Submitted:
12 July 2023
Posted:
13 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.2. Design of an Artificial Photosynthetic System
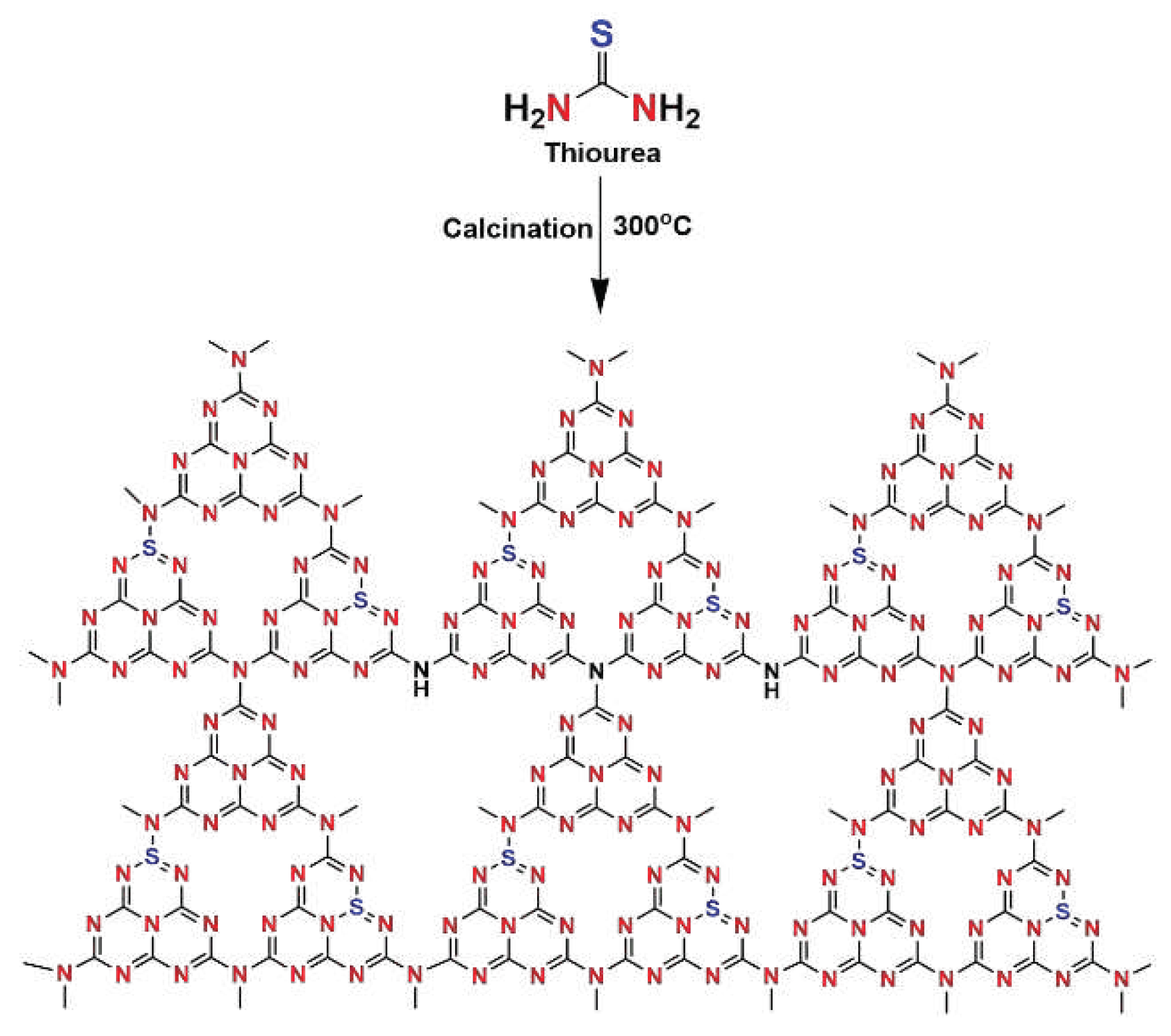
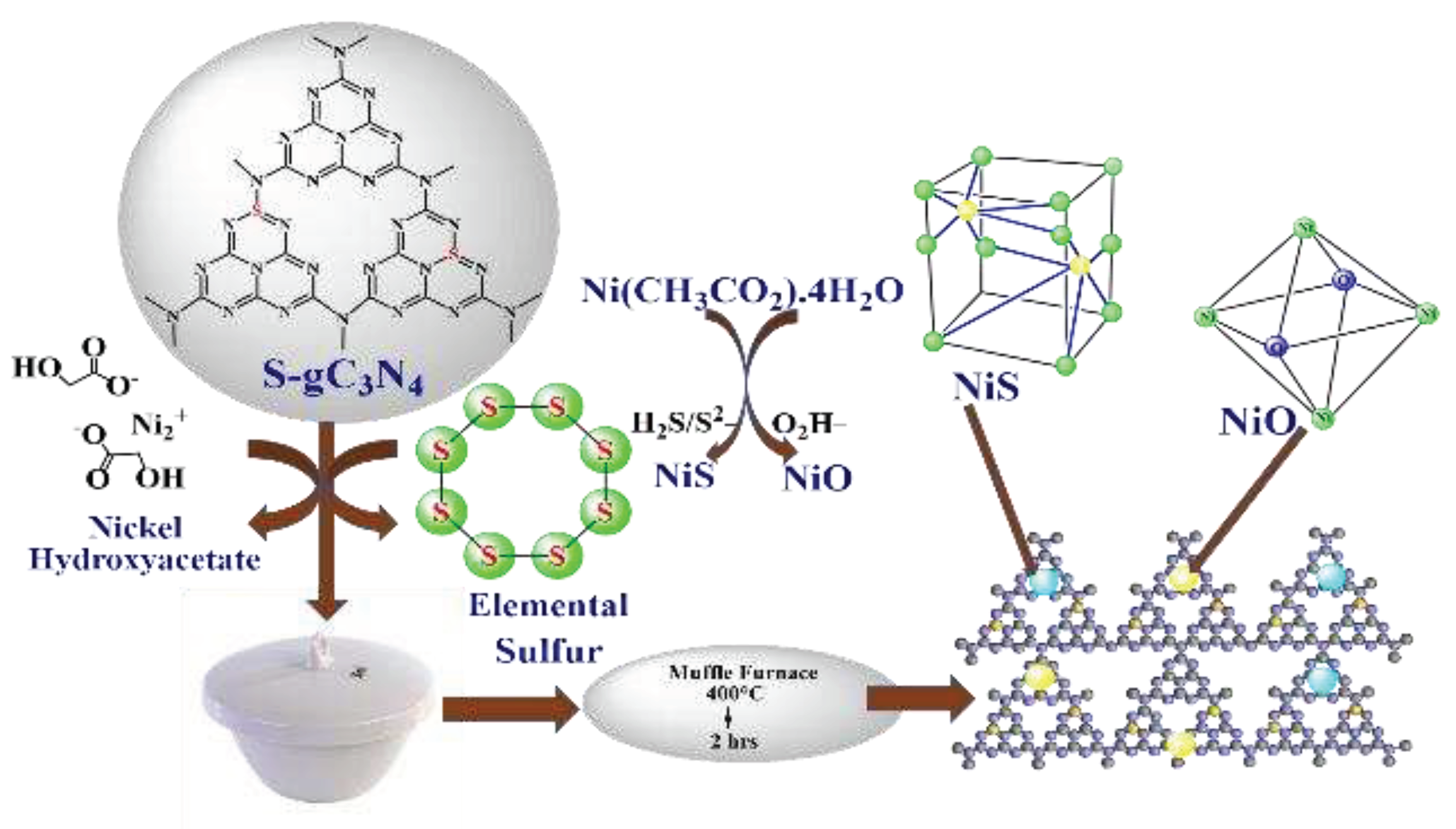
2.3. Synthesis of S-g-C3N4
2.4. Synthesis of NiS-NiO/S-g-C3N4 photocatalyst
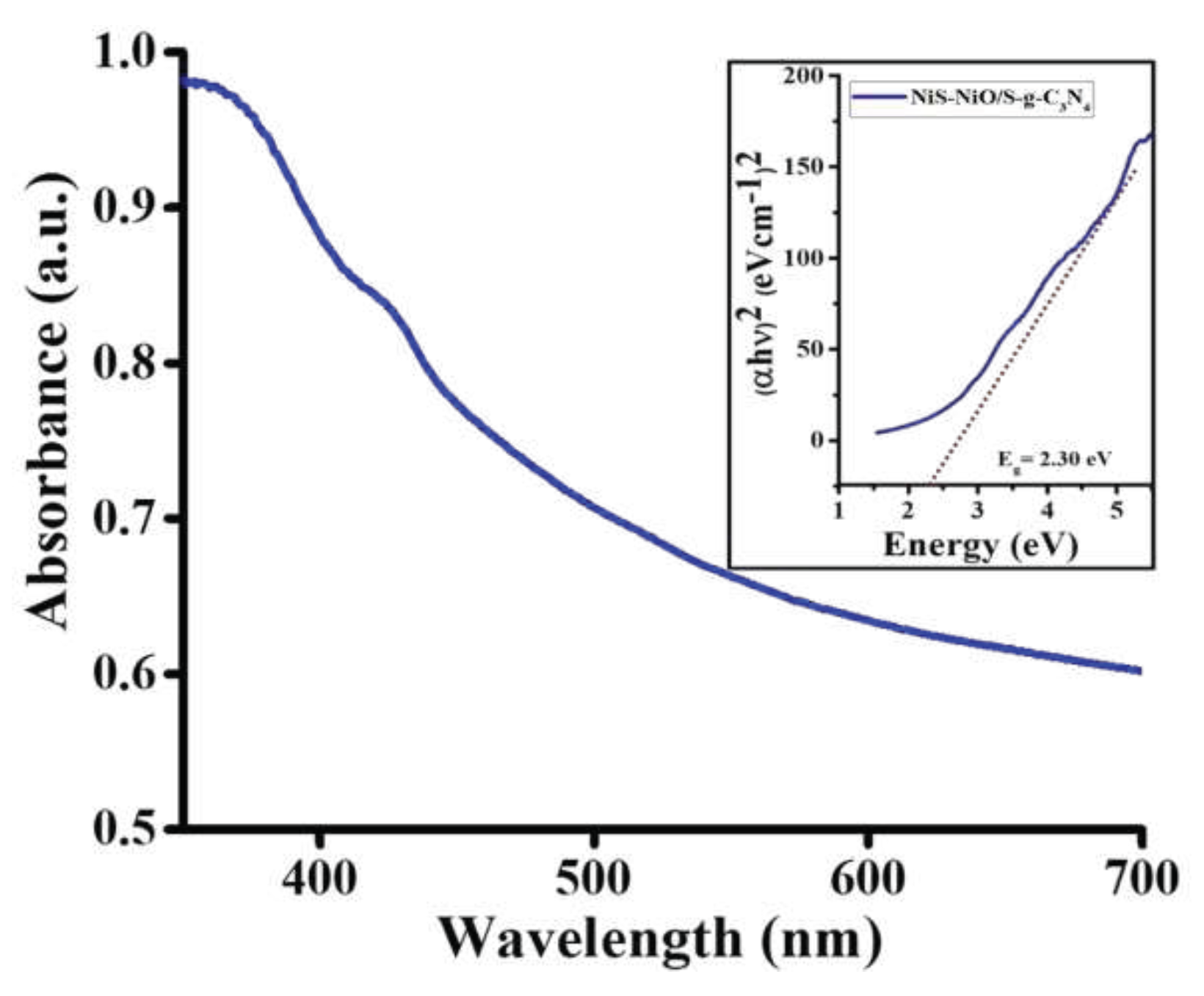
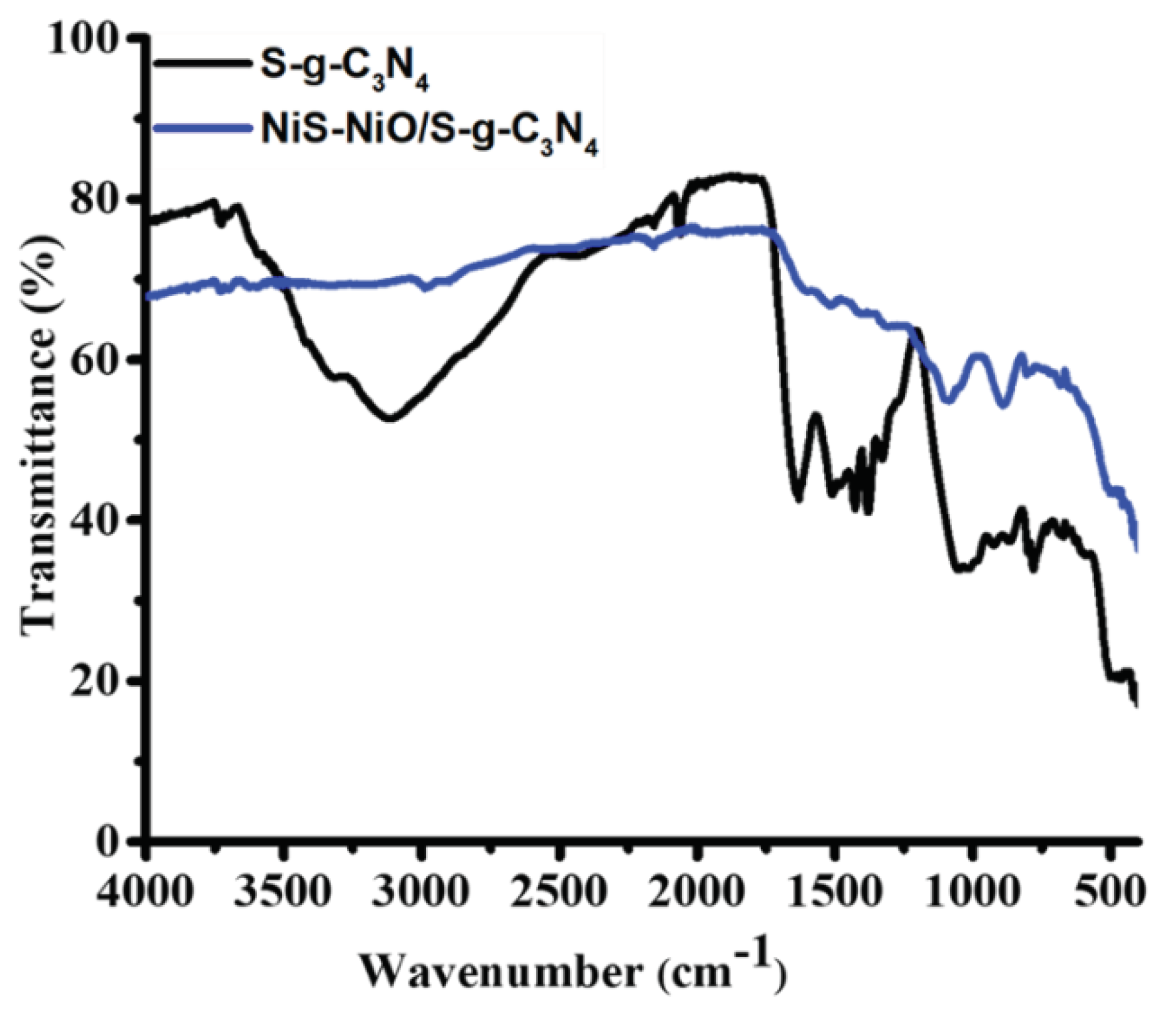
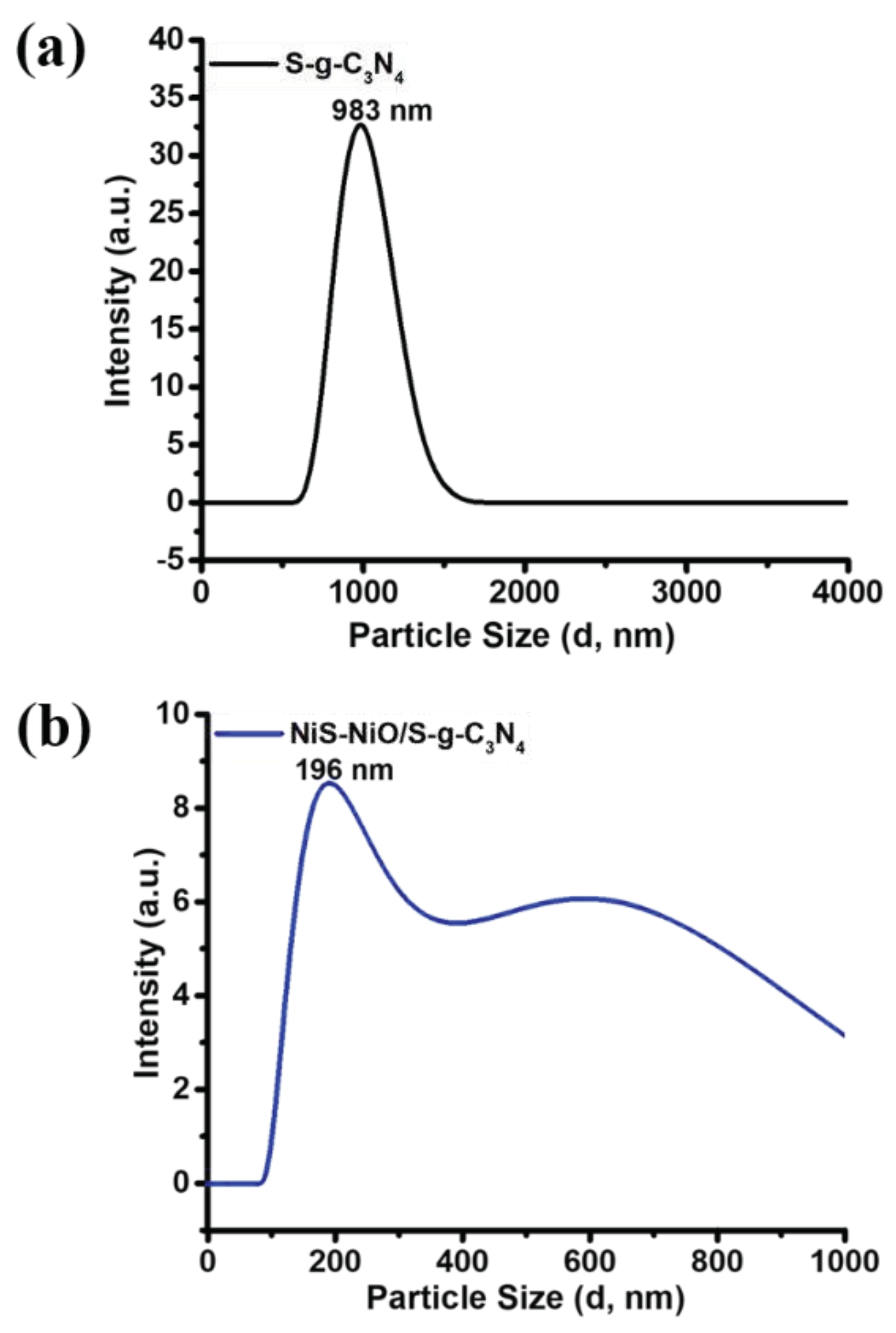
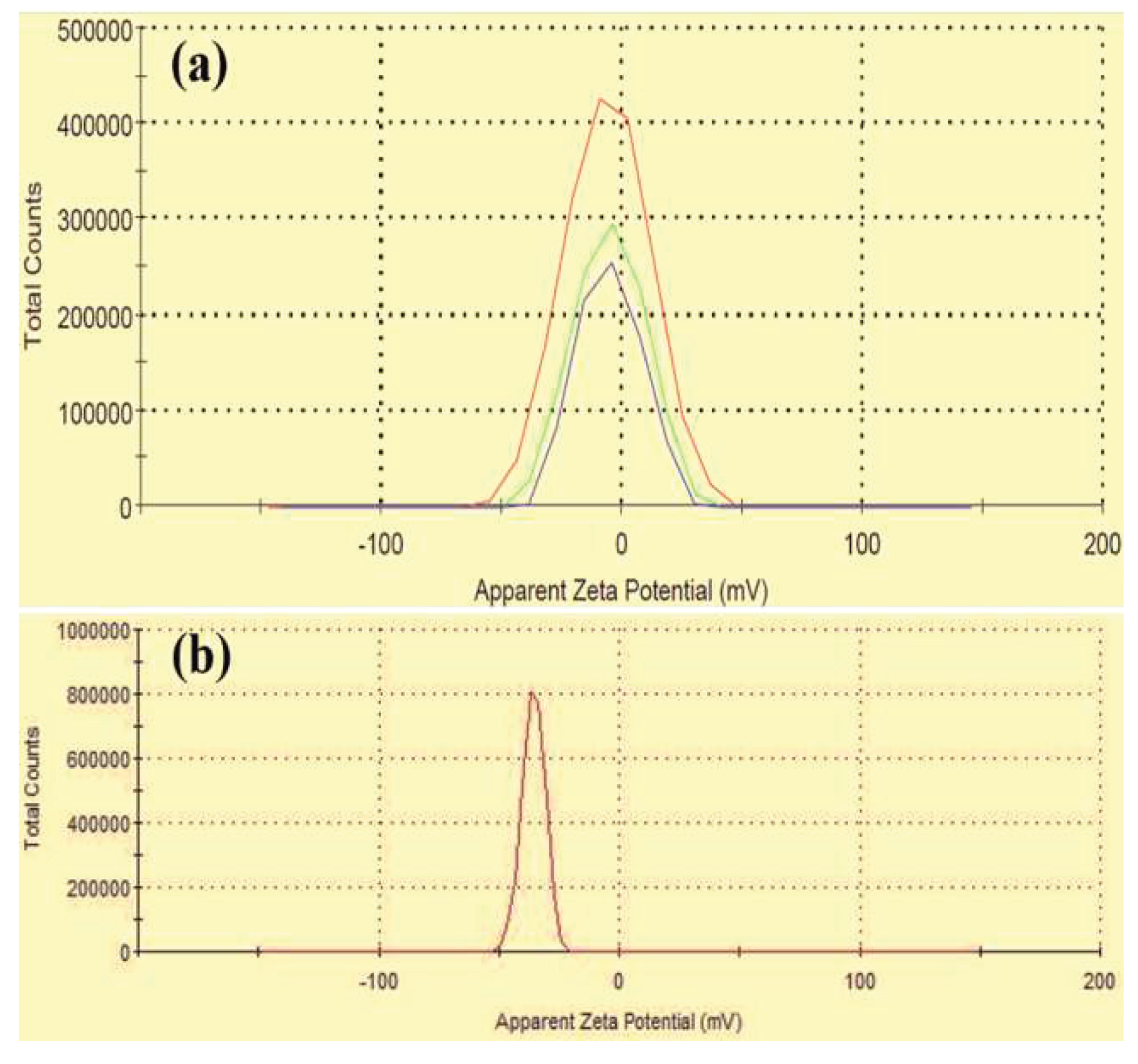
3. Results
4. Applications of NiS-NiO/S-g-C3N4 photocatalyst
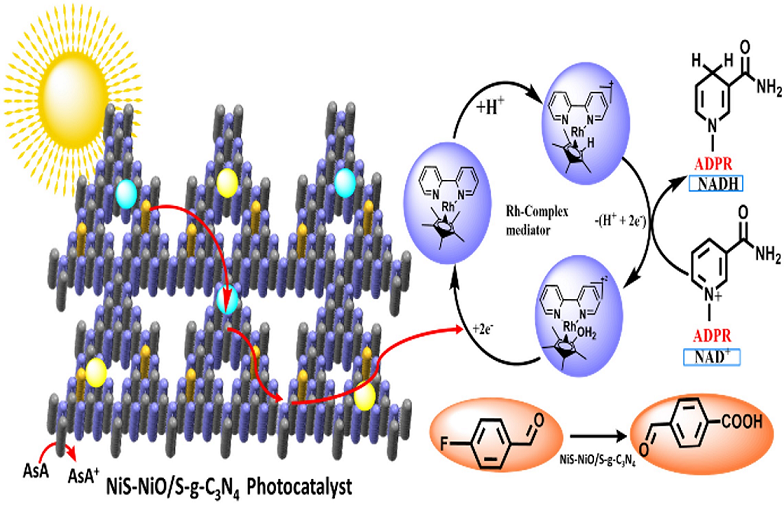
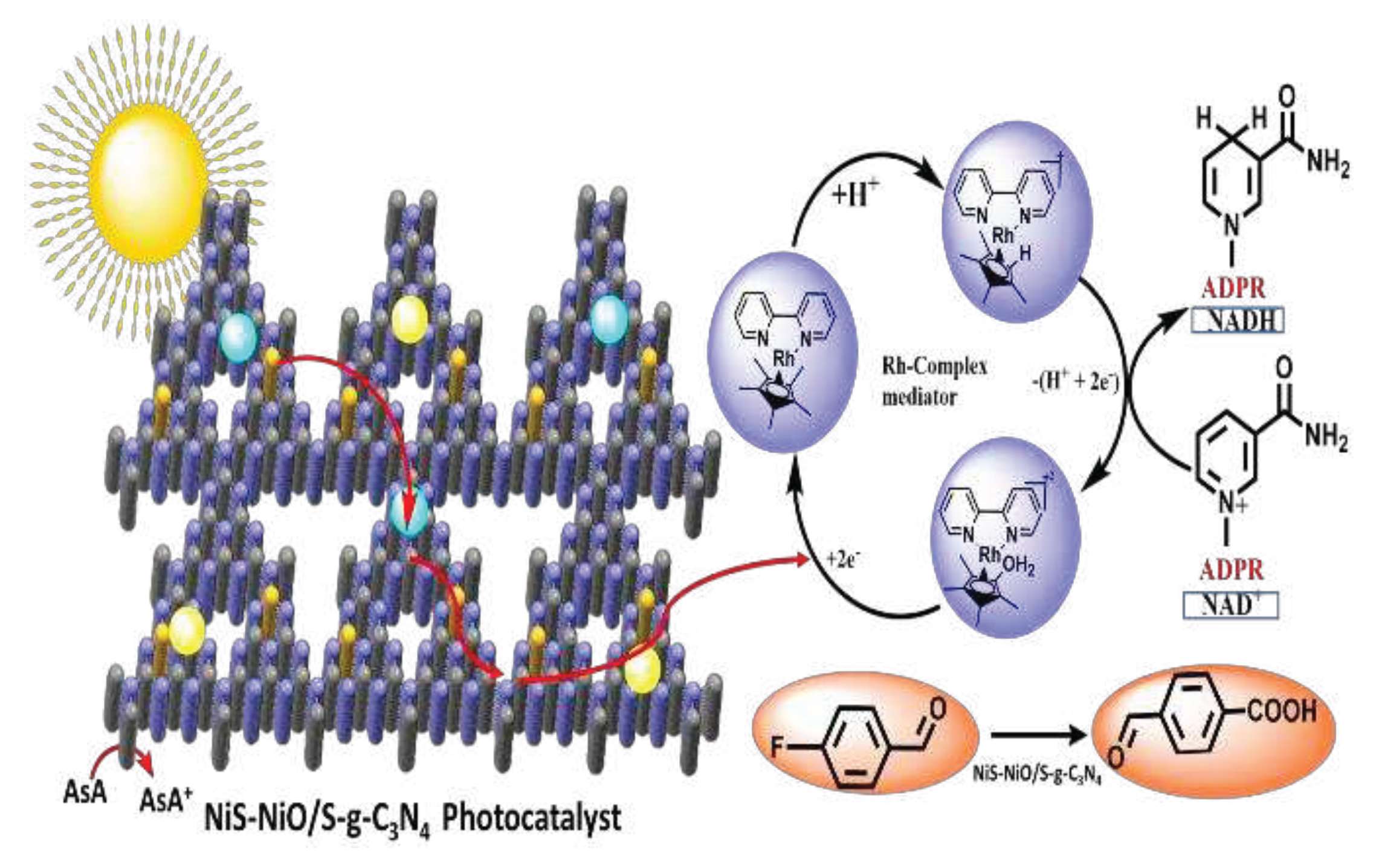
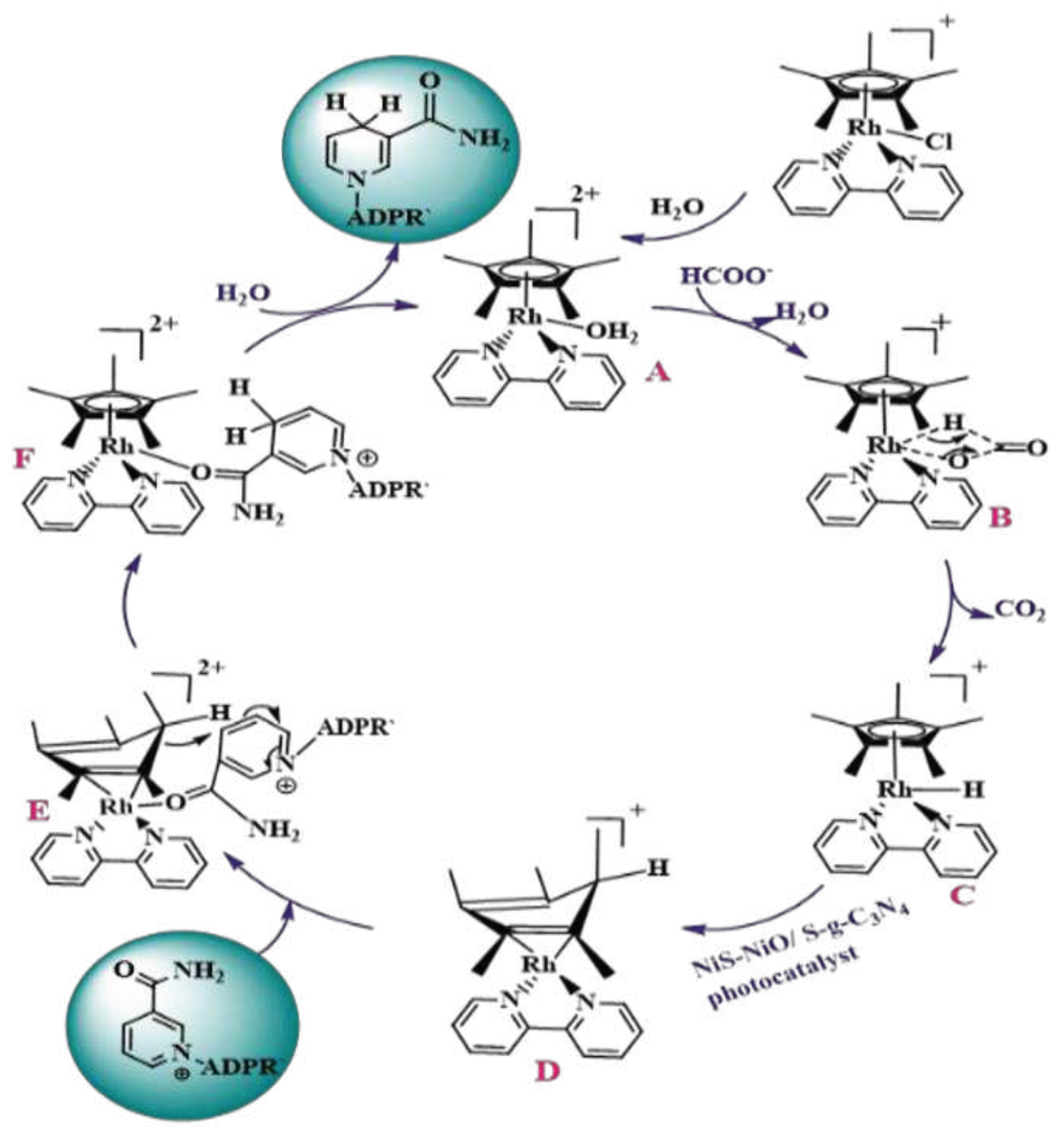
4.1. A Plausible Mechanism for the 1,4-NADH Co-Factor Regeneration through a [Cp*Rh(bpy)Cl] Cl
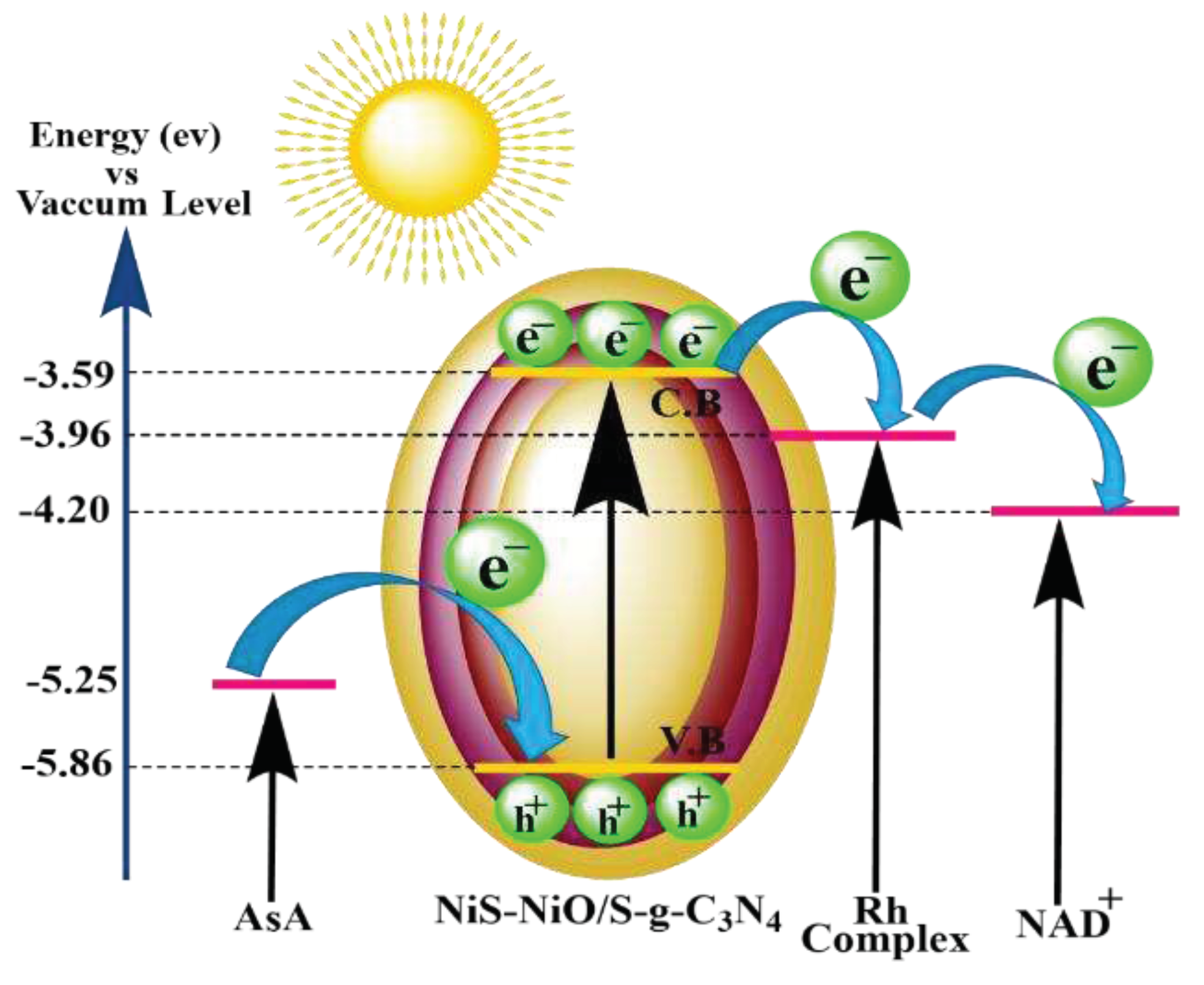
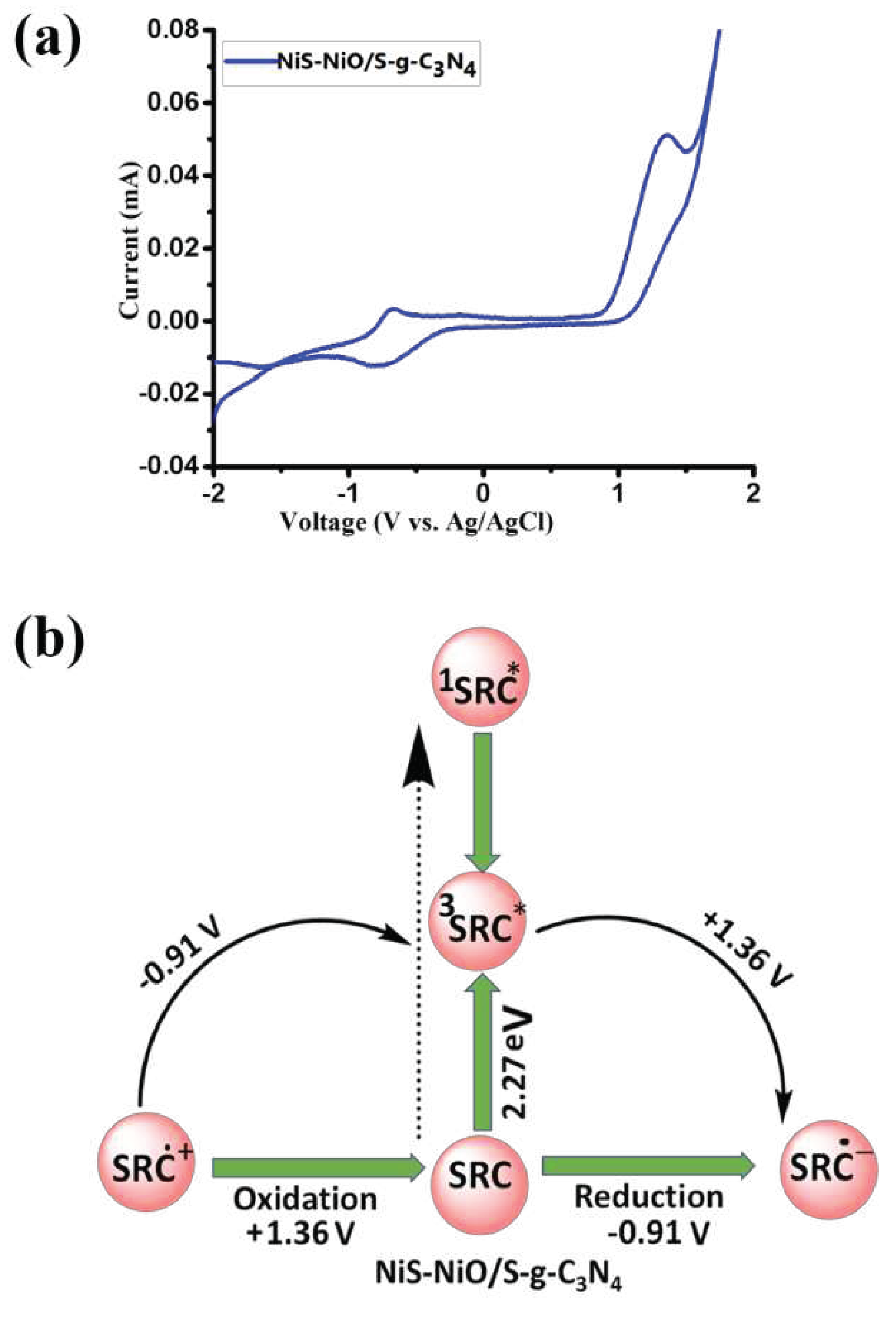
4.2. The Route of Carrier Formation and Migration in an Artificial Photocatalytic System
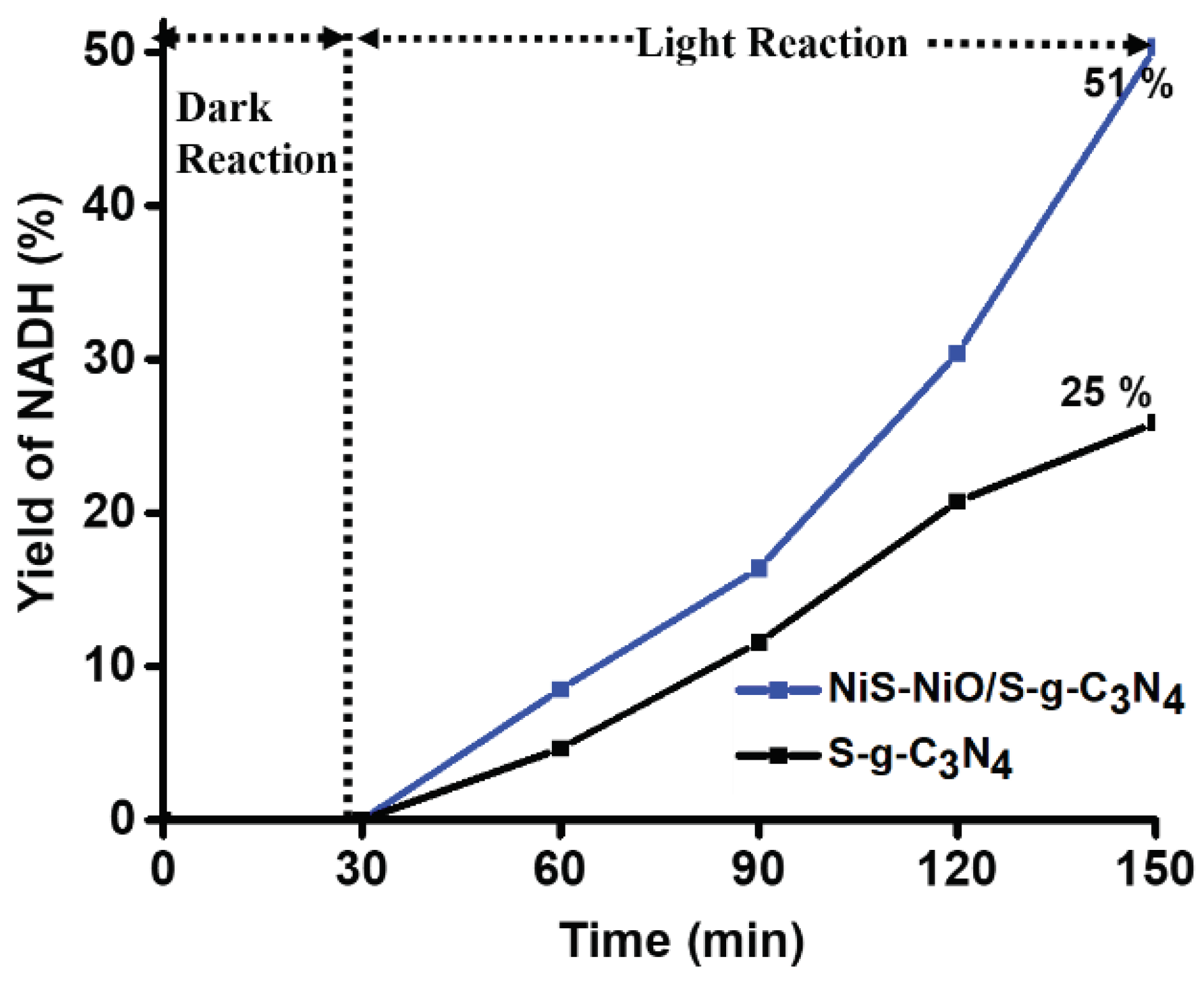
4.3. Photocatalytic Regenerations of NADH
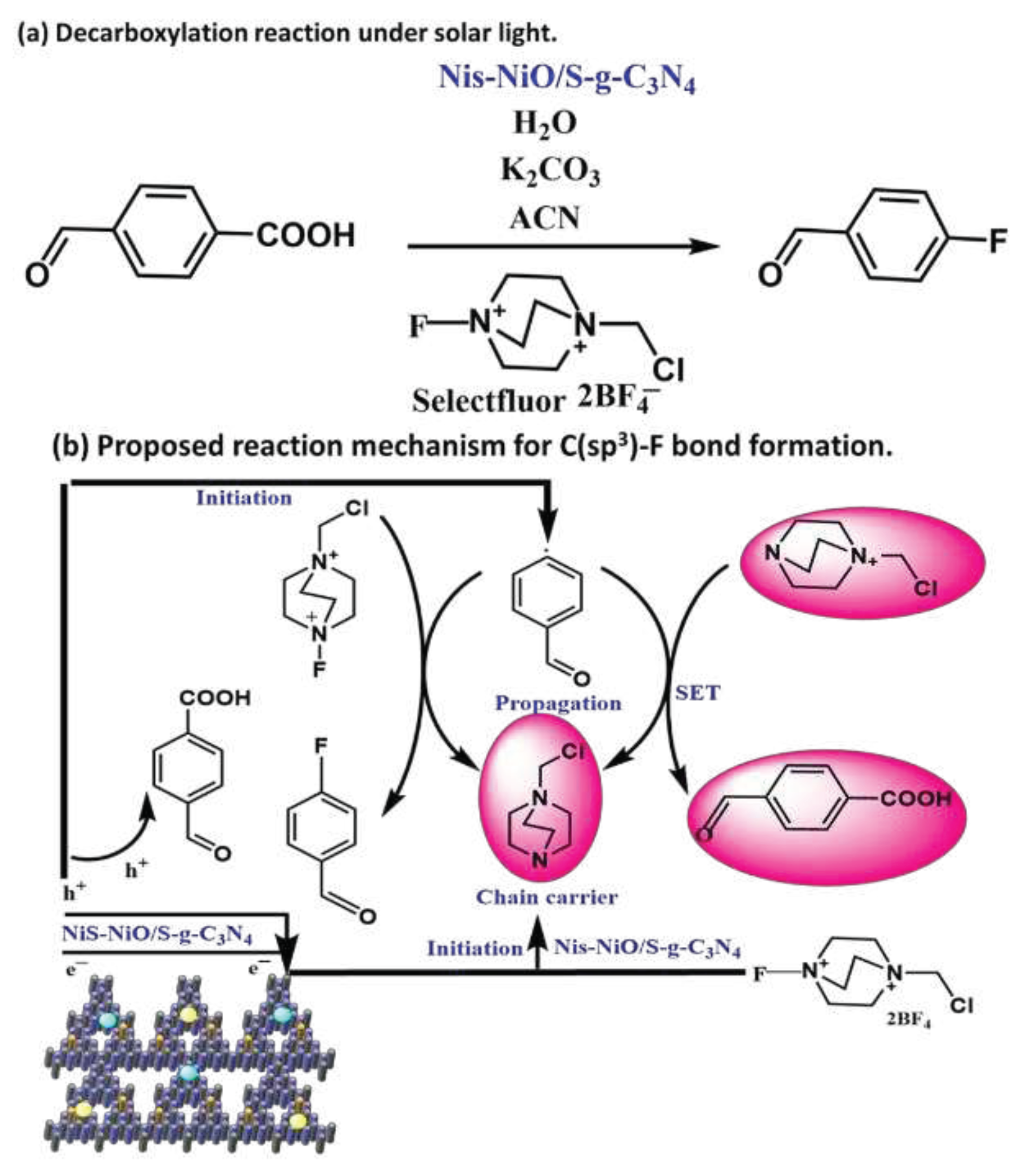
4.4. Photocatalytic Generation of C(sp3)-F Bond
4.5. Activation of C(sp3)-F Bond via NiS-NiO/S-g-C3N4 Photocatalyst: Its Mechanism
5. Conclusions
Acknowledgements
References
- M. S. Dresselhaus,I. L. Thomas, Alternative energy technologies. Nature,414, (2001), 332−337. [CrossRef]
- L. Zhang,Y. Zhu, A review of controllable synthesis and enhancement of performances of bismuth tungstate visible-lightdriven photocatalysts. Catal. Sci. Technol., 2, (2012),694−706. [CrossRef]
- T. Le Lee, V.-D. Lim, D.; Y.-C. Lin, G. M Morris,A. L. Wong, A. J Olson,J. H. Elder, C.-H. Wong, Development of a New Type of Protease Inhibitors, Efficacious against FIV and HIV Variants. J. Am. Chem. Soc.,121, (1999), 1145−1155. [CrossRef]
- C.-Y. Su, H. Li, Cheng, Z.-Q W. Liu,Li, Hou, Z. F.- Q. Bai, H.-X. Zhang, T.-Y. Ma, ZincAir Batteries: Atomic Modulation of FeCo−Nitrogen−Carbon Bifunctional Oxygen Electrodes for Rechargeable and Flexible All-Solid-State Zinc−Air Battery. Adv. Energy Mater., 7,(2017) , 13. [CrossRef]
- D. Ravelli, S. Protti, M. Fagnoni, Application of Visible and Solar Light in Organic Synthesis. Applied Photochemistry, (2016), 281–342. [CrossRef]
- S. Protti, M. Fagnoni, The sunny side of chemistry: green synthesis by solar light. Photochemical & Photobiological Sciences, 11, (2009), 1499. [CrossRef]
- Gupta, J. R. Saurav, S. Bhattacharya, Solar light-based degradation of organic pollutants using ZnOnanobrushes for water filtration, RSC Advances, 87, (2015), 71472–71481. [CrossRef]
- J. Kim, S. H. Lee, F. Tieves, D. S. Choi, F.Hollmann, C. Paul, C. B. Park,. Biocatalytic C=C Bond Reduction through Carbon Nanodot-Sensitized Regeneration of NADH Analogues.Angew. Chem. Int. Ed. (2018). [CrossRef]
- S. H. Lee, D. H. Nam, J. H. Kim, J.-O.Baeg, C. B. Park, Eosin Y-Sensitized Artificial Photosynthesis by Highly Efficient Visible-Light-Driven Regeneration of Nicotinamide Cofactor. ChemBioChem, 10, (2009), 1621–1624. [CrossRef]
- S. H. Lee, J. H. Kim, C. B. Park, Coupling Photocatalysis and Redox Biocatalysis Toward Biocatalyzed Artificial Photosynthesis. Chemistry - A European Journal, 19, (2013), 4392–4406. [CrossRef]
- D. H. Nam, S. H. Lee, C. B. Park, CdTe, CdSe, and CdS Nanocrystals for Highly Efficient Regeneration of Nicotinamide Cofactor Under Visible Light. Small, 6,(2010), 922–926. [CrossRef]
- D. H. Nam, C. B. Park, Visible Light-Driven NADH Regeneration Sensitized by Proflavine for Biocatalysis. ChemBioChem, 13, (2012), 1278–1282. [CrossRef]
- M. Rueda-Becerril, O.Mahé, M. Drouin, M. B. Majewski, J. G. West, M. O. Wolf, … J.-F. Paquin, Direct C–F Bond Formation Using Photoredox Catalysis. Journal of the American Chemical Society, 136, (2014), 2637–2641. [CrossRef]
- T. Koike, M. Akita, Photoredox Catalysis in Fluorination and Trifluoromethylation Reactions. Organofluorine Chemistry, (2021), 225–240. [CrossRef]
- M. O. Zubkov, M. Kosobokov, V. V. Levin, V. Kokorekin, A. Korlyukov, J. Hu, A. Dilman, Novel photoredox-active group for the generation of fluorinated radicals from difluorostyrenes. Chemical Science. Chem. Sci., ,11, (2020), 737-741. [CrossRef]
- G. Tarantino, C. Hammond, Catalytic formation of C(sp3)-F bonds via heterogeneous photocatalysis. ACS Catalysis. ACS Catal. 8, (2018), 10321–10330. [CrossRef]
- W. Xu, Q. Zhang, Q. Shao, C. Xia, M. Wu,Photocatalytic C−F Bond Activation of Fluoroarenes, gem -Difluoroalkenes and Trifluoromethylarenes. Asian Journal of Organic Chemistry. 10, (2021), 2454-2471. [CrossRef]
- Q. Guo, Y.Zhang, J. Qiu, G. Dong, Engineering the electronic structure and optical properties of g-C3N4 by non-metal ion doping. Journal of Materials Chemistry C, 4, (2016), 6839–6847. [CrossRef]
- Y. Yuan, L. Zhang, J. Xing, M. I. B. Utama, X. Lu, K. Du, … Q. Xiong, High-yield synthesis and optical properties of g-C3N4. Nanoscale, 7,(2015), 12343–12350. [CrossRef]
- Y.Zhang, S. Zong, C. Cheng, J. Shi, P. Guo, X.Guan, … L.Guo, Rapid high-temperature treatment on graphitic carbon nitride for excellent photocatalytic H 2 -evolution performance. Applied Catalysis B: Environmental, 233, (2018)80–87. [CrossRef]
- J. Zhang, L. Dai, Heteroatom-Doped Graphitic Carbon Catalysts for Efficient Electrocatalysis of Oxygen Reduction Reaction. ACS Catalysis, 5, (2015), 7244–7253. [CrossRef]
- M. Yourdkhani, F. Nemati, Y. Rangraz, A. Elhampour, Magnetic selenium-doped graphitic carbon nitride nanocomposite as an effective catalyst support for stabilization of Cu NPs. Diamond and Related Materials, 110, (2020) 108136. [CrossRef]
- Z. Li, G. Gu, S. Hu, X. Zou, G. Wu, Promotion of activation ability of N vacancies to N2 molecules on sulfur-doped graphitic carbon nitride with outstanding photocatalytic nitrogen fixation ability. Chinese Journal of Catalysis, 40, (2019), 1178–1186. [CrossRef]
- Y. Feng, M. Xu, P.-L. Tremblay, T. Zhang, The one-pot synthesis of a ZnSe/ZnS photocatalyst for H2 evolution and microbial bioproduction. International Journal of Hydrogen Energy, 46, (2021), 21901–21911. [CrossRef]
- S. Yu, X.-B. Fan, X. Wang, J. Li, Q. Zhang,A. Xia, …G. R. Patzke,. Efficient photocatalytic hydrogen evolution with ligand engineered all-inorganic InP and InP/ZnS colloidal quantum dots. Nature Communications, 9, (2018). [CrossRef]
- S. P. Lonkar, V. V. Pillai, S. M. Alhassan, Facile and scalable production of heterostructured ZnS-ZnO/Graphene nano-photocatalysts for environmental remediation. Scientific Reports, 8, (2018). [CrossRef]
- X. Hong, X. Wang, Y. Li, J. Fu, B.Liang, Progress in Graphene/Metal Oxide Composite Photocatalysts for Degradation of Organic Pollutants. Catalysts, 10, (2020), 921. [CrossRef]
- S.K. Gupta, A. K. Gupta, R. K. Yadav, A. Singh, B. C. Yadav, highly efficient in-situ sulfur doped graphitic carbon nitride nanoplates as an artificial photosynthetic system for NADH regeneration, Vietnam J. Chem., 59, (2021), 590-598. [CrossRef]
- S. Chaubey, C. Singh, P. Singh, A. Kumar, P. P. Pande, J.-O. Baeg, … R. K. Yadav, Efficient photocatalytic synthesis of l-glutamate using a self-assembled carbon nitride/sulfur/porphyrin catalyst. Environmental Chemistry Letters, 18, (2020), 1389–1395. [CrossRef]
- S. Singh, R. K. YadavT. W. Kim, C. Singh, P. Singh, A. P. Singh, A. K. Singh, A. K. Singh, J.-O. Baeg,Rational design of a graphitic carbon nitride catalytic–biocatalytic system as a photocatalytic platform for solar fine chemical production from CO2,React. Chem. Eng., 2022,7, 1566-1572. [CrossRef]
- J. He, C. Janáky, Recent Advances in Solar-driven Carbon Dioxide Conversion: Expectations vs. Reality. ACS Energy Letters.ACS Energy Lett., 5, (2020), 1996–2014. [CrossRef]
- R. You, H.Dou, L. Chen, S. Zheng, Y.Zhang Graphitic Carbon Nitride with S And O Codoping for Enhanced Visible Light Photocatalytic Performance, RSC Adv., 7, (2017), 15842-15850. [CrossRef]
- S. Chaubey,R. K., Yadav T. W. Kim, T. C. Yadav,A. Kumar, D.K., Dwivedi,B. K. Pandey, A.P. Singh Fabrication of Graphitic Carbon Nitride-Based Film: An Emerged Highly Efficient Catalyst for Direct C-H Arylation under Solar Light, Chinese J. Chem., 39, (2020),633-639. [CrossRef]
- Y. Li, Z. Jin, L.Zhang, K. Fan Controllable Design of Zn-Ni-P on g-C3N4 for Efficient Photocatalytic Hydrogen Production, Chinese J. Catal., 40, (2019), 390-402. [CrossRef]
- P. Singh, R. K. Yadav, K. Kumar, Y. Lee, A. K. Gupta, K. Kumar, … T. W. Kim, Eosin-Y and SulfurCodoped g-C3N4 Composite for Photocatalytic Applications: Regeneration of NADH/NADPH and Oxidation of Sulfide to Sulfoxide. Catalysis Science & Technology., Catal. Sci. Technol., 11, (2021), 6401-6410. [CrossRef]
- G. Liu, X. Qiao, M. A. Gondal, Y. Liu, K.Shen, Q. Xu,. Comparative Study of Pure g-C3N4 and Sulfur-Doped g-C3N4 Catalyst Performance in Photo-Degradation of Persistent Pollutant Under Visible Light. Journal of Nanoscience and Nanotechnology, 18, (2018),4142–4154. [CrossRef]
- M. Kaur, K. Pal, Synthesis, characterization and electrochemical evaluation of hydrogen storage capacity of graphitic carbon nitride and its nanocomposites in an alkaline environment. Journal of Materials Science: Materials in Electronics, 32, (2021), 12475–12489. [CrossRef]
- N. Matthias, F Stefan, B König, K Zeitler, Metal-Free, Cooperative Asymmetric Organophotoredox Catalysis with Visible Light. Angew. Chem. Int. Ed. 50, (2011), 951 –954. [CrossRef]
- C. L. Pitman, O. N. L. Finster, A. J. M. Miller, Cyclopentadiene-Mediated Hydride Transfer from Rhodium Complexes. Chem. Commun. 52,(2016), 9105–9108. [CrossRef]
- K. Burton, T. H. Wilson, The Free-Energy Changes for the Reduction of Diphosphopyridine Nucleotide and the Dehydrogenation of L-Malate and L-Glycerol 1-Phosphate. Biochem. J. 54, (1953), 86-94. [CrossRef]
- Y. Wu, J. Ward-Bond, D. Li, S.Zhang, J.Shi, Z.Jiang, g-C3N4@α-Fe2O3/C Photocatalysts: Synergistically Intensified Charge Generation and Charge Transfer for NADH Regeneration. ACS Catalysis, 8, (2018), 5664–5674. [CrossRef]
- S. K. Choi, H. S. Yang, J. H. Kim, H. Park, Organic Dye-Sensitized TiO2 as a Versatile Photocatalyst for Solar Hydrogen and Environmental Remediation. Appl. Catal. B Environ. 121, (2012), 206-213. [CrossRef]
- F. Hollmann, B. Witholt, A. Schmid, [Cp∗Rh(bpy)(H2O)]2+: A Versatile Tool for Efficient and Non-Enzymatic Regeneration of Nicotinamide and Flavin Coenzymes. J. Mole. Catal. B Enzym., 19, (2002), 167-176. [CrossRef]
- R. K. Yadav, J.-O. Baeg, G. H. Oh, N.-J. Park, K. Kong, J. Kim, … S. Biswas, K. A Photocatalyst–Enzyme Coupled Artificial Photosynthesis System for Solar Energy in Production of Formic Acid from CO2. Journal of the American Chemical Society, 134, (2012), 11455–11461. [CrossRef]
- R. K. Yadav, G. H. Oh, N.-J. Park, A. Kumar, K. Kong, J.-O. Baeg, Highly Selective Solar-Driven Methanol from CO2 by a Photocatalyst/Biocatalyst Integrated System. Journal of the American Chemical Society, 136, (2014), 16728–16731. [CrossRef]
- R. K. Yadav, A. Kumar, D. Yadav, N.-J. Park, J. Y. Kim, J.-O. Baeg, In Situ Prepared Flexible 3D Polymer Film Photocatalyst for Highly Selective Solar Fuel Production from CO2. ChemCatChem, 10, (2018). 2024–2029. [CrossRef]
- L. E. Oi, M.-Y. Choo, H. V. Lee, H. C. Hamid Ong, S. B. A.,J. C. Juan, Recent advances of titanium dioxide (TiO2) for green organic synthesis. RSC Adv., 6, (2016), 108741−108754. [CrossRef]
- H. Cheng, W. Xu, Recent advances on modified-TiO2 in photo-induced organic synthesis. Org. Biomol. Chem. 17, (2019), 9977−9989. [CrossRef]
- L. Ravichandran, K. Selvam, M. Muruganandham, M. Swaminathan, Photocatalytic cleavage of C–F bond in pentafluorobenzoic acid with titanium dioxide- P25. Journal of Fluorine Chemistry, 127, (2006), 1204–1210. [CrossRef]
- L. Hong, X.Penglogo, L.Nie, L. Zhou, M. Yang, F. Li, J. Hub, Z. Yao Liangxian Liu, Graphene oxide-catalyzed trifluoromethylation of alkynes with quinoxalinones and Langlois' reagent, RSC Adv., 11, (2021), 38667-38673. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




