Submitted:
14 July 2023
Posted:
17 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Immune mechanisms involved in the pathogenesis of sickle cell anaemia
Autoimmunity in Sickle cell disease
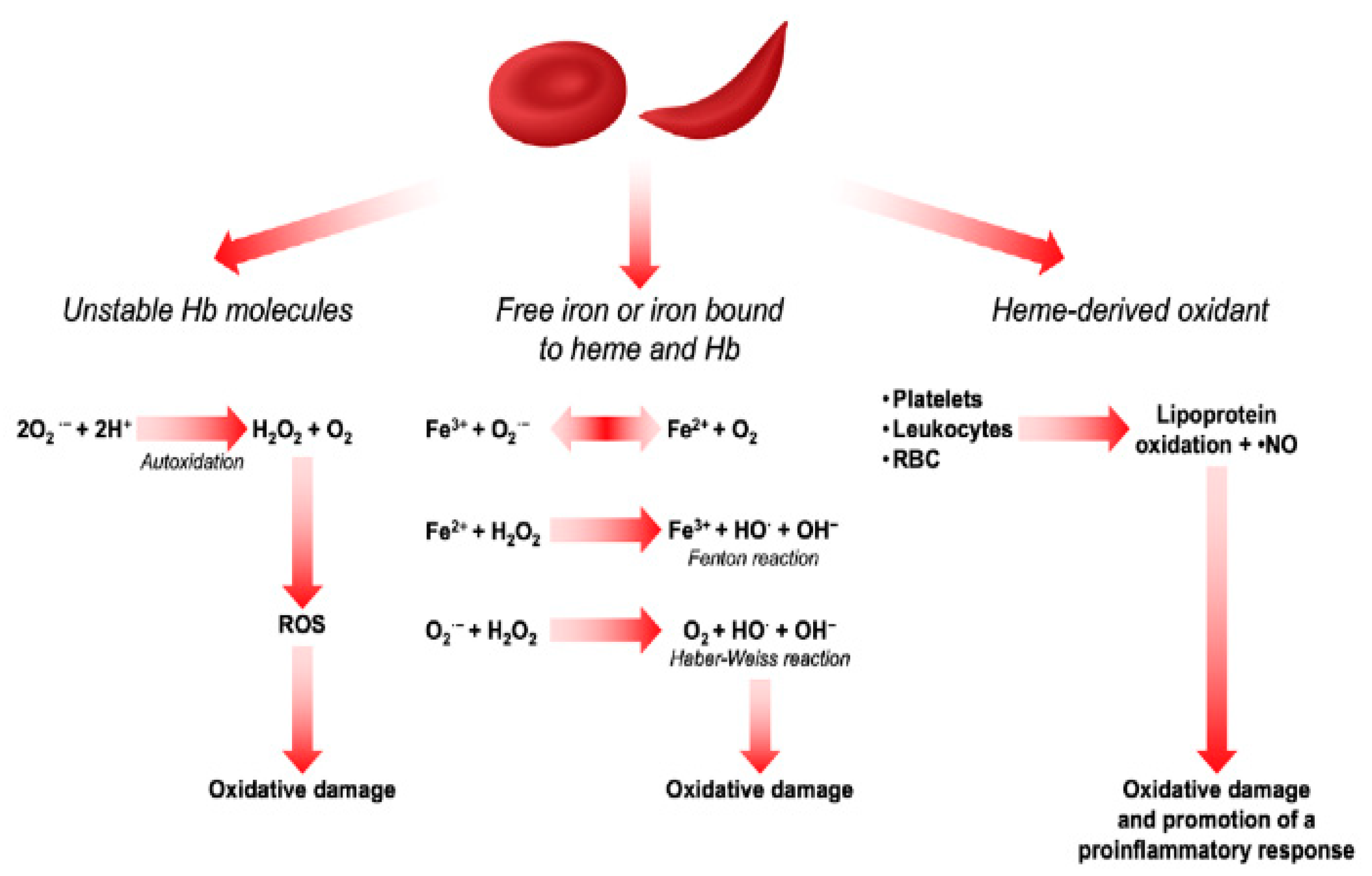
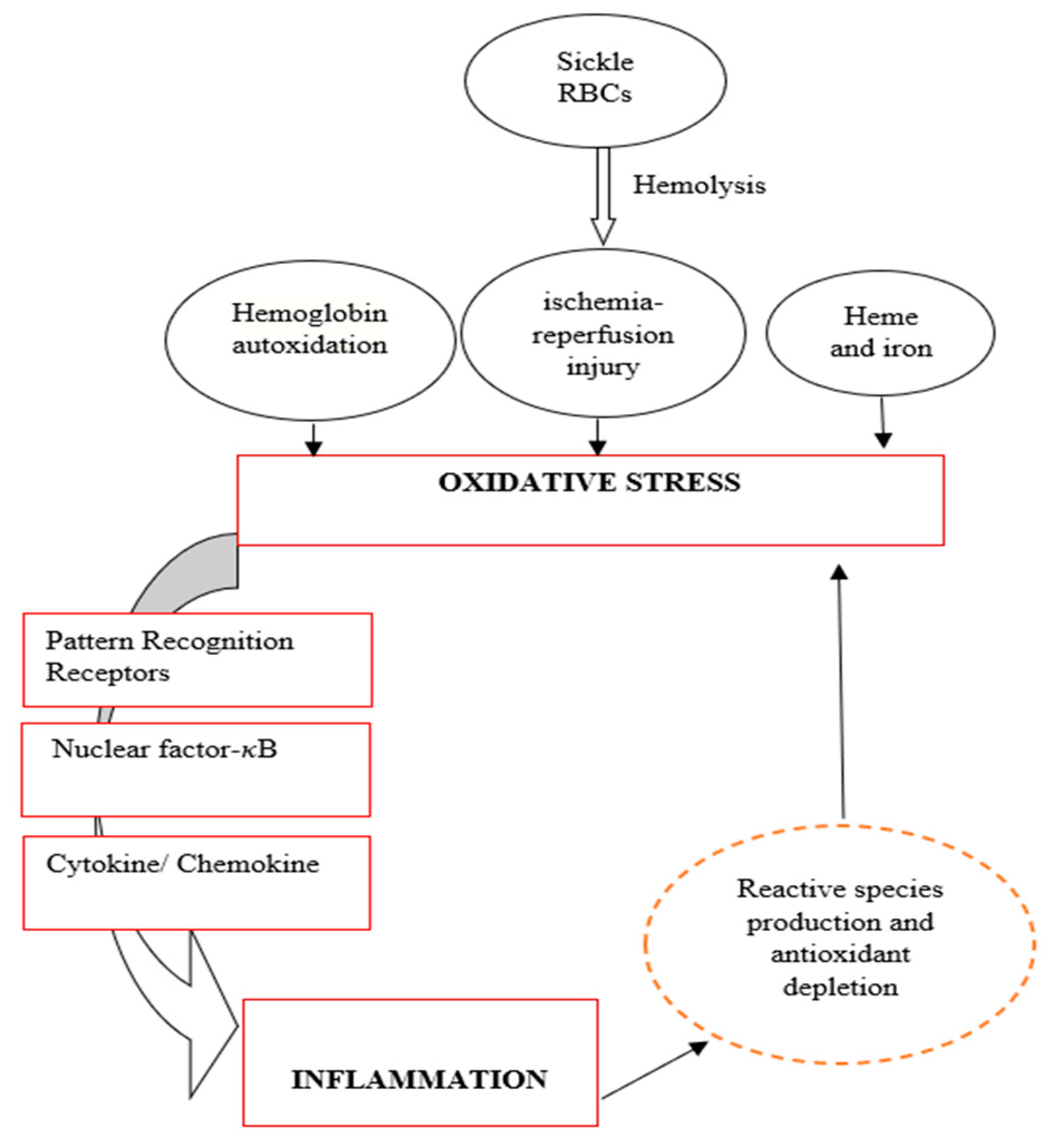
The role of oxidative stress in the pathogenesis of sickle cell anemia
The role of inflammation in the pathogenesis of sickle cell anaemia
The relationship and interdependence between inflammation and oxidative stress in SCD
Inflammation and blood transfusion in SCD
The cause of high alloimmunization in SCD patients
Other Treatment options
Conclusion
References
- Royal, C.D. , et al., Sickle cell disease is a global prototype for integrative research and healthcare. Advanced Genetics, 2021. 2(1): p. e10037. [CrossRef]
- Vona, R. , et al., Sickle cell disease: role of oxidative stress and antioxidant therapy. Antioxidants, 2021. 10(2): p. 296. [CrossRef]
- Adesina, O.A. and A.O. Opesade, Bibliometirc Analysis of Sickle Cell Anaemia Literature on Nigeria Listed in Pubmed between 2006 and 2016. Library Philosophy and Practice, 2018: p. 1.
- Fraiwan, A. , et al., Advancing healthcare outcomes for sickle cell disease in Nigeria using mobile health tools. Blood, 2019. 134: p. 2173. [CrossRef]
- Mwaiswelo, R.O. , et al., Sickle cell disease and malaria: decreased exposure and asplenia can modulate the risk from Plasmodium falciparum. Malaria Journal, 2020. 19(1): p. 1-5. [CrossRef]
- Nnodu, O. , et al., HemoTypeSC, a low-cost point-of-care testing device for sickle cell disease: promises and challenges. Blood Cells, Molecules, and Diseases, 2019. 78: p. 22-28. [CrossRef]
- Piccin, A. , et al., Insight into the complex pathophysiology of sickle cell anaemia and possible treatment. European journal of haematology, 2019. 102(4): p. 319-330. [CrossRef]
- Loggetto, S.R. , et al., Guidelines on sickle cell disease: secondary stroke prevention in children and adolescents. Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular guidelines project: Associação Médica Brasileira-2022. Hematology, Transfusion and Cell Therapy, 2022. 44: p. 246-255. [CrossRef]
- Nader, E., M. Romana, and P. Connes, The red blood cell—inflammation vicious circle in sickle cell disease. Frontiers in immunology, 2020. 11: p. 454. [CrossRef]
- Atiku, S.M., N. Louise, and D.M. Kasozi, Severe oxidative stress in sickle cell disease patients with uncomplicated Plasmodium falciparum malaria in Kampala, Uganda. BMC Infectious Diseases, 2019. 19(1): p. 1-10. [CrossRef]
- Praharaj, D.L. and A.C. Anand, Sickle hepatopathy. Journal of Clinical and Experimental Hepatology, 2021. 11(1): p. 82-96.
- Nolfi-Donegan, D. , et al., Redox signaling in sickle cell disease. Current opinion in physiology, 2019. 9: p. 26-33. [CrossRef]
- Quezado, Z.M. , et al., Mitapivat increases ATP and decreases oxidative stress and erythrocyte mitochondria retention in a SCD mouse model. Blood Cells, Molecules, and Diseases, 2022. 95: p. 102660. [CrossRef]
- Inusa, B.P. , et al., Sickle cell disease—genetics, pathophysiology, clinical presentation and treatment. International journal of neonatal screening, 2019. 5(2): p. 20.
- Bozza, M.T. and V. Jeney, Pro-inflammatory actions of heme and other hemoglobin-derived DAMPs. Frontiers in Immunology, 2020. 11: p. 1323. [CrossRef]
- Gbotosho, O.T., M. G. Kapetanaki, and G.J. Kato, The worst things in life are free: The role of free heme in sickle cell disease. Frontiers in Immunology, 2021. 11: p. 561917. [CrossRef]
- Adwas, A.A. , et al., Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng, 2019. 6(1): p. 43-47. [CrossRef]
- Tebuka, E., M. Charles, and J.O. Bhuko, Prevalence and risk factors for red blood cell alloimmunisation among sickle cell patients in Mwanza City, Tanzania. African Journal of Laboratory Medicine, 2020. 9(1): p. 1-5. [CrossRef]
- Conrath, S. , et al., Increased prevalence of alloimmunization in sickle cell disease? Should we restore blood donation in French Guiana? Frontiers in Medicine, 2021. 8: p. 681549.
- Khatun, A. , et al., Frequency of alloantibody with their specification among multitransfused patients. Global Journal of Transfusion Medicine, 2020. 5(2): p. 178. [CrossRef]
- Fasano, R.M. , et al., Impact of red blood cell antigen matching on alloimmunization and transfusion complications in patients with sickle cell disease: a systematic review. Transfusion Medicine Reviews, 2019. 33(1): p. 12-23. [CrossRef]
- El Chaer, F. , et al., Sickle cell disease complicated by iron overload: an under-recognized risk factor for Vibrio vulnificus infection. Acta Haematologica, 2018. 139(3): p. 199-200. [CrossRef]
- Allali, S. , et al., Innate immune cells, major protagonists of sickle cell disease pathophysiology. Haematologica, 2020. 105(2): p. 273. [CrossRef]
- de Azevedo, J.T.C. and K.C.R. Malmegrim, Immune mechanisms involved in sickle cell disease pathogenesis: current knowledge and perspectives. Immunology Letters, 2020. 224: p. 1-11.
- Allali, S. , et al., Innate-like T cells in children with sickle cell disease. Plos one, 2019. 14(6): p. e0219047. [CrossRef]
- Sesti-Costa, R. , et al., Inflammatory Dendritic Cells Contribute to Regulate the Immune Response in Sickle Cell Disease. Frontiers in Immunology, 2021. 11: p. 617962. [CrossRef]
- Ahmad, A. and H. Ahsan, Biomarkers of inflammation and oxidative stress in ophthalmic disorders. Journal of Immunoassay and Immunochemistry, 2020. 41(3): p. 257-271. [CrossRef]
- Engwa, G.A. , et al., Relationship of oxidative stress and antioxidant response with vaso-occlusive crisis in sickle cell anaemia. African Health Sciences, 2021. 21(1): p. 150-8. [CrossRef]
- Ochocinski, D. , et al., Life-threatening infectious complications in sickle cell disease: a concise narrative review. Frontiers in Pediatrics, 2020. 8: p. 38. [CrossRef]
- Rayes, J. , et al., The dual role of platelet-innate immune cell interactions in thrombo-inflammation. Research and practice in thrombosis and haemostasis, 2020. 4(1): p. e12266.
- Conran, N. and J.D. Belcher, Inflammation in sickle cell disease. Clinical hemorheology and microcirculation, 2018. 68(2-3): p. 263-299.
- Garcia, N.P. , et al., Sickle cell anemia patients display an intricate cellular and serum biomarker network highlighted by TCD4+ CD69+ lymphocytes, IL-17/MIP-1β, IL-12/VEGF, and IL-10/IP-10 axis. Journal of Immunology Research, 2020. 2020.
- Parsons, S.F. , et al., Erythroid cell adhesion molecules Lutheran and LW in health and disease. Best Practice & Research Clinical Haematology, 1999. 12(4): p. 729-745. [CrossRef]
- Brown, M.D., T. M. Wick, and J.R. Eckman, Activation of vascular endothelial cell adhesion molecule expression by sickle blood cells. Pediatric pathology & molecular medicine, 2001. 20(1): p. 47-72.
- Pathare, A. , et al., Cytokines in sickle cell disease. Hematology, 2003. 8(5): p. 329-337. [CrossRef]
- Antwi-Boasiako, C. , et al., Oxidative profile of patients with sickle cell disease. Medical Sciences, 2019. 7(2): p. 17. [CrossRef]
- Takeda, M. , et al., Prehospital diagnostic algorithm for acute coronary syndrome using machine learning: a prospective observational study. Scientific Reports, 2022. 12(1): p. 14593. [CrossRef]
- Belcher, J.D. , et al., Control of oxidative stress and inflammation in sickle cell disease with the Nrf2 activator dimethyl fumarate. Antioxidants & redox signaling, 2017. 26(14): p. 748-762. [CrossRef]
- Iba, T. and J. Levy, Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. Journal of Thrombosis and Haemostasis, 2018. 16(2): p. 231-241. [CrossRef]
- Darbari, D.S., V. A. Sheehan, and S.K. Ballas, The vaso-occlusive pain crisis in sickle cell disease: definition, pathophysiology, and management. European journal of haematology, 2020. 105(3): p. 237-246.
- Quintela-Carvalho, G. , et al., Heme drives oxidative stress-associated cell death in human neutrophils infected with Leishmania infantum. Frontiers in Immunology, 2017. 8: p. 1620. [CrossRef]
- Nasimuzzaman, M. and P. Malik, Role of the coagulation system in the pathogenesis of sickle cell disease. Blood Advances, 2019. 3(20): p. 3170-3180. [CrossRef]
- Annarapu, G.K. , et al., Mitochondrial reactive oxygen species scavenging attenuates thrombus formation in a murine model of sickle cell disease. Journal of Thrombosis and Haemostasis, 2021. 19(9): p. 2256-2262. [CrossRef]
- Balandya, E. , et al., Increased memory phenotypes of CD4+ and CD8+ T cells in children with sickle cell anaemia in Tanzania. Tanzania Journal of Health Research, 2017. 19(2). [CrossRef]
- Daltro, P.B. , et al., CD4+ T cell profile and activation response in sickle cell disease patients with osteonecrosis. Mediators of Inflammation, 2020. 2020: p. 1-12. [CrossRef]
- Zerra, P.E. , et al., Marginal zone B cells mediate a CD4 T-cell–dependent extrafollicular antibody response following RBC transfusion in mice. Blood, 2021. 138(8): p. 706-721. [CrossRef]
- Bernaudin, F. , et al., Immune reconstitution in 107 children with sickle cell anemia transplanted with bone marrow or cord blood from a matched-sibling donor after myeloablative conditioning regimen including 20mg/Kg ATG. Blood, 2019. 134: p. 2253. [CrossRef]
- Shokrgozar, N. , et al., Evaluation of regulatory T cells frequency and FoxP3/GDF-15 gene expression in β-thalassemia major patients with and without alloantibody; correlation with serum ferritin and folate levels. Annals of Hematology, 2020. 99: p. 421-429.
- Fasola, F. and A. Adekanmi, Haematological profile and blood transfusion pattern of patients with sickle cell anaemia vary with spleen size. Annals of Ibadan postgraduate medicine, 2019. 17(1): p. 30-38.
- Ojo, O.T. , et al., Correlation between splenic size and CD4+ T lymphocytes in sickle cell anaemia patients in a Tertiary Hospital. The Egyptian Journal of Haematology, 2018. 43(2): p. 85. [CrossRef]
- ElAlfy, M.S. , et al., Immunological role of CD4+ CD28 null T lymphocytes, natural killer cells, and interferon-gamma in pediatric patients with sickle cell disease: relation to disease severity and response to therapy. Immunologic research, 2018. 66: p. 480-490. [CrossRef]
- Boulassel, M.-R. , et al., Coexistence of sickle cell disease and systemic lupus erythematosus is associated with quantitative and qualitative impairments in circulating regulatory B cells. Human Immunology, 2022. 83(12): p. 818-825. [CrossRef]
- Fichou, Y. , et al., Defining blood group gene reference alleles by long-read sequencing: proof of concept in the ACKR1 gene encoding the Duffy antigens. Transfusion Medicine and Hemotherapy, 2020. 47(1): p. 23-32. [CrossRef]
- Thompson, K., F. Adams, and G.M. Davison, Elevated unidentified antibodies in sickle cell anaemia patients receiving blood transfusions in Cape Town, South Africa. South African Medical Journal, 2019. 109(11): p. 872-875. [CrossRef]
- Lopez, G.H., C. A. Hyland, and R.L. Flower, Glycophorins and the MNS blood group system: a narrative review. Ann Blood, 2021. 6: p. 39. [CrossRef]
- Seck, M. , et al., Transfusion practice, post-transfusion complications and risk factors in Sickle Cell Disease in Senegal, West Africa. Mediterranean Journal of Hematology and Infectious Diseases, 2022. 14(1). [CrossRef]
- Molina-Aguilar, R. , et al., Pathophysiology of Alloimmunization. Transfusion Medicine and Hemotherapy, 2020. 47(2): p. 152-159.
- Li-Thiao-Te, V. , et al., Coexistent sickle-cell anemia and autoimmune disease in eight children: pitfalls and challenges. Pediatric Rheumatology, 2018. 16(1): p. 1-6. [CrossRef]
- De Vlam, K. , et al., Detection and identification of antinuclear autoantibodies in the serum of normal blood donors. Clinical and experimental rheumatology, 1993. 11(4): p. 393-397.
- Adebajo, A. , et al., Autoantibodies in malaria, tuberculosis and hepatitis B in a west African population. Clinical & Experimental Immunology, 1993. 92(1): p. 73-76. [CrossRef]
- Baethge, B.A. , et al., Antinuclear antibodies in sickle cell disease. Acta haematologica, 1990. 84(4): p. 186-189. [CrossRef]
- Toly-Ndour, C. , et al., High titers of autoantibodies in patients with sickle-cell disease. The Journal of rheumatology, 2011. 38(2): p. 302-309. [CrossRef]
- Balsalobre, B., J. Hernández-Godoy, and D. Planelles, Autoantibodies in splenectomized patients as a consequence of abdominal trauma. Journal of Investigational Allergology & Clinical Immunology, 1992. 2(2): p. 91-95.
- Nistala, K. and K.J. Murray, Co-existent sickle cell disease and juvenile rheumatoid arthritis. Two cases with delayed diagnosis and severe destructive arthropathy. The Journal of rheumatology, 2001. 28(9): p. 2125-2128.
- Saxena, V.R. , et al., Systemic lupus erythematosus in children with sickle cell disease. Journal of pediatric hematology/oncology, 2003. 25(8): p. 668-671. [CrossRef]
- Lykavieris, P. , et al., Autoimmune liver disease in three children with sickle cell disease. Journal of pediatric gastroenterology and nutrition, 2006. 42(1): p. 104-108. [CrossRef]
- Bernini, J.C. , et al., Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood, The Journal of the American Society of Hematology, 1998. 92(9): p. 3082-3089.
- Michel, M. , et al., Characteristics and outcome of connective tissue diseases in patients with sickle-cell disease: report of 30 cases. Semin Arthritis Rheum, 2008. 38(3): p. 228-40. [CrossRef]
- Solovey, A. , et al., Interference with TNFα using long-term etanercept in S+ SAntilles sickle transgenic mice ameliorates abnormal endothelial activation, vasoocclusion, and pulmonary hypertension including its pulmonary arterial wall remodeling. Blood, 2013. 122(21): p. 728.
- Wang, Q. and R. Zennadi, The role of RBC oxidative stress in sickle cell disease: from the molecular basis to pathologic implications. Antioxidants, 2021. 10(10): p. 1608. [CrossRef]
- Cao, H. and M.A. Vickers, Oxidative stress, malaria, sickle cell disease, and innate immunity. Trends in Immunology, 2021. 42(10): p. 849-851.
- Xiang, Y. and X. Zhou, Octamer-binding transcription factor 4 correlates with complex karyotype, FLT3-ITD mutation and poorer risk stratification, and predicts unfavourable prognosis in patients with acute myeloid leukaemia. Hematology, 2018. 23(10): p. 721-728. [CrossRef]
- Bernard, K.F.C. , et al., Electrolytic and oxidative stress profile of sickle cell anaemia patients in Cameroon: the effect of some extrinsic factors. Asian Hematol Res J, 2018. 1(1): p. 1-11.
- Beri, D. , et al., Sickle cell anemia and Babesia infection. Pathogens, 2021. 10(11): p. 1435. [CrossRef]
- Bou-Fakhredin, R. , et al., Redox Balance in β-Thalassemia and Sickle Cell Disease: A Love and Hate Relationship. Antioxidants, 2022. 11(5): p. 967. [CrossRef]
- Soomro, S. , Oxidative stress and inflammation. Open Journal of Immunology, 2019. 9(01): p. 1.
- Pedrosa, A.M., L. K.A. Leal, and R.P.G. Lemes, Effects of hydroxyurea on cytotoxicity, inflammation and oxidative stress markers in neutrophils of patients with sickle cell anemia: dose-effect relationship. Hematology, Transfusion and Cell Therapy, 2021. 43: p. 468-475. [CrossRef]
- Glennon-Alty, L. , et al., Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radical Biology and Medicine, 2018. 125: p. 25-35. [CrossRef]
- Ito, F., Y. Sono, and T. Ito, Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants, 2019. 8(3): p. 72. [CrossRef]
- Piacenza, L., M. Trujillo, and R. Radi, Reactive species and pathogen antioxidant networks during phagocytosis. Journal of Experimental Medicine, 2019. 216(3): p. 501-516. [CrossRef]
- Cervantes-Gracia, K. , et al., Oxidative stress and inflammation in the development of cardiovascular disease and contrast induced nephropathy. Vessel Plus, 2020. 4: p. 27. [CrossRef]
- Ojongnkpot, T.A. , et al., Implication of Oxidative Stress and Antioxidant Defence Systems in Symptomatic and Asymptomatic Plasmodium falciparum Malaria Infection among Children Aged1 to 15 Years in the Mount Cameroon Area. Journal of Biosciences and Medicines, 2023. 11(2): p. 124-145. [CrossRef]
- Bohn, T. , Carotenoids and markers of oxidative stress in human observational studies and intervention trials: Implications for chronic diseases. Antioxidants, 2019. 8(6): p. 179. [CrossRef]
- Detterich, J.A. , et al., Erythrocyte and plasma oxidative stress appears to be compensated in patients with sickle cell disease during a period of relative health, despite the presence of known oxidative agents. Free Radical Biology and Medicine, 2019. 141: p. 408-415. [CrossRef]
- Nader, E. , et al., Association between nitric oxide, oxidative stress, eryptosis, red blood cell microparticles, and vascular function in sickle cell anemia. Frontiers in immunology, 2020: p. 2885. [CrossRef]
- El Azab, E.F. , et al., New insights into geraniol’s antihemolytic, anti-inflammatory, antioxidant, and anticoagulant potentials using a combined biological and in silico screening strategy. Inflammopharmacology, 2022. 30(5): p. 1811-1833. [CrossRef]
- Wang, Q. and R. Zennadi, Oxidative stress and thrombosis during aging: the roles of oxidative stress in RBCs in venous thrombosis. International journal of molecular sciences, 2020. 21(12): p. 4259. [CrossRef]
- Abboud, M.R. , Standard management of sickle cell disease complications. Hematology/Oncology and Stem Cell Therapy, 2020. 13(2): p. 85-90. [CrossRef]
- McMahon, T.J. , Red blood cell deformability, vasoactive mediators, and adhesion. Frontiers in physiology, 2019. 10: p. 1417.
- Kucukal, E. , et al., Whole blood viscosity and red blood cell adhesion: Potential biomarkers for targeted and curative therapies in sickle cell disease. American journal of hematology, 2020. 95(11): p. 1246-1256. [CrossRef]
- Ryter, S.W. , Heme oxygenase-1: an anti-inflammatory effector in cardiovascular, lung, and related metabolic disorders. Antioxidants, 2022. 11(3): p. 555. [CrossRef]
- Ryter, S.W. , Therapeutic potential of heme oxygenase-1 and carbon monoxide in acute organ injury, critical illness, and inflammatory disorders. Antioxidants, 2020. 9(11): p. 1153. [CrossRef]
- Kim, H. , et al., Depletion Assisted Hemin Affinity (DAsHA) Proteomics Reveals an Expanded Landscape of Heme Binding Proteins in the Human Proteome. Metallomics, 2023. [CrossRef]
- Consoli, V. , et al., Heme oxygenase-1 signaling and redox homeostasis in physiopathological conditions. Biomolecules, 2021. 11(4): p. 589. [CrossRef]
- Duvigneau, J.C., H. Esterbauer, and A.V. Kozlov, Role of heme oxygenase as a modulator of heme-mediated pathways. Antioxidants, 2019. 8(10): p. 475. [CrossRef]
- Nathan, C. , Neutrophils and immunity: challenges and opportunities. Nature reviews immunology, 2006. 6(3): p. 173-182. [CrossRef]
- Pham, C.T. , Neutrophil serine proteases: specific regulators of inflammation. Nature Reviews Immunology, 2006. 6(7): p. 541-550. [CrossRef]
- Beauvillain, C. , et al., Neutrophils efficiently cross-prime naive T cells in vivo. Blood, The Journal of the American Society of Hematology, 2007. 110(8): p. 2965-2973. [CrossRef]
- Costantini, C. and M.A. Cassatella, The defensive alliance between neutrophils and NK cells as a novel arm of innate immunity. Journal of leukocyte biology, 2011. 89(2): p. 221-233. [CrossRef]
- Lee, S.K. , et al., Response of Neutrophils to Extracellular Haemoglobin and LTA in Human Blood System. EBioMedicine, 2015. 2(3): p. 225-33.
- de Oliveira Toledo, S.L. , et al., Plasma immune mediators as laboratorial biomarkers for Sickle Cell Disease patients according to the hydroxyurea therapy and disease severity. Blood Cells, Molecules, and Diseases, 2023. 98: p. 102703. [CrossRef]
- Hendrickson, J.E. , Red blood cell alloimmunization and sickle cell disease: a narrative review on antibody induction. Annals of blood, 2020. 5. [CrossRef]
- Senchenkova, E.Y. , et al., Novel Role of T Cells and IL-6 (Interleukin-6) in angiotensin II–induced microvascular dysfunction. Hypertension, 2019. 73(4): p. 829-838. [CrossRef]
- Wasnik, R.R. , et al., Impact of Oxidative stress on Sickle cell anaemia patients: A Review. NVEO-NATURAL VOLATILES & ESSENTIAL OILS Journal| NVEO, 2021: p. 1128-1134.
- Connes, P. , et al., Oxidative stress, inflammation, blood rheology, and microcirculation in adults with sickle cell disease: Effects of hydroxyurea treatment and impact of sickle cell syndrome. European Journal of Haematology, 2021. 106(6): p. 800-807. [CrossRef]
- Netea, M.G. , et al., Innate and adaptive immune memory: an evolutionary continuum in the host’s response to pathogens. Cell host & microbe, 2019. 25(1): p. 13-26. [CrossRef]
- Tavares, W.R. and A.M. Seca, Inula L. secondary metabolites against oxidative stress-related human diseases. Antioxidants, 2019. 8(5): p. 122. [CrossRef]
- Peng, C. , et al., The NF-κB signaling pathway, the microbiota, and gastrointestinal tumorigenesis: recent advances. Frontiers in Immunology, 2020. 11: p. 1387. [CrossRef]
- Renó, C.O. , et al., Oxidative stress assessment in sickle cell anemia patients treated with hydroxyurea. Annals of Hematology, 2020. 99: p. 937-945. [CrossRef]
- Valacchi, G. , et al., OxInflammation: From subclinical condition to pathological biomarker. Frontiers in physiology, 2018. 9: p. 858. [CrossRef]
- Trevelin, S.C., A. M. Shah, and G. Lombardi, Beyond bacterial killing: NADPH oxidase 2 is an immunomodulator. Immunology Letters, 2020. 221: p. 39-48. [CrossRef]
- Gan, A.-M. , et al., Stearoyl-CoA Desaturase Regulates Angiogenesis and Energy Metabolism in Ischemic Cardiomyocytes. International Journal of Molecular Sciences, 2022. 23(18): p. 10459.
- Chou, S.T. , et al., American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood advances, 2020. 4(2): p. 327-355. [CrossRef]
- Balbuena-Merle, R. , et al., Characterization of circulating and cultured Tfh-like cells in sickle cell disease in relation to red blood cell alloimmunization status. Transfusion and Apheresis Science, 2020. 59(4): p. 102778. [CrossRef]
- Meda, E. , et al., Red blood cell alloimmunization in sickle cell disease patients in Tanzania. East African journal of public health, 2014. 11(2): p. 775.
- Firmansyah, M. and M. Abduh, Production of protein hydrolysate containing antioxidant activity from Hermetia illucens. Heliyon 5, e02005. 2019. [CrossRef]
- Cherif-Alami, S. , et al., Serum immunoglobulin levels in children with sickle cell disease: a large prospective study. Journal of Clinical Medicine, 2019. 8(10): p. 1688. [CrossRef]
- Pal, M. , et al., Hemolysis inhibits humoral B-cell responses and modulates alloimmunization risk in patients with sickle cell disease. Blood, 2021. 137(2): p. 269-280. [CrossRef]
- Boateng, L.A. , et al., Red blood cell alloimmunization in transfused patients with sickle cell disease in sub-Saharan Africa; a systematic review and meta-analysis. Transfusion Medicine Reviews, 2019. 33(3): p. 162-169. [CrossRef]
- El Fetouh, R.M.A. , et al., Frequency and specificity of Red blood cell alloantibodies in multitransfused Egyptian patients with hematological and nonhematological malignancies. Transfusion and Apheresis Science, 2020. 59(6): p. 102909.
- Adewoyin, A. , et al., Immune erythrocyte antibodies in adult patients with sickle cell disease and blood donors in Lagos, Nigeria: a comparative study. Immunohematology, 2021. 37(3): p. 131-137. [CrossRef]
- Subramaniyan, R. , Serological characteristics of Lewis antibodies and their clinical significance–A case series. Hematology, Transfusion and Cell Therapy, 2021. [CrossRef]
- Lamarre, Y. , et al., Extracellular Vesicles in Sickle Cell Disease: A Promising Tool. Bioengineering, 2022. 9(9): p. 439. [CrossRef]
- McGann, P.T. , et al., Hydroxyurea therapy for children with sickle cell anemia in sub-saharan africa: rationale and design of the REACH trial. Pediatric Blood & Cancer, 2016. 63(1): p. 98-104. [CrossRef]
- Barbu, E.A. , et al., Neutrophils remain detrimentally active in hydroxyurea-treated patients with sickle cell disease. PLoS One, 2019. 14(12): p. e0226583. [CrossRef]
- Hutchaleelaha, A. , et al., Pharmacokinetics and pharmacodynamics of voxelotor (GBT440) in healthy adults and patients with sickle cell disease. British Journal of Clinical Pharmacology, 2019. 85(6): p. 1290-1302.
- Zaidi, A.U. , et al., A reanalysis of pain crises data from the pivotal l-glutamine in sickle cell disease trial. Contemporary Clinical Trials, 2021. 110: p. 106546. [CrossRef]
- Dick, M.H. , et al., Comparing the safety and efficacy of L-glutamine, voxelotor, and crizanlizumab for reducing the frequency of vaso-occlusive crisis in sickle cell disease: a systematic review. Cureus, 2022. 14(5). [CrossRef]
- Vichinsky, E. , et al., A phase 3 randomized trial of voxelotor in sickle cell disease. New England Journal of Medicine, 2019. 381(6): p. 509-519.
- Zhang, B.-S. , et al., Comparison of the efficacy of nilotinib and imatinib in the treatment of chronic myeloid leukemia. J. Coll. Physicians Surg. Pak, 2019. 29: p. 631-634. [CrossRef]
- Kulturoglu, G. , et al., The effects of hydroxyurea on proinflammatory cytokine and tissue histopathology in an experimental sepsis model. Eur Rev Med Pharmacol Sci, 2022. 26(2): p. 526-533. [CrossRef]
- Jayasinghe, C.D. , et al., Platelet augmentation activity of mature leaf juice of Sri Lankan wild type cultivar of Carica papaya L: Insights into potential cellular mechanisms. Journal of Ethnopharmacology, 2022. 296: p. 115511. [CrossRef]
- Cominal, J.G. , et al., Bone marrow soluble mediator signatures of patients with philadelphia chromosome-negative myeloproliferative neoplasms. Frontiers in oncology, 2021. 11: p. 665037. [CrossRef]
- Cacciola, R. , et al., Impact of Anti-Endothelial Cell Antibodies (AECAs) in Patients with Polycythemia Vera and Thrombosis. Diagnostics, 2022. 12(5): p. 1077. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




