1. Background
By way of background the National health Service (NHS) in the UK is run in a devolved manner such that each of the four countries in the Union determine the level of funding, set their own policies, and manage the NHS in different ways. This author entered the English NHS in the early 1990s and was quickly involved in planning for the construction of a new hospital. I was informed that the ‘correct’ way to forecast future bed numbers was to take age-banded current admission rates (admissions per head of population) and use forecast population demographic changes (age-based forecasting) to estimate future inpatient admissions and to multiply such future admissions by the anticipated future average length of stay (LOS) to arrive at occupied bed days, and then apply an occupancy margin to arrive at the number of available beds [
1]. My experience of bed planning then led to a 30-year search for more reliable methods with many related published studies.
Throughout this study, a body of nearly 300 supporting publications will not be referenced as it is assumed that the reader will make themselves aware of the scope of this wider evidence. To facilitate access to this material the full list is given in the Supplementary Material S1. This material is broken down into 19 alphabetically labelled sections (A to S) with papers in each section having a number. The reason that there are 19 sections is that almost all the issues are interlinked. Hence capacity risk is linked to financial (cost) risk. Volatility in admissions is central to capacity and cost risk, but also to understanding the optimum bed occupancy rate, etc., etc. In the text the reader will be referred to the relevant section heading, i.e., A to R, or to a specific reference such as A.10, R.2, etc.
It should be noted that the British Journal of Healthcare Management (BJHCM) acknowledged the need for an extended research series in this poorly explored area. As part of this research BJHCM ran a monthly series called ‘Money Matters’ consisting of short mini-research pieces which explored the far wider issues of why it was so hard for healthcare organizations to achieve both bed capacity and financial balance.
Additional reference will be made to the wider body of literature using the usual bracketed reference number system. One example is the excellent systematic review of the current state of hospital bed modelling by Ravaghi et al published in 2020 [
2] which identified 11 models and 5 methods. It should be noted that some of these models/methods involve short-term or tactical forecasting while this study focusses on long-term bed planning.
I very quickly realized that this method was entirely unreliable and seemed to be deemed ‘correct’ simply because it grossly underestimated future demand, thereby ‘confirming’ the political view that England needed fewer hospital beds because the NHS was ‘inefficient’. The abject failure of age-based forecasting to predict future admissions is illustrated in the Supplementary material S2 [
3,
4,
5,
6,
7,
8,
9,
10] where the actual trend in admissions for diseases of the appendix (mostly appendicitis) is compared to the age-based forecast.
It has subsequently taken around 30 years to fully understand the intricacies of long-term bed forecasting. Any aspect of study taking 30 years is likely to indicate that the underlying issues involve a profoundly complex system. The huge divergence between actual and age-based forecast for diseases of the appendix in Supplementary material S2 is but one example of such complexity.
An additional aim is to detail how international benchmarks and readily available data can be applied to the local level of hospital bed planning. The important role of volatility in admissions and bed demand will be discussed in terms of the issue of surge capacity and the average bed occupancy. Finally, the profoundly important role of government policy will be illustrated.
Each section will discuss the limitations of current methods and suggest suitable alternatives. Many of the methods/data sources have already been covered, however, where an alternative is suggested the methods/data sources will be given in that section.
2. Introduction
It must be pointed out that the then accepted method of long-term bed forecasting using population age structure [
1] has never been proven to work, per se [A.9,C.15]. While it is true that age-standardization is a valid tool in the field of epidemiology [
11], it is mainly employed in a retrospective way but requires types of time series regression or other forecasting to extrapolate into the future. Indeed, age-banded population forecasts are themselves uncertain and contain numerous assumptions [
12,
13,
14]. My own experience is that both births and deaths, which are fundamental to population change, show profoundly high uncertainty [H.12]. Indicating ambiguity in the assumptions or that the hidden assumptions show intrinsic high variability.
The huge deficiency in this method is that the admission rate per head of population is assumed to stay constant, while the reality is that it changes (mainly increases) over time [1,A.9,A.1–20, C.1–22] as the scope of medical interventions grows, hence the threshold to admission declines due to the realization that new interventions can extend lifespan. The proportion of elderly living alone, and the availability of carers has a big impact on the ensuing hospital admissions [
15,
16]. The rate of increase also depends on the admission type, namely elective versus emergency [
17], and the specialty and specific intervention—as for diseases of the appendix, etc. In theory, this eventuality was supposed to be covered by an assessment of the likely impact of new technology [
18], however in practice this was always underestimated.
Indeed, the reality was that although the English NHS was operating with fewer and fewer available beds, the average overnight stay bed occupancy rate was rapidly rising (with associated operational problems) [19,20,L.30] and the number of occupied beds had not changed since 1998/1999 [L.49]—although the models said they were supposed to be declining.
My search eventually led to the interesting observation that occupied bed numbers appeared to be strongly influenced by the absolute number of deaths [L.10,L.16], which is termed the nearness-to-death (NTD) or time-to-death (TTD) phenomena. NTD/TTD is a very well recognized concept in the field of economics and the forecasting of future healthcare costs [
21,
22]. While NTD/TTD has been extensively researched for over 30 years it has never been incorporated into bed modelling.
This seemingly contradictory finding to the currently accepted views regarding ‘correct’ bed modelling arises for several reasons:
Around 55% of a person’s lifetime hospital bed usage occurs in the last year of life, irrespective of the age at death [
23,
24,
25].
Increased lifespan expands the opportunity for lifetime hospital admissions and hence the 55% figure (#1 above) leads to a steady inflation of bed demand with increasing longevity [
25,
26].
About half of medical bed occupancy appears to be influenced by fluctuations in the absolute number of deaths, seemingly due to local and national outbreaks of infectious agents. On this occasion deaths are serving as a proxy for the specific and nonspecific effects of wider infections, mediated by inflammatory and other processes, necessitating hospital admission but not leading to immediate death (see
Section 11). The COVID-19 pandemic is an excellent example of this relationship where there are far more admissions than deaths.
In an ageing population with increasing multimorbidity, the ‘at risk’ population [
27] where (fluctuations in) the absolute number of deaths is serving as a proxy for morbidity, is also increasing with time.
In deprived/poorer areas the population tends to die at a younger age [
26], hence, bed usage is moved forward in time due to #1 above.
As a first approximation, because everyone must die, the simple division of currently occupied bed days by the current number of deaths seems to work remarkably well [L.10,L.49]. This approximation will break down if the ratio of births to deaths substantially changes and relies on a balance between rising admissions per death (contingent on the issues raised above regarding the elderly living alone and the availability of carers) and declining average length of stay (LOS). In England, admissions seem to be rising roughly as fast as length of stay is declining.
Having established why NTD/TTD is central to bed demand the utility of the model will now be discussed before progressing to how the new model can be extended to the regional and local level within a country. The role of infectious outbreaks on the intrinsically high volatility in admissions (especially emergency) will be covered in
Section 11, where issues of surge capacity and the average bed occupancy margin needed for optimum performance will be explored—especially in the context of local health care capacity.
3. Additional Supporting Analysis
The methods used to analyze the international data and the incremental development of the model have been described previously [L.42,L.45,L.47–51, L.55–56]. Additional supporting analysis is also given in this review both in the text or in Supplementary materials. All data is publicly available, and the source is cited alongside the respective Figure or Table. A description of any methods is included in each section. Where necessary a Supplementary File has been added.
4. The New Model
4.1. Background to the Model
For any model of international bed comparison to be realistic it needs to be based on readily available international data. I first became aware of the NTD/TTD phenomena around 2010 and suggested that this was the missing ingredient in bed modelling [L.9]. Then followed two studies to demonstrate the feasibility of the concept [L.10, L.16]. The first international comparison was published in 2018 [L.42]. Thereafter followed incremental development of the model, mainly around the slope and then the relationship between the slope and the intercept.
In the absence of prior knowledge of the intricacies of each country the outputs from the model depended on the allocation of countries into broad groups (poorest through to very high). This process was conducted using initial visual observation then repeated regression studies after moving marginal countries between groups. Maximization of R-squared was used to achieve the final allocation. This process was also conducted in the absence of the potential role of the age-standardized mortality rate (ASMR), detailed in
Section 8, on the outputs. It is suggested that future development of the model involves ASMR as a variable or used to construct a wider range of groups.
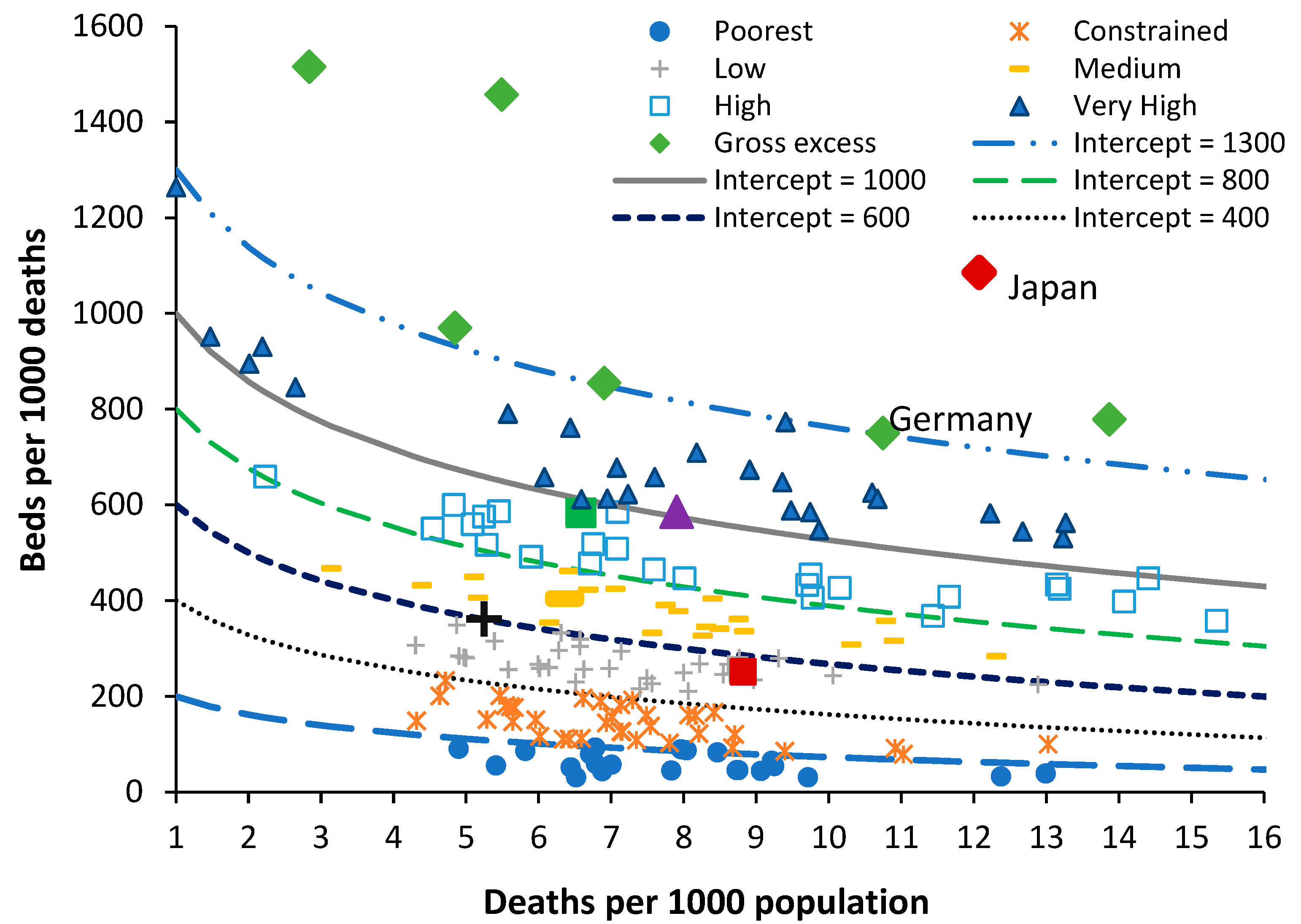
In the new model the ratio of beds per 1000 deaths is plotted against the ratio of deaths per 1000 population. The latter is called the crude mortality rate (
Figure 1) and is an approximate (and widely available) measure of the population age structure. When international bed data is plotted using this method an infinite series of lines of equivalent bed availability, as a logarithmic relationship, can be applied. The lines of equivalent bed availability reflect the different crude mortality rates between countries.
The method has been applied to investigate disparate bed numbers in the states of Australia, the USA and India [L.45,L.47,L.51]. For both total curative [L.42], medical [L.48,L.53–54,], and critical care beds [L.55], and to investigate the expressed need for beds revealed as ‘occupied’ beds in Australian states and English Clinical Commissioning Groups (CCGs) [L.49]. In the case of Australian states adjustment was applied for the proportion of indigenous people (with known higher levels of poor health) [L.56], and for the differing levels of social deprivation experienced between English CCGs [L.49]. It was finally discovered that the intercept and slope of the logarithmic relationships are directly linked such that the lines of equivalent bed availability can be defined using the value of the intercept as shown in
Figure 1 [L.56].
Countries lying along or near to a line of equivalence have similar bed numbers, however, the reasons for equivalence can be many and varied. It has been noted that the average bed occupancy for a country depends greatly on the average size of the constituent hospitals [L.12,L.49], and that acute bed demand may depend on the wider availability of nursing home beds [L.49], and factors such as the implementation of integrated care [
39,
40,
41]. A further extension of the model was to define a likely feasible region for the optimum number of critical care beds in the absence of excessive ‘futile’ intervention which can occur in fee-for-service health systems [L.55].
Figure 1 contains data for world countries in 2019 (just before the disruption caused by the COVID-19 pandemic) along with several lines of equivalent available beds. In
Figure 1 the number of beds in each country is in 2019 or the nearest year after extrapolation of the recent trend to 2019, by either linear or polynomial regression.
In terms of total beds most countries are trending down, or stationary, but a few such as China and Türkiye are trending upward. World countries have been grouped from the world’s poorest through to those with very high available beds—once again for many and varied reasons. Japan and South Korea are excessively high because they seemingly (incorrectly) count ‘nursing home’ type care as a ‘curative’ bed [L.49]. The data for North Korea is also very high but has probably been fabricated. Germany is high due to a network of smaller hospitals with potential over-utilization to offset the higher costs of running a small hospital [L.21,L.49].
Australia has a moderately higher number of available beds because there is a network of small rural hospitals and most elective surgery is conducted in smaller privately owned hospitals which flex their staffing to reflect the surgical workflow rather than staffing the beds per se. The region highlighted as ‘medium’ in
Figure 1 is probably close to the optimum number of available beds which this study seeks to identify. This number is then interpreted from patterns of operation and hospital size as per Australia, and the degree of implementation for helpful policies such as integrated care, etc.
By way of comparison countries with similar bed provision to England are (deaths per 1000 population in brackets), Fiji (8.0), Equatorial Guinea (8.5), Sweden (8.6), Kiribati (6.3), Colombia (5.4), Comoros (8.2), and Uruguay (10.1). All these countries roughly lie along a line of bed equivalence which is 28% below a line with intercept = 700 or the middle of the ‘medium’ bed number region. All these countries lie along this line for vastly different reasons. None of these countries other than Sweden can lay claim to a highly effective health care system. Sweden invests heavily in outpatient and long-term care, has a higher number of doctors and nurses per person [
31] and has a long history of policy investment in integrated care [
32]. The other countries have lower bed numbers usually due to lack of funding.
It is now useful to investigate if the widely used metric of beds per 100,000 population can also be modified along similar lines.
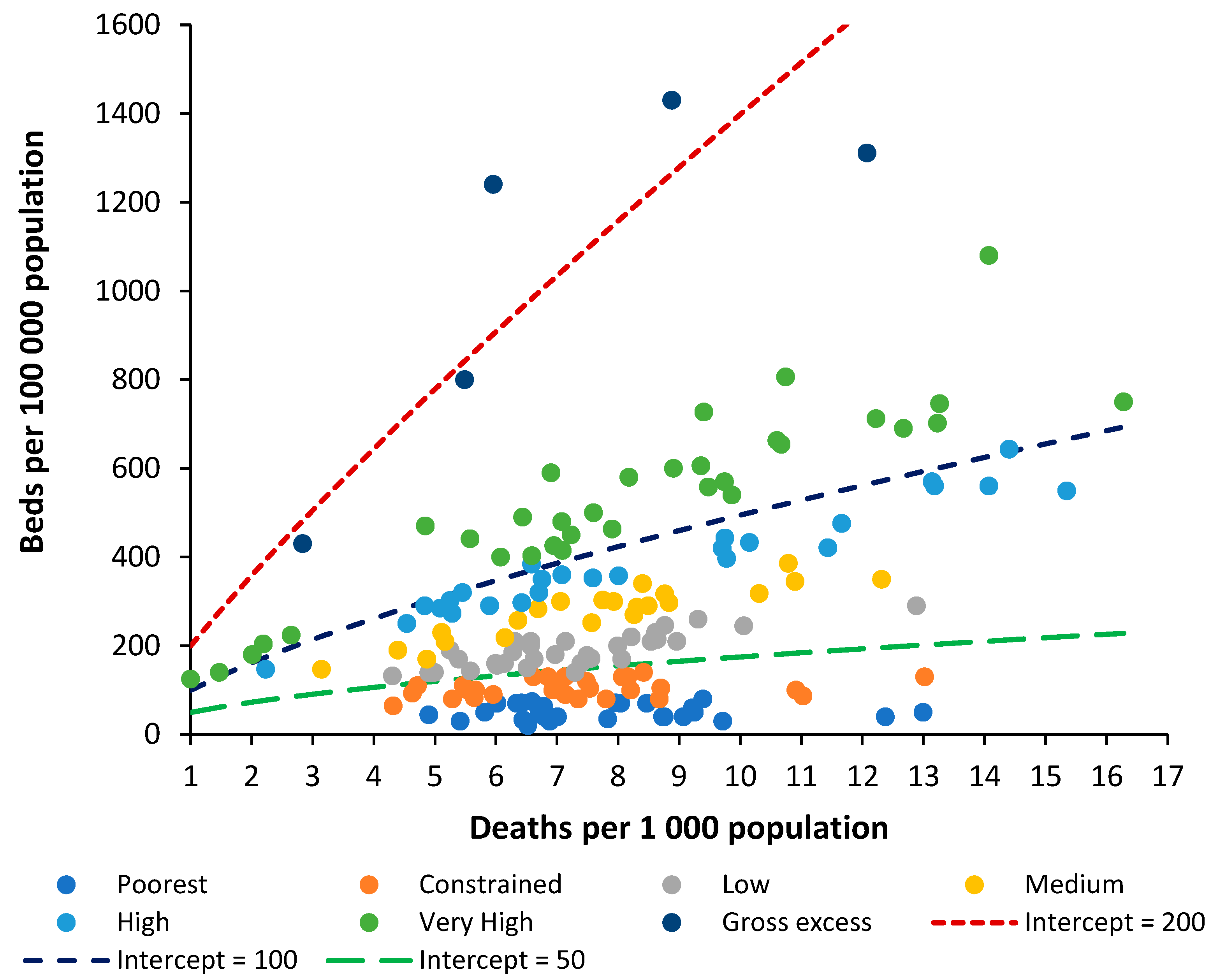
4.2. Beds per 1000 Deaths Compared to Beds per 1000 Population
For any analysis of bed numbers to be successful there must be some adjustment to reflect the population age profile, and deaths per 1000 population is the measure which is most readily available. To this end
Figure 2 shows the same data as in
Figure 1 but using the ratio of beds per 1000 population versus deaths per 1000 population.
To investigate the relationship between beds per 100,000 population and the crude mortality rate the groups used in
Figure 1 (poorest through to very high) were recalculated from beds per 1000 deaths to beds per 100,000 population. Several countries then appeared as outliers and were moved between groups. A range of functions available in the Microsoft Excel curve fit application were tested with a power law function giving the highest R-squared. The constant ‘A’ and power function ‘B’ for the power law function were then determined by curve fit for each of the groups (poorest through to very high).
Beds per 100,000 population = A × (Deaths per 100,000 population) B
The values A and B are likewise related such that the power function B equals:
B = 0.2168 × ln(A) – 0.3042 (R2 = 0.9864)
This then leads to a set of lines of equivalence defined by the intercept (the value of A) when deaths per 1000 population is equal to 1, which is the minimum international value for the crude mortality rate.
Hence, the two methods are approximately comparable. It must be pointed out that the method based on beds per 100,000 population gives slightly less discrimination between countries, and in addition has no theoretical basis as has been established for the role of deaths. The new method is therefore superior to beds per 100,000 population, even after adjustment for the role of population age structure as approximated by the crude mortality rate.
The method will now be employed to study trends in bed supply between countries.
5. Trends in Bed Supply
For the method to be valid it must be able to interpret the trends in bed supply over time. To this end
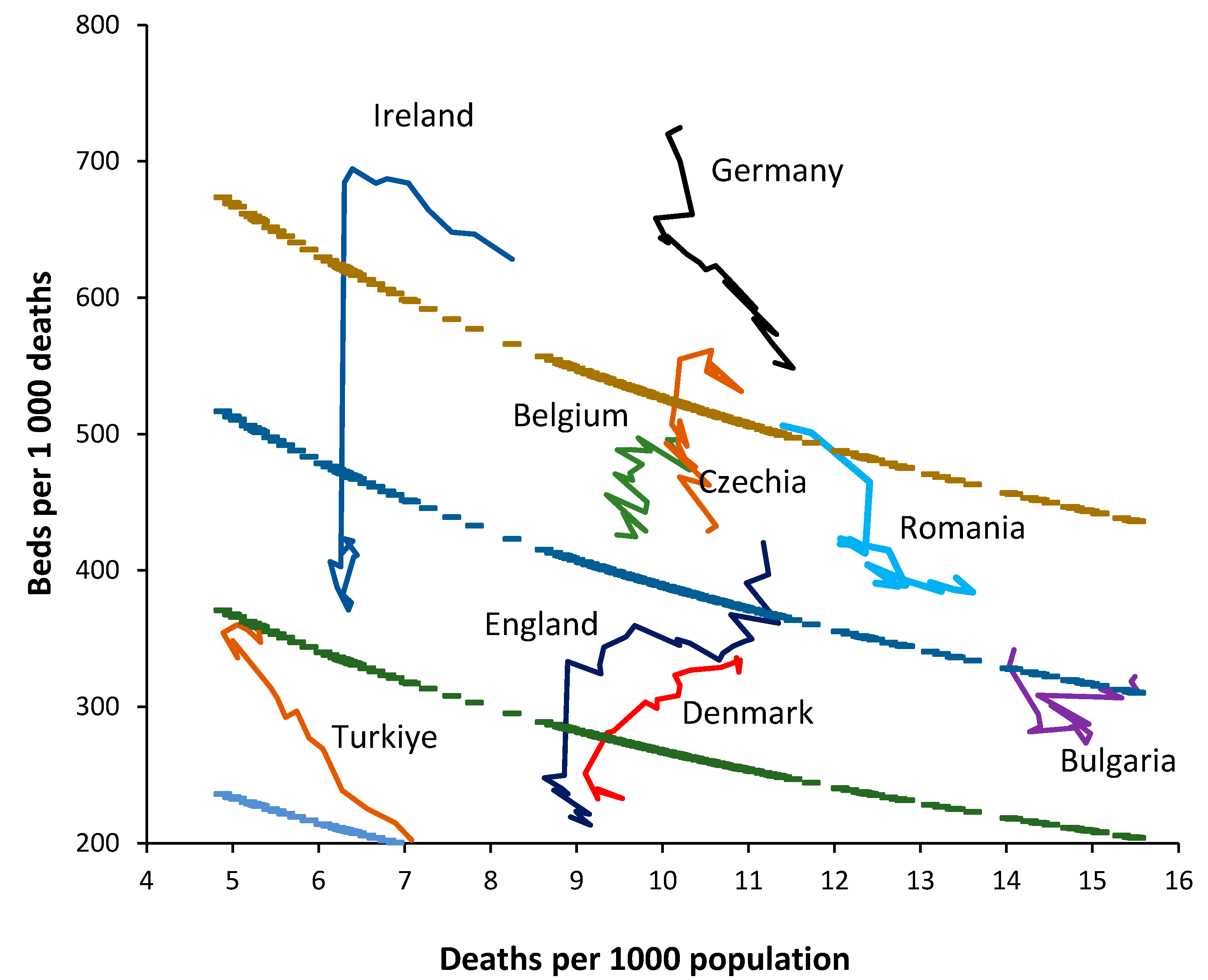
Figure 3 shows a 20-year trend in bed supply (2000–2019) for 8 randomly chosen European countries. By way of comparison a 30-year time series has been added for England.
Firstly, note that the crude mortality rate in all countries is changing with time. Declining in Türkiye through to rising in Germany. If the method is valid, in the absence of changes in bed supply each country should lie parallel to a line of equivalence. Countries with the fewest deaths will show higher levels of zig-zag movement depending on year-to-year changes as per
Figure 1. The following comments apply:
Germany commences with a very high bed supply which is rapidly reduced during the first six years. The reduction continues thereafter but at a slower pace. The rate of reduction in bed supply appears to be slowing and the most recent trend is decaying to approach a slope which is closer to a line of equivalence in an ageing population (higher crude mortality).
Ireland commences with high apparent bed supply and lies parallel to a line of equivalence for the first nine years. The sudden reduction in 2009 is due to a change in counting in which long-term-care beds are more correctly classified as ‘nursing home’ beds. Thereafter bed supply remains roughly unchanged.
Türkiye commences with very low bed supply in 2000 and then rapidly expands bed provision. By 2017 this expansion ceases and supply lies parallel to a line of equivalence.
Denmark commences with higher bed supply in 2000 and reduces bed numbers. From 2016 onward bed supply remains constant and lies parallel to a line of equivalence.
Romania commences with higher bed supply, which is then reduced. From 2010 onward supply remains constant and lies parallel to a line of equivalence.
Bulgaria shows no obvious changes in bed supply and lies roughly parallel to a line of equivalence. This country looks to be subject to higher volatility in year-to-year crude mortality suggesting higher volatility in infectious outbreaks.
Belgium commences with higher bed supply which is continuously reduced through to 2019. Belgium experiences the smallest change in crude mortality during the 20-year period with a slight trend to lower crude mortality over time.
Czechia commences with higher bed supply with slight reduction through to 2007. In this period the line is roughly parallel to a line of equivalence. Beyond 2007 bed supply is rapidly reduced. Czechia shows a U-shaped change in crude mortality reaching a minimum around 2008.
England commences in 1990 with higher beds which are rapidly reduced through to 2000. A scheme to increase bed supply then culminated in 2004, after which beds slowly declined but showed a dramatic decline due to measures to correct an NHS deficit implemented in 2010 which arose from an unfortunate coincidence of higher-than-average deaths in 2007 and 2008 and in response to austerity measures imposed on the NHS following the earlier 2008 international financial crash. The crude mortality rate declines from 1990 to 2011 after which it begins to increase.
Hence, the method does indeed appear to follow a line of equivalence under conditions when bed supply seems to be roughly adjusted to reflect the prevailing deaths. Changes in the definition of a curative bed are relatively easy to discern as for the dramatic shift seen for Ireland.
The consequences of higher deaths in 2007 and 2008 upon health care financial pressures (the morbidity-mortality relationship), plus imposed financial austerity from 2010 onwards (from the 2008 financial crash) are shown in England as hospitals attempt to save costs by cutting bed numbers.
It is apposite to turn our attention to the factors which may influence the supply of beds in different countries.
6. Wealth and Bed Supply
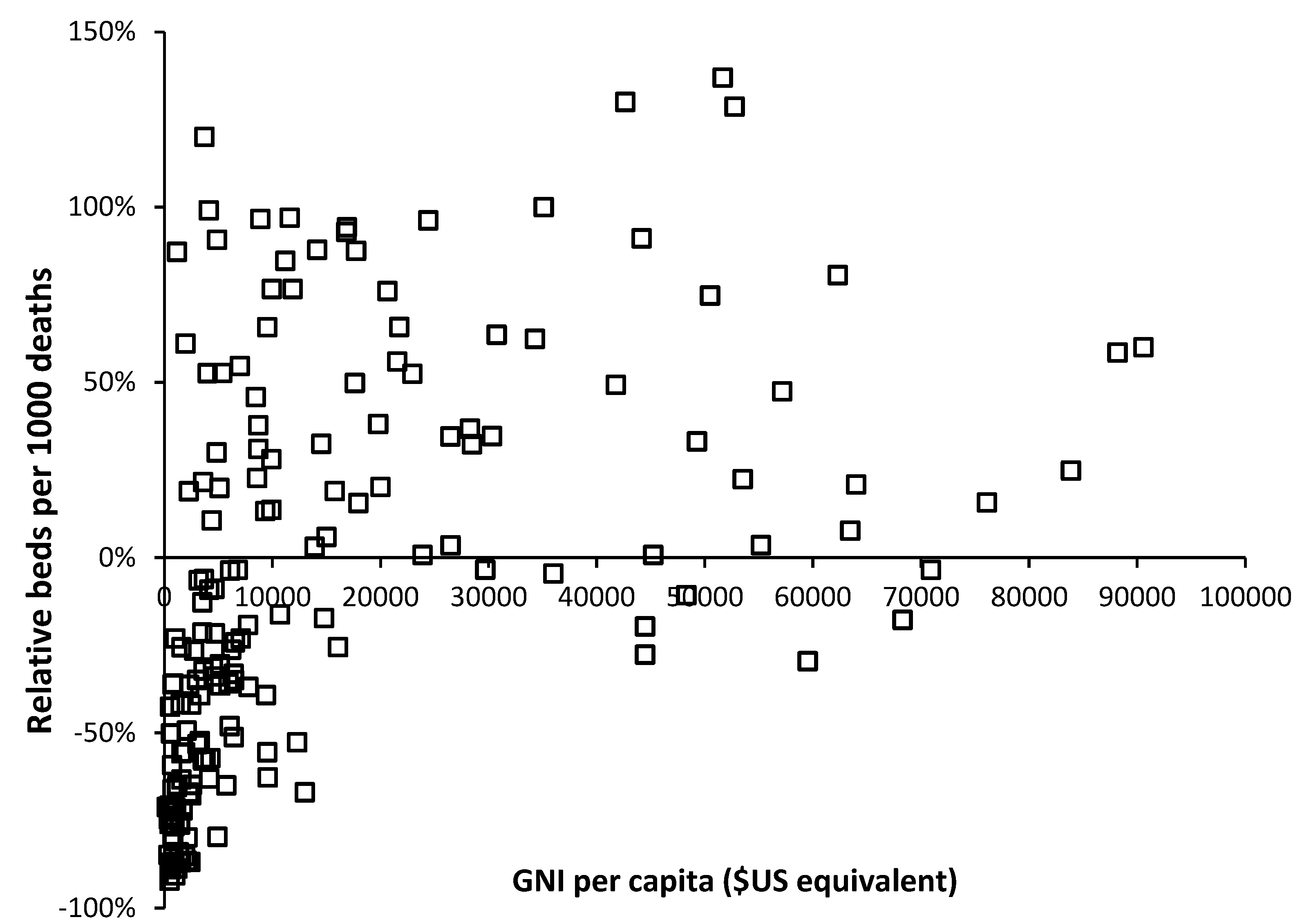
The availability of government funding for healthcare will have a profound effect on hospital bed numbers and
Figure 4 demonstrates this relationship using gross national income (GNI) per capita as a measure of wealth [
33]. GNI has been used since it is a readily available international metric.
As can be seen in
Figure 4 below
$10,000 per capita there is a strong relationship between bed supply and GNI. The most notable exception is turbulent Venezuela where a GNI of
$13,000 is associated with a relative number of beds which is 67% below that expected. This level of GNI should be associated with bed supply close to the 0% relative difference reference line.
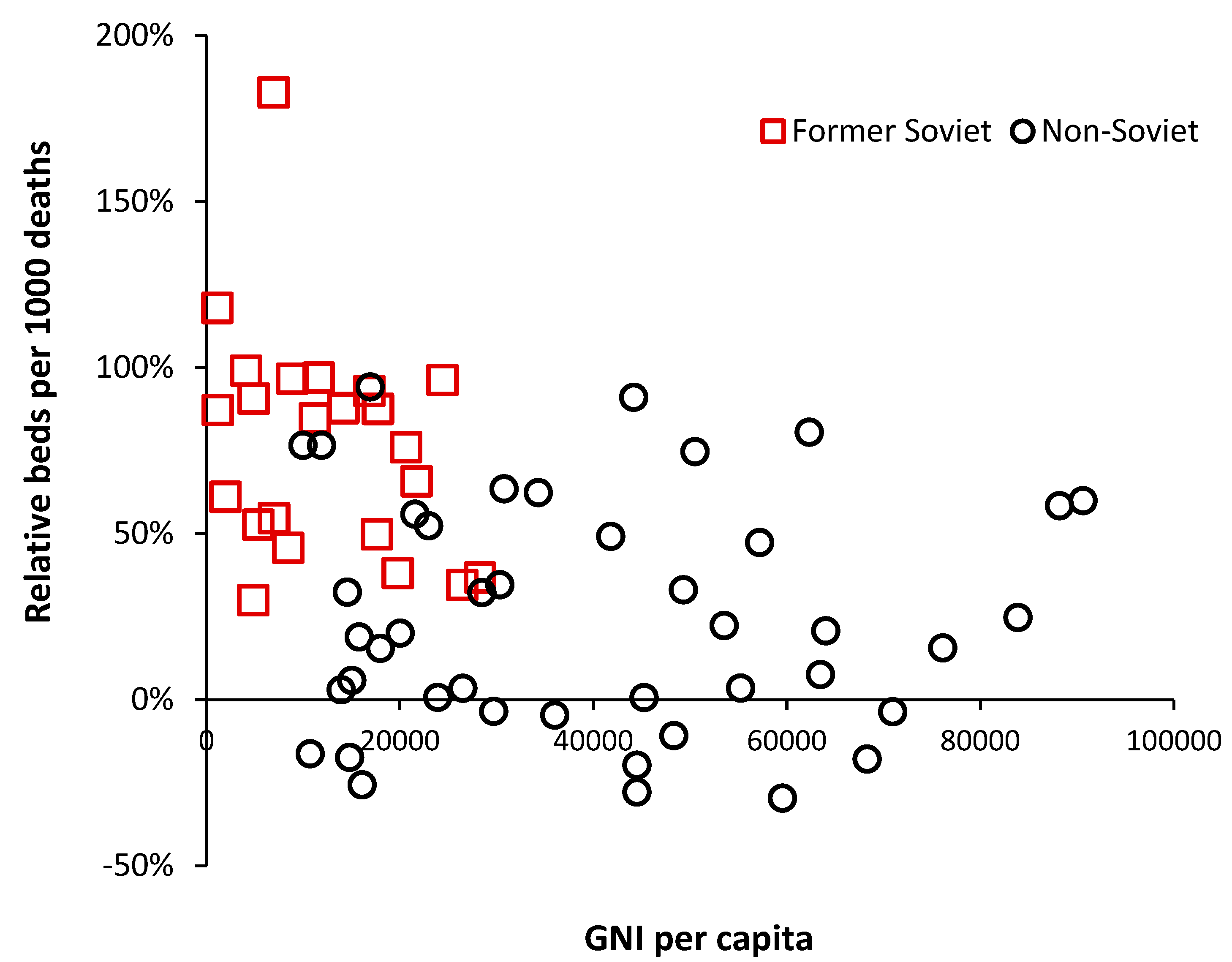
The group of countries with GNI below
$10,000 but high relative beds are of interest and inspection reveals that these are all former or current Soviet bloc countries. This issue is explored in
Figure 5 where an inherited Soviet style centrally planned health care system can be seen to be overly reliant on hospital beds. Many of the former Soviet bloc countries gained independence around 30 years ago, and after 30-years of reform and acquired higher GNI are beginning to approach levels of bed availability seen in non-Soviet countries.
However, above a GNI of $20,000 per capita there is still gross divergence between countries indicating that different health care systems run at very different levels of bed supply.
This study will now attempt to extend this new method to define a potential optimum region for different types of clinical need, i.e., acute care, medical care, mental health, longer-term (geriatric) care, etc., and to make a comparison of the levels of nurses, doctors, and surgeons.
7. Defining an Optimum Region
7.1. Medical and Critical Care Beds
A likely optimum region for adult critical care beds has been recently investigated [L.55]. It was concluded that in the absence of futile end-of-life interventions this optimum was likely to lie in the region for occupied beds between a line of equivalence with an intercept of 60 down to somewhere above 30 [L.55]. This can then be turned into available beds based on the size of the CCU. This region encompasses the available CCU beds in the wealthiest Indian states and is at the lower edge for US states. The level of futile intervention in the USA is judged to be high [L.55], hence, all but nine US states lie above the line of equivalence with an intercept of 60. All but a few wealthy Indian states lie below a line with intercept of 60.
The issue regarding medical beds was recently addressed [L.48,L.54]. A likely optimum region may lie below the line of equivalence with an intercept of 450 and above a line with intercept of 375. Occupied medical beds in England for the 21 years between 1998/1999 and 2019/2020 lie along the line of equivalence with an intercept of 375 [L.48]. Since it is known that England has too few medical beds (relative to current demand) then the optimum region will have its lowest limit above an intercept of 375.
It goes without saying that the demand for medical beds will depend on the mix of pathogens afflicting the population each year (see section 11). In this respect the level of occupied beds in England can reach as high as a line of equivalence with an intercept of 420, hence, below 420 only copes with years when the pathogen mix is less malign in its actions. This will be discussed in
Section 11.
7.2. Psychiatric Beds
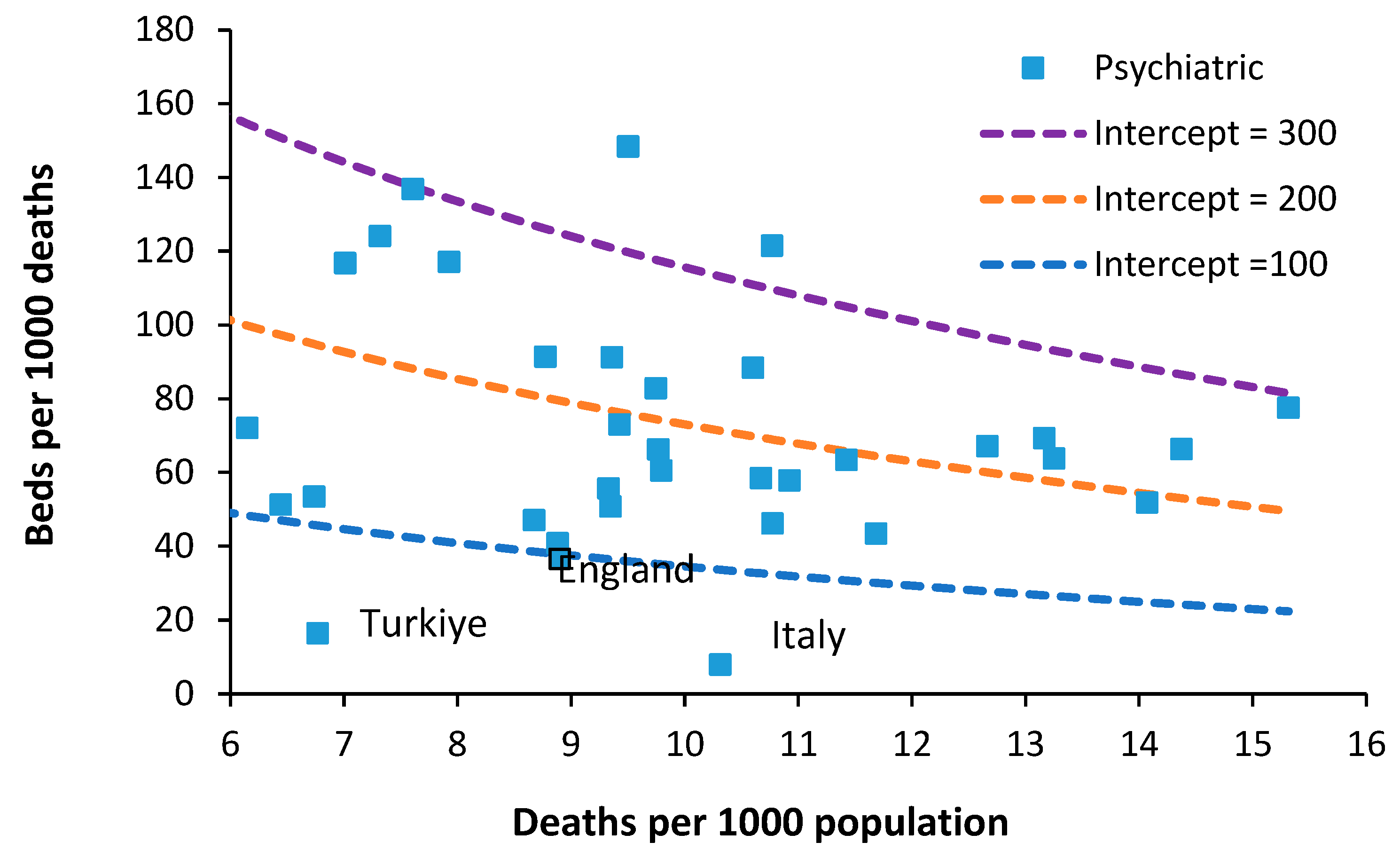
The required number of psychiatric inpatient beds is a highly debated topic. In one study an international board of advisors nominated some 65 Delphi panel members. Sixty psychiatric beds per 100,000 population were considered optimal and 30 the minimum, whilst 25–30 was regarded as mild, 15–25 as moderate, and less than 15 as severe shortage [
34]. As an approximate comparison 60 beds per 100,000 population at around 8.8 deaths per 1000 population is equivalent to 70 beds per 1000 deaths. Between 1990 and 2000 the median number of psychiatric beds in less developed countries decreased from 3.0 to 2.2 per 100,000 population (median percentage change = −16.1%). Beds in forensic and residential facilities are nonexistent in most less developed countries [
35].
Figure 6 shows the availability of Psychiatric beds for European countries in 2019.
The figure of 60 beds per 100 000 population mentioned above could feasibly lie between lines of equivalence with intercepts of 100 and 200. However, somewhere below an intercept of 100 looks possible.
In 1978 Italy implemented an aggressive shift from hospital-based to community-based care. Compulsory admissions dropped from 20,300 in 1978 to around 8800 in 2015 [
36]. Psychiatric beds fell from 160 beds per 100,000 population in 1978 down to less than 20 from 1999 onward [
36]. Hence it is possible to operate with fewer psychiatric beds provided that suitable levels of outpatient/community care are in place. However, note that this transition in Italy took about 20 years to fully implement and would require substantial investment in psychiatric community staff [
36]. This probably explains why all other countries lie above the line of equivalence with an intercept of 100.
A study in the USA calculated that at optimum levels of community mental health (CMH) workers that 35 psychiatric beds per 100,000 population (equivalent to 42 beds per 1000 deaths [
37]. This is higher than the UK and contains the inbuilt assumption of optimum levels of CMH workers.
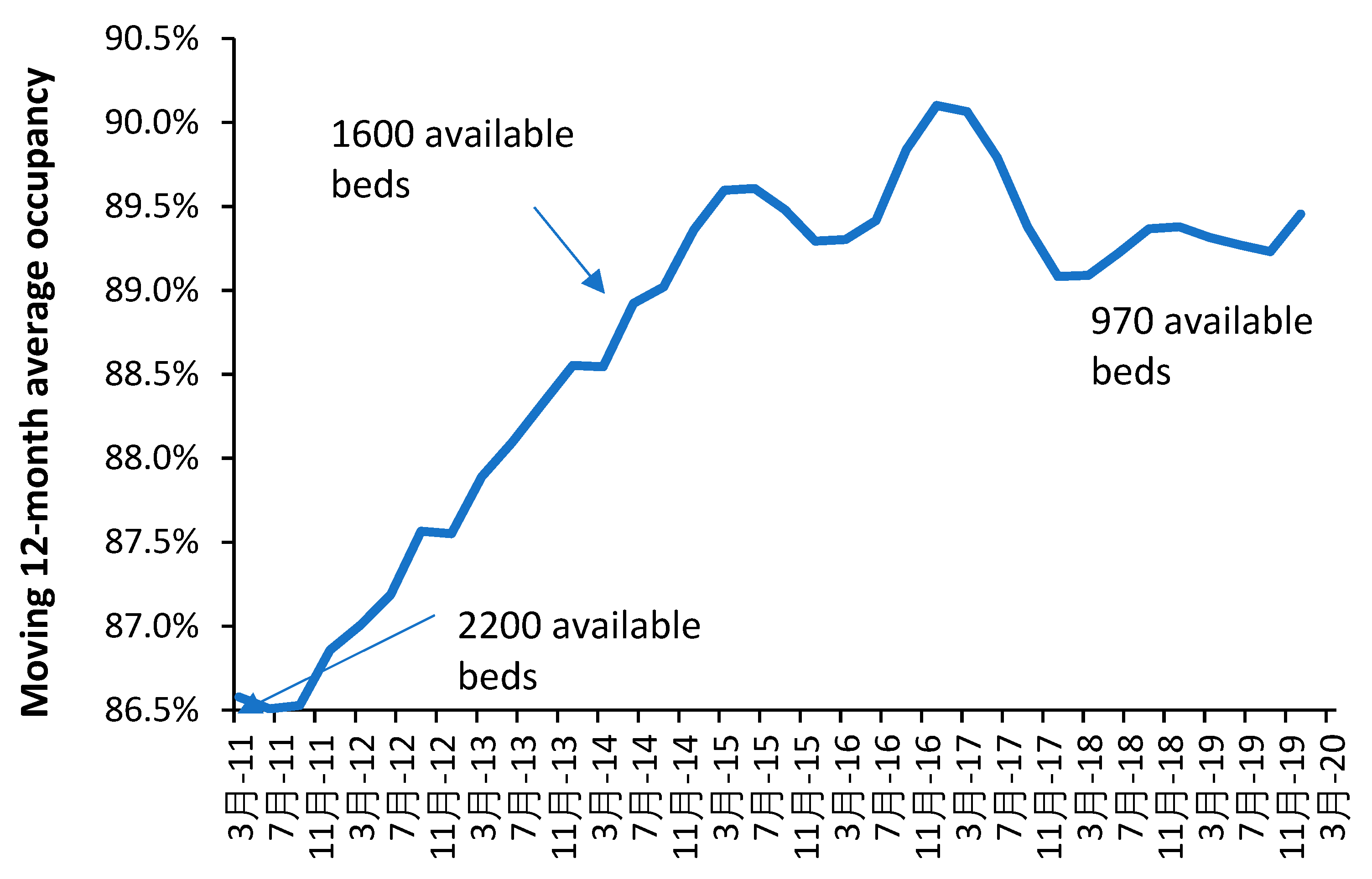
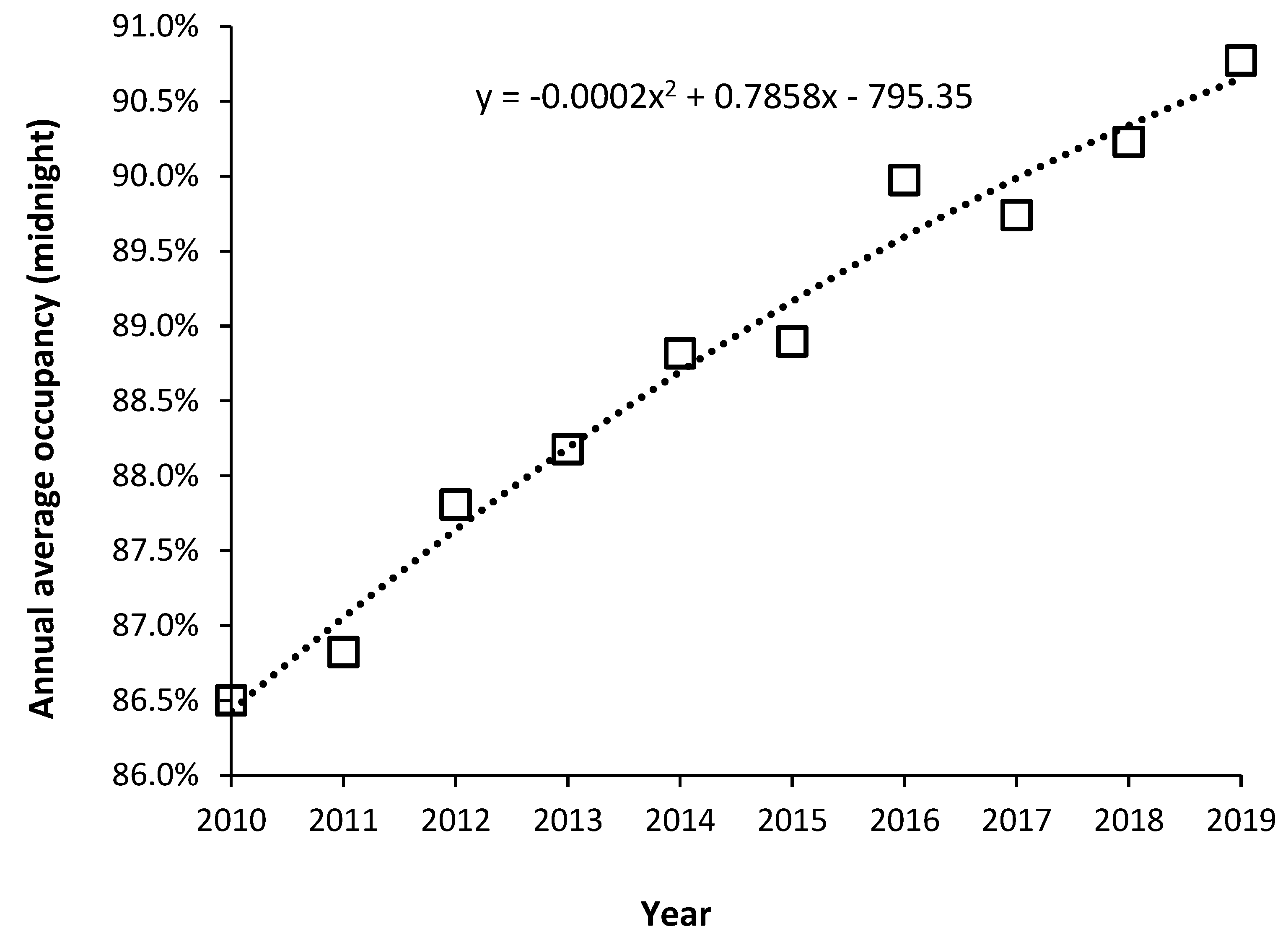
Note that the occupancy levels in English psychiatric hospitals rose from around 86.5% in 2011 to above 89.5% from 2015 onward [
19]. Given that the psychiatric bed pool encompasses everything from secure units, adolescent units, etc., the effect of size on the occupancy rate (discussed in
Section 10) means that somewhere around 90% represents the upper possible limit for average bed occupancy given that the total is made up from smaller specialist and general units. In the general psychiatric units, occupancy is often at 100% and patients are often moved to an available space which is many miles from their home. This implies that the level of outpatient/community support in England is inadequate relative to the available inpatient beds. The shift from an inpatient-based model has been hampered by under-investment in outpatient/community staffing – the importance of which was emphasized in Italy.
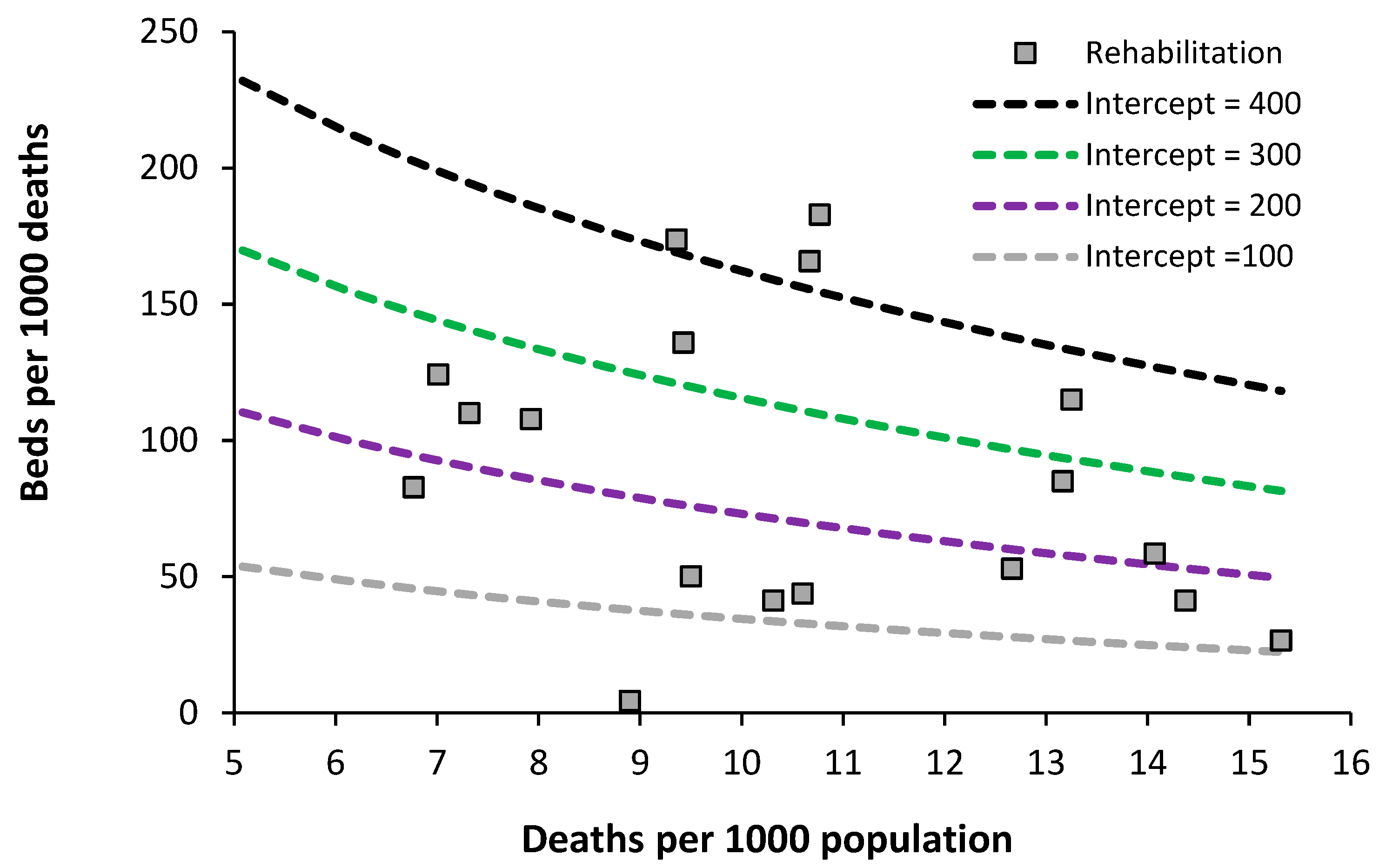
7.3. Acute Rehabilitation Beds
Data on the level of acute rehabilitation beds for European countries is presented in
Figure 7.
The usual wide difference between countries is evident. Data for the lowest country (England) only includes actual occupied bed numbers for the specialty Rehabilitation – although bed occupancy will be in the high 90% region. The issue is complicated by the fact that rehabilitation beds for army veterans are often charitably funded, and rehabilitation units can be in non-acute and private hospitals. It looks like an optimum region could exist lying between lines of equivalence with an intercept of 100 to 200. Further analysis is required on this topic to include a count of all rehabilitation beds irrespective of their location inside or outside of an acute hospital. As always this will rely on suitable investment in outpatient/community support.
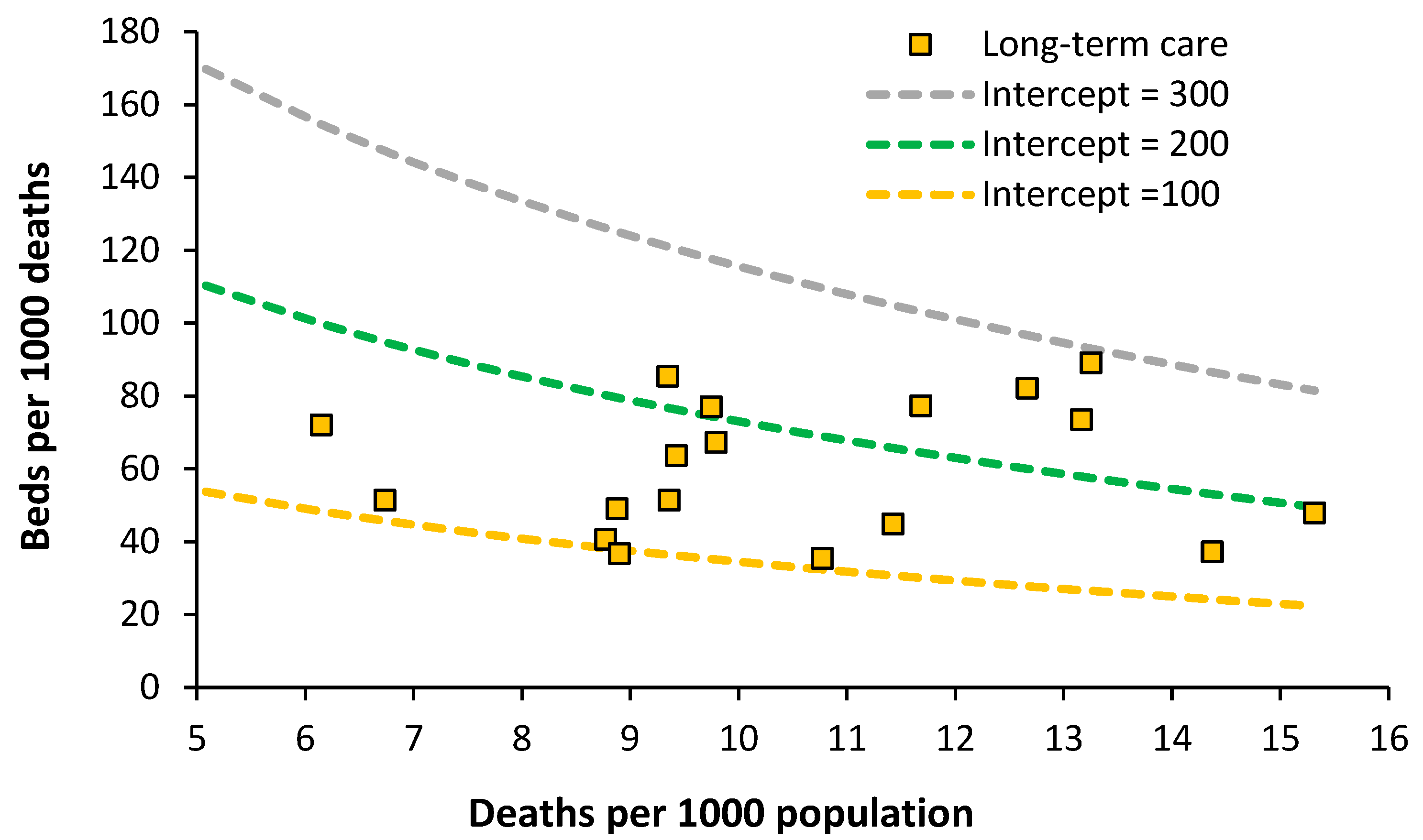
7.4. Acute Long-Term Care Beds
The situation for European countries is given in
Figure 8.
An optimum lying between lines of equivalence with an intercept of 100 to 200 looks possible. Data for England is the lowest data value along the intercept = 100 line. The England data has been taken from the inpatient specialty ‘Geriatric care’ which is delivered by consultant geriatricians. Both geriatricians and geriatric beds are in short supply relative to the demand. Patients requiring geriatric care can therefore be found in general medical beds. Impatient provision lower than an intercept of 100 may be possible with suitable investment in outpatient/community staff.
7.5. Physicians and Nurses
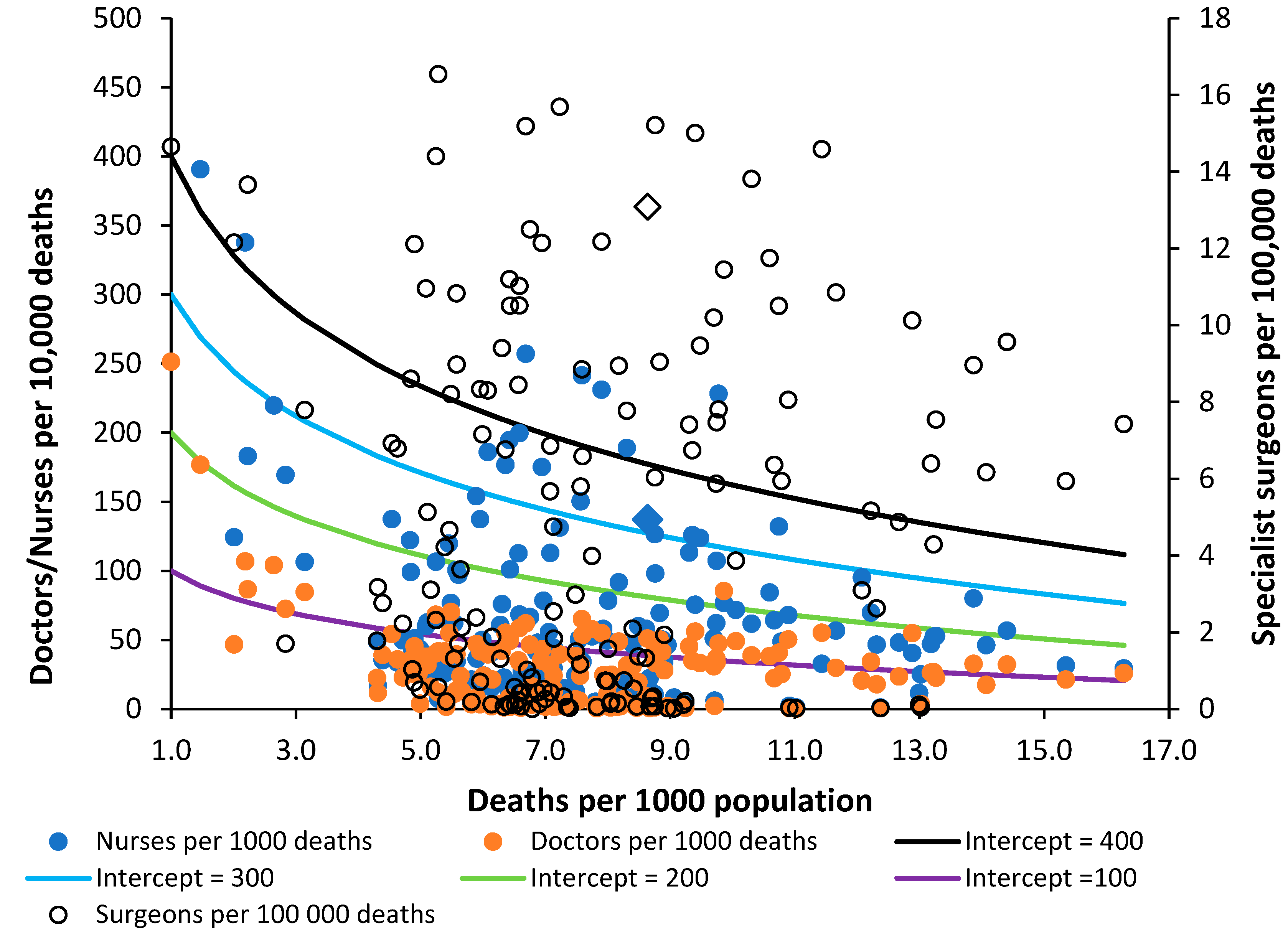
The position regarding Nurses, Doctors and Surgeons are given in
Figure 9. The data has been scaled to fit with the lines of equivalence—which are there merely as a reference point. Sweden is identified as a triangle. There are generally more nurses than doctors. The data for surgeons appears to show gross variation, which may be partly due to interpretation of the definition. The most striking outcome is that surgeons in the UK appear to have one of the highest levels in the world matched only by Greece. Overprovision appears likely—subject to validation regarding interpretation of the definition.
The provision of nurses in the UK is on the low side and is equivalent to that in Brazil, Czechia, Lithuania, Maldives, and Russia. The provision of doctors is also on the low side with the UK equivalent to Armenia, Antigua & Barbuda, Belgium, Chile, El Salvador, Malta, and Ukraine.
Compared to the UK, Sweden has 40% more nurses, 54% more doctors but 14% fewer surgeons.
The Supplementary materials S3 [
3,
41] provides further analysis using occupied rather than available beds in England. This covers a random selection of specialties. The overall conclusion is that the new method works well, provided that the historic context behind the data is considered, i.e., increasing sub specialization within general medicine and surgery, the impact of austerity following the financial crash, etc.
As I have observed over the past 30 years English politicians have made totally unsubstantiated claims that the NHS has too many beds and
Figure 1 shows that the lowest level of beds for a developed country has been achieved without the necessary investment in nurses and doctors to support such a low number of hospital beds. The UK invests around 20% less of the share of GDP into healthcare than Sweden [
42] yet expects to be able to operate with fewer beds. It should be noted that England managed to go through the COVID-19 pandemic simply because vast amounts of ongoing surgical and medical care were shut down [
43]. Which then shifts the expressed demand to a future date—leading to one crisis followed by another.
8. Adjusting International Data for Relative Population Deprivation
It has been noted that adjustment needs to be made for some measure of ‘deprivation’ [L.50,L.57]. The study relating to English Clinical Commissioning groups used the UK Index of Multiple Deprivation (IMD) [L.50], while a study in Australia used the proportion of state population which were indigenous people [L.56]. Neither of these is applicable in an international context. One readily available indicator is the age standardized mortality rate (ASMR) which can be obtained from the WHO and other sources [
44].
Supplementary materials S4 [
43,
44,
45,
46,
47,
48,
49,
50,
51]and S5 [
52,
53,
54,
55,
56] provide wider analysis supporting the notion that ASMR is an appropriate international and local variable for the effect of ‘deprivation’, and discussion on the possible range in the value of the slope for total hospital beds versus ASMR.
Figure S4.1 in the Supplementary material shows the range in ASMR for world countries and UK local authorities with different values of deaths per 1000 population [
41,
44]. As a generalization ASMR tends to increase as the raw mortality rate increases, however, the key point is that at any value of the crude mortality rate there are a wide range of values for ASMR.
As a reference point
Supplementary Table S4.1. shows the ASMR for the 50 countries with the lowest value for ASMR. Sweden is shown as the reference point for the lowest number of total curative hospital beds in a developed country. The key observation from
Table S4.1 is that England and the UK have 14% and 17% respectively higher ASMR than Sweden which suggests that the higher relative deprivation/lifestyle factors cannot justify the lower level of total curative beds noted in
Figure 1. The suggestion is that England and the UK have reached this point simply by restricting hospital bed numbers without the supporting investment in outpatient/primary care as has progressed over many years in Sweden. Hence the disruptively high bed occupancy in England to be shown later.
If we assume that higher ASMR is a measure of deprivation or hardship, then countries with a higher ASMR would have a higher intrinsic demand for hospital beds—which will go unmet in the poorer countries. Supplementary materials S5 investigates the likely slope for the relationship between total available hospital beds and ASMR. The slope is estimated to lie somewhere in the region of 13 to 34 higher total hospital beds per 1000 deaths Finally,
Table S5.1. investigates the discrepancy between bed supply and demand in US states. It is concluded that wealth and not need drives bed supply in the USA, as has been extensively reported by others [
53,
54,
55]. Having investigated the relevant factors regarding bed supply and the use of the new model we can now turn to the topic of applying the new model at the local level.
9. Defining Optimum Bed Supply at Local Level
All models contain hidden assumptions, necessitating comparison between models. It is emphasized that this method should be used alongside other methods when attempting to specify the exact size for a local hospital [
56,
57,
58,
59,
60,
61,
62,
63].
Indeed, all models based on an annual average will underestimate true bed demand simply due to the seasonal nature of demand [64,65,L.6]. Additionally, surge capacity is required to allow beds to be taken offline for a deep-clean and to cope with pandemics and catastrophes [
63].
The following sections will provide a framework for applying the international method at regional and local levels.
9.1. Data Availability at Local Level
All developed countries have a system of collecting data on deaths, births, and population at regional, local, and small-area levels. The smallest area for such aggregation is usually called a ‘census tract’ in the USA or in the UK an ‘output area’. In the UK an output area contains roughly 300 persons while a census tract in the US contains about 4000 people. Each census small area will have a population centroid (Eastings, Northings) which is essentially a grid reference (Cartesian coordinates).
Either Geographic Information System (GIS) using travel time or simple spreadsheet calculation (using straight line distance) can then be used to allocate each census area into the catchment area of a hospital [B.10]. Manual adjustment may be necessary when rivers or mountains prevent access. The relevant data can then be aggregated to give the effective deaths per 1000 population or beds per 1000 deaths for that hospital.
A sensitivity analysis should be conducted for the inclusion/exclusion of marginal census areas; however, a more pressing issue relates to forecasting future trends.
9.2. The Crude Mortality Rate at Sub-National Level
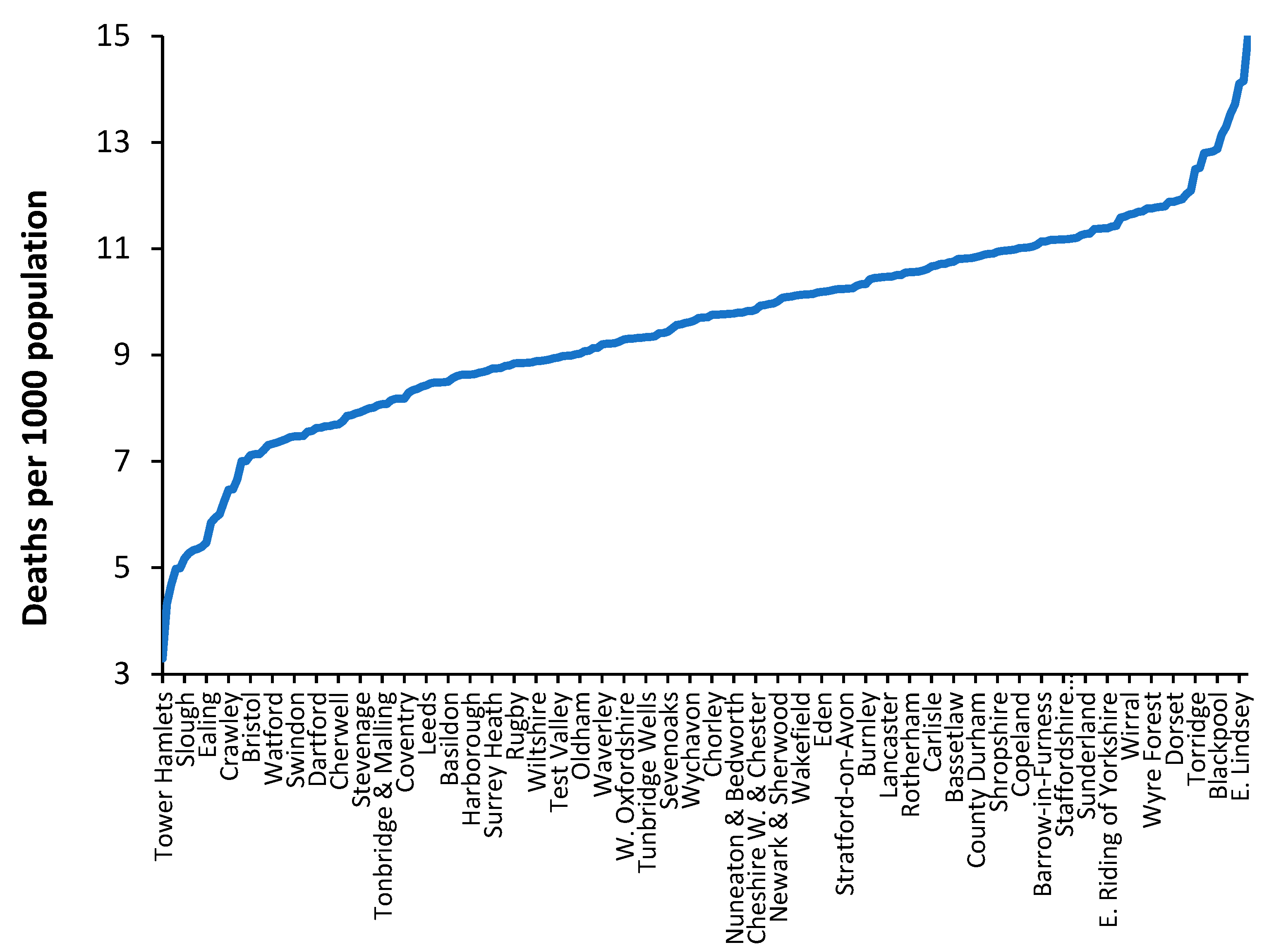
In all developed countries the crude mortality rate is available down to small-area level. This is important when attempting to take the model to a local level. As an illustration
Figure 10 shows the crude mortality rate for a selection of English and Welsh local authorities in 2019 (the pre-COVID-19 year).
While the value of the crude mortality rate for the UK was around 8.8 deaths per 1000 population in 2019 the range at local authority level is from around 3 to 15, which roughly spans the international range shown in
Figure 1. In the UK low values of crude mortality only occur in inner city locations.
From an international perspective, values of crude mortality higher than 13 deaths per 1000 population only occurs in the former Soviet countries such as Moldova, Croatia, Hungary, Ukraine, Belarus, Serbia, Lithuania, Latvia, and Bulgaria (16.3 deaths per 1000 population). Such high levels of the crude mortality rate are only approached in a minority of ‘elderly’ communities in the UK. Clearly values of crude mortality above 15 deaths per 1000 population will occur at small area level, especially areas which have high numbers of nursing home beds.
Hence, the crude mortality rate is a widely available measure which enables discrimination between communities and their associated bed demand at local level.
The same applies for ASMR where country specific ASMR calculations based on a relevant national population should be used rather than a world population, etc. Regarding ASMR it should be noted that the density of nursing home beds has a skewing effect [
66,
67], as do healthcare behaviors in urban and rural settings [68,69,L.16].
9.3. Effect of the World War II Baby Boom on Future Deaths
The cessation of WWII led to a baby boom in most participating countries. In addition, countries such as the USA and Australia initiated programs for inward immigration of the many displaced persons arising from the war. The leading edge of the baby boom turned 65 in 2011—65 being the age at which death occurs at increasing frequency. Such countries will all be experiencing rising deaths over the next 30 years [
70]. That part of bed demand relating to (changes in) deaths has therefore been activated. In addition, trends in births and migration will imply that each country will have its own unique profile of future deaths. For example, the Russian Federation has a declining population arising from the loss of young men during the 1979–1989 Afghanistan war [
71].
Such future trends are incorporated into the new model via the use of deaths per 1000 population in the x-axis, as was illustrated in
Figure 1. The effects of the WWII baby boom are then incorporated into national and local forecasts of both population and deaths.
9.4. Forecasting Future Deaths and Population
All methods for bed planning rely on forecasting the future value of the model inputs. It is the authors’ experience that even national-level forecasts of deaths are highly unreliable due to the difficulty of defining the base-year for the forecast [G.6].
Defining a base year is problematic simply because deaths as so volatile as witnessed in
Section 11. To address this problem with the base-year a method based on 11 years of previous data has been proposed [G.6]. The issue becomes more problematic at the local level and multiple scenario methods along with uncertainty intervals must be employed.
The aim of such scenarios is not to derive the minimum possible answer but to construct the likely range for the future.
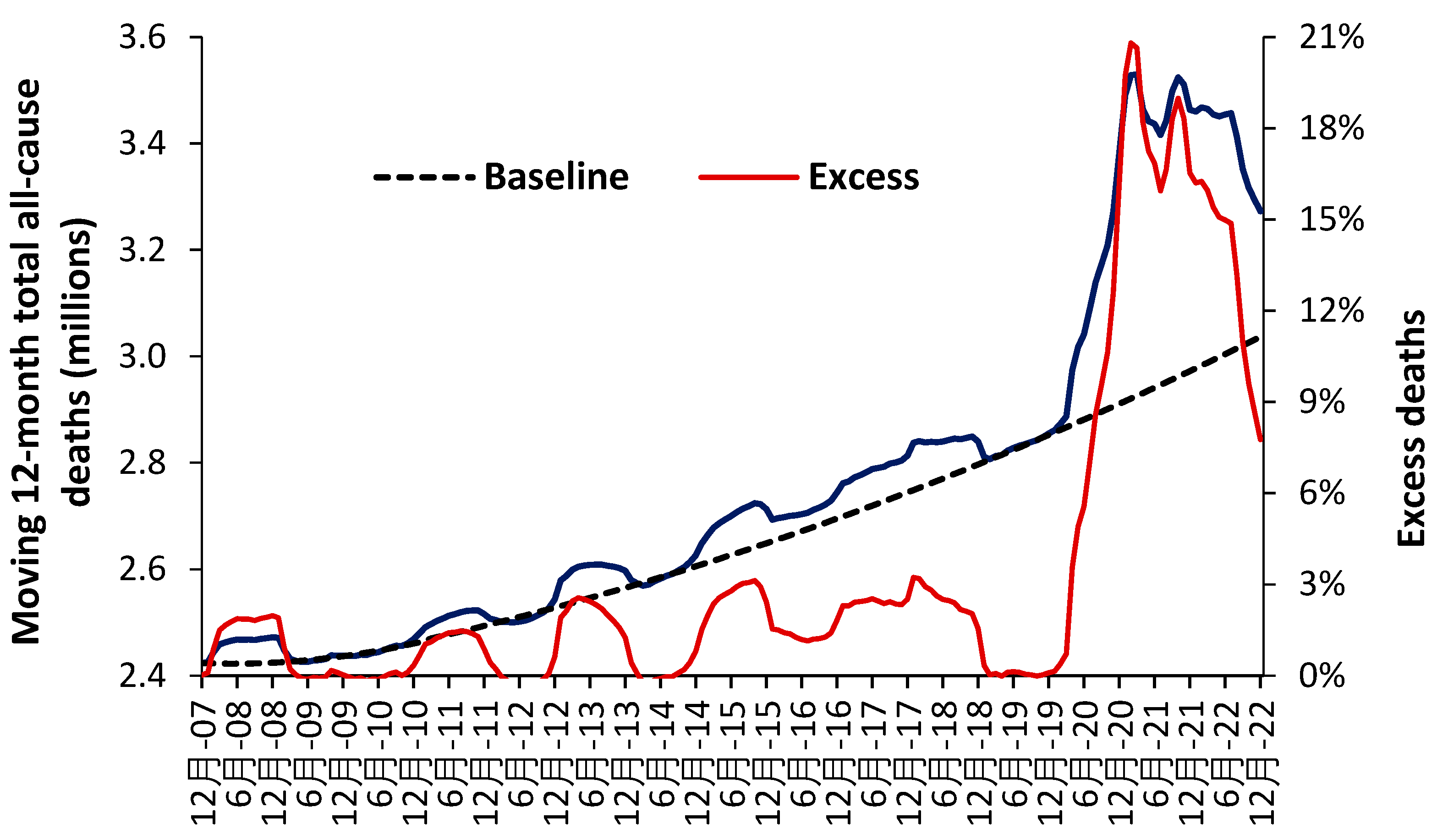
Figure 11 illustrates how such a baseline trend can be constructed [R.8]. A second order polynomial has been used to estimate the baseline trend which intersects the points of actual minimum deaths (the dark blue line). The COVID-19 pandemic illustrates a period of unanticipated ‘shock’ demand which was far larger than similar ‘shock’ increases experienced prior to this pandemic.
Figure 11 uses data for the whole of the USA, but the method can be replicated at state and smaller geographies—where local issues become more influential. Note the existence of points for minimum mortality as for the 12-months ending 7 December, 8 December to 10 December, etc. Also note the existence of a period of higher deaths lying roughly between 14 December to 19 December. This period of higher deaths is also seen in the UK [N.34].
All forecasts must reflect the reality that deaths, and associated demand, are highly volatile—with direct implications to the hospital bed occupancy margin in
Section 10.
10. Defining an Optimum Hospital Size
10.1. Average Bed Occupancy as a Measure of Operational Chaos
Hospital bed occupancy has been traditionally measured at midnight and for international comparison uses an annual average. There are several problems with this approach because:
The average occupancy rate shows seasonal changes with highest occupancy during the winter months (rainy season at the equator) due to the higher incidence of winter infectious diseases and the effects of cold [
64,
65]. Other cycles operate nearer to the equator usually based on the rainy season.
Occupancy also has a daily cycle with a minimum around midnight and a maximum during the day.
Queuing theory clearly shows that the likelihood of a patient being ‘turned away’ due to no bed being available (hence a measure of chaos, inefficiency, delays to admission and waiting lists) increases as the real or instantaneous occupancy increases [72,73,L.12].
Smaller hospitals or bed pools must operate at lower average occupancy due to the higher volatility in admissions as size decreases. Hence lower average occupancy in pediatric, maternity, and critical care bed pools, and in countries with smaller hospitals [72,73,L.12].
With these limitations aside, rising average midnight occupancy is an excellent measure that there are insufficient beds relative to the expressed demand. To this end
Figure 12 shows the trend in annual average midnight occupancy for curative beds in England from 2010 to 2019.
While 85% average occupancy is often quoted as the optimum for hospital occupancy there is no theoretical basis for this claim [72,73,L.12]. An annual average rate of 85% does, however, roughly apply to very large hospitals with greater than 1000 beds [L.12]. The reason that 85% is so often claimed to apply is that it also represents a measure of perceived busyness [L.23], which would be independent of size.
Since 2010 financial austerity in the English NHS led to the closure of beds to reduce costs.
Figure 12 is therefore the outcome of such imposed austerity. Note that the increase in average occupancy slows with time simply because it becomes increasingly difficult during the daytime to admit a patient, leading to queues for admission and cancelled elective operations. Note that over this time the national waiting list for an elective operation grew by millions of persons [
74], and widespread curtailment of elective surgery regularly occurs during the winter months (further fueling growth in the waiting list), and there are typically queues of ambulances waiting outside hospitals during the winter [
75]. English hospitals have simply descended into unfunctional chaos, even though they are generally very large by international standards. This is a by-product of high population density and government attempts to minimize costs by limiting the number of hospitals. For example, small US towns will typically have two or more hospitals due to private healthcare company competition, and a network of small community hospitals throughout the extensive rural low density US population [
76].
Each English hospital will have a large ‘bed management’ team attempting to squeeze patients into any available bed—called outlying patients who typically have higher average length of stay, receive poorer quality care, and have a higher readmission rate [
77,
78,
79,
80]. The mortality rate may not be greatly affected, although adjustment for risk factors is difficult [
77,
78,
79,
80].
By way of comparison in 26 European countries with data available for 2019 [
29,
30], the median occupancy was 73% (69% lower quartile, 79% upper quartile). Minimum average occupancy was 62% in Cyprus (small country) up to 90% in Ireland. Germany had an average of 79%. Other countries lying in the range 78% to 79% were France, Italy, Luxembourg, and Liechtenstein. Ireland has recognized that occupancy is high due to a deficit in beds with plans to expand bed numbers [
59]. Occupancy in England is seemingly the highest in Europe. High bed occupancy has consistently been associated with every possible measure of (imposed) hospital inefficiency [80-85, L.13,L.25,L.31,L.33].
As a final note, beds per se do not make a large contribution to costs, it is the staff associated with the beds which are ‘expensive’.
10.2. Optimum Hospital Size as Economy of Scale
While the average bed occupancy rate gives insight into the level of operational chaos there is also the related issue of the optimum size for technical efficiency. Economy and diseconomy of scale issues were explored in the review of Giancotti et al [
86] which gave evidence for economies of scale in hospitals with 200–300 beds. Diseconomies of scale occur below 200 beds and above 600 beds [
86]. Queuing theory shows that larger hospitals can operate at higher average occupancy [72,73,L.12] and as a hospital progresses beyond 600 beds it seems that increasing specialization leads to higher costs per patient. The diseconomy of scale below 200 beds is due to the lower average occupancy associated with small size leading to higher capital costs and higher staffing costs per patient [L.21,L.22,L.31].
10.3. Length of Stay and the Efficient Hospital
During the 1970s to the 1990s hospital length of stay (LOS) experienced a rapid decline [K.2,L.53]. This was mainly due to the simple discovery that rapid mobilization was far more effective than bed rest for recovery after injury and surgery [
87,
88,
89]. Had such rapid reduction continued the net LOS would have declined to zero well before the 2020s. Hence any model for LOS must incorporate a decline in the rate of reduction in LOS over time [
60,
61]. This effect is illustrated in Supplementary material S6.
LOS does depend on age, however, one study on LOS in the adult medical intensive care unit showed that LOS was independent of age for patients who survived but depended strongly on age for those who died [
90]. This observation could suggest that hospitals which service a population with a high crude mortality rate will experience greater pressure to high LOS.
One potential effect of the aging population on LOS is that LOS may well reach an asymptote and then start to increase as the proportion of older persons increases. I have yet to see any models for hospital bed numbers which incorporate such a possible outcome. See Supplementary material S6.
The study by Hughes et al [
77] shows that LOS ‘outliers’ make a significant contribution to the average LOS and that such ‘outliers’ should not be excluded from the LOS calculation or from the number of occupied bed days. Rachoin et al [
91] observed that medical patients with a higher LOS tended to have higher readmission rates. Indeed, the NTD/TTD phenomenon discussed earlier implies that patients in the last six months of life will be characterized by high rates of readmission—an association only realized in hindsight.
The study of Walsh et al [
92] observed a strong relationship between constrained bed supply and length of stay. Between 2010 and 2012 while length of stay fell by 6.4%, approximately 42% of this reduction was associated with the decline in bed supply. The use of length of stay as an efficiency measure should be understood on the contextual basis of other health system changes. A lower length of stay may be indicative of the lack of resources or available bed supply as opposed to reduced demand for care or the shifting of care to other settings [
92].
In the English NHS the headline LOS has been declining but closer inspection reveals that this is largely due to the inclusion of millions of zero day stay emergency admissions due to ‘admission’ of persons into short stay emergency assessment units. I would claim that the majority of such ‘admissions’ are more correctly defined as emergency department attendances. The reality was that many such ‘admissions’ were simply an attempt to circumvent the emergency department four-hour target introduced by the Blair government in 2004.
As an example, the real LOS for Shigellosis (A03) is around 5 days and is only declining at around 0.03 days per annum (0.6% p.a.) while that for Amoebiasis (A06) is around 11 days and is declining at around 0.1 days per annum (0.9% p.a.) [
3]. The reality is that the residual genuine overnight stay LOS is not declining at any great pace or is staying roughly unchanged [K.8]. See Supplementary material S6 for an example.
This does not imply that emergency assessment units should not be opened but rather that the planning for such units should incorporate separate estimates of the real-time LOS in such departments and the likely future growth in admissions. Assigning a zero LOS to any person admitted and discharged before midnight is simply an obfuscation of reality, and an open door to underestimate real future bed demand. All hospital planning must be done based on reality and not on wildly optimistic ‘concocted’ estimates of future LOS.
While it is true that poorly executed hospital processes and procedures of care can lead to a longer LOS it has been my observation that the processes of clinical coding, which includes the training of doctors on the nuances of recording patient information, often lead to spurious examples of long LOS. All hospitals are well advised to ensure that all available information is collected regarding every in-hospital death, i.e., blood biochemistry and other diagnostic test results, any imaging studies, etc. It is a fundamental risk to rely on some hastily written diagnosis by a junior doctor given that most deaths involve greater complexity of multimorbidity and other factors—all of which should be reflected in an extensive series of clinical codes for each patient. Some hospitals have an evident lack of detailed coding, which then distorts the interpretation of LOS.
It is also far too easy for a Management Consultancy to construct a plausible story (on paper) as to why future LOS will be reduced of which hospital Executives and senior Managers will be unaware of the hidden pitfalls. The series of articles under the title of ‘Understanding Length of Stay’ in Supplementary material S1 should be studied [K.1–9].
10.4. Staffing Regulates Costs Not the Physical Number of Beds
It has been the author’s observation over many years that modelling of future bed demand in the UK appears to be stricken by the fallacy that the number of beds is the primary driver of hospital costs. The capital cost of a bed plus floorspace is covered under capital costs and is amortized yearly over the expected lifespan of the building or the physical bed. The annual cost of additional beds per se is therefore surprisingly low. The initial sections of this study clearly showed that the demand for the beds is highly volatile, and this implies that the staffing of the beds needs to be highly flexible. The actual cost driver is the number of staff as full-time equivalents. A simple method is available to optimize the mix of full-time and flexible staffing requirements [L.31], however, it is never used.
My discussions with UK NHS Directors of Personnel are that flexible staffing is viewed as an ‘impossible’ task. Clearly that which is imperative to achieving minimum operating cost is viewed as a ‘no go’ area and is not reflected in overall NHS policies for NHS staffing. Part of the problem is the very high bed occupancy levels which now afflict the UK NHS. At high levels of bed occupancy, the illusion is created that the beds are always fully staffed, hence, the number of beds becomes a perceived cost and not a route to efficiency.
10.5. Delayed Transfers of Care (DTOC)
DTOCs arise at the interface between hospital and social care and can be reduced by local partnerships and discharge planning [
93]. They create increased length of stay. However, in England they are often presented as a major reason why available beds appear too low compared to the expressed demand. The problem is that when you are in a crisis all manner of superfluous reasons is given as the ‘cause’ of the problem.
Over a 30-year period it has also been my observation that DTOCs rise and fall in proportion to available funding which is influenced by the financial pressures arising from unrecognized outbreaks of infectious agents [N.9-12]. Any agent which will push a vulnerable proportion of the population toward death will simultaneously increase hospital admissions and the pressure on primary care, community nursing and social care. All parties will simultaneously experience higher demand/costs which will inevitably lead to subtle causes of DTOCs. On some occasions such insights can only be gained from experience gained over many years. This is discussed in detail in
Section 11.
DTOCs will never drop to zero and the long-term fluctuations in the level of DTOCs simply become part of the reserve or surge capacity required to run an efficient hospital. The next section is highly relevant to DTOCs and wider aspects of explaining why bed demand is so uncertain.
11. Pathogen Outbreaks, Bed Demand, and the Bed Occupancy Margin
The logic behind this line of research lay in my early observations that activities associated with hospital demand such as all types of outpatient, inpatient, and emergency department attendances/admissions showed year-to-year variation which was between 2- to 3-times higher than could arise from Poisson variation [A.13-14,N.1,N.2]. The same can be said for the length of stay [K.1-9], deaths [N.20], and healthcare budgets [N.12,N.17,N.24, N.25]. Indeed, admissions for some diagnoses and certain types of cancer show extreme volatility indicating high sensitivity to the external environment [N.18,N.26,N.27]. Supplementary material S7 illustrates the issues regarding volatility for deaths and hospital bed demand. Such high levels of inherent volatility demand an explanation.
11.1. Death as a Proxy for Wider Admissions
For individual pathogens it is possible to estimate the number of infections from the reported deaths [
94]. The action of infections upon acute demand can be very subtle. For example, persons feeling unwell due to an infection may experience higher numbers of injuries, falls and fractures—most of which may not immediately lead to death. However, in other persons the same infection may exacerbate other conditions which may involve death. If we accept the proposal that deaths are serving as a proxy for acute admissions
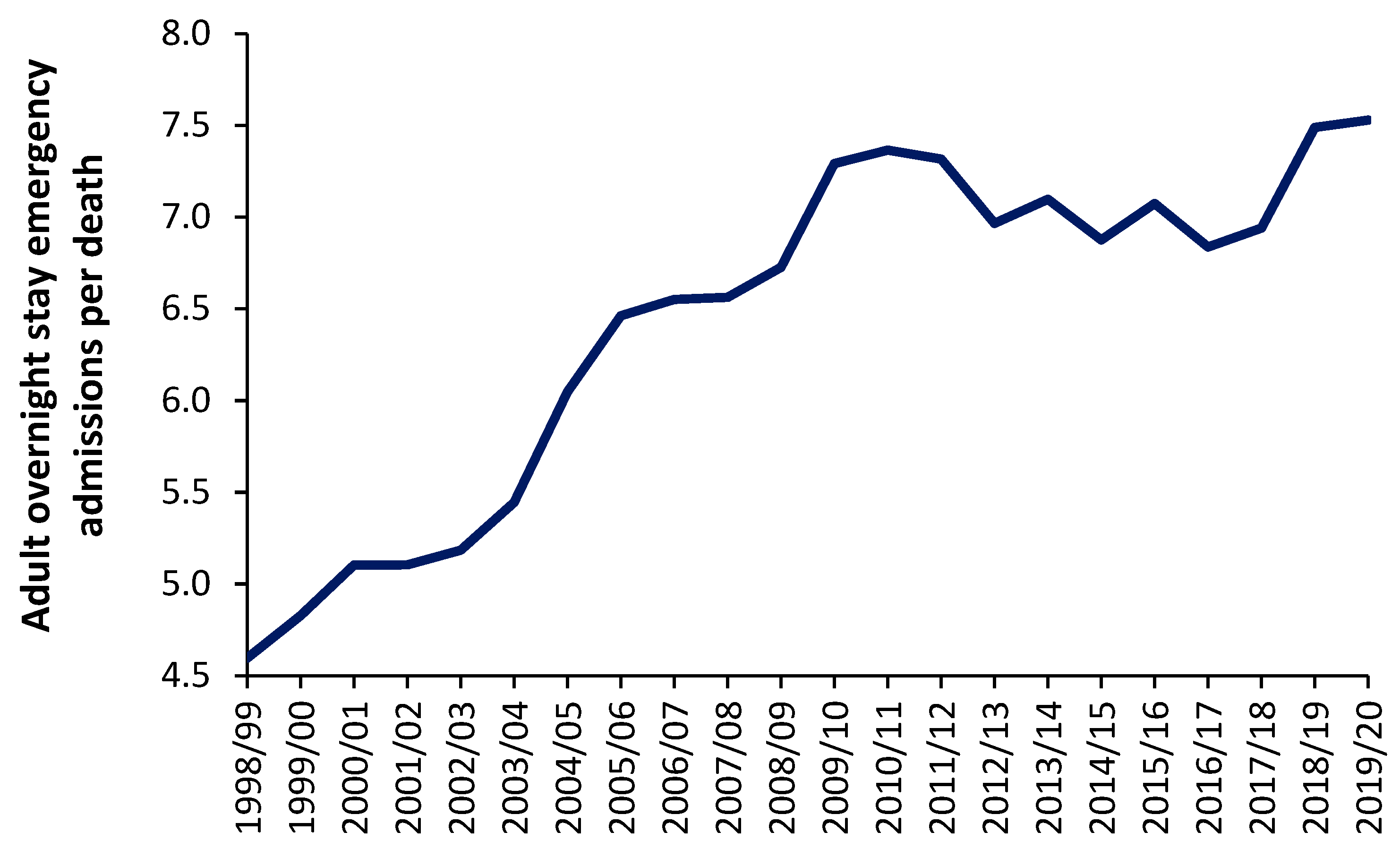
Figure 2 shows the trend in overnight stay emergency admissions per death in England from the financial year 1998/1999 through to 2019/2020 (the last financial year before the COVID-19 pandemic). Excluded from this analysis are all elective admissions, any same day stay admissions and any admissions relating to Maternity, Pediatrics, Community Care or Mental Health, i.e., acute overnight-stay hospital adult care only.
As can be seen in
Figure 13 the number of such overnight stay (adult) admissions per death appears to range from around 4.5 before 1998/1999 to 7.5 in 2019/2020. It should be noted that deaths in England reach their minimum number between the 2009/2010 and 2011/2012 financial years [
95]. The general increase in this ratio from 1998/1999 onward may reflect expansion in the range of acute medical/surgical interventions which can be offered for emergency hospital care. This range of care roughly expands at 0.12 adult emergency admissions per death per year—although perhaps more so before 2009/2010. It should be noted that admissions for certain mental health conditions are also exacerbated by infections [
96]. Indeed one-third of COVID-19 survivors receive a neurological or mental health diagnosis in the six months following infection [
97,
98].
Even if it were argued that the real ratio amenable to exacerbation by infections is only, say, 4 admissions per death the absolute number of deaths is still acting as a proxy of the demand for acute adult emergency care, and that this ratio is subject to year-to-year volatility—after correcting for underlying growth in the ratio.
In the field of statistical process control (SPC) a major observation is that ‘the variation is the voice of the process [
99]. In terms of hospital bed planning this translates into something like ‘unless you understand why healthcare is so variable you will never be able to create a credible model of bed demand’. Having discussed the role of unrecognized infectious outbreaks on human health and the consequent need for hospital beds we can now consider how the absolute number of deaths can be incorporated into hospital bed modelling.
11.2. Evidence for Such Outbreaks
From Supplementary material S2 age-based forecasting only ever delivers slightly curved continuous lines with a low slope. Yet the real-world of hospital admissions and costs shows very high volatility [M.1-29,N.1-39,P.1-6] which includes high volatility in winter deaths due to infectious outbreaks [H.1-12]. Hence the reader can view some 30 years of research publications as an attempt to introduce some understanding to the question, why?
One of the more interesting observations from my studies was that the trends in emergency admissions appeared to be behaving like a series of infectious outbreaks over and above the usual winter influenza outbreaks [A.1–20]. A series of studies [R.1–16] established that both deaths and hospital admissions (especially emergency medical admissions) showed high levels of small-area spatiotemporal behavior, or spatial granularity. Such spatiotemporal behavior is a characteristic of infectious spread [
100,
101,
102]. Also, that some diagnoses were affected while others were not [Q.7,S.2–4].
Interesting confirmation of such a possibility comes from the fact that as of 2022 there were around 3,000 species of known human pathogens [I.3]. The large majority are unexplored regarding their clinical effects, and indeed no one routinely tests for their presence—other than the more commonly known species. A study on the human DNA virome, i.e., persistent viruses, using the 17 most common persistent DNA viruses showed that individuals were infected with between 2 to 17 of these 17 most common persistent DNA viruses [
103]. Individuals were most infected with 6 to 7 of these viruses in 39% of individuals. At sub-species level for each virus there was profound genetic variation between individuals.
Table 1 demonstrates the distribution of these 17 common persistent DNA viruses in human tissues.
The top line gives a count of how many DNA viruses were found in each tissue. The percent value in red bold is for the most common virus in that tissue, while yellow cell highlight shows the highest prevalence for each DNA virus across tissues. The mechanisms behind how such persistent and other infections may interact with human health is covered in
Section 11.3. Interpret this table as the potential to influence health in each organ/tissue and that DNA viruses are only half the full picture.
Modern high speed/volume travel (air/rail/road) implies that all pathogens (persistent or occasional) are spread rapidly around the world [
104,
105,
106]. As if 3000 species of pathogen were not of sufficient concern, each species represents a wide range of sub-species, strains, and variants with a diverse range of clinical effects—the study of which is termed metagenomics [
107,
108,
109]. Also, dual infections (superinfection and coinfection) are very common [
110,
111].
Is there any evidence that unknown pathogen outbreaks are a frequent occurrence? The best evidence for such a proposal comes from the small-area behavior of deaths—which are displaying apparently ‘unexplained’ short-term trends [R.1–17]. Hence, for each area there will be a general long-term trajectory for deaths which depends on past births, changes in life expectancy, and net migration. This trajectory will be available in all developed countries and forms part of the population forecasting process. However, the shape of this calculated trajectory will be a smooth line.
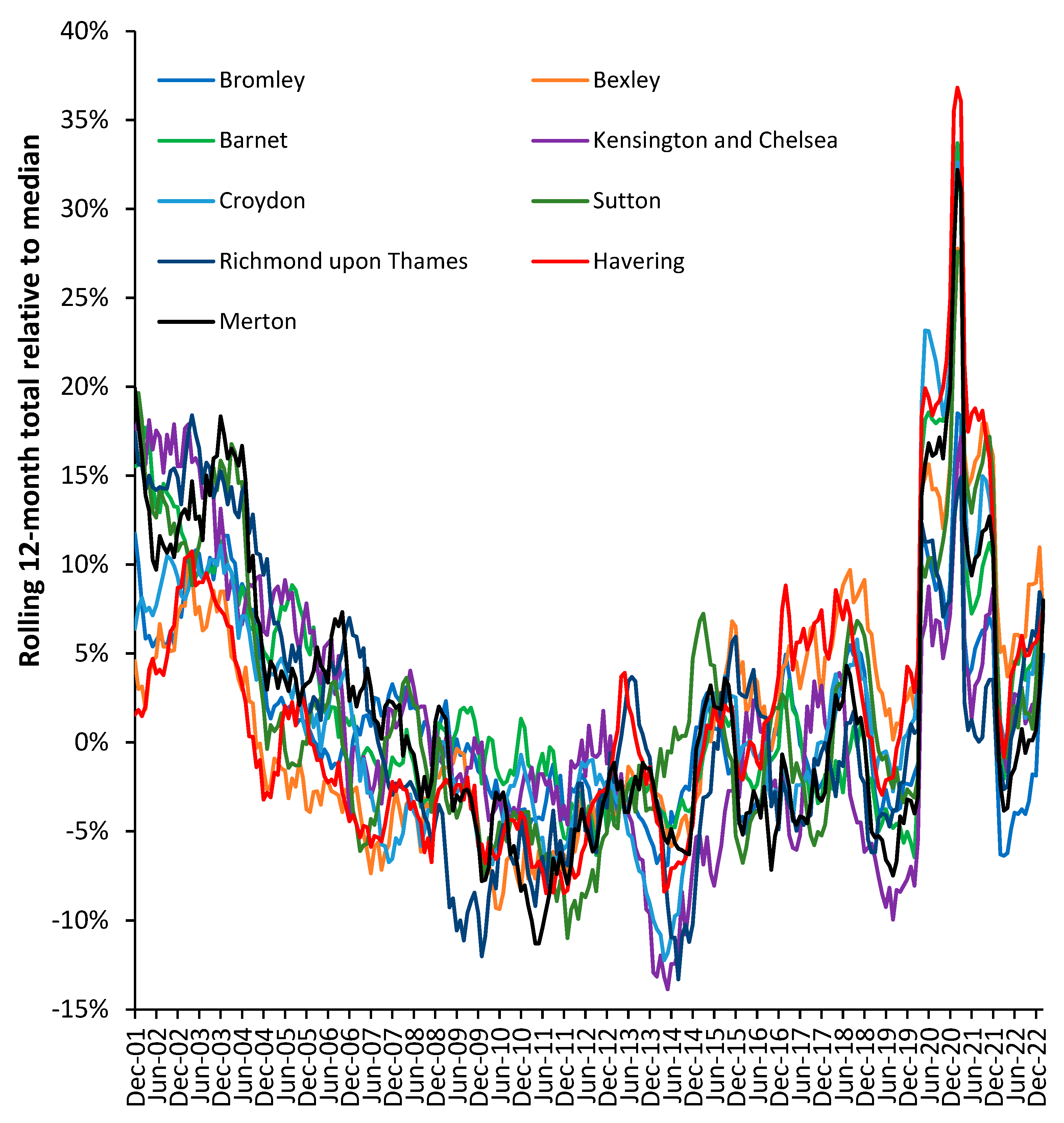
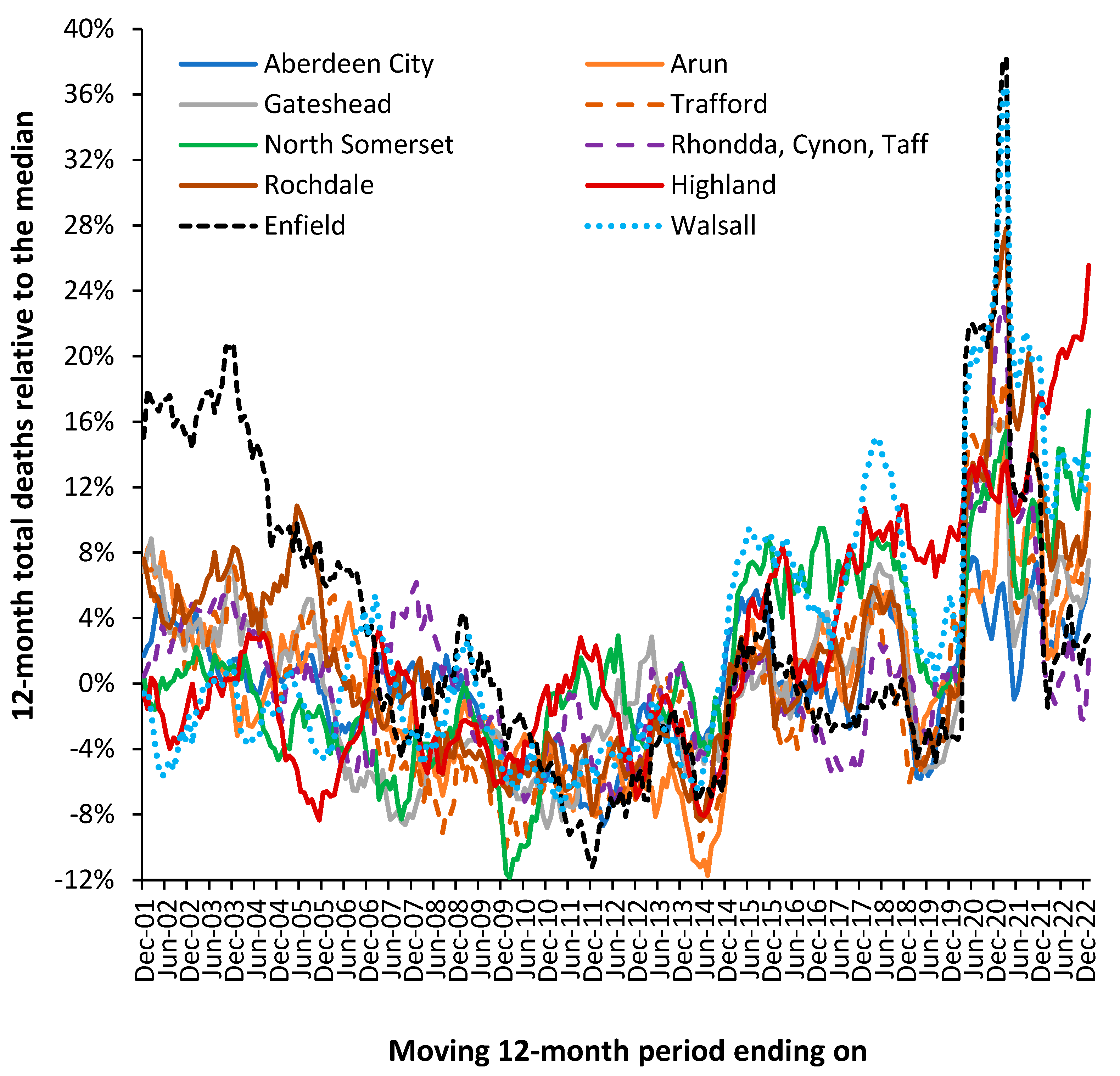
Figure 14 illustrates that the actual trajectory is far from a smooth line. The UK local authorities in
Figure 14 were randomly chosen with the criteria of having just over 2000 deaths per annum in 2001.
The displayed trajectory is a moving 12-month total commencing on 1 December which is relative to the median value of deaths in each local authority. In a moving 12-month total the usual level of seasonal cycles are removed since every point includes all 12-months. The existence of such trajectories has been largely ignored by epidemiologists because there was no obvious explanation for the observed behavior. Winter cold-snaps or summer heatwaves cannot explain the behavior since they are usually of such short duration as to make a negligible impact on the 12-month total. By choosing local authorities with above 2000 deaths per annum the role of Poisson-based randomness is likewise excluded since ±1 standard deviation of Poisson variation only accounts for ±2% variation. This is reflected in the very small background ‘wriggles’ in the trendlines.
In a moving 12-month total a spike event such as an influenza outbreak would be expected to generate a ‘tabletop’ shaped feature. The excess deaths move into the 12-month total, stay in the total for 12 months, and then exit the total. Such a spike event can be seen for Highlands commencing December 2006. Such tabletop features are somewhat less than expected and the variety of shapes indicate a more complex set of contributory factors. As has been stated, there is no such thing as a single pathogen winter [H.9-11,I.3].
In a moving 12-month chart a pyramid-shaped pattern is indicative of on/off switching. I have proposed that at a national level the net contribution from all pathogens and their interactions via pathogen interference is to produce a set of on/off switching in human health. The on-part of the cycle (the upward face of the pattern) initiates roughly 12-months of poor human health while the off-part of the cycle (the downward face) represents roughly a 12-month period of ‘better’ human health. This cycle had a longer wavelength back in the 1980s, but the frequency gradually contracted to the current 2-year pattern seen since the 2010s [S.2,Q.10].
While other explanations may be possible the pattern is real, can be followed over many years and does indeed affect different medical and psychiatric diagnoses [in 3].
To explain the residual peaks and troughs it is illustrative to point to the disruption caused by the COVID-19 pandemic where deaths peak in the 12-month total ending around March 2021. This is the outcome of a very real pathogen. However, notice the vast difference in COVID-19 deaths between Enfield (London) and Walsall (Midlands) and the almost minor effect in Aberdeen City (Scotland) or Gateshead (N.E. England). For example, note the very high deaths for the 12-month total in 2005 in Rochdale while Highland experiences a minimum. The highly granular effect in
Figure 1 arises from the role of super-spreaders and super-spreader events (SSEs) upon infectious transmission [
112,
113,
114].
Figure A1 shows a similar situation for nine London Boroughs where spatial proximity precludes wider effects due to weather patterns or other regional effects. Hence in
Figure A1 note low deaths in Richmond-on-Thames during 2009 when the other boroughs are generally high. Sutton is worst affected during an event occurring in 2014/2015 with highest moving 12-month total measured in March 2015, which barely affects Kensington and Chelsea.
Consider the implication of the high/low points. Hence a maximum implies an extended period of sustained high deaths, while a minimum implies a switch to low mortality. The low mortality extended periods can be inferred to have a low net pathogen health load and vice versa. Such switching is made possible due to pathogen interference, where infection with one pathogen modifies the frequency and clinical outcome of subsequent infections [115,116,I.3]. Pathogen interference arises from the production of interferons, which is regulated by the expression of small noncoding RNAs (miRNAs) [
117]—discussed further in the next section. Pathogen interference then amplifies/dampens the local effects on mortality depending on the local mix of pathogens circulating at the point when the new pathogen is introduced via a super-spreader or SSE.
Differences in timing and magnitude are widely evident in
Figure 1 and
Figure A1. Spatial analysis of such events shows neighborhood-area behavior characteristic of infectious transmission [R.1–17].
From
Figure 14 and
Figure A1 it is concluded that the possibility of previously undocumented (mainly local) infectious events is very real and has a direct effect upon total deaths and hence upon wider morbidity leading to hospital admission and bed demand. The key point being that the local events show high volatility, and by implication the need for an appropriate average occupancy in local hospitals to accommodate such fluctuations in demand—indeed the overwhelming bed demand seen during the early stages of the COVID-19 pandemic. The evidence has seemingly been lying right under our nose, but that traditional disease surveillance has completely failed to detect such small area impacts [R.1–17].
The central flaw in existing surveillance systems is that they are heavily biased to detect explosive disease outbreaks such as influenza or COVID-19 [F.1,R.10]. This bias is further exacerbated by the methods used to select the baseline for deaths. This problem is amply illustrated in
Figure 1 and
Figure A1 in that the ‘true’ baseline is hidden among high volatility. One solution is to select a low pathogen baseline which intersects the minimum points in the long-term trend. An example was given for the USA in
Figure 11.
At this point it must be noted that a moving 12-month total does not make any assumptions regarding a baseline and is especially useful at detecting non-standard outbreaks—which may be expected from 3000 species of pathogen [I.3]. However, it only works retrospectively.
Current disease surveillance is also heavily focused on a narrow range of pathogens with identifiable symptoms, i.e., Ebola, hemorrhagic fever, influenza, etc., whilst it is suggested that some 3000 species of pathogen are likely to have a wide range of nuanced effects against pre-existing conditions and will therefore go unreported or more correctly misreported. One example of more nuanced associations is the well-known link between influenza and acute cardiovascular events [
119].
The issue as to whether the patterns of deaths seen in
Figure 14 and
Figure A1 are impacting psychiatric bed demand needs to be explored. As was discussed earlier, infections play an important role in the initiation and exacerbation of mental health conditions. As a proxy for infectious outbreaks, deaths will therefore be one of the explanatory variables in any model for mental health beds. In the UK the specialty ‘Old Age Psychiatry’ is highly likely to be heavily influenced by nearness to death rather than age per se.
It has already been pointed out that average bed occupancy in acute hospitals in England show an unexplained pattern of occupancy which is roughly two years wide from trough to trough [L.38], even after calculating a rolling 12-month average—which would remove any seasonal patterns.
Figure 14 confirms the same pattern for Mental Illness bed occupancy. Some explanation is required in that the 12-month periods from March 2011 onward encompasses a time of rapidly declining bed supply as care for mental illness is transferred into the community.
Figure 14.
Moving 12-month average occupancy for mental illness hospital beds in England. Data from NHS England [
19]. Note that the cycle commences again in late 2019 but is interrupted by the infection control measures implemented during COVID-19.
Figure 14.
Moving 12-month average occupancy for mental illness hospital beds in England. Data from NHS England [
19]. Note that the cycle commences again in late 2019 but is interrupted by the infection control measures implemented during COVID-19.
The curious pattern is observed prior to 2014 but is suppressed due to certain conditions/diagnoses seemingly not strongly affected by the pattern being still treated in an inpatient setting. However, after the loss of 600 beds from 2011 to 2014 the pattern becomes far stronger as the range of conditions undergoing inpatient treatment has been trimmed to a core set of conditions seemingly sensitive to the strange pattern(s).
11.3. How Pathogens Interact with Existing Diseases
As per the TTD/NTD phenomena a study by Hansen et al [
119] has demonstrated that costs in the last five years of life are driven by morbidity and not age. On this occasion costs imply access to hospital and other care. How might this be influenced by pathogens? All will be familiar with the function of the immune system during infection; however, only some will be aware that immune function and many diseases are regulated by small noncoding RNAs (miRNAs). The miRNAs act to regulate gene expression which then impacts upon metabolic pathways, etc. miRNAs therefore serve as the common interface between pathogens and disease initiation and exacerbation [
120,
121,
122,
123,
124,
125,
126]—hence the seeming inexplicable trends seen in
Figure 14 and
Figure A1.
One expression of this interaction occurs in pathogen interference whereby infection with one pathogen stimulates the production of a range of miRNAs which then alter the expression of interferons which in turn then alter the ability of other pathogens to infect that individual [
117]. Changes in interferon production is only one of many induced changes in cellular function, metabolism, and immune responses such as inflammation which can include damaging cytokine storms [
120,
121,
122,
123,
124,
125,
126]. Such processes can then trigger autoimmune conditions and malignancies in genetically and epigenetically susceptible individuals [
120,
121,
122,
123,
124,
125,
126].
In such a context
Figure 14 becomes entirely understandable and implies that so-called health service’ demand management’ may be far more nuanced than has been appreciated. The COVID-19 pandemic with associated long-COVID [
97] is an excellent example of the difficulty in ‘managing’ such events given unexpected knock-on effects. Such unexpected knock-on effects are probably more widespread than anticipated and may include poorly understood conditions such as chronic fatigue syndrome or myalgic encephalomyelitis (ME/CFS) and other syndromic conditions which are surprisingly like long-COVID.
In my earlier work the issue was explored as to whether the common persistent human immune modulating virus Cytomegalovirus (HCMV) could influence the range of common diagnoses which changed during periods of unexplained higher deaths [S.1-9]. The answer was in the affirmative. However, in the light of recent developments regarding the human persistent virome [
103], the existence of 3000 species of known human pathogens [I.3], and the mechanisms of pathogen interference [I.3] it is probably better to substitute the words ‘any persistent pathogen’ for ‘CMV’, and that infection with any occasional pathogen, i.e., influenza, respiratory syncytial virus, parainfluenza, etc., etc., will trigger a cascade of events leading to the re-activation of some of the persistent human virome. This then triggers a further cascade of consequences.
The most common persistent virus, HHV-6, is alone implicated in many disease processes and malignancies [
127,
128,
129]. Reactivation of EBV, CMV and HHV-6 are common in critically ill persons with COVID-19 disease [
130].
11.4. Wider Indicators of Health are Also Affected
It should be noted that wider indicators of human health such as worker sickness absence [E.1–6] and General Practitioner (GP) referrals for a specialist opinion [D.8] also appear to be influenced by the same forces which regulate the above fluctuations in deaths. If both occasional and persistent pathogens were exerting wider than expected effects against health such associations are to be expected. The trends in sickness absence are illustrated in Supplementary material S8.
At this point it should be highlighted that fluctuations in hospital admissions for certain diagnoses also appear associated with the same root cause [L.40,Q.9]. Indeed, the unexplained fluctuations in the ratio of male to female deaths, hospital admissions [N.15,N.19, Q.4, R.13] and the costs for certain conditions may also fall into this category [N.15].
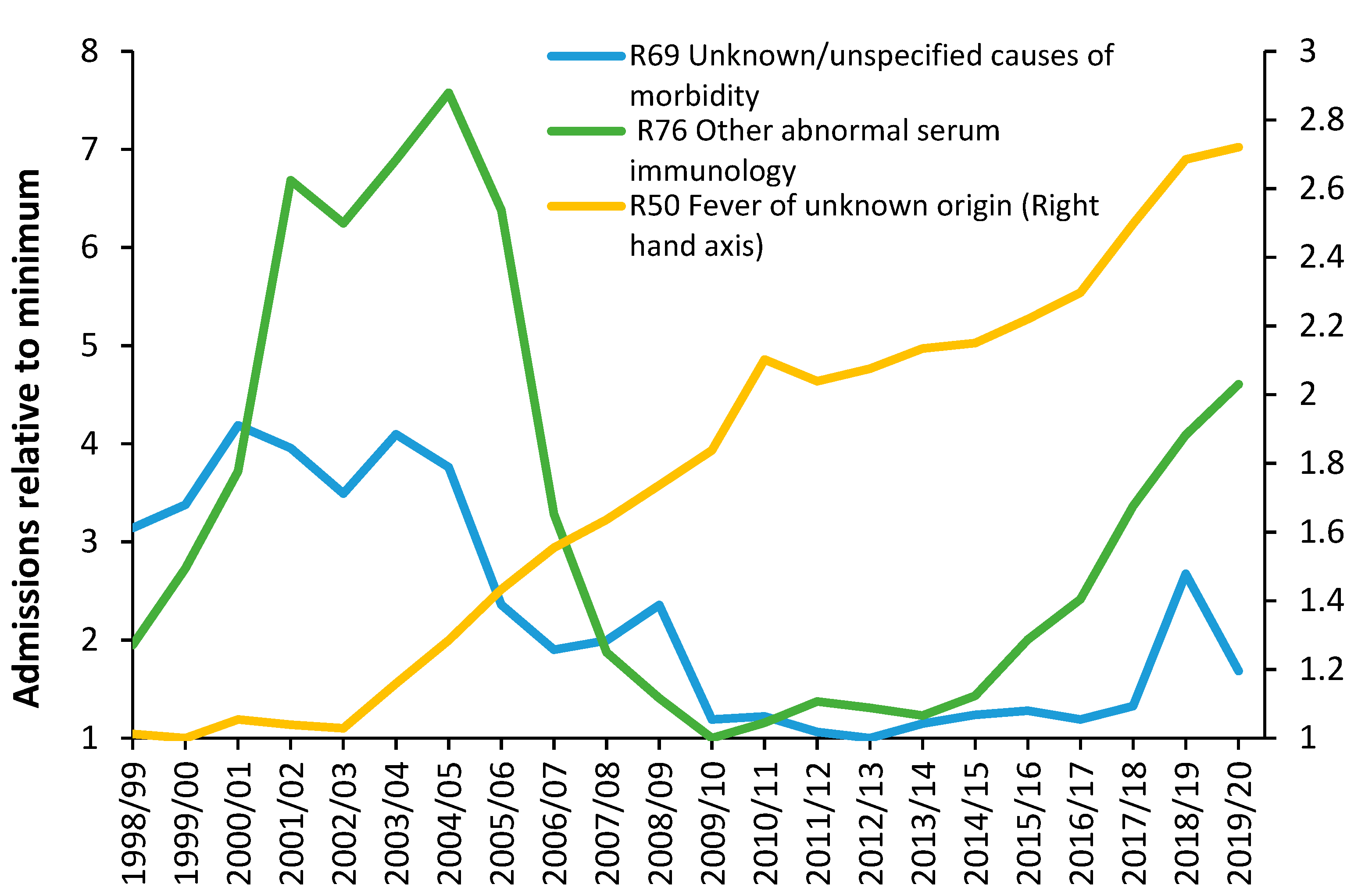
Figure 15 shows three among many unexplained trends in admissions for primary diagnoses in Chapter R (Signs and symptoms) of the International Classification of Diseases (ICD-10) primary diagnosis.
No one investigates such unexplained trends and while some of the movement may be due to changes in how symptoms are coded, the more interesting feature is the short-term fluctuations which cannot be explained by any long-term changes in the process of coding or definition of an admission. For example, why the sudden spike in admissions for unknown/unspecified cause of morbidity in 2018/2019, for fever of unknown origin in 2010/2011, or the maximum in 2004/2005 for abnormal serum immunology? Further investigation shows changes in the ratio of male to female admissions concealed in these trends—also largely unexplained [G.8].
Fluctuations in the gender ratio at birth and stillbirth rate are also likely outcomes from such infectious outbreaks [Q.4,R.13]. The hospital bed occupancy rate in England also shows otherwise unexplained patterns [L.37].
The hypothesis is that any number of unrecognized infections by the 3000 species of known pathogens is highly likely to precipitate at least part of such unexplained trends. As stated earlier, the evidence has been there for many years but has been ignored.
12. The Role of Government Policy
In theory, government policy is enacted to solve problems. However, conflicting objectives can sometimes generate unintended consequences. Hence the unintended consequences of policy when attempting to address complex problems [
131] and somewhat insidious ‘policy-based evidence’ when attempting to justify policy [
132], as opposed to evidence-based policy [
133]. At this point we need to consider how a policy imperative, namely, the Private Finance Initiative (PFI), which commenced in the UK in 1992 [
134], had a perverse effect on public hospital bed planning. The PFI initiative was initially an ‘off-balance sheet’ method to build public infrastructure [
135]. The government of the time was seeking to convince the public that national debt was being ‘prudently’ managed [
136]. Effectively a private consortium borrowed the money and built the infrastructure and then charged for the infrastructure and subsequent maintenance which was paid out of revenue. The international rules of accounting were subsequently changed to recognize that such an ‘off-balance sheet’ illusion was in fact public debt [
137], and indeed a very expensive form of public debt [
138,
139,
140,
141].
Because PFI was such an expensive way to build hospitals, and other infrastructure, the government introduced rules stipulating that the schemes should be ‘affordable’ [
142]. The only way to achieve the ‘affordability’ criteria was to effectively manipulate or fiddle the future bed demand considerably downwards. NHS managers and external Management Consultancies ‘complied’ with the rules and smaller hospitals were duly constructed. The role of external Management Consultancies was to construct a believable narrative about increasing efficiency and demand ‘management’. The reality was everyone in the NHS knew that the forecasts had been fiddled and the hospitals were far too small [
143,
144,
145,
146].
The public did not possess the detailed technical knowledge to realize that the use of LOS as an ‘efficiency’ measure is fraught with hidden dangers [K.1–9], and that the decline in the real LOS within the NHS was far lower than was being predicted on paper—as above.
The point is that such manipulation was easily achieved partly because there were no international benchmarks. Hence, claims by politicians that the hospitals were too big due to inefficiency could not be adequately dismissed.
The sad truth is that the high bed occupancy caused by too few beds relative to expressed demand directly generates chaos, harm, and inefficiency [
20,
81,
82,
83,
84,
85], thereby reinforcing the claim by politicians that the NHS is ‘inefficient’. The inefficiency is more correctly, policy imposed.
A recent analysis by the King’s Fund of the performance of the UK NHS relative to international peers has concluded that while a publicly funded NHS avoids the risk of catastrophic costs from falling ill it has suffered from substantial capital (buildings and equipment) and manpower underinvestment leading to poor health care outcomes [
147].
13. Key Recommendations
From the above discussion and observations several key recommendations can be listed:
Length of stay should be measured in real time. Occupied bed days with 2 decimal places should be sufficient (equivalent to hours and minutes).
Length of stay should be measured from the decision to admit which may include time in the emergency department waiting for a bed to be found and the time waiting in an Ambulance outside of the emergency department.
Occupied beds are the real measure of demand. Occupied bed days are the sum of all real-time LOS including time spent waiting for a bed to be found or queuing outside and inside the emergency department.
Available beds can be split into two parts:
Staffed beds—the usual measure of available beds.
Physical beds—includes staffed beds, plus beds which may be closed over the weekend, beds in day surgery units which are closed outside working hours, and beds held in reserve for infection control, deep cleaning, and surge capacity.
Hospital average bed occupancy should be measured as a real-time average and can be reported annually, monthly, weekly, or daily.
The average bed occupancy is not applicable at whole hospital level but reflects the occupancy applicable to the different sized bed pools which are specific to types of patient care, i.e., critical care, pediatrics, neonates, adult medicine, adult surgical, oncology, hematology, etc., plus the need to separate males and females in the wards.
The optimum whole hospital occupancy is therefore the weighted average of the constituent patient-type and sex-specific bed pools.
Hospital bed planning cannot be done using annual averages as this will guarantee that the bed numbers are too small. Both circadian, weekday and seasonal cycles must be included along with appropriate stand-by for infection control, deep cleaning, and surge capacity.
Hospital staffing should be flexible to allow for the natural fluctuations in demand which characterize the real-world environment, i.e., levels of local pollution, fluctuations in temperature (with extremes), and known and unknown infectious outbreaks.
Hospital planning should include a measure of unmet demand which may have accumulated in surgical waiting lists, and waiting in an outpatient queue., i.e., Demand = Admissions + change in the number waiting.
Building a hospital which is too small is a gross waste of scarce resources since it creates inefficiency, poor patient care, staff burnout and general chaos.
It has been the author’s observation over the past 30 years that all the above are regularly ignored in England in a seemingly lemming-like urge to build publicly funded hospitals which are too small to operate at maximum effectiveness and efficiency. Lastly, Supplementary material S9 illustrates the central issue, namely, hospital bed demand is the output from a complex system. All complex systems show unexpected outcomes.
14. Conclusions
The new model for acute beds appears to work well and has a relevant theoretical framework. It can also be used to compare staffing levels between countries. Both beds per 1000 deaths and beds per 1000 population give approximately similar outcomes only if the crude mortality rate is used as the x-axis. However, beds per 1000 population does not have an associated theoretical basis.
The method has been expanded to investigate the likely optimum numbers of beds and staff relating to different types of care. This feasible region depends greatly on the levels of supporting outpatient and community care and the implementation of genuinely joined-up integrated care. The latter requires a high level of supporting political will, including relevant policies and a persistent process of implementation, reevaluation, and investment.
The high volatility in deaths, probably mediated by infectious outbreaks, has profound implications to the average bed occupancy rate for optimum efficiency. This is also influenced by hospital size where smaller hospitals must operate at a lower average occupancy.
England has been used as an example of dysfunctional policy ‘management’ leading to a level of acute services which are sliding into chaos and very low levels of bed provision.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Funding
This research received no external funding
Data Availability Statement
All data is publicly available.
Conflicts of Interest
The author runs a consultancy Healthcare Analysis and Forecasting. However, this work is not deemed to constitute a conflict of interest.
Appendix A
Figure A1.
Rolling 12-month total of deaths for nine London boroughs. Each borough has been normalized to the median value of the 22-year time series. Each borough has around 2000 to 3000 deaths per annum. The average London borough is only 49 square kilometers (0.02% of the size of the UK). Monthly data is from the ONS [
95].
Figure A1.
Rolling 12-month total of deaths for nine London boroughs. Each borough has been normalized to the median value of the 22-year time series. Each borough has around 2000 to 3000 deaths per annum. The average London borough is only 49 square kilometers (0.02% of the size of the UK). Monthly data is from the ONS [
95].
References
- Farmer, R.; Emami, J. Models for forecasting hospital bed requirements in the acute sector. J. Epidemiol. Community Health 1990, 44, 307-312. [CrossRef]
- Ravaghi, H.; Alidoost, S.; Mannion, R.; Bélorgeot, V. Models and methods for determining the optimal number of beds in hospitals and regions, a systematic scoping review. BMC Health Serv. Res. 2020, 20, 186. [CrossRef]
- NHS Digital. Hospital admitted patient care activity. Available online, Hospital Admitted Patient Care Activity - NHS Digital (accessed on 20 June 2023).
- Office for National Statistics. Detailed population estimates. Available online, Estimates of the population for the UK, England, Wales, Scotland and Northern Ireland - Office for National Statistics (ons.gov.uk) (accessed on 6 July 2023).
- Soltani, S.; Kesheh, M.; Siri, G.; Faramarzi, S.; Shahbahrami, R.; Didehdar, M.; Erfani, Y.; Farahani, A. The role of viruses in human acute appendicitis: a systematic literature review. Int J Colorectal Dis. 2023, 38(1), 102. [CrossRef]
- Marcus Blohs, Alexander Mahnert, Kevin Brunnader, Christina Flucher, Christoph Castellani, Holger Till, Georg Singer & Christine Moissl-Eichinger (2023) Acute appendicitis manifests as two microbiome state types with oral pathogens influencing severity. Gut Microbes, 2023,15, 1. [CrossRef]
- Jie Yuan, Enming Qiu, Shuai Han et al. Whole metagenomic sequencing reveals different compositions of pathogenic bacteria in patients with appendicitis: Bacterial culture is not suitable as the only therapeutic guidance, 27 July 2020, PREPRINT (Version 1) available at Research Square. [CrossRef]
- Aida Vafae Eslahi, Meysam Olfatifar, Elham Houshmand, Amir Abdoli, Behzad Bijani, Sima Hashemipour, Razzagh Mahmoudi, Elham Hajialilo, Mohammad Javad Abbaszadeh Afshar, Ali Reza Mohammadzadeh & Milad Badri. Parasites in surgically removed appendices as a neglected public health concern: a systematic review and meta-analysis, Pathogens and Global Health, 2022, 116(6), 341-355. [CrossRef]
- Sarici B, Akbulut S, Ozcan M, Demyati, K., Samdanci, E. Unusual infectious agents detected in appendectomy specimens: A retrospective analysis of 42 cases. Turk J Surg. 2020, 36(3), 291-296. [CrossRef]
- Kariya, B.; Krutsri, C.; Singhatas, P.; Sumritpradit, P.; Thampongsa, T.; Lertsitthichai, P.; Phoprom, N. Incidence of complicated appendicitis during the COVID-19 pandemic: A systematic review and meta-analysis. International Journal of Surgery Open. 2022, 45, 100512. [CrossRef]
- Ahmed, O.; Boschi-Pinto, C.; Lopez, A.; Murray, C.; Lozano, R.; Inoue, M. Age standardization of rates: A new WHO standard. GPE Discussion Paper Series: No.31 EIP/GPE/EBD World Health Organization, 2001. Available online, Age Standardization of Rates: A new WHO Standard (Accessed on 2 October 2023).
- Zylstra, E.; Zipkin, E. Accounting for sources of uncertainty when forecasting population responses to climate change. J Anim Ecol. 2021, 90(3), 558-561. [CrossRef]
- Keilman, N. Uncertain population forecasts. Nature 2001, 412, 490–491. [CrossRef]
- Keilman, N. Uncertainty in Population Forecasts for the Twenty-First Century. Annual Review of Resource Economics, 2020, 12(1). [CrossRef]
- Office for National Statistics. People living alone aged 65 years old and over, by specific age group and sex, UK, 1996 to 2019. Available online, People living alone aged 65 years old and over, by specific age group and sex, UK, 1996 to 2019 - Office for National Statistics (ons.gov.uk) (Accessed on 2 October 2023).
- OECD. Who Cares? Attracting and Retaining Care Workers for the Elderly. Available online, Who Cares? Attracting and Retaining Care Workers for the Elderly | en | OECD (Accessed on 2 October 2023).
- Maguire, D.; Dunn, P.; McKenna, H. How hospital activity in the NHS in England has changed over time. The Kings Fund. 20 December 2016. Available online, How hospital activity in the NHS in England has changed over time | The King's Fund (kingsfund.org.uk) (Accessed on 3 July 2023).
- Gitsels, L.; Kulinskaya, E.; Wright, N. How medical advances and health interventions will shape future longevity. British Actuarial Journal. 2019, 24, E10. [CrossRef]
- NHS England. Bed availability and occupancy data – overnight. Available online, Statistics » Bed Availability and Occupancy Data – Overnight (england.nhs.uk) (Accessed on 13 June 2023).
- Bosque-Mercader, L.; Siciliani, L. The association between bed occupancy rates and hospital quality in the English National Health Service. Eur J Health Econ 2023, 24, 209–236. [CrossRef]
- Payne, G.; Laporte, A.; Deber, R.; Coyte, P. Counting backward to health care's future: using time-to-death modeling to identify changes in end-of-life morbidity and the impact of aging on health care expenditures. Milbank, Q. 2007, 85(2), 213-257. [CrossRef]
- Moore, P.; Bennett, K.; Normand, C. Counting the time lived, the time left or illness? Age, proximity to death, morbidity and prescribing expenditures. Soc Sci Med. 2017, 184, 1-14. [CrossRef]
- Hanlon, P.; Walsh, D.; Whyte, B.; Scott, S.; Lightbody, P.; Gilhooly, M. Hospital use by an ageing cohort: an investigation into the association between biological, behavioural and social risk markers and subsequent hospital utilization. J Public Health Med. 1998, 20(4), 467-476. [CrossRef]
- Henderson, J.; Goldacre, M.; Griffith, M. Hospital care for the elderly in the final year of life: a population-based study. BMJ 1990; 301: 17-19. [CrossRef]
- Busse, R.; Krauth, C.; Schwartz, F. Use of acute hospital beds does not increase as the population ages: results from a seven year cohort study in Germany. J Epidemiol Community Health. 2002, 56(4), 289-93. [CrossRef]
- Riumallo-Herl, C.; Canning, D.; Salomon, J. Measuring health and economic wellbeing in the Sustainable Development Goals era: development of a poverty-free life expectancy metric and estimates for 90 countries. Lancet Glob Health. 2018, 6(8), e843-e858. Erratum in: Lancet Glob Health. 2018, 6(10), e1073. [CrossRef]
- Skou, S.; Mair, F.; Fortin, M.; Guthrie, B.; Nunes, B.; Miranda, J.; Boyd, C.; Pati, S.; Mtenga, S.; Smith, S. Multimorbidity. Nat Rev Dis Primers. 2022, 8, 48. [CrossRef]
- The World Bank. Death rate, crude (per 1000 people). Available online, Death rate, crude (per 1,000 people) | Data (worldbank.org) (accessed 9 May 2023).
- World Health Organisation. Hospital beds (per 10000 population). Available online, Hospital beds (per 10 000 population) (who.int) (accessed 9 May 2023).
- Eurostat. Hospital beds by type of care - historical data (1960-2020) [HLTH_RS_BDS]. Available online, Healthcare resource statistics - beds - Statistics Explained (europa.eu) (Accessed on 20 June 2023).
- OECD/European Observatory on Health Systems and Policies. Sweden, Country Health Profile 2021, State of Health in the EU, OECD Publishing, Paris/European Observatory on Health Systems and Policies, Brussels. Available online, Sweden, Country Health Profile 2021| European Observatory on Health Systems and Policies (who.int) (accessed on 19 May 2023).
- Bäck, M.; Calltorp, J. The Norrtaelje model, a unique model for integrated health and social care in Sweden. Int J Integr Care. 2015, 15, e016. [CrossRef]
- The World Bank. GNI (current US$). Available online, GNI (current US$) | Data (worldbank.org) (accessed on 9 May 2023).
- Mundt, A.; Rozas Serri, E.; Irarrázaval, M.; O'Reilly, R.; Allison, S.; Bastiampillai, T.; Musisi, S.; Kagee, A.; Golenkov, A.; El-Khoury, J.; Park, S.; Chwastiak, L.; Priebe, S. Minimum and optimal numbers of psychiatric beds, expert consensus using a Delphi process. Mol Psychiatry. 2022, 27(4), 1873-1879. [CrossRef]
- Mundt, A.; Langerfeldt, S.; Maphisa, J.; Sourabié, O.; Yongsi, B.; Serri, E.; Bukasa Tshilonda, J.; Te, J.; Bitta, M.; Mathe, L.; et al. Changes in rates of psychiatric beds and prison populations in sub-Saharan Africa from 1990 to 2020. J Glob Health. 2022, 12, 04054. [CrossRef]
- Barbui, C.; Papola, D.; Saraceno, B. Forty years without mental hospitals in Italy. Int J Ment Health Syst. 2018, 12, 43. [CrossRef]
- Hudson, C. Benchmarks for Needed Psychiatric Beds for the United States: A Test of a Predictive Analytics Model. Int. J. Environ. Res. Public Health 2021, 18, 12205. [CrossRef]
- The World Bank. Nurses and midwives (per 1,000 people). Available online, Nurses and midwives (per 1,000 people) | Data (worldbank.org) (accessed on 9 May 2023).
- The World Bank. Physicians (per 1,000 people). Available online, Physicians (per 1,000 people) | Data (worldbank.org) (accessed on 9 May 2023).
- The World Bank. Specialist surgical workforce (per 100,000 people). Available online, Specialist surgical workforce (per 100,000 population) | Data (worldbank.org) (accessed on 9 May 2023).
- The Washington Post. The UK’s health-care breakdown demands radical thinking. 2 August 2023. Available online, The UK’s Health-Care Breakdown Demands Radical Thinking - The Washington Post (accessed 3 August 20230.
- Office for National Statistics. Deaths by usual area of residence, UK. Available online, Deaths registered by area of usual residence, UK - Office for National Statistics (ons.gov.uk) (Accessed on 6 October 2023).
- NHS England. Delivery plan for tackling the COVID-19 backlog for elective care. February 2022. Available online, Coronavirus » Delivery plan for tackling the COVID-19 backlog of elective care (england.nhs.uk) (accessed on 5 July 2023).
- Our World in Data. Age standardized deaths from all causes. Available online, Age-standardized death rate from all causes, 2019 (ourworldindata.org) (accessed on 5 July 2023).
- George, L. Neighborhood Deprivation as a Measure of Social Need and Healthcare Utilization: A Review of the Literature. Master Essay, 2021. University of Pittsburgh. Available online, Neighborhood Deprivation as a Measure of Social Need and Healthcare Utilization: A Review of the Literature - D-Scholarship@Pitt (Accessed on 6 October 2023).
- 46. GOV.UK.English indices of deprivation 2019. Available online, English indices of deprivation 2019 - GOV.UK (www.gov.uk) (Accessed on 6 October 2023).
- McCartney, G.; Tamvakas, I.; Millard, D.; Smith, L.; Renwick, L. The patterning of hospital discharges and bed-days by deprivation in Scotland (2011/12), March 2015. Available online, scotpho151119-hospital-discharges-and-bed-days-scotland-by-deprivation-2011-12-revised-october-2015.pdf (Accessed on 6 October 2023).
- Diaz, A.; Lindau, S.; Obeng-Gyasi, S.; Dimick, J.; Scott, J.; Ibrahim, A. Association of Hospital Quality and Neighborhood Deprivation with Mortality After Inpatient Surgery Among Medicare Beneficiaries. JAMA Netw Open. 2023 Jan 3;6(1):e2253620. [CrossRef]
- Office for National Statistics. Numbers of deaths and age standardised mortality rates by deprivation decile. Available online, Numbers of deaths and age standardised mortality rates by deprivation decile, month and sex: 1 January 2019 to 30 June 2022 - Office for National Statistics (ons.gov.uk) (Accessed on 6 October 2023).
- Office for National Statistics. About area classifications. Available online, About the area classifications - Office for National Statistics (Accessed on 6 October 2023).
- Institute for Government. Barnet formula. November 2020. Available online: Barnett formula | Institute for Government (accessed on 24 August 2023).
- US CDC. QuickStats: Age-Adjusted Death Rates, by State—United States, 2017. MMWR Morb Mortal Wkly Rep 2019, 68, 255. [CrossRef]
- Ginsburg, P.; Koretz, D. Bed availability and hospital utilization: estimates of the “Roemer effect”. Health Care Financ Rev. 1983, 5, 87-92.
- Dickman, S.; Himmelstein, D.; Woolhandler, S. Inequality and the health-care system in the USA. Lancet. 2017, 389(10077), 1431-1441. [CrossRef]
- McCrum, M.; Wan, N.; Han, J.; Lizotte, S.; Horns, J. Disparities in Spatial Access to Emergency Surgical Services in the US. JAMA Health Forum 2022, 3, e223633. [CrossRef]
- Najibi, S.; Seyedi, S.; Farhadi, P.; Kharazmi, E.; Shojaei, P.; Delavari, S.; Lotfi, F.; Kavosi, Z. Development of a model for predicting hospital beds shortage and optimal policies using system dynamics approach. BMC Health Serv Res. 2022, 22, 1525. [CrossRef]
- Nguyen, J.; Six, P.; Antonioli, D.; Glemain, P.; Potel, G.; Lombrail, P.; Le Beux, P. A simple method to optimize hospital beds capacity. Internat J Medical Informatics 2005, 74, 39-49. [CrossRef]
- Rachet-Jacquet, L.; Rocks, S.; Charlesworth, A. Long-term projections of health care funding, bed capacity and workforce needs in England. Health Policy 2023, 132, 104815. [CrossRef]
- Walsh, B.; Brick, A. Inpatient bed capacity requirements in Ireland in 2023: Evidence on the public acute hospital system. QEC Research Note 20230101. [CrossRef]
- Nowossadeck, E. Population aging and hospitalization for chronic disease in Germany. Deutsches Arzteblatt International 2012, 109, 151-157. [CrossRef]
- Nowossadeck, E.; Prutz, F.; Teti, A. Population change and the burden of hospitalization in Germany 2000–2040: Decomposition analysis and projection. PLoS ONE 2020, 15, e0243322. [CrossRef]
- Kramer, J.; Schreyogg, J. Demand-side determinants of rising hospital admissions in Germany: the role of ageing. Eur J Health Economics. 2019, 20, 715-728. [CrossRef]
- Sheikhbardsiri, H.; Raeisi, A.; Nekoei-Moghadam, M.; Rezaei, F. Surge Capacity of Hospitals in Emergencies and Disasters with a Preparedness Approach: A Systematic Review. Disaster Med Public Health Prep. 2017, 11, 612-620. [CrossRef]
- Moineddin, R.; Upshur, R.; Crighton, E.; Mumdani, M. Autoregression as a means of assessing the strength of seasonality in a time series. Popul Health Metrics. 2003, 1, 10. [CrossRef]
- Upshur, R.; Moineddin, R.; Crighton, E.; Kiefer, L.; Mamdani, M. Simplicity within complexity: Seasonality and predictability of hospital admissions in the province of Ontario 1988–2001, a population-based analysis. BMC Health Serv Res. 2005, 5, 13. [CrossRef]
- Shah, S.; Carey, I.; Harris, T.; DeWilde, S.; Cook, D. Mortality in older care home residents in England and Wales, Age and Ageing. 2013, 42(2), 209–215. [CrossRef]
- Boucaud-Maitre, D.; Letenneur, L.; Dramé, M.; Taubé-Teguo, N.; Dartigues, J.; et al. Comparison of mortality and hospitalizations of older adults living in residential care facilities versus nursing homes or the community. A systematic review. PLOS ONE 2023, 18, e0286527. [CrossRef]
- Nuako A, Liu J, Pham G, Smock, N., James, A., et al. Quantifying rural disparity in healthcare utilization in the United States: Analysis of a large midwestern healthcare system. PLOS ONE 2022, 17, e0263718. [CrossRef]
- Kim, I.; Lim, H.-K.; Kang, H.-Y.; Khang, Y-H. Comparison of three small-area mortality metrics according to urbanity in Korea: the standardized mortality ratio, comparative mortality figure, and life expectancy. Popul Health Metrics. 2020, 18, 3. [CrossRef]
- Song, Z.; Ferris, T. Baby Boomers and Beds: A Demographic Challenge for the Ages. J Gen Intern Med. 2018, 33, 367-369. [CrossRef]
- Thayer, B. Considering population and war: a critical and neglected aspect of conflict studies. Philos Trans R Soc Lond B Biol Sci. 2009, 364(1532), 3081-3092. [CrossRef]
- Kakad, M.; Utley, M.; Rugkåsa, J.; Dahl, F. Erlang could have told you so-A case study of health policy without maths. Health Policy 2019, 123, 1282-1287. [CrossRef]
- Proudlove NC. The 85% bed occupancy fallacy: The use, misuse and insights of queuing theory. Health Serv Manage Res. 2020, 33, 110-121. [CrossRef]
- NHS England. Referral to treatment (RTT) waiting times. Available online, Statistics » Referral to Treatment (RTT) Waiting Times (england.nhs.uk) (accessed on 7 July 2023).
- NHS England. Reducing ambulance handover delays. Available online, NHS England » Reducing ambulance handover delays: key lines of enquiry (accessed on 6 October 2023).
- American Hospital Association. Rural report. Available online, rural-report-2019.pdf (aha.org) (Accessed on 10 October, 2023).
- Hughes, A.; Horrocks, D. Jr.; Leung, C.; Richardson, M.; Sheehy, A.; Locke, C. The increasing impact of length of stay “outliers” on length of stay at an urban academic hospital. BMC Health Serv Res. 2021, 21, 940. [CrossRef]
- Stowell, A.; Claret, P.; Sebbane, M. et al. Hospital out-lying through lack of beds and its impact on care and patient outcome. Scand J Trauma Resusc Emerg Med 2013, 21, 17. [CrossRef]
- Nardi, R.; Mazzone, A.; Fontanella, A.; Campanini, M.; Manfellotto, D.; Bellandi, T.; Gussoni, G.; Tartaglia, R.; Squizzato, A. What Quality and Safety of Care for Patients Admitted to Clinically Inappropriate Wards, a Systematic Review. J Gen Intern Med. 2019, 34, 1314-1321. [CrossRef]
- Stylianou, N.; Fackrell, R.; Vasilakis, C. Are medical outliers associated with worse patient outcomes? A retrospective study within a regional NHS hospital using routine data. BMJ Open 2017, 7, e015676. [CrossRef]
- Madsen, F.; Ladelund, S.; Linneberg, A. High levels of bed occupancy associated with increased inpatient and thirty-day hospital mortality in Denmark. Health Aff (Millwood) 2014, 33, 1236-1244. [CrossRef]
- Friebel, R.; Fisher, R.; Deeny, S.; Gardner, T.; Molloy, A.; Steventon, A. The implications of high bed occupancy rates on readmission rates in England, A longitudinal study. Health Policy 2019, 123, 765-772. [CrossRef]
- Wyatt, S.; Joshi, R.; Mortimore, J.; Mohammed, M. Relationship between emergency department and inpatient occupancy and the likelihood of an emergency admission, a retrospective hospital database study. Emergency Medicine Journal 2022, 39, 174-180. [CrossRef]
- Jones, S.; Moulton, C.; Swift, S.; Molyneux, P.; Black, S.; Mason, N.; Oakley, R., Mann, C. Association between delays to patient admission from the emergency department and all-cause 30-day mortality. Emerg Med J. 2022, 39, 168-173. [CrossRef]
- Bosque-Mercader, L.; Siciliani, L. The association between bed occupancy rates and hospital quality in the English National Health Service. Eur J Health Econ. 2023, 24, 209–236. [CrossRef]
- Giancotti, M.; Guglielmo, A.; Mauro, M. Efficiency and optimal size of hospitals, Results of a systematic search. PLoS One 2017, 12, e0174533. [CrossRef]
- Epstein, N. A review article on the benefits of early mobilization following spinal surgery and other medical/surgical procedures. Surg Neurol Int. 2014, 16, 5(Suppl 3), S66-73. [CrossRef]
- Yakkanti, R.; Miller, A.; Smith, L.; Feher, A.; Mont, M.; Malkani, A. Impact of early mobilization on length of stay after primary total knee arthroplasty. Ann Transl Med. 2019, 7, 69. [CrossRef]
- Tazreean, R.; Nelson, G.; Twomey, R. Early mobilization in enhanced recovery after surgery pathways: current evidence and recent advancements. J Comp Eff Res. 2022, 11, 121-129. [CrossRef]
- Gavilanes, E.; Gavilanes, N.; Fleming, R. The effect of age on length of stay in the medical intensive care unit. Chest. 2010, 138, S225A. [CrossRef]
- Rachoin JS, Aplin KS, Gandhi S, Kupersmith E, Cerceo, E. Impact of Length of Stay on Readmission in Hospitalized Patients. Cureus. 2020, 12, e10669. [CrossRef]
- Walsh, B.; Smith, S.; Wren, M.; Eighan, J.; Lyons, S. The impact of inpatient bed capacity on length of stay. Eur J Health Econ. 2022, 23, 499-510. [CrossRef]
- Malisauskaite, G.; Jones, K.; Allan, S.; Birks, Y.; Baxter, K.; Gridley, K. How local partnerships to improve urgent and emergency care have impacted delayed transfers of care from hospitals in England, an analysis based on a synthetic control estimation method. BMJ Open 2022, 12, e054568. [CrossRef]
- Brännström, Å.; Sjödin, H.; Rocklöv, J. A Method for Estimating the Number of Infections from the Reported Number of Deaths. Front Public Health 2022, ;9, 648545. [CrossRef]
- Office for National Statistics. Deaths registered monthly in England and Wales. Available online, Deaths registered monthly in England and Wales - Office for National Statistics (ons.gov.uk) (accessed on 9 May 2023).
- Tucci, V.; Moukaddam, N.; Meadows, J.; Shah, S.; Galwankar, S.; Kapur, G. The Forgotten Plague, Psychiatric Manifestations of Ebola, Zika, and Emerging Infectious Diseases. J Glob Infect Dis. 2017, 9, 151-156. [CrossRef]
- O’Mahoney; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; Wilkinson, T.; et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations, A systematic review and meta-analysis. EClinicalMedicine 2022, 55, 101762. [CrossRef]
- Taquet, M.; Geddes, J.; Husain, M.; Luciano, S.; Harrison, P. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19, a retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416-427. [CrossRef]
- Wheeler, D.; Chambers, D. Understanding statistical process control, 2nd Edition.1992. SPC Press, Knoxville, Tennessee. ISBN 0-945320-13-2.
- Lu, W.; Ren, H. Diseases spectrum in the field of spatiotemporal patterns mining of infectious diseases epidemics: A bibliometric and content analysis. Front Public Health 2023, 10. [CrossRef]
- Changruenngam, S.; Bicout, D.; Modchang, C. How the individual human mobility spatio-temporally shapes the disease transmission dynamics. Sci Rep. 2020, 10, 11325. [CrossRef]
- Coletti, P.; Poletto, C.; Turbelin, C.; Blanchon, T.; Colizza, V. Shifting patterns of seasonal influenza epidemics. Sci Rep 2018, 8, 12786. [CrossRef]
- Pyöriä, L.; Pratas, D.; Toppinen, M.; Hedman, K.; Sajantila, A.; Perdomo, M. Unmasking the tissue-resident eukaryotic DNA virome in humans. Nucleic Acids Res. 2023, 51, 3223-3239. [CrossRef]
- Hertzberg, V.; Weiss, H.; Elon, L.; Si, W.; Norris, S.; FlyHealthy Research Team. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci USA. 2018, 115, 3623-3627. [CrossRef]
- Mathai, V.; Das, A.; Bailey, J.; Breuer, K. Airflows inside passenger cars and implications for airborne disease transmission. Sci Adv. 2021, 7,eabe0166(2021). [CrossRef]
- Yang, X.; Ou, C.; Yang, H.; Liu, L.; Song, T.; Kang, M.; Lin, H.; Hang, J. Transmission of pathogen-laden expiratory droplets in a coach bus. J Hazardous Materials 2020, 397, 122609. [CrossRef]
- Miller RR, Montoya V, Gardy JL, Patrick DM, Tang, P. Metagenomics for pathogen detection in public health. Genome Med. 2013, 5, 81. [CrossRef]
- Fournier, P.; Dubourg, G.; Raoult, D. Clinical detection and characterization of bacterial pathogens in the genomics era. Genome Med. 2014, 6, 114. [CrossRef]
- Song, S.; Ma, L.; Xu, X.; Shi, H.; Li, X.; Liu, Y.; Hao, P. Rapid screening and identification of viral pathogens in metagenomic data. BMC Med Genomics. 2021, 14(Suppl 6), 289. [CrossRef]
- Wark, P. Viral and bacterial interactions in pneumonia. Expert Review Resp Med. 2010, 4, 221-228. [CrossRef]
- Griffiths, E.; Pedersen, A.; Fenton, A.; Petchey, O. The nature and consequences of coinfection in humans. J Infect. 2011, 63, 200-206. [CrossRef]
- Wong, G.; Liu, W.; Liu, Y.; Zhou, B.; Bi, Y.; Gao, G. MERS, SARS, and Ebola, The Role of Super-Spreaders in Infectious Disease. Cell Host Microbe. 2015, 18, 398-401. [CrossRef]
- Wanelik, K.; Begon, M.; Fenton, A.; Norman, R.; Beldomenico, P. Positive feedback loops exacerbate the influence of superspreaders in disease transmission. iScience. 2023, 26, 106618. [CrossRef]
- Kyriakopoulos, A.; Papaefthymiou, A.; Georgilas, N.; Doulberis, M.; Kountouras, J. The Potential Role of Super Spread Events in SARS-COV-2 Pandemic; a Narrative Review. Arch Acad Emerg Med. 2020, 8, e74.
- Opatowski, L.; Baguelin, M.; Eggo, R. Influenza interaction with cocirculating pathogens and its impact on surveillance, pathogenesis, and epidemic profile: A key role for mathematical modelling. PLOS Pathogens 2018, 14, e1006770. [CrossRef]
- Brodsky, I.; Medzhitov, R. Targeting of immune signalling networks by bacterial pathogens. Nat Cell Biol. 2009, 11, 521–526. [CrossRef]
- Sedger, L. microRNA control of interferons and interferon induced anti-viral activity. Mol Immunol. 2013, 56, 781-93. [CrossRef]
- Chow, E.; Rolfes, M.; O'Halloran, A.; Anderson, E.; Bennett, N.; Billing, L.; Chai, S.; Dufort, E.; Herlihy, R.; Kim, S.; Lynfield, R.; et al. Acute Cardiovascular Events Associated With Influenza in Hospitalized Adults. Ann Intern Med. 2020, 173, 605-613. [CrossRef]
- Hansen, A.; Mortensen, L.; Trompet, S.; Westendorp, R. Health care expenditure in the last five years of life is driven by morbidity, not age: A national study of spending trajectories in Danish decedents over age 65. PLoS One 2020, 15, e0244061. [CrossRef]
- Vishnoi, A.; Rani, S. MiRNA Biogenesis and Regulation of Diseases, An Overview. Methods Mol Biol. 2017, 1509, 1-10. [CrossRef]
- Riahi Rad, Z.; Riahi Rad, Z.; Goudarzi, H.; Goudarzi, M.; Mahmoudi, M.; Yasbolaghi Sharahi, J.; Hashemi, A. MicroRNAs in the interaction between host-bacterial pathogens, A new perspective. J Cell Physiol. 2021, 236, 6249-6270. [CrossRef]
- Zhou, X.; Li, X.; Wu, M. miRNAs reshape immunity and inflammatory responses in bacterial infection. Sig Transduct Target Ther 3, 14 (2018). [CrossRef]
- Chandan, K.; Gupta, M.; Sarwat, M. Role of Host and Pathogen-Derived MicroRNAs in Immune Regulation During Infectious and Inflammatory Diseases. Front Immunol. 2020 Jan 24; 10: 3081. [CrossRef]
- Vaghf, A.; Khansarinejad, B.; Ghaznavi-Rad, E.; et al. The role of microRNAs in diseases and related signaling pathways. Mol Biol Rep 49, 6789–6801 (2022). [CrossRef]
- Di Marco, M.; Ramassone, A.; Pagotto, S.; Anastasiadou, E.; Veronese, A.; Visone, R. MicroRNAs in Autoimmunity and Hematological Malignancies. Int J Mol Sci. 2018, 19, 3139. [CrossRef]
- Lee, B.; Li, JL.; Marchica, J.; et al. Mapping genetic variability in mature miRNAs and miRNA binding sites in prostate cancer. J Hum Genet 2021, 66, 1127–1137. [CrossRef]
- Aimola, G.; Beythien, G.; Aswad, A., Kaufer, B. Current understanding of human herpesvirus 6 (HHV-6) chromosomal integration. Antiviral Res. 2020, 176, 104720. [CrossRef]
- Eliassen, E.; Lum, E.; Pritchett, J.; Ongradi, J.; Krueger, G.; Crawford, J.; Phan, T.; Ablashi, D.; Hudnall, S. Human Herpesvirus 6 and Malignancy: A Review. Front Oncol. 2018, 8, 512. [CrossRef]
- Romanescu, C.; Schreiner, T.; Mukovozov, I. The Role of Human Herpesvirus 6 Infection in Alzheimer's Disease Pathogenicity-A Theoretical Mosaic. J Clin Med. 2022, 11, 3061. [CrossRef]
- Simonnet, A.; Engelmann, I.; Moreau, A.; Garcia, B.; Six, S.; El Kalioubie, A.; Robriquet, L.; Hober, D.; Jourdain, M. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect Dis Now. 2021, 51, 296-299. [CrossRef]
- Oliver, K.; Lorenc, T.; Tinkler, J.; Bonell, C. Understanding the unintended consequences of public health policies: the views of policymakers and evaluators. BMC Public Health 2019, 19, 1057. [CrossRef]
- Cairney, P. The UK government’s imaginative use of evidence to make policy. Br Polit. 2019, 14, 1–22. [CrossRef]
- Phillips, P.; Castle, D.; Smyth, S. Evidence-based policy making: determining what is evidence. Heliyon. 2020, 6, e04519. [CrossRef]
- Broadbent, J.; Laughlin, R. Control and legitimation in government accountability processes, the private finance initiative in the UK. Critical Perspectives on Accounting. 2003, 14, 23-48. [CrossRef]
- Hodges, R.; Mellett, H. The U.K. private finance initiative, An accounting retrospective. The British Accounting Review 2012, 44, 235-247. [CrossRef]
- Grout, P. The economics of the private finance initiative. Oxford Review of Economic Policy 1997, 13, 53-66. [CrossRef]
- Holt, G. Off balance sheet activities. CPD technical article 1 July 2010. Available online, Off balance sheet activities | ACCA Global (accessed 7 April 2023).
- Hellowell, M.; Pollock, A. New Development, The PFI, Scotland’s plan for expansion and its implications. Public Money and Management 2007, 27, 351-354. [CrossRef]
- Hellowell, M.; Pollock, A. Do PPPs in Social Infrastructure Enhance the Public Interest? Evidence from England's National Health Service. Aust J Public Admin. 2010, 69, S23-S34. [CrossRef]
- Gaffney, D.; Pollock, A. PFI in the NHS—is there an economic case? BMJ 1999, 319, 116. [CrossRef]
- Gaffney, D.; Pollock, A. Pump-priming the PFI, why are privately financed hospital schemes being subsidized? Public Money and Management, 1999, 19, 55-62.
- GOV.UK. PFI/PPP finance guidance. Available online, PFI/PPP finance guidance - GOV.UK (www.gov.uk) (accessed 7 April 2023).
- Pollock, A.; Dunnigan, M.; Gaffney, D.; Macfarlane, A.; Majeed, F. What happens when the private sector plans hospital services for the NHS, three case studies under the private finance initiative. BMJ 1997, 314, 1266. [CrossRef]
- Dunnigan, M.; Pollock, A. Downsizing of acute inpatient beds associated with private finance initiative, Scotland’s case study. BMJ 2003, 326, 905. [CrossRef]
- Pollock, A. The NHS goes private. The Lancet. 1995, 346, 683-684.
- Pollock, A.; Shaoul, J.; Vickers, N. Private finance and “value for money” in NHS hospitals, a policy in search of a rationale? BMJ 2002, 324, 1205-1209. [CrossRef]
- The King’s Fund. How does the NHS compare to the health care systems of other countries? June 2023. Available online, How does the NHS compare to the health care systems of other countries (kingsfund.org.uk) (accessed on 30 June 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).