1. Introduction
The development of modern "practical chemistry", that is, chemistry that enabled humans to develop the first chemical structures, and then their dynamic development and application in pharmacy, for the production of cosmetics, for obtaining plant protection products (pesticides), or in the production of food additives, has been possible due to chemical substances naturally occurring in nature [
1].
Arthropods, and among them insects, form the largest group of organisms living on Earth in terms of both taxon diversity and biomass. About one million species of insects have been described to date, making them more than half of the organisms known today [
2]. Insects inhabit almost every environment except the marine, which is in turn dominated by another group of arthropods, the crustaceans. Due to their diversity and abundance, insects have a tremendous impact on the functioning of ecosystems and the circulation of elements in nature. Inhabiting a variety of habitats, subject to various abiotic factors, and interacting with other species (defense against predation, parasitism, herbivory), insects have had to produce an extraordinary different metabolites that have enabled them to survive in the environment and achieve such enormous evolutionary success. The chemical compounds produced by insects, both small and macromolecular substances, are involved in such processes as communication (ants, termites), mimicry - chemical mimicry (myrmecophilous insects), defense (runner beetles), food recognition, or neutralization of plant toxins [
1,
3]. Chemical compounds produced by insects can be synthesized by themselves, can be taken from host plants, or from symbiotic microorganisms hosted in internal organs [
4].
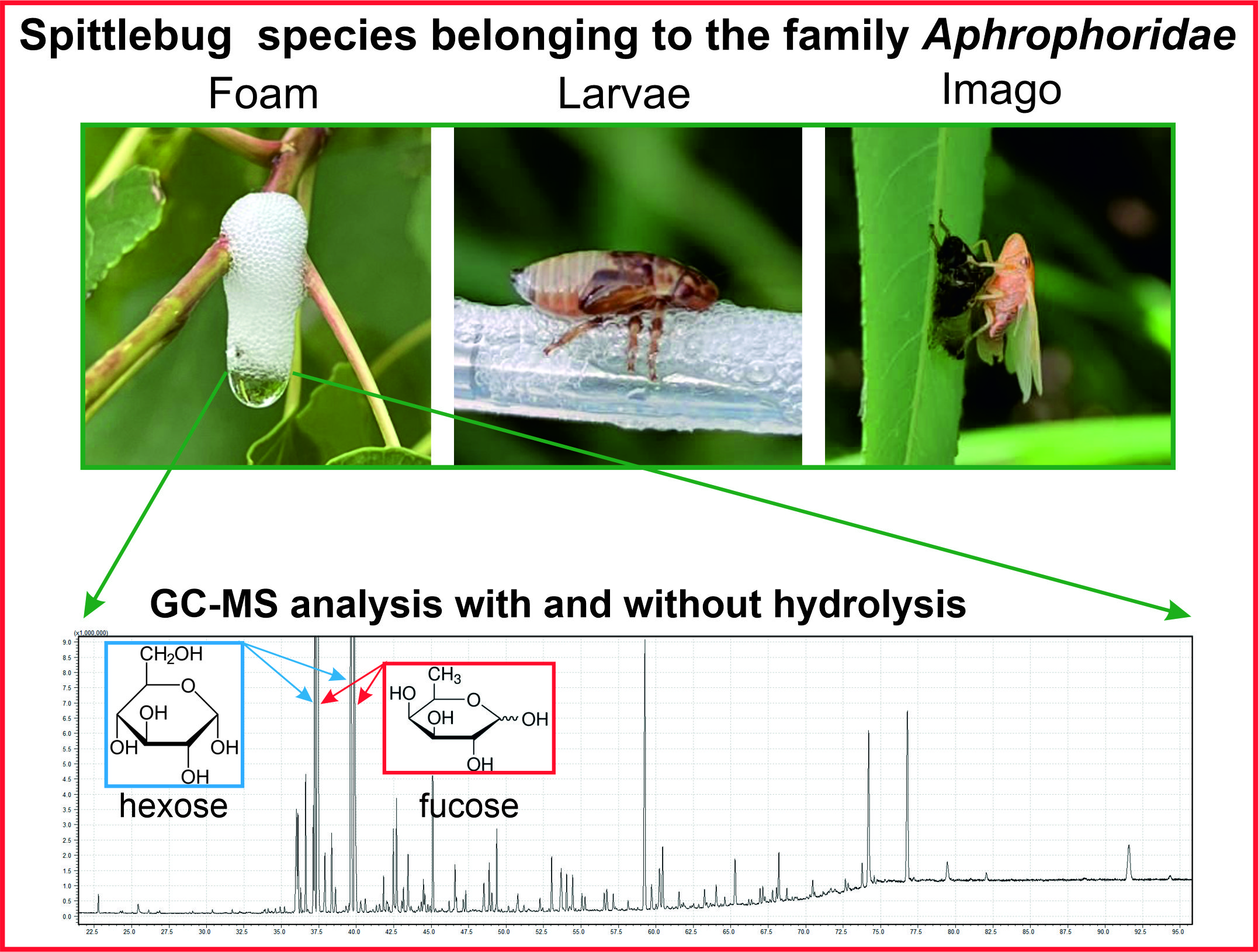
Hemipterans belonging to the spittlebug (Hemiptera: Aphrophoridae) (
Figure 1) produce a variety of secretions with which they cover their bodies. These secretions are produced in the Malpighi tubules, excreted from the digestive system and then spread on the surface of the cuticle [
5,
6]. This behavior is exhibited by both mature forms and nymphs. The nymphs of spittlebugs from the family Aphrophoridae exhibit a very unusual behavior. They produce a foam (
Figure 1A), which they coat themselves with and under which they feed. The excreted fluid moves under the larvae, whose spiracles are located along a groove in the abdominal side of the body. By exhaling, the larva causes foam to form (
Figure 1B). This foam is thought to protect the nymphs from predators, parasites and adverse weather conditions [
7,
8]. Recent studies have shown that the foam produced by the nymphs plays a very important thermoregulatory role by maintaining the optimal temperature for the nymphs to develop [
9,
10]. It has also been suggested that components of the foam produced by the nymphs have antibacterial and antifungal properties [
8,
10], but it is difficult to find studies in the modern literature that are conclusive on these issues due to the lack of ongoing research in this area. The fact, however, is that spittlebug nymphs are very rarely attacked by vertebrates (birds) as well as invertebrates [
11,
12]. To date, one species of parasitic nematode that feeds on spittlebug nymphs has been identified [
13].
Nymphal foams produced by spittlebugs of the family Aphrophoridae, although they have attracted some attention from researchers, have so far not been thoroughly analyzed in terms of chemical composition. Only a few papers on the chemical composition of the foam produced can be found in the literature. For instance, the foam of the cercopid Deois sp. contains at least ten different polypeptides (mostly glycopolypeptides), and acidic proteoglycans [
14]. Another work showed the presence of fatty acid-derived alcohols, γ-lactones, 1-monoacylglycerol, pinitol and poly-3-hydroxy butyric acid in the foam of Aphrophora cribrata [
11]. The foam produced by spittlebug depending on the type of plant on which the nymphs were feeding consisted of carbohydrates, proteins and enzymes however, these compounds were not identified [
10]. The same team showed the presence of fatty acids (9-Octadecanoic acid and 6-Octadecanoic acid) in the foam, additionally benzo(h)quinoline, 2-4-dimethyle-2,4,6-Cycloheptatrien-1-one and 3,5-bis-trimethylsilyl. The presence of palmitic acid and stearic acid was confirmed [
9]. The components of the nymphs's secretions have also been shown to act as an effective repellent against ants [
11].
Yellow biotechnology based on definition presented by Vilcinskas is still a little-known though increasingly thriving branch of biotechnology [
15,
16]
.The objects of interest in this field are all kinds of products related to insects or their symbionts with applications in medicine and pharmacy [
15], plant protection or industry [
16]. The growing interest, especially in Asian markets (China, Japan, South Korea), in insect-derived products is creating an increasingly favorable environment for research into insect biology and evolution, as well as insect-derived substances with potential human applications [
17]. Also the establishment in 2014 of the LOEWE Center for Insect Biotechnology & Bioresources in Giessen, Germany, the first insect biotechnology center in Europe, is proof of this. The enormous diversity of insects offers completely new and completely unexplored possibilities for obtaining new biologically active substances with promising properties. However, the chemical composition of the substances under study must first be known. The nymphal envelopes of Aphrophoridae are unexplored, and their chemical composition is very mysterious, and little information is available in the literature as to what chemicals constitute its composition. Therefore, the aim of this study was to investigate the chemical composition of nymphal envelopes produced by
A. alni (Fallén, 1805) collected in Poland in Mazowieckie Voivodeship in 2020-2022
2. Material and methods
2.1. Chemicals
Analytical standards: L-rhamnose monohydrate (cat. number 41651), L-(-)-fucose (cat. number 93183), D(-)-ribose (cat. number 1603108), xylose (cat. number X0200000), D-(+)-mannose (cat. number 92683), D-(+)-glucose (cat. number 1370481000), myo-inositol (cat. number I5125), 1,5-anhydro-D-sorbitol (cat. number A7165) and D-pinitol (cat. number 74948) were bought from MERCK Poland. All the standards were >99.0 % pure.
Other reagents: Hexamethyldisilazane (cat. number 52619), chlorotrimethylsilane (cat. number 89595), pyridine anhydrous (cat. number 270970), methanol anhydrous (cat. number 322415) and trifluoroacetic acid(cat. Numer T6508) were bought from MERCK Poland. All the reagents were >99 % pure.
2.2. Field Work
The foam analyzed in this research was collected in springtime, when juveniles of A. alni were active. In 2020 and 2021, material was collected in April - May, while in 2022 the collection occurred later, in May and early June nymphal. The foam produced by the spittlebug was collected from young black alders (Alnus glutinosa), on which the insects were feeding. The material was collected for three consecutive years from the same sites to check the variation in the chemical composition of the foam produced by the nymphal forms. Day and night air temperature and relative humidity were also recorded during the collection.
2.3. Foam collection method
Foam collection lasted for about two weeks and began in the morning around 5-6 am. The foam was collected into sterile glass bottles. The bottles were capped with a cap with a silicone-Teflon septum, which was pierced with a sterile needle with a syringe. A sterile glass cotton wool was placed at the bottom of the syringe, through which the collected foam was passed. The foam was collected with a sterile Pasteur pipette in such a way as not to damage the insect and to leave enough of the envelope to keep the insect covered with foam. About 100 drops of secretion had to be collected in order to gather 10 ml of foam (shown in
Figure 1A). The secretion was collected from one region but from 12 different points/areas about 100m apart. Foam samples were combined only within a single collection point and therefore n = 12 for each collection period. The collected foam was transported to the laboratory, and centrifuged at 1000 rpm, 20 °C for 10 min to remove solids - environmental contaminants mainly flower pollen. After centrifugation, the secretion was frozen at -80 °C, and then lyophilized. Analysis of chemical composition was carried out on lyophilized material.
2.4. Lyophilization
The collected secretion was weighed and then frozen at -80 °C and lyophilized (Telstar, model: LyoQuest -85) with the following parameters: condenser temperature -90 °C, shelf temperature 15 °C, lyophilization pressure 0.2 mbar, lyophilization time 48 h. After 48 h, re-drying was conducted for another 6 h with the following parameters: shelf temperature 30 °C, lyophilization pressure 0.01 mbar. After the process was completed, the dried samples were weighed again. From the difference in weight before and after the lyophilization process, the dry matter content was calculated.
2.5. Protein content analysis
The protein content of the collected material was performed according to the manufacturer's procedure using the Pierce™ Rapid Gold BCA Protein Assay Kit (Thermo Scientific, Warsaw, Poland).
2.6. Gas Chromatography–Mass Spectroscopy GC-MS
GC-MS analysis was carried out using a gas chromatograph coupled to a GCMS-QP2010 Ultra mass spectrometer from Shimadzu (City, State). A Zebron ZB-FFAP 60 m x 0.25 mm x 0.25 μm chromatography column from Phenomenex Ltd. was used in the analysis. The lyophilized foam was subjected to GC-MS analysis after and without acid hydrolysis. The following chromatographic system parameters were used: Injection temperature was 300 °C, the interface set at 320 °C, and the ion source adjusted to 200°C. The carrier gas was helium (flow rate 2 mL min-1). The temperature program was 5 min isothermal heating at 40 °C, followed by a 3 °C/min oven temperature ramp to 250 °C, then held for 45 min. Mass spectra were recorded at scans/s (m/z 50-1000). Samples (4 μL) were injected in split mode, with split ratio: 2.0. The analysis ware done immediately after lyophilization process.
2.7. Sample preparation without acid hydrolysis
1-2 mg of lyophilized foam was weighed into glass reaction bottles and then 1.2 ml of anhydrous pyridine was added. The bottles were incubated for 30 min at 60 °C with constant stirring. Then 0.2 ml of hexamethyldisilazane (HMDS), 0.1 ml of chlorotrimethylsilane (TMCS) were added and then stirred vigorously for 20 min at room temperature. Excess amounts of silanizing agents were neutralized by adding 0.5 ml of methanol. The sample thus prepared was filtered through a 0.45-μm syringe filter and subjected to GC-MS analysis.
2.8. Sample preparation after acid hydrolysis
1-2 mg of lyophilized foam was weighed into reaction bottles, and then 1 ml each of 2 mol dm3 of aqueous trifluoroacetic acid (TFA) solution was added. The samples were tightly capped and incubated at 120 °C for 2 h with constant stirring. After acid hydrolysis, the contents of the bottles were evaporated to dryness in a stream of nitrogen at 60 °C. Then 1.2 ml of anhydrous pyridine was added. The bottles were incubated for 30 min at 60 °C with constant stirring. Then 0.2 ml of HMDS, 0.1 ml of TMCS were added and stirred vigorously for 20 min at room temperature. Excess amounts of silanizing agents were neutralized by adding 0.5 ml of methanol. The sample prepared in this way was filtered through a syringe filter with a 0.45 μm pore and subjected to GC-MS analysis.
2.9. Preparation of calibration curves of the analyzed substances
Into a 10-ml volumetric flask, 1-10 mg of the analytes to be examined were weighed (the analyte weight was adjusted according to its approximate content in the samples), dissolved in anhydrous pyridine at 60 °C. From the solution thus prepared, 0.01, 0.025, 0.05, 0.1 and 0.2 ml were taken into 5 ml reaction bottles. Anhydrous pyridine was then added to a final volume of 1.2 ml. The bottles were incubated for 30 min at 60 °C with constant stirring. Then 0.2 ml of HMDS, 0.1 ml of TMCS were added, the whole was stirred intensively and left for 20 min at room temperature. Excess amounts of silanizing agents were neutralized by adding 0.5 ml of methanol. The sample thus prepared was filtered through a syringe filter with 0.45 μm pores and subjected to GC-MS analysis. Five calibration points were determined against which the identified compounds were determined in the samples of secretions.
2.10. Statistical analysis of the results
The results obtained from the quantitative study were subjected to statistical analysis using one-way analysis variance with Duncan post-hoc test using Statgraphic Centurion 18 Version 18.1.16 software (Statgraphics Technologies inc., USA Virginia). The data were normally distributed. The purpose of the analysis was to test the hypothesis H0, which assumed that the chemical composition of Aphrophora alni nymphal envelopes varies between collection years. Tables with results include: the arithmetic mean value , standard deviation (SD) while letter subscripts at the top of the arithmetic mean value indicate groups statistically different from each other at α =0.05, n = 12 in each collection year.
3. Results
Table 1 provides data on the amount of foam collected in the years 2020, 2021 and 2022 . For each sample, the protein content and dry matter calculated after the lyophilization process are provided.
In 2020 and 2021, nymphal envelopes were collected in a similar period, i.e., April-May. The environmental parameters, i.e., temperature, were also similar. The temperature range during the day was 24-26 °C, while the temperature at night did not fall below 10 °C. Dry matter content was 0.05-0.06 %. The situation was completely different in 2022, as the insects appeared very late. The daytime temperature was lower than in previous years, and the nighttime temperature fell below 10 °C. The dry matter content of the collected foam was almost twice as high as in previous years. The protein content of the nymphal secretions is very low, averaging about 1.1 mg/g, which is only 0.11 % of the substances contained in the test material. The collected test material was subjected to chemical analysis using gas chromatography.
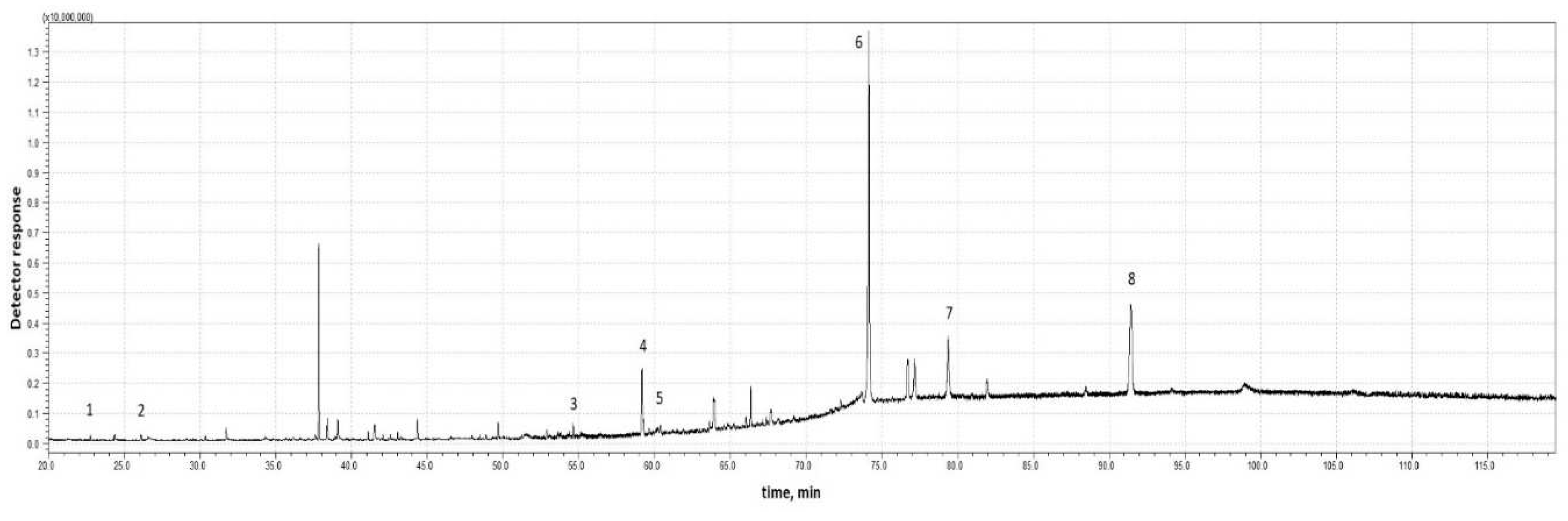
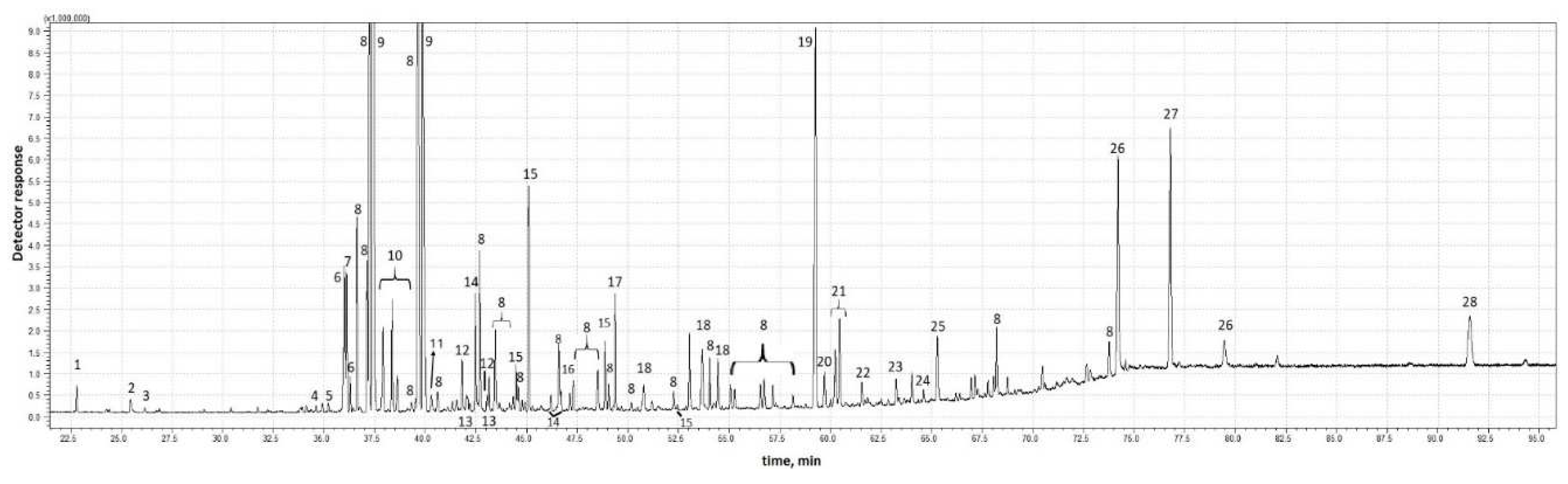
Figure 2 and
Figure 3 show representative chromatograms obtained for the analyzed secretion without hydrolysis (
Figure 2) and after acid hydrolysis with TFA (
Figure 3).
The following chemical substances were identified in the foam without hydrolysis (
Figure 2): 1 - glycerol, 2 - 1-pentadecene, 3 - (9Z)-hexadec-9-enoic acid (palmitoleic acid), 4 - tetratriacontane, 5 - octadecanoic acid (stearic acid), 6: - 9-octadecenoic acid (oleic acid), 7 - hexacosyl alcohol, 8 - 5-henicosyldihydrofuran-2(3H)-one. A much richer profile of chemical compounds was obtained when the foam was subjected to a hydrolysis process.
The following chemical substances were successfully identified in the hydrolyzed foam (
Figure 3): 1 - glycerol, 2 - formamide, 3 - 1-pentadecene, 4 - acetamide, 5 - 1,5-anhydro-D-sorbitol, 6 - pentoses, 7 - rhamnose, 8 - hexoses, 9 - fucose, 10 - ribose, 11 - xylose, 12 - fructose, 13 - pinitol, 14 - mannose, 15 - glucose, 16 - tetradecanoic acid (myristic acid), 17: myo-inositol, 18: trimethyl citrate, 19: tetratriacontane, 20: octadecanoic acid (stearic acid), 21 - 9-octadecenoic acid (oleic acid), 22 - 9,12-octadecadienoic acid (linoleic acid), 23 - docosyl trifluoroacetate, 24 - proline, 25 - 11-eicosenoic acid, 26 - hexacosyl alcohol, 27 - hexatriacontane, 28 - 5-Henicosyldihydrofuran-2(3H)-one. Definitely most of the substances contained in the nymphal envelopes produced by
Aphrophora alni are chemically bound. The identified compounds were determined quantitatively in order to check what proportion of the chemical substances contained in the analyzed nymphal envelopes could be identified.
Table 2 shows the results of quantitative analysis of the foam after and without hydrolysis.
Chromatographic analysis shows (
Figure 3 and
Table 2) that the secretion of
A. alni nymphal envelopes consists mainly of sugar polymers. It was possible to identify the main sugar units building polysaccharides contained in the foam of spittlebugs. Of the substances identified and labeled, fucose accounts for the largest share, ranging from about 17.0 % to about 35.6 % of the secretion by weight. Glucose accounts from about 6.2 % to about 16.2 %, followed by mannose from about 1.6 to about 10.0 %. Rhamnose, ribose, xylose account for less than 5 % of the secretion by weight. The presence of fairly large amounts of myo-inositol was found, with its content ranging from about 0.2 % to about 1.0 %. It should be noted that the profile of sugar units in the studied insect secretions is more complex, as various hexoses (from about 28 % up to about 43 %) and pentoses (from about 1.2 % to about 1.6 %) were found in the samples; however, these are isomeric forms of various sugars, which the analytical technique used was unable to identify. In
Figure 3, the identified hexoses are marked with the number 15 while pentoses are marked with the number 6, and
Table 2 gives their sum content by calculating the content of hexoses relative to glucose, and pentoses relative to ribose. In addition to sugar units, five fatty acids (0.01 mg/g - 0.21 mg/g), pinitol (0.02 mg/g - 0.04 mg/g), high-molecular-weight aliphatic hydrocarbons (0.01 mg/g - 0.55 mg/g), alcohols (0.3 mg/g - 0.51 mg/g) and hydrocarbon derivatives of furan-2(3H)-one (0.12 mg/g - 0.35 mg/g), were noted in the analyzed secretions of spittlebugs. In addition, glycerol (0.01 mg/g - 0.04 mg/g), formamide (about 0.01 mg/g), acetamide (about 0.01 mg/g) and 1,5-anhydro-D-sorbitol (about 0.01 mg/g) were found. The chemical composition of foam collected in different years varied greatly and the quantity of almost all the identified compounds was statistically different between the three years. The largest number of chemicals was identified in 2022, as the mass balance of identified substances was as high as about 93 %, while in earlier years, i.e., 2021, about 75 %, and in 2020 about 74 %. Referring to the content of individual identified substances in the analyzed secretions, an increasing trend in their content in subsequent years starting from 2020 through 2021 until 2022 can be observed.
4. Discussion
The results presented here can be considered a kind of breakthrough over the study of the chemical composition of the nymphal envelopes of a rather large group of insects commonly called spittlebugs. The most evident finding is that the foam produced by nymphs of A. alni is composed mainly of carbohydrate polymers while proteins are minor components. This is due to the fact that for the first time all the compounds identified in the secretions have been quantified. It was possible to identify and quantify from 73.7 % to over 93.2 % of the chemical substances contained in the secretions produced by the nymphal forms of A. alni. The key point is that the secretion produced by nymphal forms of A. alni is nit composed mainly of protein but mainly of carbohydrate polymers.
The protein content of the analyzed secretion did not exceed 0.12 % on a dry weight basis, while sugar units identified after acid hydrolysis accounted for as much as 92 % in the case of foam collected in 2022. The obtained results are inconsistent with the results of the chemical composition of foam produced by
M. fimbriolata nymphs, which showed a correlation opposite to the results obtained in our study. The protein content of the foam of
M. fimbriolata was about 320 µg ml
-1, while the carbohydrate content was only 0.579 µg ml
-1 [
9]. Chemical composition of the foam produced by the spittlebug
Poophilus costalis consists mainly of carbohydrates although it also contains protein structures [
10,
18]. Differently from our research, the authors based their findings on qualitative rather than quantitative analysis, so it is difficult to say how much more carbohydrates the foam they studied contained.
It is interesting to note that the main identified sugar unit in the foam produced by
A. alni is fucose, a sugar characteristic of carbohydrate polymers produced mainly by marine bacteria[
18,
19,
20] algae and fungi, where it occurs in a mostly sulfated form as fucoidan [
21]. The fucose content of the analyzed secretion samples ranged from about 17.0 % to 35.6 %, while the fucose content ranges from 19.0 % (
Ecklonia radiata) to 51.2 % (
Cladosiphon sp.) depending on the species of algae from which the fucoidate was isolated [
21]. A typical fucoidan isolated from various seaweeds, in addition to fucose, also contains other sugar units such as xylose (2.1 % - 11.0 %), glucose (1.3 % - 25.2 %), arabinose (0.0 % - 6.2 %) and rhamnose (0.0 % - 1.7 %). Analyzing the results obtained after acid hydrolysis of samples of secretions produced by
A. alni, it is clear that they contain typical sugar units such as in the case of fucoidians isolated from marine algae. It is likely that the polymer or polymer mixture found in the analyzed foam samples is produced by symbiotic bacteria that produce a polymer to some extent similar to marine fucoidians.
It should be noted that spittlebugs, both as imago and nymphs, are phytophages and feed on sap. The sap taken by spittlebugs contain only a few essential amino acids, while they contain large amounts of polysaccharides. In order to provide the organism with the dietary components missing from the food, spittlebugs have a number of symbiotic organisms located in different areas of the digestive tract or specially adapted tissues – bacteriomes [
6,
22,
23]. These organisms produce a whole range of biologically active compounds that pass into the foam produced [
11]. It can be presumed that as a result of digesting the juice of the plant on which the nymphs feed, the symbiotic organisms produce a polymer that is necessary for the insect to produce the protective nymphal envelope necessary for its survival. This element needs to be studied further to determine the exact chemical structure including the type of glycosidic bonds present in the insect-derived polymer.
Another remarkable result is the very low dry matter content of the analyzed samples. The dry matter content is in the range of 0.05 % - 0.1 %. Unfortunately, it is not possible to relate the obtained results to the literature data, since no one has previously studied the secretions of insects belonging to spittlebugs in this regard. Such a low content of dry matter in the secretion may indicate the amazing properties of the carbohydrate polymer in it, which even has ideal foaming properties including increasing the surface tension of water, thus enabling the formation of "bubbles" of foam around the insect's feeding site. Chemical compounds probably helpful in producing foam bubbles are the fatty acids like tetradecanoic acid (myristic acid), (9Z)-hexadec-9-enoic acid (palmitoleic acid) octadecanoic acid (stearic acid), 9-octadecenoic acid (oleic acid), 9,12-octadecadienoic acid (linoleic acid) and 11-eicosenoic acid. Similar acids were found in the secretions produced by spittlebugs nymphs, recognizing that they play some role in the production of nymphal envelope bubbles by changing the surface tension of water occurring in the form of sodium or potassium salts – soaps [
9,
11]. We agree with the attributed role of fatty acids found in the secretions produced by spittlebugs, but additionally, in our opinion, they may have other functions, e.g. protective - antibacterial, anti-inflammatory, etc.
In the foam samples analyzed, we also found the presence of other substances, which were also identified when studying the secretions of
Aphrophora cribrata a species closely related to
A. alni [
11]. We also found the presence of glycerol, pintol, myo-inositol, hexacosyl alcohol, hexatriaconane and 5-henicosyldihydrofuran-2(3H)-one[
11]. We also agree with the thesis that these substances are the result of an activity of symbiotic organisms whose metabolites enter the foam. Similar results were obtained, with the additional demonstration of variability in the chemical composition of nymphal envelopes depending on the host plant on which the nymphs were feeding [
10]. It is also interesting to note the highly variable chemical composition of nymphal envelopes depending on the year of collection. Nymphal envelopes produce and cover themselves with foam as a thermoregulatory adaptation [
9] , so we decided to test whether the chemical composition of foam harvested in different years is different. The research hypothesis set forth was confirmed as shown by the results presented in
Table 2. The variation in chemical composition at different collection periods is likely to have a strong connection to aspects of thermoregulation and the maintenance of a constant temperature of the larva surrounded by foam bubbles. With the above in mind, the values of daytime and nighttime temperatures were recorded, and the foam was always collected in the morning (5-6 am), mainly to avoid heating the feeding grounds of spittlebugs during the day. The results obtained absolutely showed that the chemical composition of the foam varied in the successive years analyzed. The main factors that changed during these periods were air temperature. A tendency was observed that the colder the nights and the air temperatures during the day, the higher was the dry matter content of the foam and the content of sugar units building the carbohydrate polymer increased significantly. In 2022 where the night temperature fell below 10 °C and the average daytime temperature was 20 °C, the sugar units building the polymer of insect secretions accounted for as much as 92 % of the compounds identified in the foam. We have to underline that not only temperature can play important role in chemical composition changes of nymphal envelopes. We cannot forgot about host plant species indirectly plays an important role in the chemical composition of the produced froth. Additionally plant growth, soil mineral and organic composition composition, amount of sunny days ect can play important role in change of chemical composition of nymphal envelopes and it could be next aim of another studies to understand mentioned factors.
5. Conclusion
The results emphasize how the chemical composition of spittlebugs' secretions is important for thermoregulation of the larva's environment, and the organism itself attempts to adapt to the changing environment. For the first time, the chemical composition of the foam produced by the spittlebug was quantitatively analyzed. Significant changes in the chemical composition of nymphal foam was found depending on the year of collection, which is probably strongly related to environment temperature. We have to underline that not only temperature can play important role in chemical composition changes of nymphal envelopes. We cannot forgot about host plant species indirectly plays an important role in the chemical composition of the produced froth. Additionally plant growth, soil mineral and organic composition composition, amount of sunny days ect can play important role in change of chemical composition of nymphal envelopes and it could be next aim of another studies to understand mentioned factors. The analyzed samples consisted mainly of a carbohydrate polymer similar in sugar unit composition to fucoidate isolated from brown seaweed. The most important sugar units identified were fucose, glucose, mannose, rhamnose and xylose. In addition, various fatty acids, myo-inositol, pinitol, aliphatic hydrocarbons, waxes and aliphatic derivatives of 2(3H)-furanone were found.
Author Contributions
Conceptualization, Methodology, Formal analysis, Writing—original draft preparation, Supervision, Project administration, and Funding acquisition, A.S.; Software, B.S.; Validation, K.O., B.S.; Investigation, A.S., K.O., B.S.; Resources, A.S., S.F.; Data curation, A.S., K.O.; Writing—review and editing, S.F.; Visualization, K.O.; All authors have read and agreed to the published version of the manuscript.
Data Availability
The data that support the findings of this study are available from the corresponding author (ASz) upon reasonable request
Acknowledgments
This work was supported by the National Center of Science Twardowskiego 16 st, 30-312 Kraków, Poland, grant ID 407548, grant number 2018/29/B/NZ7/00380.
Conflicts of Interest
The authors declare no competing interests.
References
- Pietra, F. Biodiversity and Natural Product Diversity; Pergamon Press: Amsterdam, 2002. [Google Scholar]
- Chapman, A.D. Numbers of Living Species in Australia and the World, 2nd ed.; Australian Biological Resources Study: Toowoomba, 2009; ISBN 978 0 642 56861 8. [Google Scholar]
- Gronquist, M.; Schroeder, F.C. Insect Natural Products. In Comprehensive Natural Products II; Elsevier, 2010; pp. 67–108. [Google Scholar]
- Pankewitz, F.; Hilker, M. Polyketides in Insects: Ecological Role of These Widespread Chemicals and Evolutionary Aspects of Their Biogenesis. Biol. Rev. 2008, 83, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Rakitov, R.A. Post-Moulting Behavior Associated with Malphigian Tubule Secretions in Leafhoppers and Treehoppers. Eur. J.Entomol. 1996, 93, 167–184. [Google Scholar]
- Maryańska-Nadachowska, A.; Drosopoulos, S.; Lachowska, D.; Kajtoch, Ł.; Kuznetsova, V.G. Molecular Phylogeny of the Mediterranean Species of Philaenus (Hemiptera: Auchenorrhyncha: Aphrophoridae) Using Mitochondrial and Nuclear DNA Sequences. Syst. Entomol. 2010, 35, 318–328. [Google Scholar] [CrossRef]
- Whittaker, J.B. Cercopid Spittle as a Microhabitat. Oikos 1970, 21, 59–64. [Google Scholar] [CrossRef]
- Mello, M.L.S. Effect of Some Enzymes, Chemicals and Insecticides on the Macromolecular Structure of the Froth of the Spittlebug, the Cercopid Deois Sp. Entomol. Exp. Appl. 1987, 44, 139–144. [Google Scholar] [CrossRef]
- Tonelli, M.; Gomes, G.; Silva, W.D.; Magri, N.T.C.; Vieira, D.M.; Aguiar, C.L.; Bento, J.M.S. Spittlebugs Produce Foam as a Thermoregulatory Adaptation. Sci. Rep. 2018, 8, 4729. [Google Scholar] [CrossRef] [PubMed]
- Sahayaraj, K.; Saranya, B.; Sayed, S.; Estelle, L.Y.L.; Madasamy, K. Biofoam of Spittlebug, Poophilus Costalis (Walker): Preferential Sites, Temperature Regulation, Chemical Composition and Antimicrobial Activity. Insects 2021, 12. [Google Scholar] [CrossRef]
- del Campo, M.L.; King, J.T.; Gronquist, M.R. Defensive and Chemical Characterization of the Froth Produced by the Cercopid Aphrophora Cribrata. Chemoecology 2011, 21, 1–8. [Google Scholar] [CrossRef]
- Balzani, P.; Nencioni, A.; Grillini, M.; Masoni, A.; Zuri, F.; Picchi, S.; Frizzi, F.; Sacchetti, P.; Cantini, C.; Santini, G. Spittlebug Invisibility Cloak: Experimental Tests on the Antipredatory Effect of the Froth of Philaenus Spumarius. Bull. Insectology 2023, 76, 21–28. [Google Scholar]
- Carvalho, G.S.; Webb, M. Cercopid Spittle Bugs of the New World (Hemiptera, Auchenorrhyncha, Cercopidae); Pensoft, 2005; ISBN 954-642-246-0. [Google Scholar]
- Mello, M.L.S.; Pimentel, E.R.; Yamada, A.T.; Storopoli-Neto, A. Composition and Structure of the Froth of the Spittlebug, Deois SP. Insect Biochem. 1987, 17, 493–502. [Google Scholar] [CrossRef]
- Yellow Biotechnology I. Vilcinskas, A. (Ed.) In Advances in Biochemical Engineering/Biotechnology; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; Volume 135, ISBN 978-3-642-39862-9. [Google Scholar]
- Yellow Biotechnology II. Vilcinskas, A. (Ed.) In Advances in Biochemical Engineering/Biotechnology; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; Volume 136, ISBN 978-3-642-39901-5. [Google Scholar]
- Tonk, M.; Vilcinskas, A. The Medical Potential of Antimicrobial Peptides from Insects. Curr. Top. Med. Chem. 2016, 17, 554–575. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.T. Spittle-Production and Tube-Building by Cercopid Larvae (Homoptera)-IV. Mucopolysaccharide Associated with Spittle-Production. J. Insect Physiol. 1966, 12, 635–644. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Rasin, A.B.; Zueva, A.O.; Kusaykin, M.I.; Zvyagintseva, T.N.; Kalinovsky, A.I.; Kurilenko, V. V.; Ermakova, S.P. Fucoidan Sulfatases from Marine Bacterium Wenyingzhuangia Fucanilytica CZ1127T. Biomolecules 2018, 8, 98. [Google Scholar] [CrossRef]
- Marshall, A.T. Protein Synthesis and Secretion by the Malpighian Tubules of Cercopoid Larvae (Homoptera). J. Insect Physiol. 1973, 19, 2317–2326. [Google Scholar] [CrossRef]
- Fitton, J.; Stringer, D.; Karpiniec, S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.A.; Dorsey, C.K. Studies on the Composition and Microbiology of Insect Spittle1. Ann. Entomol. Soc. Am. 1957, 50, 399–406. [Google Scholar] [CrossRef]
- Nencioni, A.; Pastorelli, R.; Bigiotti, G.; Cucu, M.A.; Sacchetti, P. Diversity of the Bacterial Community Associated with Hindgut, Malpighian Tubules, and Foam of Nymphs of Two Spittlebug Species (Hemiptera: Aphrophoridae). Microorganisms 2023, 11. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).