Submitted:
13 July 2023
Posted:
14 July 2023
You are already at the latest version
Abstract
Keywords:
1. Concept of scoliosis
2. Research progress on the pathogenesis of scoliosis
3. Animal models for IS research
4. The advantages and disadvantages of zebrafish as a scoliosis research model
5. Two uses of zebrafish model in IS research
| Related diseases | Gene | Animal model validation | Year | Reference | |
|---|---|---|---|---|---|
| Reissner Fiber | AIS | scospondin | zebrafish | 2018, 2020 | [89,90,91] |
| dnah10 | zebrafish | 2022 | [92] | ||
| spine curvature | camel | zebrafish | 2021 | [93] | |
| vangl2 | zebrafish | 2022 | [94] | ||
| IS | scospondin | zebrafish | 2020 | [95] | |
| katnb1 | zebrafish | 2022 | [96] | ||
| ccdc57 | zebrafish | 2023 | [97] | ||
| cilia | AIS | rpgrip1l | zebrafish | 2019 | [98] |
| cfap298 | zebrafish | 2021 | [99] | ||
| dnah10 | zebrafish | 2022 | [100] | ||
| ccdc57 | zebrafish | 2023 | [101] | ||
| IS | ttll11 | zebrafish | 2021 | [102] | |
| katnb1 | zebrafish | 2022 | [103] | ||
| ccdc57 | zebrafish | 2023 | [104] | ||
| CS, IS | ptk7 | zebrafish | 2014, 2016 | [26,105] | |
| IS, CSF stasis | kif6 | mouse, zebrafish | 2014, 2018 | [106,107] | |
| PCD | ccdc40 | zebrafish | 2011 | [108] | |
| dyx1c1 | mouse, zebrafish | 2013 | [109] | ||
| ccdc151 | mouse, zebrafish | 2014 | [110] | ||
| zmynd10 | medaka, zebrafish | 2017, 2018 | [111,112] | ||
| ciliopathies | c21orf59 | zebrafish | 2013 | [113] | |
| cep290 | zebrafish | 2019, 2022 | [114,115] | ||
| ciliopathy syndromes | bbs-5 | C. elegans, mouse, zebrafish | 2022 | [116] | |
| nphp-4 | |||||
| others | AIS | gpr126 | zebrafish | 2013 | [117] |
| mouse | 2019 | [118] | |||
| bnc2 | zebrafish | 2015 | [119] | ||
| mapk7 | zebrafish | 2018 | [120] | ||
| lbx1 | mouse, zebrafish | 2016, 2022, 2023 | [121,122,123] | ||
| uncx | zebrafish | 2023 | [124] | ||
| CS | Foxo4 | rat | 2019 | [125] | |
| IS | poc5 | zebrafish | 2015 | [126] | |
| kif7 | zebrafish | 2021 | [127] | ||
| ppp2r3b | zebrafish | 2023 | [128] | ||
| PCD | zmynd10 | zebrafish | 2018 | [129] | |
| kif3b | |||||
| uts2ra | |||||
| thoracic aortic aneurysm | col1a2 | zebrafish | 2022 | [130] | |
| col5a1 | |||||
| col5a2 | |||||
| fbn1 | |||||
| spine curvature | pkd2l1 | zebrafish | 2018 | [131] | |
| urp1 | zebrafish | 2022, 2023 | [132,133] | ||
| urp2 |
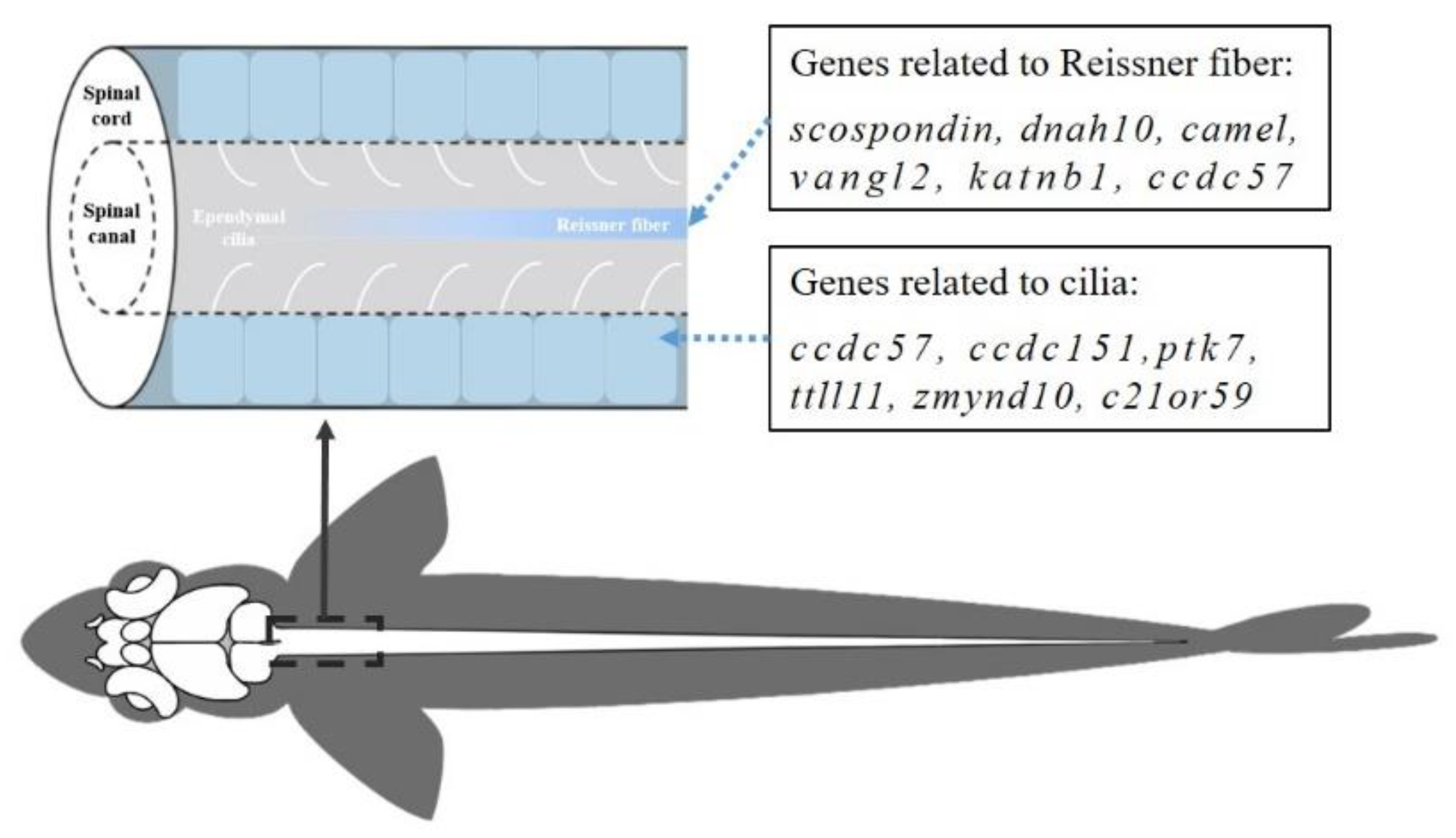
6. The relationship between cilia and IS was studied by zebrafish model
7. Conclusions and perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trobisch, P.; Suess, O.; Schwab, F. Idiopathic Scoliosis. Deutsches Ärzteblatt international 2010. [Google Scholar] [CrossRef] [PubMed]
- Vialle, R.; Thévenin-Lemoine, C.; Mary, P. Neuromuscular Scoliosis. Orthopaedics & Traumatology: Surgery & Research 2013, 99, S124–S139. [Google Scholar] [CrossRef]
- Konieczny, M.R.; Senyurt, H.; Krauspe, R. Epidemiology of Adolescent Idiopathic Scoliosis. J Child Orthop 2013, 7, 3–9. [Google Scholar] [CrossRef]
- Fadzan, M.; Bettany-Saltikov, J. Etiological Theories of Adolescent Idiopathic Scoliosis: Past and Present. TOORTHJ 2017, 11, 1466–1489. [Google Scholar] [CrossRef]
- Latalski, M.; Danielewicz-Bromberek, A.; Fatyga, M.; Latalska, M.; Kröber, M.; Zwolak, P. Current Insights into the Aetiology of Adolescent Idiopathic Scoliosis. Arch Orthop Trauma Surg 2017, 137, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Huebert, H.T. Scoliosis. A Brief History. Manit Med Rev 1967, 47, 452–456. [Google Scholar] [PubMed]
- Yan, B.; Lu, X.; Qiu, Q.; Nie, G.; Huang, Y. Association Between Incorrect Posture and Adolescent Idiopathic Scoliosis Among Chinese Adolescents: Findings From a Large-Scale Population-Based Study. Frontiers in Pediatrics 2020, 8. [Google Scholar] [CrossRef]
- Gréalou, L.; Aubin, C.-É.; Labelle, H. Rib Cage Surgery for the Treatment of Scoliosis: A Biomechanical Study of Correction Mechanisms. J. Orthop. Res. 2002, 20, 1121–1128. [Google Scholar] [CrossRef]
- Thillard, M.J. Vertebral column deformities following epiphysectomy in the chick. C R Hebd Seances Acad Sci 1959, 248, 1238–1240. [Google Scholar]
- O’Kelly, C.; Wang, X.; Raso, J.; Moreau, M.; Mahood, J.; Zhao, J.; Bagnall, K. The Production of Scoliosis after Pinealectomy in Young Chickens, Rats, and Hamsters. Spine (Phila Pa 1976) 1999, 24, 35–43. [Google Scholar] [CrossRef]
- Machida, M.; Dubousset, J.; Yamada, T.; Kimura, J.; Saito, M.; Shiraishi, T.; Yamagishi, M. Experimental Scoliosis in Melatonin-Deficient C57BL/6J Mice without Pinealectomy. J Pineal Res 2006, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.M.C.; Wang, T.; Poon, A.M.S.; Carl, A.; Tranmer, B.; Hu, Y.; Luk, K.D.K.; Leong, J.C.Y. The Effect of Pinealectomy on Scoliosis Development in Young Nonhuman Primates. Spine (Phila Pa 1976) 2005, 30, 2009–2013. [Google Scholar] [CrossRef]
- Day, G.; McPhee, I.; Tuffley, J.; Tomlinson, F.; Chaseling, R.; Kellie, S.; Torode, I.; Sherwood, M.; Cutbush, K.; Geddes, A.; et al. Idiopathic Scoliosis and Pineal Lesions in Australian Children. J Orthop Surg (Hong Kong) 2007, 15, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Gorman, K.F.; Julien, C.; Moreau, A. The Genetic Epidemiology of Idiopathic Scoliosis. Eur Spine J 2012, 21, 1905–1919. [Google Scholar] [CrossRef]
- Nowak, R.; Szota, J.; Mazurek, U. Vitamin D Receptor Gene (VDR) Transcripts in Bone, Cartilage, Muscles and Blood and Microarray Analysis of Vitamin D Responsive Genes Expression in Paravertebral Muscles of Juvenile and Adolescent Idiopathic Scoliosis Patients. BMC Musculoskelet Disord 2012, 13, 259. [Google Scholar] [CrossRef]
- Zhao, D.; Qiu, G.; Wang, Y.; Zhang, J.; Shen, J.; Wu, Z. Association between Adolescent Idiopathic Scoliosis with Double Curve and Polymorphisms of Calmodulin1 Gene/Estrogen Receptor-α Gene. Orthop Surg 2009, 1, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Burwell, R.G.; Clark, E.M.; Dangerfield, P.H.; Moulton, A. Adolescent Idiopathic Scoliosis (AIS): A Multifactorial Cascade Concept for Pathogenesis and Embryonic Origin. Scoliosis Spinal Disord 2016, 11, 8. [Google Scholar] [CrossRef]
- Raggio, C.L.; Giampietro, P.F.; Dobrin, S.; Zhao, C.; Dorshorst, D.; Ghebranious, N.; Weber, J.L.; Blank, R.D. A Novel Locus for Adolescent Idiopathic Scoliosis on Chromosome 12p. J Orthop Res 2009, 27, 1366–1372. [Google Scholar] [CrossRef]
- Alden, K.J.; Marosy, B.; Nzegwu, N.; Justice, C.M.; Wilson, A.F.; Miller, N.H. Idiopathic Scoliosis: Identification of Candidate Regions on Chromosome 19p13. Spine (Phila Pa 1976) 2006, 31, 1815–1819. [Google Scholar] [CrossRef]
- Gurnett, C.A.; Alaee, F.; Bowcock, A.; Kruse, L.; Lenke, L.G.; Bridwell, K.H.; Kuklo, T.; Luhmann, S.J.; Dobbs, M.B. Genetic Linkage Localizes an Adolescent Idiopathic Scoliosis and Pectus Excavatum Gene to Chromosome 18 q. Spine (Phila Pa 1976) 2009, 34, E94–100. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Z.; Zhuang, Q.; Fei, Q.; Zhang, J.; Liu, Y.; Wang, Y.; Ding, Y.; Qiu, G. Association Study of Tryptophan Hydroxylase 1 and Arylalkylamine N-Acetyltransferase Polymorphisms with Adolescent Idiopathic Scoliosis in Han Chinese. Spine (Phila Pa 1976) 2008, 33, 2199–2203. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qiu, Y.; Zhang, L.; Sun, Q.; Qiu, X.; He, Y. Association of Estrogen Receptor Gene Polymorphisms with Susceptibility to Adolescent Idiopathic Scoliosis. Spine (Phila Pa 1976) 2006, 31, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.S.; Tang, N.L.S.; Yeung, H.Y.; Lee, K.-M.; Hung, V.W.Y.; Ng, B.K.W.; Ma, S.L.; Kwok, R.H.K.; Qin, L.; Qiu, Y.; et al. Melatonin Receptor 1B (MTNR1B) Gene Polymorphism Is Associated With the Occurrence of Adolescent Idiopathic Scoliosis. Spine 2007, 32, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kou, I.; Takahashi, A.; Johnson, T.A.; Kono, K.; Kawakami, N.; Uno, K.; Ito, M.; Minami, S.; Yanagida, H.; et al. A Genome-Wide Association Study Identifies Common Variants near LBX1 Associated with Adolescent Idiopathic Scoliosis. Nat Genet 2011, 43, 1237–1240. [Google Scholar] [CrossRef]
- Hayes, M.; Gao, X.; Yu, L.X.; Paria, N.; Henkelman, R.M.; Wise, C.A.; Ciruna, B. Ptk7 Mutant Zebrafish Models of Congenital and Idiopathic Scoliosis Implicate Dysregulated Wnt Signalling in Disease. Nat Commun 2014, 5, 4777. [Google Scholar] [CrossRef]
- Hayes, M.; Gao, X.; Yu, L.X.; Paria, N.; Henkelman, R.M.; Wise, C.A.; Ciruna, B. Ptk7 Mutant Zebrafish Models of Congenital and Idiopathic Scoliosis Implicate Dysregulated Wnt Signalling in Disease. Nat Commun 2014, 5, 4777. [Google Scholar] [CrossRef]
- Su, Z.; Yang, Y.; Wang, S.; Zhao, S.; Zhao, H.; Li, X.; Niu, Y.; Qiu, G.; Wu, Z.; et al.; Deciphering Disorders Involving Scoliosis and COmorbidities (DISCO) Study Group The Mutational Landscape of PTK7 in Congenital Scoliosis and Adolescent Idiopathic Scoliosis. Genes 2021, 12, 1791. [Google Scholar] [CrossRef]
- Roshono, Y.; Kaneda, K.; Yamamoto, I. A Biomechanical Analysis of Zielke, Kaneda, and Cotrel-Dubousset Instrumentations in Thoracolumbar Scoliosis A Calf Spine Model. Spine 1991, 16, 1305–1311. [Google Scholar] [CrossRef]
- Braun, J.T.; Akyuz, E. Prediction of Curve Progression in a Goat Scoliosis Model. J Spinal Disord Tech 2005, 18, 272–276. [Google Scholar]
- Langenskiöld, A.; Michelsson, J.-E. EXPERIMENTAL PROGRESSIVE SCOLIOSIS IN THE RABBIT. The Journal of Bone and Joint Surgery. British volume 1961, 43-B, 116–120. [Google Scholar] [CrossRef]
- Kasuga, K. Experimental scoliosis in the rat spine induced by binding the spinous processes. Nihon Seikeigeka Gakkai Zasshi 1994, 68, 798–807. [Google Scholar]
- Bobyn, J.D.; Little, D.G.; Gray, R.; Schindeler, A. Animal Models of Scoliosis. J. Orthop. Res. 2015, 33, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Gorman, K.F.; Breden, F. Teleosts as Models for Human Vertebral Stability and Deformity. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2007, 145, 28–38. [Google Scholar] [CrossRef]
- Yoshihara, H.; Kawakami, N.; Matsuyama, Y.; Inoh, H.; Imagama, S.; Ishiguro, N. A Histomorphologic Study of Scoliosis in Pinealectomized Chickens. Spine (Phila Pa 1976) 2005, 30, 2244–2251. [Google Scholar] [CrossRef]
- Castelein, R.M.; van Dieën, J.H.; Smit, T.H. The Role of Dorsal Shear Forces in the Pathogenesis of Adolescent Idiopathic Scoliosis--a Hypothesis. Med Hypotheses 2005, 65, 501–508. [Google Scholar] [CrossRef]
- Boswell, C.W.; Ciruna, B. Understanding Idiopathic Scoliosis: A New Zebrafish School of Thought. Trends in Genetics 2017, 33, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Witten, P.E.; Harris, M.P.; Huysseune, A.; Winkler, C. Small Teleost Fish Provide New Insights into Human Skeletal Diseases. In Methods in Cell Biology; Elsevier, 2017; Volume 138, pp. 321–346. ISBN 978-0-12-803473-6. [Google Scholar]
- Fleming, A.; Keynes, R.; Tannahill, D. A Central Role for the Notochord in Vertebral Patterning. Development 2004, 131, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Bird, N.C.; Hernandez, L.P. Building an Evolutionary Innovation: Differential Growth in the Modified Vertebral Elements of the Zebrafish Weberian Apparatus. Zoology (Jena) 2009, 112, 97–112. [Google Scholar] [CrossRef]
- Grande, T.; Young, B. The Ontogeny and Homology of the Weberian Apparatus in the Zebrafish Danio Rerio (Ostariophysi: Cypriniformes). Zoological Journal of the Linnean Society 2004, 140, 241–254. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Harris, M.P.; Henke, K.; Hawkins, M.B.; Witten, P.E. Fish Is Fish: The Use of Experimental Model Species to Reveal Causes of Skeletal Diversity in Evolution and Disease. J. Appl. Ichthyol. 2014, 30, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Apschner, A.; Schulte-Merker, S.; Witten, P.E. Not All Bones Are Created Equal – Using Zebrafish and Other Teleost Species in Osteogenesis Research. In Methods in Cell Biology; Elsevier, 2011; Volume 105, pp. 239–255. ISBN 978-0-12-381320-6. [Google Scholar]
- Andoniadou, C.L.; Martinez-Barbera, J.P. Developmental Mechanisms Directing Early Anterior Forebrain Specification in Vertebrates. Cell. Mol. Life Sci. 2013, 70, 3739–3752. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/β-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, S.M.K.; Neuhauss, S.C.F. Whole-Genome Duplication in Teleost Fishes and Its Evolutionary Consequences. Mol Genet Genomics 2014, 289, 1045–1060. [Google Scholar] [CrossRef]
- Busse, B.; Galloway, J.L.; Gray, R.S.; Harris, M.P.; Kwon, R.Y. Zebrafish: An Emerging Model for Orthopedic Research. J. Orthop. Res. 2020, 38, 925–936. [Google Scholar] [CrossRef]
- Bergen, D.J.M.; Kague, E.; Hammond, C.L. Zebrafish as an Emerging Model for Osteoporosis: A Primary Testing Platform for Screening New Osteo-Active Compounds. Front. Endocrinol. 2019, 10, 6. [Google Scholar] [CrossRef]
- Kwon, R.Y.; Watson, C.J.; Karasik, D. Using Zebrafish to Study Skeletal Genomics. Bone 2019, 126, 37–50. [Google Scholar] [CrossRef]
- Kou, I.; Takahashi, Y.; Johnson, T.A.; Takahashi, A.; Guo, L.; Dai, J.; Qiu, X.; Sharma, S.; Takimoto, A.; Ogura, Y.; et al. Genetic Variants in GPR126 Are Associated with Adolescent Idiopathic Scoliosis. Nat Genet 2013, 45, 676–679. [Google Scholar] [CrossRef]
- Patten, S.A.; Margaritte-Jeannin, P.; Bernard, J.-C.; Alix, E.; Labalme, A.; Besson, A.; Girard, S.L.; Fendri, K.; Fraisse, N.; Biot, B.; et al. Functional Variants of POC5 Identified in Patients with Idiopathic Scoliosis. J. Clin. Invest. 2015, 125, 1124–1128. [Google Scholar] [CrossRef]
- Guo, L.; Yamashita, H.; Kou, I.; Takimoto, A.; Meguro-Horike, M.; Horike, S.; Sakuma, T.; Miura, S.; Adachi, T.; Yamamoto, T.; et al. Functional Investigation of a Non-Coding Variant Associated with Adolescent Idiopathic Scoliosis in Zebrafish: Elevated Expression of the Ladybird Homeobox Gene Causes Body Axis Deformation. PLoS Genet 2016, 12, e1005802. [Google Scholar] [CrossRef]
- Ogura, Y.; Kou, I.; Miura, S.; Takahashi, A.; Xu, L.; Takeda, K.; Takahashi, Y.; Kono, K.; Kawakami, N.; Uno, K.; et al. A Functional SNP in BNC2 Is Associated with Adolescent Idiopathic Scoliosis. The American Journal of Human Genetics 2015, 97, 337–342. [Google Scholar] [CrossRef]
- Sharma, S.; Londono, D.; Eckalbar, W.L.; Gao, X.; Zhang, D.; Mauldin, K.; Kou, I.; Takahashi, A.; et al.; TSRHC Scoliosis Clinical Group; Japan Scoliosis Clinical Research Group A PAX1 Enhancer Locus Is Associated with Susceptibility to Idiopathic Scoliosis in Females. Nat Commun 2015, 6, 6452. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, C.; Xu, C.; Zhou, H.; Gao, B.; Su, D.; Liao, Z.; Li, Y.; Yang, S.; Su, P. Mutant MAPK7-Induced Idiopathic Scoliosis Is Linked to Impaired Osteogenesis. Cell Physiol Biochem 2018, 48, 880–890. [Google Scholar] [CrossRef]
- Mathieu, H.; Patten, S.A.; Aragon-Martin, J.A.; Ocaka, L.; Simpson, M.; Child, A.; Moldovan, F. Genetic Variant of TTLL11 Gene and Subsequent Ciliary Defects Are Associated with Idiopathic Scoliosis in a 5-Generation UK Family. Sci Rep 2021, 11, 11026. [Google Scholar] [CrossRef]
- Su, Z.; Yang, Y.; Wang, S.; Zhao, S.; Zhao, H.; Li, X.; Niu, Y.; Qiu, G.; Wu, Z.; et al.; Deciphering Disorders Involving Scoliosis and COmorbidities (DISCO) Study Group The Mutational Landscape of PTK7 in Congenital Scoliosis and Adolescent Idiopathic Scoliosis. Genes 2021, 12, 1791. [Google Scholar] [CrossRef]
- Bradley, E.C.; Cunningham, R.L.; Wilde, C.; Morgan, R.K.; Klug, E.A.; Letcher, S.M.; Schöneberg, T.; Monk, K.R.; Liebscher, I.; Petersen, S.C. In Vivo Identification of Small Molecules Mediating Gpr126/Adgrg6 Signaling during Schwann Cell Development. Ann. N.Y. Acad. Sci. 2019, 1456, 44–63. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Z.; Zhuang, Q.; Fei, Q.; Zhang, J.; Liu, Y.; Wang, Y.; Ding, Y.; Qiu, G. Association Study of Tryptophan Hydroxylase 1 and Arylalkylamine N-Acetyltransferase Polymorphisms With Adolescent Idiopathic Scoliosis in Han Chinese. Spine 2008, 33, 2199–2203. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gao, X.; Londono, D.; Devroy, S.E.; Mauldin, K.N.; Frankel, J.T.; Brandon, J.M.; Zhang, D.; Li, Q.-Z.; Dobbs, M.B.; et al. Genome-Wide Association Studies of Adolescent Idiopathic Scoliosis Suggest Candidate Susceptibility Genes. Human Molecular Genetics 2011, 20, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Kou, I.; Takahashi, Y.; Johnson, T.A.; Takahashi, A.; Guo, L.; Dai, J.; Qiu, X.; Sharma, S.; Takimoto, A.; Ogura, Y.; et al. Genetic Variants in GPR126 Are Associated with Adolescent Idiopathic Scoliosis. Nat Genet 2013, 45, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Lv, F.; Zhu, Z.; Qian, B.; Wang, B.; Yu, Y.; Qiu, Y. Lack of Association between the CHL1 Gene and Adolescent Idiopathic Scoliosis Susceptibility in Han Chinese: A Case-Control Study. BMC Musculoskelet Disord 2014, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Kou, I.; Miura, S.; Takahashi, A.; Xu, L.; Takeda, K.; Takahashi, Y.; Kono, K.; Kawakami, N.; Uno, K.; et al. A Functional SNP in BNC2 Is Associated with Adolescent Idiopathic Scoliosis. The American Journal of Human Genetics 2015, 97, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Tang, N.L.-S.; Xu, L.; Qin, X.; Mao, S.; Song, Y.; Liu, L.; Li, F.; Liu, P.; Yi, L.; et al. Genome-Wide Association Study Identifies New Susceptibility Loci for Adolescent Idiopathic Scoliosis in Chinese Girls. Nat Commun 2015, 6, 8355. [Google Scholar] [CrossRef] [PubMed]
- Chettier, R.; Nelson, L.; Ogilvie, J.W.; Albertsen, H.M.; Ward, K. Haplotypes at LBX1 Have Distinct Inheritance Patterns with Opposite Effects in Adolescent Idiopathic Scoliosis. PLoS ONE 2015, 10, e0117708. [Google Scholar] [CrossRef]
- Nada, D.; Julien, C.; Samuels, M.E.; Moreau, A. A Replication Study for Association of LBX1 Locus With Adolescent Idiopathic Scoliosis in French–Canadian Population. Spine 2018, 43, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Kou, I.; Otomo, N.; Takeda, K.; Momozawa, Y.; Lu, H.-F.; Kubo, M.; Kamatani, Y.; Ogura, Y.; Takahashi, Y.; Nakajima, M.; et al. Genome-Wide Association Study Identifies 14 Previously Unreported Susceptibility Loci for Adolescent Idiopathic Scoliosis in Japanese. Nat Commun 2019, 10, 3685. [Google Scholar] [CrossRef]
- Xia, C.; Xu, L.; Xue, B.; Sheng, F.; Qiu, Y.; Zhu, Z. Rare Variant of HSPG2 Is Not Involved in the Development of Adolescent Idiopathic Scoliosis: Evidence from a Large-Scale Replication Study. BMC Musculoskelet Disord 2019, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Sheng, F.; Xia, C.; Xu, L.; Qin, X.; Tang, N.L.-S.; Qiu, Y.; Cheng, J.C.-Y.; Zhu, Z. New Evidence Supporting the Role of FBN1 in the Development of Adolescent Idiopathic Scoliosis. Spine 2019, 44, E225–E232. [Google Scholar] [CrossRef]
- Xu, L.; Sheng, F.; Xia, C.; Li, Y.; Feng, Z.; Qiu, Y.; Zhu, Z. Common Variant of POC5 Is Associated With the Susceptibility of Adolescent Idiopathic Scoliosis. Spine 2018, 43, E683–E688. [Google Scholar] [CrossRef]
- Mathieu, H.; Spataru, A.; Aragon-Martin, J.A.; Child, A.; Barchi, S.; Fortin, C.; Parent, S.; Moldovan, F. Prevalence of POC5 Coding Variants in French-Canadian and British AIS Cohort. Genes 2021, 12, 1032. [Google Scholar] [CrossRef]
- Terhune, E.A.; Wethey, C.I.; Cuevas, M.T.; Monley, A.M.; Baschal, E.E.; Bland, M.R.; Baschal, R.; Trahan, G.D.; Taylor, M.R.G.; Jones, K.L.; et al. Whole Exome Sequencing of 23 Multigeneration Idiopathic Scoliosis Families Reveals Enrichments in Cytoskeletal Variants, Suggests Highly Polygenic Disease. Genes 2021, 12, 922. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, S.; Liu, L.; Du, H.; Zhao, H.; Wang, S.; Niu, Y.; Li, X.; Qiu, G.; Wu, Z.; et al. Aberrant Interaction between Mutated ADAMTSL2 and LTBP4 Is Associated with Adolescent Idiopathic Scoliosis. Gene 2022, 814, 146126. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-R.; Chou, P.-H.; Huang, K.-J.; Ting, J.; Liu, C.-Y.; Chou, W.-H.; Lin, G.-H.; Chang, J.-G.; Ikegawa, S.; Wang, S.-T.; et al. Whole-Exome Sequencing Identifies Genetic Variants for Severe Adolescent Idiopathic Scoliosis in a Taiwanese Population. JPM 2022, 13, 32. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Shu, L.; Meng, Y.; Ma, J.; Gao, R.; Zhou, X. A Genetic Variant of the ROBO3 Gene Is Associated With Adolescent Idiopathic Scoliosis in the Chinese Population. Spine 2023, 48, E20–E24. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Londono, D.; Eckalbar, W.L.; Gao, X.; Zhang, D.; Mauldin, K.; Kou, I.; Takahashi, A.; Matsumoto, M.; Kamiya, N.; et al. A PAX1 Enhancer Locus Is Associated with Susceptibility to Idiopathic Scoliosis in Females. Nat Commun 2015, 6, 6452. [Google Scholar] [CrossRef]
- Einarsdottir, E.; Grauers, A.; Wang, J.; Jiao, H.; Escher, S.A.; Danielsson, A.; Simony, A.; Andersen, M.; Christensen, S.B.; Åkesson, K.; et al. CELSR2 Is a Candidate Susceptibility Gene in Idiopathic Scoliosis. PLoS ONE 2017, 12, e0189591. [Google Scholar] [CrossRef] [PubMed]
- Terhune, E.A.; Cuevas, M.T.; Monley, A.M.; Wethey, C.I.; Chen, X.; Cattell, M.V.; Bayrak, M.N.; Bland, M.R.; Sutphin, B.; Trahan, G.D.; et al. Mutations in KIF7 Implicated in Idiopathic Scoliosis in Humans and Axial Curvatures in Zebrafish. Human Mutation 2021, 42, 392–407. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Liu, S.; Song, X.; Yang, X.-Z.; Fan, Y.; Chen, W.; Akdemir, Z.C.; Yan, Z.; et al.; DISCO (Deciphering disorders Involving Scoliosis and COmorbidities) Study The Coexistence of Copy Number Variations (CNVs) and Single Nucleotide Polymorphisms (SNPs) at a Locus Can Result in Distorted Calculations of the Significance in Associating SNPs to Disease. Hum Genet 2018, 137, 553–567. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, Y.; Li, J.; Zhu, Z.; Qiu, Y.; Liu, Z. Bioinformatics Analysis and Experimental Verification Identify Downregulation of COL27A1 in Poor Segmental Congenital Scoliosis. Computational and Mathematical Methods in Medicine 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Lu, C.; Yang, D.; Lei, C.; Wang, R.; Guo, T.; Luo, H. Identification of Two Novel DNAAF2 Variants in Two Consanguineous Families with Primary Ciliary Dyskinesia. PGPM 2021, Volume 14, 1415–1423. [Google Scholar] [CrossRef]
- Tan, Z.; Shek, H.T.; Chen, P.; Dong, Z.; Zhou, Y.; Yin, S.; Qiu, A.; Dong, L.; Gao, B.; To, M.K.T. Clinical Features and Molecular Characterization of Chinese Patients with FKBP10 Variants. Molec Gen & Gen Med 2023, 11, e2122. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, S.; Hu, W.; Jin, P. Analysis of a child with Marfan syndrome due to a novel variant of FBN1 gene. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2023, 40, 62–65. [Google Scholar] [CrossRef]
- Hendrickx, G.; Boudin, E.; Steenackers, E.; Collet, C.; Mortier, G.R.; Geneviève, D.; Van Hul, W. A Recessive Form of Craniodiaphyseal Dysplasia Caused by a Homozygous Missense Variant in SP7/Osterix. Bone 2023, 167, 116633. [Google Scholar] [CrossRef]
- Hjeij, R.; Onoufriadis, A.; Watson, C.M.; Slagle, C.E.; Klena, N.T.; Dougherty, G.W.; Kurkowiak, M.; Loges, N.T.; Diggle, C.P.; Morante, N.F.C.; et al. CCDC151 Mutations Cause Primary Ciliary Dyskinesia by Disruption of the Outer Dynein Arm Docking Complex Formation. Am J Hum Genet 2014, 95, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Tarkar, A.; Loges, N.T.; Slagle, C.E.; Francis, R.; Dougherty, G.W.; Tamayo, J.V.; Shook, B.; Cantino, M.; Schwartz, D.; Jahnke, C.; et al. DYX1C1 Is Required for Axonemal Dynein Assembly and Ciliary Motility. Nat Genet 2013, 45, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Becker-Heck, A.; Zohn, I.E.; Okabe, N.; Pollock, A.; Lenhart, K.B.; Sullivan-Brown, J.; McSheene, J.; Loges, N.T.; Olbrich, H.; Haeffner, K.; et al. The Coiled-Coil Domain Containing Protein CCDC40 Is Essential for Motile Cilia Function and Left-Right Axis Formation. Nat Genet 2011, 43, 79–84. [Google Scholar] [CrossRef]
- Austin-Tse, C.; Halbritter, J.; Zariwala, M.A.; Gilberti, R.M.; Gee, H.Y.; Hellman, N.; Pathak, N.; Liu, Y.; Panizzi, J.R.; Patel-King, R.S.; et al. Zebrafish Ciliopathy Screen Plus Human Mutational Analysis Identifies C21orf59 and CCDC65 Defects as Causing Primary Ciliary Dyskinesia. The American Journal of Human Genetics 2013, 93, 672–686. [Google Scholar] [CrossRef] [PubMed]
- Cantaut-Belarif, Y.; Sternberg, J.R.; Thouvenin, O.; Wyart, C.; Bardet, P.-L. The Reissner Fiber in the Cerebrospinal Fluid Controls Morphogenesis of the Body Axis. Current Biology 2018, 28, 2479–2486. [Google Scholar] [CrossRef]
- Troutwine, B.R.; Gontarz, P.; Konjikusic, M.J.; Minowa, R.; Monstad-Rios, A.; Sepich, D.S.; Kwon, R.Y.; Solnica-Krezel, L.; Gray, R.S. The Reissner Fiber Is Highly Dynamic In Vivo and Controls Morphogenesis of the Spine. Current Biology 2020, 30, 2353–2362. [Google Scholar] [CrossRef]
- Rose, C.D.; Pompili, D.; Henke, K.; Van Gennip, J.L.M.; Meyer-Miner, A.; Rana, R.; Gobron, S.; Harris, M.P.; Nitz, M.; Ciruna, B. SCO-Spondin Defects and Neuroinflammation Are Conserved Mechanisms Driving Spinal Deformity across Genetic Models of Idiopathic Scoliosis. Current Biology 2020, 30, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Troutwine, B.R.; Zhang, H.; Gray, R.S. The Axonemal Dynein Heavy Chain 10 Gene Is Essential for Monocilia Motility and Spine Alignment in Zebrafish. Developmental Biology 2022, 482, 82–90. [Google Scholar] [CrossRef]
- Yang, S.; Emelyanov, A.; You, M.-S.; Sin, M.; Korzh, V. Camel Regulates Development of the Brain Ventricular System. Cell Tissue Res 2021, 383, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Jussila, M.; Boswell, C.W.; Griffiths, N.W.; Pumputis, P.G.; Ciruna, B. Live Imaging and Conditional Disruption of Native PCP Activity Using Endogenously Tagged Zebrafish SfGFP-Vangl2. Nat Commun 2022, 13, 5598. [Google Scholar] [CrossRef]
- Lu, H.; Shagirova, A.; Goggi, J.L.; Yeo, H.L.; Roy, S. Reissner Fibre-Induced Urotensin Signalling from Cerebrospinal Fluid-Contacting Neurons Prevents Scoliosis of the Vertebrate Spine. Biology Open 2020, 9, bio052027. [Google Scholar] [CrossRef]
- Meyer-Miner, A.; Van Gennip, J.L.M.; Henke, K.; Harris, M.P.; Ciruna, B. Resolving Primary Pathomechanisms Driving Idiopathic-like Spinal Curvature Using a New Katnb1 Scoliosis Model. iScience 2022, 25, 105028. [Google Scholar] [CrossRef]
- Xie, H.; Kang, Y.; Liu, J.; Huang, M.; Dai, Z.; Shi, J.; Wang, S.; Li, L.; Li, Y.; Zheng, P.; et al. Ependymal Polarity Defects Coupled with Disorganized Ciliary Beating Drive Abnormal Cerebrospinal Fluid Flow and Spine Curvature in Zebrafish. PLoS Biol 2023, 21, e3002008. [Google Scholar] [CrossRef]
- Vesque, C.; Anselme, I.; Pezeron, G.; Cantaut-Belarif, Y.; Eschstruth, A.; Djebar, M.; Santos, D.L.; Ribeuz, H.L.; Jenett, A.; Khoury, H.; et al. Loss of the Reissner Fiber and Increased URP Neuropeptide Signaling Underlie Scoliosis in a Zebrafish Ciliopathy Mutant; Developmental Biology, 2019.
- Marie-Hardy, L.; Cantaut-Belarif, Y.; Pietton, R.; Slimani, L.; Pascal-Moussellard, H. The Orthopedic Characterization of Cfap298tm304 Mutants Validate Zebrafish to Faithfully Model Human AIS. Sci Rep 2021, 11, 7392. [Google Scholar] [CrossRef]
- Wang, Y.; Troutwine, B.R.; Zhang, H.; Gray, R.S. The Axonemal Dynein Heavy Chain 10 Gene Is Essential for Monocilia Motility and Spine Alignment in Zebrafish. Developmental Biology 2022, 482, 82–90. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Ou, Y.; Wu, J.; Li, H.; Wang, X.; Tang, L.; Dai, X.; Yang, C.; Wei, Z.; et al. Ccdc57 Is Required for Straightening the Body Axis by Regulating Ciliary Motility in the Brain Ventricle of Zebrafish. Journal of Genetics and Genomics 2023, 50, 253–263. [Google Scholar] [CrossRef]
- Mathieu, H.; Patten, S.A.; Aragon-Martin, J.A.; Ocaka, L.; Simpson, M.; Child, A.; Moldovan, F. Genetic Variant of TTLL11 Gene and Subsequent Ciliary Defects Are Associated with Idiopathic Scoliosis in a 5-Generation UK Family. Sci Rep 2021, 11, 11026. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Miner, A.; Van Gennip, J.L.M.; Henke, K.; Harris, M.P.; Ciruna, B. Resolving Primary Pathomechanisms Driving Idiopathic-like Spinal Curvature Using a New Katnb1 Scoliosis Model. iScience 2022, 25, 105028. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Kang, Y.; Liu, J.; Huang, M.; Dai, Z.; Shi, J.; Wang, S.; Li, L.; Li, Y.; Zheng, P.; et al. Ependymal Polarity Defects Coupled with Disorganized Ciliary Beating Drive Abnormal Cerebrospinal Fluid Flow and Spine Curvature in Zebrafish. PLoS Biol 2023, 21, e3002008. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.T.; Boswell, C.W.; Morante, N.F.C.; Henkelman, R.M.; Burdine, R.D.; Ciruna, B. Zebrafish Models of Idiopathic Scoliosis Link Cerebrospinal Fluid Flow Defects to Spine Curvature. Science 2016, 352, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Buchan, J.G.; Gray, R.S.; Gansner, J.M.; Alvarado, D.M.; Burgert, L.; Gitlin, J.D.; Gurnett, C.A.; Goldsmith, M.I. Kinesin Family Member 6 (Kif6) Is Necessary for Spine Development in Zebrafish: KIF6 in Zebrafish Spine Development. Dev. Dyn. 2014, 243, 1646–1657. [Google Scholar] [CrossRef]
- Konjikusic, M.J.; Yeetong, P.; Boswell, C.W.; Lee, C.; Roberson, E.C.; Ittiwut, R.; Suphapeetiporn, K.; Ciruna, B.; Gurnett, C.A.; Wallingford, J.B.; et al. Mutations in Kinesin Family Member 6 Reveal Specific Role in Ependymal Cell Ciliogenesis and Human Neurological Development. PLoS Genet 2018, 14, e1007817. [Google Scholar] [CrossRef]
- Becker-Heck, A.; Zohn, I.E.; Okabe, N.; Pollock, A.; Lenhart, K.B.; Sullivan-Brown, J.; McSheene, J.; Loges, N.T.; Olbrich, H.; Haeffner, K.; et al. The Coiled-Coil Domain Containing Protein CCDC40 Is Essential for Motile Cilia Function and Left-Right Axis Formation. Nat Genet 2011, 43, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Tarkar, A.; Loges, N.T.; Slagle, C.E.; Francis, R.; Dougherty, G.W.; Tamayo, J.V.; Shook, B.; Cantino, M.; Schwartz, D.; et al.; UK10K DYX1C1 Is Required for Axonemal Dynein Assembly and Ciliary Motility. Nat Genet 2013, 45, 995–1003. [Google Scholar] [CrossRef]
- Hjeij, R.; Onoufriadis, A.; Watson, C.M.; Slagle, C.E.; Klena, N.T.; Dougherty, G.W.; Kurkowiak, M.; Loges, N.T.; Diggle, C.P.; Morante, N.F.C.; et al. CCDC151 Mutations Cause Primary Ciliary Dyskinesia by Disruption of the Outer Dynein Arm Docking Complex Formation. The American Journal of Human Genetics 2014, 95, 257–274. [Google Scholar] [CrossRef]
- Kobayashi, D.; Asano-Hoshino, A.; Nakakura, T.; Nishimaki, T.; Ansai, S.; Kinoshita, M.; Ogawa, M.; Hagiwara, H.; Yokoyama, T. Loss of Zinc Finger MYND-Type Containing 10 (Zmynd10) Affects Cilia Integrity and Axonemal Localization of Dynein Arms, Resulting in Ciliary Dysmotility, Polycystic Kidney and Scoliosis in Medaka (Oryzias Latipes). Developmental Biology 2017, 430, 69–79. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, S.; Chen, Z.; Chong, Y.L.; Xie, H.; Feng, D.; Wu, X.; Song, D.Z.; Roy, S.; Zhao, C. Cilia-Driven Cerebrospinal Fluid Flow Directs Expression of Urotensin Neuropeptides to Straighten the Vertebrate Body Axis. Nat Genet 2018, 50, 1666–1673. [Google Scholar] [CrossRef]
- Austin-Tse, C.; Halbritter, J.; Zariwala, M.A.; Gilberti, R.M.; Gee, H.Y.; Hellman, N.; Pathak, N.; Liu, Y.; Panizzi, J.R.; Patel-King, R.S.; et al. Zebrafish Ciliopathy Screen Plus Human Mutational Analysis Identifies C21orf59 and CCDC65 Defects as Causing Primary Ciliary Dyskinesia. The American Journal of Human Genetics 2013, 93, 672–686. [Google Scholar] [CrossRef]
- Lessieur, E.M.; Song, P.; Nivar, G.C.; Piccillo, E.M.; Fogerty, J.; Rozic, R.; Perkins, B.D. Ciliary Genes Arl13b, Ahi1 and Cc2d2a Differentially Modify Expression of Visual Acuity Phenotypes but Do Not Enhance Retinal Degeneration Due to Mutation of Cep290 in Zebrafish. PLoS ONE 2019, 14, e0213960. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Thomas, H.R.; Thompson, R.G.; Waldrep, S.C.; Fogerty, J.; Song, P.; Li, Z.; Ma, Y.; Santra, P.; Hoover, J.D.; et al. Variable Phenotypes and Penetrance between and within Different Zebrafish Ciliary Transition Zone Mutants. Disease Models & Mechanisms 2022, 15, dmm049568. [Google Scholar] [CrossRef]
- Bentley-Ford, M.R.; LaBonty, M.; Thomas, H.R.; Haycraft, C.J.; Scott, M.; LaFayette, C.; Croyle, M.J.; Andersen, R.S.; Parant, J.M.; Yoder, B.K. Evolutionarily Conserved Genetic Interactions between Nphp-4 and Bbs-5 Mutations Exacerbate Ciliopathy Phenotypes. Genetics 2022, 220, iyab209. [Google Scholar] [CrossRef] [PubMed]
- Kou, I.; Takahashi, Y.; Johnson, T.A.; Takahashi, A.; Guo, L.; Dai, J.; Qiu, X.; Sharma, S.; Takimoto, A.; Ogura, Y.; et al. Genetic Variants in GPR126 Are Associated with Adolescent Idiopathic Scoliosis. Nat Genet 2013, 45, 676–679. [Google Scholar] [CrossRef]
- Musa, G.; Cazorla-Vázquez, S.; Amerongen, M.J.; Stemmler, M.P.; Eckstein, M.; Hartmann, A.; Braun, T.; Brabletz, T.; Engel, F.B. Gpr126 (Adgrg6) Is Expressed in Cell Types Known to Be Exposed to Mechanical Stimuli. Ann. N.Y. Acad. Sci. 2019, 1456, 96–108. [Google Scholar] [CrossRef]
- Ogura, Y.; Kou, I.; Miura, S.; Takahashi, A.; Xu, L.; Takeda, K.; Takahashi, Y.; Kono, K.; Kawakami, N.; Uno, K.; et al. A Functional SNP in BNC2 Is Associated with Adolescent Idiopathic Scoliosis. The American Journal of Human Genetics 2015, 97, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, C.; Xu, C.; Zhou, H.; Gao, B.; Su, D.; Liao, Z.; Li, Y.; Yang, S.; Su, P. Mutant MAPK7-Induced Idiopathic Scoliosis Is Linked to Impaired Osteogenesis. Cell Physiol Biochem 2018, 48, 880–890. [Google Scholar] [CrossRef]
- Guo, L.; Yamashita, H.; Kou, I.; Takimoto, A.; Meguro-Horike, M.; Horike, S.; Sakuma, T.; Miura, S.; Adachi, T.; Yamamoto, T.; et al. Functional Investigation of a Non-Coding Variant Associated with Adolescent Idiopathic Scoliosis in Zebrafish: Elevated Expression of the Ladybird Homeobox Gene Causes Body Axis Deformation. PLoS Genet 2016, 12, e1005802. [Google Scholar] [CrossRef]
- Decourtye, L.; McCallum-Loudeac, J.A.; Zellhuber-McMillan, S.; Young, E.; Sircombe, K.J.; Wilson, M.J. Characterization of a Novel Lbx1 Mouse Loss of Function Strain. Differentiation 2022, 123, 30–41. [Google Scholar] [CrossRef]
- Matsuhashi, Y.; Horiuchi, K.; Nakagawa, T.; Takahashi, Y.; Imabayashi, H.; Hosogane, N.; Watanabe, K.; Matsumoto, M.; Chiba, K. Abrogation of LBX1 in Skeletal Muscle Results in Hypoplastic Limbs and Progressive Kyphosis in Mice. Journal Orthopaedic Research 2023, 41, 884–890. [Google Scholar] [CrossRef]
- Yonezawa, Y.; Guo, L.; Kakinuma, H.; Otomo, N.; Yoshino, S.; Takeda, K.; Nakajima, M.; Shiraki, T.; Ogura, Y.; Takahashi, Y.; et al. Identification of a Functional Susceptibility Variant for Adolescent Idiopathic Scoliosis That Upregulates Early Growth Response 1 (EGR1)-Mediated UNCX Expression. J of Bone & Mineral Res 2023, 38, 144–153. [Google Scholar] [CrossRef]
- Chen, C.; Tan, H.; Bi, J.; Li, L.; Rong, T.; Lin, Y.; Sun, P.; Liang, J.; Jiao, Y.; Li, Z.; et al. LncRNA-SULT1C2A Regulates Foxo4 in Congenital Scoliosis by Targeting Rno-miR-466c-5p through PI3K-ATK Signalling. J Cell Mol Med 2019, 23, 4582–4591. [Google Scholar] [CrossRef]
- Patten, S.A.; Margaritte-Jeannin, P.; Bernard, J.-C.; Alix, E.; Labalme, A.; Besson, A.; Girard, S.L.; Fendri, K.; Fraisse, N.; Biot, B.; et al. Functional Variants of POC5 Identified in Patients with Idiopathic Scoliosis. J. Clin. Invest. 2015, 125, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Terhune, E.A.; Cuevas, M.T.; Monley, A.M.; Wethey, C.I.; Chen, X.; Cattell, M.V.; Bayrak, M.N.; Bland, M.R.; Sutphin, B.; Trahan, G.D.; et al. Mutations in KIF7 Implicated in Idiopathic Scoliosis in Humans and Axial Curvatures in Zebrafish. Human Mutation 2021, 42, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Seda, M.; Crespo, B.; Corcelli, M.; Osborn, D.P.; Jenkins, D. A CRISPR/Cas9-Generated Mutation in the Zebrafish Orthologue of PPP2R3B Causes Idiopathic Scoliosis. Sci Rep 2023, 13, 6783. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, S.; Chen, Z.; Chong, Y.L.; Xie, H.; Feng, D.; Wu, X.; Song, D.Z.; Roy, S.; Zhao, C. Cilia-Driven Cerebrospinal Fluid Flow Directs Expression of Urotensin Neuropeptides to Straighten the Vertebrate Body Axis. Nat Genet 2018, 50, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.; Ziganshin, B.A.; Papanikolaou, D.; Zafar, M.A.; Nicoli, S.; Mukherjee, S.; Elefteriades, J.A. Phenotyping Zebrafish Mutant Models to Assess Candidate Genes Associated with Aortic Aneurysm. Genes 2022, 13, 123. [Google Scholar] [CrossRef]
- Sternberg, J.R.; Prendergast, A.E.; Brosse, L.; Cantaut-Belarif, Y.; Thouvenin, O.; Orts-Del’Immagine, A.; Castillo, L.; Djenoune, L.; Kurisu, S.; McDearmid, J.R.; et al. Pkd2l1 Is Required for Mechanoception in Cerebrospinal Fluid-Contacting Neurons and Maintenance of Spine Curvature. Nat Commun 2018, 9, 3804. [Google Scholar] [CrossRef]
- Bearce, E.A.; Irons, Z.H.; O’Hara-Smith, J.R.; Kuhns, C.J.; Fisher, S.I.; Crow, W.E.; Grimes, D.T. Urotensin II-Related Peptides, Urp1 and Urp2, Control Zebrafish Spine Morphology. eLife 2022, 11, e83883. [Google Scholar] [CrossRef]
- Gaillard, A.-L.; Mohamad, T.; Quan, F.B.; De Cian, A.; Mosimann, C.; Tostivint, H.; Pézeron, G. Urp1 and Urp2 Act Redundantly to Maintain Spine Shape in Zebrafish Larvae. Developmental Biology 2023, 496, 36–51. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, Y.; Zhang, R.; Wang, Z.; Xu, M.; Zhang, D.; Huang, J.; Luo, F.; Li, F.; Ni, Z.; et al. Dstyk Mutation Leads to Congenital Scoliosis-like Vertebral Malformations in Zebrafish via Dysregulated MTORC1/TFEB Pathway. Nat Commun 2020, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Bagwell, J.; Norman, J.; Ellis, K.; Peskin, B.; Hwang, J.; Ge, X.; Nguyen, S.V.; McMenamin, S.K.; Stainier, D.Y.; Bagnat, M. Notochord Vacuoles Absorb Compressive Bone Growth during Zebrafish Spine Formation. Elife 2020, 9, e51221. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhang, X.; Jia, S.; Yelick, P.C.; Zhao, C. Zebrafish as a Model for Human Ciliopathies. Journal of Genetics and Genomics 2016, 43, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Scholey, J.M. Assembly, Functions and Evolution of Archaella, Flagella and Cilia. Current Biology 2018, 28, R278–R292. [Google Scholar] [CrossRef]
- Klena, N.; Pigino, G. Structural Biology of Cilia and Intraflagellar Transport. Annu. Rev. Cell Dev. Biol. 2022, 38, annurev. [Google Scholar] [CrossRef]
- Van De Weghe, J.C.; Rusterholz, T.D.S.; Latour, B.; Grout, M.E.; Aldinger, K.A.; Shaheen, R.; Dempsey, J.C.; Maddirevula, S.; Cheng, Y.-H.H.; Phelps, I.G.; et al. Mutations in ARMC9, Which Encodes a Basal Body Protein, Cause Joubert Syndrome in Humans and Ciliopathy Phenotypes in Zebrafish. The American Journal of Human Genetics 2017, 101, 23–36. [Google Scholar] [CrossRef]
- Lessieur, E.M.; Song, P.; Nivar, G.C.; Piccillo, E.M.; Fogerty, J.; Rozic, R.; Perkins, B.D. Ciliary Genes Arl13b, Ahi1 and Cc2d2a Differentially Modify Expression of Visual Acuity Phenotypes but Do Not Enhance Retinal Degeneration Due to Mutation of Cep290 in Zebrafish. PLoS ONE 2019, 14, e0213960. [Google Scholar] [CrossRef]
- Bentley-Ford, M.R.; LaBonty, M.; Thomas, H.R.; Haycraft, C.J.; Scott, M.; LaFayette, C.; Croyle, M.J.; Andersen, R.S.; Parant, J.M.; Yoder, B.K. Evolutionarily Conserved Genetic Interactions between Nphp-4 and Bbs-5 Mutations Exacerbate Ciliopathy Phenotypes. Genetics 2022, 220, iyab209. [Google Scholar] [CrossRef]
- Masek, M.; Etard, C.; Hofmann, C.; Hülsmeier, A.J.; Zang, J.; Takamiya, M.; Gesemann, M.; Neuhauss, S.C.F.; Hornemann, T.; Strähle, U.; et al. Loss of the Bardet-Biedl Protein Bbs1 Alters Photoreceptor Outer Segment Protein and Lipid Composition. Nat Commun 2022, 13, 1282. [Google Scholar] [CrossRef]
- Yu, X.; Ng, C.P.; Habacher, H.; Roy, S. Foxj1 Transcription Factors Are Master Regulators of the Motile Ciliogenic Program. Nat Genet 2008, 40, 1445–1453. [Google Scholar] [CrossRef]
- Grimes, D.T.; Boswell, C.W.; Morante, N.F.C.; Henkelman, R.M.; Burdine, R.D.; Ciruna, B. Zebrafish Models of Idiopathic Scoliosis Link Cerebrospinal Fluid Flow Defects to Spine Curvature. Science 2016, 352, 1341–1344. [Google Scholar] [CrossRef]
- Mathieu, H.; Patten, S.A.; Aragon-Martin, J.A.; Ocaka, L.; Simpson, M.; Child, A.; Moldovan, F. Genetic Variant of TTLL11 Gene and Subsequent Ciliary Defects Are Associated with Idiopathic Scoliosis in a 5-Generation UK Family. Sci Rep 2021, 11, 11026. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Kang, Y.; Liu, J.; Huang, M.; Dai, Z.; Shi, J.; Wang, S.; Li, L.; Li, Y.; Zheng, P.; et al. Ependymal Polarity Defects Coupled with Disorganized Ciliary Beating Drive Abnormal Cerebrospinal Fluid Flow and Spine Curvature in Zebrafish. PLoS Biol 2023, 21, e3002008. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Ou, Y.; Wu, J.; Li, H.; Wang, X.; Tang, L.; Dai, X.; Yang, C.; Wei, Z.; et al. Ccdc57 Is Required for Straightening the Body Axis by Regulating Ciliary Motility in the Brain Ventricle of Zebrafish. Journal of Genetics and Genomics 2023, 50, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Yang, G.; Gao, Q.; Xiao, L.; Li, J.; Guo, C.; Troutwine, B.R.; Gray, R.S.; Xie, L.; et al. Coding Variants Coupled With Rapid Modeling in Zebrafish Implicate Dynein Genes, Dnaaf1 and Zmynd10, as Adolescent Idiopathic Scoliosis Candidate Genes. Front Cell Dev Biol 2020, 8, 582255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, S.; Chen, Z.; Chong, Y.L.; Xie, H.; Feng, D.; Wu, X.; Song, D.Z.; Roy, S.; Zhao, C. Cilia-Driven Cerebrospinal Fluid Flow Directs Expression of Urotensin Neuropeptides to Straighten the Vertebrate Body Axis. Nat Genet 2018, 50, 1666–1673. [Google Scholar] [CrossRef]
- Aboitiz, F.; Montiel, J.F. The Enigmatic Reissner’s Fiber and the Origin of Chordates. Front. Neuroanat. 2021, 15, 703835. [Google Scholar] [CrossRef]
- Troutwine, B.R.; Gontarz, P.; Konjikusic, M.J.; Minowa, R.; Monstad-Rios, A.; Sepich, D.S.; Kwon, R.Y.; Solnica-Krezel, L.; Gray, R.S. The Reissner Fiber Is Highly Dynamic In Vivo and Controls Morphogenesis of the Spine. Curr Biol 2020, 30, 2353–2362. [Google Scholar] [CrossRef]
- Gobron, S.; Creveaux, I.; Meiniel, R.; Didier, R.; Dastugue, B.; Meiniel, A. SCO-Spondin Is Evolutionarily Conserved in the Central Nervous System of the Chordate Phylum. Neuroscience 1999, 88, 655–664. [Google Scholar] [CrossRef]
- Cantaut-Belarif, Y.; Sternberg, J.R.; Thouvenin, O.; Wyart, C.; Bardet, P.-L. The Reissner Fiber in the Cerebrospinal Fluid Controls Morphogenesis of the Body Axis. Current Biology 2018, 28, 2479–2486. [Google Scholar] [CrossRef]
- Troutwine, B.R.; Gontarz, P.; Konjikusic, M.J.; Minowa, R.; Monstad-Rios, A.; Sepich, D.S.; Kwon, R.Y.; Solnica-Krezel, L.; Gray, R.S. The Reissner Fiber Is Highly Dynamic In Vivo and Controls Morphogenesis of the Spine. Current Biology 2020, 30, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Driever, W. Developmental Biology: Reissner’s Fiber and Straightening of the Body Axis. Current Biology 2018, 28, R833–R835. [Google Scholar] [CrossRef] [PubMed]
- Castañeyra-Perdomo, A.; Carmona Calero, E.; H, P.-G.; Valenzuela, I.; P, P.-M.; C, O.-R.; González-Marrero, I.; A, T.-D.; R, F.-T. Ontogenic Development of the Human Subcommissural Organ. European Journal of Anatomy 2004, 8, 107–120. [Google Scholar]
| Related diseases | Gene | Sample source | Year | Reference |
|---|---|---|---|---|
| AIS | TPH1 | 103 AIS cases and 108 controls (Chinese Han population) | 2008 | [59] |
| DSCAM | 419 AIS families | 2011 | [60] | |
| CNTNAP2 | ||||
| GPR126 | 1,819 AIS cases and 25,939 controls | 2013 | [61] | |
| CHL1 | 500 AIS cases and 500 controls (10-18 years old females in a Han Chinese population) | 2014 | [62] | |
| BNC2 | 2,109 AIS cases and 11,140 controls | 2015 | [63] | |
| AJAP1 | 4,317 AIS cases and 6,016 controls (Chinese Han population) | 2015 | [64] | |
| PAX3 | ||||
| EPHA4 | ||||
| BCL-2 | ||||
| LBX1 | 620 AIS cases and 1,287 controls (female Caucasian) | 2015 | [65] | |
| 667 AIS cases and 901 controls (French-Canadian population) | 2018 | [66] | ||
| TBX1 | 79,211 individuals (Japanese population) | 2019 | [67] | |
| DSE | ||||
| FTO | ||||
| BOC | ||||
| HSPG2 | 1752 AIS cases and 1584 controls (China) | 2019 | [68] | |
| FBN1 and FBN2 | 952 AIS cases and 1499 controls | 2019 | [69] | |
| POC5 | 2432 AIS cases and 2292 controls (Chinese population) | 2018 | [70] | |
| French-Canadian and British families and sporadic cases | 2021 | [71] | ||
| COL8A2 | 23 Multigeneration Idiopathic Scoliosis Families | 2021 | [72] | |
| COL4A3 | ||||
| COL6A5 | ||||
| COL27A1 | ||||
| COL7A1 | ||||
| COL21A1 | ||||
| COL9A2 | ||||
| COL9A3 | ||||
| COL4A6 | ||||
| HSPG2 | ||||
| FBN2 | ||||
| ADAMTSL2 | 302 AIS cases and 818 controls | 2022 | [73] | |
| LTBP4 | ||||
| TTN | 11 AIS cases (Taiwan) | 2022 | [74] | |
| CLCN1 | ||||
| SOX8 | ||||
| ROBO3 | 135 AIS cases and 267 controls | 2023 | [75] | |
| IS | PAX1 | 3,102 individuals | 2015 | [76] |
| CELSR2 | 1739 IS cases and 1812 controls(Swedish-Danish) 3 IS cases in a multigenerational family |
2017 2020 |
[77] [78] |
|
| KIF7 | ||||
| CS | TBX6 | 161 CS cases and 166 controls (PUMCH in China, Oct. 2010 – Jun. 2014) | 2018 | [79] |
| PSCS | COL27A1 | GSE11854 expression dataset associated with somite formation in the GEO database | 2022 | [80] |
| PCD | DNAAF2 | patients from two consanguineous families | 2021 | [81] |
| OI | FKBP10 | Patients diagnosed with OI | 2023 | [82] |
| MFS | FBN1 | a child with Marfan syndrome | 2023 | [83] |
| CDD | SP7 | 2 CDD cases from a large consanguineous family | 2023 | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





