Submitted:
14 July 2023
Posted:
17 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characterization
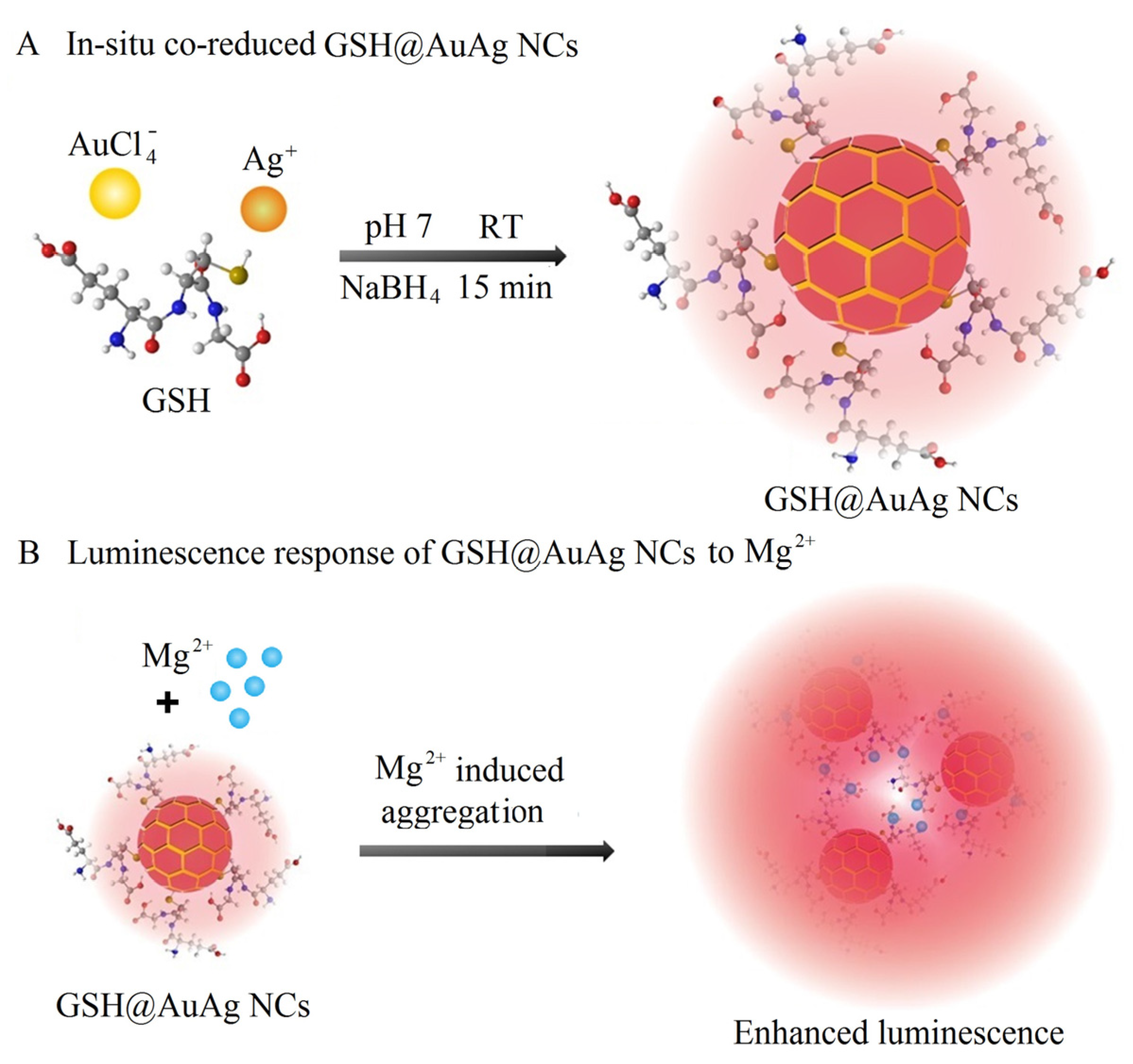
2.3. Synthesis of GSH@AuAg NCs
2.4. Selective and Sensitive Detection of Mg2+
3. Results and Discussion
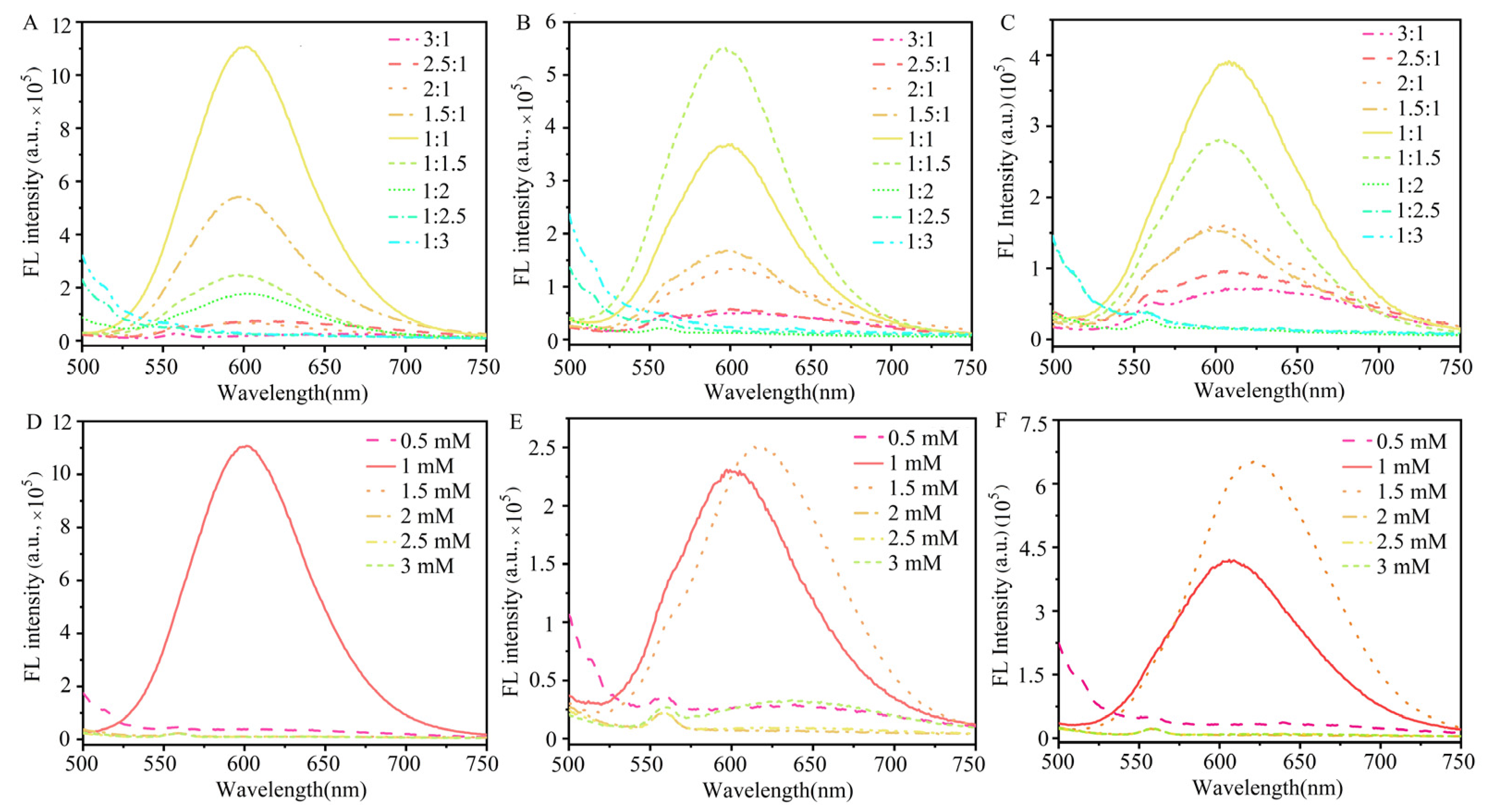
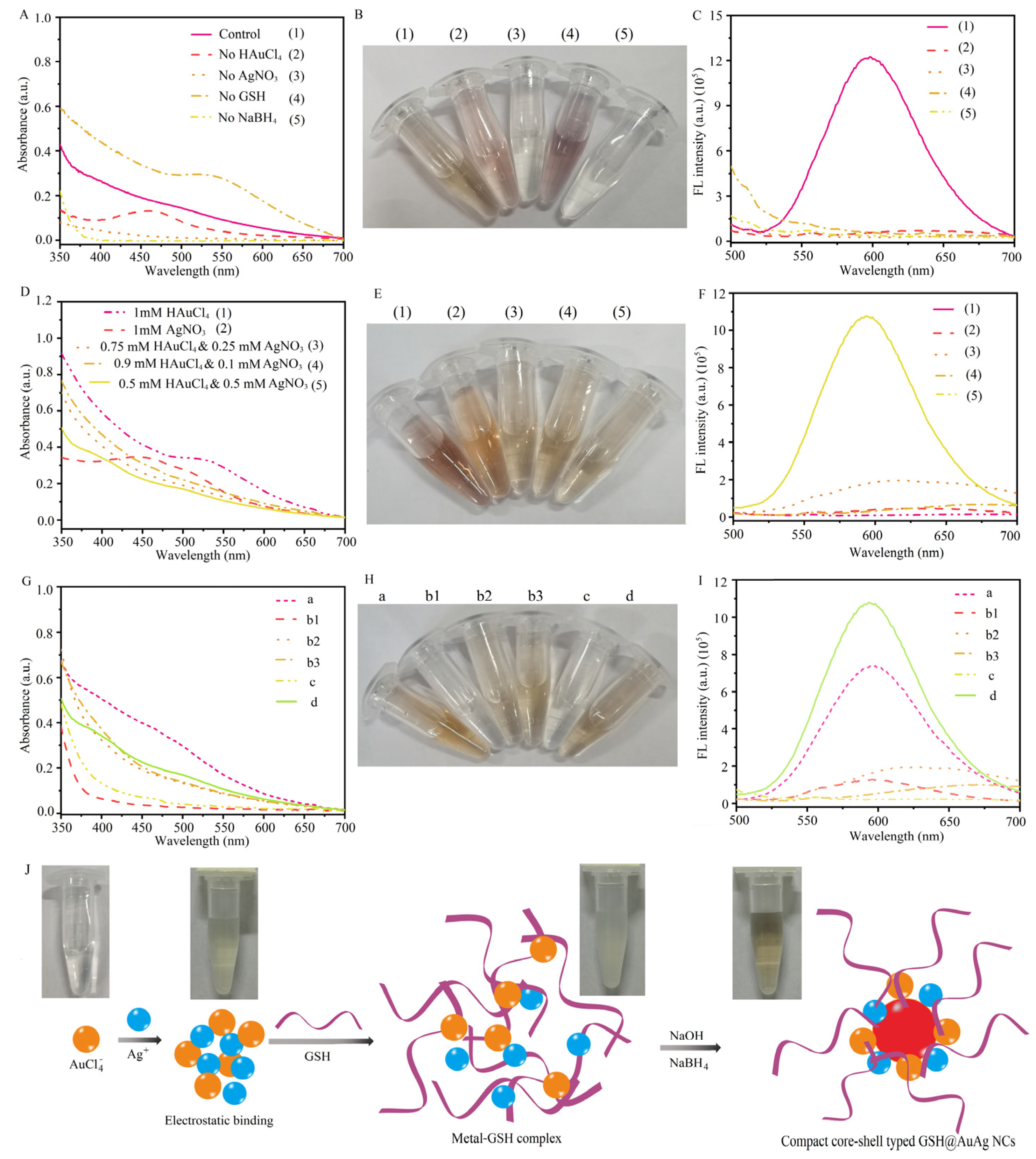
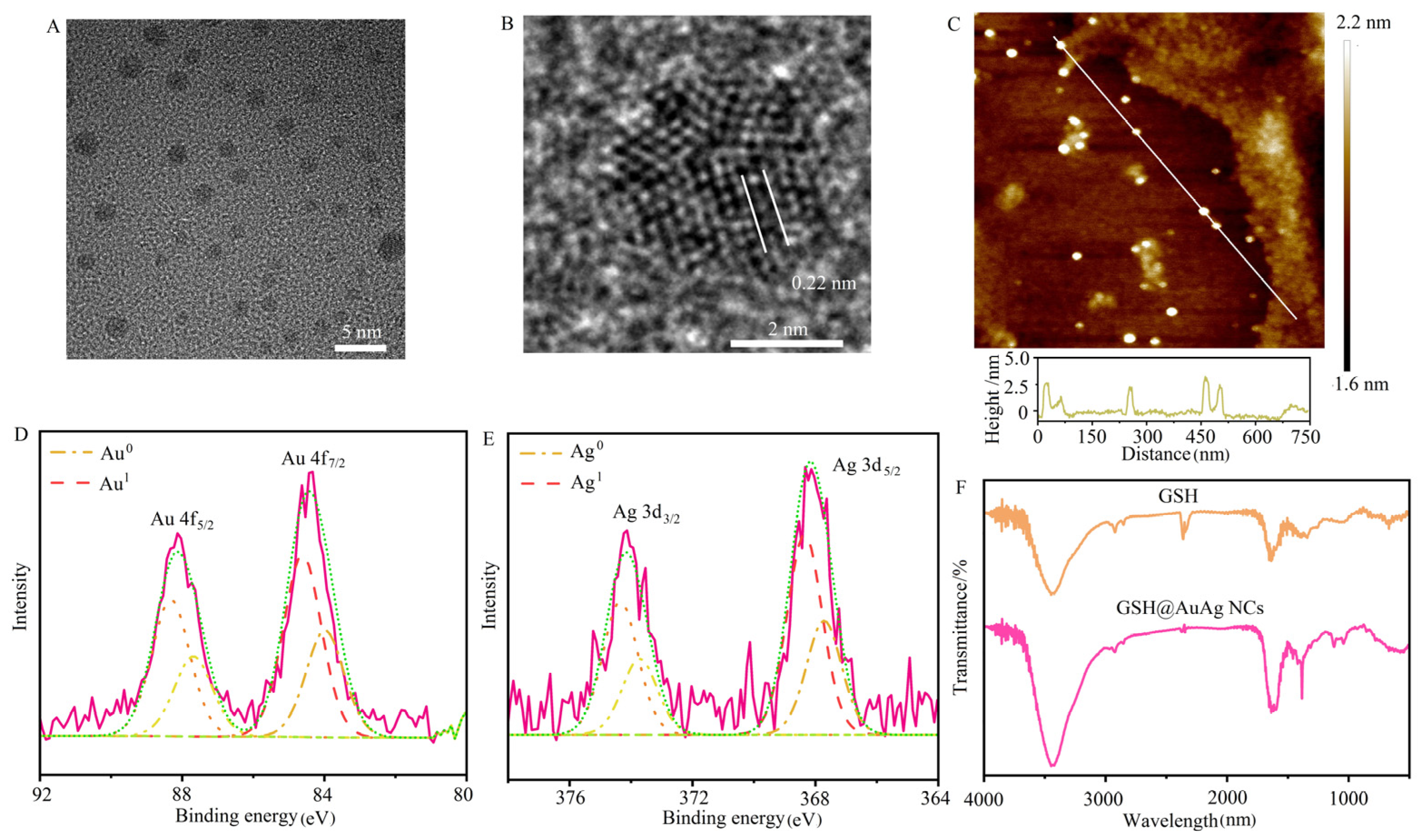
3.1. Synthesis and Characterization of GSH@AuAg NCs
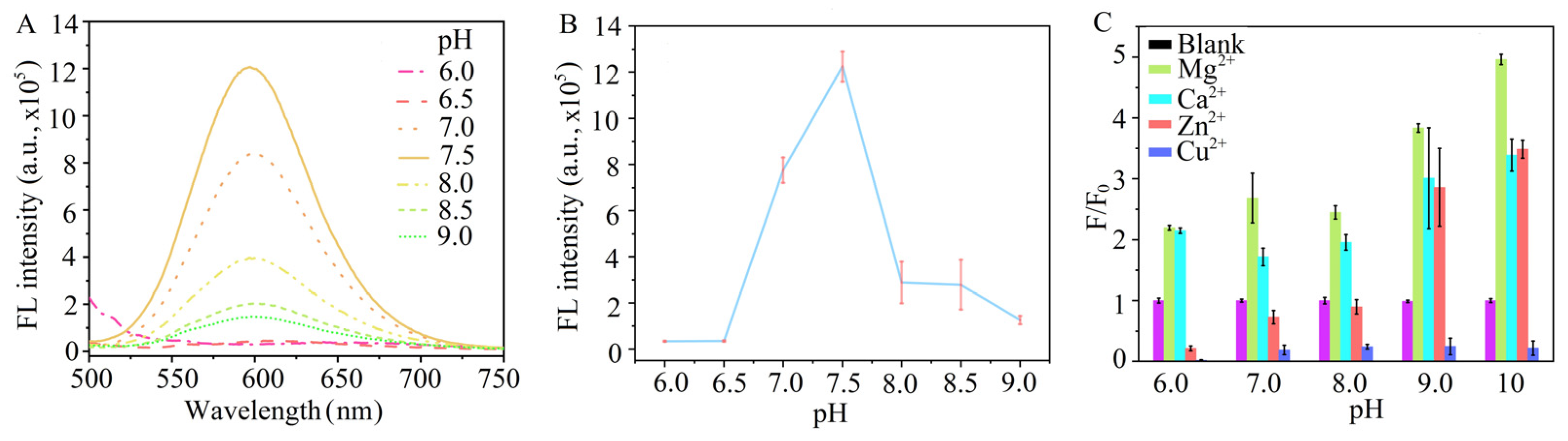
3.2. Optimization of pH for Recognition of GSH@AuAg NCs to Mg2+
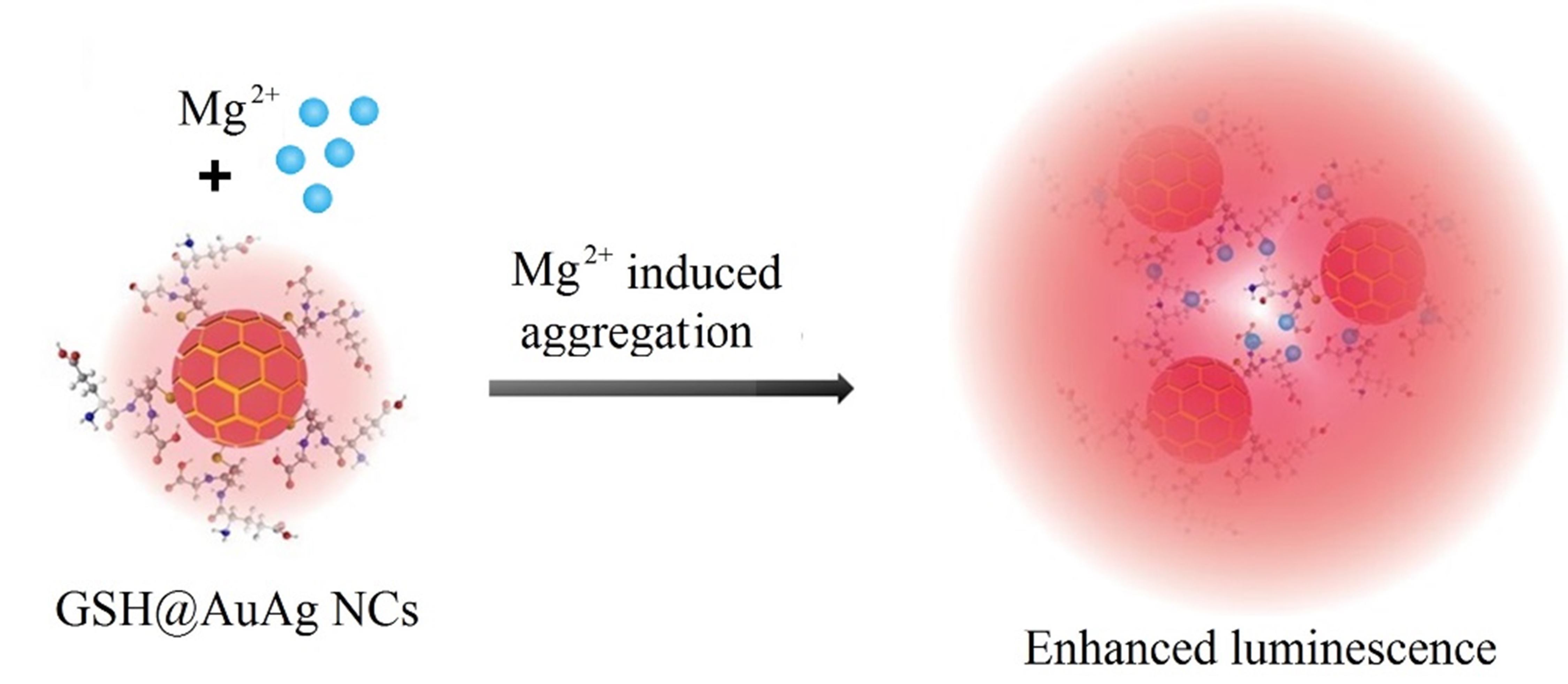
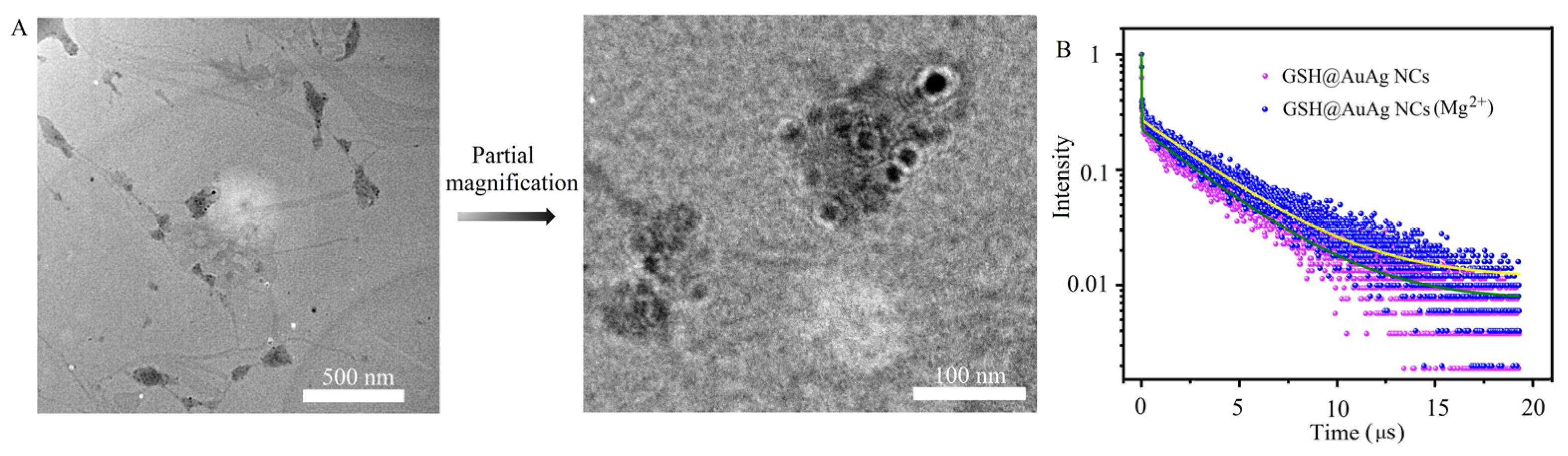
3.3. Mechanism of Mg2+-Mediated Fluorescence Enhancement of GSH@AuAg NCs
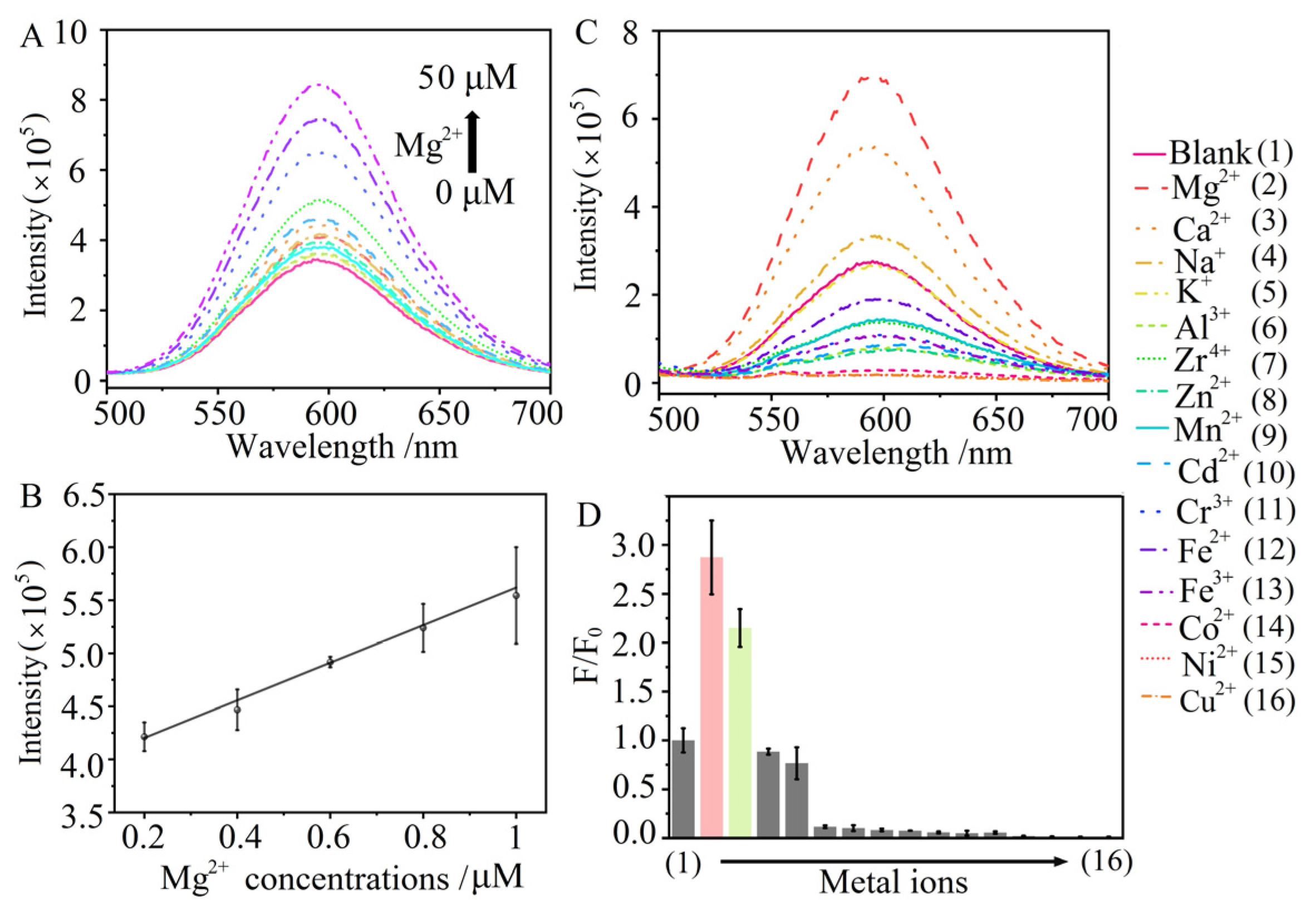
3.4. Sensing Performance towards Mg2+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, Q.; Chen, T.; Yuan, X.; Xie, J. Toward Total Synthesis of Thiolate-Protected Metal Nanoclusters. Acc. Chem. Res. 2018, 51, 1338–1348. [Google Scholar] [CrossRef]
- Jia, H.; Yu, S.; Yang, L.; Wei, Q.; Ju, H. Near-Infrared Electrochemiluminescence of Dual-Stabilizer-Capped Au Nanoclusters for Immunoassays. ACS Appl. Nano Mater. 2021, 4, 2657–2663. [Google Scholar] [CrossRef]
- Jia, H.; Yang, L.; Fan, D.; Kuang, X.; Sun, X.; Wei, Q.; Ju, H. Cobalt ion doping to improve electrochemiluminescence emisssion of gold nanoclusters for sensitive NIR biosensing. Sens. Actuators B Chem. 2022, 367, 132304. [Google Scholar] [CrossRef]
- Srinivasulu, Y.G.; Goswami, N.; Yao, Q.; Xie, J. High-Yield Synthesis of AIE-type Au22(SG)18 Nanoclusters through Precursors Engineering and Its pH-Dependent Size Transformation. J. Phys. Chem. C 2021, 125, 4066–4076. [Google Scholar] [CrossRef]
- Mastracco, P.; Gonzalez-Rosell, A.; Evans, J.; Bogdanov, P.; Copp, S.M. Chemistry-Informed Machine Learning Enables Discovery of DNA-Stabilized Silver Nanoclusters with Near-Infrared Fluorescence. ACS Nano 2022, 16, 16322–16331. [Google Scholar] [CrossRef]
- Aires, A.; Sousaraei, A.; Moller, M.; Cabanillas-Gonzalez, J.; Cortajarena, A.L. Boosting the Photoluminescent Properties of Protein-Stabilized Gold Nanoclusters through Protein Engineering. Nano Lett 2021, 21, 9347–9353. [Google Scholar] [CrossRef]
- Li, J.; Peng, G.; Yu, Y.; Lin, B.; Zhang, L.; Guo, M.; Cao, Y.; Wang, Y. Cu2+-mediated turn-on fluorescence biosensor based on DNA-templated silver nanoclusters for label-free and sensitive detection of adenosine triphosphate. Microchim. Acta 2023, 190, 41. [Google Scholar] [CrossRef]
- Zanetti-Polzi, L.; Charchar, P.; Yarovsky, I.; Corni, S. Origins of the pH-Responsive Photoluminescence of Peptide-Functionalized Au Nanoclusters. ACS Nano 2022, 16, 20129–20140. [Google Scholar] [CrossRef]
- Li, X.; Luo, J.; Jiang, X.; Yang, M.; Rasooly, A. Gold nanocluster-europium(III) ratiometric fluorescence assay for dipicolinic acid. Microchim. Acta 2021, 188, 26. [Google Scholar] [CrossRef]
- Mi, W.; Tang, S.; Guo, S.; Li, H.; Shao, N. In situ synthesis of red fluorescent gold nanoclusters with enzyme-like activity for oxidative stress amplification in chemodynamic therapy. Chinese Chem. Lett. 2022, 33, 1331–1336. [Google Scholar] [CrossRef]
- Mi, W.; Tang, S.; Jin, Y.; Shao, N. Au/Ag Bimetallic Nanoclusters Stabilized by Glutathione and Lysozyme for Ratiometric Sensing of H2O2 and Hydroxyl Radicals. ACS Appl. Nano Mater. 2021, 4, 1586–1595. [Google Scholar] [CrossRef]
- Yin, M-M. ; Chen, W-Q.; Hu, Y-J.; Liu, Y, Jiang, F-L. Rapid preparation of water-soluble Ag@Au nanoclusters with bright deep-red emission. Chem. Commun. 2022, 58, 2492–2495. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, C.; Chen, B.; Huang, H.; Xia, Q.; Li, X.; Shen, Z.; Ge, Z.; Wang, Y. Surface functionalized red fluorescent dual-metallic Au/Ag nanoclusters for endoplasmic reticulum imaging. Microchim. Acta 2022, 187, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, Y.; Zhou, X.; Hu, J. A label-free turn-on-off fluorescent sensor for the sensitive detection of cysteine via blocking the Ag+-enhancing glutathione-capped gold nanoclusters. Talanta 2018, 179, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Farrag, M.; Tschurl, M.; Heiz, U. Chiral Gold and Silver Nanoclusters: Preparation, Size selection, and Chiroptical Properties. Chem. Mater. 2013, 25, 862–870. [Google Scholar] [CrossRef]
- Pontes, M.H.; Yeom, J.; Groisman, E.A. Reducing ribosome biosynthesis promotes translation during low Mg2+ stress. Mol. Cell 2016, 64, 480–492. [Google Scholar] [CrossRef]
- Kim, D. Y.; Shinde, S.; Ghodake, G. Colorimetric detection of magnesium (II) ions using tryptophan functionalized gold nanoparticles. Sci. Rep. 2017, 7, 3966. [Google Scholar] [CrossRef]
- Li, L.; Ding, Y.; Zhang, C.; Xian, H.; Chen, S.; Dai, G.; Wang, X.; Ye, C. Ratiometric Fluorescence Detection of Mg2+ based on Regulating Crown-Ether Modified Annihilators for Triplet-Triplet Annihilation Upconversion. J. Phys. Chem. B 2022, 126, 3276–3282. [Google Scholar] [CrossRef]
- Gruskos, J. J.; Zhang, G.; Buccella, D. Visualizing Compartmentalized Cellular Mg2+ on Demand with Small-Molecule Fluorescent Sensors. J. Am. Chem. Soc. 2016, 138, 14639–14649. [Google Scholar] [CrossRef]
- Fujii, T.; Shindo, Y.; Hotta, K.; Citterio, D.; Nishiyama, S.; Suzuki, K.; Oka, K. Design and Synthesis of a FlAsH-Type Mg2+ Fluorescent Probe for Specific Protein Labeling. J. Am. Chem. Soc. 2014, 136, 2374–2381. [Google Scholar] [CrossRef]
- Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.; Xie, J. Luminescent Metal Nanoclusters with Aggregation-Induced Emission. J. Phys. Chem. Lett. 2016, 7, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From Aggregation-Induced Emission of Au(I)-Thiolate Complexes to Ultrabright Au (0) @Au (I)-Thiolate Core-Shell Nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, R.; Hao, Y.; Pang, J.; Gao, H.; Qu, F.; Tian, M.; Guo, C.; Mao, B.; Chai, F. Portable smartphone-integrated AuAg nanoclusters electrospun membranes for multivariate fluorescent sensing of Hg2+, Cu2+ and L-histidine in water and food samples. Food Chem. 2023, 418, 135961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X-L. ; Li, X.; Li, X-T.; Gao, Y.; Feng, F.; Yang, G-J. Electrochemiluminescence sensor for pentoxifylline detection using Au nanoclusters@graphene quantum dots as an amplified electrochemiluminescence luminophore. Sens. Actuators B Chem. 2019, 282, 927–935. [Google Scholar] [CrossRef]
- Jia, H.; Yang, L.; Dong, X.; Zhou, L.; Wei, Q.; Ju, H. Cysteine Modification of Glutathione-Stabilized Au Nanoclusters to Red-Shift and Enhance the Electrochemiluminescence for Sensitive Bioanalysis. Anal. Chem. 2022, 94, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, L.; Zhang, M.; Deng, C.; Yang, X. A Gold-silver bimetallic nanocluster-based fluorescent probe for cysteine detection in milk and apple. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 2022, 278, 121345. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Yu, J.; Gong, X.; Liang, W.; Fan, L.; Dong, C. A turn-off-on near-infrared photoluminescence sensor for sequential detection of Fe3+ and ascorbic acid based on glutathione-capped gold nanoclusters. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 2021, 247, 119085. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, A.; Wen, Q.; Wu, B.; Wang, J.; Hu, Y.; Pu, Z.; Ling, J.; Cao, Q. Glutathione stabilized green-emission gold nanoclusters for selective detection of cobalt ion. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 2021, 254, 119628. [Google Scholar] [CrossRef]
- Sadhanala, H.K.; Aryal, S.; Sharma, K.; Orpaz, Z.; Michaeli, S.; Gedanken, A. Nitrogen-doped carbon dots as a highly selective and sensitive fluorescent probe for sensing Mg2+ ions in aqueous solution, and their application in the detection and imaging of intracellular Mg2+ ions. Sens. Actuators B Chem. 2022, 366, 131958. [Google Scholar] [CrossRef]
- Chang, H.; Karan, N.S.; Shin, K.; Bootharaju, M.S.; Nah, S.; Chae, S.I.; Baek, W.; Lee, S.; Kim, J.; Son, Y.J.; Kang, T.; Ko, G.; Kwon, S-H.; Hyeon, T. Highly Fluorescent Gold Cluster Assembly. J.Am. Chem. Soc. 2021, 143, 326–334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




