Introduction
PF is a disease characterized by purpuric lesions and is often associated with ecchymosis and necrosis due to diffuse thrombosis and disseminated intravascular coagulation. [
1,
2,
3]. About 90% of the cases that have been described in the pediatric literature have been fatal. Three forms of PF are described as follows: neonatal, idiopathic and acute infection. First is the patient with abnormalities of the protein C or protein S anticoagulant course. [
3,
4].The second condition is the rarest form of the disease, and the third form is the most common type, and it manifests as hemorrhagic necrosis due to thrombosis of the microvascular system of the skin. [
2,
3,
4,
6]. Streptococcus pneumoniae, although it is a very uncommon agent, which can cause PF [
1,
4,
5,
6]. Pneumococcus, often known as
Streptococcus pneumoniae, is a Gram-positive, extracellular pathogen, highly invasive, that is contagious through droplets. Blood, cerebrospinal fluid, and other body fluids (peritoneal, synovial, pleural, or pericardial) are examples of typically sterile places where S. pneumoniae can be isolated from. [
1,
2,
6,
7,
8]. Other bacteria such as Proteus Mirabilis, Pseudomonas aeruginosa, Mycobacterium tuberculosis, and Haemophilus influenzae, group A and B streptococcal have been linked to both children and adults. Pneumococcal infections are dangerous and often require hospitalization, with mortality rates between 8 to 15%. [
7,
8,
9,
10]. As of clinical presentations, PF first develops cutaneous macules, which become indurated and then evolve into irreversible necrosis of the skin, sepsis, and secondary infections it may occur as gangrene. As necrosis extends to deeper tissues, it is associated with a high incidence of amputations, skin grafts and decreases in survival rate.[
11,
12,
13,
14,
15]. We herein report the rare case of PF due to acute infections caused by S. pneumoniae, in a healthy child. We recommend rapid diagnosis and treatment to minimize limb necrosis, which can evolve unfavourably, especially in children with risk factors.[
15,
16,
17,
18].
Case Report
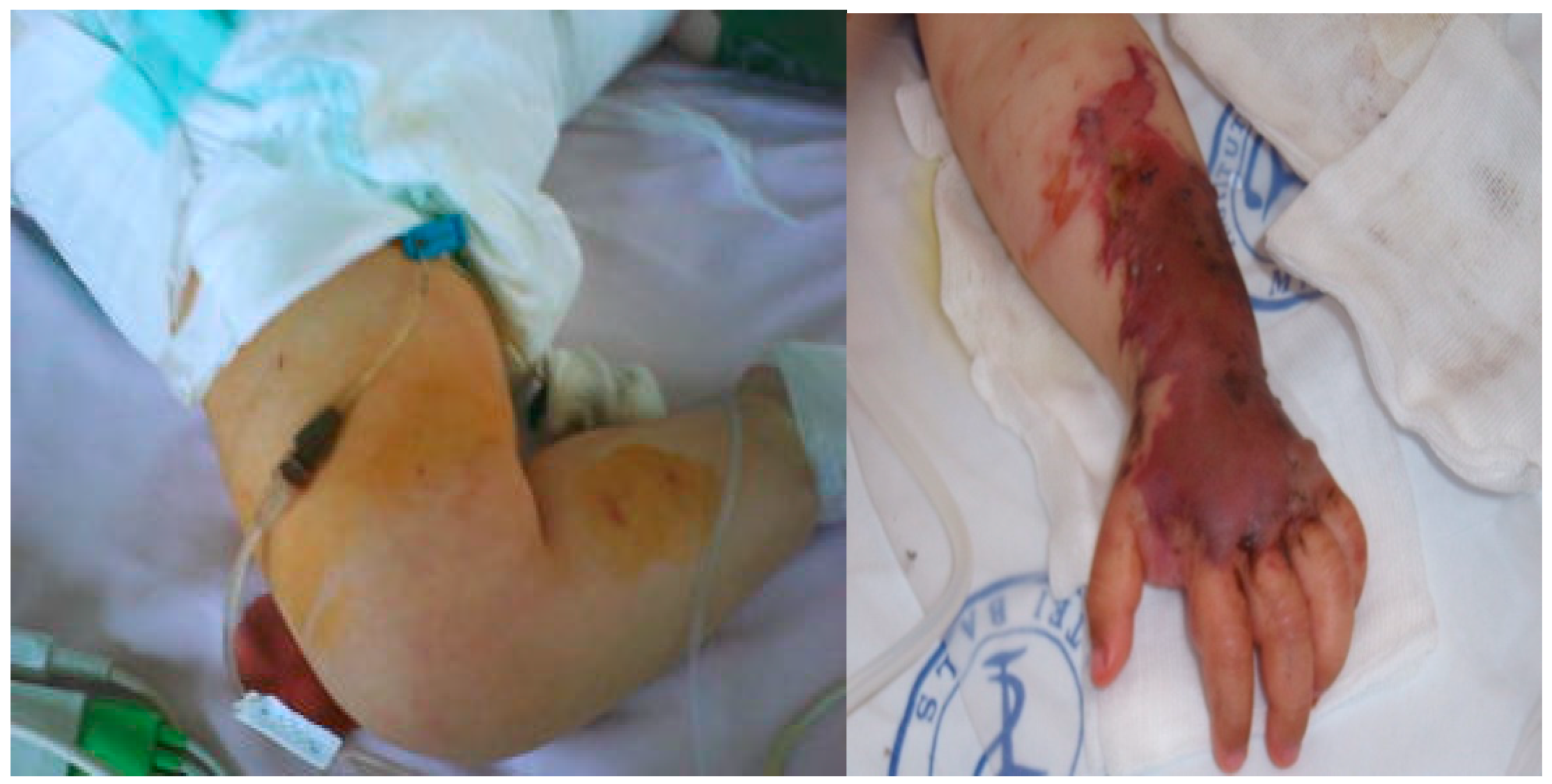
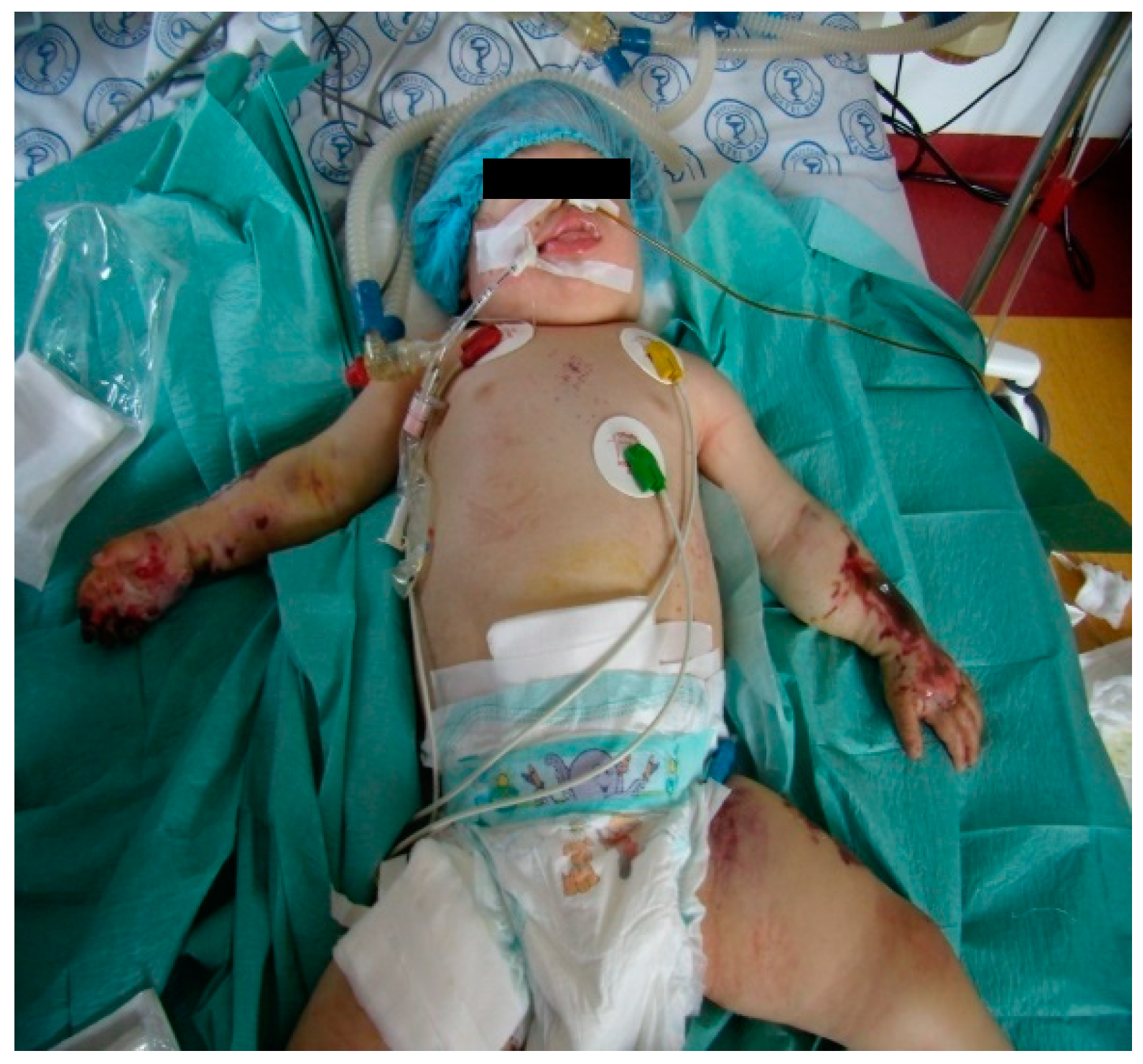
This previously healthy one year and nine months old girl, with a history of fever, nasal obstruction, mucopurulent rhinorrhea, otorrhea, and productive cough and was discharged home. She developed fever (39.8°C), and became lethargic, so her parents returned to the Paediatric Intensive Care Unit at National Institute for Infectious Diseases, „Prof. Dr Matei Bals”, Bucharest, Romania. From initial examination in our Pediatric department, she was in an altered general condition, coma, dehydrated, hypotension (55/30mmHg), tachycardia (188 beats/min), delayed capillary refill, and was found to have fever (38.8°C), and petechial rash on both her arms and trunk that quickly progressed to purpura. (See
Figure 1).
Abdominal examination did not reveal hepatosplenomegaly or other peritoneal signs. At the moment, the diagnosis revealed pneumonia with sepsis condition. The left-hand lesions were diagnosed as PF. Abnormalities in laboratory studies included tests as follows: elevated C reactive protein 97mg/L, leukocytosis 34.000/µL, anemia (Hb 9.7 g/dL), thrombocytopenia - platelet:23,000/µL, impaired renal function (Creatinine 4.45 mg/dL, hyponatremia and hyperkalemia). Coagulation tests revealed disseminated intravascular coagulation (DIC) with prolonged prothrombin time (international normalized ratio >5.59), activated partial thromboplastin time over 160 s, thrombin time over 140s, and increased D-dimer levels >89000µg/L. Clinical and laboratory measurements showed signs of septic shock. Standardized sepsis therapy was applied according to the surviving sepsis guidelines. Local treatment and dressing of skin lesions were also carried out.
Clinically and biologically, the diagnosis of severe sepsis with multiple organ failure (cardio-respiratory, hematological, hepatic, neurological, renal) is established. Laboratory data confirm severe sepsis with MSOF (leukocytosis with neutrophilia, anemia, thrombocytopenia, inflammatory syndrome present, positive Serum procalcitonin (PCT-Q), hepatic cytolysis syndrome, increasing values of serum urea and creatinine). Lumbar puncture reveals cloudy, purulent CSF, latex agglutination positive for pneumococcus; positive CSF culture – Streptococcus pneumoniae serotype 1a.
The child was intubated and mechanically ventilated, and complex therapy was administered, supporting vital functions, hydro-electrolytic and acid-base rebalancing, pathogenic (corticosteroids), a broad-spectrum antibiotic, symptomatic therapy, and parenteral nutrition.
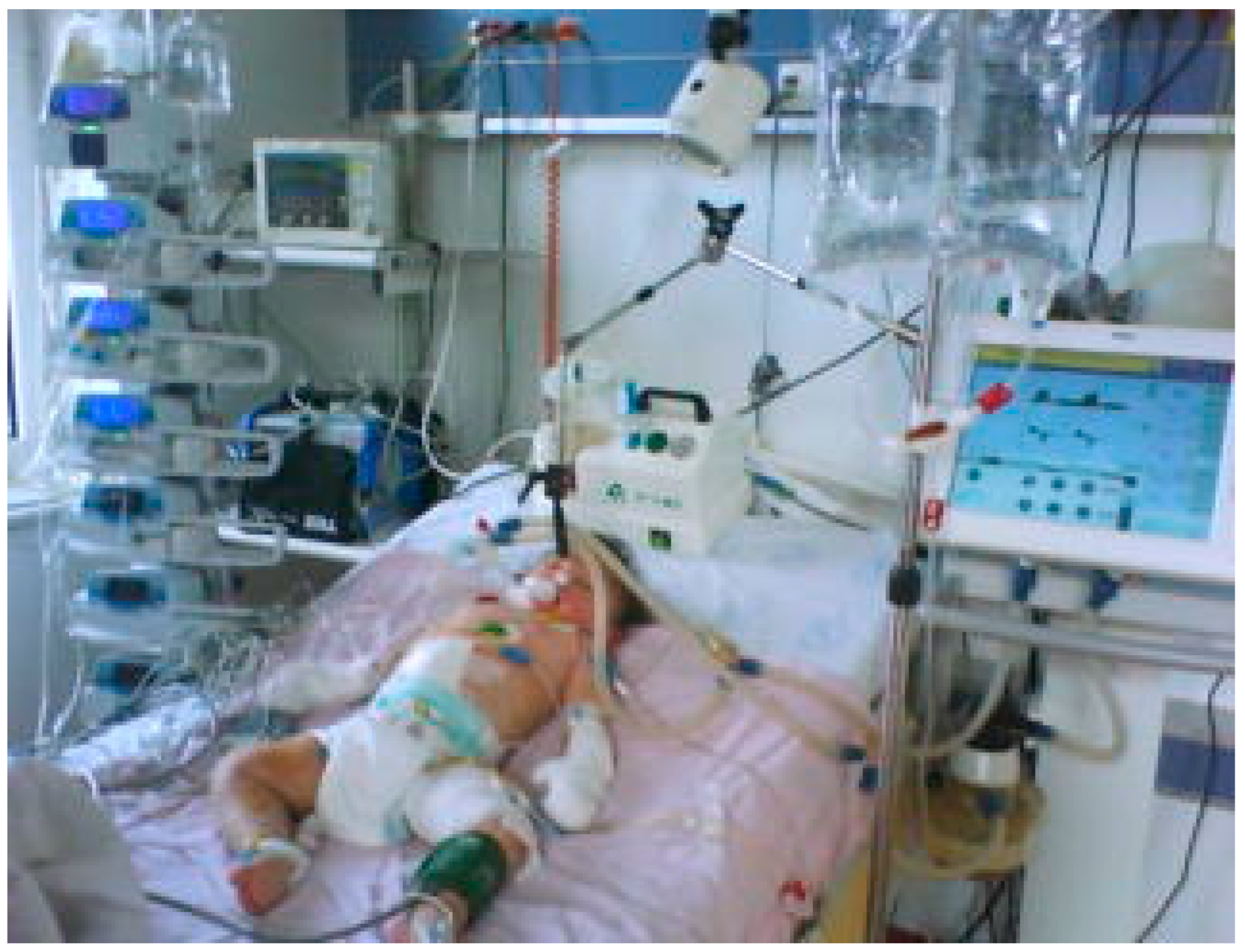
Because renal function did not resume after the specific hydro electrolytic and acid-base rebalancing treatment, it was decided to initiate peritoneal dialysis. (See
Figure 3). Heparin administration could not be started due to thrombocytopenia.
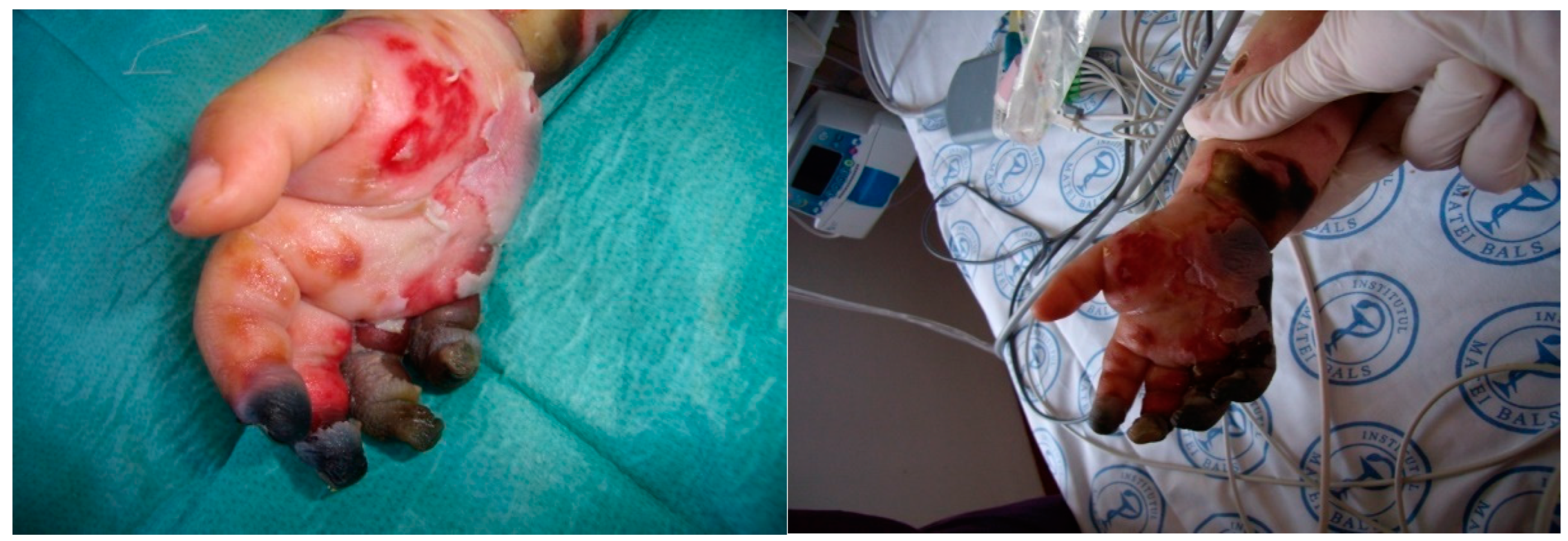
The plastic surgery consultant indicated the intervention to remove the necrotic and devitalized areas from the fingers, and the patient was transferred to the Plastic Surgery Clinic. (See
Figure 4). After plastic surgery, the child becomes anuric, without the resumption of diuresis, with the worsening of multiple organ failure and death by biological exhaustion one month after the onset of the disease. This clinical case highlights the severity of invasive pneumococcal disease complicated with multiple organ failure, which, although presented early at the hospital, the evolution was severe with renal failure initially remitted by peritoneal dialysis. The child's death was unfortunately found, despite complex and complete treatment due to complications of PF and biological exhaustion.
Discussion
Purpura fulminans, also known as purpura gangrenosa, was first described by Guelliot in 1884 and is a rapidly progressive syndrome of intravascular thrombosis and hemorrhagic infarction of the skin. It often occurs in infants and small children and is accompanied by vascular collapse, fever and DIC. [
11,
12,
13,
18,
19]. Our patient developed a dramatic complication of pneumococcal bacteremia, including septic shock, multiorgan failure, disseminated intravascular coagulation, and PF. [
18,
19,
20]. The prognosis for this age category is poor once soft tissue ischemia evolves to necrosis because it is associated with arterial and venous thrombosis leading to serious events and death. Aggressive resuscitation, antibiotics, and volume expansion are essential in acute infectious PF. [
8,
9,
15,
21].Correction of electrolyte and acid-base imbalances is beneficial. We compared our data with similar articles published on children with PF and the condition was complicated by necrosis of the skin. In our opinion, early removal of limb necrosis could probably attenuate multiple organ dysfunction and reduced mortality. In terms of etiology, Neisseria meningitidis is the most common agent in children and adolescents with PF. [
15,
22]. This is in accordance with other studies published by Sharip and colleagues, Jensen and colleagues, and Spanjaard and colleagues. [
23,
24,
25,
26,
27,
28]. We found S.pneumoniae associated with the course of sepsis and PF. Given that septic shock started abruptly and that blood purification therapy takes time to take effect, it is unclear if this would have prevented the onset of purpura fulminans and severe necrosis.[
29,
30,
31]. We believe that early endotoxin and inflammatory cytokine elimination could likely lower multiple organ dysfunction and the severity of organ failure. Her extremely complicated case of hemodynamic collapse with skin involvement raised questions about several differential diagnoses, including drug-related skin reactions like Steven-Johnson syndrome, toxic epidermal necrolysis, cryoglobulinaemia as well as autoimmune vasculitis.[
15,
19,
31,
32]. None of these was less likely if there had been recent drug use and if there were no symptoms such as mucosal involvement, skin peeling, or blistering. Autoimmune, immunological, and hepatitis labs were collected for further assessment of cryoglobulinaemia's and autoimmune vasculitis. Obtaining cultures and conducting a thorough infectious disease workup for septic shock was necessary. Her cultures and infectious disease workup resulted positive for Streptococcus pneumoniae, explaining the sepsis. Autoimmune and immunological tests were unspecific. The patient was fully vaccinated for her age. It is difficult to explain where treatment interventions altered the outcomes in this case report.
Conclusions
Pneumococcal sepsis in the presented case started as purpura fulminans-like, associated with AKI, a less common clinical form of the disease as the etiology is usually meningococcal. Peritoneal dialysis was beneficial, with the resumption of diuresis, but late surgical intervention to remove the necroses (sources of precipitates in the renal tubules) led to the onset of AKI and, subsequently, death by biological exhaustion.
This clinical case reveals the severity of systemic infection with Streptococcus pneumoniae serotype 1a, caused by a germ for which there is specific prophylaxis and vaccination. We emphasize once again the importance of anti-pneumococcal vaccination of the pediatric population and ask for rapid management of the case, which may be lifesaving, especially in children.
Author Contributions
Conceptualization, G.J., M.L., C.P. and M.M.M.; Methodology, G.J.; Software, G.J.,,M.L., and C.P.; Validation, G.J., M.L., C.P. and M.M.M.; Formal Analysis, G.J.,C.P.; Investigation, G.J. and C.P.; Resources, G.J. and M.L.; Data Curation, G.J. and M.M.M.; Writing—Original Draft Preparation, G.J.; Writing—Review & Editing, G.J., M.L., C.P. and M.M.M.; Visualization, G.J. and C.P.; Supervision, G.J.; Project Administration, G.J., M.L., C.P. and M.M.M.; funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the institutional ethical review committee, C0133/10.06.2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is unavailable.
Conflicts of Interest
The authors declare no conflict of interest.
Statement of Ethics
The study was conducted ethically in accordance with the Declaration of Helsinki. Written, informed consent was taken from the patient's parents for reporting this case.
References
- Irfan Kazi SG, Siddiqui E, Habib I, Tabassum S, Afzal B, Khan IQ. Neonatal Purpura Fulminans, a rare genetic disorder due to protein C deficiency: A case report. J Pak Med Assoc. 2018 Mar;68(3):463-465.
- Maat M, Buysse CM, Emonts M, Spanjaard L, Joosten KF, de Groot R, Hazelzet JA. Improved survival of children with sepsis and purpura: effects of age, gender, and era. Crit Care. 2007;11:R112. [CrossRef]
- Gurgey A, Aytac S, Kanra G, Secmeer G, Ceyhan M, Altay C. Outcome in children with purpura fulminans: report on 16 patients. Am J Hematol. 2005;80:20–25. [CrossRef]
- Wheeler JS, Anderson BJ, De Chalain TM. Surgical interventions in children with meningococcal purpura fulminans--a review of 117 procedures in 21 children. J Pediatr Surg. 2003;38:597–603. [CrossRef]
- Findley T, Patel M, Chapman J, Brown D, Duncan AF. Acquired Versus Congenital Neonatal Purpura Fulminans: A Case Report and Literature Review. J Pediatr Hematol Oncol. 2018 Nov;40(8):625-627. [CrossRef]
- Gheorghiță Jugulete, Mădălina Merișescu, Alexandra Eugenia Bastian, Monica Luminos - Clinical aspects and medico-legal implications of purpura fulminans in children, Rom J Leg Med [25] 364-368 [2017]. © 2017 Romanian Society of Legal Medicine, www.rjml.ro. [CrossRef]
- D. M. . Morens, J. K. Taubenberger, and A. S. Fauci, “Predominant Role of Bacterial Pneumonia as a Cause of Death in Pandemic Influenza: Implications for Pandemic Influenza Preparedness,” J. Infect. Dis., vol. 198, no. 7, pp. 962–970, Oct. 2008. [CrossRef]
- Dutta AK, Swaminathan, Abitbol V, Kolhapure S, Sathyanarayanan S. A Comprehensive Review of Meningococcal Disease Burden in India. Infect Dis Ther 2020;9(3):537–59.
- Daniela Pițigoi, Oana Săndulescu, Maria Dorina Crăciun, Gheorghiță Jugulete, Anca Streinu-Cercel, Angelica Vișan, Claudia Rîciu, Alexandru Rafila, Victoria Aramă, Monica Luminos & Adrian Streinu-Cercel - Measles in Romania – clinical and epidemiological characteristics of hospitalized measles cases during the first three years of the 2016-ongoing epidemic, Virulence 2020 Vol 11, No 1, 11:1, 686-694. [CrossRef]
- Mădălina Merișescu, Gheorghiță Jugulete, Alexandra Eugenia Bastian, Monica Luminos - Evaluation of sepsis mortality in children, Rom J Leg Med [26] 37-41 [2018]. © 2018 Romanian Society of Legal Medicine, www.rjml.ro. [CrossRef]
- Silvia Maria Stoicescu, Ramona Mohora, Monica Luminos, Madalina Maria Merisescu, Gheorghiță Jugulete, Leonard Nastase - PRESEPSIN - New Marker of SEPSIS Romanian Neonatal Intensive Care Unit Experience, REV CHIM-BUCHAREST, Vol. 70, Nr. 8, 2019, pag. 3008-3013, www.revistadechimie.
- Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 2007;369(9580):2196–210.
- WHO. Meningococcal meningitis. [Internet]. World Health Organization 2018. [cited 2019 30 Sep]. Available from: https://www.who.int/en/newsroom/fact-sheets/detail/meningococcal-meningitis.
- Victor Daniel Miron, Oana Sandulescu, Constanta Angelica Visan, Maria Madalina Merisescu, Anca Streinu Cercel, Daniela Pitigoi, Alexandru Rafila, Olga Mihaela Dorobat, Gheorghiță Jugulete, Adrian Streinu Cercel, Silvia Stoicescu, Monica Luminita Luminos - Pneumococcal Colonization and Pneumococcal Disease in Children with Influenza Clinical, Laboratory and Epidemiological features REV CHIM-BUCHAREST, Vol. 69, Nr. 10, October 2018, pag. 2749-53,www.revistadechimie.ro.
- Warner PM, Kagan RJ, Yakuboff KP, Kemalyan N, Palmieri TL, Greenhalgh DG, Sheridan RL, Mozingo DW, Heimbach DM, Gibran NS, Engrav L, Saffle JR, Edelman LS, Warden GD. Current management of purpura fulminans: a multicenter study. J Burn Care Rehabil. 2003 May-Jun;24(3):119-26. [CrossRef] [PubMed]
- Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J 2004;23(12 Suppl):S274–9. 6.Tekin RT, Dinleyici EC, Ceyhan M, Karbuz A, Salman N, Sutçu M, et al. The prevalence, serogroup distribution and risk factors of meningococcal carriage in adolescents and young adults in Turkey. Hum Vaccin Immunother 2017;13(5):1182–9.
- Factsheet about meningococcal disease 2019. [Internet]. European Centre for Disease Prevention and Control. [cited 2019 July 11]. Available from: https://ecdc.europa. eu/en/meningococcal-disease/factsheet.
- Wang B, Santoreneos R, Giles L, Afzali HH, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: A systematic review and meta-analysis. Vaccine 2019;37(21):2768–82. [CrossRef]
- Sinclair D, Preziosi MP, Jacob John T, Greenwood B. The epidemiology of meningococcal disease in India. Trop Med Int Health 2010;15(12):1421–35. [CrossRef]
- Aye AMM, Bai X, Borrow R, Bory S, Carlos J, Caugant DA, et al. Meningococcal disease surveillance in the Asia-Pacific region (2020): The global meningococcal initiative. J Infect 2020;81(5):698–711. [CrossRef]
- Azzari C, Nieddu F, Moriondo M, Indolfi G, Canessa C, Ricci S, et al. Underestimation of Invasive Meningococcal Disease in Italy. Emerg Infect Dis 2016;22(3):469–75. [CrossRef]
- Jugulete, G.; Merisescu, M.M.; Bastian, A.; Zurac, S.A.; Pavelescu, C.; Luminos, M.L. Zoonotic Infection with Rabies Virus Leading to Fatal Encephalitis in Pediatric Patient – Case Report. Preprints.org 2022, 2022110294. [CrossRef]
- Sharip A, Sorvillo F, Redelings MD, Mascola L, Wise M, Nguyen DM: Population-based analysis of meningococcal disease mortality in the United States: 1990–2002. Pediatr Infect Dis J 2006, 25: 191-194. [CrossRef]
- Ouldali N, Levy C, Varon E, Bonacorsi S, Béchet S, Cohen R, et al. Incidence of paediatric pneumococcal meningitis and emergence of new serotypes: a time-series analysis of a 16-year French national survey. Lancet Infect Dis. 2018;18:983–91. [CrossRef]
- Jensen ES, Schonheyder HC, Lind I, Berthelsen L, Norgard B, Sorensen HT: Neisseria meningitidis phenotypic markers and septicaemia, disease progress and case-fatality rate of meningococcal disease: a 20-year population-based historical follow-up study in a Danish county. J Med Microbiol 2003,52(Pt 2):173-179. [CrossRef]
- Green MS, Schwartz N, Peer V. A meta-analytic evaluation of sex differences in meningococcal disease incidence rates in 10 countries. Epidemiol Infect 2020;148:e246. 14.Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine 2012;30(Suppl 2):B3–9. [CrossRef]
- Stein-Zamir C, Shoob H, Sokolov I, Kunbar A, Abramson N, Zimmerman D. The clinical features and long-term sequelae of invasive meningococcal disease in children. Pediatr Infect Dise J 2014;33(7):777–9. [CrossRef]
- Bloch D, Murray K, Peterson E, Ngai S, Rubinstein I, Halse TA, et al. Sex difference in meningococcal disease mortality, New York City, 2008–2016. Clin Infect Dis 2018;67(5):760–9. [CrossRef]
- Andreasen TJ, Green SD, Childers BJ. Massive infectious soft-tissue injury: diagnosis and management of necrotizing fasciitis and purpura fulminans. Plast Reconstr Surg. 2001;107:1025–1034. [CrossRef]
- Chalmers E, Cooper P, Forman K, Grimley C, Khair K, Minford A, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066–1071. [CrossRef]
- Plüß M, Zeisberg M, Müller GA, Vasko R, Korsten P. Therapeutic response to glucocorticoids, anticoagulation and plasma exchange in a patient with primary antiphospholipid syndrome presenting with purpura fulminans. Lupus. 2018;27:2170–2173. [CrossRef]
- Cooper JS, Allinson P, Keim L, Sisson J, Schuller D, Sippel J, et al. Hyperbaric oxygen: a useful adjunct for purpura fulminans: case report and review of the literature. Undersea Hyperb Med. 2014;41:51–57.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).