1. Introduction
When describing the conductivity of a suspension of various stabilized solutions, in particular, when studying transport phenomena in dilute colloidal electrolytes containing magnetic nanoparticles, Maxwell’s theory [
1,
2,
3], which has long become a classic, is successfully used. The formula proposed by the latter for the conductivity of an inhomogeneous medium, consisting of a matrix with a conductivity
, containing randomly located spherical inclusions with a conductivity of
, has the form:
where
is the relative volume occupied by the inclusions. In the case of inclusions which conductivity exceeds that one of the solvent one can easily see that the Maxwell’s formula demonstrates the linear growth of effective conductivity
in the region of weak concentrations (
) with its saturation as
grows in case
.
Application of the Maxwell’s formula to dilute colloidal electrolytes requires caution. Being immersed (or synthesized within) in electrolyte solution, the colloidal particles of the bare radius
, acquire surface ions (e.g., hydroxyl groups, citrate, etc. [
4,
5,
6] resulting in a very large structural charge eZ
[
7]. Its sign can be both positive and negative, depending on the surface group type. The latter in return, attracts counterions from the surrounding solvent creating an electrostatic screening coat of the size of the Debye length
with an effective charge
. In these conditions, nano-particles approaching between them to the distances
begin to repel each other without floculation [
8,
9,
10]. The region of an essential interaction between them corresponds to the condition
where
is the density of colloidal particles and superscript
c indicates its critical value. In order for the electrolyte to remain homogeneous, it is necessary to avoid overlapping of the screening shells surrounding the core of the colloidal particles, that is, to limit ourselves to concentrations that satisfy the condition of applicability of the theory of Deryagin-Landau-Verwey-Overbeek [
8,
9,
10]:
When Eq. (
1) is applied to a colloidal suspension, the dimensionless parameter of the theory
has to be identified namely with this quantity:
and, hence, only the linear regime in the concentration dependence of conductivity turns out to be available.
The characteristic features of the experimental works [
11,
12,
13,
14], where the Maxwell theory was used for description of the obtained results, are the following:
A. The validity of Eq. (1) implicitly requires the uniformity (lack of dispersion) of individual characteristics of inclusions. The methods of preparation of dilute colloidal electrolytes containing maghemite nanoparticles in Ref.[
11,
12,
13,
14] allow to monitor the quality of this component with good accuracy and to control the fulfillment of the requirement
. This is a qualitative difference of the studies [
11,
12,
13,
14] from the most of transport experiments with the suspensions prepared mechanically (see, for example, reviews [
15,
16]).
B. In all experiments of Ref. [
11,
12,
13,
14] the linear change of conductivity versus relative volume occupied by the inclusions is observed in the region
. This characteristic feature gives grounds to use the Maxwell theory in interpretation of the transport properties of various suspensions (see Refs. [
15,
16]). Yet, it is necessary to attract attention to the fact, that in the most of such successful linear fittings of the observed
the slope of this dependence dramatically exceeds that one allowed by Maxwell theory. Indeed, the maximal slope
of the linear approximation of Eq. (1) is reached when
and is equal to
while in the measurements of different years for mechanically prepared suspensions it exceeds this theoretical limit by three orders: for example, in suspension aluminum/water this value reaches
[
17]. This is an overwhelming inconsistency indicates the inapplicability of Maxwell’s formalism to explain the citing experiment.
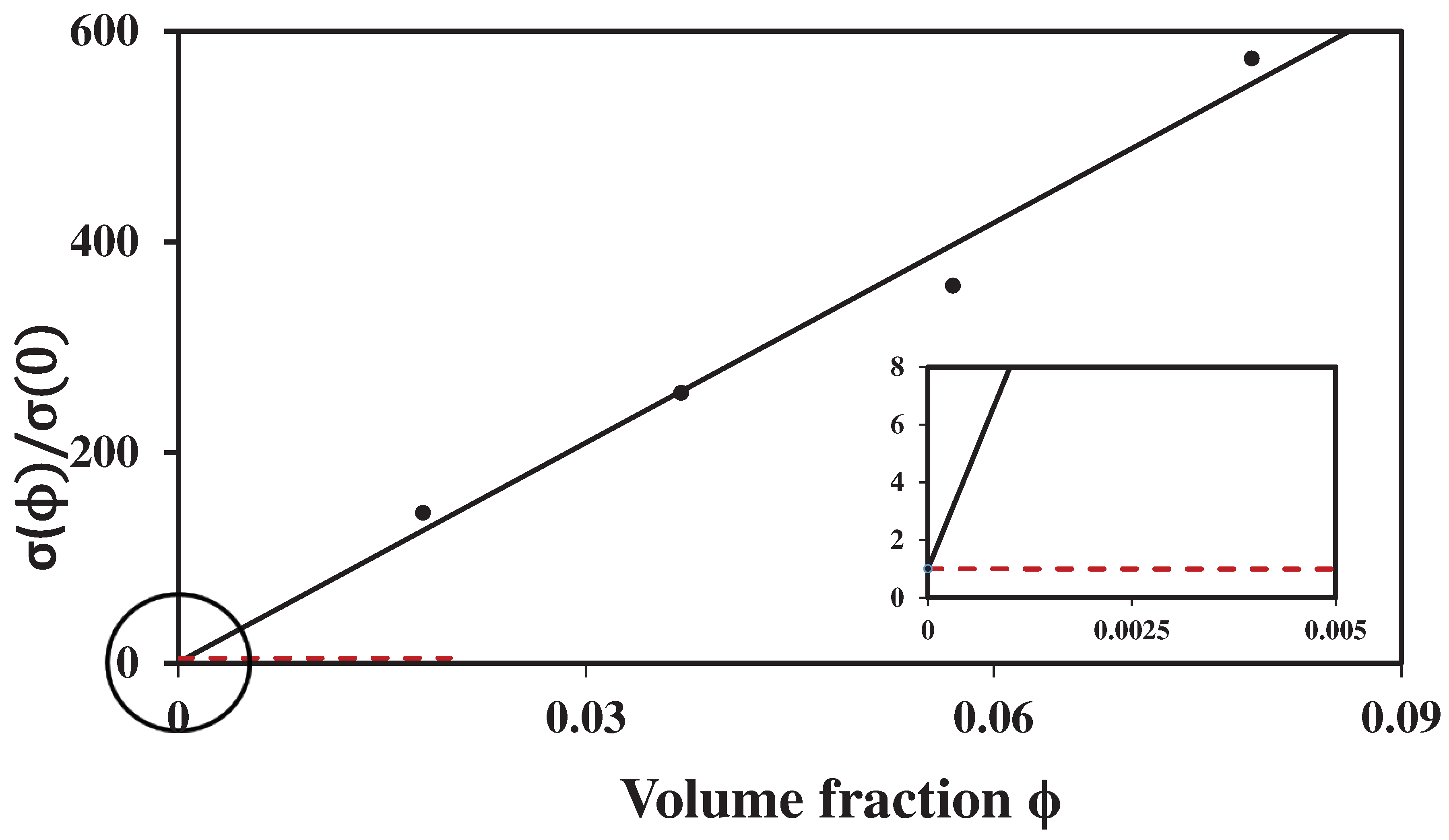
The lack of consistency is deepened also by the available data for obviously dielectric suspensions (see Ref. [
18]). Namely, the conductivity
of coats in
nanoparticles (fumed silica) in ethylene glycol is less than the conductivity of the alcohol (
nanoparticles are good dielectrics). As a consequence, the expected effect of such a powder inclusions on the conductivity of ethylene, where it is added, should be negative. Yet, as we see, the corresponding effect is positive (see
Figure 1).
A similar situation takes place in suspensions containing the nanotubes. Namely, the aqueous suspension of nanotubes [
19] demonstrates an increase in the conductivity of the suspension, although carbon nanotubes are wide-gap semiconductors. At room temperature, their conductive properties are similar to those of classical dielectrics, hence, the influence of such additives on the effective conductivity of the suspension should be negative.
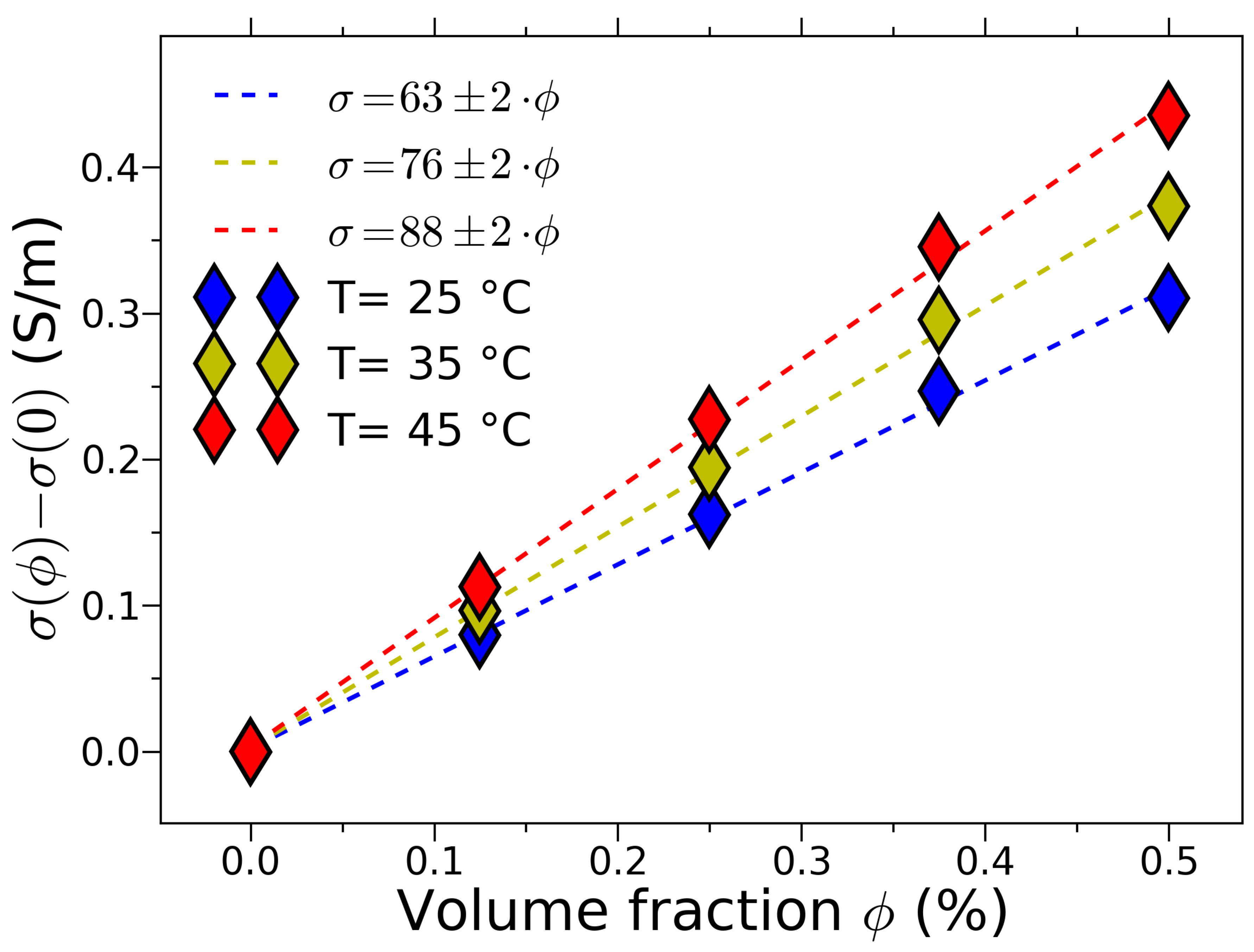
Fortunately, within the low concentration limit, the Maxwell’s formalism turns out to be suitable for description of the properties of dilute solutions containing the maghemite nanoparticles (see [
11,
12,
13,
20] and
Figure 2). The reasons for such discrepancy in the interpretation of the data from Ref. [
11,
12,
13,
20] will be discussed below (see Section II).
In this relation let’s stress the striking difference in conductivity values
in experiments with mechanical suspensions [
17,
18] and electrolytes containing magnetic nanoparticles [
13]. Thus, in the paper [
18] conductivity
, while in the case of electrolytes [
13] this value turns out to be four orders greater:
.
C. An important “mark of distinction” which illustrates the applicability of Maxwell theory to suspension of stabilized solutions is the non-linearity of
in the region
. According to Refs. [
1,
2,
3], the behavior of
in this region loses its linearity, demonstrating the systematic deviation of the observed values of conductivity above the linear increase. At the same time the data of Ref. [
11] demonstrates the reverse trend in this region: the conductivity at high enough concentrations
deviates below the linear asymptotic. The reasons of this discrepancy require special attention and will be discussed below (see Section II).
D. Ionic liquids. The paper [
14] reported the structural and transport properties of the dilute solutions of maghemite nanoparticles in ionic liquids
Ethylammonium nitrate. To elucidate the role of the solvent (water or ionic liquid) in the properties of dilute electrolytes, it is useful to compare what happens with maghemite nanoparticles in ionic liquids and aqueous stabilizing electrolytes. The details of corresponding discussion are presented in Section III.
2. ac Impedance Diagnostics
Impedance ac (alternative current) diagnostics is regularly involved in the study of transport phenomena in conductive media. A common reason for resorting to ac-complications against the background of relatively simple methodological possibilities in the dc-mode (direct current mode) is the desire to exclude the influence on the IVC (current-volt characteristic) of contact phenomena accompanying dc measurements. In some cases (2d - electrons over helium), dc transport measurements are impossible by definition. In lightly doped semiconductors (dilute electrolytes), the situation is less critical, but problems with contact resistance persist (see [
21,
22]).
In the simplest case, impedance diagnostics deals with an RC circuit that obeys the equation [
23]
where
is the value of the charge at the electrodes at the moment
t and
is the ac-voltage applied to them. Other parameters: the resistance of the electrolyte filling the volume between the electrodes of a flat capacitor
, the distance between them
L, the conductivity of the electrolyte
, the area of the electrodes
S, the capacitance of a flat capacitor composed of plates
C are time independent. Usually in the electrical engineering the resistor and the capacitor are considered as two separate elements of the circuit. Let us stress, that in the problem under consideration both these values characterize the same cuvette. The specifics of its capacitance
C is the essence of the present analysis and it will be discussed in details below.
Corresponding current
generated in the cell is equal to
The lock in-tester is capable to measure simultaneously both real and imaginary parts of the impedance. In result it provides information about the phase shift
between the current
and the voltage
Measuring the dependence one can extract the value of the product . Assuming the capacitance C to be known, one determines the resistance R.
In “real” liquid electrolytes and in charged colloidal solutions, however, the measured complex impedance contains contributions such as the leakage resistance, slow diffusions (related to the micro-structure of the electrodes, and/or large ions and particles) in addition to the constant phase element, which refers to the ion motions within the Debye length (electronic double layer) at the electrode/liquid interface. Consequently, Eq. (6) cannot fully describe such multi-electrochemical phenomena, and it is customary to use more phenomenological models such as the Havriiliak-Negami model [
24].
When the measurements are performed at so high frequencies that the phase shift becomes negligible, one can use an alternative way of the
measurement. Namely, let us follow the method of complex impedance and formally assume that the complex harmonic signal
is applied to the cuvette filled by electrolyte. Looking at Eq. (5) with such “driving force” in right-hand-side one can easily see that after some relaxation period the stationary oscillations of the“complex charge” (which can be considered as the integral of complex current)
will set in the system. The amplitude
itself is the complex function of frequency and properties of the circuit, reflecting the existence of the retard of the charge oscillations at the capacitor plates with respect to the voltage applied. The corresponding imaginary part
with
as the characterizing the inertial properties of the circuit time constant, demonstrates the maximum at the frequency
.
Here it is necessary to recall that we are interested in the dependence of the electrolyte resistance in function of the nanoparticles concentration in it. Hence, to be consistent in use of Eq. (7), one has to take into account also the effect of nanoparticles presence on the capacity of the cuvette. When the role of capacitor plays the cell filled by pure water, it acquires well known in radio engineering literature specifics. In this case the electric field penetrates between plates only at the distances of the order of the Debye length
, which is much less than the distance between electrodes
L (
):
Hence, the effective capacity turns out to be much larger than expected .
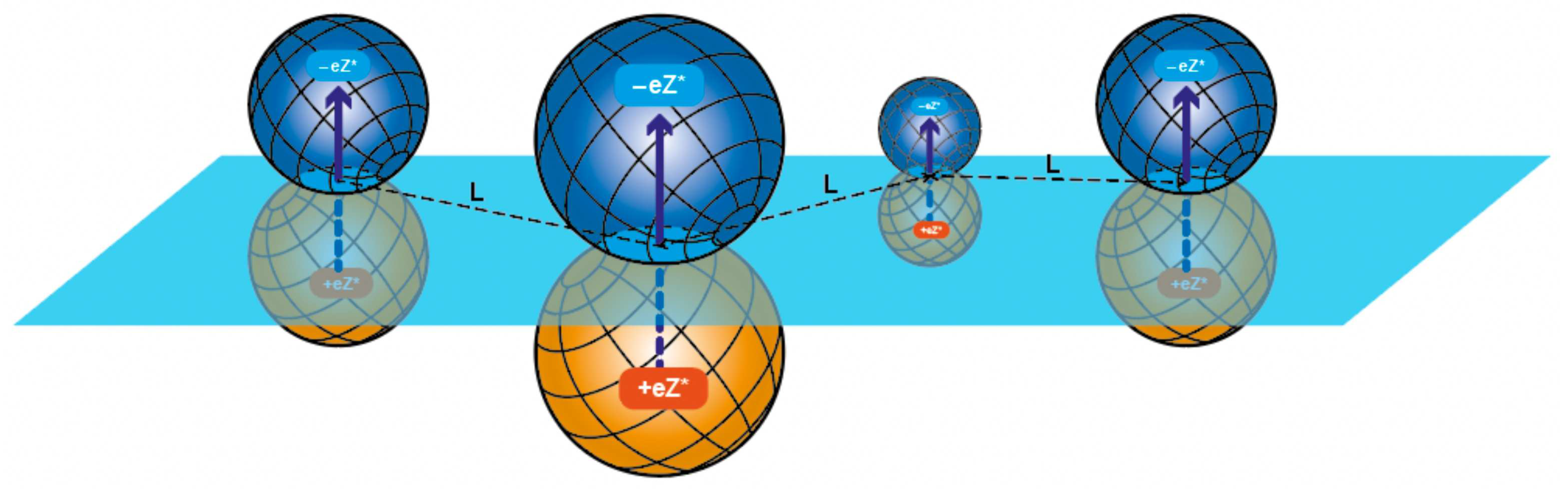
Turning to a well conducting suspension, i.e. adding DLVO type inclusions of the radius
to water (
), one creates the ground for intensive adsorption of colloids at the metal-electrolyte interface (this effect was studied in details in Ref. [
25]). Indeed, as colloidal particles concentration in the bulk of electrolyte increases, grows also their number in the vicinity of the electrode, grows the number of colloidal particles mirror images behind the metallic interface. More and more dense dipole gas of colloidal particles with corresponding images in the vicinity of electrode is formed (see
Figure 3).
The described process [
25]) is nothing but the adsorption of such colloidal particles, which can dramatically change the value of cell capacitance. In fact, the presence of each adsorbed colloid results in cutting of a “hole” of area
at the metal boundary (see
Figure 3, where such areas are shown as light circles in the contact zone of each colloid with its mirror image).
In result, the value of capacitance
C of the cell filled by water containing the DLVO inclusions considerably reduces with respect to that one of the cell filled by pure water:
where
is the density of adsorbed colloids. In Ref. [
25] was demonstrated that the latter is exponentially related to the volume one
by means of the colloid’s binding energy
:
where
Let us see how the discussed above peculiarities of the colloidal solution affect the procedure of measuring its conductivity
The area S does not enter more in this expression, but the cell length L remains, since the electrolytic capacity does not depend on it.
The idea of the above story is obvious. A standard instrument for measuring the conductivity of electrolytes, is tuned to the specifics of contactless measurements of the ac-conductivity of charged liquids. The algorithm for processing of the experimental information of this device does not contain information concerning the ability of the capacitor in the RC - chain to abrupt changes when the density of the suspension varies in the region of its small values. As a consequence, the measured phase shift is controlled by the product , where the suspension’s conductivity is determined by the Maxwell relation (1).
As wee have demonstrated above the screening length (see Eq. (11)) starts its exponential growth already at so small volume fractions as . Since the values and enter in Eq. (10) in the form of product, one, analyzing the experimental data, can formally attribute the exponential growth of the thickness of accumulation layer to that one of conductivity.
3. The applicability of the Maxwell model for ionic liquid dilute suspension
The compressibility and conductivity of colloidal solutions in ionic liquids was studied in Ref. [
14] and compared with the analogous characteristics of aqueous solutions of the same colloids (see also Refs. [
11,
12,
13,
26]).
The obtained in Refs. [
13,
26] results for compressibility suggest that colloids in dilute ionic liquids behave similarly to
-clusters in aqueous solutions. What concerns the behavior of conductivity
of dilute aqueous solutions [
13] and that one of ionic liquids
[
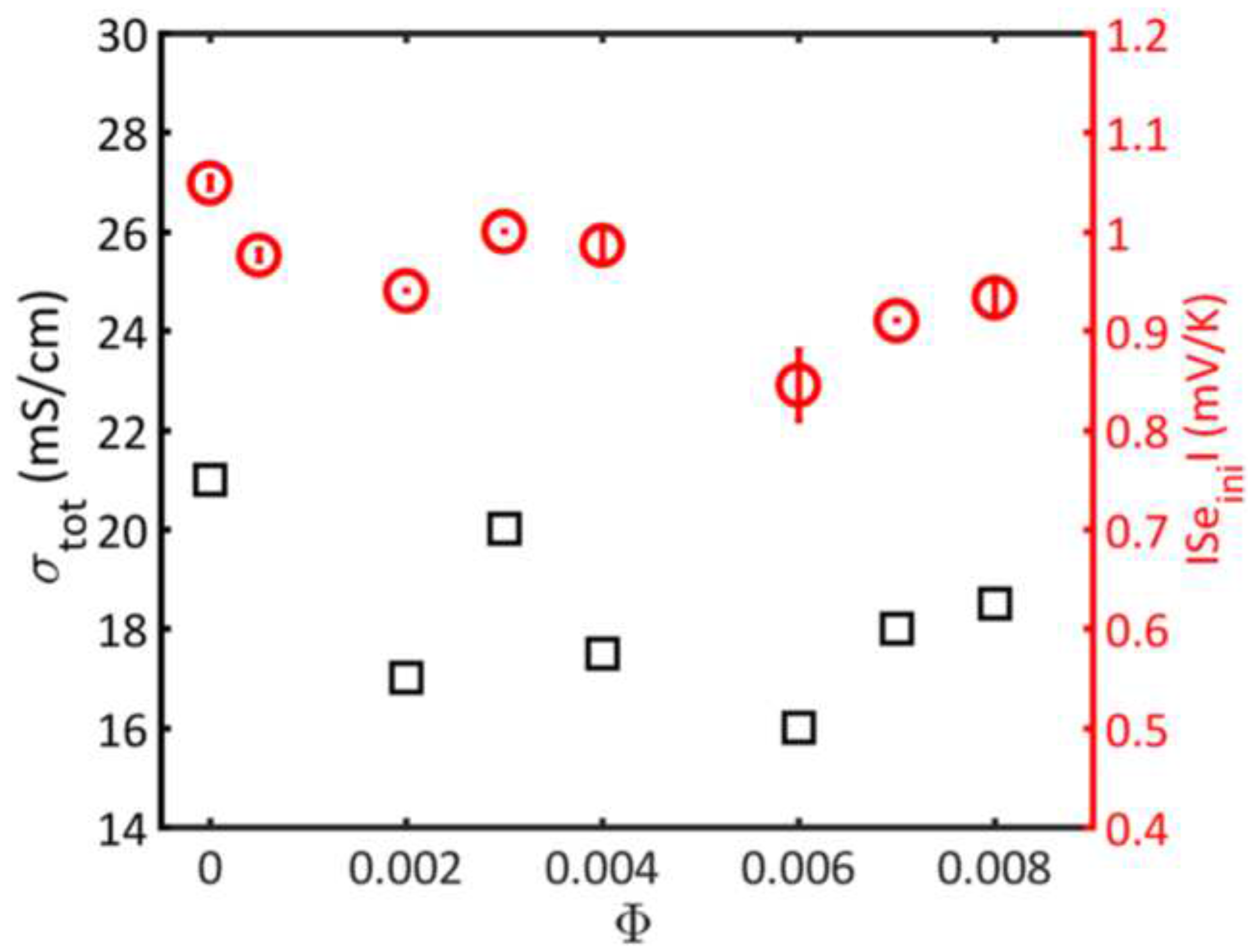
14] one can easily notice qualitative differences. The former shows a well-defined linear increase versus colloidal concentration (see
Figure 2), what corresponds to predictions of the Maxwell model (see Eq. (1)), while the latter remains unchanged in limits of the measurements accuracy(see
Figure 4).
To recognize the source of this discrepancy let us recall that the conductivity measurements of ionic liquid doped by magnetic colloids were performed by means of the ac-impedance diagnostics. The measured phase shift (see Eq. (6)) contains the product of two
-dependent characteristics of the electrolyte:
. While
with the growth of colloidal concentration increases ([
14]), the cell capacity
decreases and the resulting slope
may well be close to zero. Moreover, there are examples when the slope
even in aqueous solutions of magnetic colloids( see
Figure 4a from [
12]). The consistent interpretation of the discussed discrepancy requires some additional information regarding the capacitive properties of the cell used at the metal-ion liquid interfaces. However, the observed difference in the data of
Figure 2 and
Figure 4 does not give grounds to refuse from use of the Maxwell model in interpretation of the properties of the ionic liquids.
Among bunch of colloidal solutions conductivity measurements the data of Ref. [
14] take quite a worthy place, supplementing the existing variants of experiments with the actual scenario with ionic liquid as a conductive basis (along with pure water, its diluted solutions (acidic or alkaline), various alcohols, etc.)
It is useful to note that the data on the initial stage of the Seebeck effect for massive colloidal particles in an ionic liquid present in
Figure 4, satisfactorily correlate with the behavior of conductivity
under the same conditions. This fact, noted in Ref. [
27], confirms the general consistency of the proposed interpretation of the details of
- transport in colloidal media based on the Maxwell model. The mechanisms of conductivity involving the Brownian motion, or aggregation phenomena are less clear, especially in the linear
domain.