Submitted:
10 July 2023
Posted:
12 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
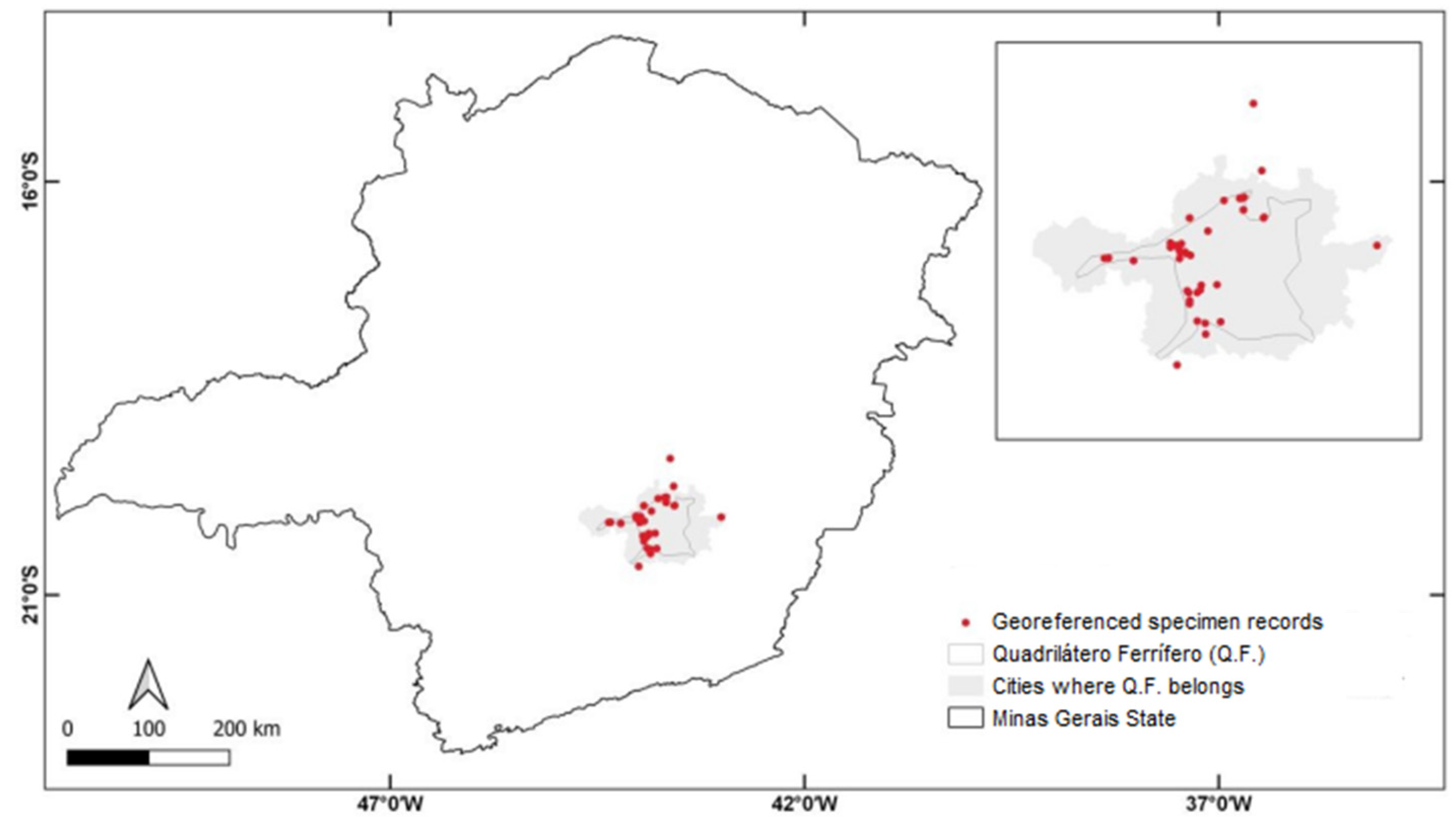
2.1. Study species
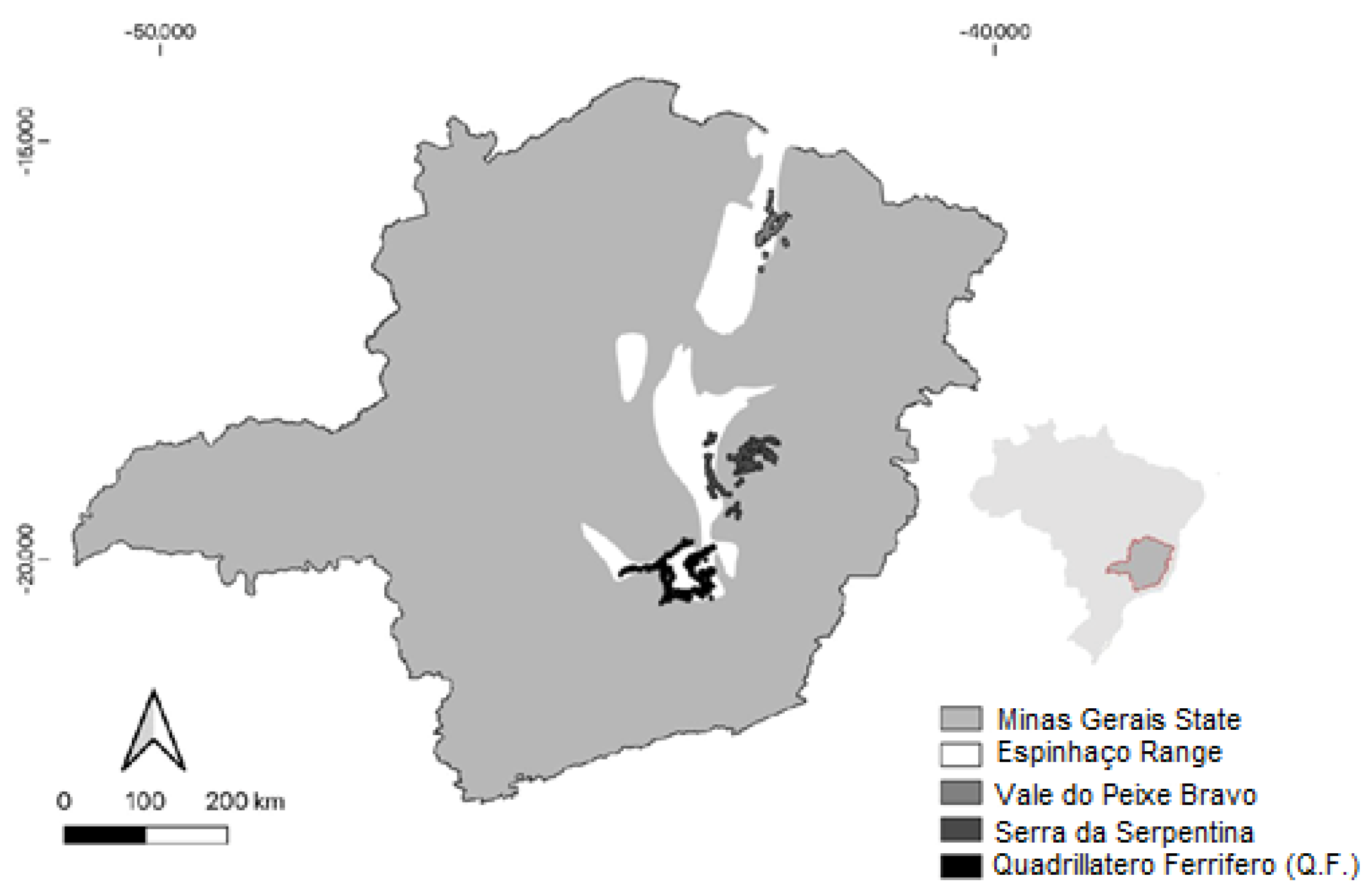
2.2. Study area
2.3. Species distribution modeling
2.4. Climatic and edaphic data
2.5. Modeling and Evaluation Procedures
2.4. Mining titles
3. Results
3.1. Selected climatic and edaphic variables
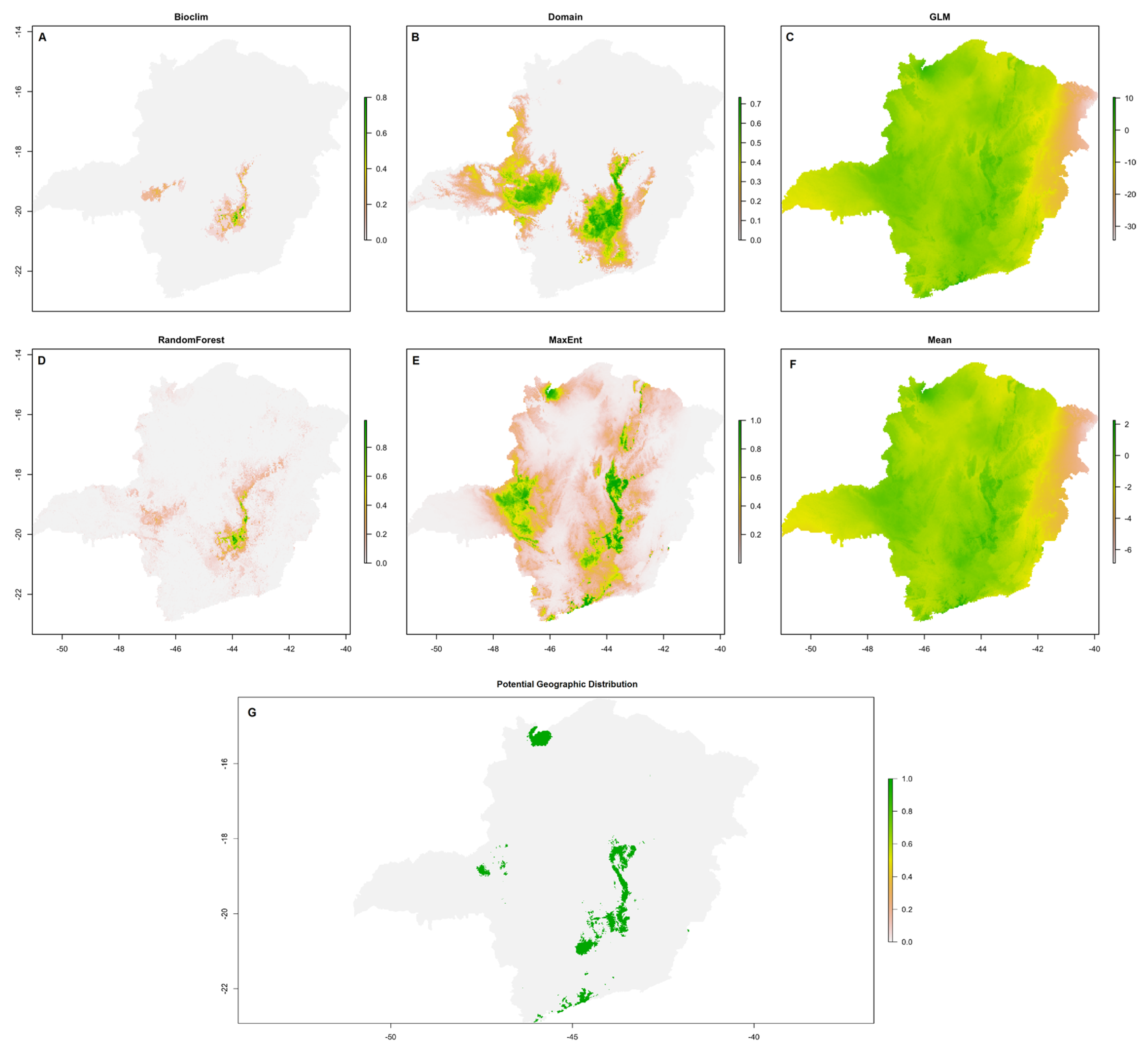
3.2. Current potential environmental suitability for Arthrocereus glaziovii
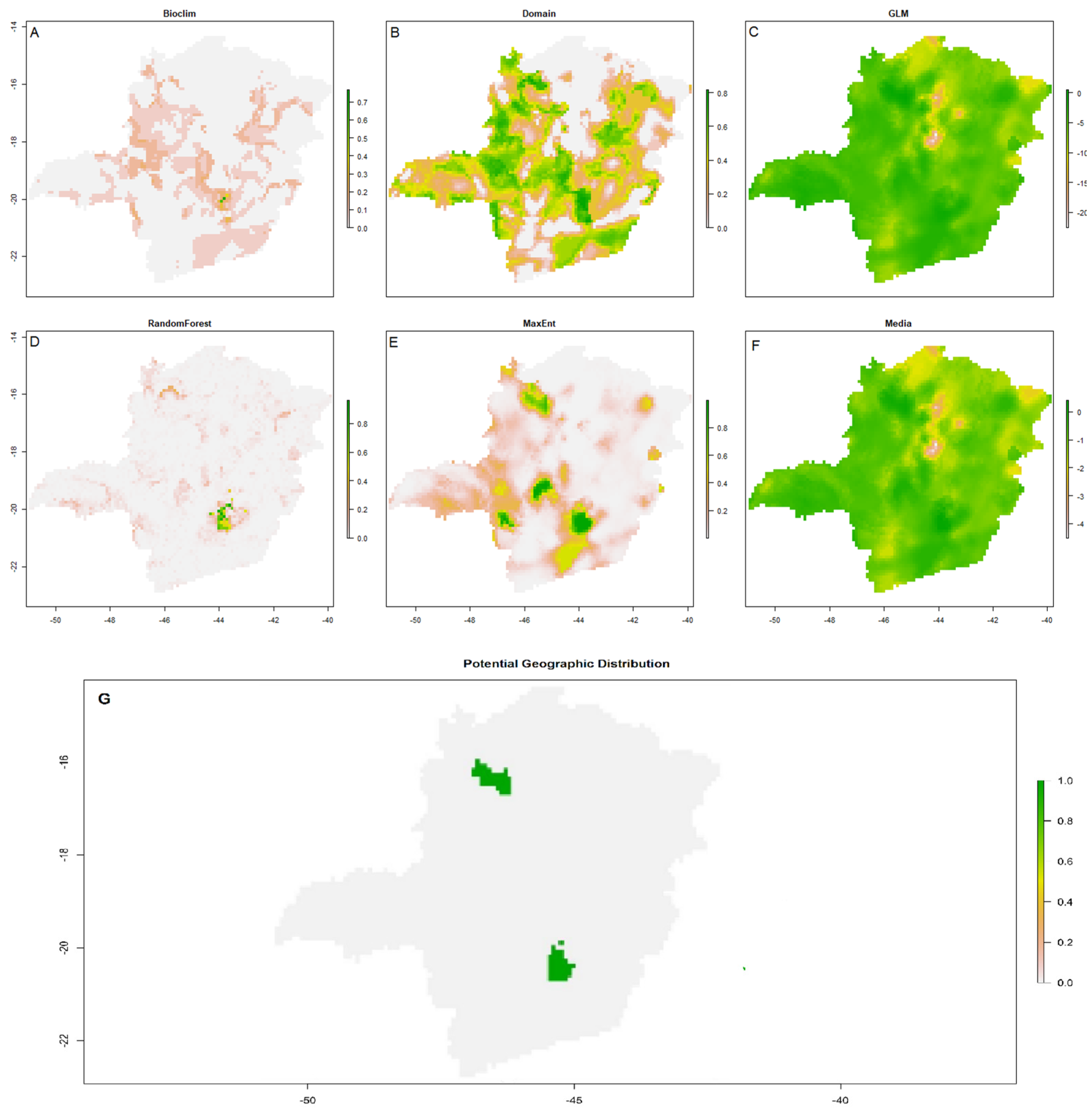
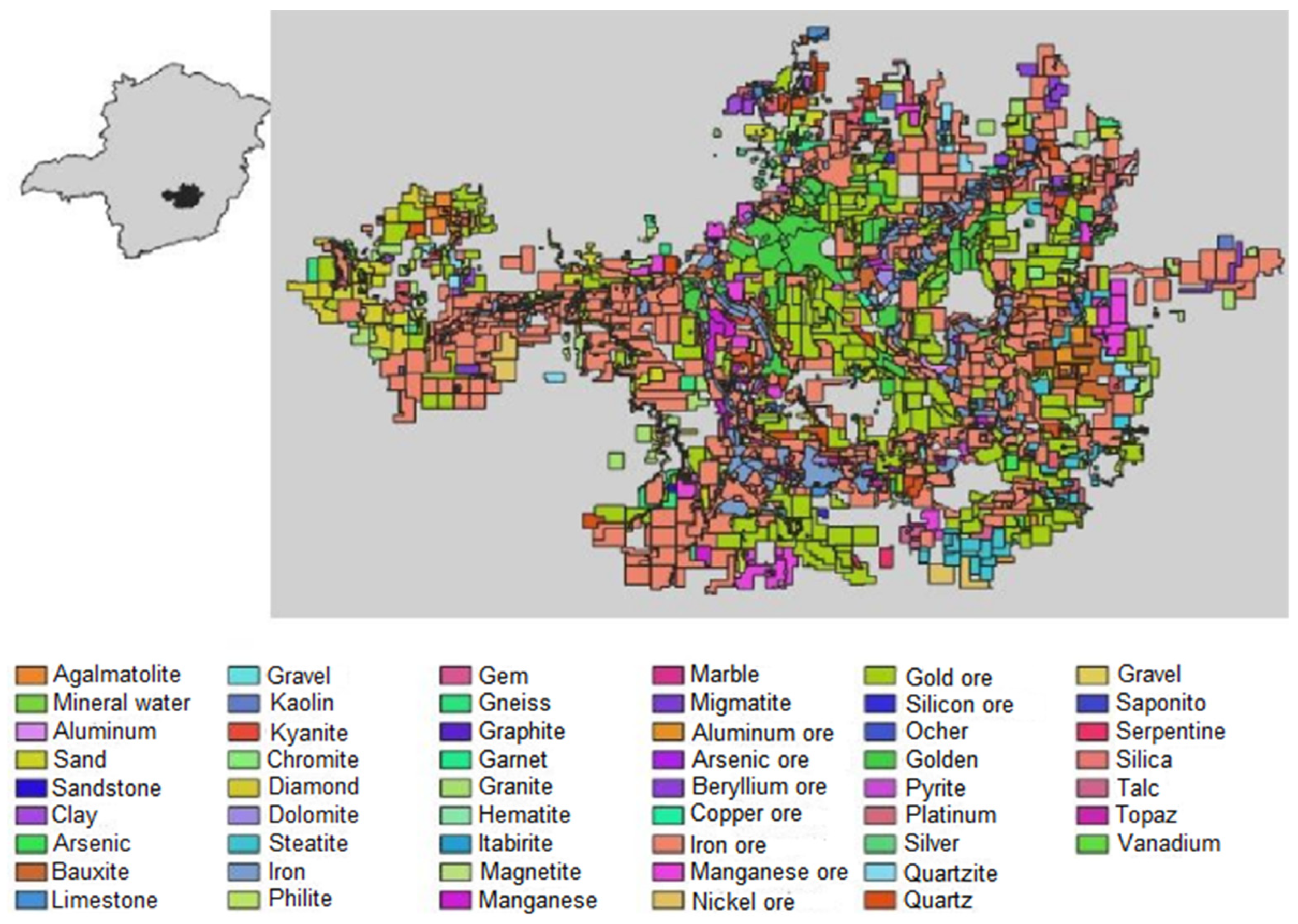
3.3. Mining titles
4. Discussion
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Cheib, A.L. Ecologia da germinação e potencial para formação de banco de sementes de espécies de Arthrocereus A. Berger (Cactaceae) endêmicas dos campos rupestres de Minas Gerais, Brasil. Dissertação de Mestrado, Universidade Federal de Minas Gerais, Belo Horizonte, 2009.
- Giulietti, A.M.; Pirani, J.R.; Harley, R.M. Espinhaço range region, eastern Brazil. In: Centres of plant diversity: a guide and strategy for their conservation, 1st ed.; Davis, S.D.; Heywood, V.H.; Herrera-MacBryde, O.; Villa-Lobos, J.; Hamilton, A.C., Eds.; WWF/IUCN: Cambridge, UK, 1997; pp. 397–404.
- Porto, M. L.; Silva, M. F. F. Tipos de vegetação metalófila em áreas da Serra de Carajás e de Minas Gerais. Acta Botanica Brasilica 1989, 3, 13-21. [CrossRef]
- Jacobi, C.M.; Carmo, F.F. Diversidade dos campos rupestres ferruginosos no Quadrilátero Ferrífero, MG. Megadiversidade 2008, 4, 1-12.
- Jacobi C.M.; Carmo F.F. Plantas Vasculares sobre Cangas. In Diversidade Florística nas cangas do Quadrilátero Ferrífero, 1st ed.; Jacobi C.M., Carmo F.F., Eds.; Código Editora: Belo Horizonte, Brazil, 2012; pp. 31– 42.
- Alvares C.A., Stape J.L., Sentelhas P.C., Gonçalves J.L.M., Sparovek G. Köppen’s climate classification map for Brazil. Meteorologische Zeitschrift 2013, 22, 711-728. [CrossRef]
- Gianotti, A.R.C.; Souza, M.J.H.; Machado, L.M.; Pereira, I.M.; Vieira, A.D. Magalhães, M. R. Análise microclimática em duas fitofisionomias do cerrado no alto Vale do Jequitinhonha, Minas Gerais. Revista Brasileira de Meteorologia 2013, 28, 246-256. [CrossRef]
- Jacobi, C.M.; Carmo, F.F.; Vincent, R.C.; Stehmann, J. R. Plant communities on the ironstone outcrops - a diverse and endangered Brazilian ecosystem. Biodiversity and Conservation 2007, 16, 2185-2200. [CrossRef]
- Lima, L. R.; Pirani, J.R. O gênero Croton L. (Euphorbiaceae) na cadeia do espinhaço, Minas Gerais, Brasil. Bol Bot. 2003, 21, 299-344. [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853-858. [CrossRef]
- Oliveira, A.S.; Resende, M.G.; Silva, W.H.O. Análise da fragmentação florestal da região centro sul do estado de Minas Gerais através de estimativa de métricas de paisagem, Proceedings of the Congresso Nacional de Botânica, Belo Horizonte, Brazil, 2013; Belo Horizonte, Brazil, 2013, Abstract Number id6220.
- Carmo, F.F. Importância Ambiental e Estado de Conservação dos Ecossistemas de Cangas no Quadrilátero Ferrífero e Proposta de Áreas-Alvo para a Investigação e Proteção da Biodiversidade em Minas Gerais. Dissertação de Mestrado, Universidade Federal de Minas Gerais, Belo Horizonte, 2010.
- Salles, D.M.; Carmo, F.F.; Jacobi, C.M. Habitat loss challenges the conservation of endemic plants in mining-targeted Brazilian mountains. Environmental Conservation 2018, 46, 140-146. [CrossRef]
- Jacobi, C.M.; Carmo, F.F.; Campos, I.C. Soaring extinction threats to endemic plants in Brazilian metal-rich regions. AMBIO 2011, 40, 540-543. [CrossRef]
- Negreiros, D.; Fernandes, G.W; Silveira, F.A.O.; Chalub, C. Seedling growth and biomass allocation of endemic and threatened shrubs of rupestrian fields. Acta Oecologica 2009, 35, 301-310. [CrossRef]
- Silveira, F.A.O.; Negreiros, D.; Barbosa, N.P.U.; Buisson, E.; Carmo, F.F.; Carstensen, D.W.; Conceição, A.A.; Cornelissen, T.G.; Echternacht, L.; Fernandes, G.W.; Garcia, Q.S.; Guerra, T.J.; Jacobi, C.M.; Lemos-Filho, J.P.; Le Stradic, S.; Morellato, L.P.C.; Neves, F.S.; Oliveira, R.S.; Schaefer, C.E.; Viana, P.L.; Lambers, H. Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant and Soil 2016, 403, 129–152. [CrossRef]
- Jacobi, C.M.; Carmo, F.F. The contribution of ironstone outcrops to plant diversity in the Iron Quadrangle, a threatened Brazilian landscape. AMBIO 2008, 37, 324-326. [CrossRef]
- Fernandes, G.W.; Ribeiro, S.P. Deadly conflicts: mining, people, and conservation. Perspectives in Ecology and Conservation 2017, 15, 141–144. [CrossRef]
- Zappi, D.; Taylor, N.; Silva, S.R.; Machado, M.; Moraes, E.M.; Calvente, A.; Cruz, B.; Correia, D.; Larocca, J.; Assis, J.G.A.; Aona, L.; Menezes, M.O.T.; Meiado, M.; Marchi, M.N.; Santos, M.R.; Bellintani, M.; Coelho, P.; Nahoum, P.I.; Resende, S. Plano de ação nacional para a conservação das Cactaceas. 1st ed.; Instituto Chico Mendes de Conservação da Biodiversidade, ICMBio: Brasília, Brazil, 2011, pp. 1-58.
- Nabout, J.C.; Júnior, P.M.; Bini, L.M.; Diniz-Filho, J.A.F. Distribuição geográfica potencial de espécies americanas do caranguejo "violinista"; (Uca spp.) (Crustacea, Decapoda) com base em modelagem de nicho ecológico. Iheringia 2009, 92-98. [CrossRef]
- Sousa, N.P.R. Estrutura hierárquica na resposta das distribuições geográficas de plantas do Cerrado a mudanças climáticas. Dissertação de Mestrado, Universidade Federal de Goiás, Goiânia, 2011.
- Carvalho, M.C.; Gomide, L.R., Acerbi-Júnior, F.W.; Tng, D. Potential and future geographical distribution of Eremanthus erythropappus (DC.) MacLeish: a tree threatened by climate change. Floresta e Ambiente 2019, 26, e20180455. [CrossRef]
- REFLORA - Plantas do Brasil: resgate histórico e herbário virtual para o conhecimento e conservação da flora brasileira. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB1417 (acessed in 21/06/2019).
- Gonzaga, D.R.; Souza, M.A.; Neto, L.M.; Peixoto, A.L.; Mendonça, C.B.F.; Gonçalves-Esteves, V. The systematic value of pollen morphology in Arthrocereus A. Berger (Cactaceae, Cactoideae). Review of Palaeobotany and Palynology 2019, 269, 33-41. [CrossRef]
- Zappi, D.C.; Taylor, N.P. Cactaceae. In Diversidade Florística nas Cangas do Quadrilátero Ferrífero, 1st ed.; Jacobi, C.M.; Carmo, F.F., Eds.; Código Editora: Belo Horizonte, Brazil, 2012.
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/search?query=Arthrocereus%20glaziovii&searchType=species (acessed in 22/07/2019).
- Skirycz, A.; Castilho A.; Chaparro, C.; Carvalho N.; Tzotzos G., Siqueira J.O. (2014). Canga biodiversity, a matter of mining. Frontiers in Plant Science 2014, 5, 1-9. [CrossRef]
- King, L.C. A geomorfologia do Brasil Oriental. Rio de Janeiro, Revista Brasileira de Geografia 1956, 18, 147-265.
- Stannard, B.L.; Harvey, Y.B; Harley, R.M. Flora of the Pico das Almas: Chapada Diamantina - Bahia, Brazil, 1st ed.; Royal Botanic Gardens: Kew, London, England, pp. 1-853.
- Giulietti, A.M.; Pirani, J.R.; Harley, R.M. Espinhaço range region, eastern Brazil. In Centres of plant diversity: a guide and strategy for their conservation, Davis, S.D.; Heywood, V.H., Eds.; Gland : IUCN / WWF: Cambridge, England, v.3, pp. 1-562.
- IBRAM - Instituto Brasileiro de Mineração. Minério de Ferro. In Informações Sobre Economia Mineral Brasileira, 1st ed.; Rodrigues, C.P., Costa, E.R., Eds.; Instituto Brasileiro de Mineração: Brasília, Brazil, 2015; pp. 22–25.
- Bianchetti, M. Vale contabiliza recuo de 41,2% na extração de minério em MG. In: Diário do comércio. Available online: https://diariodocomercio.com.br (acessed in 20/01/2021).
- Global Biodiversity Information Facility (GBIF). Available online: https://www.gbif.org/ (acessed in 13/01/2019).
- speciesLink. Available online: https://specieslink.net/ (acessed in 13/01/2019).
- Climaco, L.F.S. Variabilidade fenotípica da espécie microendêmica Arthrocereus glaziovii Zappy & Taylor (Cactaceae) em campos rupestres ferruginosos. Dissertação de Mestrado, Universidade Federal de Ouro Preto, Ouro Preto, 2017.
- Hijmans, R.J.; Etten, J. V. Raster: Geographic analysis and modeling with raster data. R package version 2.0-12. 2012. Available online: http://CRAN.R-project.org/package=raster (acessed in 20/12/2022).
- Worldclim 2020. Available online: http://www.worldclim.org (acessed in 15/05/2019).
- Arruda, D. M.; Fernandes-Filho, E. I.; Solar, R.R.; Schaefer, C. E. Combining climatic and soil properties better predicts covers of Brazilian biomes. The Science of Nature 2017, 104, 1-10. [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/. 2017.
- Genuer, R.; Poggi, J. M.; Malot, T. M. VSURF: Variable Selection Using Random Forests. R package version 1.0.4. 2018. Available online: https://CRAN.R-project.org/package=VSURF (acessed in 20/12/2022).
- Instituto Pristino’s Geoenvironmental Digital Atlas database. Available online: https://www.institutopristino.org.br (acessed in 20/12/2019).
- Petit, S.The reproductive phenology of three sympatric species of columnar cacti on Curaçao. Journal of Arid Environments 2001, 49, 521–531. [CrossRef]
- Lima, A.L.A. Padrões fenológicos de espécies lenhosas e cactáceas em uma área do semi-árido do Nordeste do Brasil. Dissertação de Mestrado, Universidade Federal Rural de Pernambuco, Recife, 2007.
- Alencar, J.C.; Almeida, R.A.; Fernandes, N.P. Fenologia de espécies florestais em floresta tropical úmida de terra firme na Amazônia Central. Acta Amazônica 1979, 9, 163-198. [CrossRef]
- Oliveira, D.V. Aspectos da história de vida de Arthrocereus glaziovii (K.Schum.) N.P.Taylor & Zappi (Cactaceae), uma espécie endêmica do Quadrilátero Ferrífero, Minas Gerais, Brasil. Dissertação de Mestrado, Universidade Federal de Ouro Preto, Ouro Preto, 2017.
- Cheib, A.L.; Garcia, Q.S. Longevity and germination ecology of seeds of endemic Cactaceae species from high-altitude sites in southeastern Brazil. Seed Science Research 2012, 22, 45-53. [CrossRef]
- Gonçalves, L.N. Campos de altitude do maciço Marins-Itaguaré, Serra da Mantiqueira SP/MG: composição florística, fitogeografia e estrutura da vegetação. Dissertação de Mestrado. Universidade Federal de Juíz de Fora, Juíz de Fora, 2019.
- Sá Júnior, A. Aplicação da classificação de Köppen para o zoneamento climático do estado de Minas Gerais. Dissertação de Mestrado. Universidade Federal de Lavras, Lavras, 2009.
- Costa, S.A.D. Caracterização química, física, mineralógica e classificação de solos ricos em ferro do Quadrilátero Ferrífero. Dissertação de Mestrado (Solos e Nutrição de Plantas). Universidade Federal de Viçosa, Viçosa, 2003.
- Souza, V. N. R.; Neto, J. E. E.; Matrangolo, C. A. R.; Magalhães, W. T.; Fogaça, C. A.; Figueiredo, M. A. P.; Figueiredo, L. H. A. Caracterização de diferentes solos eutróficos na região norte de Minas Gerais. Revista Intercâmbio 2019, 15, 106-122. [CrossRef]
- Fernandes, G.W.; Barbosa, N.P.U.; Alberton, B.; Barbieri, A.; Dirzo, R.; Goulart, F.; Guerra, T.J.; Morellato, L.P.C.; Solar, R.R.C. The deadly route to collapse and the uncertain fate of Brazilian rupestrian grasslands. Biodiversity and Conservation 2018, 27, 2587-2603.
- Durán, A.P.; Rauch, J.; Gaston, K.J. Global spatial coincidence between protected areas and metal mining activities. Biological Conservation 2013, 160, 272-278. [CrossRef]
- Pena, J.C.C.; Goulart, F.; Fernandes, G.W.; Hoffmann, D.; Leite, F.S.F.; Santos, N.B.; Soares-Filho, B.; Sobral-Souza, T.; Vancine, M.H.; Rodrigues, M. Impacts of mining activities on the potential geographic distribution of eastern Brazil mountaintop endemic species. Perspectives in Ecology and Conservation 2017, 15, 172–178. [CrossRef]
| Climatics1 | Edaphics2 |
|---|---|
| Seasonal Precipitation (BIO15) | Silt |
| Mean Annual Temperature (BIO1) | Potassium (K) |
| Annual Precipitation (BIO12) | Nitrogen (N) |
| Temperature Seasonality (BIO4) | Hydrogen (H) |
| Annual Temperature Variation (BIO7) | Clay |
| Sand | |
| Water pH | |
| Magnesium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





