Submitted:
10 July 2023
Posted:
11 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
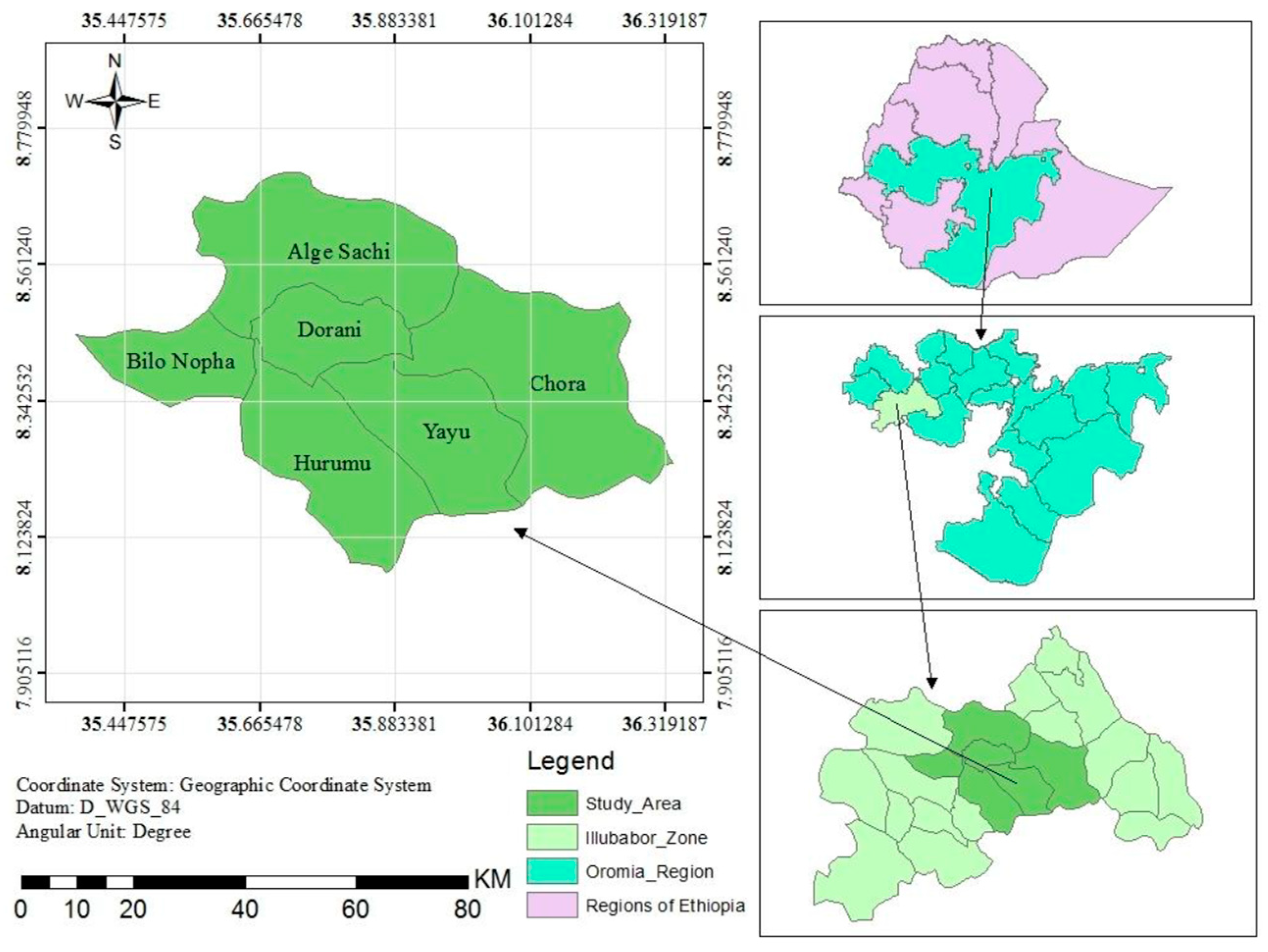
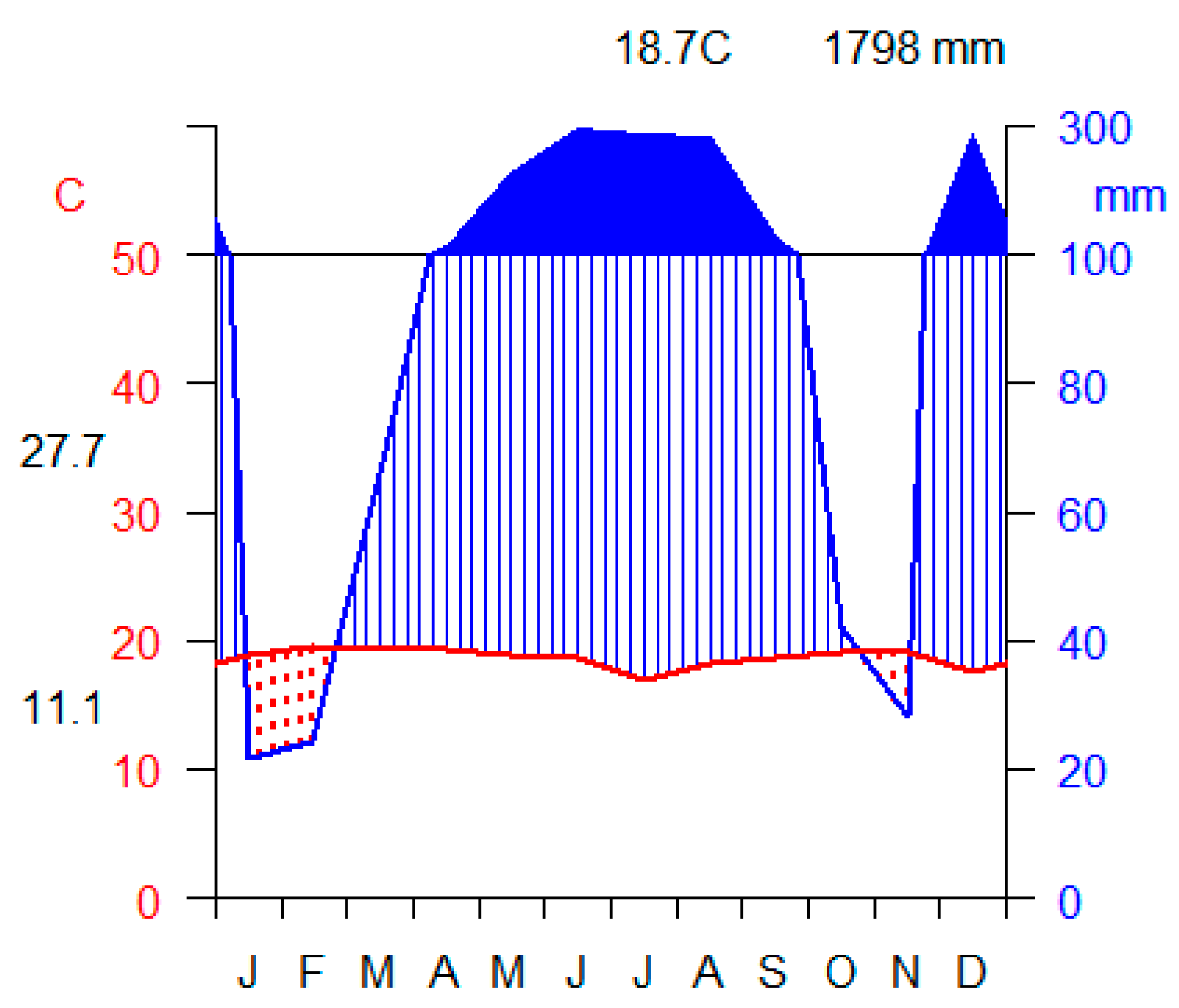
2.1. Study Area Descriptions
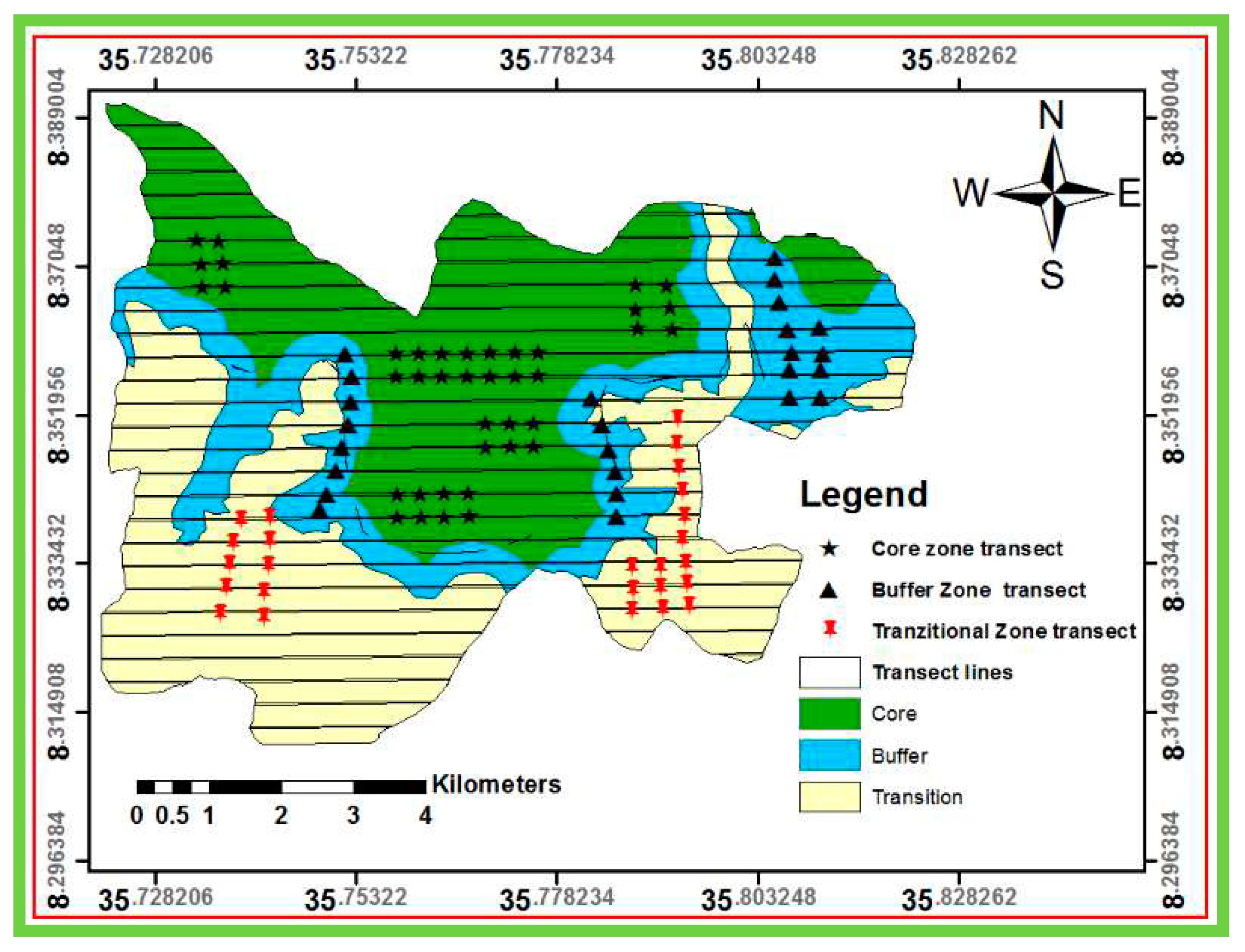
2.2. Sampling Design
2.3. Vegetation Data Collection and Environmental Factors
2.4. Quantification of Functional Diversity and Taxonomic Diversity Indices
2.5. Statistical Analysis
3. Results
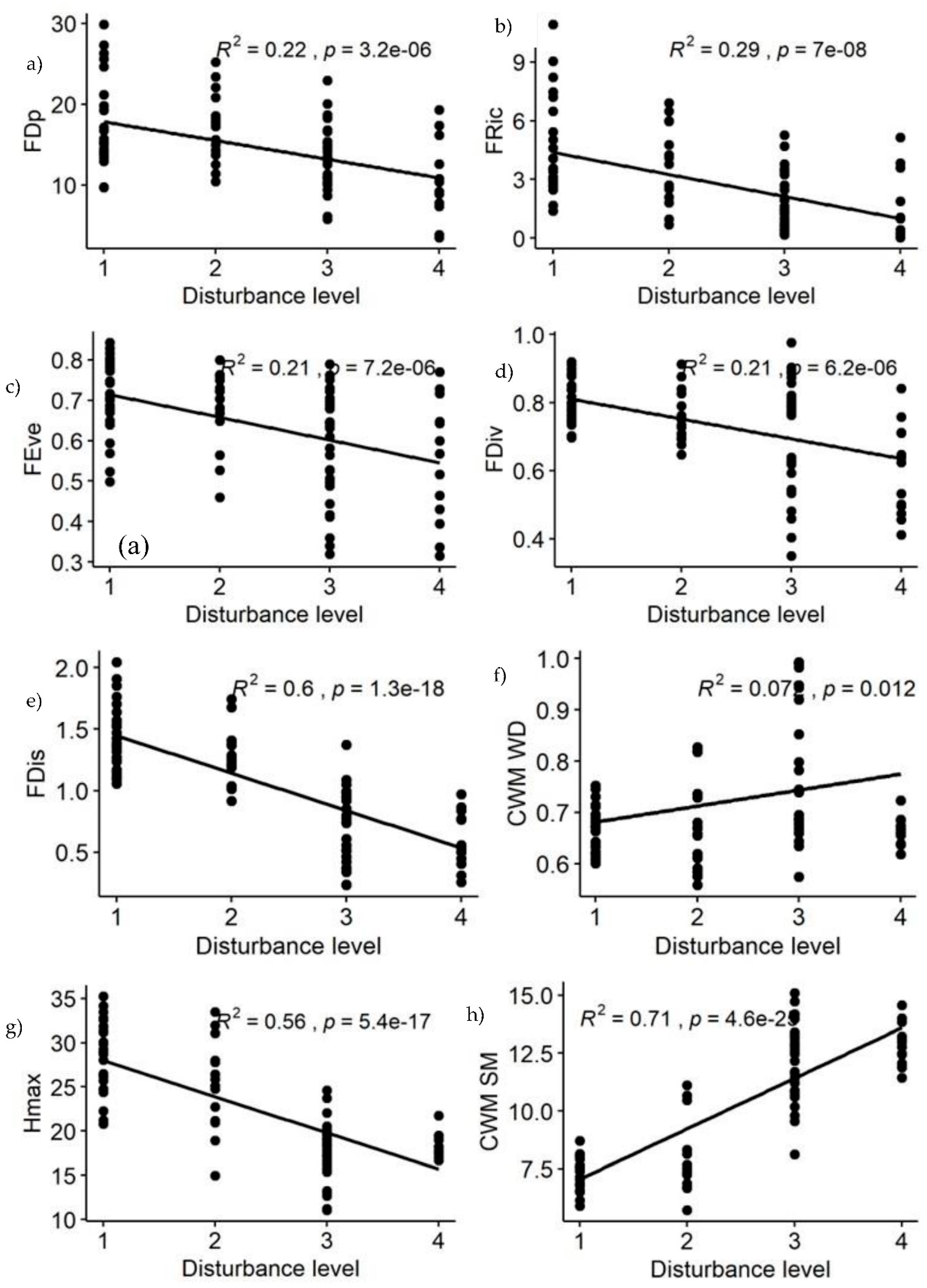
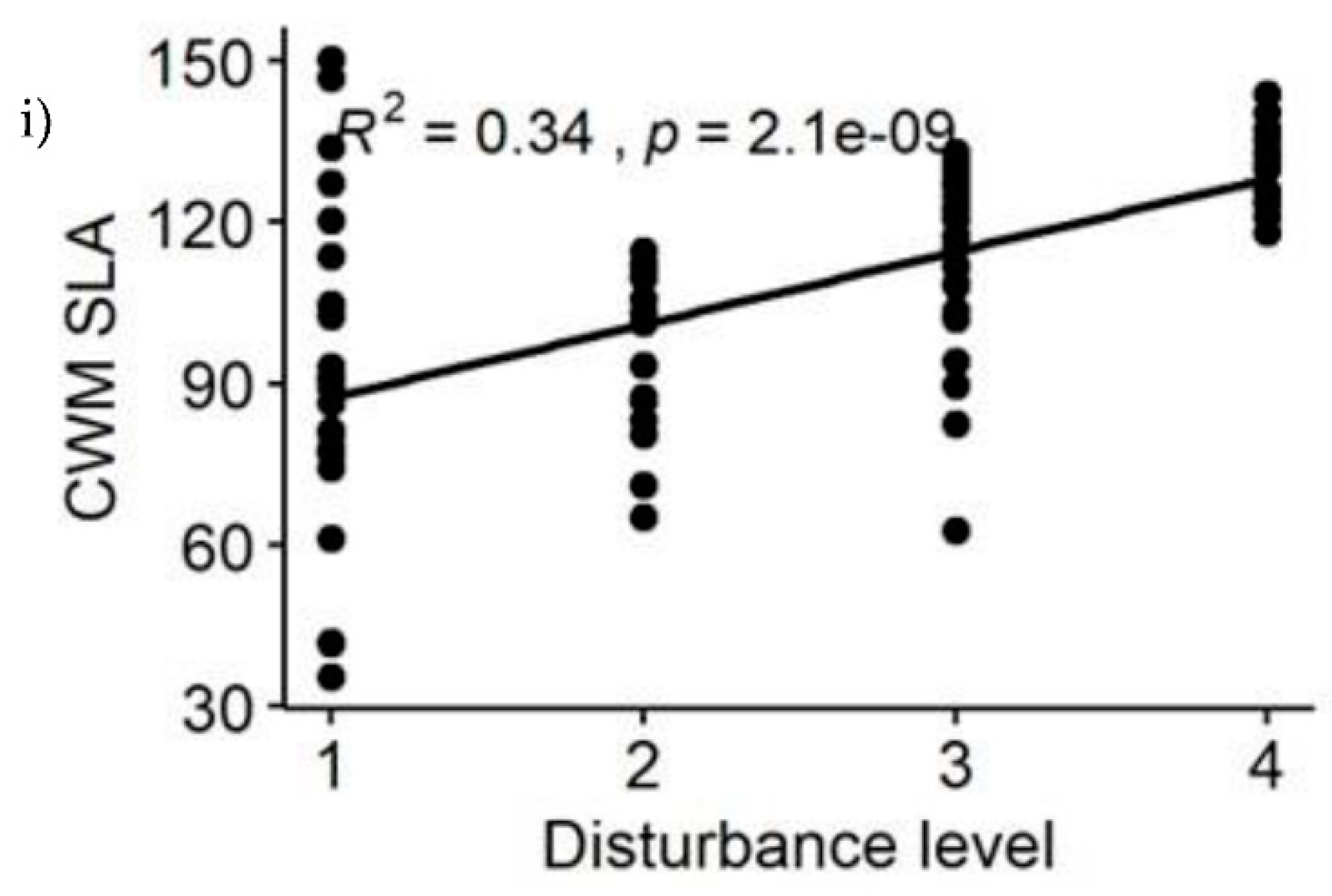
3.1. Variation of Plant Functional Diversity along Disturbance Gradients
3.2. Relationships between Functional Diversity and Environmental Factors of YCFBR
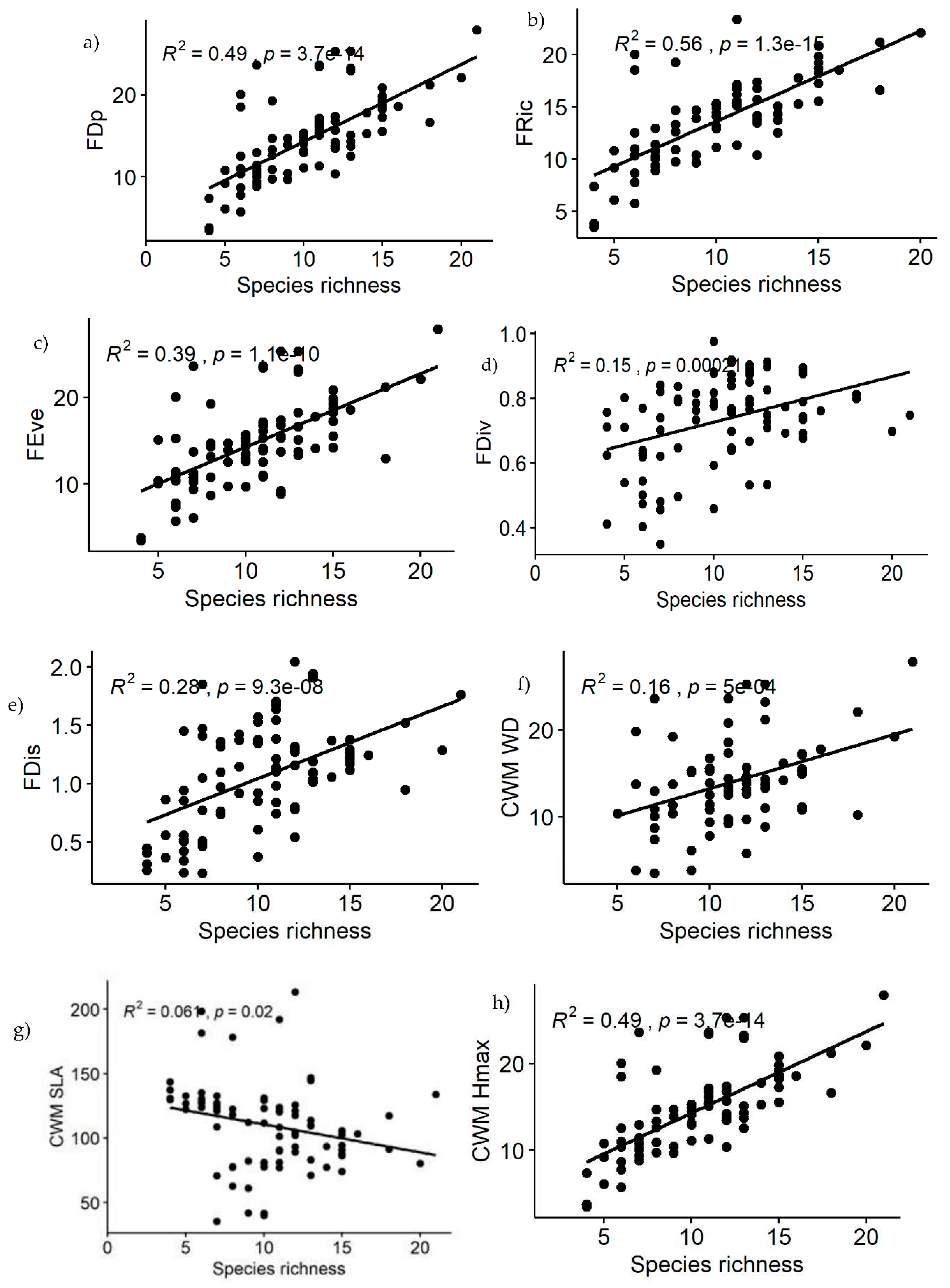
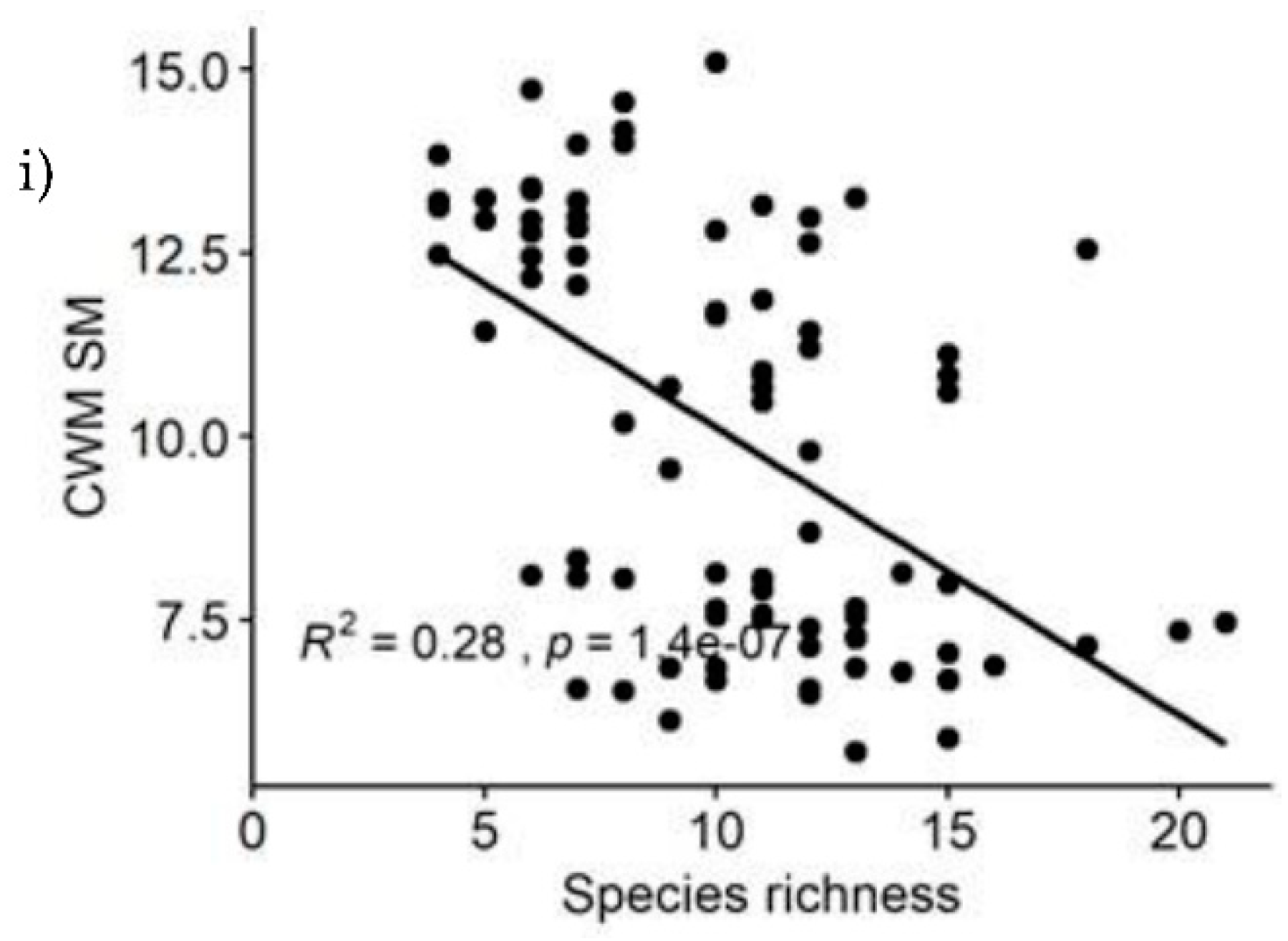
3.3. Functional Diversity Indices and Species Diversity Relationships
4. Discussions
4.1.Variations of Plant Functional Diversity along Disturbance Gradients
4.2. Effects of Environmental Factors on Functional Diversity
4.3. Functional Diversity and Species Diversity Relationships
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lewis, C.C. , Weiner, B.J., Stanick, C. et al. Advancing Implementation Science through Measure Development and Evaluation: A Study Protocol. implemenation Sci. 2015, 102. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, Jesús. , Erika Berenguer, Imma Oliveras Menor, David Bauman, Jose Javier Corral-Rivas, M.G.N.-M. et al. Functional Susceptibility of Tropical Forests to Climate Change. Ecol. Evol. 2022, 1–26.
- IPCC Tropical Forests In Climate Change: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Trop. For. 2022, CCP7. [Google Scholar]
- Geneletti, D. Integrating Ecosystem Services in Land Use Planning: Concepts and Applications; 2012;
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; MacE, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity Loss and Its Impact on Humanity. Nature 2012, 486. [Google Scholar] [CrossRef]
- Devries, B.; Pratihast, A.K.; Verbesselt, J.; Kooistra, L.; Herold, M. Characterizing Forest Change Using Community-Based Monitoring Data and Landsat Time Series. PLoS One 2016. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Lowto Moderate-Level Forest Disturbance Effects on Plant Functional Traits and Associated Soil Microbial Diversity in Western Himalaya. Front. For. Glob. Chang. 2021, 4, 1–12. [Google Scholar] [CrossRef]
- Friis, I. Forests and Forest Trees of Northeast Tropical Africa. Their Natural Habitats and Distribution Patterns in Ethiopia, Djibouti and Somalia. Kew Bulletin Additional Series XV. Kew, United Kingdom: HMSO [Her Majesty’s Stationery Office]. 1992.
- Mengesha Asefa, Min Cao, Yunyun He, Ewuketu Mekonnen, and X.S.; Yang, J. Ethiopian Vegetation Types, Climate and Topography. Sci. direct 2020, 42, 302–311, doi:http: //www.keaipubl ishing.com/en/journals/plant-diversity/ http:// journal.kib.ac.cn.
- Semegnew Tadese, Teshome Soromessa, Tesefaye Bekele, and G.G. Biosphere Reserves in the Southwest of Ethiopia: Review Article. Hindawi Adv. Agric. 2021, 1–7. [CrossRef]
- MEFCC National Potential and Priority Maps for TreeBased Landscape Restoration in Ethiopia (Version 0.0): Technical Report. Addis Ababa: Ministry of Environment, Forest and Climate Change. 2017.
- Yitebitu Moges, Z.E. and S.N. ETHIOPIAN FOREST RESOURCES: CURRENT STATUS AND FUTURE MANAGEMENT OPTIONS IN VIEW OF ACCESS TO CARBON FINANCES: REVIEW. 2010, 1–55.
- DeVries Ben, Jan Verbesselt, Lammert Kooistra, M. H. Robust Monitoring of Small-Scale Forest Disturbances in a Tropical Montane Forest Using Landsat Time Series. Sci. direct 2015, 161, 107–121. [Google Scholar]
- DeVries B, Pratihast AK, V. J.; Kooistra L, H.M. Characterizing Forest Change Using Community-Based Monitoring Data and Landsat Time Series. PLoS One 2016, 11, 1–25. [Google Scholar] [CrossRef]
- Hundera, K.; Aerts, R.; Fontaine, A.; Van Mechelen, M.; Gijbels, P.; Honnay, O.; Muys, B. Effects of Coffee Management Intensity on Composition, Structure, and Regeneration Status of Ethiopian Moist Evergreen Afromontane Forests. Environ. Manage. 2013. [Google Scholar] [CrossRef]
- YCFBRMP Yayu Coffee Forest Biosphere Reserve Management Plan:Oromia Environment, Forest and Climate Change Authority and Oromia Forest and WildLife Enterprise. 2018.
- Hou, Z.; Lv, G.; Jiang, L. Functional Diversity Can Predict Ecosystem Functions Better Than Dominant Species: The Case of Desert Plants in the Ebinur Lake Basin. sustainability 2021, 13, 1–13. [Google Scholar] [CrossRef]
- Shipra, V. Plant Functional Traits in Tropical Dry Forests: A Review. 2020, 1–86.
- Kattge, Jens, Gerhard Bönisch, Sandra Díaz, S. et al. TRY Plant Trait Database – Enhanced Coverage and Open Access. Glob. chnage Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef] [PubMed]
- Jesse, R. Laskya, María Uriarteb, Vanessa K. Boukilic, and R.L.C. Trait-Mediated Assembly Processes Predict Successional Changes in Community Diversity of Tropical Forests. Ecol. Appl. 2016, 111, 5616–5621. [Google Scholar]
- Nicola Kühn, Carolina Tovar, J.C.; Vigdis Vandvik, B.J.E. and K.J.W. Globally Important Plant Functional Traits for Coping with Climate Change. Front. Biogeogr. 2021, 1–18. [CrossRef]
- Sarah Lueder, Kaushik Narasimhan, Jorge Olivo, D. C.; Juana G. Jurado, L.G. and J.K. Functional Traits, Species Diversity and Species Composition of a Neotropical Palm Community Vary in Relation to Forest Age. Front. Ecol. Evol. 2022, 10, 1–13. [Google Scholar]
- Tadesse Woldemariam, T.W. , Feyera, Senbeta, Kassahun, T. and Fite, G. Yayu Coffee Forest Biosphere Reserve Nomination Form. Ethiopian MAB National Committee, Addis Ababa. 2009.
- Beyene, A.D.; Mekonnen, A.; Hirons, M.; Robinson, E.J.Z.; Gonfa, T.; Gole, T.W.; Demissie, S. Contribution of Non-Timber Forest Products to the Livelihood of Farmers in Coffee Growing Areas: Evidence from Yayu Coffee Forest Biosphere Reserve. J. Environ. Plan. Manag. 2020, 63, 1633–1654. [Google Scholar] [CrossRef]
- Gole, T.W. Vegetation of the Yayu Forest in SW Ethiopia: Impacts of Human Use and Implications for in Situ Conservation of Wild Coffea Arabica L. Populations. Ecol. Dev. Ser. 2003, 10. [Google Scholar]
- Jemal, O.; Callo-Concha, D.; van Noordwijk, M. Local Agroforestry Practices for Food and Nutrition Security of Smallholder Farm Households in Southwestern Ethiopia. Sustain. 2018. [Google Scholar] [CrossRef]
- NABU, T.N. and B.C.U. NABU’s Biodiversity Assessment at the Kafa Biosphere Reserve. Berlin, Addis Ababa. 2017.
- UNFCCC Measurements for Estimation of Carbon Stocks in Afforestation and Reforestation Project Activities under the Clean Development Mechanism: A Field Manual. A F. Man. 2015, 1–72.
- Djomo, A.N.; Picard, N.; Fayolle, A.; Henry, M.; Ngomanda, A.; Ploton, P.; McLellan, J.; Saborowski, J.; Adamou, I.; Lejeune, P. Tree Allometry for Estimation of Carbon Stocks in African Tropical Forests. Forestry 2016. [Google Scholar] [CrossRef]
- Pearson Timothy, Sarah Walker, and S.B. Source Book for Land Use, Land-Use Change and Fores Try Proje Cts. 2005, 1–64.
- Snowdon, P. , Raison, J., Keith, H., Ritson, P., Grierson, P., Adams, M.; Montagu, K., Bi, H.Q., Burrows, W. & Eamus, D. Protocol for Sampling Tree and Stand Biomass. Natl. Carbon Account. Syst. Tech. Rep. no. 31. Aust. Greenh. Off. Canberra 2002. [Google Scholar]
- EFAP EFAP, Ethiopian Forestry Action Program, Volume III.Summary Final Report, Ethiopia, Addis Ababa. 2020.
- Edwards, S. , Tadesse, M. , Demissew, S. and Hedberg, I. Flora of Ethiopia and Eritrea: Magnoliaceae to Flacourtiaceae. The National Herbarium, Addis Ababa and the Department of Systematic Botany, Uppsala. 2000, 2. [Google Scholar]
- Hedberg, I. , Kelbessa, E. , Edwards, S., Demissew, S. and Persson, E. Flora of Ethiopia and Eritrea, Gentianaceae to Cyclocheilaceae. The National Herbarium, Addis Ababa University, Addis Ababa and Uppsala. 2006, 5. [Google Scholar]
- Yuan, Z. , Wang, S., Ali, A. et al Aboveground Carbon Storage Is Driven by Functional Trait Composition and Stand Structural Attributes Rather than Biodiversity in Temperate Mixed Forests Recovering from Disturbances. Ann. For. Sci. 2018, 75. [Google Scholar] [CrossRef]
- Woldu, G.; Solomon, Negasi, Hadgu Hishe, Hailemariam Gebrewahid, M. A.G.; Birhane, E. Topographic Variables to Determine the Diversity of Woody Species in the Exclosure of Northern Ethiopia. Sci. direct 2019, 6, 1–7. [Google Scholar]
- Wekesa, C. , Leley, N., Maranga, E., Kirui, B., Muturi, G., Mbuvi, M., & Chikamai, B. Effects of Forest Disturbance on Vegetation Structure and Above-Ground Carbon in Three Isolated Forest Patches of Taita Hills. Open J. For. 2016, 6, 142–161. [Google Scholar] [CrossRef]
- Girma Shumi, Ha¨rdtle, P. R.. J.H.. W.; Schultner, K.H.. F.S.. J.F.. J. Woody Plant Species Diversity as a Predictor of Ecosystem Services in a Social–Ecological System of Southwestern Ethiopia. Landsc. Ecol. 2021, 36, 373–391. [Google Scholar] [CrossRef]
- Girma Shumi, Ha¨rdtle, P.R.. J.H.. W.; Schultner, K.H.. F.S.. J.F.. J. Land Use Legacy Effects on Woody Vegetation in Agricultural Landscapes of South-Western Ethiopia. WILEY Biodivers. Distrib. 2018, 1–13. [CrossRef]
- Melese Genete Muluneh, Motuma Tolera Feyissa, and T. M.W. Effect of Forest Fragmentation and Disturbance on Diversity and Structure of Woody Species in Dry Afromontane Forests of Northern Ethiopia. Biodivers. Conserv. 2021, 30, 1753–1779. [Google Scholar] [CrossRef]
- Technologies), W. (World O. of C.A. and Where the Land Is Greener: Case Studies and Analysis of Soil and Water Conservation Initiatives Worldwide. Berne, Switzerland: WOCAT. 2007.
- Lujin Hu, Zongyi He, Jiping Liu, C. Z. Method for Measuring the Information Content of Terrain from Digital Elevation Models. open J. entropy 2015, 17, 7021–7051. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of Plant Diversity to Ecosystems: Immediate, Filter and Founder Effects. J. Ecol. 1998, 86, 901–1076. [Google Scholar] [CrossRef]
- McGill BJ, Enquist BJ, Weiher E, W. M. Rebuilding Community Ecology from Functional Traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Westoby, M. A Leaf-Height-Seed (LHS) Plant Ecology Strategy Scheme. Plant Soil 1998, 199, 213–227. [Google Scholar] [CrossRef]
- Mason, N. , Mouillot, D., Lee, W., et al. Functional Rich_ Ness, Functional Evenness and Functional Divergence: The Primary Components of Functional Diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- EFRL ETHIOPIA’S FOREST REFERENCE LEVEL SUBMISSION TO THE UNFCCC. 2017.
- Sylvanus Mensah, Vale`re K. Salako, Achille E. Assogbadjo Kakaı, R.G. Differential Responses of Taxonomic, Structural, and Functional Diversity to Local-Scale Environmental Variation in Afromontane Forests in South Africa. Trop. Conserv. Sci. 2018.
- Magurran, A.E. Ecological Diversity and Its Measurements.Chapman & Hall, London. 1988, 179.
- Pielou, E.C. Ecological Diversity, London: Wiley. 1975.
- Casanoves, F. , Pla, L., Di Rienzo, J., and Diaz, S. FDiver_ Sity: A Software Package for the Integrated Analysis of Func_ Tional Diversity, Methods. Col. Evol. 2011, 2, 233–237. [Google Scholar]
- Pla, L., Casanoves, F., & Rienzo, J.D. Quantifying Functional Biodiversity. Springer, Dordrecht, Heidelberg, London, New York. 2012.
- Kuznetsova, A. , Brockhoff, P. B., & Christensen, R.H.B. LmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Software, 2017, 82, 1–26. [Google Scholar]
- Bates, D, Mächler, M, Bolker, B. , & Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar]
- Feyera Senbeta, Christine Schmitt, Tadese Woldemariam, H. J.B. and M.D. PLANT DIVERSITY, VEGETATION STRUCTURE AND RELATIONSHIP BETWEEN PLANT COMMUNITIES AND ENVIRONMENTAL VARIABLES IN THE AFROMONTANE FORESTS OF ETHIOPIA. SINET Ethiop. J. Sci., 2014, 37, 113–130. [Google Scholar]
- Garuma, G.C. Plant Diversity and Ethnobotanical Study of Traditional Medicinal and Wild Edible Plants in Yayo and Hurumu Districts, Ilu Abba Bor Zone of Oromia Region, Southwest Ethiopia:A. Ph.D. Thesis, ; Addis Ababa University Addis Ababa, Ethiopia, 2020. [Google Scholar]
- Gautam M.K , R. K. Manhas, and A.K.T. Overstory Structure and Soil Nutrients Effect on Plant Diversity in Unmanaged Moist Tropical Forest. Acta Oecologica 2016, 75, 43–53. [CrossRef]
- Wilkinson, D.M. The Disturbing History OfIntermediate Disturbance. Oikos 1999, 84, 145–7., doi:10.2307/3546874 (https://doi.org/10.2307%2F35468 74) . JSTOR 3546874 (https://www.jstor.org/stable/35468 74). [CrossRef]
- Kong, L.; Xiong, K. . Z.; S.; Zhang, Y.; Deng, X. Review on Driving Factors of Ecosystem Services: Its Enlightenment for the Improvement of Forest Ecosystem Functions in Karst Desertification Control. forest 2023, 14, 1–20. [Google Scholar] [CrossRef]
- Biswas, S. R. , and A.U.M. Species Diversity and Functional Diversity Relationship Varies with Disturbance Intensity. Ecosphere 2011, 2, 1–10. [Google Scholar] [CrossRef]
- Levine, N.M.; Zhang, K.; Longo, M.; Baccini, A.; Phillips, O.L.; Lewis, S.L.; Alvarez-Dávila, E.; De Andrade, A.C.S.; Brienen, R.J.W.; Erwin, T.L.; et al. Ecosystem Heterogeneity Determines the Ecological Resilience of the Amazon to Climate Change. Proc. Natl. Acad. Sci. U. S. A. 2016. [Google Scholar] [CrossRef]
- Zhang Jin-Tun, Xiaohang Bai, D. S. FUNCTIONAL DIVERSITY AND ITS ATTRIBUTE FACTORS IN THE YUNMENG MOUNTAIN NATIONAL FOREST PARK IN BEIJING, CHINA. CERNE 2017, 23, 75–84. [Google Scholar] [CrossRef]
- Bertrand Fournier, Francois Gillet, Renee-Claire Le Bayon, Edward A. D. Mitchell, M.M. Functional Responses of Multitaxa Communities to Disturbance and Stress Gradients in a Restored Floodplain. J. Appl. Ecol. 2015, 1–19. [CrossRef]
- Adler Peter, Roberto Salguero-Gómez, Aldo Compagnoni, and M. F. Functional Traits Explain Variation in Plant Life History Strategies. Biol. Conserv. 2014, 111, 740–745. [Google Scholar]
- Hongwei Zhang, F.L.L. Adaptive Cooperative Tracking Control of Higher-Order Nonlinear Systems with Unknown Dynamics. Sci. direct 2012, 48, 1432–1439. [Google Scholar] [CrossRef]
- Ying Li, Jihua Hou, Li Xu, Mingxu Li, Zhi Chen, Zihao Zhang, N. H. Variation in Functional Trait Diversity from Tropical to Cold-Temperate Forests and Linkage to Productivity. Ecol. Indic. 2022, 138, 1–22. [Google Scholar] [CrossRef]
- Papanikolaou, A.D. , Fyllas, N.M., Mazaris, A.D., D.; trakopoulos, P.G., Kallimanis, A.S., and Pantis, J.D. Grazing Effects on Plant Functional Group Diversity in Mediterra_nean Shrublands. Biodiv. Conserv 2011, 20, 2831–2843. [Google Scholar] [CrossRef]
- Zhang Jin Tun, Jitian Xiao, L. L. Variation of Plant Functional Diversity Along a Disturbance Gradient in Mountain Meadows of the Donglingshan Reserve, Beijing, China. Russ. J. Ecol. 2015, 46, 157–166. [Google Scholar] [CrossRef]
- Mensaha Sylvanus, Kolawolé Valère Salakoa, Achille Assogbadjob, R. G.K.; Brice Sinsinb, T.S. Functional Trait Diversity Is a Stronger Predictor of Multifunctionality than Dominance: Evidence from an Afromontane Forest in South Africa. Ecol. Indic. 2020, 115, 1–11. [Google Scholar]
- Mengistu Teshome, Zebene, Asfaw, & Mohammed, M. Pattern of Functional Diversity along the Elevation Gradient in the Dry Evergreen Afromontane Forest of Hararghe Highland, Southeast Ethiopia. biosytems Divers. 2020, 28, 257–264. [Google Scholar] [CrossRef]
- Zhang Zihao, Hou Jihua, and H. N. Predictability of Functional Diversity Depends on the Number of Traits. J. Resour. Ecol. 2021, 12, 332–345. [Google Scholar] [CrossRef]
- Laliberte, E. and Legendre, P. A Distance_based Framework for Measuring Functional Diversity from Multiple Traits. Ecology 2010, 91, 295–305. [Google Scholar] [CrossRef]
- Ruksan Bose, B.R.R.R.P.; Munoz, F. Phylogenetic Diversity in the Western Ghats Biodiversity Hotspot Reflects Environmental Filtering and Past Niche Diversification of Trees. J. Biogeogr. 2018, 1–13. [Google Scholar] [CrossRef]
- Mayfield1, M. M., S. P.B.; Morgan, J.W.; Aubin, I.; And, S.M.; Vesk, P.A. What Does Species Richness Tell Us about Functional Trait Diversity? Predictions and Evidence for Responses of Species and Functional Trait Diversity to Land-Use Change. Glob. Ecol. Biogeogr. 2010, 19, 423–431. [Google Scholar] [CrossRef]
| FDp | FRic | FEve | FDiv | FDis | CWM WD | CWM SLA | CWM Hmax | CWM SM | |
|---|---|---|---|---|---|---|---|---|---|
| Disturbance | -0.46*** | -0.54*** | -0.51*** | -0.44*** | -0.70*** | 0.11 | 0.61*** | -0.75*** | 0.81*** |
| Slope | 0.19 | 0.20 | 0.03 | 0.15 | 0.25* | 0.10 | -0.00 | 0.11 | -0.12 |
| Aspect | 0.00 | -0.02 | 0.02 | 0.09 | -0.02 | 0.13 | 0.01 | -0.03 | -0.02 |
| Elevation | -0.44*** | -0.49*** | -0.34** | -0.43*** | -0.52*** | -0.016 | 0.39*** | -0.44*** | 0.52*** |
| FunctionalDiversity Component | Effect | Estimate | d.f. | SE | P Value |
|---|---|---|---|---|---|
| FDp | Aspect | 0.015 | 81.615 | 0.007 | 0.029 * |
| Disturbance | 2.311 | 83.147 | 0.565 | 9.84e-05 *** | |
| FRic | Disturbance | 1.400 | 83.104 | 0.520 | 0.009 ** |
| FEve | Aspect | 0.001 | 82.947 | 0.0004 | 0.027 * |
| disturbance | 0.069 | 16.181 | 0.029 | 0.031 * | |
| FDiv | Aspect | 0.002 | 82.960 | 0.0007 | 0.047 * |
| FDis | disturbance | 0.277 | 83.05 | 0.032 | 2.38e-13 *** |
| CWM WD | disturbance | -0.079 | 83.928 | 0.030 | 0.011 * |
| CWM Hmax | Disturbance | 1.541 | 74.251 | 0.758 | 0.046 * |
| CWM SM | Disturbance | -2.196 | 83.033 | 0.144 | < 2e-16 *** |
| Functional Diversity Components | Species Richness (S) | Shannon Weiner Diversity (H') | Evenness Index (E) |
|---|---|---|---|
| FDp | 0.70 *** | 0.48*** | 0.40*** |
| FRic | 0.64 *** | 0.51*** | 0.52*** |
| FEve | 0.41*** | 0.50*** | 0.37*** |
| FDiv | 0.33** | 0.39*** | 0.33** |
| FDis | 0.46*** | 0.63**** | 0.72*** |
| CWM SLA | -0.36*** | -0.56*** | -0.51*** |
| CWM WD | -0.036 | -0.30** | 0.028 |
| CWM Hmax | 0.42*** | 0.71*** | 0.59*** |
| CWM SM | -0.55*** | -0.86*** | -0.57*** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





