H.; Tuon, F.F. Evaluation of Staphylococcus aureus and Candida albicans biofilms adherence to PEEK and titanium-alloy prosthetic spine devices. Eur J Orthop Surg Traumatol 2021, 981-989. [CrossRef]
- Sladowski, D.; Grabska-Liberek, I.; Olkowska-Truchanowicz, J.; Lipski, K.; Gut, G. An evaluation of sterilisation processes. Altern Lab Anim 2008, 36, 585-590. [CrossRef]
- Wittmann, C.; Vanvelk, N.; Furst, A.E.; Moriarty, T.F.; Zeiter, S. Development and Characterization of a Subcutaneous Implant-Related Infection Model in Mice to Test Novel Antimicrobial Treatment Strategies. Biomedicines 2022, 11, 40. [CrossRef]
Figure 1.
Histological analysis stained with hematoxylin-eosin to evaluate the structure of impregnated pericardium with AgNP (left), Vancomycin (middle), and control group (right).
Figure 1.
Histological analysis stained with hematoxylin-eosin to evaluate the structure of impregnated pericardium with AgNP (left), Vancomycin (middle), and control group (right).
Figure 2.
Macroscopic characteristic of bovine pericardium with AgNP (A), vancomycin (B), without impregnation (C) and polypropylene mesh (D). The scanning electronic miscroscopy demonstrate the maintenance of collagen fibers despite of impregantation with AgNP (E), vancomycin (F) and letter G is the pericardium without impregnation.
Figure 2.
Macroscopic characteristic of bovine pericardium with AgNP (A), vancomycin (B), without impregnation (C) and polypropylene mesh (D). The scanning electronic miscroscopy demonstrate the maintenance of collagen fibers despite of impregantation with AgNP (E), vancomycin (F) and letter G is the pericardium without impregnation.
Figure 3.
Left: Scanning electronic microscopy (x 50.000) of bovine pericardium impregnated with AgNPs. Right: Energy Dispersive Spectroscopy showing peak of silver (Ag) in the pericardial tissue.
Figure 3.
Left: Scanning electronic microscopy (x 50.000) of bovine pericardium impregnated with AgNPs. Right: Energy Dispersive Spectroscopy showing peak of silver (Ag) in the pericardial tissue.
Figure 4.
Left: Agar test of impregnated bovine pericardium with vancomycin (upper left), AgNP (upper right) and control (below). Right: The median with 25% interquartile range of inhibition halo in millimeters tested with methicillin-resistant S. aureus (n=10) and methicillin-susceptible S. aureus (n=10).
Figure 4.
Left: Agar test of impregnated bovine pericardium with vancomycin (upper left), AgNP (upper right) and control (below). Right: The median with 25% interquartile range of inhibition halo in millimeters tested with methicillin-resistant S. aureus (n=10) and methicillin-susceptible S. aureus (n=10).
Figure 5.
Biofilm test with S. aureus. T0 is the immediate inoculation of bacteria in the tissue model (bovine pericardium). T24 is the plate 24 hours after bacterial inoculation in the well. The arrow 1 is showing a biofilm formation in the well, and the arrow 2 is well without biofilm formation. Group A is the polypropylene mesh, B is bovine perciardium without impregnation, C is vancomycin impregnated and D is AgNP impregnated bovine pericardium. The graphic with collumns represents the quantitative analysis os biofilm in the tissue in cfu per gram.
Figure 5.
Biofilm test with S. aureus. T0 is the immediate inoculation of bacteria in the tissue model (bovine pericardium). T24 is the plate 24 hours after bacterial inoculation in the well. The arrow 1 is showing a biofilm formation in the well, and the arrow 2 is well without biofilm formation. Group A is the polypropylene mesh, B is bovine perciardium without impregnation, C is vancomycin impregnated and D is AgNP impregnated bovine pericardium. The graphic with collumns represents the quantitative analysis os biofilm in the tissue in cfu per gram.
Figure 6.
The histological analysis of the implanted membranes in mice demonstrated that in animals without induced infection, there was minimal tissue inflammatory response, only fibroblast cells developing a perimembrane capsule. This response was lower in the AgNP group, suggesting an anti-inflammatory activity of the nanoparticles (figures above). In the group of animals with induced S. aureus infection, an intense inflammatory response was observed in the control group, characterized by neutrophilic infiltrate and microabscess formation (black arrow). In the VAN (vancomycin) group, there was no inflammatory response, and in the AgNP group, the inflammatory response was less pronounced than in the control group, with no formation of microabscesses.
Figure 6.
The histological analysis of the implanted membranes in mice demonstrated that in animals without induced infection, there was minimal tissue inflammatory response, only fibroblast cells developing a perimembrane capsule. This response was lower in the AgNP group, suggesting an anti-inflammatory activity of the nanoparticles (figures above). In the group of animals with induced S. aureus infection, an intense inflammatory response was observed in the control group, characterized by neutrophilic infiltrate and microabscess formation (black arrow). In the VAN (vancomycin) group, there was no inflammatory response, and in the AgNP group, the inflammatory response was less pronounced than in the control group, with no formation of microabscesses.
Figure 7.
Quantitative bacterial load (cfu/g) in the pericardium removed from mice five days after infection. The bacterial load was considerable lower in the group VAN and AgNP in comparison with pericardium without impregnation (positive control).
Figure 7.
Quantitative bacterial load (cfu/g) in the pericardium removed from mice five days after infection. The bacterial load was considerable lower in the group VAN and AgNP in comparison with pericardium without impregnation (positive control).
Figure 8.
Biomechanical assays. Left: The dynamic curves of time and tensile stress (N). Right: The tensile stress measured (in MPA) with median and IQR in different impregnations of bovine pericardium, including a control group (pericardium without impregnation) and a polypropylene mesh. Only the propylene group showed a significant difference with pericardium, and all pericardium group were similar in the biomechanical assay of tensile stress.
Figure 8.
Biomechanical assays. Left: The dynamic curves of time and tensile stress (N). Right: The tensile stress measured (in MPA) with median and IQR in different impregnations of bovine pericardium, including a control group (pericardium without impregnation) and a polypropylene mesh. Only the propylene group showed a significant difference with pericardium, and all pericardium group were similar in the biomechanical assay of tensile stress.
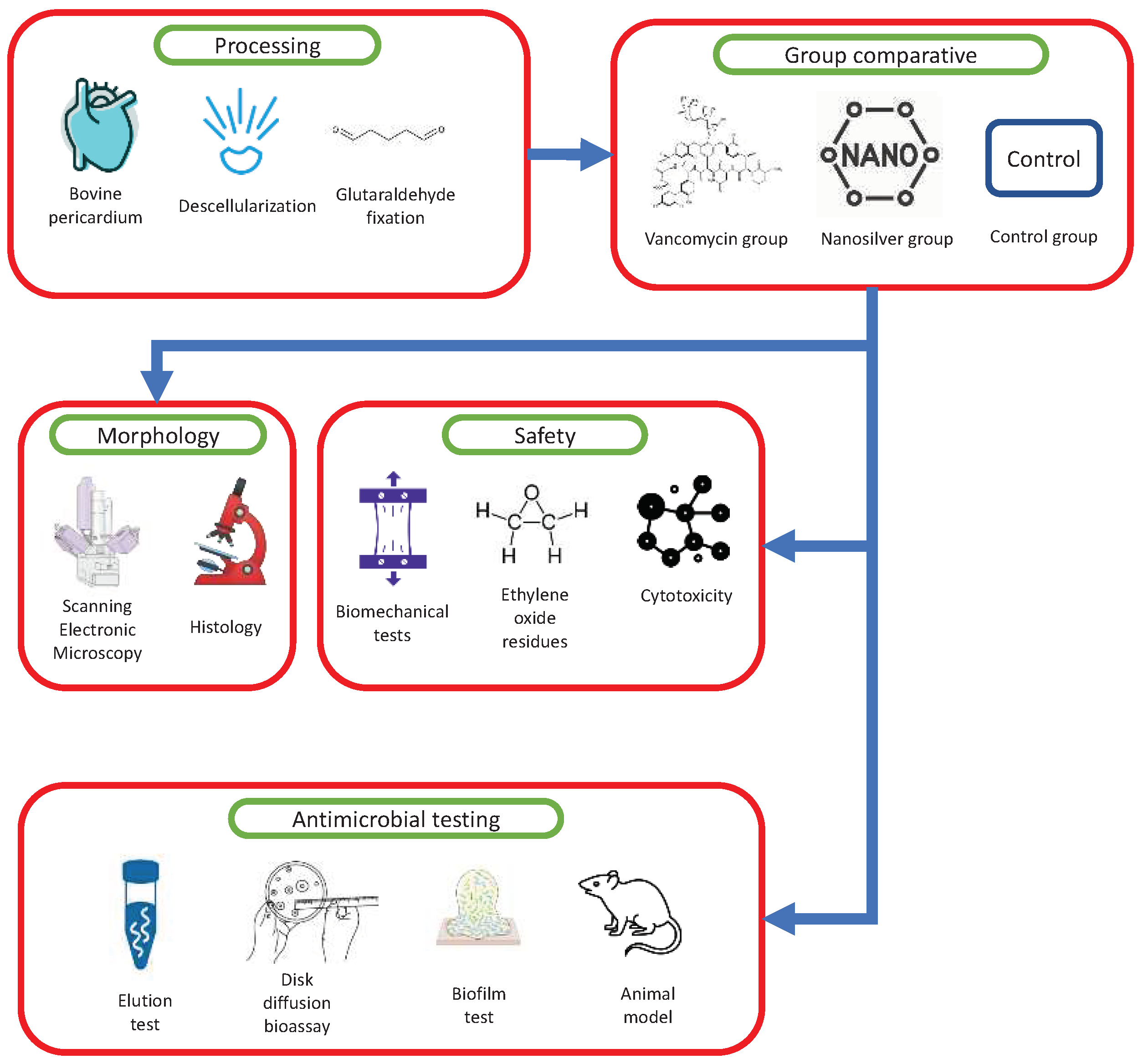
Figure 9.
Sequential steps involved in the processing of bovine pericardium for the development of a biological membrane. These steps include processing, as well as testing analysis encompassing morphology assessment, safety evaluation, and examination of the antimicrobial activity of vancomycin and AgNP impregnation.
Figure 9.
Sequential steps involved in the processing of bovine pericardium for the development of a biological membrane. These steps include processing, as well as testing analysis encompassing morphology assessment, safety evaluation, and examination of the antimicrobial activity of vancomycin and AgNP impregnation.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).