1. Introduction
The clinical definition of infertility is a disease of male or female reproductive system defined by the failure to achieve a pregnancy after 1 year of regular unprotected sexual intercourse. Infertility affects approximately 10-15% of reproductive age couples worldwide [
1]. The primary causes of female infertility are typically related to ovulation disorders, tubal problems, and endometriosis. There are, however, other factors (such as metabolic disorder related factors and insulin resistance) that can be responsible for “idiopathic infertility” when the cause of infertility is unknown. By understanding the metabolic risk factors, we can gain more information on idiopathic infertility in women reducing the proportion of unexplained infertility [
2].
As a result of unfavorable lifestyle and dietary changes in modern civilizations, there is an increase in the global prevalence of metabolic syndrome [
3]. Metabolic syndrome (MetS) is a term used to describe the simultaneous presence of several cardiovascular risk factors such as insulin resistance, obesity, dyslipidemia, and hypertension [
4]. The most widely accepted hypothesis for the pathophysiology of the metabolic syndrome is centered around insulin resistance which is believed to be partially triggered by excessive fatty acid levels resulting from inappropriate lipolysis [
5]. Insulin resistance (IR) is a metabolic disorder with impaired insulin signaling and reduced glucose uptake by the target tissues. It is characterized by obesity, type 2 diabetes and hyperinsulinemia that is a compensatory response to the target tissue insulin resistance [
6]. There is strong evidence that obesity-associated hyperinsulinemia and insulin resistance have a negative effect on fertility. For instance, the decreased weight in obese women experiencing infertility is linked to an improved frequency of ovulation and increased chances of achieving pregnancy. Even among ovulatory women, the higher body mass index (BMI) is associated with reduced rates of spontaneous pregnancy. The underlying mechanism is believed to involve the adverse impact of elevated insulin levels on ovarian function [
7]. The prevalence of obesity is increasing because of the combination of reduced exercise, dietary changes, and high calorie intake [
8]. Obesity influences all regulatory systems in the human body and can cause reproductive system related problems and infertility [
9]. The increasing BMI and obesity can be associated with higher risk to develop reproductive problems such as menstrual irregularities, anovulation, subfertility, miscarriage, and negative pregnancy outcomes [
10]. Numerous studies indicated that obese women undergoing in vitro fertilization (IVF) experience a decreased ovarian response to controlled ovarian stimulation [
11]. Other studies reported significant impairments in the quality of the oocytes and embryos including lower number of oocytes retrieved [
12], lower number of mature oocytes [
13], poorer oocyte quality with lower fertilization rates [
14] and decreased embryo quality [
15]. In addition, there is a causal association between maternal obesity and pregnancy complications, with the risk of pregnancy complications increasing with obesity [
7]. Maternal complications during the second and third trimester of pregnancy are often attributed to the metabolic syndrome of obesity.
Recently, the role of oxidative stress in IR was also recognized as a key factor. Oxidative stress is characterized by an excessive presence of endogenous oxidative species that can damage cells and disrupt signal pathways [
16]. Produced primarily in the mitochondria and peroxisomes, reactive oxygen species (ROS) such as superoxide, hydrogen peroxide and hydroxyl radical ions are the main molecules of oxidative stress. Emerging evidence from recent studies concluded that ROS-induced damage directly contributes to the development and progression of various chronic diseases such as IR and type 2 diabetes [
17]. It was also reported that both low and high levels of ROS have negative effects on the fertility, embryo quality and outcome of the pregnancy. These findings are consistent with the concept of a “quiet metabolism”, suggesting that there are specific upper and lower thresholds of metabolic activity within which the embryo remains under optimal conditions [
18].
Metabolomics, a relatively new subfield within the broader field of “omics” focuses on the analysis of low molecular weight metabolites, their presence and concentration in different biological fluids. In recent years it has been utilized to explore the underlying biological pathways in different diseases and to identify metabolites that can be used as biomarkers for certain diseases. Metabolomic studies have employed various matrices, including blood, urine, saliva and more specific biofluids such as follicular fluid [
19]. The follicular fluid (FF) is formed by the passage of blood plasma constituents across the blood-follicular barrier. This mechanism is influenced by the secretory actions of granulosa and theca cells [
20]. FF serves as a critical microenvironment for the development and maturation of oocytes. It contains essential metabolites such as growth factors, cytokines, energy substrates, amino acids, steroids, and various lipids including cholesterol. These metabolites accumulate within the oocytes and play a vital role in their growth and development [
21]. The composition of FF has been found to have an important effect on the oocyte quality and embryo development. The amino acid composition of the FF might be related to the developmental competence of the oocytes [
22]. Metabolic alterations observed in the follicular fluid can arise from changes in the plasma metabolites or be influenced by the selective filtering of the granulosa cells. The FF comprises crucial metabolites necessary for oocyte growth and development, serving as an indicator of oocyte quality and embryo viability [
20]. Recent studies have reported that IR is strongly related to the amino acid metabolism, and it seems that plasma amino acid levels may vary during IR. Due to obesity and IR there are alterations in the levels of amino acids in the plasma in the early stage of the lifestyle-related diseases but fortunately these alterations can be reversed by interventions that improve insulin sensitivity [
3].
Since metabolic disorders, especially IR, have great effects on the health of oocytes and fertility, we planned to investigate how the metabolic alterations change the amino acid composition of the follicular fluid. We also surveyed the literature to support our results related to metabolic changes on the quality of oocytes and on fertility.
2. Results
2.1. Demographic and Clinical Data of Patients
In
Table 1. the demographic and clinical features of the patients enrolled to our study are shown.
2.2. Amino Acid (AA) Analysis of the Follicular Fluid Samples
A total of 20 amino acids that are the building blocks of the proteins were measured in all FF samples. The concentration values are shown in
Supplementary Table S1 (
Table S1). Glutamine, alanine, and glycine appeared to be the most abundant AAs in the FF which finding is supported by previous studies [
23] showing the physiological roles of these AAs in oocyte development [
24].
2.2.1. Comparison of Amino Acid Content According to Insulin Resistance and Non-insulin Resistance Groups
The quantitative AA results after statistical comparison were expressed as p values and are shown in
Table 2. The patients were separated into two groups based on their insulin resistance. One group (IR, n=11) was defined as patients with insulin resistance and the members of the other group (NIR, n=36) were in this regard apparently healthy. Concentrations of three amino acids were found to be significantly altered in the IR group. These were glycine (p<0.001), cysteine (p=0.037) and aspartate. Aspartate level was significantly (p=0.02) higher in the IR group.
2.2.2. Comparison of Amino Acid Contents Based on Body Mass Index (BMI)
The patients were divided into two groups according to their BMI. BMI is calculated as BMI=kg/m2, where kg is the person’s weight (in kg) and m2 is the height of the person in meters squared. Between 8.5 and 24.9 BMI the patients were considered to belong to the normal group (n=21) while in the overweight group the BMI score was above 25 (n=22). All the 11 IR patients were in the overweight group and comparison of the IR/NIR groups showed that the patients in the IR group had significantly higher BMI (p<0.001). The concentration of three amino acids were identified as statistically significant namely aspartate (p=0.02), glutamate (p=0.008) and glycine (p=0.009). Glycine was present in lower concentration in the overweight group and the concentration of aspartate and glutamate was higher.
2.2.3. Comparison of Amino Acid Contents Based on the Age of the Patients
In this comparison, patients were separated into two groups by their age. One group (younger, n=20) was determined as patients aged 34 and below and the other group was the older group (n=27) where the age of the patients was 35 or above. There was no significant alteration in this comparison.
2.3. Multivariate PCA and PLS-DA Analysis
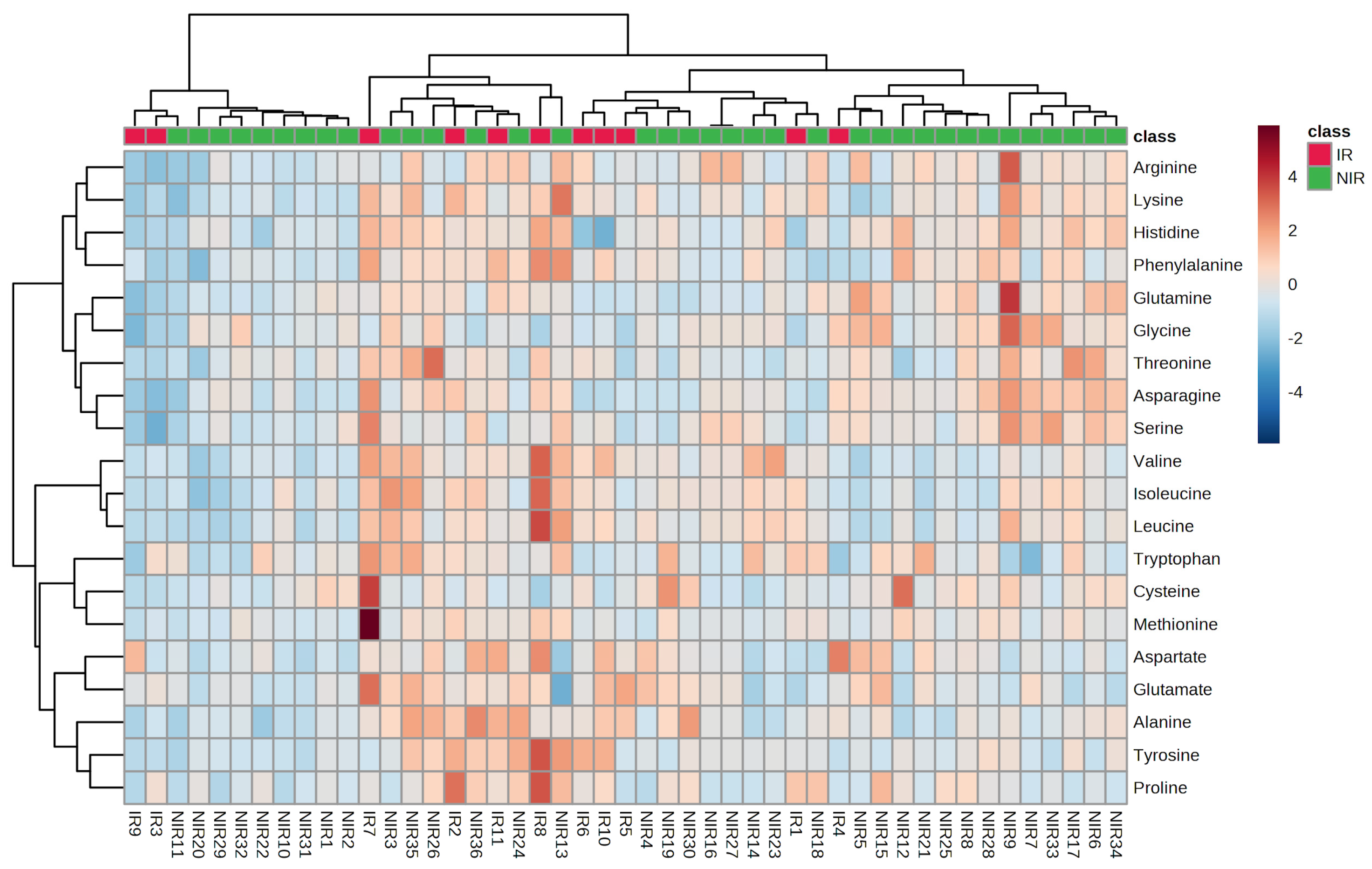
The heatmap (
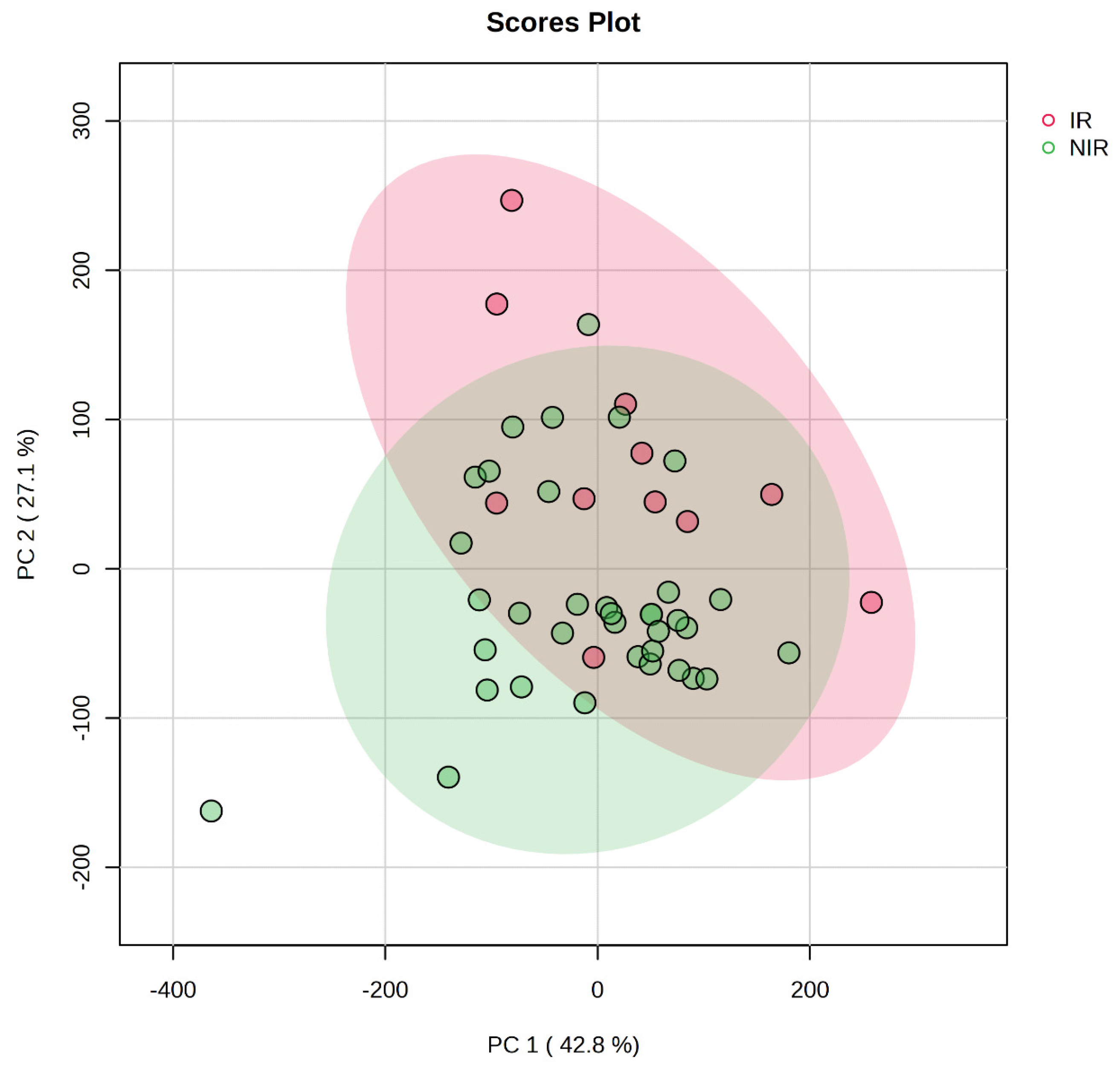
Figure 1) in PCA analysis is a visual representation of the relationship between variables and PCs in a multivariate dataset. In this heatmap, each row represents a variable, and each column represents a PC. The cells of the heatmap display the strength of the relationship between the variable and a PC. The indication of the relationship is based on color gradient or intensity. Strong positive association between the variable and PC is indicated by darker or intense red or blue colors and weak or negative associations are represented by lighter or less intense colors. Heatmaps can reveal groups of variables that exhibit similar relationships with PCs. Variables that are close to each other in the heatmap and share similar color patterns are likely to have similar effects on the PCs. Principal component analysis scores were evaluated using PLS-DA analysis for IR and NIR patients. The scores plots are illustrated in
Figure 2.
The score plot provides valuable insights into the grouping, the patterns, and relationships among samples in a multivariate data analysis. Each follicular fluid sample is represented as a data point and the position of the data point in the plot corresponds to its scores on the principal components. An overlap can be observed between the IR and NIR groups in the score plot. It suggests that there is a similarity between the samples from these groups in terms of the measured variables. The overlap indicates that samples in the IR group may share characteristics that are also present in the NIR group.
2.4. Potentially Important Metabolites – Biomarker Analysis
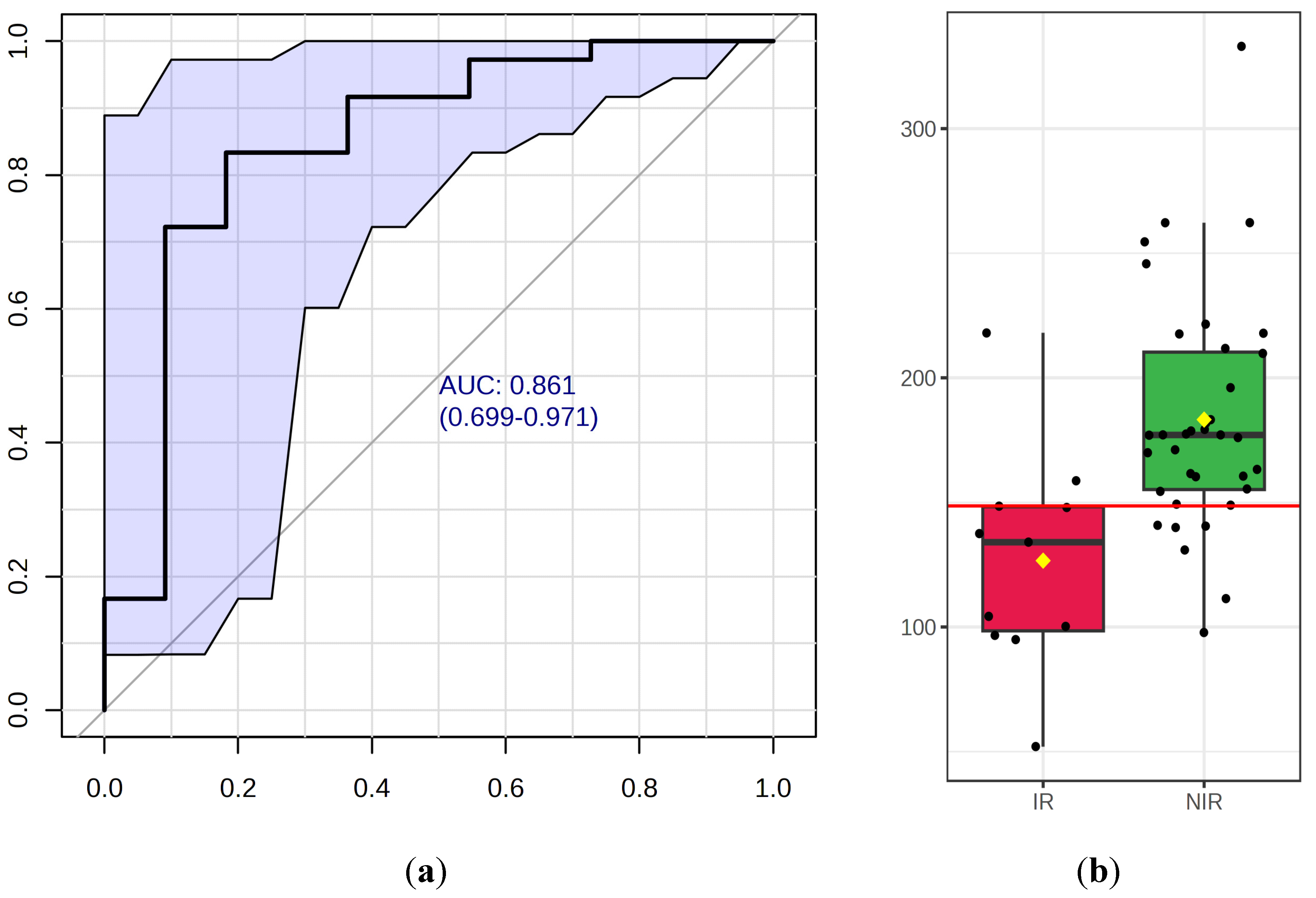
Biomarker analysis aims to identify a metabolite or a set of metabolites capable of classifying conditions or disease with high sensitivity (true-positive) and specificity (true negative). The amino acids with significant between-group differences were further tested using ROC curve analyses. Of the 20 important amino acids three (glycine, aspartate, and cysteine) were found to have an AUC above 0.7 (
Table 3). Glycine and aspartate had p values below 0.05 indicating significant differences between the IR and NIR groups.
When analyzing glycine concentrations in the IR and NIR groups we performed a receiver operating curve (ROC) analysis to see the predictive value of glycine and to determine a diagnostic cutoff value of it. The results are shown in
Figure 3a,b.
2.5. Metabolic Pathway Analysis
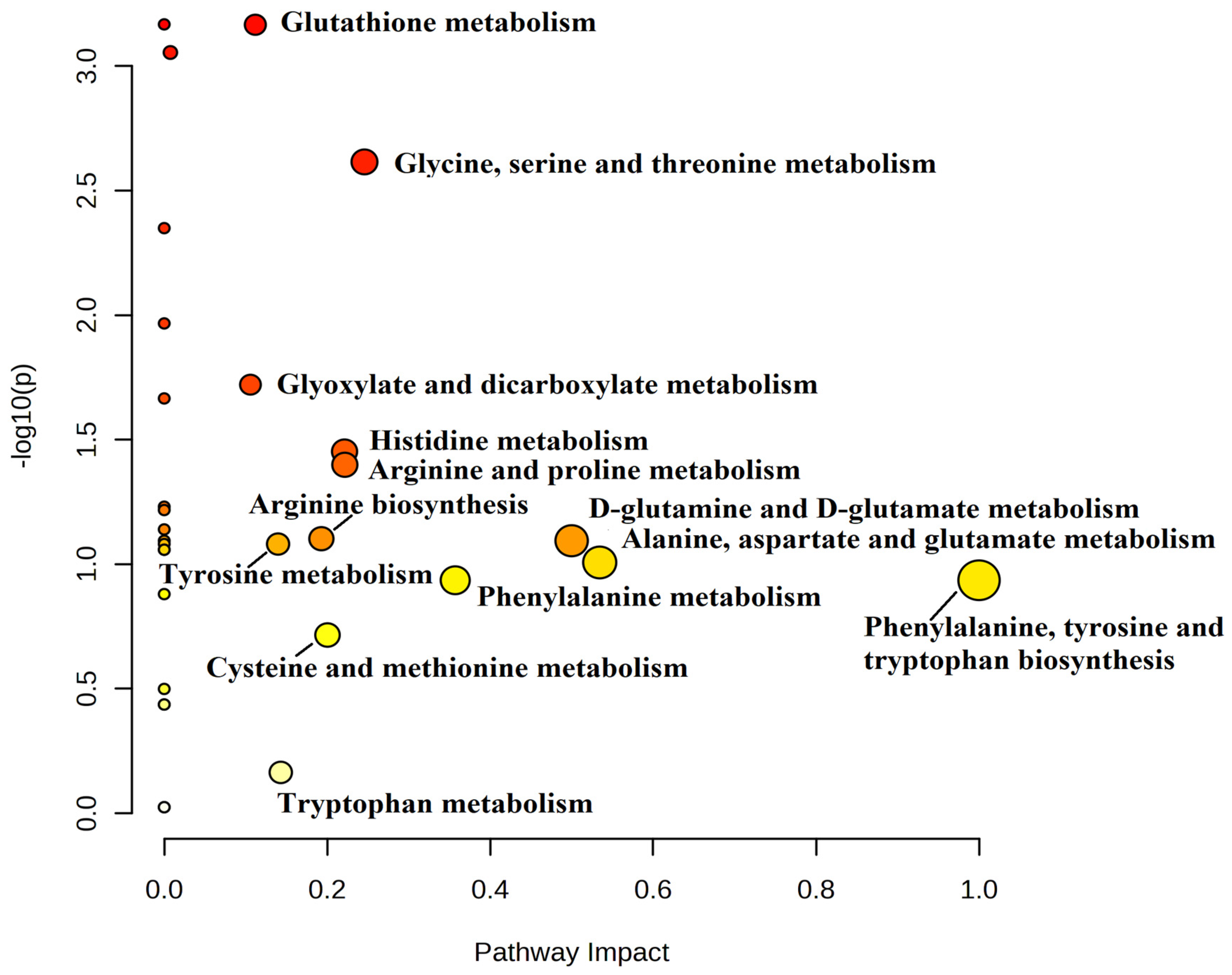
We analyzed certain metabolic pathways between the IR and NIR groups. Using topological analysis, the cutoff value of the metabolic pathway involvement was set to 0.1 and the pathways with the value above 0.01 were selected as potential key metabolic pathways (
Figure 4). A total of 13 metabolic pathways were above this value (
Table 4). Based on the significance level (p<0.05) glutathione metabolism (p<0.001) had the lowest value but four other pathways were identified as target pathways namely the glycine, serine and threonine metabolism, the arginine and proline metabolism, the histidine metabolism and the glyoxylate and dicarboxylate metabolism.
3. Discussion
In this study we quantified the 20 main amino acids in the follicular fluid of IVF patients and compared the amino acid profile of patients between the IR and NIR groups. Based on the comparison of FF amino acid concentration of patients in the IR and NIR groups several amino acids were found to be significantly altered. Two amino acids (aspartate and glycine) showed differences both in the IR/NIR and normal/overweight groups. In the follicular fluid samples the concentration of
aspartate was higher in the IR group correlating well with the previously reported data that in the plasma of MetS patients the level of aspartate is significantly higher [
3]. Recently, it was also found that in obese children the plasma concentration of aspartate was higher due to the impaired glucose tolerance and the aggravated metabolite metabolism. [
25]. Another study concluded that the elevated levels of aspartate in the plasma can be a strong predictor for prediabetes [
26]. Some evidence suggests that aspartate plays a vital role in the energy metabolism of the oocytes by being converted into oxaloacetate that is a key intermediate in the tricarboxylic acid cycle. This metabolic pathway is involved in generating energy for cellular processes [
27]. It was observed that there is a significant increase in aspartate levels within the cumulus cells throughout the maturation progress and it is utilized directly for energy production within the cumulus cells or is transferred to the oocytes for energy production [
28].
The other amino acid that was significantly altered between the IR/NIR and normal/overweight groups was
glycine. The follicular fluid samples of IR patients had lower levels of glycine and in the overweight group similar results were found. Additionally, this amino acid was found to be the best metabolite candidate for classification of IR from follicular fluid samples. Glycine is a non-essential amino acid that plays a crucial role in various biological processes such as neurotransmitter, controlling epigenetics, reproduction, fertility, and metabolic regulation. Glycine is also precursor for many important metabolites such as glutathione, porphyrins, purines, hem, and creatine [
29]. Glycine is an important amino acid for fully grown oocytes as well and it is transported into the cell by glycine transporter (GLYT1). Glycine and cysteine transport increases at the time of oocyte maturation which may result the need for glutathione [
30]. Previous studies have concluded that the plasma glycine level is significantly lower in patients with obesity and IR compared to healthy individuals [
31]. Our results, based on the measurement of amino acid concentrations in the follicular fluid, showed similar characteristics. In other studies, observations suggested that in long term, mild glycine deficiency may facilitate the development of metabolic disorders [
32]. Studies based on glycine supplementation reported that by adding glycine to the diet increased the insulin response and glucose tolerance and with proper dose it was very successful in decreasing other metabolic disorders, many inflammatory diseases, a few types of cancers and obesity [
29]. Experiments on animals found that glycine supplementation can also improve embryo quality and implantation rates suggesting its potential role in enhancing fertility [
33]. The levels of glycine in the FF have been shown to be a good indicator of post-fertilization development [
34]. Glycine has an important role also in pregnancy and it should be taken into consideration that the de novo synthesis is inadequate to supply the metabolic demand in late pregnancy [
35].
Cysteine was also found to have significantly lower concentration in the IR group compared to the NIR group. Cysteine can be obtained from the diet but is also synthetized in the body. It is an important source of sulfur in the human metabolism and although it is a non-essential AA elderly, children, and individuals with certain types of metabolic diseases need to obtain it from the diet. Cysteine itself is a major extracellular antioxidant and together with glycine and glutamate they form the glutathione molecule which is a vital antioxidant [
36]. The availability of cysteine is recognized as a rate-limiting factor in the synthesis of glutathione and this relationship has been extensively documented in clinical and animal studies. Cysteine, alone or together with glutamate and glycine raises glutathione levels in the oocytes and cumulus cells promoting maturation [
37]. Cysteine supplementation has been shown to enhance the synthesis and levels of glutathione thus lowering the oxidative stress and the insulin resistance [
38]. The analysis of IR patients’ plasma revealed that cysteine was found to be in lower concentration [
38], and we experienced the same in the follicular fluid.
Glutamate was the only amino acid that was significantly altered in the overweight group but not in the IR group. The concentration of this amino acid was higher in the overweight group than in the normal group. Glutamate is the most abundant amino acid, and it has a fundamental role in the metabolism of amino acids, and it is also a key molecule of the synthesis of glutathione [
39]. It was already reported that the plasma levels of glutamate were higher in obese patients and the elevated levels of this amino acid were associated with increased risk of cardiovascular diseases, dyslipidemia, and IR. In the follicular fluid samples, we experienced that the levels of glutamate were higher in the IR group but there was no significant difference between the IR/NIR groups. Another study concluded that the elevated glutamate level in the plasma was associated with higher liver fat content and lower insulin sensitivity and the higher level of glutamate is related to metabolic dysfunctions [
40]. Our findings in the follicular fluid were similar to those in the literature and overweight patients had significantly higher concentration of glutamate in their follicular fluid samples. High glutamate levels may be the results of altered metabolic function due to obesity.
The pathway analysis revealed that the most significant alterations between the IR and NIR groups are the glutathione metabolic pathway and the glycine, serine, and threonine metabolism pathway. Glutathione (GSH) is a vital antioxidant that is produced from three amino acids: cysteine (Cys), glycine (Gly), and glutamate (Glu). It was already claimed that the stability of reproductive cells and tissues relies on maintaining a balance between the production of free radicals and the presence of scavenging antioxidants [
41]. GSH plays a crucial role in the maturation of oocytes, fertilization, and the early development of embryos [
42]. It was previously reported that GSH protects eggs from damage caused by oxidative stress and therefore oocytes with higher levels of GSH produce healthier embryos [
43]. GSH deficiency was reported to be related to premature ovarian aging [
44]. In another study it was revealed that GSH has antiaging antioxidant property, therefore has impact on the egg health [
44]. In our experiments in the IR group significantly lower levels of glycine and cysteine were reported and this can give rise to lower glutathione levels as well. It was concluded earlier that in metabolic conditions associated with enhanced oxidative stress (such as IR) the availability of glycine can be too low to sustain the optimal rate of GSH synthesis. [
32] and the glycine related metabolic pathways can also be affected as we illustrated in
Figure 4. In this study we found several amino acids that are altered in patients with IR and obesity. These alterations may be the results of the metabolic changes due to the aforementioned disorders.
4. Materials and Methods
4.1. Patient Enrollment
The study was conducted between May 2021 and February 2023 at the Department of Obstetrics and Gynecology (FF sampling), and at the National Laboratory on Human Reproduction (analytical studies), University of Pécs, Hungary. Detailed information was given to all patients or their next-of-kin regarding our study protocol while written consent was obtained from all. Exclusion criteria were patients under 18 years of age, unobtainable or withdrawn consent and autoimmune diseases or overt diabetes. The study protocol was approved by the Regional Research Ethics Committee of the University of Pécs (no. 5273-2/2012/EHR) conforming to the 7th revision of the Helsinki Declarations (2013). All the 47 patients enrolled in this study received assisted reproductive treatment (ART). The members of the IR group (n=11) were diagnosed by endocrinologists based on the homeostatic model assessment of insulin resistance (HOMA IR) formula.
4.2. Collection of Follicular Fluid
The follicular fluid collection was performed during oocyte retrieval procedure with transvaginal ultrasound-guided puncture. The samples were centrifuged immediately at 6700 g for 10 minutes at room temperature to remove the erythrocytes and white blood cells. The supernatant was collected and stored at -80°C until further analyses.
4.3. Sample Processing and Measurement
4.3.1. Reagents
For the amino acid analyses the following chemicals were used: 3-mercaptopropionic acid ≥99.0% (HPLC grade), orto-phtalaldehyde ≥99% (HPLC grade), 9-fluorenylmethyloxycarbonyl chloride (FMOC chloride) ≥99.0% (HPLC grade both from Merck KGaA, Darmstadt, Germany), acetonitrile, ≥ 99.9 % HPLC gradient grade, methanol, ≥ 99.8 % HPLC grade and water HPLC gradient grade, both from Fisher Chemical Pittsburgh, Pennsylvania, United States, 20 mM phosphate buffer of pH 6.2. For the UHPLC elution the mobile phase was acetonitrile/methanol/water solution: 400 ml acetonitrile, 450 ml methanol, 150 ml water. L-Norvaline (Merck, KgaA, Darmstadt, Germany) was used as an internal standard.
4.3.2. Sample Preparation for the UHPLC Measurement
The quantitative amino acid analyses of the FF samples were performed after precipitation of the proteins, fluorescence derivatization of the amino acids and UHPLC chromatography (Shimadzu Nexera X2 UHPLC System) using a fluorescence detector (RF-20A XS, both from Shimadzu Europa GmbH Duisburg, Germany) and an internal standard (250 µmol/L L-Norvaline). 300 µL ice-cold acetonitrile solution was added to each 200 µL of follicular fluid sample. The samples were vortexed and centrifuged for 4 minutes at 6100 g (ScanSpeed Mini, Labogene, Allerod, Denmark). After centrifugation 600 µL of phosphate buffer was added to 300 µL supernatant and the samples were filtered (Millex® GV 4mm Durapore PVDF 0.22 µm, Merck KGaA, Darmstadt, Germany) and inserted into the autosampler module of the device (SIL-30AC Autosampler) in which the temperature was set to 20˙C.
4.3.3. Derivatization
For the derivatization of the amino acids 3-mercaptoproprionic acid (MPA) and orto-phtalaldehyde (OPA) were used. In case of proline, 9-fluorenylmethyloxycarbonyl chloride (FMOC) was applied. As an internal standard 250 µmol/L L-Norvaline was utilized. 7.5 µL of sample, 45 µL of MPA, 22 µL of OPA and 3 µL of L-Norvaline was mixed and incubated for 1 minute. After the incubation 10 µL of FMOC reagent was added to the mixture and incubated for 2 minutes. At the end 5 µL of derivatized sample was injected into the loop of the injector.
4.3.4. Parameters of the UHPLC Method
Aliquots of 5 μL of the samples were injected into the UHPLC system. A reverse-phase 100 x 3.0 mm Kinetex 2.6 μm EVO C18 100Å (Phenomenex, Torrance, CA, USA) column was used for the separation. The gradient mobile phase is composed of 20 mmol/L phosphate buffer (A) and 40:45:15 acetonitrile: methanol: water solution (B). The flow rate was 1.3 mL/min and the column temperature was set to 27°C. The total running time was 15.1 min. The amino acids (except proline) were detected at 450 nm in RF-20A xs module. In case of proline the detection was achieved at 305 nm. The evaluation was performed by Shimadzu LabSolutions 5.97 SP1 software. Each amino acid was identified by the retention time (RT). The concentration of each amino acid was calculated based on the area under curve of the internal standard. The samples were measured in duplicates and the final concentration was calculated from their average.
4.4. Data Analysis
The statistical analysis was performed using SPSS for Windows (version 28.0.0.0, IBM SPSS Statistics, USA). The distribution of data was first checked for the normal distribution but since most of the amino acids did not follow that pattern, non-parametric statistical tests were chosen. To determine the differences between the groups, Mann-Whitney U-test was performed. Values of p< 0.05 were considered statistically significant.
To evaluate the amino acid concentration results MetaboAnalyst (version 5.0, RRID:SCR_015539, Alberta, Canada) web-based tool was utilized. With this tool multivariate PCA analysis, biomarker analysis, and metabolic pathway analysis were performed. Based on the potential amino acid candidates in the insulin resistance (IR) and non-insulin resistance (NIR) groups a heatmap was created for unsupervised clustering. To explore the underlying structure and patterns in the dataset, Principal Component Analysis (PCA) was performed. PCA transformed the original variables into a set of uncorrelated components (principal components -PCs) that captured the maximum variance in the data. Following PCA, PLS-DA was employed to examine the discrimination between the IR and NIR groups. PLS-DA combines the concept of PCA and linear regression to model the relationship between the independent (features) and dependent (class membership) variables. For the biomarker analysis classical univariate ROC curve analysis was performed to identify potential biomarkers and evaluate their performance. ROC curve was generated, and the AUC was calculated to compute the optimal cutoffs of the different amino acids. AUC value over 0.7 was established as discriminative power. For the metabolic pathway analysis, the list of the amino acids measured was added to Metaboanalyst 5.0. The metabolomic pathway related to the amino acids was found by analyzing the topological characteristics of the pathway. The metabolic pathways related to the IR were obtained and mapped and the diagram of each metabolic pathway was obtained. The impact threshold was set to 0.10. Any pathway beyond this value was classified as a potential target pathway.
5. Conclusions
Follicular fluid is a complex biological fluid that surrounds the oocytes and is derived mainly from plasma. It is a very important microenvironment for the development of the oocytes and the quality of these oocytes are linked to amino acid metabolism. Any alteration in the metabolic processes due to IR will alter the amino acid composition of the follicular fluid as well and the oocytes may undergo metabolism-induced changes and hence it can result in impaired oocyte quality and decreased fertility. IR can cause many negative effects in patients so it would be useful to improve the state of this disease. The combined dietary changes increased physical exercises and glycine/cysteine supplementation may lead to positive metabolic changes in the serum and alterations in the follicular fluid as well that can have positive impact on the quality of the oocytes and on fertility.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, T.K., C.K. and G.L.K.; methodology, T.K., C.K., V.L., D.H., P.M., K.G.; software, T.K., C.K. and R.H.; validation, T.K., C.K. and G.L.K.; formal analysis, T.K., C.K. and R.H.; investigation, C.K.; resources, T.K., Á.V., G.L.K. A.L. and Á.L.; data curation, T.K. and C.K.; writing—original draft preparation, T.K. and C.K.; writing—review and editing, T.K., C.K. and G.L.K.; visualization, C.K.; supervision, T.K. and G.L.K.; project administration, C.K.; funding acquisition, T.K. and G.L.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financed by the RRF-2.3.1-21-2022-00012 “National Laboratory on Human Reproduction” program and by the EFOP 3.6.1-16-2016-00004 project (Comprehensive Development for Implementing Smart Specialization Strategies), University of Pécs, Hungary.
Institutional Review Board Statement
5273-2/2012/EHR.
Informed Consent Statement
Detailed information was given to all patients or their next-of-kin regarding our study protocol while written consent was obtained from all.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- G. Simionescu et al., “The complex relationship between infertility and psychological distress (Review),” Exp Ther Med, vol. 21, no. 4, Feb. 2021. https://doi.org/10.3892/etm.2021.9737. [CrossRef]
- R. Westerman and A. K. Kuhnt, “Metabolic risk factors and fertility disorders: A narrative review of the female perspective,” Reproductive Biomedicine and Society Online, vol. 14. Elsevier Ltd, pp. 66–74, Mar. 01, 2022. [CrossRef]
- S. Sun et al., “Metabolic Syndrome and Its Components Are Associated With Altered Amino Acid Profile in Chinese Han Population,” Front Endocrinol (Lausanne), vol. 12, Jan. 2022 . [CrossRef]
- P. L. Huang, “A comprehensive definition for metabolic syndrome,” DMM Disease Models and Mechanisms, vol. 2, no. 5–6. pp. 231–237, May 2009 . [CrossRef]
- E. McCracken, M. Monaghan, and S. Sreenivasan, “Pathophysiology of the metabolic syndrome,” Clin Dermatol, vol. 36, no. 1, pp. 14–20, Jan. 2018 . [CrossRef]
- T. Sakumoto et al., “Insulin resistance/hyperinsulinemia and reproductive disorders in infertile women,” Reproductive Medicine and Biology, vol. 9, no. 4. John Wiley and Sons Ltd, pp. 185–190, 2010 . [CrossRef]
- J. H. Sliwowska, C. Fergani, M. Gawałek, B. Skowronska, P. Fichna, and M. N. Lehman, “Insulin: Its role in the central control of reproduction,” Physiology and Behavior, vol. 133. Elsevier Inc., pp. 197–206, Jun. 22, 2014 . [CrossRef]
- R. J. Norman, M. Noakes, R. Wu, M. J. Davies, L. Moran, and J. X. Wang, “Improving reproductive performance in overweight/obese women with effective weight management,” Human Reproduction Update, vol. 10, no. 3. pp. 267–280, May 2004 . [CrossRef]
- Z. Ö. Dağ and B. Dilbaz, “Impact of obesity on infertility in women,” Journal of the Turkish German Gynecology Association, vol. 16, no. 2. AVES Ibrahim Kara, pp. 111–117, 2015 . [CrossRef]
- E. S. Jungheim, J. L. Travieso, K. R. Carson, and K. H. Moley, “Obesity and Reproductive Function,” Obstetrics and Gynecology Clinics of North America, vol. 39, no. 4. pp. 479–493, Dec. 2012 . [CrossRef]
- A. Maheshwari, L. Stofberg, and S. Bhattacharya, “Effect of overweight and obesity on assisted reproductive technology - A systematic review,” Human Reproduction Update, vol. 13, no. 5. pp. 433–444, Sep. 2007 . [CrossRef]
- P. É. Ter, F. F. Fedorcsa´k, R. Storeng, O. Dale, T. Tanbo, and T. Åbyholm, “Acta Obstetricia et Gynecologica Scandinavica Obesity is a risk factor for early pregnancy loss after IVF or ICSI,” C Acta Obstet Gynecol Scand, vol. 79, pp. 43–48, 2000.
- A. Dokras, L. Baredziak, J. Blaine, C. Syrop, B. J. Vanvoorhis, and A. Sparks, “Obstetric Outcomes After In Vitro Fertilization in Obese and Morbidly Obese Women LEVEL OF EVIDENCE: II-2,” 2006. [Online]. Available: http://journals.lww.com/greenjournal .
- E. C. A. M. Van Swieten, L. Van Der Leeuw-Harmsen, E. A. Badings, and P. J. Q. Van Der Linden, “Obesity and clomiphene challenge test as predictors of outcome of in vitro fertilization and intracytoplasmic sperm injection,” Gynecol Obstet Invest, vol. 59, no. 4, pp. 220–224, May 2005 . [CrossRef]
- M. Metwally, R. Cutting, A. Tipton, J. Skull, W. L. Ledger, and T. C. Li, “Effect of increased body mass index on oocyte and embryo quality in IVF patients,” Reprod Biomed Online, vol. 15, no. 5, pp. 532–538, 2007 . [CrossRef]
- S. Hurrle and W. H. Hsu, “The etiology of oxidative stress in insulin resistance,” Biomedical Journal, vol. 40, no. 5. Elsevier B.V., pp. 257–262, Oct. 01, 2017 . [CrossRef]
- M. Schieber and N. S. Chandel, “ROS function in redox signaling and oxidative stress,” Current Biology, vol. 24, no. 10. Cell Press, May 19, 2014 . [CrossRef]
- Á. Várnagy et al., “Levels of total antioxidant capacity and 8-hydroxy-2′-deoxyguanosine of serum and follicular fluid in women undergoing in vitro fertilization: focusing on endometriosis,” Hum Fertil, vol. 23, no. 3, pp. 200–208, Jul. 2020 . [CrossRef]
- R. B. Hood et al., “Characterizing the follicular fluid metabolome: quantifying the correlation across follicles and differences with the serum metabolome,” Fertil Steril, vol. 118, no. 5, pp. 970–979, Nov. 2022 . [CrossRef]
- B. K. Arya, A. U. Haq, and K. Chaudhury, “Oocyte quality reflected by follicular fluid analysis in poly cystic ovary syndrome (PCOS): A hypothesis based on intermediates of energy metabolism,” Med Hypotheses, vol. 78, no. 4, pp. 475–478, Apr. 2012 . [CrossRef]
- Y. Wei et al., “Nontargeted metabolomics analysis of follicular fluid in patients with endometriosis provides a new direction for the study of oocyte quality,” MedComm (Beijing), vol. 4, no. 3, Jun. 2023 . [CrossRef]
- K. D. Sinclair, L. A. Lunn, W. Y. Kwong, K. Wonnacott, R. S. T. Linforth, and J. Craigon, “Amino acid and fatty acid composition of follicular fluid as predictors of in-vitro embryo development,” Reprod Biomed Online, vol. 16, no. 6, pp. 859–868, 2008 . [CrossRef]
- M. Józwik, M. Józwik, C. Teng, and F. C. Battaglia, “Amino acid, ammonia and urea concentrations in human pre-ovulatory ovarian follicular fluid,” Human Reproduction, vol. 21, no. 11, pp. 2776–2782, 2006 . [CrossRef]
- T. Kirsipuu, K. Laks, A. Velthut-Meikas, L. Levkov, A. Salumets, and P. Palumaa, “Comprehensive elucidation of amino acid profile in human follicular fluid and plasma of in vitro fertilization patients,” Gynecological Endocrinology, vol. 31, pp. 9–17, Oct. 2015 . [CrossRef]
- Y. Suzuki, J. Kido, S. Matsumoto, K. Shimizu, and K. Nakamura, “Associations among amino acid, lipid, and glucose metabolic profiles in childhood obesity,” BMC Pediatr, vol. 19, no. 1, Aug. 2019 . [CrossRef]
- Owei, N. Umekwe, F. Stentz, J. Wan, and S. Dagogo-Jack, “Amino acid signature predictive of incident prediabetes: A case-control study nested within the longitudinal pathobiology of prediabetes in a biracial cohort,” Metabolism, vol. 98, pp. 76–83, Sep. 2019 . [CrossRef]
- P. Cetica, L. Pintos, G. Dalvit, and M. Beconi, “Involvement of enzymes of amino acid metabolism and tricarboxylic acid cycle in bovine oocyte maturation in vitro,” 2003.
- K. Uhde, H. T. A. Van Tol, T. A. E. Stout, and B. A. J. Roelen, “Metabolomic profiles of bovine cumulus cells and cumulus-oocyte-complex-conditioned medium during maturation in vitro,” Sci Rep, vol. 8, no. 1, Dec. 2018 . [CrossRef]
- M. A. Razak, P. S. Begum, B. Viswanath, and S. Rajagopal, “Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review,” Oxidative Medicine and Cellular Longevity, vol. 2017. Hindawi Limited, 2017 . [CrossRef]
- E. Seli, E. Babayev, S. C. Collins, G. Nemeth, and T. L. Horvath, “Minireview: Metabolism of female reproduction: Regulatory mechanisms and clinical implications,” Molecular Endocrinology, vol. 28, no. 6. Endocrine Society, pp. 790–804, 2014 . [CrossRef]
- R. Yan-Do and P. E. MacDonald, “Impaired ‘glycine’-mia in type 2 diabetes and potential mechanisms contributing to glucose homeostasis,” Endocrinology, vol. 158, no. 5. Endocrine Society, pp. 1064–1073, May 01, 2017 . [CrossRef]
- A. Alves, A. Bassot, A. L. Bulteau, L. Pirola, and B. Morio, “Glycine metabolism and its alterations in obesity and metabolic diseases,” Nutrients, vol. 11, no. 6, Jun. 2019 . [CrossRef]
- S. Li et al., “Glycine treatment enhances developmental potential of porcine oocytes and early embryos by inhibiting apoptosis,” J Anim Sci, vol. 96, no. 6, pp. 2427–2437, Jun. 2018. [CrossRef]
- Z. Liu and R. H. Foote, “Effects of amino acids on the development of in-vitro matured/in-vitro fertilization bovine embryos in a simple protein-free medium,” 1995. [Online]. Available: https://academic.oup.com/humrep/article-abstract/10/11/2985/674301 .
- B. F. Rasmussen, M. A. Ennis, R. A. Dyer, K. Lim, and R. Elango, “Glycine, a Dispensable Amino Acid, Is Conditionally Indispensable in Late Stages of Human Pregnancy,” Journal of Nutrition, vol. 151, no. 2, pp. 361–369, Feb. 2021 . [CrossRef]
- V. Otasevic and B. Korac, “Amino Acids: Metabolism,” in Encyclopedia of Food and Health, Elsevier Inc., 2015, pp. 149–155 . [CrossRef]
- X. Y. Byeong-Seon Jeong, “Cysteine, glutathione, and percoll treatments improve porcine oocyte maturation and fertilization in vitro,” Mol Reprod Dev, vol. 59, no. 3, pp. 330–335, 2001 . [CrossRef]
- S. K. Jain, D. Micinski, L. Huning, G. Kahlon, P. F. Bass, and S. N. Levine, “Vitamin D and L-cysteine levels correlate positively with GSH and negatively with insulin resistance levels in the blood of type 2 diabetic patients,” Eur J Clin Nutr, vol. 68, no. 10, pp. 1148–1153, Jan. 2014 . [CrossRef]
- J. T. Brosnan and M. E. Brosnan, “Glutamate: A truly functional amino acid,” Amino Acids, vol. 45, no. 3, pp. 413–418, Sep. 2013 . [CrossRef]
- P. Gumus Balikcioglu et al., “Branched-chain α-keto acids and glutamate/glutamine: Biomarkers of insulin resistance in childhood obesity,” Endocrinol Diabetes Metab, vol. 6, no. 1, Jan. 2023 . [CrossRef]
- L. H. Sekhon, S. Gupta, Y. Kim, and A. Agarwal, “Female Infertility and Antioxidants,” 2010.
- J. P. Anchordoquy et al., “Effect of cysteine, glutamate and glycine supplementation to in vitro fertilization medium during bovine early embryo development,” Reprod Biol, vol. 19, no. 4, pp. 349–355, Dec. 2019. [CrossRef]
- A. Mukherjee et al., “Resveratrol treatment during goat oocytes maturation enhances developmental competence of parthenogenetic and hand-made cloned blastocysts by modulating intracellular glutathione level and embryonic gene expression,” J Assist Reprod Genet, vol. 31, no. 2, pp. 229–239, 2014 . [CrossRef]
- J. Lim et al., “Glutathione-deficient mice have increased sensitivity to transplacental benzo[a]pyrene-induced premature ovarian failure and ovarian tumorigenesis,” Cancer Res, vol. 73, no. 2, pp. 908–917, Jan. 2013 . [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).