1. Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease. Cases of UC have increased globally and are expected to continue to increase [
1]. In recent years, new UC drugs have become available [
2]. However, intractable and severe cases are treated with either tacrolimus (TAC), a calcineurin inhibitor, or infliximab, a biological product (Bio). TAC is a very potent drug [
2,
3]. Because it is used to treat intractable and severe UC, the rate of colectomy due to relapse at 52 weeks after remission induction is high, i.e., 20% to 30
% [4].
In intractable UC, an important treatment goal is preventing relapse after achieving remission with TAC. Maintenance therapy is often performed with azathioprine (AZA), which is generally used as an immunomodulator [
5]. AZA has long been used as maintenance therapy, and its effectiveness has been confirmed [
6].
However, it may cause serious adverse events (AEs), such as cytopenia, hepatic disorder, and alopecia totalis [
7]. The gene
NUDT15 was shown to be involved in AEs during AZA treatment, so serious AEs can now be avoided by examining
NUDT15 [
8]. However, in clinical practice, it can be difficult to predict mild to intermediate AEs during AZA treatment solely by assessing
NUDT15. When patients who have been using AZA as maintenance therapy develop relapse, some may have to switch from AZA to an antibody preparation, e.g., a Bio.
The new drugs in UC include anti-TNF-α inhibitors (adalimumab [ADA]), anti-integrin agents (vedolizumab [VED]) and interleukin 12/23 antagonists (ustekinumab [UST]) [
2]. Therefore, this retrospective study aimed to examine the rate of remission induction with TAC, general and drug-specific maintenance rates, and safety.
2. Methods
2.1. Participants
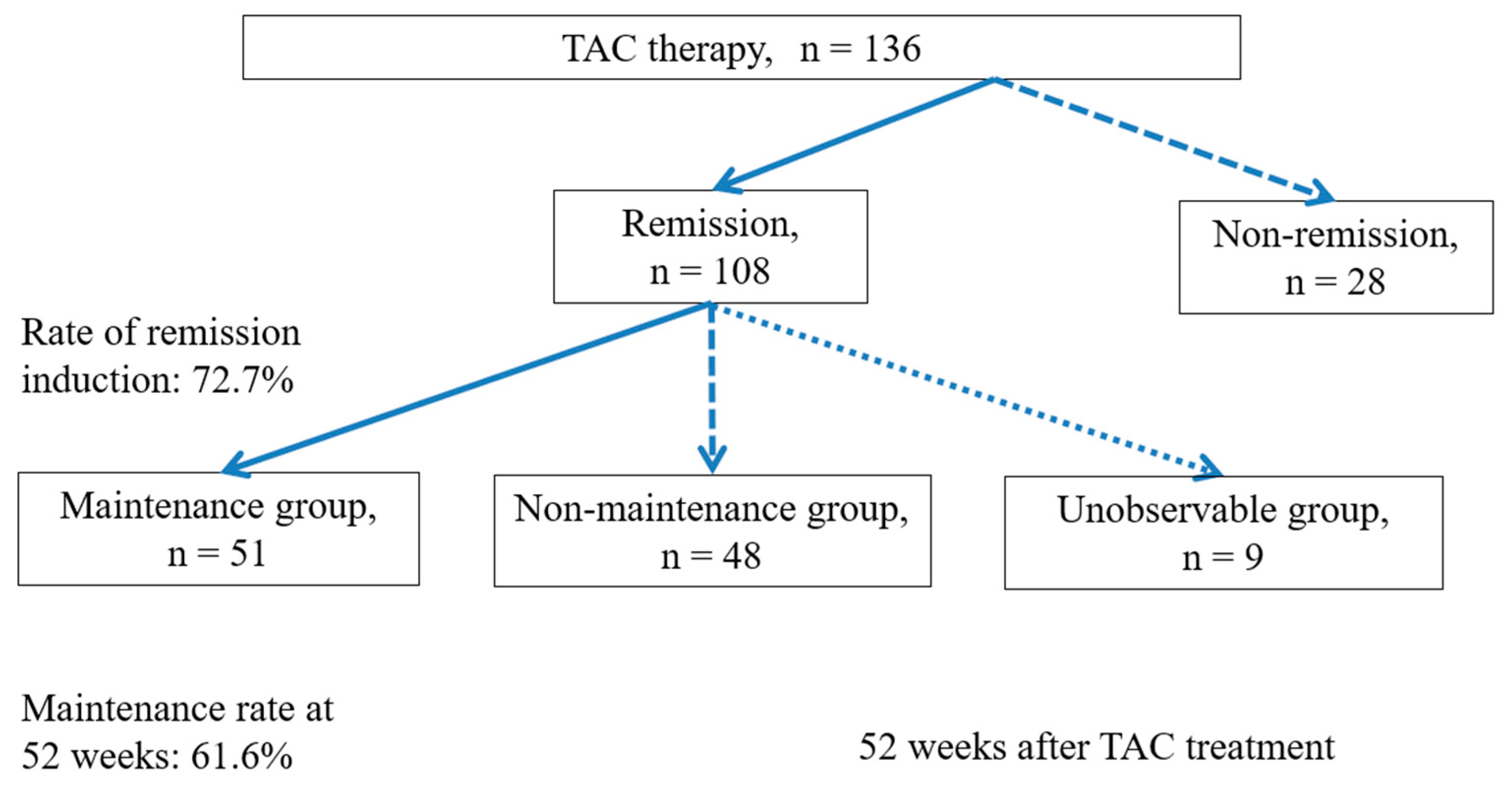
Study 1 included patients who used TAC from April 2009 to December 2021 (N = 136); Study 2 included patients who achieved remission and were followed for 52 weeks (N = 99) (
Figure 1); and Studies 3 and 4 examined drug-specific maintenance and included patients who used a single maintenance drug (N = 78).
2.2. Methods
Study 1 evaluated the rate of remission induction with TAC. In patients who achieved remission (remission group, n = 108) and those who did not (non-remission group, n = 28), the following background factors were compared: sex, age at onset, affected area, duration of disease, clinical activity index (CAI, Lichtiger score) before TAC therapy, hemoglobin (Hb), albumin (Alb), C-reactive protein (CRP), endoscopic scores (Mayo, Ulcerative Colitis Endoscopic Index of Severity [UCEIS]), total prednisolone (PSL) dose during hospitalization, time (days) to trough, and endoscopic scores at 2 weeks after achieving trough levels [
9,
10,
11]. Study 2 evaluated maintenance rate at 52 weeks after remission induction with TAC. In patients who achieved maintenance up to 52 weeks (maintenance group, n = 51) and those who did not (non-maintenance group, n = 48), the following background factors were examined: sex, age at onset, affected area, duration of disease, presence or absence of 5-aminosalicylic acid (5-ASA) allergy and AZA intolerance, history of AZA use, CAI before TAC therapy, Hb, Alb, CRP, endoscopic scores (Mayo, UCEIS), duration of hospitalization, and total PSL dose during hospitalization. Study 3 compared patients according to the maintenance drug used: AZA (n = 58) vs. TAC (n = 12) and AZA vs. Bio (n = 8); the Bio group included the following drugs: ADA (n = 2), VED (n = 5), and UST (n = 1). The following maintenance rates and patient characteristics were examined by drug: sex, age at onset, age, affected area, duration of disease, presence or absence of 5-ASA allergy and AZA intolerance, history of AZA use (yes/no), CAI (Lichtiger score) before TAC therapy, Hb, Alb, CRP, endoscopic scores (Mayo, UCEIS), duration of hospitalization, and total PSL dose during hospitalization. Study 4 examined AEs by drug.
TAC was given at 0.025 to 0.075 mg/kg body weight twice daily before breakfast and dinner. Blood samples were collected every other day for measurement of TAC levels until the target blood concentration was reached. The TAC dose was adjusted to reach the target trough concentration of 10 to 15 ng/mL blood within two weeks of starting TAC remission induction therapy. Then, at 2 to 3 weeks after the TAC concentration was within the target range, the dose was adjusted again to reach a new lower target concentration of 5 to 10 ng/mL. The target concentration was also set at 5 to 10 ng/mL, when TAC was used for longer than 90 days as maintenance therapy [
3].
Remission was defined as a CAI score less than or equal to 4 at 4 weeks after TAC induction therapy and thereafter. Maintenance was defined as requiring none of the following during the course of observation: high-dose IV PSL (0.5 kg/day); switch to a Bio or Janus kinase inhibitor; TAC re-administration; re-induction of remission by escalating doses to obtain high blood trough concentrations (≥ 10 ng/dL); or surgery. Maintenance drugs were defined as drugs used as maintenance therapy after remission induction (within 180 days of TAC therapy), and the following comparisons were performed: AZA group (n = 58) vs. TAC group (n = 12) and AZA group vs. Bio group (n = 8, ADA, n = 2; VED, n = 5; UST, n = 1). The TAC and Bio groups included only patients without concomitant AZA use.
2.3. Statistical analysis
Results are presented as number of patients or mean ± standard deviation (SD). Groups were compared with the Mann-Whitney and chi-square tests. Maintenance rates were determined by the Kaplan-Meier estimator and compared by the log-rank test. A p value less than 0.05 was considered significant. Statistical analysis was performed with JMP Pro16 (Statistical Discovery, SAS).
2.4. Ethical considerations
The study protocol was reviewed and approved by the ethics review committee of Tokyo Women’s Medical University (approval number: 2022-0143). Written and verbal informed consent was obtained from all patients.
3. Results
3.1. Observation period
The mean observation period was 1230 ± 175 days.
3.2. Remission induction and background factors
Study 1 remission was induced in 72.7% of patients (
Figure 1). In the non-remission maintenance group, a significant difference in remission maintenance was observed between the sexes (M/F; remission group 62/46, non-remission group 23/5; P = 0.016).
Significant differences were observed in pretreatment endoscopic scores: Mayo scores were 2.9 ± 0.2 in the remission group and 2.7 ± 0.4 in the non-remission group (P = 0.0018), and the respective UCEIS scores were 6.5 ± 0.7 and 4.7 ± 6.9 (P = 0.025). Endoscopic scores 2 weeks after achieving TAC trough were also significantly different: Mayo scores were 1.7 ± 0.7 in the remission group and 2.5 ± 0.7 in the non-remission group (P = 0.0009), and the respective UCEIS scores were 2.5 ± 0.7 and 6.0 ± 0.7 (P = 0.0047). No significant differences were observed in any background factors (
Table 1). In summary, for remission induction, significant intergroup differences were observed in sex and endoscopic scores before treatment and 2 weeks after achieving TAC trough.
3.3. Maintenance at 52 weeks and patient characteristics
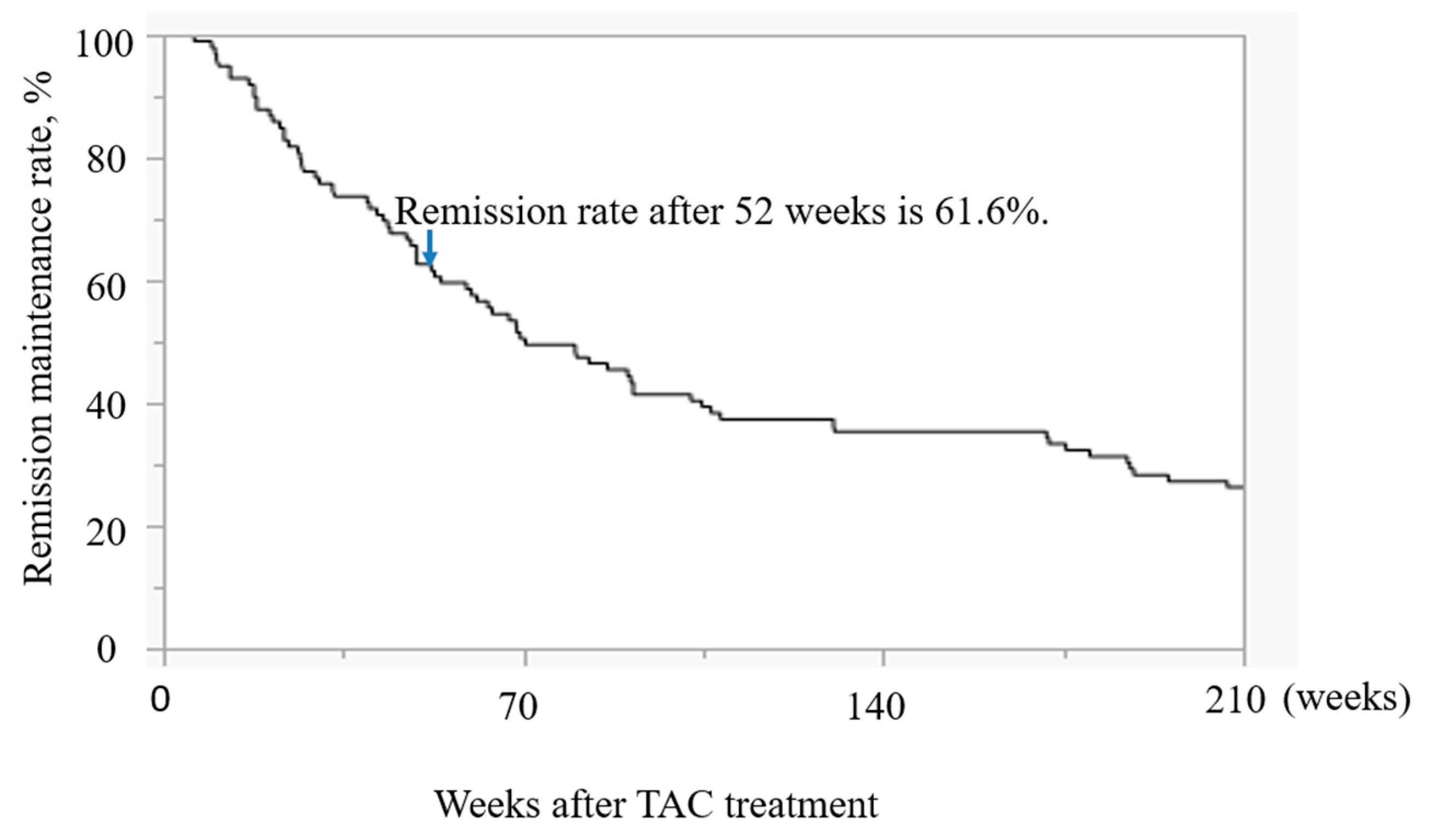
Study 2 in the Kaplan-Meier analysis, the 52-week maintenance rate was 61.6% (
Figure 2). In the non-remission maintenance group, significant differences were observed in sex, total PSL dose during hospitalization, and duration of hospitalization (
Table 2). No significant intergroup differences were observed in any other background factors (
Table 2).
3.4. Maintenance rates and patient characteristics by drug
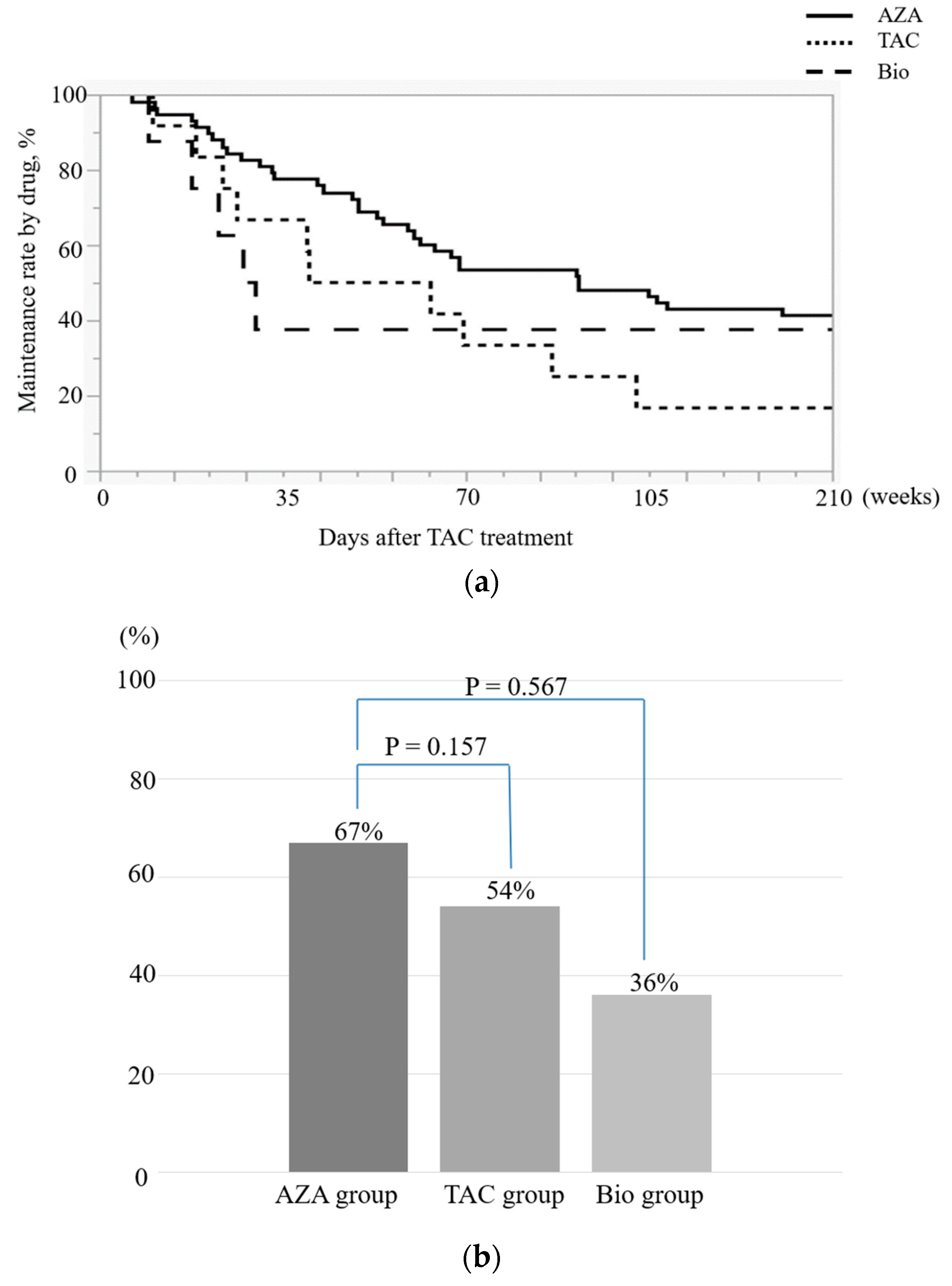
Study 2 no significant differences were observed in the 52-week maintenance rates in the AZA vs. TAC group or in the AZA vs. Bio group (
Table 3,
Figure 3-1,2). With respect to patient characteristics, a significant difference was found in the numbers of patients with AZA intolerance (P = 0.0043,
Table 3). No significant intergroup differences were observed in the other background factors (
Table 3).
3.5. AEs
Study 4 AEs observed at the time of remission induction with TAC included tremors (23 patients), renal disorder (9 patients), and headache (4 patients). During maintenance therapy, in the AZA group 4 patients had headache, 7 had cytopenia, 5 had nausea, 4 had hepatic disorder, 3 had hair loss (1 of these patients had alopecia totalis), and 2 had pneumonia; in the TAC group, 6 patients had tremors, and 4 had renal disorder; and in the Bio group, 2 patients had pneumonia, 1 had influenza, and 2 had pneumomediastinum. Breakdown of adverse effects associated with biologics: pneumonia (n=1) and influenza (n=1) in ADA, pneumonia (n=1) in VED and (n=2) in UST resulted in pneumomediastinum.
4. Discussion
4.1. Rate of remission induction and background factors
The rate of remission induction was higher than that reported in a previous study [
3]. Remission was induced in female patients with high endoscopic scores at the time of remission, long hospitalization periods, high total steroid doses, and low endoscopic scores 2 weeks after achieving TAC trough levels. Various studies have evaluated sex-dependent differences in UC; however, whether such differences are involved in the prognosis of the disease remains unclear. Although no differences between the sexes were observed in the drug treatment of UC, women have been reported to avoid surgery more often than men do [
12]. In addition, women maintain compliance with oral administration better than men [
13]. These aspects might have affected the high rate of remission induction observed among female patients.
In addition, with respect to long hospitalization periods and high total steroid doses, remission induction might be affected by adequate treatment with steroids when inflammation is most prominent. Steroids are very effective in UC and are often used to treat moderate to severe UC, with proven benefits [
14,
15]. However, they are associated with various AEs, so it is important to avoid performing steroid treatment aimlessly over a long period of time [
2,
16].
The present study revealed that remission can be induced in patients with reduced endoscopic scores 2 weeks after achieving TAC trough levels, even if pretreatment endoscopic scores were high. These findings suggest that remission may be more easily induced in patients with good response to TAC from an early stage. Endoscopic scores 2 weeks after achieving TAC trough levels may be a potential prognostic factor for remission induction.
4.2. Maintenance rate at 52 weeks and patient characteristics
Reports on medium- to long-term maintenance with TAC are scarce. Most studies focused on the avoidance of surgery and reported rates of 49.9% at 1 year after TAC induction therapy and 37.8% at 2 years [
17]. Compared with these reports, the 52-week maintenance rate in the present study (61.6%) is considered to be high; it may have been higher because of the relatively long-term use of TAC (about 180 days). Various studies have examined the duration of TAC use [
4]. Generally, TAC is used to induce remission and AZA is used as maintenance therapy [
3]. The duration of TAC use for inducing remission is likely to change depending on how remission induction is perceived. In Japan, guidelines recommend roughly 90 days’ TAC use, whereas European Crohn’s and Colitis Organisation guidelines recommend about 180 days’ use [
2]. Based on previous reports, adequate duration of TAC may promote colonic mucosal healing and maintain long-term remission [
3,
18]. However, long-term use of TAC requires careful follow-up.
One study found that infliximab, which is also used as remission induction therapy for severe UC, maintained clinical response after a median of 33 months in 68% of patients who had initially responded [
19]. Although the observation period was shorter in that than in the present study, the results were almost the same. In the present study, background factors with significant differences in the remission maintenance group were female sex, high total PSL dose during hospitalization, and long hospitalization period. As mentioned above, these findings are likely associated with the high rate of surgery avoidance and high compliance with oral administration among female patients. In addition, adequate use of steroids in the initial stage of remission induction may also benefit the long-term prognosis.
We found no significant differences in the presence or absence of 5-ASA allergy and AZA intolerance or the history of AZA use (
Table 2). In general, 5-ASA allergy and AZA intolerance are associated with poor prognosis, and in many such cases, make maintenance therapy difficult [
20,
21]. In the present study, these factors were not different between the maintenance and non-maintenance groups. Remission induction with TAC may be an effective option even in patients with 5-ASA allergy and AZA intolerance.
Drug allergy and intolerance are difficult to diagnose. Therefore, it is important to continue treatment while keeping discussions open between the patient and medical staff. In the treatment of inflammatory bowel disease, a chronic disease, it is important to maintain compliance with oral treatment [
22].
4.3. Drug-specific maintenance rates and patient characteristics
No significant differences were observed in 52-week maintenance rates between drugs. However, the maintenance rates differed between the AZA and Bio groups, suggesting the need to re-examine this topic in a larger number of patients. In general, maintenance with AZA is attempted after remission induction is achieved with TAC [
2].
As mentioned above, AZA intolerance is observed in roughly 10% of patients. In such cases, TAC use is considered to be difficult. In the present study, maintenance could be achieved with TAC or Bio even in patients in whom AZA use was not feasible. In patients on maintenance therapy with TAC after remission induction with TAC, one study reported a relapse rate at 300 days after remission induction of 32% [
23]. Thus, we believe it is possible to achieve maintenance with TAC alone. However, long-term use of TAC carries a risk of renal disorder [
24].
Regarding maintenance therapy with a Bio, patients in the present study were treated with ADA (n = 2), VED (n = 5), and UST (n = 1). A previous study on maintenance therapy with VED after TAC therapy found that VED was effective as maintenance therapy, with a rate of surgery avoidance of 67% after 1 year; the study reported no serious AEs, suggesting good safety [
25]. Another study on the use of VED as maintenance therapy after TAC found a rate of avoidance of intestinal resection at 11 months after remission induction with TAC of 68% [
26]. VED can be used in older patients with suspected infections and in those with a history of anti-TNF-α use because anti-drug antibodies are not easily produced [
27]; it is also used in patients with AZA intolerance. UST is used for remission induction and maintenance in moderate to serious cases [
3]. In the presen study, it was used as secondary therapy when ADA and VED were ineffective. The choice of UST as maintenance therapy was based on a report that clinical remission was achieved also in patients with insufficient response to VED and that remission was maintained at 44 weeks [
28]. In this study, maintenance therapy with anti-TNF-α (N=2) used ADA. In these two cases, ADA was used prior to tacrolimus treatment. However, since remission could not be achieved with ADA alone, tacrolimus was added to induce remission. Therefore, ADA was continued as maintenance therapy after induction of remission
As for patient characteristics by drug, AZA intolerance was significantly lower in the AZA group than in the TAC and Bio groups and was mild in many cases in the AZA group. In patients for whom AZA could not be used, maintenance was performed with TAC or a Bio. The advantages of AZA include not only its efficacy in maintaining remission, but also its ability to suppress the production of anti-drug antibodies. Moreover, when AZA is added to therapy with a Bio, e.g., VED, it can be expected to have additional effects in maintaining remission [
29]. AZA is considered to be an important drug also for long-term maintenance. Maintenance after TAC therapy requires further investigation in future studies.
4.4. AEs
AEs observed at the time of remission induction with TAC included tremors, renal disorder, and headache. Adjusting the trough levels of TAC allowed the patients with these AEs to continue treatment. Previous studies also reported tremors, renal disorder, and headache as AEs associated with TAC [
3,
24].
During maintenance therapy with AZA, the reported AEs were headache, cytopenia, nausea, hepatic disorder, hair loss/alopecia totalis, and pneumonia. The AZA dose was reduced in the patients with headache and nausea. Among the patients with cytopenia, AZA was either discontinued (in the patients with hepatic disorder, alopecia totalis, and pneumonia) or the dose was reduced.
As described above, AEs during AZA treatment can now be predicted to some extent by examining
NUDT15, at least in serious cases [
8]. However, in clinical practice, AEs can sometimes develop during continued AZA treatment [
30]. For this reason, the AEs that may occur during AZA treatment must be discussed thoroughly with patients.
During maintenance therapy with TAC, tremors and renal disorder occurred as AEs. The patients who developed renal disorder were encouraged to drink water. Patients with a decrease in glomerular filtration rate of 30% or more require appropriate examination of renal function because subsequent transition to chronic nephropathy can be expected [
24]. In patients with TAC tremors, a wait-and-see approach was taken, and improvement was observed by reducing TAC trough levels.
In the Bio group, AEs were pneumonia, influenza, and pneumomediastinum. The patients with pneumonia and influenza discontinued use of the Bio. Breakdown of adverse effects associated with biologics: pneumonia (n=1) and influenza (n=1) in ADA, pneumonia (n=1) in VED and (n=2) in UST resulted in pneumomediastinum. In the patients who developed pneumomediastinum, a wait-and-see approach was taken. Among infections that develop as AEs during Bio use, 50% are respiratory. In particular, anti-TNF-α agents reduce the immune defense against
Mycobacterium tuberculosis, leading to the development of tuberculosis. One study found that caution must be taken when patients with rheumatism and existing pulmonary disease use a Bio [
31].
4.5. Limitations
The limitations of the present study are that it was a retrospective study performed at a single facility, so some bias can be expected. Future challenges include increasing the number of patients and performing a multi-center prospective study.
5. Conclusions
TAC is highly effective in intractable UC, especially female, good responder and high dose steroid. This study suggests that after remission induction with TAC, TAC or a Bio can be used for long-term maintenance if patients cannot use AZA as maintenance therapy.
Author Contributions
Ayumi Ito and Katsutoshi Tokushige introduced the concept and design of the treatment protocol. Ayumi Ito and Shun Murasugi compiled and processed the patients’ data. Ayumi Ito wrote the paper’s draft. Ayumi Ito, Omori, Shinichi Nakamura, and Katsutoshi Tokushige read the paper, undertook critical revisions, and approved the final version of the paper.
Funding
The authors received no funding.
Ethics approval
The study protocol was reviewed and approved by the ethics review committee of Tokyo Women’s Medical University [approval number: 2022-0143].
Patient consent
Written and verbal informed consent was obtained from all patients
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 29, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Harbord, M.; Eliakim, R.; Bettenworth, D.; et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2, Current management. J Crohns Colitis 2017, 11, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Matsui, T.; Nakamura, M.; et al. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut 2006, 55, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- KomakiY; Komaki, F. ; Ido, A.; Sakurada, A. Efficacy and safety of tacrolimus therapy for active ulcerative colitis; a systematic review and meta-analysis. J Crohns Colitis 2016, 10, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Kobayashi, T.; Ueno, F.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol 2018, 53, 305–353. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.G.; Orchard, T.R.; Jewell, D.P. The efficacy of azathioprine for the treatment of inflammatory bowel disease: A 30 year review. Gut 2002, 50, 485–489. [Google Scholar] [CrossRef]
- Gearry, R.B.; Barclay, M.L.; Burt, M.J.; Collett, J.A.; Chapman, B.A. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol Drug Saf 2004, 13, 563–567. [Google Scholar] [CrossRef]
- Kakuta, Y.; Kawai, Y.; Okamoto, D.; et al. NUDT15 codon 139 is the best pharmacogenetic marker for predicting thiopurine-induced severe adverse events in Japanese patients with inflammatory bowel disease: A multicenter study. J Gastroenterol 2018, 53, 1065–1078. [Google Scholar] [CrossRef]
- Lichtiger, S.; Present, D.H.; Kornbluth, A.; et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994, 330, 1841–1845. [Google Scholar] [CrossRef]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Travis, S.P.; Schnell, D.; Krzeski, P.; et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology 2013, 145, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, S.D.; Kayal, M.; Shah, S.C. Sex-based differences in inflammatory bowel diseases: A review. Therap Adv Gastroenterol 2020, 13, 1756284820915043. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.L. Prevalence, predictors, and clinical consequences of medical adherence in IBD: How to improve it? World J Gastroenterol 2009, 15, 4234–4239. [Google Scholar] [CrossRef] [PubMed]
- Truelove, S.C.; Watkinson, G.; Draper, G. Comparison of corticosteroid and sulphasalazine therapy in ulcerative colitis. Br Med J 1962, 2, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.H.; Connell, A.M.; Kanaghinis, T.G.; Lennard-Jones, J.E.; Jones, A.F. Out-patient treatment of ulcerative colitis. Comparison between three doses of oral prednisone. Br Med J 1962, 2, 441–443. [Google Scholar] [CrossRef]
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, complications, and practice delivery issues. Ochsner J 2014, 14, 203–207. [Google Scholar]
- Miyoshi, J.; Matsuoka, K.; Inoue, N.; et al. Mucosal healing with oral tacrolimus is associated with favorable medium- and long-term prognosis in steroid-refractory/dependent ulcerative colitis patients. J Crohns Colitis 2013, 7, e609–e614. [Google Scholar] [CrossRef]
- Ito, A.; Murasugi, S.; Omori, T.; Nakamura, S.; Tokushige, K. Relationship between mucosal healing by tacrolimus and relapse of refractory ulcerative colitis: A retrospective study. BMC Gastroenterol 2020, 20, 203. [Google Scholar] [CrossRef]
- Ferrante, M.; Vermeire, S.; Fidder, H.; et al. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis 2008, 2, 219–225. [Google Scholar] [CrossRef]
- Mizuno, S.; Ono, K.; Mikami, Y.; et al. 5-aminosalicylic acid intolerance is associated with a risk of adverse clinical outcomes and dysbiosis in patients with ulcerative colitis. Intest Res 2020, 18, 69–78. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Gardezi, A.S.; Santharam, V.; Boyd, J.; Lanzon-Miller, S. Effect of azathioprine intolerance on outcomes of inflammatory bowel disease: A cross-sectional study. Frontline Gastroenterol 2014, 5, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Dasharathy, S.S.; Long, M.D.; Lackner, J.M.; et al. Psychological factors associated with adherence to oral treatment in ulcerative colitis. Inflamm Bowel Dis 2023, 29, 97–102. [Google Scholar] [CrossRef]
- Ito, A.; Iizuka, B.; Omori, T.; Nakamura, S.; Tokushige, K. Tacrolimus for remission induction and maintenance therapy in patients with ulcerative colitis: A retrospective evaluation study. Gastroenterol Res Pract 2016, 2016, 5956316. [Google Scholar] [CrossRef]
- Haga, K.; Shibuya, T.; Nomura, K.; et al. Effectiveness and nephrotoxicity of long-term tacrolimus administration in patients with ulcerative colitis. J Clin Med 2020, 9, 1771. [Google Scholar] [CrossRef] [PubMed]
- Ollech, J.E.; Dwadasi, S.; Rai, V.; et al. Efficacy and safety of induction therapy with calcineurin inhibitors followed by vedolizumab maintenance in 71 patients with severe steroid-refractory ulcerative colitis. Aliment Pharmacol Ther 2020, 51, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Baert, F.; Danese, S.; et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology 2020, 158, 562–572. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; Danese, S.; Targan, S.; Abreu, M.T.; Hisamatsu, T.; Szapary, P.; Marano, C.; UNIFI Study Group. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, M.; Watanabe, K.; Motoya, S.; et al. Potential benefits of immunomodulator use with vedolizumab for maintenance of remission in ulcerative colitis. J Gastroenterol Hepatol 2022, 37, 81–88. [Google Scholar] [CrossRef]
- Lamers, C.B.; Griffioen, G.; van Hogezand, R.A.; Veenendaal, R.A. Azathioprine: An update on clinical efficacy and safety in inflammatory bowel disease. Scand J Gastroenterol Suppl 1999, 230, 111–115. [Google Scholar]
- Tokuda, H.; Harigai, M.; Kameda, H.; et al. Consensus statements for medical practice: Biological agents and lung disease [Abridged English translation by the Japanese Respiratory Society]. Respir Investig 2017, 55, 229–251. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).