Submitted:
12 July 2023
Posted:
13 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
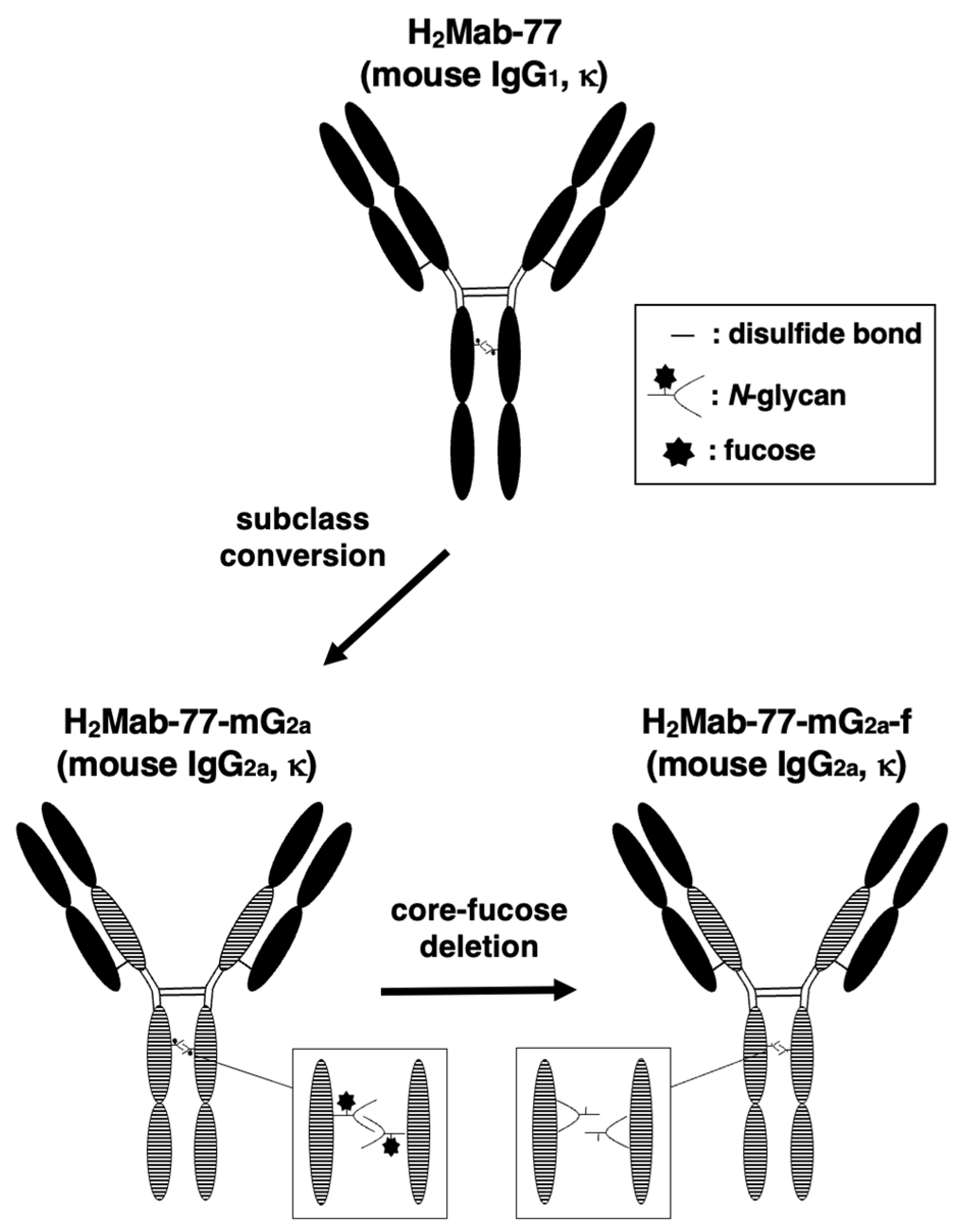
2.1. Development of a Core-Fucose-Deficient Anti-HER2 mAb, H2Mab-77-mG2a-f
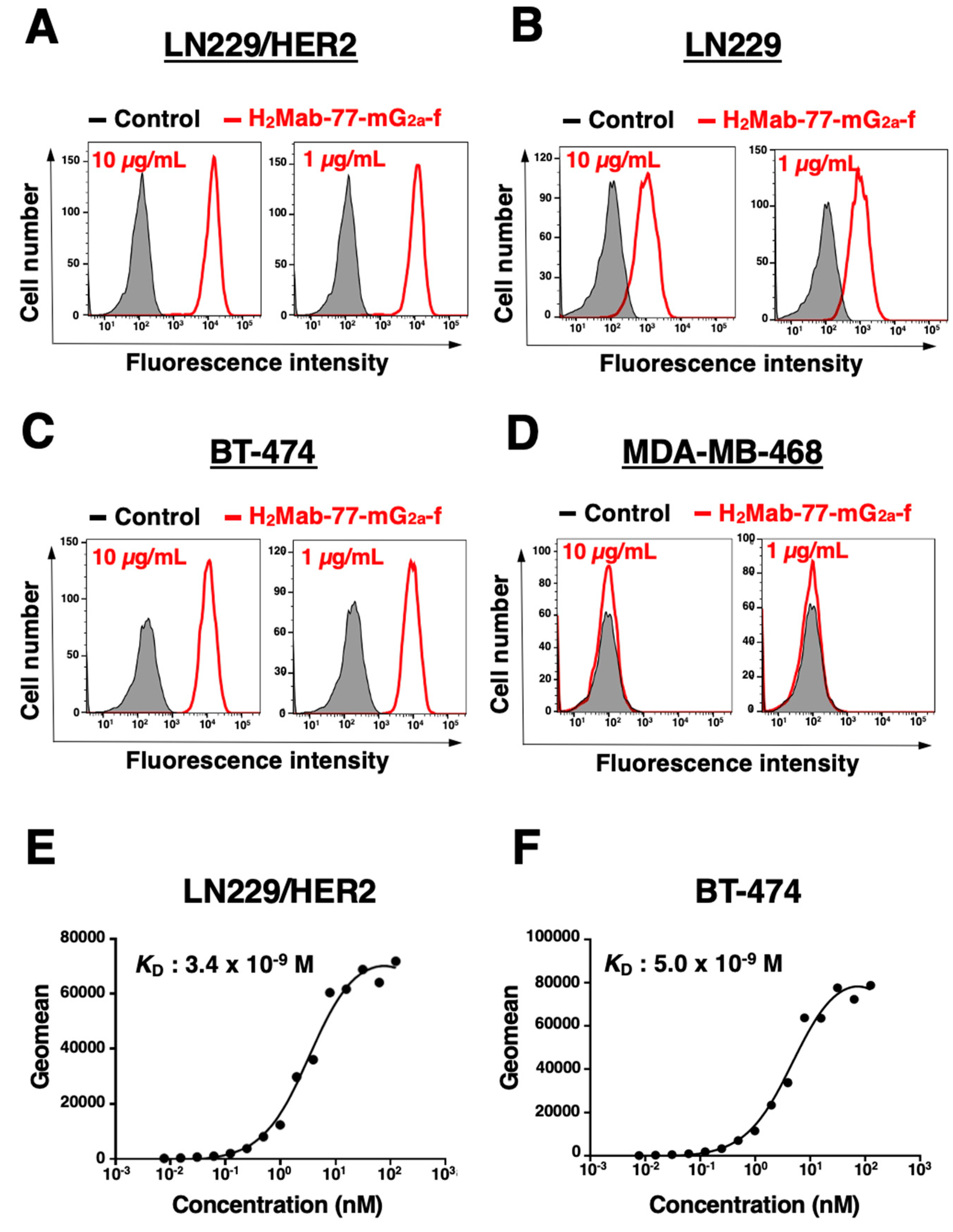
2.2. Analysis of H2Mab-77-mG2a-f Reactivity against Breast Adenocarcinoma Cells by Flow Cytometry
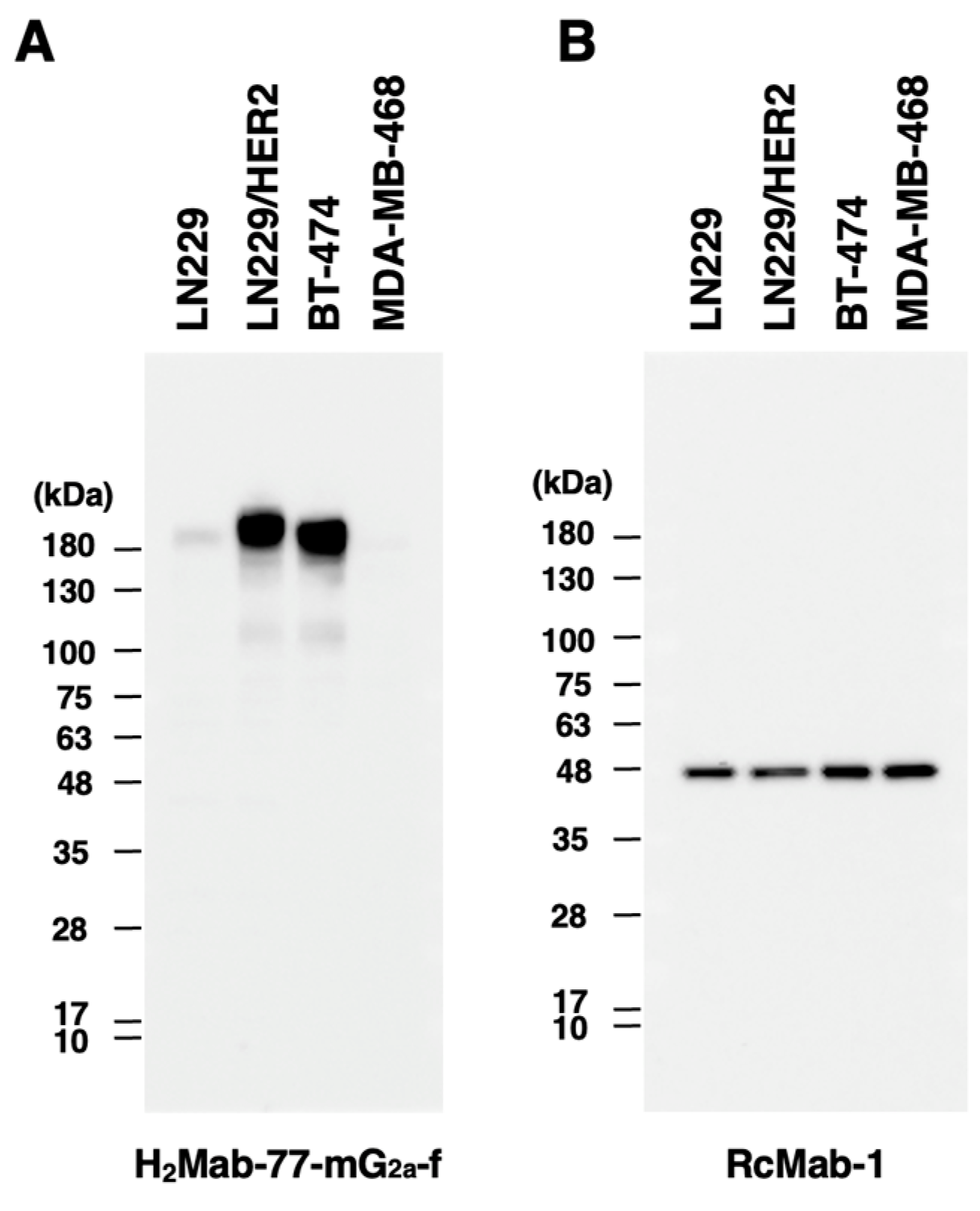
2.3. Western Blotting Using H2Mab-77-mG2a-f
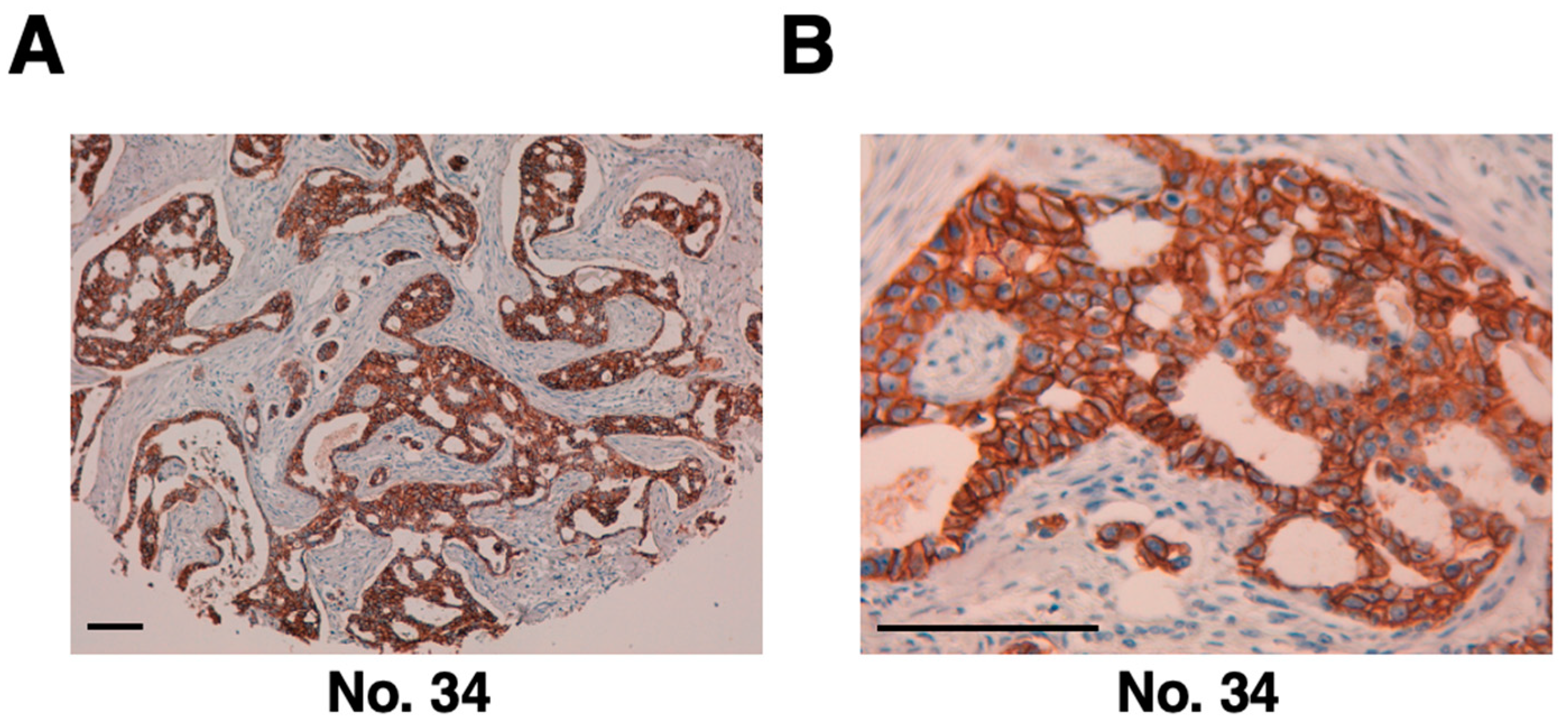
2.4. Immunohistochemical Analyses Using H2Mab-77-mG2a-f against Breast Cancer Tissue
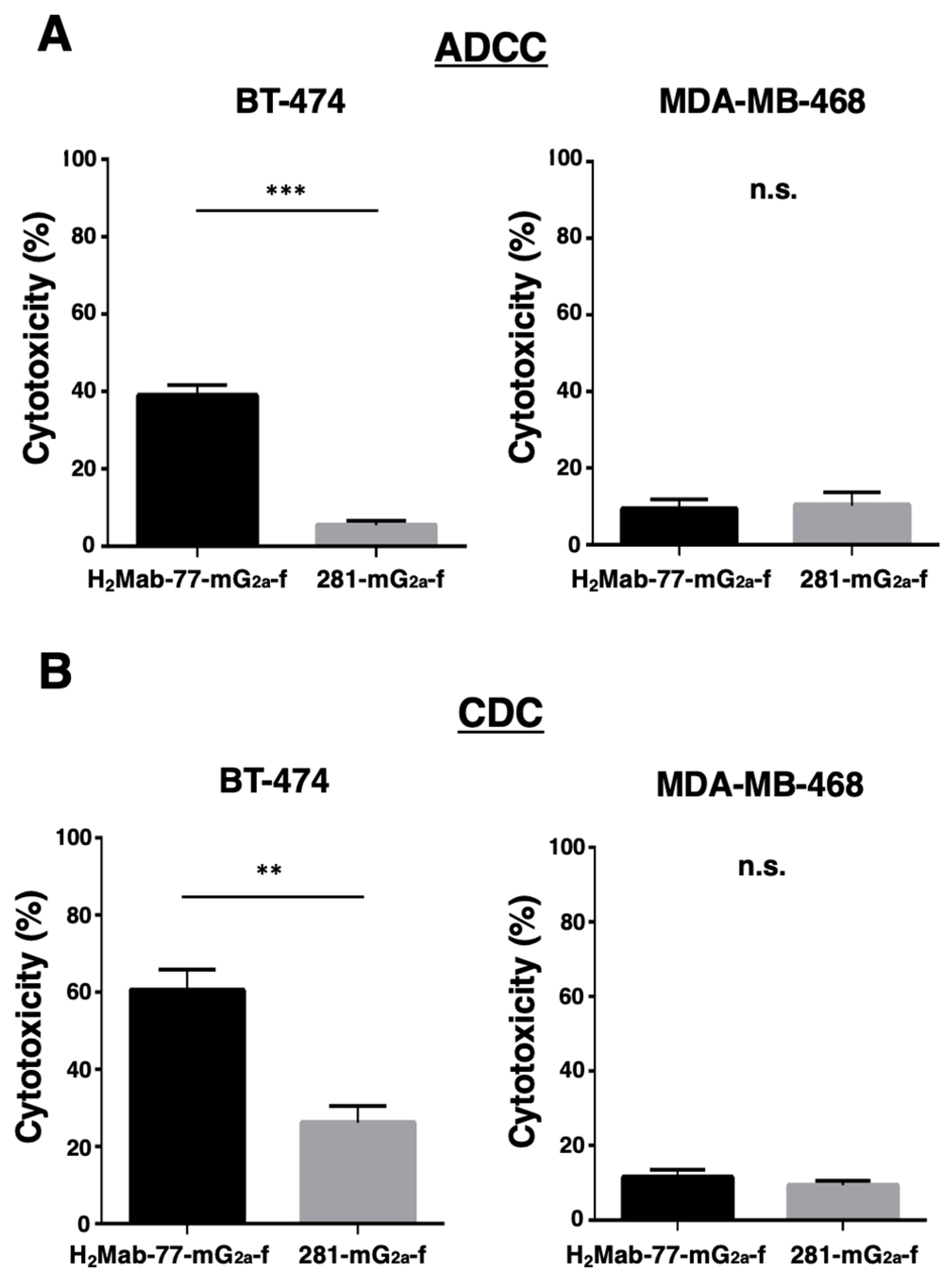
2.5. ADCC and CDC Activities of H2Mab-77-mG2a-f -Mediated in Breast Cancer
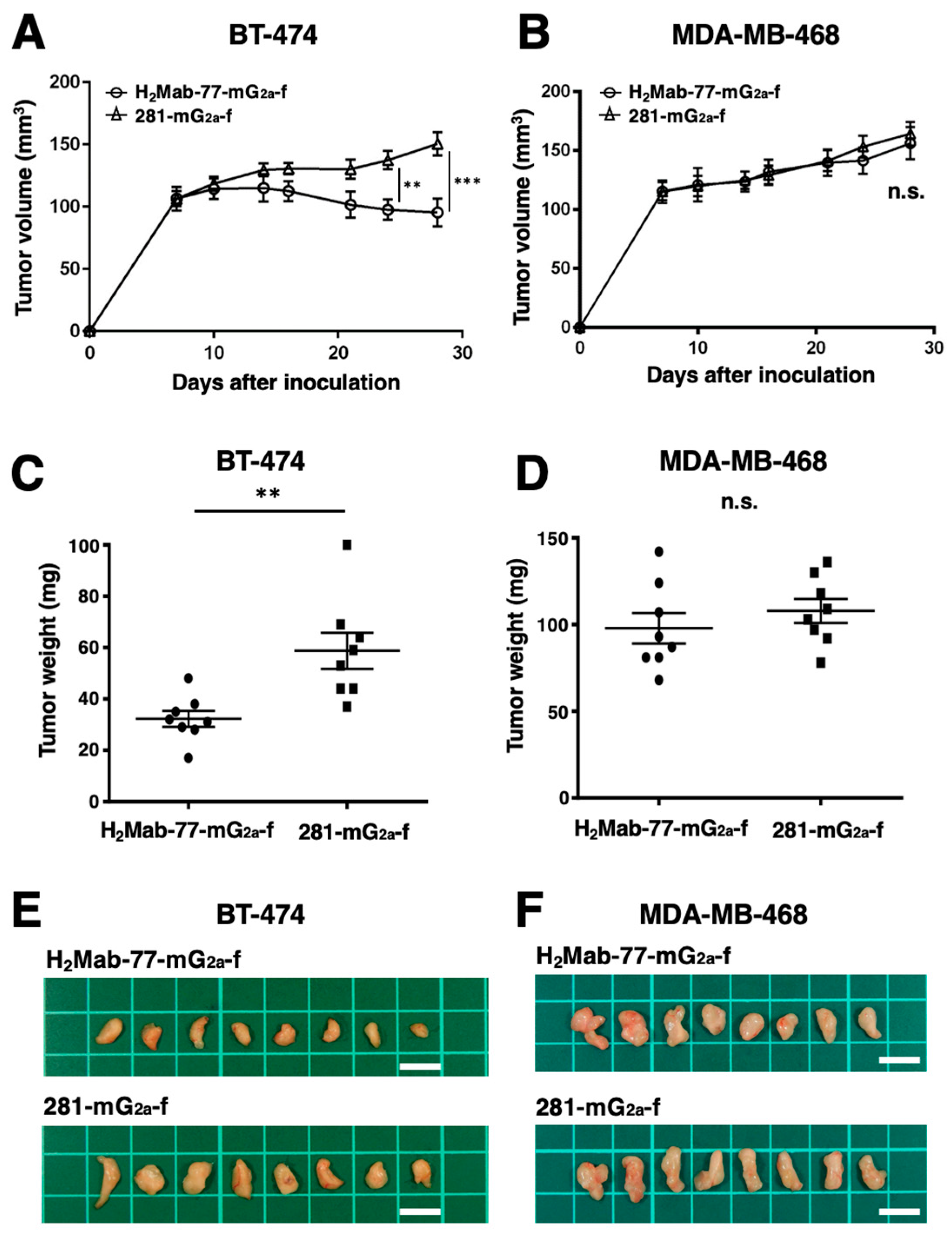
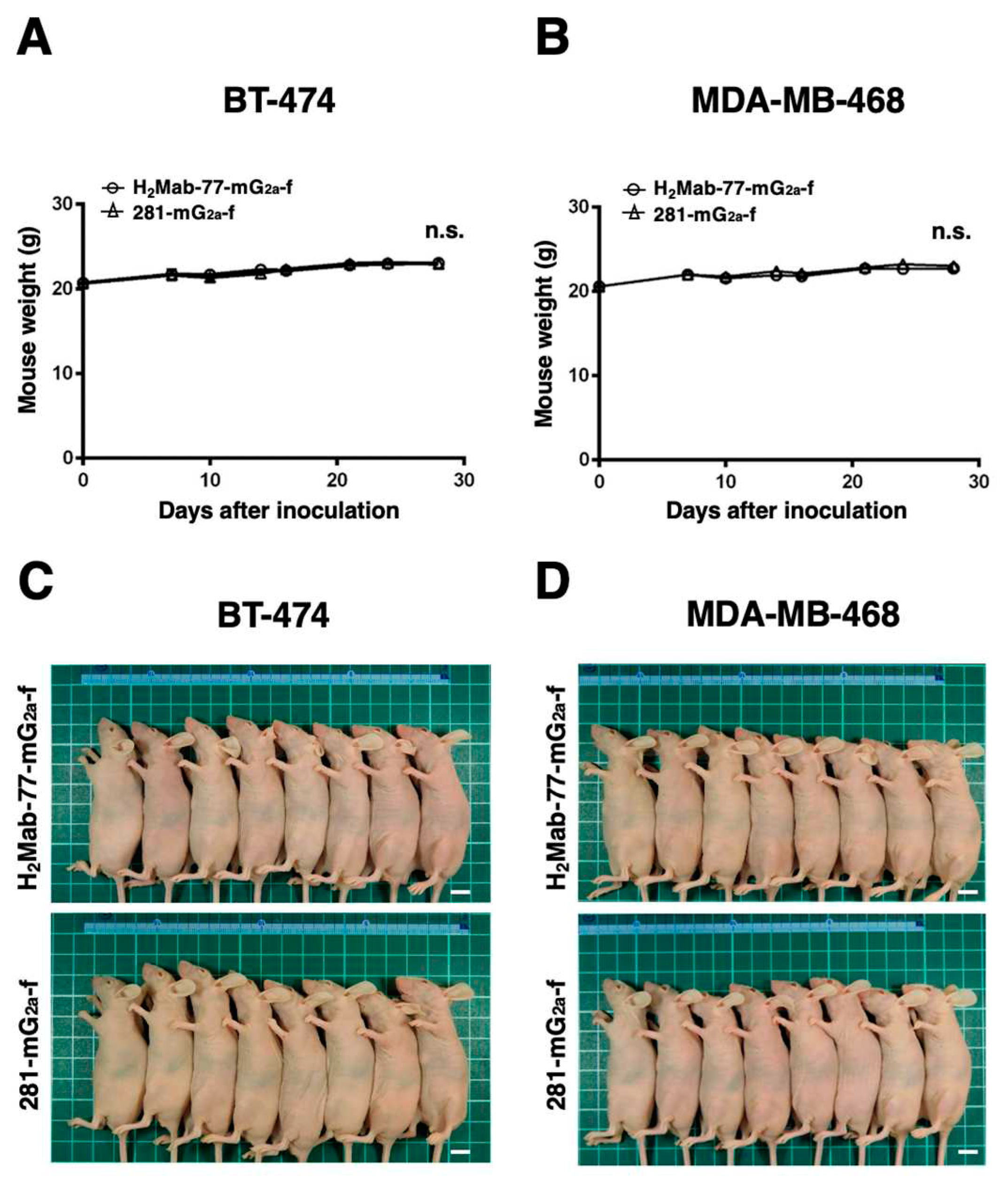
2.6. Antitumor Activities of H2Mab-77-mG2a-f in the Mouse Xenografts of Breast Tumor Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Animals
4.3. Antibodies
4.4. Flow Cytometry
4.5. Determination of the Binding Affinity by Flow Cytometry
4.6. Western Blotting
4.7. Immunohistochemical Analysis
4.8. ADCC of H2Mab-77-mG2a-f
4.9. CDC of H2Mab-77-mG2a-f
4.10. Antitumor Activities of H2Mab-77-mG2a-f in Xenografts of Breast Cancer
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pal, S.K.; Pegram, M. Targeting HER2 Epitopes. Semin Oncol 2006, 33, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Agus, D.B.; Akita, R.W.; Fox, W.D.; Lewis, G.D.; Higgins, B.; Pisacane, P.I.; Lofgren, J.A.; Tindell, C.; Evans, D.P.; Maiese, K.; et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.P.; McKern, N.M.; Lou, M.; Elleman, T.C.; Adams, T.E.; Lovrecz, G.O.; Kofler, M.; Jorissen, R.N.; Nice, E.C.; Burgess, A.W.; et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell 2003, 11, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Wallasch, C.; Weiss, F.U.; Niederfellner, G.; Jallal, B.; Issing, W.; Ullrich, A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. Embo j 1995, 14, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Alimandi, M.; Romano, A.; Curia, M.C.; Muraro, R.; Fedi, P.; Aaronson, S.A.; Di Fiore, P.P.; Kraus, M.H. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 1995, 10, 1813–1821. [Google Scholar]
- Pinkas-Kramarski, R.; Soussan, L.; Waterman, H.; Levkowitz, G.; Alroy, I.; Klapper, L.; Lavi, S.; Seger, R.; Ratzkin, B.J.; Sela, M.; et al. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. Embo j 1996, 15, 2452–2467. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J Clin 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Cronin, K.A.; Harlan, L.C.; Dodd, K.W.; Abrams, J.S.; Ballard-Barbash, R. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest 2010, 28, 963–968. [Google Scholar] [CrossRef]
- Guarneri, V.; Barbieri, E.; Dieci, M.V.; Piacentini, F.; Conte, P. Anti-HER2 neoadjuvant and adjuvant therapies in HER2 positive breast cancer. Cancer Treat Rev 2010, 36 Suppl 3, S62–66. [Google Scholar] [CrossRef]
- Abd El-Rehim, D.M.; Pinder, S.E.; Paish, C.E.; Bell, J.A.; Rampaul, R.S.; Blamey, R.W.; Robertson, J.F.; Nicholson, R.I.; Ellis, I.O. Expression and co-expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. Br J Cancer 2004, 91, 1532–1542. [Google Scholar] [CrossRef]
- Purdie, C.A.; Baker, L.; Ashfield, A.; Chatterjee, S.; Jordan, L.B.; Quinlan, P.; Adamson, D.J.; Dewar, J.A.; Thompson, A.M. Increased mortality in HER2 positive, oestrogen receptor positive invasive breast cancer: a population-based study. Br J Cancer 2010, 103, 475–481. [Google Scholar] [CrossRef]
- Herter-Sprie, G.S.; Greulich, H.; Wong, K.K. Activating Mutations in ERBB2 and Their Impact on Diagnostics and Treatment. Front Oncol 2013, 3, 86. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, F.; Fontanella, C.; Amoroso, V.; Bianchi, G.V.; Bisagni, G.; Falci, C.; Fontana, A.; Generali, D.; Gianni, L.; Grassadonia, A.; et al. Current challenges in HER2-positive breast cancer. Crit Rev Oncol Hematol 2016, 98, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.F. HER2 and Breast Cancer - A Phenomenal Success Story. N Engl J Med 2019, 381, 1284–1286. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Engelman, J.A. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014, 25, 282–303. [Google Scholar] [CrossRef]
- Riecke, K.; Witzel, I. Targeting the Human Epidermal Growth Factor Receptor Family in Breast Cancer beyond HER2. Breast Care (Basel) 2020, 15, 579–585. [Google Scholar] [CrossRef]
- Martin, A.M.; Cagney, D.N.; Catalano, P.J.; Warren, L.E.; Bellon, J.R.; Punglia, R.S.; Claus, E.B.; Lee, E.Q.; Wen, P.Y.; Haas-Kogan, D.A.; et al. Brain Metastases in Newly Diagnosed Breast Cancer: A Population-Based Study. JAMA Oncol 2017, 3, 1069–1077. [Google Scholar] [CrossRef]
- Kuksis, M.; Gao, Y.; Tran, W.; Hoey, C.; Kiss, A.; Komorowski, A.S.; Dhaliwal, A.J.; Sahgal, A.; Das, S.; Chan, K.K.; et al. The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007, 25, 118–145. [Google Scholar] [CrossRef]
- Ross, J.S.; Slodkowska, E.A.; Symmans, W.F.; Pusztai, L.; Ravdin, P.M.; Hortobagyi, G.N. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 2009, 14, 320–368. [Google Scholar] [CrossRef] [PubMed]
- Cobleigh, M.A.; Vogel, C.L.; Tripathy, D.; Robert, N.J.; Scholl, S.; Fehrenbacher, L.; Wolter, J.M.; Paton, V.; Shak, S.; Lieberman, G.; et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999, 17, 2639–2648. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.L.; Cobleigh, M.A.; Tripathy, D.; Gutheil, J.C.; Harris, L.N.; Fehrenbacher, L.; Slamon, D.J.; Murphy, M.; Novotny, W.F.; Burchmore, M.; et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002, 20, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Amiri-Kordestani, L.; Blumenthal, G.M.; Xu, Q.C.; Zhang, L.; Tang, S.W.; Ha, L.; Weinberg, W.C.; Chi, B.; Candau-Chacon, R.; Hughes, P.; et al. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin Cancer Res 2014, 20, 4436–4441. [Google Scholar] [CrossRef]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The Latest Research and Development into the Antibody-Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem Pharm Bull (Tokyo) 2019, 67, 173–185. [Google Scholar] [CrossRef]
- Zheng, Y.; Zou, J.; Sun, C.; Peng, F.; Peng, C. Ado-tratuzumab emtansine beyond breast cancer: therapeutic role of targeting other HER2-positive cancers. Front Mol Biosci 2023, 10, 1165781. [Google Scholar] [CrossRef]
- Hudis, C.A. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007, 357, 39–51. [Google Scholar] [CrossRef]
- Cho, H.S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W., Jr.; Leahy, D.J. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003, 421, 756–760. [Google Scholar] [CrossRef]
- Shi, Y.; Fan, X.; Deng, H.; Brezski, R.J.; Rycyzyn, M.; Jordan, R.E.; Strohl, W.R.; Zou, Q.; Zhang, N.; An, Z. Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcγ receptors on macrophages. J Immunol 2015, 194, 4379–4386. [Google Scholar] [CrossRef]
- Molina, M.A.; Codony-Servat, J.; Albanell, J.; Rojo, F.; Arribas, J.; Baselga, J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res 2001, 61, 4744–4749. [Google Scholar]
- Loo, L.; Capobianco, J.A.; Wu, W.; Gao, X.; Shih, W.Y.; Shih, W.H.; Pourrezaei, K.; Robinson, M.K.; Adams, G.P. Highly sensitive detection of HER2 extracellular domain in the serum of breast cancer patients by piezoelectric microcantilevers. Anal Chem 2011, 83, 3392–3397. [Google Scholar] [CrossRef]
- Junttila, T.T.; Li, G.; Parsons, K.; Phillips, G.L.; Sliwkowski, M.X. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 2011, 128, 347–356. [Google Scholar] [CrossRef]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016, 107, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med 2022, 386, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med 2020, 382, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, C.I.; Ghetie, M.A.; Uhr, J.; Marches, R.; Li, J.L.; Shen, G.L.; Vitetta, E.S. Targeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin Cancer Res 2002, 8, 1720–1730. [Google Scholar] [PubMed]
- Harbeck, N.; Beckmann, M.W.; Rody, A.; Schneeweiss, A.; Müller, V.; Fehm, T.; Marschner, N.; Gluz, O.; Schrader, I.; Heinrich, G.; et al. HER2 Dimerization Inhibitor Pertuzumab - Mode of Action and Clinical Data in Breast Cancer. Breast Care (Basel) 2013, 8, 49–55. [Google Scholar] [CrossRef]
- Barthélémy, P.; Leblanc, J.; Goldbarg, V.; Wendling, F.; Kurtz, J.E. Pertuzumab: development beyond breast cancer. Anticancer Res 2014, 34, 1483–1491. [Google Scholar]
- Franklin, M.C.; Carey, K.D.; Vajdos, F.F.; Leahy, D.J.; de Vos, A.M.; Sliwkowski, M.X. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004, 5, 317–328. [Google Scholar] [CrossRef]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000, 6, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Nitta, H.; Li, Z. HER2 Intratumoral Heterogeneity in Breast Cancer, an Evolving Concept. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Itai, S.; Fujii, Y.; Kaneko, M.K.; Yamada, S.; Nakamura, T.; Yanaka, M.; Saidoh, N.; Chang, Y.W.; Handa, S.; Takahashi, M.; et al. H2Mab-77 is a Sensitive and Specific Anti-HER2 Monoclonal Antibody Against Breast Cancer. Monoclon Antib Immunodiagn Immunother 2017, 36, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Tsao, L.C.; Force, J.; Hartman, Z.C. Mechanisms of Therapeutic Antitumor Monoclonal Antibodies. Cancer Res 2021, 81, 4641–4651. [Google Scholar] [CrossRef]
- Shinkawa, T.; Nakamura, K.; Yamane, N.; Shoji-Hosaka, E.; Kanda, Y.; Sakurada, M.; Uchida, K.; Anazawa, H.; Satoh, M.; Yamasaki, M.; et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 2003, 278, 3466–3473. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Ohishi, T.; Nakamura, T.; Inoue, H.; Takei, J.; Sano, M.; Asano, T.; Sayama, Y.; Hosono, H.; Suzuki, H.; et al. Development of Core-Fucose-Deficient Humanized and Chimeric Anti-Human Podoplanin Antibodies. Monoclon Antib Immunodiagn Immunother 2020, 39, 167–174. [Google Scholar] [CrossRef]
- Song, K.H.; Trudeau, T.; Kar, A.; Borden, M.A.; Gutierrez-Hartmann, A. Ultrasound-mediated delivery of siESE complexed with microbubbles attenuates HER2+/- cell line proliferation and tumor growth in rodent models of breast cancer. Nanotheranostics 2019, 3, 212–222. [Google Scholar] [CrossRef]
- Valabrega, G.; Montemurro, F.; Aglietta, M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol 2007, 18, 977–984. [Google Scholar] [CrossRef]
- Amiri-Kordestani, L.; Wedam, S.; Zhang, L.; Tang, S.; Tilley, A.; Ibrahim, A.; Justice, R.; Pazdur, R.; Cortazar, P. First FDA approval of neoadjuvant therapy for breast cancer: pertuzumab for the treatment of patients with HER2-positive breast cancer. Clin Cancer Res 2014, 20, 5359–5364. [Google Scholar] [CrossRef]
- Swain, S.M.; Kim, S.B.; Cortés, J.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Knott, A.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2013, 14, 461–471. [Google Scholar] [CrossRef]
- Derakhshani, A.; Rezaei, Z.; Safarpour, H.; Sabri, M.; Mir, A.; Sanati, M.A.; Vahidian, F.; Gholamiyan Moghadam, A.; Aghadoukht, A.; Hajiasgharzadeh, K.; et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J Cell Physiol 2020, 235, 3142–3156. [Google Scholar] [CrossRef]
- Vivekanandhan, S.; Knutson, K.L. Resistance to Trastuzumab. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Khoury, R.; Saleh, K.; Khalife, N.; Saleh, M.; Chahine, C.; Ibrahim, R.; Lecesne, A. Mechanisms of Resistance to Antibody-Drug Conjugates. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Nagy, P.; Friedländer, E.; Tanner, M.; Kapanen, A.I.; Carraway, K.L.; Isola, J.; Jovin, T.M. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res 2005, 65, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Carraway, K.L.; Perez, A.; Idris, N.; Jepson, S.; Arango, M.; Komatsu, M.; Haq, B.; Price-Schiavi, S.A.; Zhang, J.; Carraway, C.A. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog Nucleic Acid Res Mol Biol 2002, 71, 149–185. [Google Scholar] [CrossRef] [PubMed]
- Royce, M.; Osgood, C.L.; Amatya, A.K.; Fiero, M.H.; Chang, C.J.G.; Ricks, T.K.; Shetty, K.A.; Kraft, J.; Qiu, J.; Song, P.; et al. FDA Approval Summary: Margetuximab plus Chemotherapy for Advanced or Metastatic HER2-Positive Breast Cancer. Clin Cancer Res 2022, 28, 1487–1492. [Google Scholar] [CrossRef]
- Nordstrom, J.L.; Gorlatov, S.; Zhang, W.; Yang, Y.; Huang, L.; Burke, S.; Li, H.; Ciccarone, V.; Zhang, T.; Stavenhagen, J.; et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res 2011, 13, R123. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Cortés, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escrivá-de-Romaní, S.; et al. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2021, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Mandó, P.; Rivero, S.G.; Rizzo, M.M.; Pinkasz, M.; Levy, E.M. Targeting ADCC: A different approach to HER2 breast cancer in the immunotherapy era. Breast 2021, 60, 15–25. [Google Scholar] [CrossRef]
- Ko, B.K.; Lee, S.Y.; Lee, Y.H.; Hwang, I.S.; Persson, H.; Rockberg, J.; Borrebaeck, C.; Park, D.; Kim, K.T.; Uhlen, M.; et al. Combination of novel HER2-targeting antibody 1E11 with trastuzumab shows synergistic antitumor activity in HER2-positive gastric cancer. Mol Oncol 2015, 9, 398–408. [Google Scholar] [CrossRef]
- Yamada, S.; Itai, S.; Nakamura, T.; Chang, Y.W.; Harada, H.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Establishment of H(2)Mab-119, an Anti-Human Epidermal Growth Factor Receptor 2 Monoclonal Antibody, Against Pancreatic Cancer. Monoclon Antib Immunodiagn Immunother 2017, 36, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.K.; Yamada, S.; Itai, S.; Kato, Y. Development of an Anti-HER2 Monoclonal Antibody H2Mab-139 Against Colon Cancer. Monoclon Antib Immunodiagn Immunother 2018, 37, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ohishi, T.; Takei, J.; Nakamura, T.; Sano, M.; Asano, T.; Sayama, Y.; Hosono, H.; Kawada, M.; Kaneko, M.K. An Anti-Human Epidermal Growth Factor Receptor 2 Monoclonal Antibody H2Mab-19 Exerts Antitumor Activity in Mouse Colon Cancer Xenografts. Monoclon Antib Immunodiagn Immunother 2020, 39, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ohishi, T.; Yamada, S.; Itai, S.; Takei, J.; Sano, M.; Nakamura, T.; Harada, H.; Kawada, M.; Kaneko, M.K. Anti-Human Epidermal Growth Factor Receptor 2 Monoclonal Antibody H2Mab-41 Exerts Antitumor Activity in a Mouse Xenograft Model of Colon Cancer. Monoclon Antib Immunodiagn Immunother 2019, 38, 157–161. [Google Scholar] [CrossRef]
- Kato, Y.; Ohishi, T.; Sano, M.; Asano, T.; Sayama, Y.; Takei, J.; Kawada, M.; Kaneko, M.K. H(2)Mab-19 Anti-Human Epidermal Growth Factor Receptor 2 Monoclonal Antibody Therapy Exerts Antitumor Activity in Pancreatic Cancer Xenograft Models. Monoclon Antib Immunodiagn Immunother 2020, 39, 61–65. [Google Scholar] [CrossRef]
- Kato, Y.; Ohishi, T.; Takei, J.; Nakamura, T.; Kawada, M.; Kaneko, M.K. An Antihuman Epidermal Growth Factor Receptor 2 Monoclonal Antibody (H(2)Mab-19) Exerts Antitumor Activity in Glioblastoma Xenograft Models. Monoclon Antib Immunodiagn Immunother 2020, 39, 135–139. [Google Scholar] [CrossRef]
- Azim, H.A.; Azim, H.A., Jr. Systemic treatment of brain metastases in HER2-positive breast cancer: current status and future directions. Future Oncol 2012, 8, 135–144. [Google Scholar] [CrossRef]
- Leone, J.P.; Leone, B.A. Breast cancer brain metastases: the last frontier. Exp Hematol Oncol 2015, 4, 33. [Google Scholar] [CrossRef]
- Turini, M.; Chames, P.; Bruhns, P.; Baty, D.; Kerfelec, B. A FcγRIII-engaging bispecific antibody expands the range of HER2-expressing breast tumors eligible to antibody therapy. Oncotarget 2014, 5, 5304–5319. [Google Scholar] [CrossRef]
- Diermeier-Daucher, S.; Ortmann, O.; Buchholz, S.; Brockhoff, G. Trifunctional antibody ertumaxomab: Non-immunological effects on Her2 receptor activity and downstream signaling. MAbs 2012, 4, 614–622. [Google Scholar] [CrossRef]
- Haense, N.; Atmaca, A.; Pauligk, C.; Steinmetz, K.; Marmé, F.; Haag, G.M.; Rieger, M.; Ottmann, O.G.; Ruf, P.; Lindhofer, H.; et al. A phase I trial of the trifunctional anti Her2 × anti CD3 antibody ertumaxomab in patients with advanced solid tumors. BMC Cancer 2016, 16, 420. [Google Scholar] [CrossRef]
- Schram, A.M.; Odintsov, I.; Espinosa-Cotton, M.; Khodos, I.; Sisso, W.J.; Mattar, M.S.; Lui, A.J.W.; Vojnic, M.; Shameem, S.H.; Chauhan, T.; et al. Zenocutuzumab, a HER2xHER3 Bispecific Antibody, Is Effective Therapy for Tumors Driven by NRG1 Gene Rearrangements. Cancer Discov 2022, 12, 1233–1247. [Google Scholar] [CrossRef] [PubMed]
- Fontana, E.; Torga, G.; Fostea, R.; Cleator, S.; Wasserman, E.; Murat, A.; Arkenau, H.T. Sustained Tumor Regression With Zenocutuzumab, a Bispecific Antibody Targeting Human Epidermal Growth Factor Receptor 2/Human Epidermal Growth Factor Receptor 3 Signaling, in NRG1 Fusion-Positive, Estrogen Receptor-Positive Breast Cancer After Progression on a Cyclin-Dependent Kinase 4/6 Inhibitor. JCO Precis Oncol 2022, 6, e2100446. [Google Scholar] [CrossRef]
- McDonagh, C.F.; Huhalov, A.; Harms, B.D.; Adams, S.; Paragas, V.; Oyama, S.; Zhang, B.; Luus, L.; Overland, R.; Nguyen, S.; et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther 2012, 11, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Cui, Y.; Liu, X.; Liu, G.; Dong, X.; Tang, L.; Hung, Y.; Wang, C.; Feng, M.Q. A bispecific antibody targeting HER2 and PD-L1 inhibits tumor growth with superior efficacy. J Biol Chem 2021, 297, 101420. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.L.; Zhu, H.X.; Deng, L.; Meng, X.Q.; Li, K.; Xu, W.; Zhao, L.; Liu, Y.Q.; Zhu, Z.P.; Huang, H.M. Bispecific antibody simultaneously targeting PD1 and HER2 inhibits tumor growth via direct tumor cell killing in combination with PD1/PDL1 blockade and HER2 inhibition. Acta Pharmacol Sin 2022, 43, 672–680. [Google Scholar] [CrossRef]
- Boulch, M.; Cazaux, M.; Loe-Mie, Y.; Thibaut, R.; Corre, B.; Lemaître, F.; Grandjean, C.L.; Garcia, Z.; Bousso, P. A cross-talk between CAR T cell subsets and the tumor microenvironment is essential for sustained cytotoxic activity. Sci Immunol 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Lugtenburg, P.J.; Kersten, M.J.; Subklewe, M.; Spanjaart, A.; Shah, N.N.; Kerbauy, L.N.; Roddie, C.; Pennings, E.R.A.; Mahuad, C.; et al. Cellular therapy in lymphoma. Hematol Oncol 2023. [Google Scholar] [CrossRef]
- Shin, M.H.; Oh, E.; Kim, Y.; Nam, D.H.; Jeon, S.Y.; Yu, J.H.; Minn, D. Recent Advances in CAR-Based Solid Tumor Immunotherapy. Cells 2023, 12. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves first CAR T therapy. Nat Rev Drug Discov 2017, 16, 669. [Google Scholar] [CrossRef]
- Shiina, S.; Ohno, M.; Ohka, F.; Kuramitsu, S.; Yamamichi, A.; Kato, A.; Motomura, K.; Tanahashi, K.; Yamamoto, T.; Watanabe, R.; et al. CAR T Cells Targeting Podoplanin Reduce Orthotopic Glioblastomas in Mouse Brains. Cancer Immunol Res 2016, 4, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Waseda, M.; Ishii, T.; Kaneko, M.K.; Kato, Y.; Kaneko, S. Improved anti-solid tumor response by humanized anti-podoplanin chimeric antigen receptor transduced human cytotoxic T cells in an animal model. Genes Cells 2022, 27, 549–558. [Google Scholar] [CrossRef]
- Fujii, Y.; Kaneko, M.K.; Kato, Y. MAP Tag: A Novel Tagging System for Protein Purification and Detection. Monoclon Antib Immunodiagn Immunother 2016, 35, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kaneko, M.K.; Ogasawara, S.; Yamada, S.; Yanaka, M.; Nakamura, T.; Saidoh, N.; Yoshida, K.; Honma, R.; Kato, Y. Development of RAP Tag, a Novel Tagging System for Protein Detection and Purification. Monoclon Antib Immunodiagn Immunother 2017, 36, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Ikota, H.; Nobusawa, S.; Arai, H.; Kato, Y.; Ishizawa, K.; Hirose, T.; Yokoo, H. Evaluation of IDH1 status in diffusely infiltrating gliomas by immunohistochemistry using anti-mutant and wild type IDH1 antibodies. Brain Tumor Pathol 2015, 32, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y. Specific monoclonal antibodies against IDH1/2 mutations as diagnostic tools for gliomas. Brain Tumor Pathol 2015, 32, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Nanamiya, R.; Suzuki, H.; Takei, J.; Li, G.; Goto, N.; Harada, H.; Saito, M.; Tanaka, T.; Asano, T.; Kaneko, M.K.; et al. Development of Monoclonal Antibody 281-mG(2a)-f Against Golden Hamster Podoplanin. Monoclon Antib Immunodiagn Immunother 2022, 41, 311–319. [Google Scholar] [CrossRef]
| No. | Sex | Age | Pathological Diagnosis | Differentiation | TNM | H2Mab-77-mG2a-f |
|---|---|---|---|---|---|---|
| 1 | F | 44 | Invasive ductal carcinoma | Moderately | T2N2M1 | - |
| 2 | F | 58 | Medullary carcinoma | Moderately | T2N2M1 | - |
| 3 | F | 40 | Invasive ductal carcinoma | Moderately | T2N1M0 | 2+ |
| 4 | F | 52 | Invasive ductal carcinoma | Moderately | T2N2M1 | - |
| 5 | F | 60 | Invasive ductal carcinoma | Moderately | T2N1M1 | - |
| 6 | F | 57 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 7 | F | 48 | Invasive ductal carcinoma | Moderately | T2N0M0 | 3+ |
| 8 | F | 66 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 9 | F | 58 | Adenocarcinoma | Moderately | T2N2M1 | - |
| 10 | F | 63 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 11 | F | 32 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 12 | F | 59 | Invasive lobular carcinoma | Well | T2N2M0 | - |
| 13 | F | 44 | Invasive lobular carcinoma | Well | T2N2M0 | - |
| 14 | F | 60 | Invasive lobular carcinoma | Moderately | T2N1M0 | - |
| 15 | F | 44 | Invasive ductal carcinoma | Moderately | T2N2M0 | 3+ |
| 16 | F | 82 | Invasive ductal carcinoma | Moderately | T2N1M1 | - |
| 17 | F | 58 | Adenocarcinoma | Moderately | T2N1M1 | 2+ |
| 18 | F | 57 | Invasive ductal carcinoma | Poorly | T3N3M0 | - |
| 19 | F | 41 | Invasive ductal carcinoma | Moderately | T2N1M0 | - |
| 20 | F | 44 | Invasive ductal carcinoma | Moderately | T2N2M0 | 1+ |
| 21 | F | 78 | Invasive ductal carcinoma | Moderately | T2N1M0 | - |
| 22 | F | 60 | Invasive ductal carcinoma | Moderately | T2N0M0 | 1+ |
| 23 | F | N/A | Invasive ductal carcinoma | Moderately | T2N1M1 | 2+ |
| 24 | F | 46 | Invasive ductal carcinoma | Moderately | T2N3M1 | - |
| 25 | F | 41 | Invasive ductal carcinoma | Moderately | T2N2M0 | - |
| 26 | F | 59 | Invasive ductal carcinoma | Poorly | T2N0M0 | - |
| 27 | F | 45 | Invasive ductal carcinoma | Poorly | T2N0M0 | - |
| 28 | F | 43 | Invasive ductal carcinoma | N/A | T2N1M1 | - |
| 29 | F | 26 | Fibroadenoma | N/A | T1N0M0 | - |
| 30 | F | 40 | Invasive ductal carcinoma | N/A | T1N0M0 | - |
| 31 | F | 38 | Fibroadenoma | N/A | T2N0M0 | - |
| 32 | F | 51 | Invasive ductal carcinoma | Moderately | T2N2M0 | - |
| 33 | F | 45 | Invasive ductal carcinoma | Poorly | T2N0M0 | 2+ |
| 34 | F | 45 | Invasive ductal carcinoma | Poorly | T2N1M0 | 3+ |
| 35 | F | 47 | Invasive ductal carcinoma | Moderately | T2N1M0 | - |
| 36 | F | 55 | Invasive ductal carcinoma | Moderately | T2N3M1 | 1+ |
| 37 | F | 58 | Invasive ductal carcinoma | Moderately | T3N3M0 | - |
| 38 | F | 47 | Invasive ductal carcinoma | Moderately | T2N0M0 | 1+ |
| 39 | F | 38 | Invasive ductal carcinoma | Poorly | T2N0M0 | - |
| 40 | F | 40 | Invasive ductal carcinoma | Poorly | T2N0M0 | - |
| 41 | F | 57 | Invasive ductal carcinoma | Poorly | T2N0M0 | - |
| 42 | F | 42 | Invasive ductal carcinoma | Moderately | T2N0M0 | 3+ |
| 43 | F | 60 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 44 | F | 58 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 45 | F | 41 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 46 | F | 50 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 47 | F | 60 | Invasive ductal carcinoma | Moderately | T2N2M1 | - |
| 48 | F | 53 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 49 | F | 65 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 50 | F | 43 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 51 | F | 57 | Invasive ductal carcinoma | Moderately | T2N0M0 | 3+ |
| 52 | F | 37 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 53 | F | 50 | Invasive ductal carcinoma | Moderately | T2N3M0 | - |
| 54 | F | 48 | Invasive ductal carcinoma | Poorly | T2N1M0 | - |
| 55 | F | 50 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 56 | F | 53 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 57 | F | 49 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 58 | F | 65 | Invasive ductal carcinoma | Moderately | T2N1M0 | - |
| 59 | F | 43 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 60 | F | 58 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 61 | F | 48 | Invasive ductal carcinoma | Moderately | T2N0M0 | - |
| 62 | F | N/A | Invasive ductal carcinoma | Moderately | N/A | 1+ |
| 63 | F | N/A | Invasive ductal carcinoma | Moderately | N/A | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





