Submitted:
12 July 2023
Posted:
14 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Human vessels culture
2.2. Cell culture
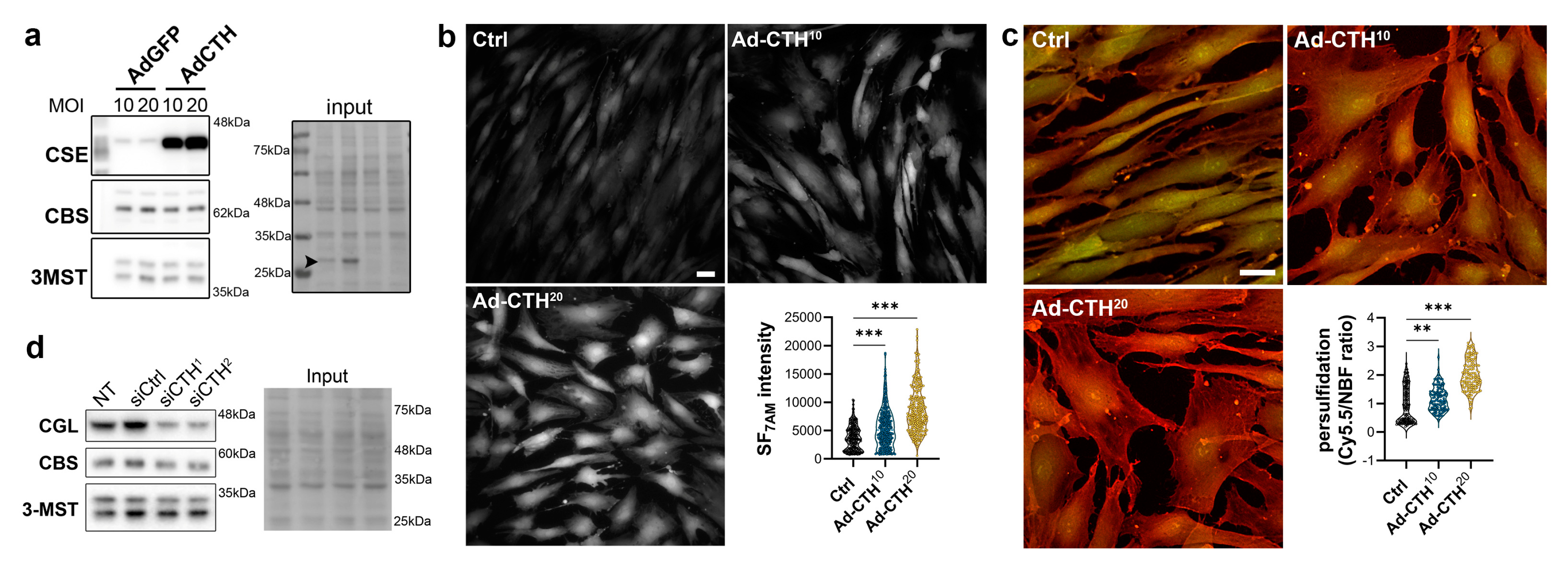
2.3. siRNA-mediated knock-down and adenoviral-mediated overexpression
2.4. Histology
2.5. Western blotting
2.6. Reverse transcription and quantitative polymerase chain reaction (RT-qPCR)
2.7. Lead acetate (CSE activity assay)
2.8. H2S and persulfidation measurement
2.9. BrdU assay
2.10. Wound healing assay
2.11. Statistical analyses
2.12. Ethics Statement
3. Results
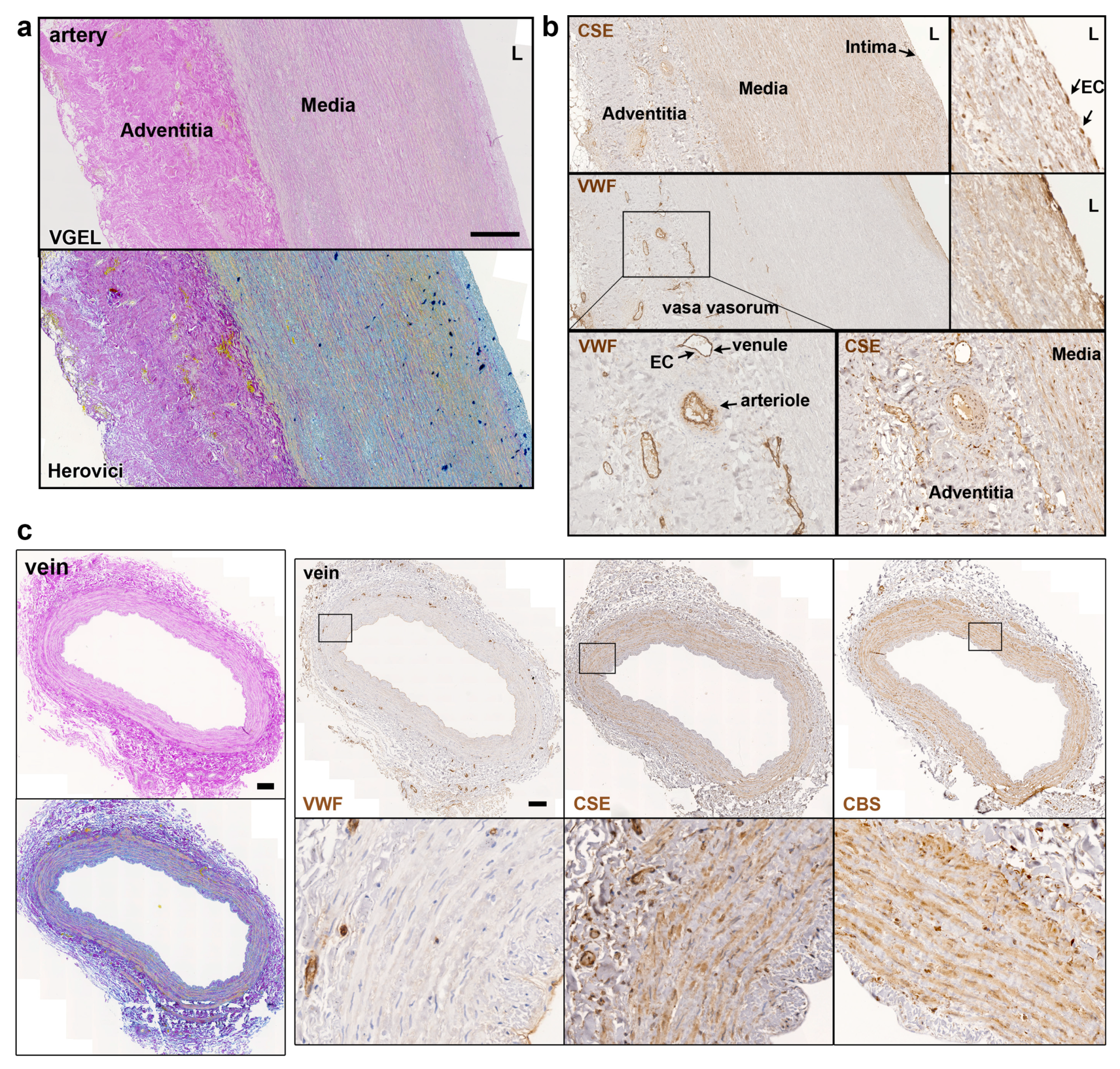
3.1. CSE is expressed in the media and intima of human saphenous vein and artery segments
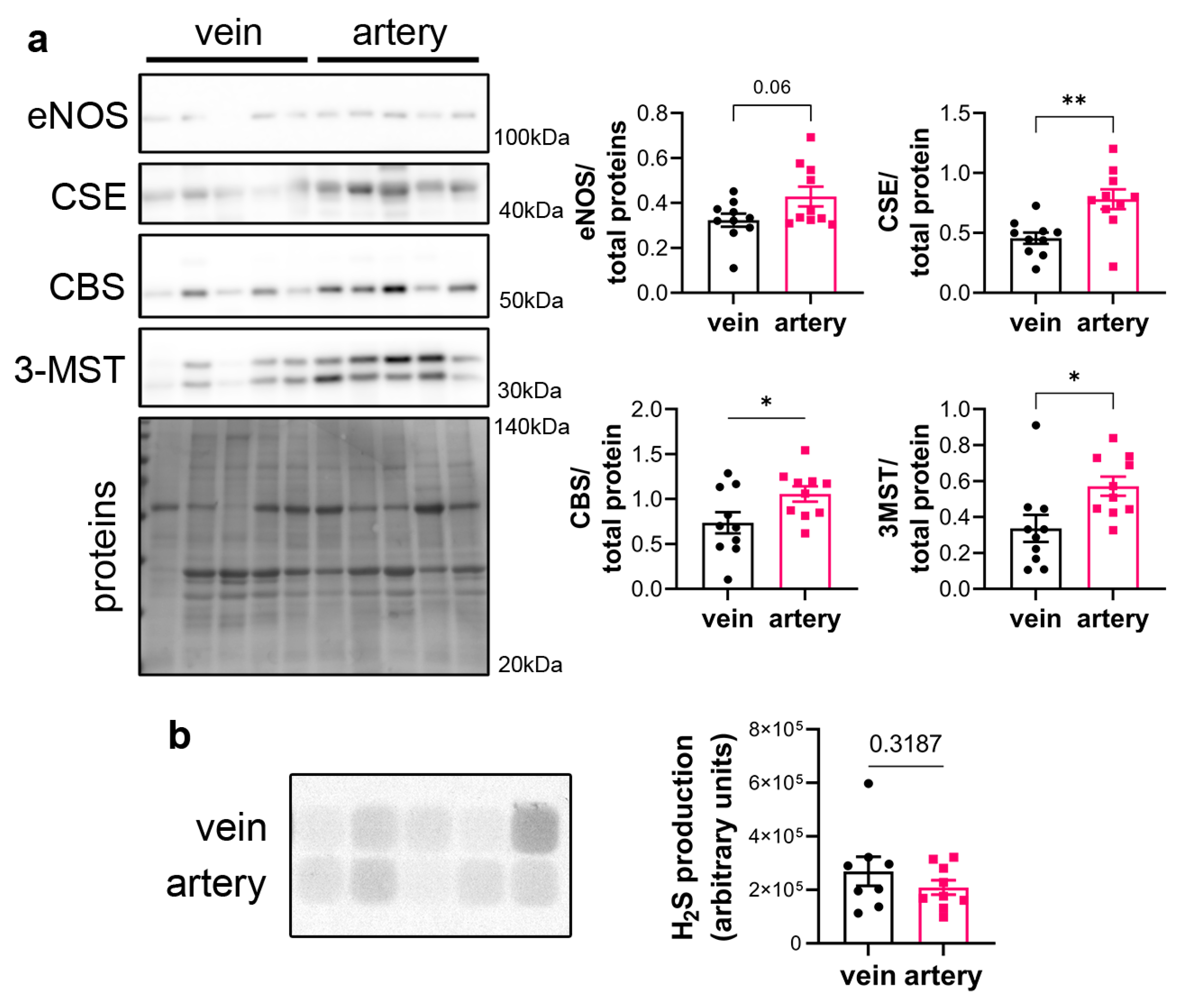
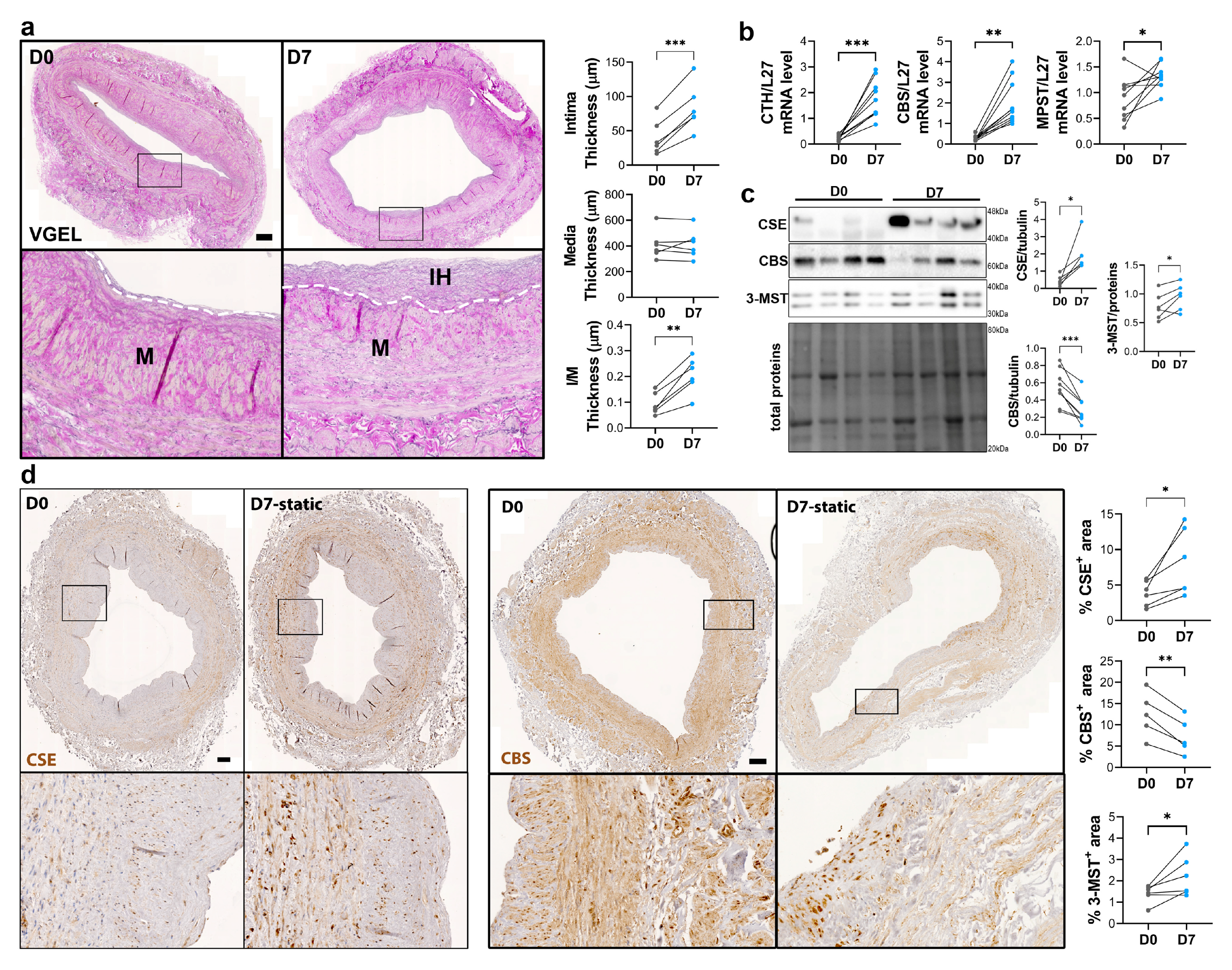
3.2. CSE expression is regulated by flow
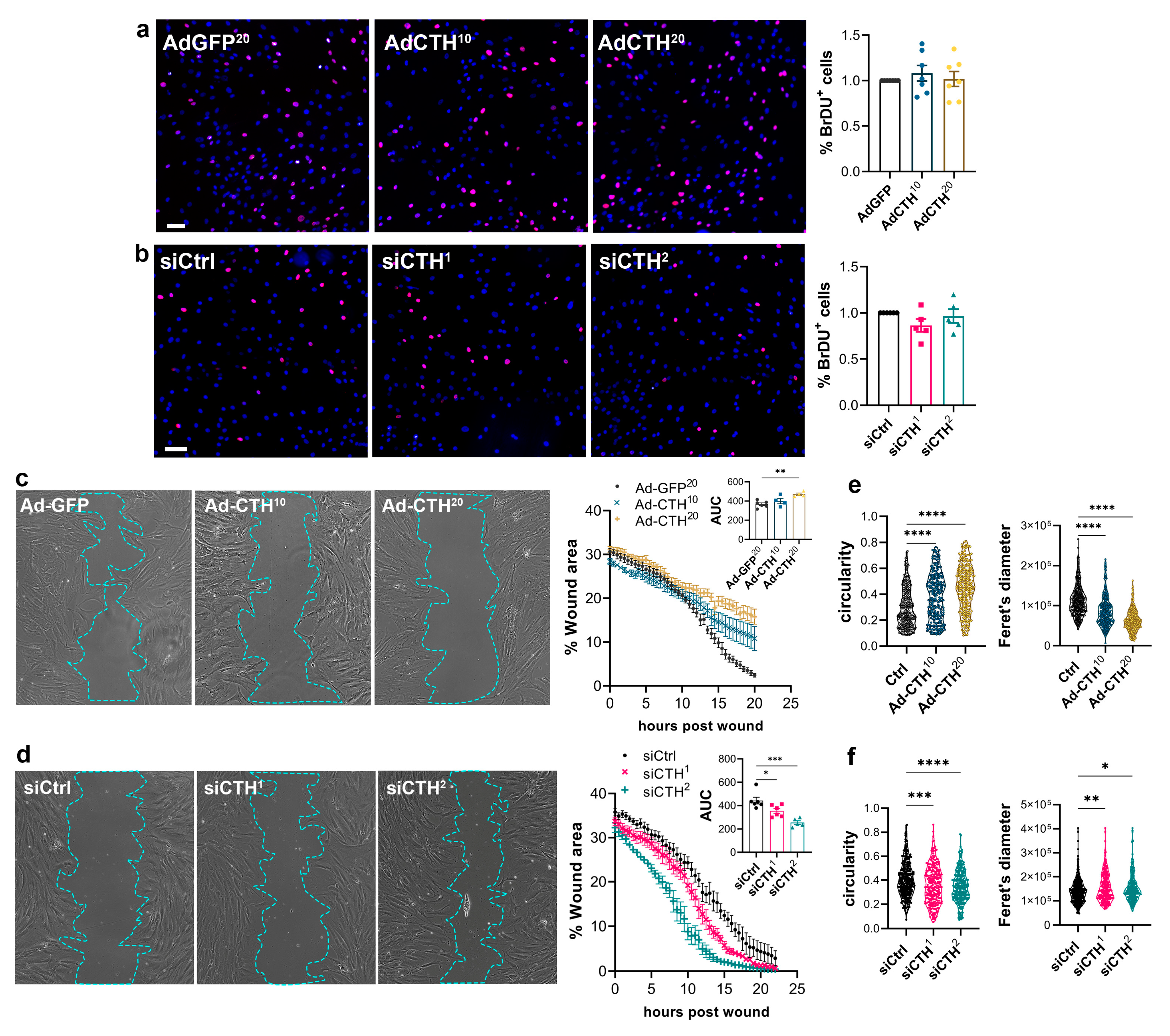
3.3. CSE regulates human VSMC migration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Déglise S, Bechelli C, Allagnat F. Vascular Smooth Muscle Cells in Intimal Hyperplasia, an Update. Frontiers in Physiology (2023) 13. [CrossRef]
- Simpson EL, Kearns B, Stevenson MD, Cantrell AJ, Littlewood C, Michaels JA. Enhancements to Angioplasty for Peripheral Arterial Occlusive Disease: Systematic Review, Cost-Effectiveness Assessment and Expected Value of Information Analysis. Health Technol Assess (2014) 18(10):1-252. [CrossRef]
- Cirino G, Szabo C, Papapetropoulos A. Physiological Roles of Hydrogen Sulfide in Mammalian Cells, Tissues and Organs. Physiol Rev (2022). Epub 2022/04/19. [CrossRef]
- Jain SK BR, Rains JL, Bass PF, Levine SN, Reddy S, McVie R, Bocchini JA. Low Levels of Hydrogen Sulfide in the Blood of Diabetes Patients and Streptozotocin-Treated Rats Causes Vascular Inflammation? Antioxid Redox Signal (2010) 12:1333-7. [CrossRef]
- Islam KN, Polhemus DJ, Donnarumma E, Brewster LP, Lefer DJ. Hydrogen Sulfide Levels and Nuclear Factor-Erythroid 2-Related Factor 2 (Nrf2) Activity Are Attenuated in the Setting of Critical Limb Ischemia (Cli). J Am Heart Assoc (2015) 4(5). Epub 2015/05/16. 2015. [CrossRef]
- Beard RS, Jr., Bearden SE. Vascular Complications of Cystathionine Beta-Synthase Deficiency: Future Directions for Homocysteine-to-Hydrogen Sulfide Research. Am J Physiol Heart Circ Physiol (2011) 300(1):H13-26. [CrossRef]
- Longchamp A, MacArthur MR, Trocha K, Ganahl J, Mann CG, Kip P, et al. Plasma Hydrogen Sulfide Is Positively Associated with Post-Operative Survival in Patients Undergoing Surgical Revascularization. Front Cardiovasc Med (2021) 8:750926. Epub 2021/11/12. [CrossRef]
- Yang G, Li H, Tang G, Wu L, Zhao K, Cao Q, et al. Increased Neointimal Formation in Cystathionine Gamma-Lyase Deficient Mice: Role of Hydrogen Sulfide in Alpha5beta1-Integrin and Matrix Metalloproteinase-2 Expression in Smooth Muscle Cells. J Mol Cell Cardiol (2012) 52(3):677-88. [CrossRef]
- Macabrey D, Longchamp A, MacArthur MR, Lambelet M, Urfer S, Deglise S, et al. Sodium Thiosulfate Acts as a Hydrogen Sulfide Mimetic to Prevent Intimal Hyperplasia Via Inhibition of Tubulin Polymerisation. EBioMedicine (2022) 78:103954. Epub 20220322. [CrossRef]
- Trocha KM, Kip P, Tao M, MacArthur MR, Trevino-Villarreal JH, Longchamp A, et al. Short-Term Preoperative Protein Restriction Attenuates Vein Graft Disease Via Induction of Cystathionine Gamma-Lyase. Cardiovasc Res (2020) 116(2):416-28. Epub 2019/03/30. [CrossRef]
- Meng QH, Yang G, Yang W, Jiang B, Wu L, Wang R. Protective Effect of Hydrogen Sulfide on Balloon Injury-Induced Neointima Hyperplasia in Rat Carotid Arteries. Am J Pathol (2007) 170(4):1406-14. [CrossRef]
- Ma B, Liang G, Zhang F, Chen Y, Zhang H. Effect of Hydrogen Sulfide on Restenosis of Peripheral Arteries after Angioplasty. Mol Med Rep (2012) 5(6):1497-502. Epub 2012/04/04. [CrossRef]
- Macabrey D, Deslarzes-Dubuis C, Longchamp A, Lambelet M, Ozaki CK, Corpataux JM, et al. Hydrogen Sulphide Release Via the Angiotensin Converting Enzyme Inhibitor Zofenopril Prevents Intimal Hyperplasia in Human Vein Segments and in a Mouse Model of Carotid Artery Stenosis. Eur J Vasc Endovasc Surg (2022) 63(2):336-46. Epub 20211213. [CrossRef]
- Longchamp A, Kaur K, Macabrey D, Dubuis C, Corpataux JM, Deglise S, et al. Hydrogen Sulfide-Releasing Peptide Hydrogel Limits the Development of Intimal Hyperplasia in Human Vein Segments. Acta Biomater (2019) 97:374-84. Epub 20190726. [CrossRef]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. H2s as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine G-Lyase. Science (2008) 322:587-90. [CrossRef]
- Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen Sulfide-Induced Relaxation of Resistance Mesenteric Artery Beds of Rats. Am J Physiol Heart Circ Physiol (2004) 287(5):H2316-23. Epub 2004/06/12. [CrossRef]
- Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, et al. Hydrogen Sulfide as Endothelium-Derived Hyperpolarizing Factor Sulfhydrates Potassium Channels. Circ Res (2011) 109(11):1259-68. [CrossRef]
- Kiesworo K, MacArthur MR, Kip P, Agius T, Macabrey D, Lambelet M, et al. Cystathionine-Gamma-Lyase Overexpression Modulates Oxidized Nicotinamide Adenine Dinucleotide Biosynthesis and Enhances Neovascularization. JVS Vasc Sci (2023) 4:100095. Epub 20230113. [CrossRef]
- Yang G, Wu L, Bryan S, Khaper N, Mani S, Wang R. Cystathionine Gamma-Lyase Deficiency and Overproliferation of Smooth Muscle Cells. Cardiovasc Res (2010) 86(3):487-95. [CrossRef]
- Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular Endothelium Expresses 3-Mercaptopyruvate Sulfurtransferase and Produces Hydrogen Sulfide. J Biochem (2009) 146(5):623-6. Epub 2009/07/17. [CrossRef]
- Bibli SI, Hu J, Leisegang MS, Wittig J, Zukunft S, Kapasakalidi A, et al. Shear Stress Regulates Cystathionine Gamma Lyase Expression to Preserve Endothelial Redox Balance and Reduce Membrane Lipid Peroxidation. Redox Biol (2020) 28:101379. Epub 20191113. [CrossRef]
- Yuan S, Yurdagul A, Jr., Peretik JM, Alfaidi M, Al Yafeai Z, Pardue S, et al. Cystathionine Gamma-Lyase Modulates Flow-Dependent Vascular Remodeling. Arterioscler Thromb Vasc Biol (2018) 38(9):2126-36. Epub 2018/07/14. [CrossRef]
- Macabrey D, Joniova J, Gasser Q, Bechelli C, Longchamp A, Urfer S, et al. Sodium Thiosulfate, a Source of Hydrogen Sulfide, Stimulates Endothelial Cell Proliferation and Neovascularization. Front Cardiovasc Med (2022) 9:965965. Epub 20221003. [CrossRef]
- Saucy F, Probst H, Alonso F, Berard X, Deglise S, Dunoyer-Geindre S, et al. Ex Vivo Pulsatile Perfusion of Human Saphenous Veins Induces Intimal Hyperplasia and Increased Levels of the Plasminogen Activator Inhibitor 1. European surgical research Europaische chirurgische Forschung Recherches chirurgicales europeennes (2010) 45(1):50-9. [CrossRef]
- Berard X, Deglise S, Alonso F, Saucy F, Meda P, Bordenave L, et al. Role of Hemodynamic Forces in the Ex Vivo Arterialization of Human Saphenous Veins. J Vasc Surg (2013) 57(5):1371-82. [CrossRef]
- Longchamp A, Allagnat F, Berard X, Alonso F, Haefliger JA, Deglise S, et al. Procedure for Human Saphenous Veins Ex Vivo Perfusion and External Reinforcement. J Vis Exp (2014) (92):e52079. Epub 20141001. [CrossRef]
- Longchamp A, Alonso F, Dubuis C, Allagnat F, Berard X, Meda P, et al. The Use of External Mesh Reinforcement to Reduce Intimal Hyperplasia and Preserve the Structure of Human Saphenous Veins. Biomaterials (2014) 35(9):2588-99. Epub 20140113. [CrossRef]
- Allagnat F, Dubuis C, Lambelet M, Le Gal L, Alonso F, Corpataux JM, et al. Connexin37 Reduces Smooth Muscle Cell Proliferation and Intimal Hyperplasia in a Mouse Model of Carotid Artery Ligation. Cardiovasc Res (2017) 113(7):805-16. [CrossRef]
- Longchamp A, Kaur K, Macabrey D, Dubuis C, Corpataux JM, Deglise S, et al. Hydrogen Sulfide-Releasing Peptide Hydrogel Limits the Development of Intimal Hyperplasia in Human Vein Segments. Acta Biomater (2019). Epub 2019/07/29. [CrossRef]
- Longchamp A, Mirabella T, Arduini A, MacArthur MR, Das A, Trevino-Villarreal JH, et al. Amino Acid Restriction Triggers Angiogenesis Via Gcn2/Atf4 Regulation of Vegf and H2s Production. Cell (2018) 173(1):117-29 e14. Epub 2018/03/24. [CrossRef]
- Teuscher AC, Statzer C, Pantasis S, Bordoli MR, Ewald CY. Assessing Collagen Deposition During Aging in Mammalian Tissue and in Caenorhabditis Elegans. Methods Mol Biol (2019) 1944:169-88. Epub 2019/03/07. [CrossRef]
- Allagnat F, Haefliger JA, Lambelet M, Longchamp A, Berard X, Mazzolai L, et al. Nitric Oxide Deficit Drives Intimal Hyperplasia in Mouse Models of Hypertension. Eur J Vasc Endovasc Surg (2016) 51(5):733-42. Epub 20160319. [CrossRef]
- Lin VS, Lippert AR, Chang CJ. Cell-Trappable Fluorescent Probes for Endogenous Hydrogen Sulfide Signaling and Imaging H2o2-Dependent H2s Production. Proc Natl Acad Sci U S A (2013) 110(18):7131-5. Epub 2013/04/17. [CrossRef]
- Suarez-Arnedo A, Torres Figueroa F, Clavijo C, Arbelaez P, Cruz JC, Munoz-Camargo C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS One (2020) 15(7):e0232565. Epub 20200728. [CrossRef]
- Dubuis C, May L, Alonso F, Luca L, Mylonaki I, Meda P, et al. Atorvastatin-Loaded Hydrogel Affects the Smooth Muscle Cells of Human Veins. The Journal of pharmacology and experimental therapeutics (2013) 347(3):574-81. Epub 2013/09/28. [CrossRef]
- Yang G, Wu L, Wang R. Pro-Apoptotic Effect of Endogenous H2s on Human Aorta Smooth Muscle Cells. FASEB J (2006) 20(3):553-5. [CrossRef]
- Bibli SI, Hu J, Sigala F, Wittig I, Heidler J, Zukunft S, et al. Cystathionine Gamma Lyase Sulfhydrates the Rna Binding Protein Human Antigen R to Preserve Endothelial Cell Function and Delay Atherogenesis. Circulation (2019) 139(1):101-14. Epub 2018/07/05. [CrossRef]
- Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Zaid A, Giannogonas P, et al. Cgmp-Dependent Protein Kinase Contributes to Hydrogen Sulfide-Stimulated Vasorelaxation. PLoS One (2012) 7(12):e53319. [CrossRef]
- Owens GK, Kumar MS, Wamhoff BR. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiol Rev (2004) 84(3):767-801. Epub 2004/07/23. [CrossRef]
- Chakraborty R, Chatterjee P, Dave JM, Ostriker AC, Greif DM, Rzucidlo EM, et al. Targeting Smooth Muscle Cell Phenotypic Switching in Vascular Disease. JVS Vasc Sci (2021) 2:79-94. Epub 2021/10/08. [CrossRef]
- Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, et al. Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models. Circ Res (2016) 119(12):1313-23. Epub 2016/09/30. [CrossRef]
- Worssam MD, Lambert J, Oc S, Taylor JC, Taylor AL, Dobnikar L, et al. Cellular Mechanisms of Oligoclonal Vascular Smooth Muscle Cell Expansion in Cardiovascular Disease. Cardiovasc Res (2022). Epub 2022/08/23. [CrossRef]
- Wang Y, Nanda V, Direnzo D, Ye J, Xiao S, Kojima Y, et al. Clonally Expanding Smooth Muscle Cells Promote Atherosclerosis by Escaping Efferocytosis and Activating the Complement Cascade. Proc Natl Acad Sci U S A (2020) 117(27):15818-26. Epub 2020/06/17. [CrossRef]
- Longchamp A, Allagnat F, Alonso F, Kuppler C, Dubuis C, Ozaki CK, et al. Connexin43 Inhibition Prevents Human Vein Grafts Intimal Hyperplasia. PLoS One (2015) 10(9):e0138847. Epub 20150923. [CrossRef]
- Peleli M, Bibli SI, Li Z, Chatzianastasiou A, Varela A, Katsouda A, et al. Cardiovascular Phenotype of Mice Lacking 3-Mercaptopyruvate Sulfurtransferase. Biochem Pharmacol (2020) 176:113833. Epub 2020/02/07. [CrossRef]
- Ngowi EE, Afzal A, Sarfraz M, Khattak S, Zaman SU, Khan NH, et al. Role of Hydrogen Sulfide Donors in Cancer Development and Progression. Int J Biol Sci (2021) 17(1):73-88. Epub 20210101. [CrossRef]
- Kiesworo K, MacArthur MR, Kip P, Agius T, Macabrey D, Lambelet M, et al. Cystathionine Gamma Lyase Overexpression Modulates Nad+ Biosynthesis and Enhances Neovascularization. JVS-Vascular Science (2023). [CrossRef]
- Bibli SI, Hu J, Looso M, Weigert A, Ratiu C, Wittig J, et al. Mapping the Endothelial Cell S-Sulfhydrome Highlights the Crucial Role of Integrin Sulfhydration in Vascular Function. Circulation (2021) 143(9):935-48. Epub 2020/12/15. [CrossRef]
- Misra A, Sheikh AQ, Kumar A, Luo J, Zhang J, Hinton RB, et al. Integrin Beta3 Inhibition Is a Therapeutic Strategy for Supravalvular Aortic Stenosis. J Exp Med (2016) 213(3):451-63. Epub 20160208. [CrossRef]
- Slepian MJ, Massia SP, Dehdashti B, Fritz A, Whitesell L. Beta3-Integrins Rather Than Beta1-Integrins Dominate Integrin-Matrix Interactions Involved in Postinjury Smooth Muscle Cell Migration. Circulation (1998) 97(18):1818-27. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




