Submitted:
14 July 2023
Posted:
17 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Theoretical perspective
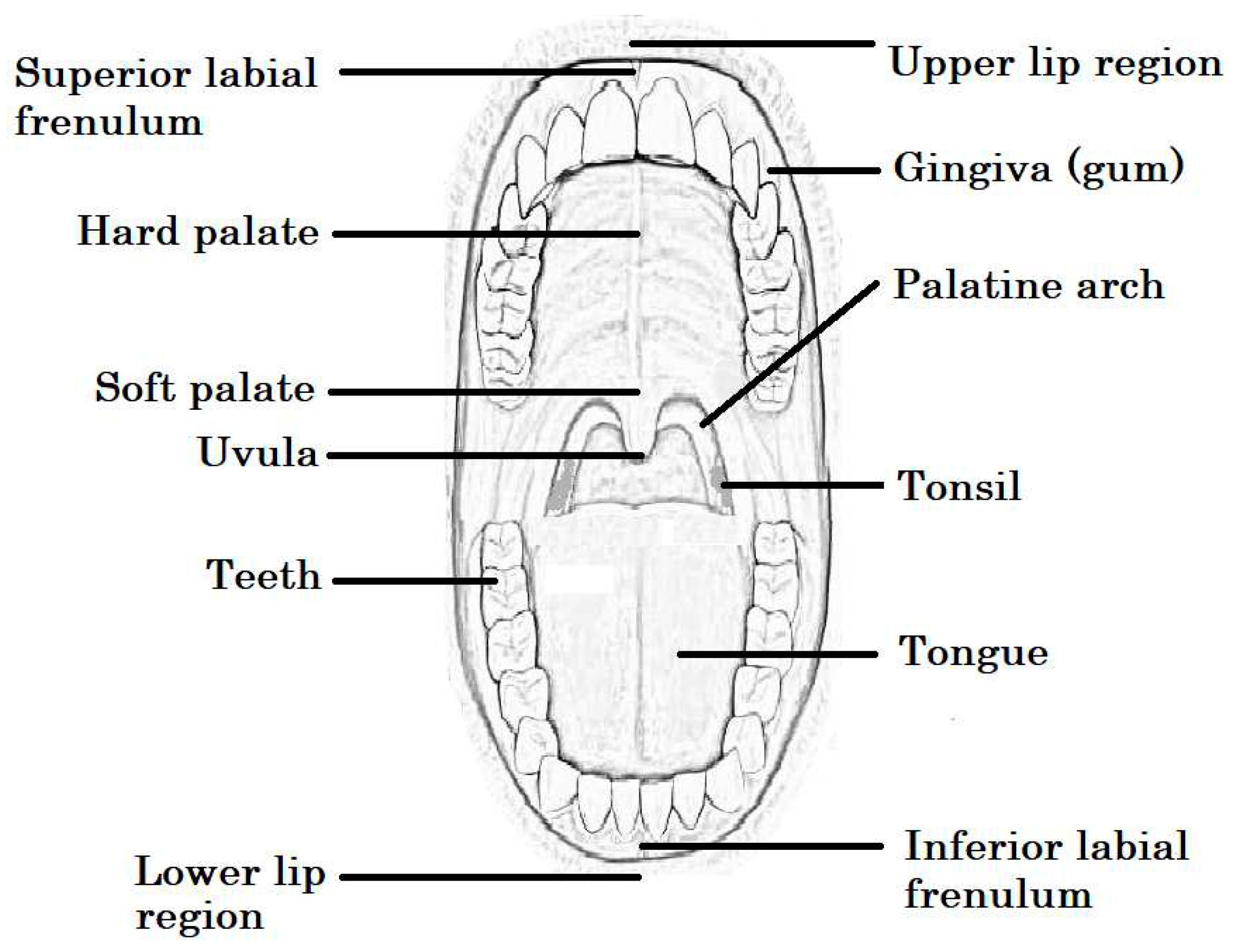
Anatomy and physiology
Diseases of the oral cavity
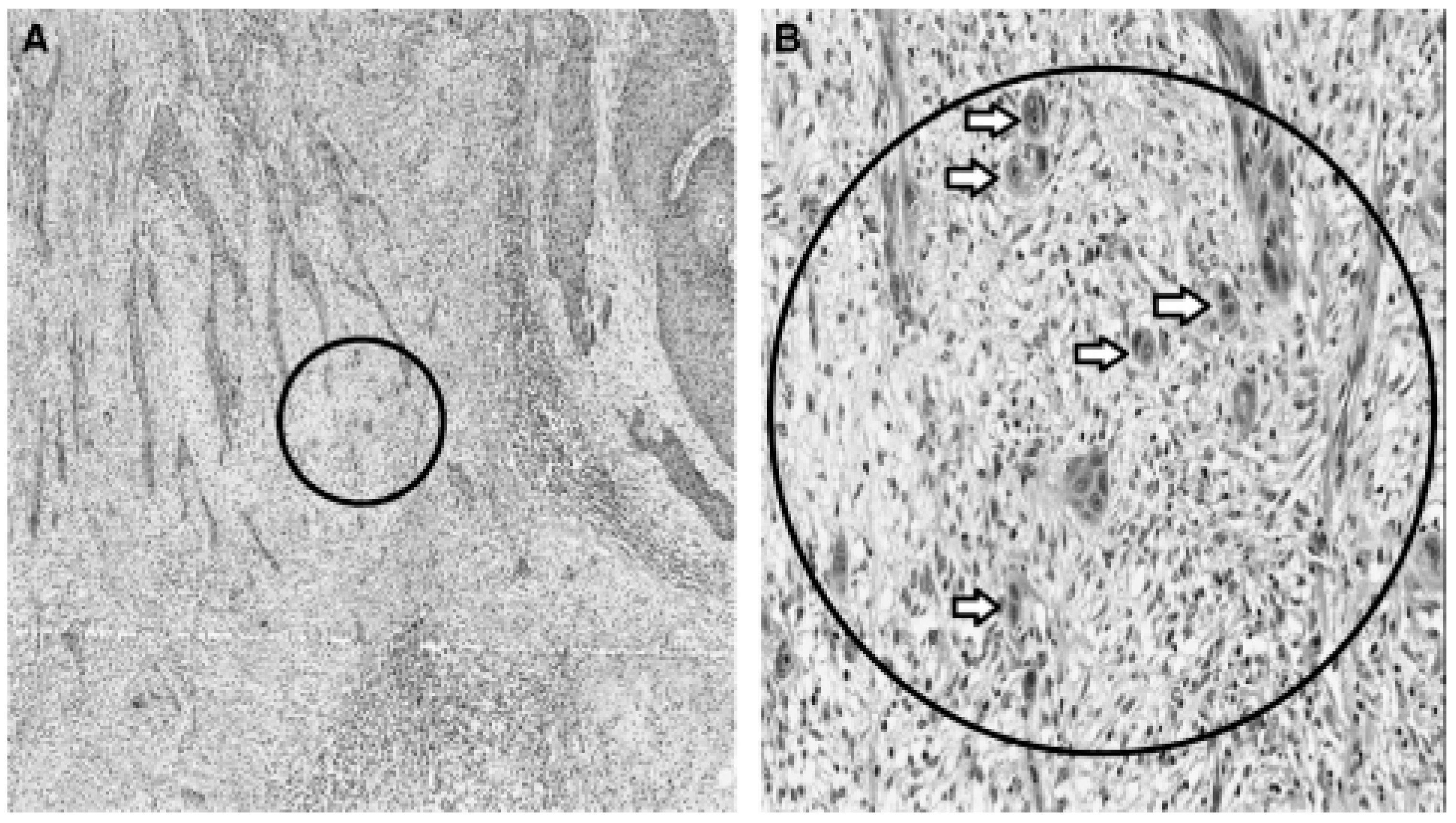
Oral cancer
Microbial infections
Gingivitis
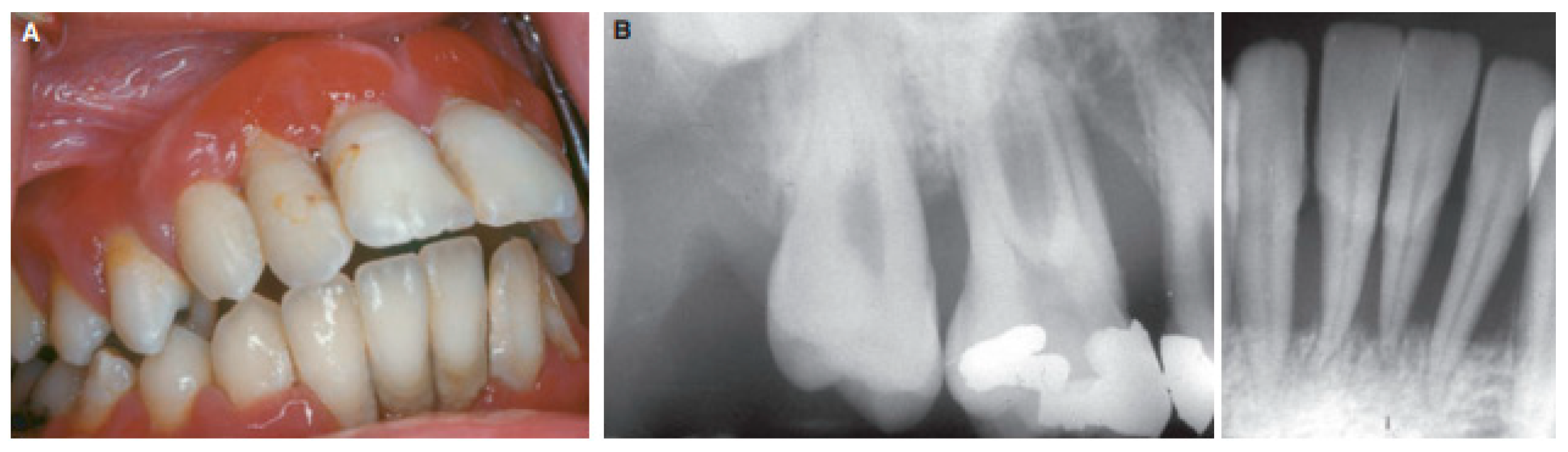
Pain and inflammation control
Local anesthetics
Steroidal anti-inflammatory agents
Other drug classes
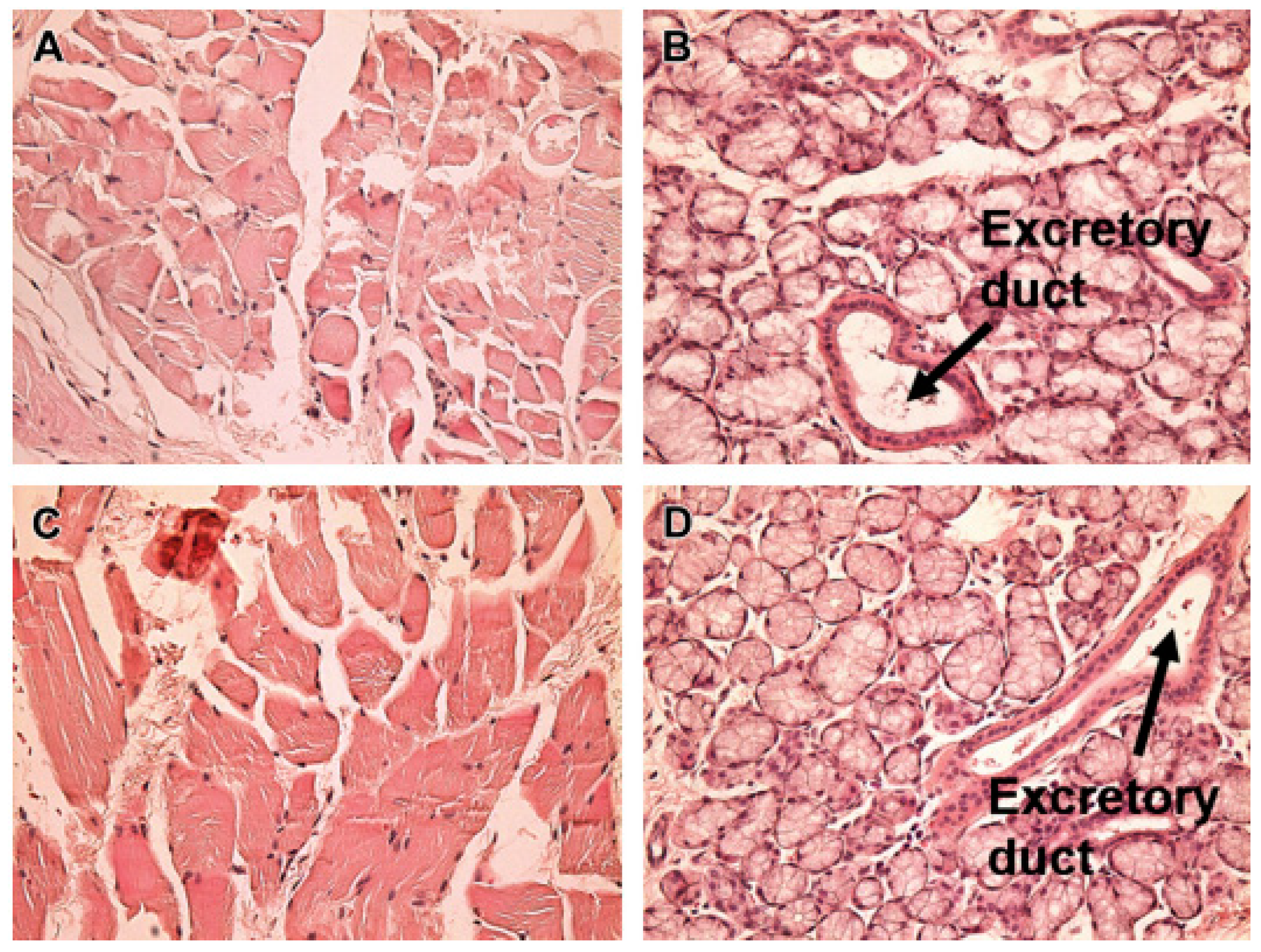
Nanotechnological formulations
Nanoparticles (NPs)
| Dosage/ Delivery | Drug | Polymer(s) | Method of preparation | Oral pathology | Reference |
|---|---|---|---|---|---|
| Nanoparticles | Curcumin | Chitosan (coating) polycaprolactone |
Nanoprecipitation | Oral cancer | [2] |
| Nanogels | Lignocaine hydrochloride monohydrate | Catechol, chitosan, genipin | Conjugation, polymerization | Local anesthetic | [99] |
| Electrospun fiber in patch | Clobetasol-17- propionate | Polyethylene oxide, polyvinyl pyrollidone, eudragit, polycaprolactone | Electrospinning | Anti-inflammatory | [67] |
| Nanoparticles | Doxorubicin | poly (ethylene glycol)-block-poly (4-vinylbenzylphosphonate | Solvent evaporation | Anti-cancer | [92] |
| Nanoparticles | Genistein | (D,L) Lactic acid | Emulsion diffusion method | Anti-cancer and phytoestrogen | [94] |
| Nanoparticles | Gene delivery | poly(DLlactide-co-glycolide) | Probe sonication | Drug and gene delivery | [95] |
| Nanoparticles in film | Calcium fluoride and lignocaine | Thiolated chitosan | Co-precipitation and polymer synthesis | Dental caries | [71] |
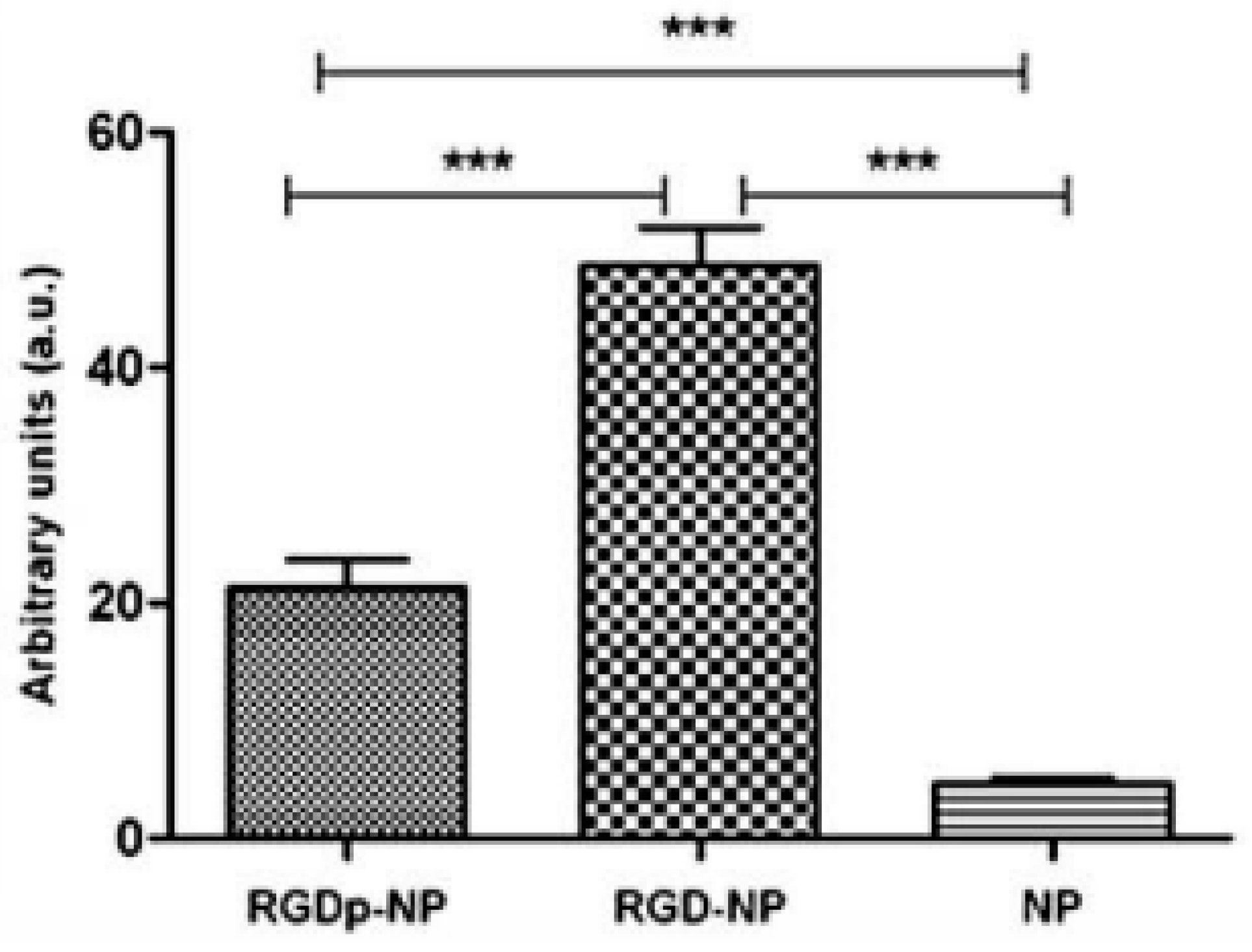
| Peptide grafting nanoparticle | Paclitaxel | Polylactide co-glycolide, RGD-peptide, polyethylene glycol | Polymer synthesis and peptide grafting | Oral cancer | [98] |
Nanogels
| Drug | Dosage form | Subjects | Settings | Clinical outcomes | References |
|---|---|---|---|---|---|
| Triamcinolone | Nanogels | Diseased volunteers | Triple blind, randomized | Nano delivery accelerated healing | [103] |
| Clobetasol | Patch | Healthy subjects | Single center | Better adherence and less irritation | [67] |
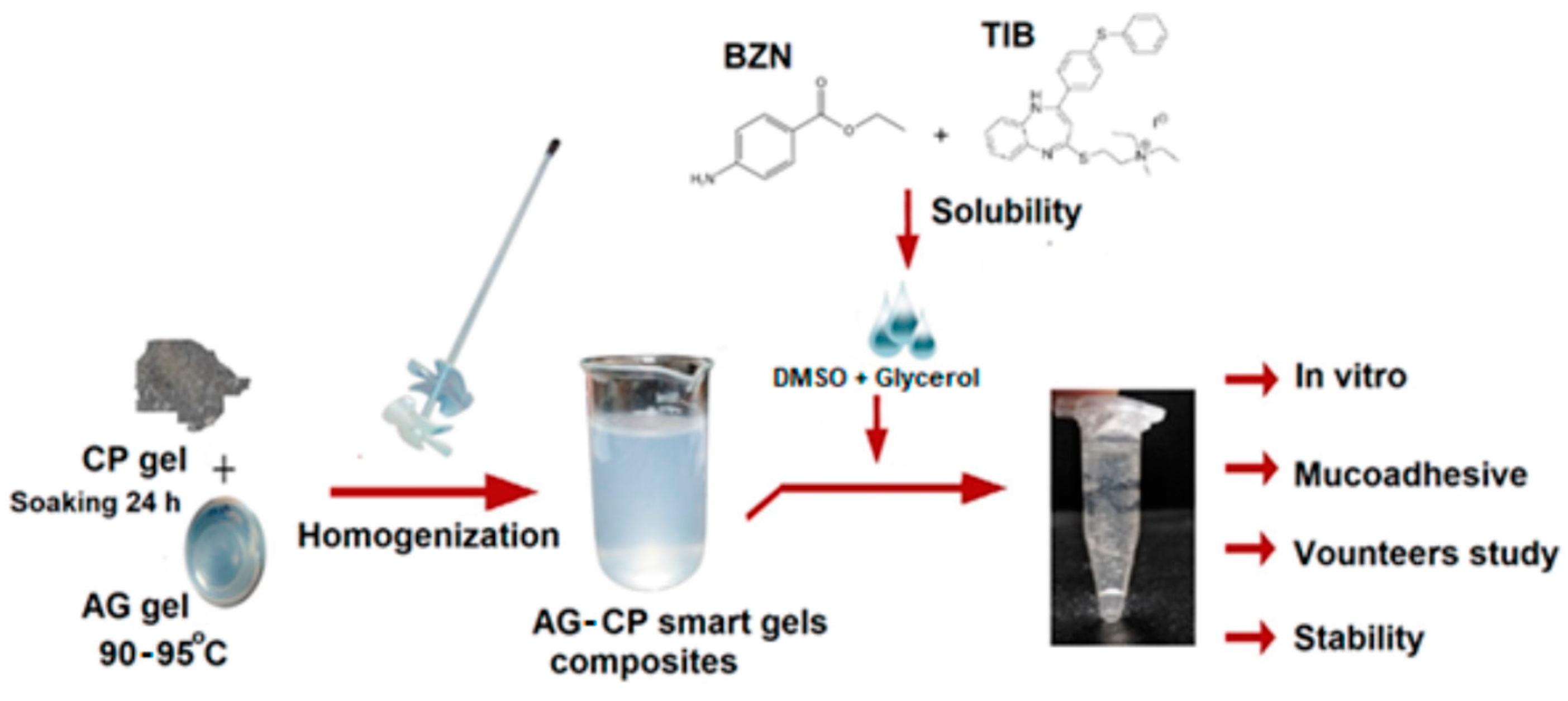
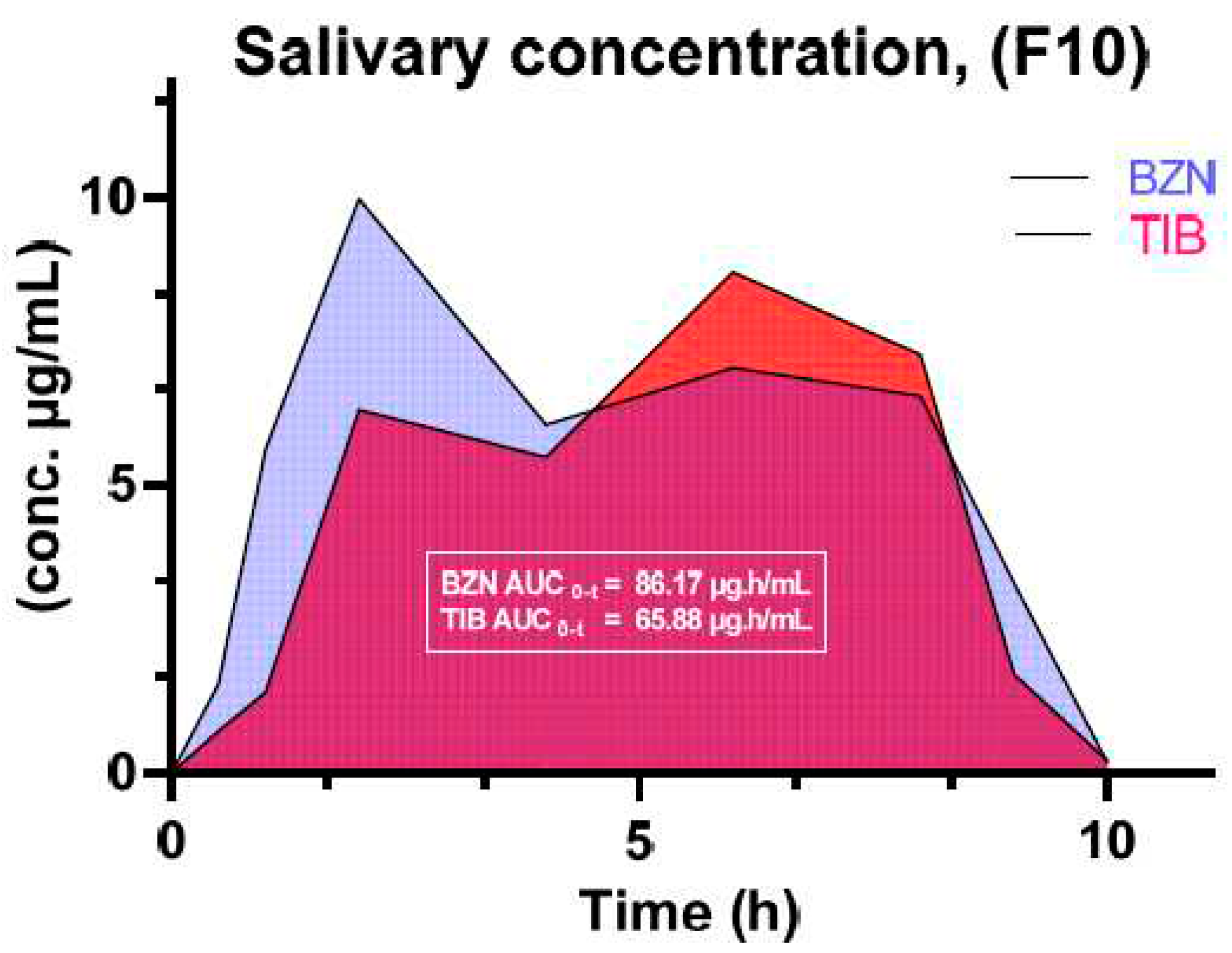
| Tibezonium iodide | Gels | Healthy volunteers | Single center | Agarose-carbopol showed superior mucoadhesion Dosage form adaptable to volunteers Stable for up to 6 months |
[90] |
Buccal film-loaded nanotechnology
Patches
Nanofibers
Preclinical and clinical findings
Conclusions
Funding
References
- Kenechukwu, F.C.; Attama, A.A.; Ibezim, E.C. Novel solidified reverse micellar solution-based mucoadhesive nano lipid gels encapsulating miconazole nitrate-loaded nanoparticles for improved treatment of oropharyngeal candidiasis. Journal of Microencapsulation 2017, 34, 592–609. [Google Scholar] [CrossRef] [PubMed]
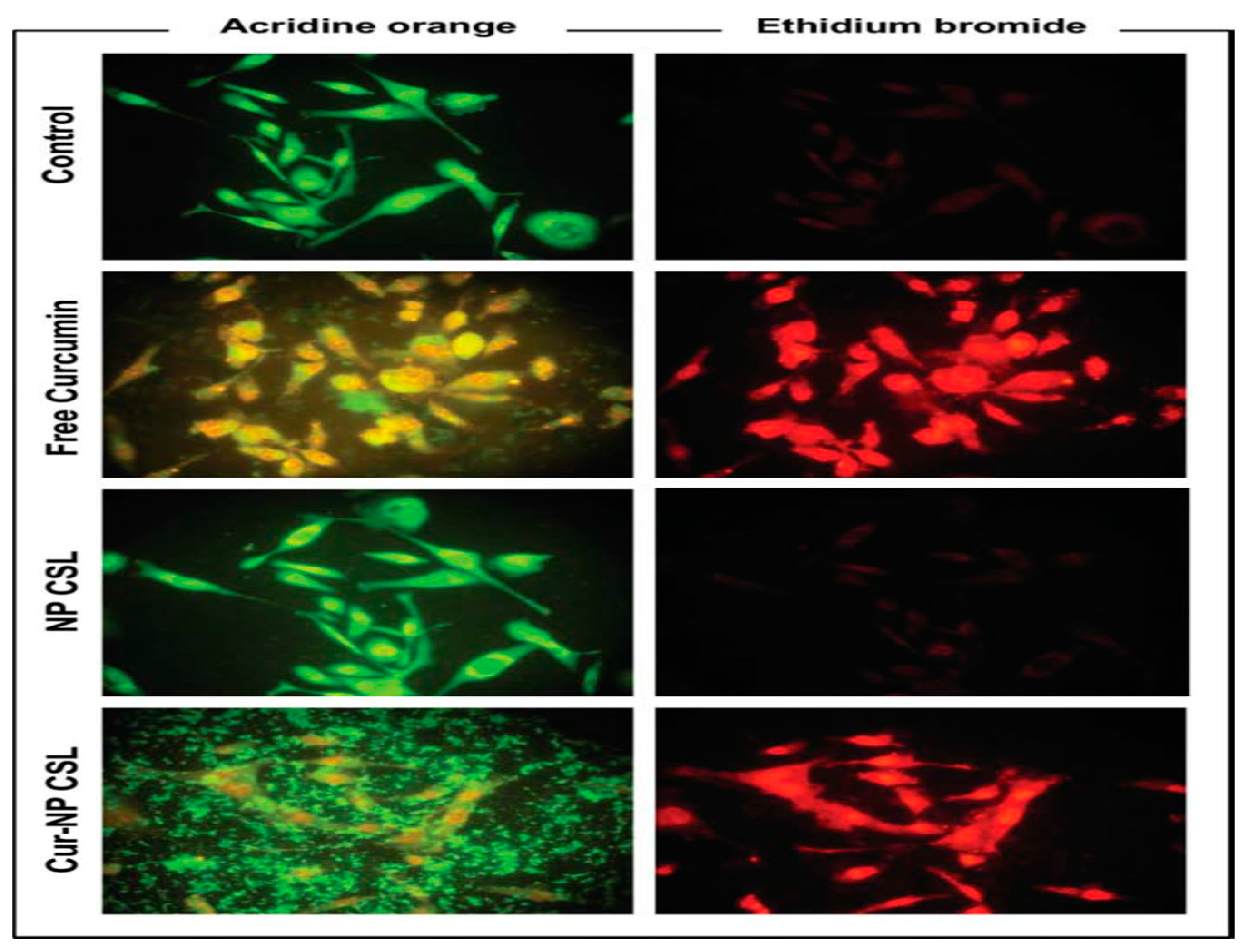
- Mazzarino, L.; Loch-Neckel, G.; Bubniak, L.d.S.; Mazzucco, S.; Santos-Silva, M.C.; Borsali, R.; Lemos-Senna, E. Curcumin-loaded chitosan-coated nanoparticles as a new approach for the local treatment of oral cavity cancer. Journal of nanoscience and nanotechnology 2015, 15, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Boden, W.E.; Padala, S.K.; Cabral, K.P.; Buschmann, I.R.; Sidhu, M.S. Role of short-acting nitroglycerin in the management of ischemic heart disease. Drug Design, Development and Therapy 2015, 9, 4793. [Google Scholar] [PubMed]
- Hanif, S.; Sarfraz, R.M.; Syed, M.A.; Ali, S.; Iqbal, Z.; Shakir, R.; Iqbal, J. Formulation and evaluation of chitosan-based polymeric biodegradable mucoadhesive buccal delivery for locally acting drugs: In vitro, ex vivo and in vivo volunteers characterization. 2021.
- Pather, S.I.; Rathbone, M.J.; Şenel, S. Current status and the future of buccal drug delivery systems. Expert Opinion on Drug Delivery 2008, 5, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kaul, M.; Rawat, A.; Saini, S. An overview on buccal drug delivery system. International journal of pharmaceutical sciences and research 2011, 2, 1303. [Google Scholar]
- Hassan, N.; Ahad, A.; Ali, M.; Ali, J. Chemical permeation enhancers for transbuccal drug delivery. Expert opinion on drug delivery 2010, 7, 97–112. [Google Scholar] [CrossRef]
- Razzaq, S.; Syed, M.A.; Irfan, M.; Khan, I.; Sarfraz, R.M.; Shakir, R.; Ali, S.; Iqbal, Z.; Niaz, Y.; Mujtaba, S.H. Optimization of metronidazole SR buccal tablet for gingivitis using genetic algorithm. Pakistan Journal of Pharmaceutical Sciences 2021, 34. [Google Scholar]
- Marques, A.C.; Rocha, A.I.; Leal, P.; Estanqueiro, M.; Lobo, J.M.S. Development and characterization of mucoadhesive buccal gels containing lipid nanoparticles of ibuprofen. International Journal of Pharmaceutics 2017, 533, 455–462. [Google Scholar] [CrossRef]
- Pham, M.N.; Van Vo, T.; Tran, V.-T.; Tran, P.H.-L.; Tran, T.T.-D. Microemulsion-based mucoadhesive buccal wafers: wafer formation, in vitro release, and ex vivo evaluation. AAPS PharmSciTech 2017, 18, 2727–2736. [Google Scholar] [CrossRef]
- Abruzzo, A.; Cerchiara, T.; Bigucci, F.; Gallucci, M.C.; Luppi, B. Mucoadhesive buccal tablets based on chitosan/gelatin microparticles for delivery of propranolol hydrochloride. Journal of pharmaceutical sciences 2015, 104, 4365–4372. [Google Scholar] [CrossRef]
- Nafee, N.A.; Boraie, M.A.; Ismail, F.A.; Mortada, L.M. Design and characterization of mucoadhesive buccal patches containing cetylpyridinium chloride. Acta pharmaceutica (Zagreb, Croatia) 2003, 53, 199–212. [Google Scholar] [PubMed]
- Silva, B.M.; Borges, A.F.; Silva, C.; Coelho, J.F.; Simões, S. Mucoadhesive oral films: the potential for unmet needs. International journal of pharmaceutics 2015, 494, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Poonia, M.; Ramalingam, K.; Goyal, S.; Sidhu, S.K. Nanotechnology in oral cancer: A comprehensive review. Journal of oral and maxillofacial pathology: JOMFP 2017, 21, 407. [Google Scholar]
- Hafez, M.M.; Aboulwafa, M.M.; Yassien, M.A.; Hassouna, N.A. Activity of some mucolytics against bacterial adherence to mammalian cells. Applied biochemistry and biotechnology 2009, 158, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, J.; Shan, W.; Huang, Y. Developments of mucus penetrating nanoparticles. Asian Journal of Pharmaceutical Sciences 2015, 10, 275–282. [Google Scholar] [CrossRef]
- Ensign, L.M.; Lai, S.K.; Wang, Y.-Y.; Yang, M.; Mert, O.; Hanes, J.; Cone, R. Pretreatment of human cervicovaginal mucus with pluronic F127 enhances nanoparticle penetration without compromising mucus barrier properties to herpes simplex virus. Biomacromolecules 2014, 15, 4403–4409. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.-Y.; Hida, K.; Cone, R.; Hanes, J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proceedings of the National Academy of Sciences 2010, 107, 598–603. [Google Scholar] [CrossRef]
- Gandhi, R.B.; Robinson, J.R. Oral cavity as a site for bioadhesive drug delivery. Advanced drug delivery reviews 1994, 13, 43–74. [Google Scholar] [CrossRef]
- Sudhakar, Y.; Kuotsu, K.; Bandyopadhyay, A. Buccal bioadhesive drug delivery—a promising option for orally less efficient drugs. Journal of Controlled Release 2006, 114, 15–40. [Google Scholar] [CrossRef]
- Nanci, A. Ten Cate's Oral Histology-Pageburst on VitalSource: Development, Structure, and Function; Elsevier Health Sciences: 2007.
- Silk, H. Diseases of the mouth. Primary care: Clinics in office practice 2014, 41, 75–90. [Google Scholar] [CrossRef]
- Prince, A.; Aguirre-Ghizo, J.; Genden, E.; Posner, M.; Sikora, A. Head and neck squamous cell carcinoma: new translational therapies. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine 2010, 77, 684–699. [Google Scholar] [CrossRef] [PubMed]
- Rapidis, A.D.; Wolf, G.T. Immunotherapy of head and neck cancer: current and future considerations. Journal of oncology 2009, 2009. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.P.; Gil, Z. Current concepts in management of oral cancer–surgery. Oral oncology 2009, 45, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Shtenberg, Y.; Goldfeder, M.; Prinz, H.; Shainsky, J.; Ghantous, Y.; El-Naaj, I.A.; Schroeder, A.; Bianco-Peled, H. Mucoadhesive alginate pastes with embedded liposomes for local oral drug delivery. International journal of biological macromolecules 2018, 111, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Almangush, A.; Pirinen, M.; Heikkinen, I.; Mäkitie, A.A.; Salo, T.; Leivo, I. Tumour budding in oral squamous cell carcinoma: a meta-analysis. British journal of cancer 2018, 118, 577–586. [Google Scholar] [CrossRef]
- Addy, M. Local delivery of antimicrobial agents to the oral cavity. Advanced drug delivery reviews 1994, 13, 123–134. [Google Scholar] [CrossRef]
- Rindom Schiött, C.; Löe, H.; Børglum Jensen, S.; Kilian, M.; Davies, R.; Glavind, K. The effect of chlorhexidine mouthrinses on the human oral flora. Journal of Periodontal Research 1970, 5, 84–89. [Google Scholar] [CrossRef]
- Edgar, W. Saliva: its secretion, composition and functions. British dental journal 1992, 172, 305–312. [Google Scholar] [CrossRef]
- Marsh, P.D. Role of the oral microflora in health. Microbial ecology in health and disease 2000, 12, 130–137. [Google Scholar] [CrossRef]
- Lamont, R.; Jenkinson, H. Adhesion as an ecological determinant in the oral cavity. Oral bacterial ecology: the molecular basis 2000, 131-168.
- Puy, C.L. The role of saliva in maintaining oral health and as an aid to diagnosis. Med Oral Patol Oral Cir Bucal 2006, 11, 449–455. [Google Scholar]
- Bernimoulin, J.P. Recent concepts in plaque formation. Journal of Clinical Periodontology 2003, 30, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.; Furgang, D.; Lieb, R.; Korik, I.; Vincent, J.; Barnett, M. Effects of sublethal exposure to an antiseptic mouthrinse on representative plaque bacteria. Journal of clinical periodontology 1996, 23, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Tatakis, D.N.; Scapoli, C.; Bottega, S.; Orlandini, E.; Tosi, M. Modulation of clinical expression of plaque-induced gingivitis. Journal of clinical periodontology 2004, 31, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.A.; Avery, D.R.; McDonald, R.E. McDonald and Avery dentistry for the child and adolescent; Elsevier Health Sciences: 2010.
- Barnett, M.L. The role of therapeutic antimicrobial mouthrinses in clinical practice: control of supragingival plaque and gingivitis. The Journal of the American Dental Association 2003, 134, 699–704. [Google Scholar] [CrossRef]
- Armitage, G. Diagnosis of periodontal diseases. Journal of periodontology 2003, 74, 1237–1247. [Google Scholar] [CrossRef]
- Ohlrich, E.; Cullinan, M.; Seymour, G. The immunopathogenesis of periodontal disease. Australian dental journal 2009, 54, S2–S10. [Google Scholar] [CrossRef]
- Armitage, G.C.; Cullinan, M.P. Comparison of the clinical features of chronic and aggressive periodontitis. Periodontology 2000 2010, 53, 12–27. [Google Scholar] [CrossRef]
- Salvi, G.E.; Aglietta, M.; Eick, S.; Sculean, A.; Lang, N.P.; Ramseier, C.A. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clinical oral implants research 2012, 23, 182–190. [Google Scholar] [CrossRef]
- Lang, N.P.; Schätzle, M.A.; Löe, H. Gingivitis as a risk factor in periodontal disease. Journal of clinical periodontology 2009, 36, 3–8. [Google Scholar] [CrossRef]
- Plancak, D.; Jorgic–Srdjak, K.; Curilovic, Z. New Classification of Periodontal Diseases. Acta Stomat Croat 2001, 35, s9–s93. [Google Scholar]
- Szpaderska, A.; Zuckerman, J.; DiPietro, L. Differential injury responses in oral mucosal and cutaneous wounds. Journal of dental research 2003, 82, 621–626. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.A. Wound healing: the role of the macrophage and other immune cells. Shock (Augusta, Ga.) 1995, 4, 233–240. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.A.; Burdick, M.; Low, Q.E.; Kunkel, S.L.; Strieter, R.M. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. The Journal of clinical investigation 1998, 101, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Lei, K.; Jin, W.; Longenecker, G.; Kulkarni, A.B.; Greenwell-Wild, T.; Hale-Donze, H.; McGrady, G.; Song, X.-Y.; Wahl, S.M. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nature medicine 2000, 6, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Zewail, M.B.; Asaad, G.F.; Swellam, S.M.; Abd-Allah, S.M.; Hosny, S.K.; Sallah, S.K.; Eissa, J.E.; Mohamed, S.S.; El-Dakroury, W.A. Design, characterization and in vivo performance of solid lipid nanoparticles (SLNs)-loaded mucoadhesive buccal tablets for efficient delivery of Lornoxicam in experimental inflammation. International Journal of Pharmaceutics 2022, 624, 122006. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cui, Y.; Zhang, L.; Zhu, H.-p.; Guo, Y.-S.; Zhong, B.; Hu, X.; Zhang, L.; Wang, X.-h.; Chen, L. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. International journal of pharmaceutics 2012, 430, 114–119. [Google Scholar] [CrossRef]
- Hussain, A.; Syed, M.A.; Abbas, N.; Hanif, S.; Arshad, M.S.; Bukhari, N.I.; Hussain, K.; Akhlaq, M.; Ahmad, Z. Development of ann optimized mucoadhesive buccal tablet containing flurbiprofen and lidocaine for dental pain. Acta Pharmaceutica 2016, 66, 245–256. [Google Scholar] [CrossRef]
- Daneshmehr, M.A.; Adibpour, H.; Ataie, Z. Formulation and Evaluation of Hydrocortisone Sodium Succinate Mucoadhesive Buccal Tablet. International Journal of Pharmaceutical Investigation 2020, 10, 300–304. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Boetker, J.; Rantanen, J.; Jacobsen, J.; Vizirianakis, I.S.; Fatouros, D.G. Fabrication of mucoadhesive buccal films for local administration of ketoprofen and lidocaine hydrochloride by combining fused deposition modeling and inkjet printing. Journal of Pharmaceutical Sciences 2020, 109, 2757–2766. [Google Scholar] [CrossRef]
- Preis, M.; Woertz, C.; Schneider, K.; Kukawka, J.; Broscheit, J.; Roewer, N.; Breitkreutz, J. Design and evaluation of bilayered buccal film preparations for local administration of lidocaine hydrochloride. European Journal of Pharmaceutics and Biopharmaceutics 2014, 86, 552–561. [Google Scholar] [CrossRef]
- Hasan, A.A.; Samir, R.M.; Abu-Zaid, S.S.; Lila, A.S.A. Revitalizing the local anesthetic effect of Mebeverine hydrochloride via encapsulation within ethosomal vesicular system. Colloids and Surfaces B: Biointerfaces 2020, 194, 111208. [Google Scholar] [CrossRef]
- Kottke, D.; Majid, H.; Breitkreutz, J.; Burckhardt, B.B. Development and evaluation of mucoadhesive buccal dosage forms of lidocaine hydrochloride by ex-vivo permeation studies. International journal of pharmaceutics 2020, 581, 119293. [Google Scholar] [CrossRef]
- Pleguezuelos-Villa, M.; Nácher, A.; Hernández, M.J.; Busó, M.; Barrachina, M.; Peñalver, N.; Díez-Sales, O. A novel lidocaine hydrochloride mucoadhesive films for periodontal diseases. Journal of Materials Science: Materials in Medicine 2019, 30, 1–7. [Google Scholar] [CrossRef]
- Clitherow, K.H.; Murdoch, C.; Spain, S.G.; Handler, A.M.; Colley, H.E.; Stie, M.B.; Mørck Nielsen, H.; Janfelt, C.; Hatton, P.V.; Jacobsen, J. Mucoadhesive electrospun patch delivery of lidocaine to the oral mucosa and investigation of spatial distribution in a tissue using MALDI-mass spectrometry imaging. Molecular pharmaceutics 2019, 16, 3948–3956. [Google Scholar] [CrossRef]
- Jelvehgari, M.; Rashidi, M.R.; Samadi, H. Mucoadhesive and drug release properties of benzocaine gel. 2006.
- Jose, J.; Sisira, M.; Prasanth, M.L.; Prasanth, C.S.; Pradeep, P. Design and Characterization of Buccal Films of Benzocaine for Mouth Ulcer. Polymer 2021, 10, 250. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Khan, S.K.A.; Dehghan, M.H. Evaluation of polaxomer-based in situ gelling system of articaine as a drug delivery system for anesthetizing periodontal pockets–an in vitro study. Indian Journal of Dentistry 2012, 3, 201–208. [Google Scholar] [CrossRef]
- Hanif, S.; Sarfraz, R.M.; Syed, M.A.; Mahmood, A.; Hussain, Z. Smart mucoadhesive buccal chitosan/HPMC scaffold for sore throat: In vitro, ex vivo and pharmacokinetic profiling in humans. Journal of Drug Delivery Science and Technology 2022, 71, 103271. [Google Scholar] [CrossRef]
- Javed, Q.u.A.; Syed, M.A.; Arshad, R.; Rahdar, A.; Irfan, M.; Raza, S.A.; Shahnaz, G.; Hanif, S.; Díez-Pascual, A.M. Evaluation and Optimization of Prolonged Release Mucoadhesive Tablets of Dexamethasone for Wound Healing: In Vitro–In Vivo Profiling in Healthy Volunteers. Pharmaceutics 2022, 14, 807. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, Z.; Rabiee, M.; Houshmand, B.; Eslahi, N.; Khoshroo, K.; Rasoulianboroujeni, M.; Tahriri, M.; Tayebi, L. Development of chitosan/gelatin/keratin composite containing hydrocortisone sodium succinate as a buccal mucoadhesive patch to treat desquamative gingivitis. Drug development and industrial pharmacy 2018, 44, 40–55. [Google Scholar] [CrossRef]
- Kumria, R.; Nair, A.B.; Goomber, G.; Gupta, S. Buccal films of prednisolone with enhanced bioavailability. Drug delivery 2016, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Colley, H.; Said, Z.; Santocildes-Romero, M.; Baker, S.; D'Apice, K.; Hansen, J.; Madsen, L.S.; Thornhill, M.; Hatton, P.; Murdoch, C. Pre-clinical evaluation of novel mucoadhesive bilayer patches for local delivery of clobetasol-17-propionate to the oral mucosa. Biomaterials 2018, 178, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, E.; Hill, D.; Doudin, K. Chlorhexidine Mucoadhesive Buccal Tablets: The Impact of Formulation Design on Drug Delivery and Release Kinetics Using Conventional and Novel Dissolution Methods. Pharmaceuticals 2021, 14, 493. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Vohra, S.; Gedeon, C.; Akoury, H.; Bernstein, P.; Pairaudeau, N.; Cormier, J.; Dontigny, L.; Arsenault, M.-Y.; Fortin, C. The fetal safety of hydrocortisone-pramoxine (Proctofoam-HC) for the treatment of hemorrhoids in late pregnancy. Journal of Obstetrics and Gynaecology Canada 2011, 33, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, B.; Domb, A.J. Mucoadhesive polymers for delivery of drugs to the oral cavity. Recent patents on drug delivery & formulation 2008, 2, 108–119. [Google Scholar]
- Ghafar, H.; Khan, M.I.; Sarwar, H.S.; Yaqoob, S.; Hussain, S.Z.; Tariq, I.; Madni, A.U.; Shahnaz, G.; Sohail, M.F. Development and characterization of bioadhesive film embedded with lignocaine and calcium fluoride nanoparticles. Aaps Pharmscitech 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Tsibouklis, J.; Middleton, A.M.; Patel, N.; Pratten, J. Toward mucoadhesive hydrogel formulations for the management of xerostomia: The physicochemical, biological, and pharmacological considerations. Journal of Biomedical Materials Research Part A 2013, 101, 3327–3338. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Li, W.; Gao, P.; Xiang, D.; Ren, X.; Liu, D. Mucoadhesive buccal film containing ornidazole and dexamethasone for oral ulcers: in vitro and in vivo studies. Pharmaceutical development and technology 2019, 24, 118–126. [Google Scholar] [CrossRef]
- Tejada, G.; Piccirilli, G.; Sortino, M.; Salomón, C.; Lamas, M.; Leonardi, D. Formulation and in-vitro efficacy of antifungal mucoadhesive polymeric matrices for the delivery of miconazole nitrate. Materials Science and Engineering: C 2017, 79, 140–150. [Google Scholar] [CrossRef]
- Hassan, M.; Barakat, N.; El-Badry, M.; Shehata, S. Formulation and in vitro/in vivo evaluation of naproxen mucoadhesive buccal patches for local effect. Journal of drug delivery science and technology 2011, 21, 423. [Google Scholar] [CrossRef]
- Rencber, S.; Karavana, S.Y.; Yılmaz, F.F.; Eraç, B.; Nenni, M.; Özbal, S.; Pekcetin, C.; Gurer-Orhan, H.; Hoşgör-Limoncu, M.; Güneri, P. Development, characterization, and in vivo assessment of mucoadhesive nanoparticles containing fluconazole for the local treatment of oral candidiasis. International journal of nanomedicine 2016, 11, 2641. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Choudhury, H.; Ying, J.N.S.; Ling, J.F.S.; Ting, J.; Ting, J.S.S.; Zhia Hwen, I.K.; Suen, H.W.; Samsul Kamar, H.S.; Gorain, B. Mucoadhesive nanocarriers as a promising strategy to enhance intracellular delivery against oral cavity carcinoma. Pharmaceutics 2022, 14, 795. [Google Scholar] [CrossRef] [PubMed]
- Ghafar, H.; Khan, M.I.; Sarwar, H.S.; Yaqoob, S.; Hussain, S.Z.; Tariq, I.; Madni, A.U.; Shahnaz, G.; Sohail, M.F. Development and characterization of bioadhesive film embedded with lignocaine and calcium fluoride nanoparticles. Aaps Pharmscitech 2020, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Elkanayati, R.M.; Chambliss, W.G.; Omari, S.; Almutairi, M.; Repka, M.A.; Ashour, E.A. Mucoadhesive buccal films for treatment of xerostomia prepared by coupling HME and 3D printing technologies. Journal of Drug Delivery Science and Technology 2022, 75, 103660. [Google Scholar] [CrossRef]
- Abha, D.; Sheeja, K.; Bhagyashri, J. Design and evaluation of buccal film of diclofenac sodium. Int J pharm Bio sci 2011, 1, 17–30. [Google Scholar]
- Hearnden, V.; Sankar, V.; Hull, K.; Juras, D.V.; Greenberg, M.; Kerr, A.R.; Lockhart, P.B.; Patton, L.L.; Porter, S.; Thornhill, M.H. New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Advanced drug delivery reviews 2012, 64, 16–28. [Google Scholar] [CrossRef]
- Jelvehgari, M.; Rashidi, M.R.; Samadi, H. Mucoadhesive and drug release properties of benzocaine gel. Iranian journal of pharmaceutical sciences 2006, 2, 185–194. [Google Scholar]
- Yehia, S.A.; El-Gazayerly, O.N.; Basalious, E.B. Fluconazole mucoadhesive buccal films: in vitro/in vivo performance. Current drug delivery 2009, 6, 17–27. [Google Scholar] [CrossRef]
- Van Roey, J.; Haxaire, M.; Kamya, M.; Lwanga, I.; Katabira, E. Comparative efficacy of topical therapy with a slow-release mucoadhesive buccal tablet containing miconazole nitrate versus systemic therapy with ketoconazole in HIV-positive patients with oropharyngeal candidiasis. JAIDS Journal of Acquired Immune Deficiency Syndromes 2004, 35, 144–150. [Google Scholar] [CrossRef]
- Razzaq, S.; Hanif, S.; Syed, M.A.; Iqbal, J.; Raza, S.A.; Riaz, H.; Abid, F. Development and evaluation of mucoadhesive buccal tablet containing metronidazole for the treatment of periodontitis and gingivitis. Pakistan Journal of Pharmaceutical Sciences 2018, 31. [Google Scholar]
- Ahn, J.-S.; Choi, H.-K.; Chun, M.-K.; Ryu, J.-M.; Jung, J.-H.; Kim, Y.-U.; Cho, C.-S. Release of triamcinolone acetonide from mucoadhesive polymer composed of chitosan and poly (acrylic acid) in vitro. Biomaterials 2002, 23, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Samani, S.; Bahri-Najafi, R.; Yousefi, G. Formulation and in vitro evaluation of prednisolone buccoadhesive tablets. Il Farmaco 2005, 60, 339–344. [Google Scholar] [CrossRef]
- Bender, L.; Boostrom, H.M.; Varricchio, C.; Zuanon, M.; Celiksoy, V.; Sloan, A.; Cowpe, J.; Heard, C.M. A novel dual action monolithic thermosetting hydrogel loaded with lidocaine and metronidazole as a potential treatment for alveolar osteitis. European Journal of Pharmaceutics and Biopharmaceutics 2020, 149, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Azadi Boroujeni, A.; Talebi Ardakani, M.; Houshmand, B.; Moscowchi, A. Designing a novel chitosan-based periofilm containing metronidazole–ciprofloxacin. SN Applied Sciences 2020, 2, 1–8. [Google Scholar]
- Syed, M.A.; Aziz, G.; Jehangir, M.B.; Tabish, T.A.; Zahoor, A.F.; Khalid, S.H.; Khan, I.U.; Hosny, K.M.; Rizg, W.Y.; Hanif, S. Evaluating Novel Agarose-Based Buccal Gels Scaffold: Mucoadhesive and Pharmacokinetic Profiling in Healthy Volunteers. Pharmaceutics 2022, 14, 1592. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.S.; Castro, P.M.; Roque, L.; Thomé, N.G.; Reis, C.P.; Pintado, M.E.; Fonte, P. Novel and revisited approaches in nanoparticle systems for buccal drug delivery. Journal of Controlled Release 2020, 320, 125–141. [Google Scholar] [CrossRef]
- Kamimura, M.; Furukawa, T.; Akiyama, S.-i.; Nagasaki, Y. Enhanced intracellular drug delivery of pH-sensitive doxorubicin/poly (ethylene glycol)-block-poly (4-vinylbenzylphosphonate) nanoparticles in multi-drug resistant human epidermoid KB carcinoma cells. Biomaterials science 2013, 1, 361–367. [Google Scholar] [CrossRef]
- Du, F.; Meng, H.; Xu, K.; Xu, Y.; Luo, P.; Luo, Y.; Lu, W.; Huang, J.; Liu, S.; Yu, J. CPT loaded nanoparticles based on beta-cyclodextrin-grafted poly (ethylene glycol)/poly (L-glutamic acid) diblock copolymer and their inclusion complexes with CPT. Colloids and Surfaces B: Biointerfaces 2014, 113, 230–236. [Google Scholar] [CrossRef]
- NR, R.; Tiyaboonchai, W.; Madhusudhan, B. Fabrication and characterization of genistein encapsulated poly (D, L) lactic acid nanoparticles for pharmaceutical application. Current Nanoscience 2013, 9, 293–302. [Google Scholar]
- Panyam, J.; Zhou, W.Z.; Prabha, S.; Sahoo, S.K.; Labhasetwar, V. Rapid endo-lysosomal escape of poly (DL-lactide-coglycolide) nanoparticles: implications for drug and gene delivery. The FASEB journal 2002, 16, 1217–1226. [Google Scholar] [CrossRef]
- Ren, F.; Chen, R.; Wang, Y.; Sun, Y.; Jiang, Y.; Li, G. Paclitaxel-loaded poly (n-butylcyanoacrylate) nanoparticle delivery system to overcome multidrug resistance in ovarian cancer. Pharmaceutical research 2011, 28, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Gharat, S.A.; Momin, M.M.; Bhavsar, C. Oral squamous cell carcinoma: current treatment strategies and nanotechnology-based approaches for prevention and therapy. Critical Reviews™ in Therapeutic Drug Carrier Systems 2016, 33. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Vroman, B.; Lecouturier, N.; Crokart, N.; Pourcelle, V.; Freichels, H.; Jérôme, C.; Marchand-Brynaert, J.; Feron, O.; Préat, V. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. Journal of Controlled Release 2009, 140, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Strandman, S.; Zhu, J.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Li, L.D.; Crouzier, T.; Sarkar, A.; Dunphy, L.; Han, J.; Ribbeck, K. Spatial configuration and composition of charge modulates transport into a mucin hydrogel barrier. Biophysical journal 2013, 105, 1357–1365. [Google Scholar] [CrossRef]
- Krampe, R.; Visser, J.C.; Frijlink, H.W.; Breitkreutz, J.; Woerdenbag, H.J.; Preis, M. Oromucosal film preparations: points to consider for patient centricity and manufacturing processes. Expert opinion on drug delivery 2016, 13, 493–506. [Google Scholar] [CrossRef]
- Syed, M.A.; Hanif, S.; Ain, N.u.; Syed, H.K.; Zahoor, A.F.; Khan, I.U.; Abualsunun, W.A.; Jali, A.M.; Qahl, S.H.; Sultan, M.H. Assessment of Binary Agarose–Carbopol Buccal Gels for Mucoadhesive Drug Delivery: Ex Vivo and In Vivo Characterization. Molecules 2022, 27, 7004. [Google Scholar] [CrossRef]
- Sadeghian, R.; Rohani, B.; Golestannejad, Z.; Sadeghian, S.; Mirzaee, S. Comparison of therapeutic effect of mucoadhesive nano-triamcinolone gel and conventional triamcinolone gel on oral lichen planus. Dental research journal 2019, 16, 277. [Google Scholar]
- Begum, M.Y.; Alqahtani, A.; Ghazwani, M.; Ramakrishna, M.; Hani, U.; Atiya, A.; Rahamathulla, M. Preparation of Carbopol 934 based ketorolac tromethamine buccal mucoadhesive film: in vitro, ex vivo, and in vivo assessments. International Journal of Polymer Science 2021, 2021. [Google Scholar] [CrossRef]
- Reid, G. Probiotics: definition, scope and mechanisms of action. Best practice & research Clinical gastroenterology 2016, 30, 17–25. [Google Scholar]
- Meurman, J.H. Probiotics: do they have a role in oral medicine and dentistry? European journal of oral sciences 2005, 113, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.L.; Hubbard, N.E. Probiotic immunomodulation in health and disease. The Journal of nutrition 2000, 130, 403S–409S. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Pavan, S.; Kleerebezem, M. Towards understanding molecular modes of probiotic action. Current opinion in biotechnology 2006, 17, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Vitali, B.; Lombardi, F.; Guerrini, L.; Cinque, B.; Parolin, C.; Bigucci, F.; Cerchiara, T.; Arbizzani, C.; Gallucci, M.C. Mucoadhesive buccal films for local delivery of lactobacillus brevis. Pharmaceutics 2020, 12, 241. [Google Scholar] [CrossRef]
- Palo, M.; Rönkönharju, S.; Tiirik, K.; Viidik, L.; Sandler, N.; Kogermann, K. Bi-layered polymer carriers with surface modification by electrospinning for potential wound care applications. Pharmaceutics 2019, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Shirvan, A.R.; Bashari, A.; Hemmatinejad, N. New insight into the fabrication of smart mucoadhesive buccal patches as a novel controlled-drug delivery system. European Polymer Journal 2019, 119, 541–550. [Google Scholar] [CrossRef]
- Cid, Y.P.; Pedrazzi, V.; de Sousa, V.P.; Pierre, M.B.R. In vitro characterization of chitosan gels for buccal delivery of celecoxib: influence of a penetration enhancer. AAPS PharmSciTech 2012, 13, 101–111. [Google Scholar] [CrossRef]
- Samprasit, W.; Kaomongkolgit, R.; Sukma, M.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Mucoadhesive electrospun chitosan-based nanofibre mats for dental caries prevention. Carbohydrate polymers 2015, 117, 933–940. [Google Scholar] [CrossRef]
- Voronova, A.; Prieto, C.; Pardo-Figuerez, M.; Lagaron, J.M.; Sanyal, A.; Demir, B.; Hubert, T.; Plaisance, V.; Pawlowski, V.; Vignoud-Despond, S. Photothermal Activatable Mucoadhesive Fiber Mats for On-Demand Delivery of Insulin via Buccal and Corneal Mucosa. ACS Applied Bio Materials 2022, 5, 771–778. [Google Scholar] [CrossRef]
- Samprasit, W.; Rojanarata, T.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Kaomongkolgit, R.; Opanasopit, P. Fabrication and in vitro/in vivo performance of mucoadhesive electrospun nanofiber mats containing α-mangostin. Aaps Pharmscitech 2015, 16, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Drávavölgyi, G.; Faragó, K.; Farkas, A.; Vigh, T.; Sóti, P.L.; Wagner, I.; Madarász, J.; Pataki, H.; Marosi, G. Plasticized drug-loaded melt electrospun polymer mats: characterization, thermal degradation, and release kinetics. Journal of pharmaceutical sciences 2014, 103, 1278–1287. [Google Scholar] [CrossRef]
- Garg, K.; Tirgar, P. Assessment of Effect of Intra-pocket Delivery of Metronidazole Loaded NTrimethyl Quaternary Ammonium Chitosan Nanoparticles in Treatment of Periodontal Disease. Current Drug Therapy 2023, 18, 49–67. [Google Scholar] [CrossRef]
- Hanif, S.; Sarfraz, R.M.; Syed, M.A.; Mahmood, A.; Minhas, M.U.; Irfan, M. Development and optimization of tibezonium iodide and lignocaine hydrochloride containing novel mucoadhesive buccal tablets: A pharmacokinetic investigation among healthy humans. Drug Development and Industrial Pharmacy 2021, 47, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
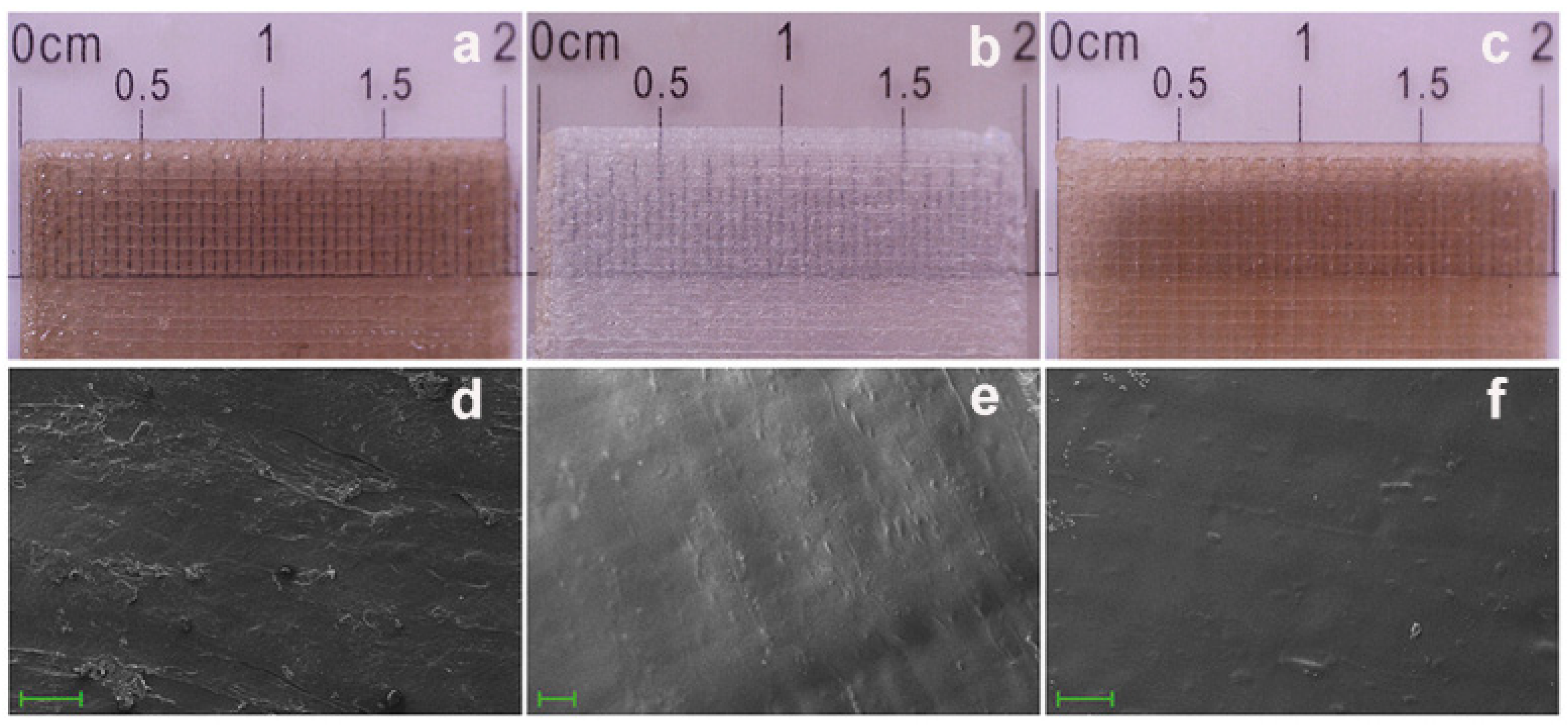
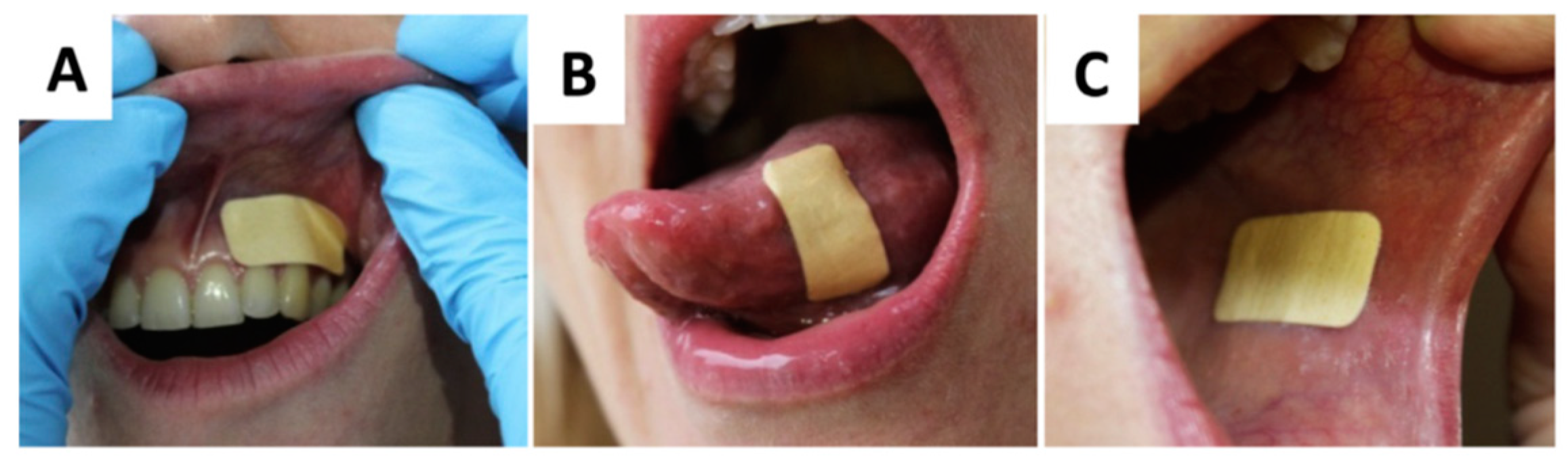
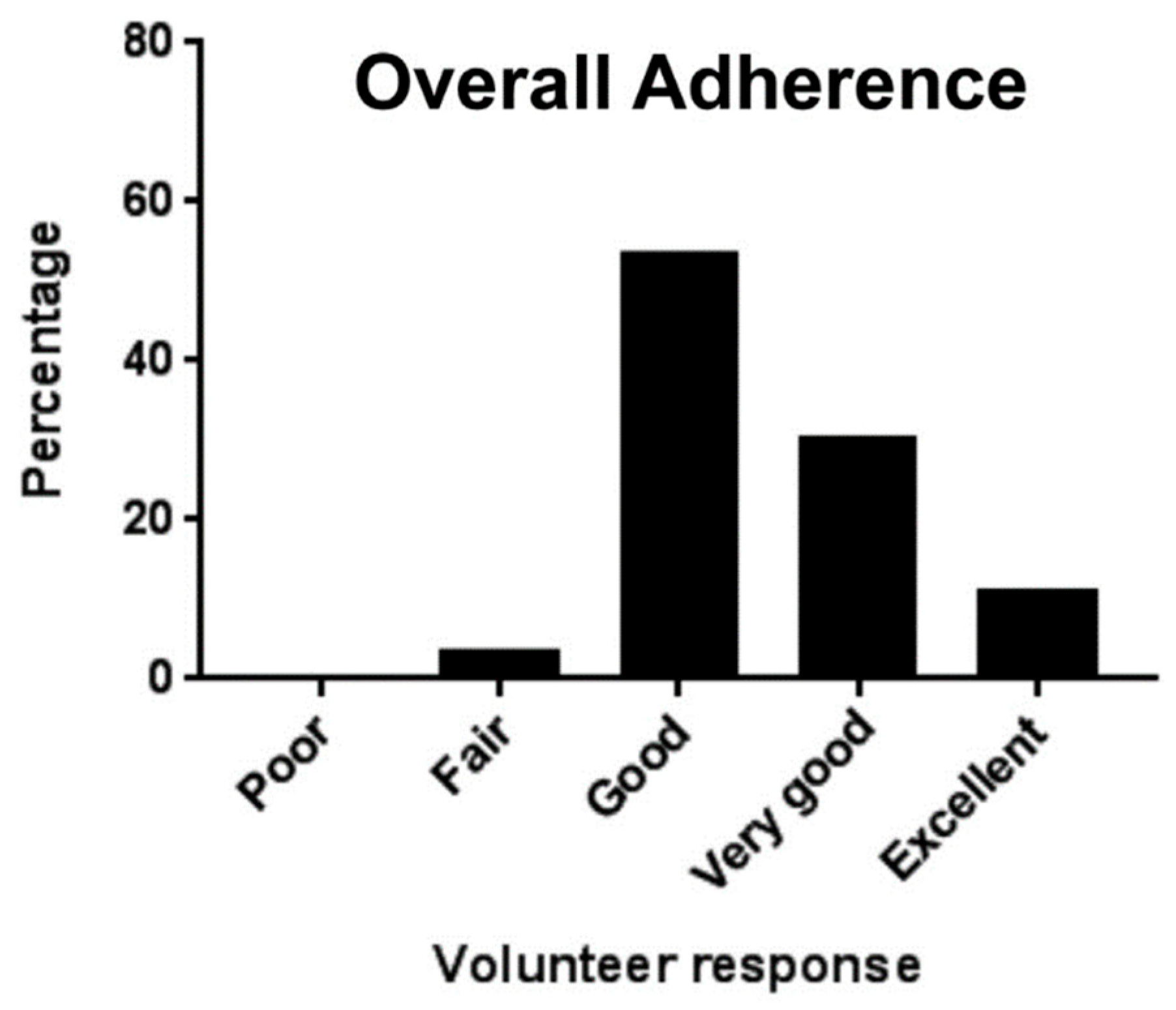
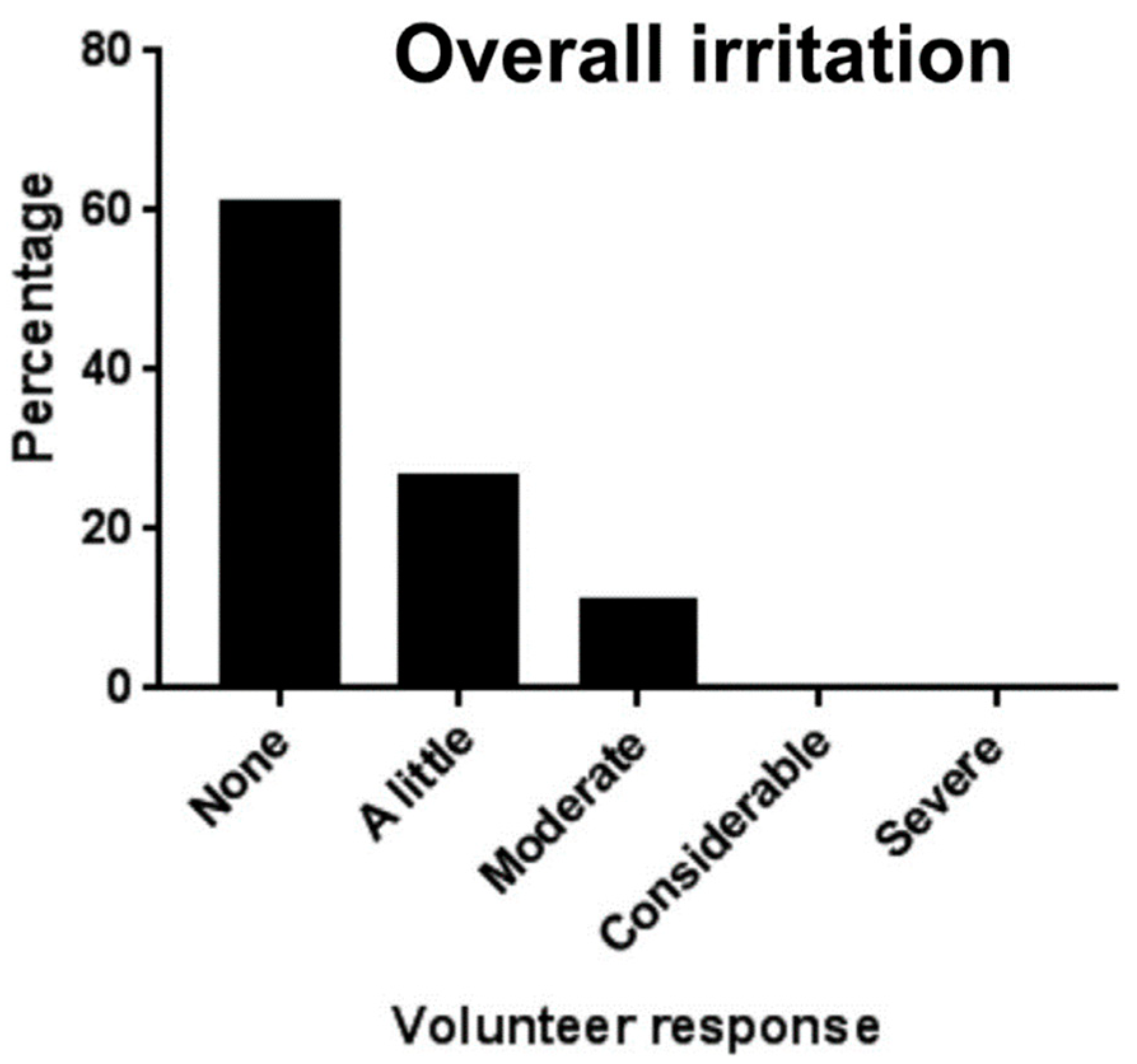
| Anaerobic Gram-negative bacteria | Anaerobic Gram-positive bacteria |
|---|---|
| Actinobacillus actinomycetemcomitans | Actinomyces viscosus |
| Porphyromonas gingivalis | Peptostreptococcus micros |
| Prevotella intermedia | |
| Tannerella forsythensis |
| Gingivitis | Periodontitis |
|---|---|
| Dental plaque-induced gingival disease | Chronic periodontitis (CP) |
| Acute necrotizing ulcerative gingivitis (ANUG) | Aggressive periodontitis (AP) |
| Steroid hormone-induced gingival enlargement | Periodontitis as a manifestation of systemic diseases |
| Drug-induced gingival enlargement | Necrotizing periodontal diseases |
| Gingivitis associated with blood disorders, nutritional deficits, tumors, genetic factors, viral infection | Periodontal abscesses |
| Desquamative gingivitis | Periodontitis with endodontic lesions |
| Gingivitis associated with malnutrition | Developmental and acquired deformation and conditions |
| Non-plaque induced gingival disease | |
| Gingival disease of bacterial, viral, fungal origin | |
| Gingival lesions of genetic origin | |
| Gingival manifestation of systemic conditions | |
| Traumatic lesions |
| Disease | Local therapy | Reference(s) |
|---|---|---|
| Oral sores | Antimicrobial, antifungal, antiseptic, adjunct therapy, anti-inflammatory drugs | [73,74] |
| Swelling | Non-steroidal and steroidal anti-inflammatory drugs | [64,75] |
| Microbial infection | Antimicrobial, antifungal, antiseptic | [68,74,76] |
| Pain | Local anesthetic agent | [52] |
| Tumor | Anti-cancer drugs | [77] |
| Dietary calcium supplement | Calcium supplements | [78] |
| Xerostomia | Secretagogues | [72,79] |
| Monotherapy | Dual therapy |
|---|---|
| Diclofenac [80] Lignocaine [78] Minocycline [81] Dexamethasone [73] Benzocaine [82] Chlorhexidine [62] Articaine [62] Fluconazole [83] Ketoconazole [84] Metronidazole [85] Triamcinolone acetonide [86] Prednisolone [87] Calcium supplement [78] |
Metronidazole and lignocaine [88] Ciprofloxacin and metronidazole [89] Flurbiprofen and lignocaine [52] Tibezonium iodide and lignocaine [63] Tibezonium iodide and benzocaine [90] Ketoprofen and lignocaine hydrochloride [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





