Submitted:
12 July 2023
Posted:
13 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Chemical characteristics of the study soils
| Parameters / Locality |
C/N | M.O .% |
pHeau (1/2,5) |
pHKCl (1/2,5) |
P ass (mg/Kg) |
Ca éch .méq/100g |
Mg méq/100g |
K éch.méq/100g |
Na éch.méq/100g |
Sum of cations méq/100g |
CEC | %V=S/T *100 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ouénou | 15,5 | 1,5 | 6,6 | 6,3 | 6,2 | 4,3 | 0,7 | 0,2 | 0,4 | 5,6 | 6,5 | 84,3 |
| Bagou | 13,4 | 1,3 | 6,4 | 5,9 | 7,7 | 5,5 | 1,0 | 0,2 | 0,4 | 7,1 | 10,3 | 71,1 |
| Kokey | 12,0 | 1,2 | 6,7 | 6,2 | 4,8 | 4,0 | 0,9 | 0,4 | 0,3 | 5,6 | 7,8 | 72,8 |
2.2. Effect of CMA-based biostimulant on growth parameters
2.2.1. Height of maize plants
| Area | Treatment | Height of the plants | Plant diameter | Leaf area of the plants | |||
| Mean | SE | Mean | SE | Mean | SE | ||
| Bagou | T0 | 156.01b | 1.73 | 1.48c | 0.04 | 1279.60 | 29.78 |
| T1 | 190.77d | 2.14 | 1.80d | 0.03 | 1659.88 | 32.87 | |
| T2 | 188.62d | 1.99 | 1.91de | 0.04 | 1702.36 | 31.34 | |
| Kokey | T0 | 156.00b | 3.30 | 1.44c | 0.05 | 1339.36 | 29.64 |
| T1 | 203.19e | 2.15 | 2.06ef | 0.02 | 1652.78 | 28.19 | |
| T2 | 212.72e | 1.98 | 2.15f | 0.03 | 1796.85 | 25.38 | |
| Ouénou | T0 | 133.20a | 3.42 | 1.02a | 0.03 | 1329.83 | 36.05 |
| T1 | 165.79bc | 2.21 | 1.23b | 0.03 | 1567.12 | 36.76 | |
| T2 | 169.20c | 2.62 | 1.36bc | 0.04 | 1660.69 | 40.92 | |
| P. Value | 6.289e-06 *** | 7.706e-09 *** | |||||
2.2.2. Neck diameter of maize plants
2.2.3. Leaf area of maize plants
2.2.4. Maize Yield Assessment
| ZONE | TREATMENT | Plant yield (t/ha) | ||
| Average | Standard error | IC-95% | ||
| Bagou | T0 | 1,13 | 0,4 | [0.86 ; 1.40] |
| T1 | 1,59 | 0,41 | [1.32 ; 1.87] | |
| T2 | 1,55 | 0,26 | [1.37 ; 1.72] | |
| Kokey | T0 | 1,08 | 0,36 | [0.85 ; 1.31] |
| T1 | 1,43 | 0,33 | [1.22 ; 1.64] | |
| T2 | 1,44 | 0,4 | [1.19 ; 1.70] | |
| Ouénou | T0 | 1,35 | 0,45 | [1.04 ; 1.65] |
| T1 | 1,94 | 0,35 | [1.71 ; 2.18] | |
| T2 | 1,79 | 0,4 | [1.53 ; 2.06] | |
2.2.5. Nutritional status of the plants
| Area | Treatment | Nitrogen (N) % | Phosphorus (P) % | Potassium (K) % | |||
| Mean | SE | Mean | SE | Mean | SE | ||
| Bagou | T0 | 1,63ab | 0,023 | 1,8bc | 0,026 | 1,71ab | 0,026 |
| T1 | 1,98d | 0,046 | 2,3e | 0,053 | 2,1e | 0,061 | |
| T2 | 1,82c | 0,033 | 1,92cd | 0,038 | 1,88cd | 0,047 | |
| Kokey | T0 | 1,72ac | 0,021 | 1,66ab | 0,024 | 1,7ab | 0,021 |
| T1 | 2d | 0,055 | 2,01d | 0,047 | 1,94d | 0,025 | |
| T2 | 1,81c | 0,032 | 2d | 0,045 | 1,93d | 0,044 | |
| Ouénou | T0 | 1,6a | 0,03 | 1,61a | 0,018 | 1,59a | 0,017 |
| T1 | 1,74ac | 0,014 | 1,98d | 0,045 | 1,83bd | 0,026 | |
| T2 | 1,75bc | 0,017 | 1,81bc | 0,014 | 1,73abc | 0,017 | |
| P. Value | 0,005 ** | 0,0002 *** | 0,023 * | ||||
2.2.6. Evaluation of mycorrhization frequency and intensity
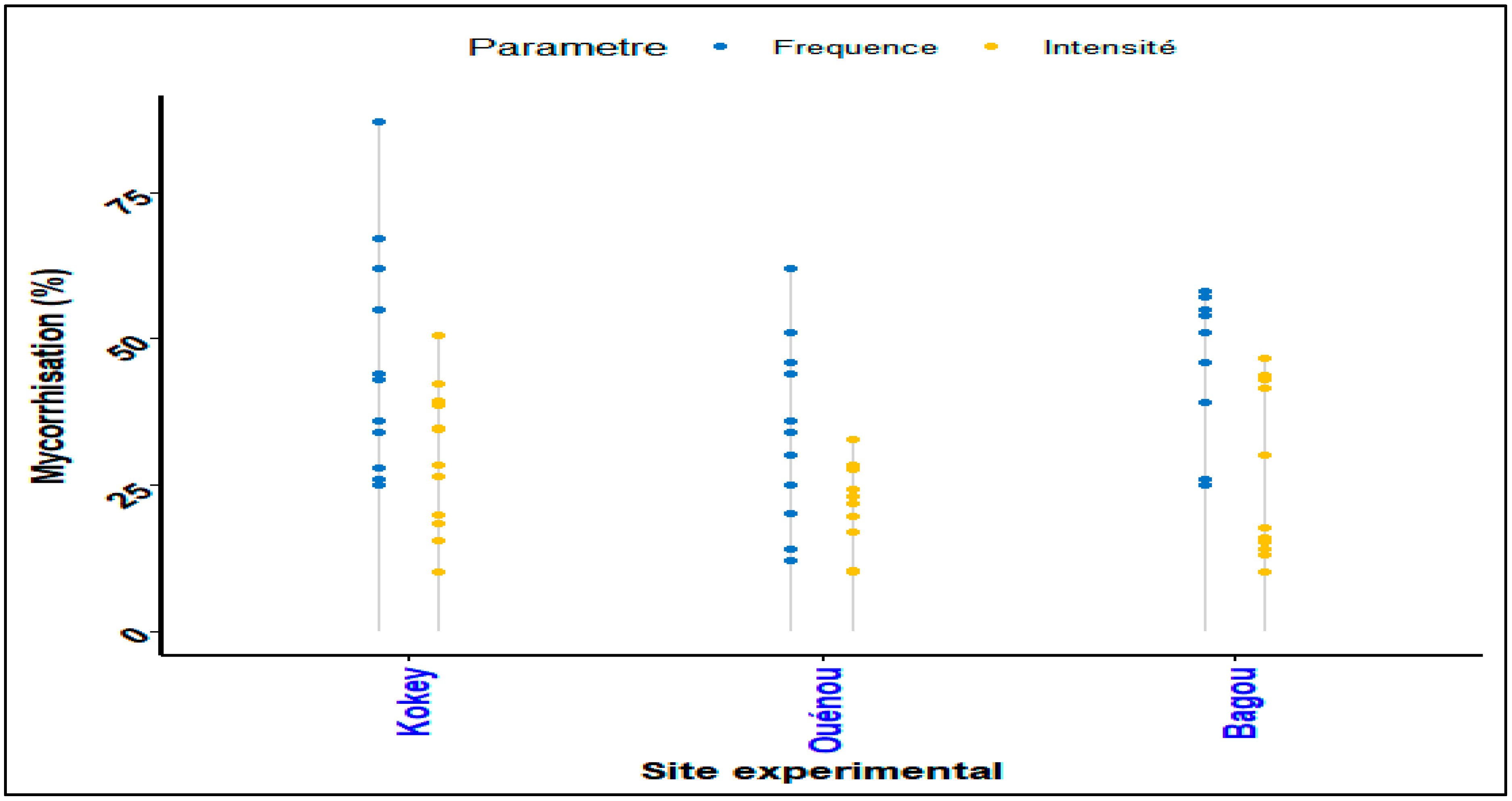
2.2.7. Participatory component analysis
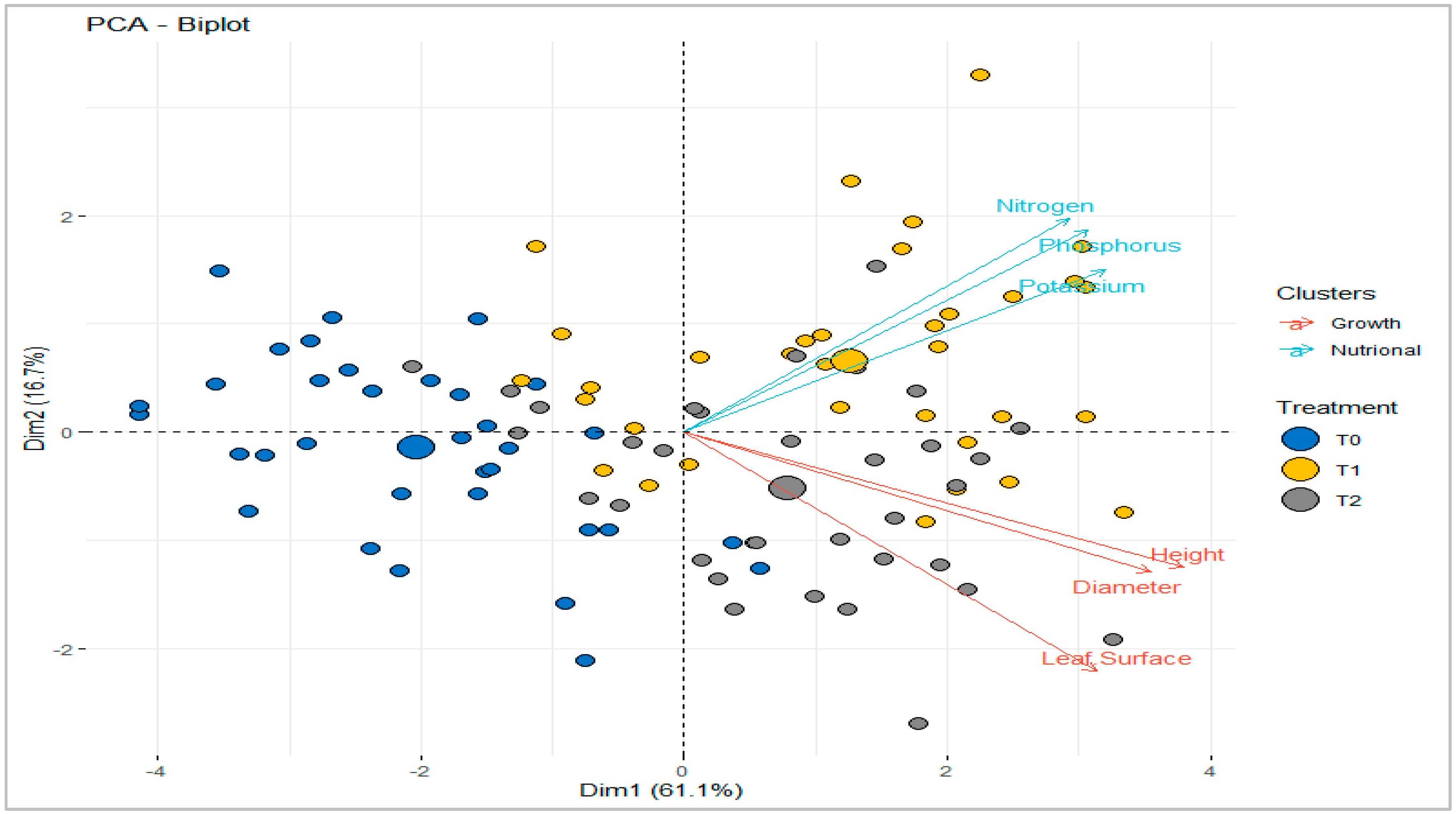
3. Discussion
4. Materials and Methods
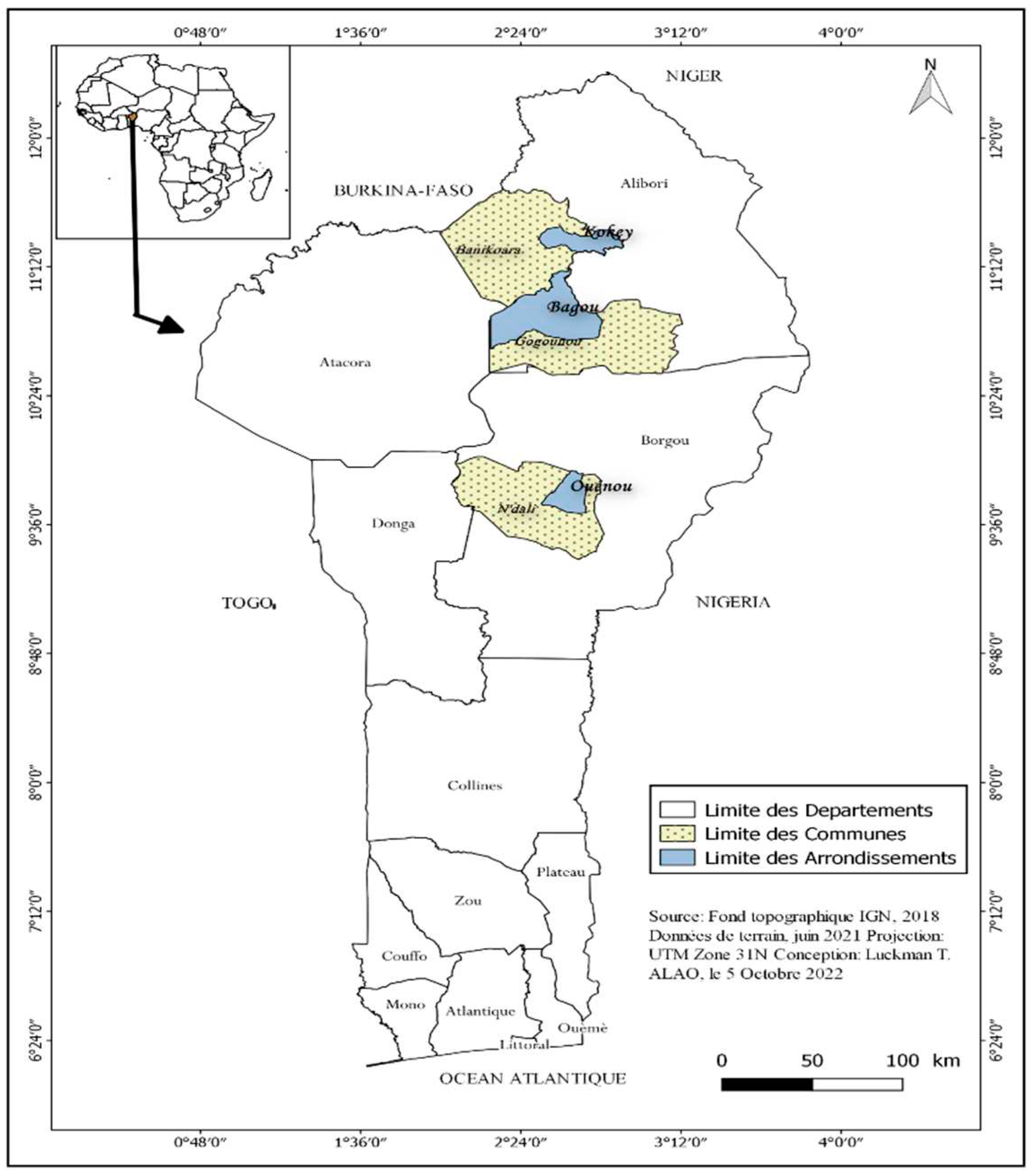
4.1. Experimental site
4.2. Materials
4.3. Methods
4.3.1. Preparation of the inoculum
4.3.2. Experimental set-up
4.3.3. Collection of soil samples
4.3.4. Evaluation of growth parameters
4.3.5. Grain Yield Assessment
4.3.6. Assessment of the nutritional status of corn plants
4.3.7. Determination of mycorrhization frequency and intensity
4.4. Statistical analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ProSOL. Analysis of framework conditions for sustainable soil management in Benin. 2015, p. 106.
- INSAE. Fifth general population and housing census, 2015. Cotonou, Benin.
- SNCA. National Agricultural Council Strategy 2018-2025, pp 15.
- Adjanohoun, A. ; Noumavo, P. A. ; Sikirou, R. ; Allagbe, M. ; Gotoechan-Hodonou, H. ; Dossa, K. K. ; ... Baba-Moussa, L. Effects of PGPR rhizobacteria on maize yield and macroelement content on undegraded ferralitic soil in South Benin. International Journal of Biological and Chemical Sciences. 2012, 6(1), 279-288.
- Amogou, O. ; Dagbénonbakin, G.; Agbodjato, N. A.; Noumavo, P.A.; Salami, H. A.; Salako, V.; Aguégué, M. R.; Assogba A. S.; Koda, A. D.; Adjanohoun, A.; Baba -Moussa, L. Influence of Isolated PGPR Rh izobacteria in Central and Northern Benin on Maize Germination and Greenhouse Growth. American Journal of Plant Sciences. 2018, 9, 2775-2793.
- Directorate of Agricultural Statistics, final figures for the 2021-2022 crop year, May 2022.
- Timothy, G.; Reeves, G. T.; Gordon, R. Producing more with less in practice corn - rice - wheat. A guide to sustainable grain production. Food and Agriculture Organization of the United Nations (FAO). 2016, ISBN 978-92-5-208519-5.
- Di Falco, S.; Véronesie, M.; Yesuf, M. Does climate change adaptation ensure food security? A micro-perspective from Ethiopia. American Journal of Agricultural Economics. 2011, 93, 829846.
- Willy, D. K. ; Holm-Müller, K. Social influence and collective action effects on farm level soil conservation effort in rural Kenya. Ecological Economics. 2013, 90, 94-103.
- ProSOL. Proposals for better integration of agriculture and livestock for sustainable land management in Alibori, Benin. Survey report. 2016, 64p.
- Agbodjato NA, Adoko MY, Babalola OO, Amogou O, Badé FT, Noumavo PA, Adjanohoun A, Baba-Moussa L Efficacy of biostimulants formulated with Pseudomonas putida and clay, peat, clay-peat binders on maize productivity in a farming environment in southern Benin. Frontiers in Sustainable Food Systems. 2021, 5:666718. https://doi.org/10.3389/fsufs.2021.666718. [CrossRef]
- Bindraban, P. S.; Van der Velde, M.; Ye, L.; Van den Berg, M.; Materechera, S.; Kiba, D. I.; Van Lynden, G. Assessing the impact of soil degradation on food production. Current Opinion in Environmental Sustainability. 2012, 4(5), 478-488.https://doi.org/10.1016/j.cosust.2012.09.015. [CrossRef]
- Obalum, S. E.; Buri, M. M.; Nwite, J. C.; Hermansah, Watanabe, Y.; Igwe, C. A.; Wakatsuki, T. Soil degradationinduced decline in productivity of sub-saharan african soils: The prospects of looking downwards the lowlands with the sawah ecotechnology. Applied and Environmental Soil Science. 2012. https://doi.org/10.1155/2012/673926. [CrossRef]
- Gomiero, T. Soil degradation, land scarcity and food security: Reviewing a complex challenge. Sustainability. 2016, 8(3), 1–41. https://doi.org/10.3390/su8030281. [CrossRef]
- Diogo, R. V. C.; Houédégnon, P.; Djédjé, M. Characterization of conventional cotton production and improvement of the ecological sustainability of the production system in northern Benin. Annales de l’Université de Parakou, Série ’Sciences Naturelles et Agronomie. 2018, 8(2), 1-14. ISSN 1840-8494, eISSN 1840-8508.
- Henao, J.; Baanante, C. Agricultural production and soil nutrient extraction in Africa: Implications for resource conservation and policy development. IFDC Tech, Taurus, International Fertilizer Development Center, Muscle Shoals, AL, USA. 2006.
- Igiehon, O. N.; Babalola, O. O. Rhizobium and mycorrhizal fungal species improved soybean yield under drought stress conditions. Current Microbiology. 2021 78(4) 1615-1627. https://doi.org/10.1007/s00284-021-02432-w. [CrossRef]
- Agbodjato, N.A.; Assogba, S. A.; Babalola, O. O.; Koda, A. D.; Aguegue, R. M; Sina, H.; Dagbenonbakin, G.D.; Adjanohoun, A.; Baba-Moussa, L. Formulation of biostimulants based on arbuscular mycorrhizal fungi for maize growth and yield (Research Topic: Soil-Plant-Microbe Interactions: An Innovative Approach Towards Improving Soil Health and Plant Growth. Frontiers in Agronomy. 2022, 4:894489. https://doi.org/10.3389/fagro.2022.894489. [CrossRef]
- Fadiji, A.E.; Babalola, O.O.; Santoyo, G.; Perazzolli, M. The potential role of microbial biostimulants in the amelioration of climate change associated abiotic stresses on crops. Frontiers in Microbiology.2022, 12:829099. https://doi.org/10.3389/fmicb.2021.829099. [CrossRef]
- Aguegue, R. M.; Akpode, C.; Adjobimey, T.; Sina, H.; Assogba, S.A.; Koda, A. D.; Agbodjato, N. A.; Ahoyo Adjovi, N. R.; Adjanohoun, A.; Babalola, O. O.; Baba-Moussa, L."Mycorrhizal symbiosis for sustainable optimization of tropical agriculture: a review of research" "Symbiosis in Nature" Editor Prof. Everlon Rigobelo. 2023, ISBN: 978-1-83768-638-4 IntechOpen https://www.intechopen.com/online-first/mycorrhizal-symbiosis-for-sustainable-optimization-of-tropical-agriculture-a-review-of-research https://doi.org/10.5772/intechopen.110084. [CrossRef]
- Xin, Y.; Fan, Y.; Babalola, OO.; Zhang, X.; Yang, W. Legacy effects of biochar and compost addition on arbuscular mycorrhizal fungal community and co-occurrence network in black soil. Microorganisms. 2022, 10, 2137. https://doi.org/10.3390/microorganisms10112137. [CrossRef]
- Alori, E.T.; Dare, M. O. ; Babalola O. O. Microbial inoculants for soil quality and plant health, Review of sustainable agriculture Springer. 2017, pp.281-307.
- Du Jardin, P., 2012. The science of plant biostimulants-A literature review. Ad hoc study report to the European Commission DG ENTR. 2012; http://ec.europa.eu/enterprise/sectors/chemicals/files/fertilizers/final_report_bio_2012_en.pdf.
- Bossou, L. D. R.; Houngnandan, H. B.; Adandonon A.; Zoundji C.; Houngnand, P. Diversity of arbuscular mycorrhizal fungi associated with maize (Zea mays L.) cultivation in Benin. International Journal Biological and Chemical Sciences. 2019,13(2):597-609. https://doi.org/10.4314/ijbcs.v13i2.2. [CrossRef]
- Igiehon, N.O.; Babalola, O.O.; Cheseto, X.; Torto, B. Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiol. Res. 2021, 242, 126640. [CrossRef].
- Fotso, B.; Nandjui, J.; Voko, B.D.R.R.; Amoa, J.A.; Brou, Y.C.; Niemenak N. Native arbuscular mycorrhizal fungi increased resistance of two plantain varieties (Fhia 21 and Orishele), under water deficit conditions in Cote d’Ivoire. Microbiology and Nature. 2019, 1, pp. 16-28.
- Beltrano, J.; Ruscitti, M.; Arango, M.C.; Ronco, M. Effects of arbuscular mycorrhiza inoculation on plant growth, biological and physiological parameters and mineral nutrition in pepper grown under different salinity and levels. Journal of Soil Science and Plant Nutrition. 2013, 13, pp. 123-141.
- Crossay, T. Taxonomic characterization of arbuscular mycorrhizal fungi native to the ultramafic soils of New Caledonia; analysis of their synergy allowing plant adaptation to these extreme environments. PhD thesis. University of New Caledonia. 2018, 293 p.
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: exploring arbuscular mycorrhizal fungi. Applied Microbiology and Biotechnology. 2017, 101:4871-4881. https://doi.org/10.1007/s00253-017-8344-z. [CrossRef]
- Igiehon, N.O; Babalola, O.O. Below-ground-above-ground plant-microbial interactions: focusing on soybean, rhizobacteria, and Mycorrhizal fungi". The Open Microbiology Journal. 2018 12:261-279. https://doi.org/10.2174/1874285801812010261. [CrossRef]
- Ndoye, F.; Diedhiou, A. ; Gueye, M. ; Fall, D. ; Barnaud, A. ; Sy, M. O. ; Noba, K. ; Diouf, D. ; Kane, A. Réponse du fonio blanc (Digitaria exilis Stapf) à l’inoculation avec des champignons mycorhiziens à arbuscules en conditions semi-contrôlées. Journal of Applied Biosciences. 2016, 103:9784-9799 ISSN 1997-5902.
- Haro, H. ; Semde, K. ; Bahadio, K. ; Sanon, K. B. Effet de l’inoculation mycorhizienne avec des souches des champignons mycorhiziens arbusculaires sur la croissance de Mucuna pruriens (L.) DC en condition contrôlée. International Journal of Biological and Chemical Sciences. 2020, Sci. 14(3): 1065-1073, ISSN 1997-342X (Online), ISSN 1991-8631 Print. https://doi.org/10.4314/ijbcs.v14i3.32. [CrossRef]
- Zoungrana, A. ; Zi, Y. ; Sanou, A. K. ; Savadogo, P. W. Comparison of the effect of two arbuscular mycorrhizal fungi on the growth and productivity of sesame (Sesamum indicum L.) in Burkina Faso. International Journal of Biological and Chemical Sciences. 2022, 16(1): 201-212, ISSN 1997-342X (Online), ISSN 1991-8631 (Print). https://doi.org/10.4314/ijbcs.v16i1.17. [CrossRef]
- Aguégué, M. R.; Assogba, S. A.; Salami, H. A. A.; Koda, A. D.; Agbodjato, N. A.; Amogou, O.; Sina, H. K.; Salako, V.; Ahoyo Adjovi, N. R.; Dagbénonbakin, G.; Glélé Kakai, R.; Adjanohoun, A.; Baba-Moussa, L. Organic Fertilizer Based on Rhizophagus intraradices: Valorization in a Farming Environment for Maize in the South, Center and North of Benin. Front. Sustain. Food Syst. 2021, 5:605610. https://doi.org/10.3389/fsufs.2021.605610. [CrossRef]
- Aguégué, M. R.; Ahoyo Adjovi, N. R.; Noumavo, A. P.; Agbodjato, A. N.; Assogba, A. S.; Koda, A. D.; de la Noval Pons, B. M.; Rivera, R.; Adjanohoun, A.; Baba-Moussa, L. Evaluation of the efficacy of the mychorizal fungus Rhizophagus intraradices on maize (Zea mays L.) in South Benin. Proceedings of the Special National Scientific Workshop of the West African Agricultural Productivity Program (PPAAO-Benin). Legal deposit N°12002 of January 24, 2020, 1st Quarter. pp. xx-xx, January 2020. Legal deposit N°12002 of January 24, 2020, 1st Quarter. ISBN : 978-99982-53-77-3.
- Davet, P. Soil Microbial Life and Plant Production. Editions Quae. Paris: National Institute for Agronomic Research. 1996.
- Parent, L.E.; Gagné, G. Fertilization reference guide. Reference center in agriculture and agri-food in Quebec. 2010.
- Coughlan, A. P.; Dalpé, Y.; Lapointe, L.; Piché, Y. Soil pH- induced changes in root colonization, diversity, and reproduction of symbiotic arbuscular mycorrhizal fungi from healthy and declining maple forests. Can. J. For. Res. 2000, 30, 1543-1554. https://doi.org/10.1139/x00-09.
- Yamawaki, K.; Matsumura, A.; Hattori, R.; Tarui, A.; Hossain, M. A.; Ohashi, Y.; Daimon, H. Effect of inoculation with arbuscular mycorrhizal fungi on growth, nutrient uptake and curcumin production of turmeric (Curcuma longa L.). Agricultural Sciences. 2013, 4(2): 66–71.
- Koda, A. D.; Dagbenonbakin, G.; Assogba , F.; Agbodjato, N.,;N’tcha, C.; Assogba, A. S.; Aguégué, M. R.; Kelomey, A. E.; Adjanohoun, A.; Baba -Moussa, L. Impact of native arbuscular mycorhizal fungi based fertilizers on to increase maize productivity in North Benin. African Journal of Agricultural Research. 2020, Vol. 16 (9). pp. 1298-1306. http://www.academicjournals.org/AJAR.
- Sampath Kumar, G.; Murugesh, S.; Rajendran, A.; Madhumathi, B.; Ganesh Kumar, A. Association of vam fungi with sesame and its influence on growth. Sesame and Safflower. 2002, Newsletter No. 17.
- Haro, H.; Sanon, K.B.; Krasova-Wade, T.; Kane, A.; N’doye, I.; Traoré, A.S. Response to double mycorrhizal and rhizobial inoculation of cowpea (variety, KVX396-4-5-2D) grown in Burkina Faso. International Journal of Biological and Chemical Science. 2015, 9(3): 1485-1493. https://doi.org/10.4314/ijbcs.v9i3.31. [CrossRef]
- Smith, S. E.; Read, D. J. Mycorrhizal symbiosis. Third Edition, London, UK, Academic press. 2008, 787 pp.
- Haro, H. ; Sanon, K. B. ; Le Roux, C. ; Duponnois, R. ; Traoré, A. S. Improvement of cowpea productivity by rhizobial and mycorrhizal inoculation in Burkina Faso. Symbiosis. 2017, 1-14. https://doi.org/10.1007/s13199-017-0478-3. [CrossRef]
- Gnamkoulamba, A.; Tounou, A. K.; Tchao, M.; Adjevi, A. K. M.; Batawila, K. Field evaluation of growth potential and production of rice (Oryza Sativa L.) variety IR841 inoculated in nursery with four strains of Arbuscular Mycorrhizal Fungi. 2018, URL:http://dx.doi.org/10.19044/esj.2018.v14n12p452. [CrossRef]
- Emmanuel, O.C.; Babalola, O.O Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth-promoting bacteria. Microbiology Research. 2020, 239: 126569. https://doi.org/10.1016/j.micres.2020.126569. [CrossRef]
- Garbaye, J. Mycorrhizal Symbiosis: An Association Between Plants and Fungi. Mycorrhizal Symbiosis. 2013, pp. 1-280.
- Houngnandan, P.; Yemadje, R.G.H.; Kane, A.; Boeckx, P.; Van Cleemput, O. Native glomales of the Isober- linia doka (Craib and Stapf) open forest at Wari-Maro in central Benin. Tropicultura. 2009, 27, 83-87.
- Tawaraya, K. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Science and Plant Nutrition. 2003, 49(5), 655-668. https://doi.org/10.1080/00380768.2003.10410323. [CrossRef]
- Hijri, M.; Sycorova, Z.; Oehl, F.; Ineichen, K.; Mader, P.; Wiemken, A.; Redecker, D. Communities of arbuscular mycorrhizal fungi in arable soils are not necessary low in diversity. Molecular Ecology. 2006, 15(8): 2277-2289. https://doi.org/10.1111/j.1365-294X.2006.02921.x. [CrossRef]
- Breuillin, F.; Schramm, J.; Hajirezaei, M.; Ahkami, A.; Favre, P.; Druege, U.; Hause, B.; Bucher, M.; Kretzschmar, T.; Bossolini, E. Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. The Plante Journal: for Cell and Molecular Biology. 2010, 64(6): 1002-1017. doi.org/10.1111/j.1365-313X.2010.04385.x. [CrossRef]
- MAEP. Catalogue Béninois des Espèces et Variétés végétales (CaBeV), 2ème Edn. Ministère de l’Agriculture de l’Elevage et de la Pêche ; INRAB/DPVPPAAO/ProCAD/MAEP et CORAF/WAAPP, Dépôt légal N 8982 du 21 octobre 2016, Bibliothèque Nationale du Bénin, 4ème trimestre. Disponible en ligne à l’adresse : http:/inrab.org/wp-content/uploads/2018/01/CaBEV – interactif-2.pdf (consulté le 25 janvier 2021).
- Fernández, F. ; Gómez, R. ; Vanegas, L.F. ; De la Noval, B.M. ; Martínez, M.A. Producto inoculante micorrizógenov. Oficina Nacional de Propiedad Industrial. Cuba. 2000, Patente No. 22641.
- Boudoudou, H. ; Hassikou, R.; Ouazzani Touhami, A.; Badoc, A.; Douria, A. Physicochemical parameters and fungal flora of Moroccan rice field soils. Bull. Soc. Pharm. Bordeaux. 2009, 148, 17-44.
- Bray, R.H.; Kurtz, T.L. Determination of total, organic and available forms of phosphorus in soils. Soil Science. 1945, 59(1): 39-45. View at Google Scholar | View at Publisher.
- Thomas, G.W. Exchangeable cations. In: Methods of soil analysis. (Page AL, RH Miller and DR Keeney, 2nd eds) (Madison). Agronomy. 1982, 9: 154-157. View at Google Scholar | View at Publisher.
- Walkley, A.; Black, C.A. An examination of the degtjareff method for determining soil organic matter and a proposal modification of the chromic acid titration method. Soil Science.1934, 37(1): 29-38. View at Google Scholar | View at Publisher.
- Metson, A.J. Methods of chemical analysis for soil survery samples. Soil Science. 1956, 83(3): 245. View at Publisher.
- Kjeldahl, J. Neue Methode zur Bestimmung des Stickstoffs in organischen Körpern. Z. Analytical Chemistry. 1883, 22(1): 366-382. View at Google Scholar | View at Publisher.
- Ruget, F. B.; Chartier, M. Simple estimation of leaf area of growing corn plants. Agronomy. 1996, 16, 553-562.
- Valdés, E.M.F.; González, E.C.; Serrano, M.M.; Labrada, H.R.; Báez, E.M.; Hernández, F.G.; Hernández, F.A. Experiencias obtenidas en el desarrollo participativo de híbridos lineales simples de maíz (Zea Mays, L.) en condiciónes de bajos insumos agrícolas. Cultivos Tropicales. 2013, 34(2): 61-69.
- Phillips, J.M.; Hayman, D.S. Improved procedures for cleaning roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society. 1970, 55(1): 158 – 161.
- Trouvelot, A. ; Kough, J.L. ; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherches et méthodes d’estimation ayant une signification fonctionnelle. In: Aspects physiologiques et génétiques des mycorhizes, Dijon, 1986: INRA.
- Douglas, C. E. ; Fligner, M. A. On distribution-free multiple comparaisons in the one-way analysis of variance. Commun. Stat. Theory Methods. 1991, 20, 127-139. doi : 10.1080/03610929108830487. [CrossRef]
- Core Team, R. R : Un langage et un environnement pour le calcul statistique. Vienne : R : Foundation for Statistical Computing. 2020.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





