1. Introduction
Sodium-glucose cotransporter inhibitors (SGLT2i) represent a cornerstone in the treatment of patients with heart failure with reduced ejection fraction (HFrEF) [1-6]. This has led to a change in the recommended treatment of patients with HFrEF [
4]. Currently, four classes of drugs should be introduced as soon as possible to maximize their benefit, i.e. SGLT2i, beta-blockers, mineralcorticoid receptor antagonists (MRAs), and angiotensin receptor neprilysin inhibitors (ARNi) or, if not tolerated, ACE inhibitors (ACEi) or angiotensin receptor blockers (ARBs) [
4]. However, there are not many about the feasibility of these recommendations and the potential interaction between some of these classes, such as SGLT2i and ARNi [
7,
8].
Moreover, the possibility of prescribing SGLT2i is based not only on evidence and guideline recommendations but also on the indications provided by regulatory agencies and the reimbursement policies of different national healthcare systems. For example, in Italy, the reimbursement for SGLT2i has been limited to patients with type 2 diabetes mellitus (T2DM) until February 2022, and only later was it possible to prescribe with reimbursement for patients with HFrEF first dapaglifozin and then empaglifozin. Furthermore, until June 2023, no reimbursement was allowed in patients with mildly reduced ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF) despite the available evidence [9-10].
On the basis of these considerations, the aim of this study was to evaluate, in a real-world population from a single center, the feasibility of introducing SGLT2i and their interaction with other recommended drug classes.
2. Materials and Methods
We evaluated patients referred to the Heart Failure Unit of the University Policlinic Hospital of Foggia for diagnosis of chronic heart failure (CHF) since the February of 2022, when the reimbursement of SGLT2i was allowed by Italian National Health System. In accordance with the indications of the Italian Ministry of Health, dapagliflozin (since February 2022) or empagliflozin (since June 2022) could be prescribed and reimbursed in presence of NYHA class II-II, left ventricular ejection fraction (LVEF) ≤40%, already treated, if not contraindicated or not tolerated, with ARNi (sacubitril/valsartan) or ACEi or ARB, MRA and beta-blockers [
4]. Moreover, independently from LVEF, all SGLT2i could be prescribed and reimbursed if CHF patients were also affected by type 2 diabetes mellitus (T2DM). All patients were enrolled in the Daunia registry, among whose end-points there is the study of the effects of new therapeutic approaches on the clinical. The registry has been approved by local Ethic Committees, and all enrolled patients gave their written informed consent.
Baseline evaluations. The baseline evaluation was considered as the first recorded medical visit after February 2022. Physical examination, 12-lead electrocardiogram, one- and two-dimensional echocardiographic evaluations were performed, and peripheral blood samples were taken. At the medical visit, the presence of ischaemic cardiomyopathy, cerebrovascular disease or stroke, arterial hypertension, atrial fibrillation, diabetes mellitus, and dyslipidaemia were recorded as well as HF, NYHA class and antidiabetic therapy. Echocardiographic recordings were done using a phased-array echo-Doppler system (EPIQ CVx system, Philips, Amsterdam, the Netherlands) to evaluate LVEF by the Simpson method. Creatinine serum concentrations (mg/dL) levels were measured at the baseline evaluation and then the GFR (mL/min) was calculated using the CKD-EPI formula [
11]. The dose of heart failure classes of drugs was evaluated as follows: for ACEi the equivalent enalapril dose was calculated according to the following proportions: enalapril 20 mg /die equivalent to ramipril 10 mg/die, zofenopril 30 mg/die, lisinopril 20 mg/die. For ARBs it was calculated the equivalent valsartan dose according to the following proportions: valsartan 320 mg /die equivalent to losartan 100 mg/die, candesartan 32 mg/die [
4]. For beta-blockers, the equivalent bisoprolol dose was calculated according to the following proportions: bisoprolol 10 mg /die equivalent to carvedilol 50 mg/die, nebivolol 10 mg/die, metoprolol tartrate 200 mg/die. Finally sacubitril/valsartan the dose of 24/26 mg b.i.d. was computed as 100 mg/die, that of 49/51 mg b.i.d. as 200 mg/die, that of 97/103 mg b.i.d. as 400 mg/die.
Follow-up. The patients were followed up with scheduled visits according to the protocol of our outpatient HF clinic: at least one evaluation within 6 months. Every effort was taken in order to introduce and uptitrate the disease modifiers’ drugs currently recommended for HFrEF [
4] and to introduce SGLT2i in patients with LVEF>40% when affected by T2DM. For each patient, the 6 or 12 month re-evaluations were analyzed to evaluate the changes in the studied parameters and in therapy.
Statistical analysis. Continuous variables are expressed as mean values ± standard deviation. Discrete variables were summarized as frequencies and percentages. to compare patients with and without LVEF <40%. t-student test and exact Fisher test were used. to test the changes of the study parameters in patients with SGLT2i therapy, t Student test for paired samples and McNemar’s Test were used for continuous and cathegorical variables, respectively. Statistica 6.1 software (StatSoft Inc., Tulsa, Okla-homa). A p value of <0.05 was considered statistically significant.
3. Results
The patients who underwent an evaluation since the indexed period were 350. Among these, 213 (61%) showed a LVEF
<40% (HFrEF), 59 (17%) between 41 and 49% (heart failure with mild reduced ejection fraction, HFmrEF) and 78 (22%)
>50% (heart failure with preserved ejection fraction, HFpEF).
Table 1 shows baseline clinical characteristics of all the enrolled patients as well as of those with and without HFrEF. Patients with HFrEF were more frequently males, with ischemic etiology, more frequently taking therapy with beta-blockers and ARNi and carrying a cardioverter defibrillator with or without CRT. They were also less frequently hypertensive with lower baseline systolic blood pressure. Finally, they showed a significantly worse NYHA class.
Table 1.
Patient baseline clinical characteristics.
Table 1.
Patient baseline clinical characteristics.
| |
All
patients |
HFrEF
patients |
HFrEF/HFpEF
patients |
| Number |
350 |
213 |
137 |
| Age (years) |
66±12 |
65±12 |
67±12 |
| Males, n (%) |
280 (80) |
181 (85) |
99 (72) |
| De novo HF, n (%) |
16 (5) |
13 (6) |
3 (2) |
| Ischemic etiology n, (%) |
147 (42) |
104 (49) |
42 (31) |
| Diabetes mellitus n, (%) |
122 (35) |
75 (35) |
47 (34) |
| Arterial Hypertension n, (%) |
230 (66) |
128 (60) |
102 (74) |
| Atrial Fibrillation n, (%) |
48 (14) |
28 (13) |
20 (15) |
NYHA class I, n (%)
II, n (%)
III, n (%)
|
27 (7.7)
193 (55.1)
130 (37.2) |
10 (4.7)
124 (64.3)
79 (31) |
17 (12.4)
69 (50.4)*
51 (37.2) |
| SAP (mm Hg) |
124±19 |
121±18 |
130±20* |
| Heart rate (beats/minute) |
68±12 |
68±12 |
69±13 |
| LVEF (%) |
39±9 |
32±6 |
49±5* |
| Creatinine (mg/dl) |
1.30±0.8 |
1.30±0.6 |
1.30±1.0 |
| GFR-EPI (ml/min/1.73 m2) |
62.5±24.7 |
61±21 |
65±27 |
| Concomitant therapy at the enrollment |
|
|
|
| ARNi, n (%)
|
179 (51) |
139 (65) |
40 (29) |
| Sacubitril/Valsartan dose (mg/die)
|
191±134 |
175±130 |
251±145* |
| ACE-I, n (%)
|
66 (19) |
33 (16) |
33 (24) |
| Enalapril equivalent dose (mg/die)
|
9.9±6.7 |
9.2±6.7 |
10.8±6.7 |
| ARB, n (%)
|
52 (15) |
20 (9) |
32 (23) |
| Valsartan equivalent dose (mg/die)
|
139±103 |
90±67 |
170±111 |
| Beta-blockers n, (%)
|
330 (94) |
207 (97) |
123 (90)* |
| Bisoprolol equivalent dose (mg/die)
|
4.8±6.2 |
4.9±3.3 |
4.6±2.9 |
| MRA n, (%)
|
235 (67) |
150 (70) |
85 (62) |
| MRA dose
|
38.4±30.1 |
39±29 |
37±31 |
| Loop diuretics n, (%)
|
250 (71) |
156 (73) |
94 (69) |
| Furosemide equivalent dose (mg/die)
|
71±86 |
66±74 |
78±103 |
| ICD and/or CRT, n (%) |
191 (55) |
143 (67) |
48 (35)* |
| |
|
|
|
| SGLT2i |
|
|
|
| Before baseline evaluation, n (%) |
17 (5) |
13 (6) |
4 (3) |
| After baseline evaluation, n (%) |
203 (58) |
165 (77) |
37 (27) |
| |
|
|
|
3.1. SGLT2 inhibitor therapy
At baseline only 17 (5%) patients were already taking SGLT2i, 13 with HFrEF, 2 with HFmrEF and 2 with HFpEF. After baseline evaluation, SGLT2i were prescribed to 220 (63%) patients, among whom 178 (84%) with HFrEF, 25 (42%) with HFmrEF and 17 (22%) with HFpEF. In 183 (83%) patients, Dapaglifozin was prescribed, in 35 (16%) Empaglifozin and in 2 (1%) Canaglifozin.
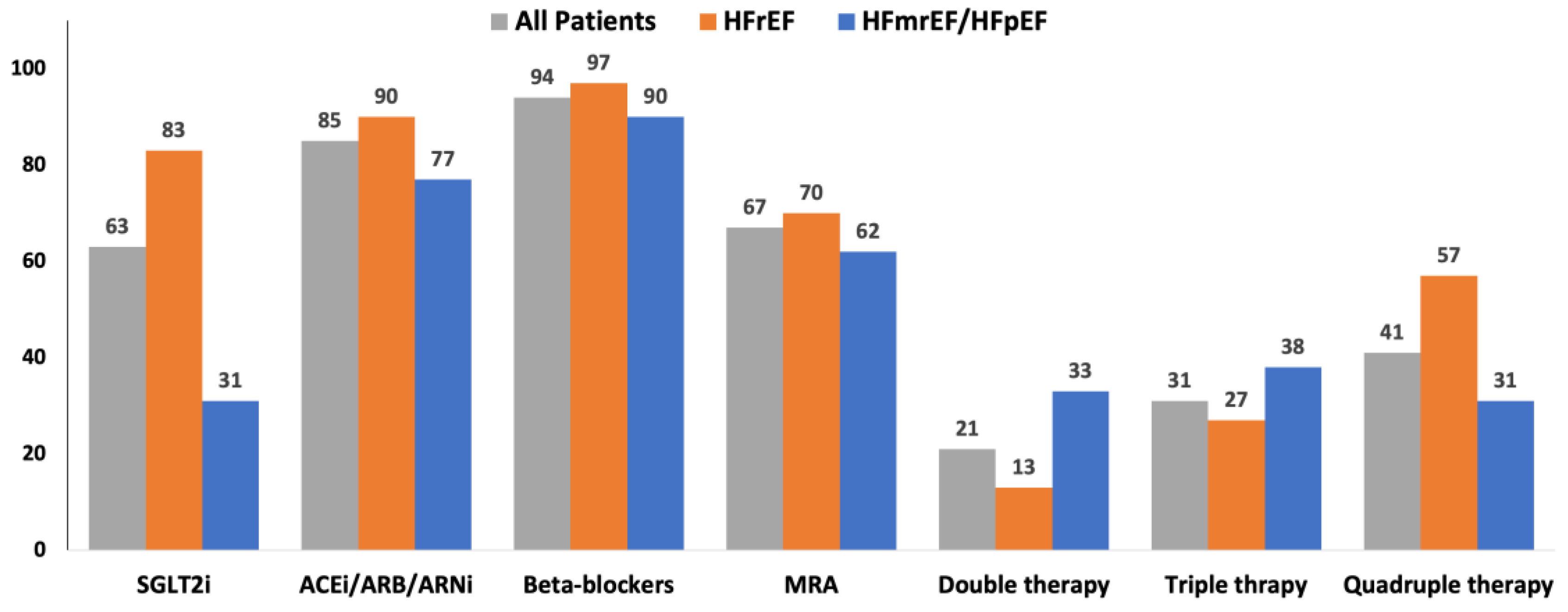
Figure 1 shows the main classes of drugs prescribed at baseline. After 6/12 months of follow-up, only 7 of 220 patients withdrawn SGLT2i due to hypotension (2), acute kidney injury (1), intolerance (2) and urinary tract infection (2).
Figure 1.
Therapy prescribed at baseline. The main classes of drugs prescribed at baseline in all the enrolled patients, in those with HFrEF and in those with HFmrEF or HFpEF. ACE: Angiotensin converting enzyme; ARB: Angiotensin II receptor blockers; ARNi: angiotensin II receptor and neprylisin inibithor; HFmrEF: heart failure with mild reduced left ventricular ejection fraction; HFmrEF: heart failure with preserved left ventricular ejection fraction; HFrEF: heart failure with reduced left ventricular ejection fraction; MRA: mineralcorticoid receptor antagonists; SGLT2i: inhibitors of type 2 sodium-glucose cotransporter.
Figure 1.
Therapy prescribed at baseline. The main classes of drugs prescribed at baseline in all the enrolled patients, in those with HFrEF and in those with HFmrEF or HFpEF. ACE: Angiotensin converting enzyme; ARB: Angiotensin II receptor blockers; ARNi: angiotensin II receptor and neprylisin inibithor; HFmrEF: heart failure with mild reduced left ventricular ejection fraction; HFmrEF: heart failure with preserved left ventricular ejection fraction; HFrEF: heart failure with reduced left ventricular ejection fraction; MRA: mineralcorticoid receptor antagonists; SGLT2i: inhibitors of type 2 sodium-glucose cotransporter.
3.2. Changes in patients with SGLT2 inhibitor therapy
As shown in
Table 2, when all patients in whom SGLT2i was prescribed at baseline were considered, a significant reduction in weight, systolic arterial pressure, NYHA class and a slight significant increase in creatinine serum levels were observed. Moreover, a significant greater proportion of patients taking ARNi as well as of its dose was present during follow-up. In concomitance, there was a significant reduction in the proportion of patients taking ACEi/ARB as well as diuretics. No differences were found when beta-blocker and MRA therapy were analyzed.
The results were similar when only patients with HFrEF were considered, as shown in the second part of
Table 2. In
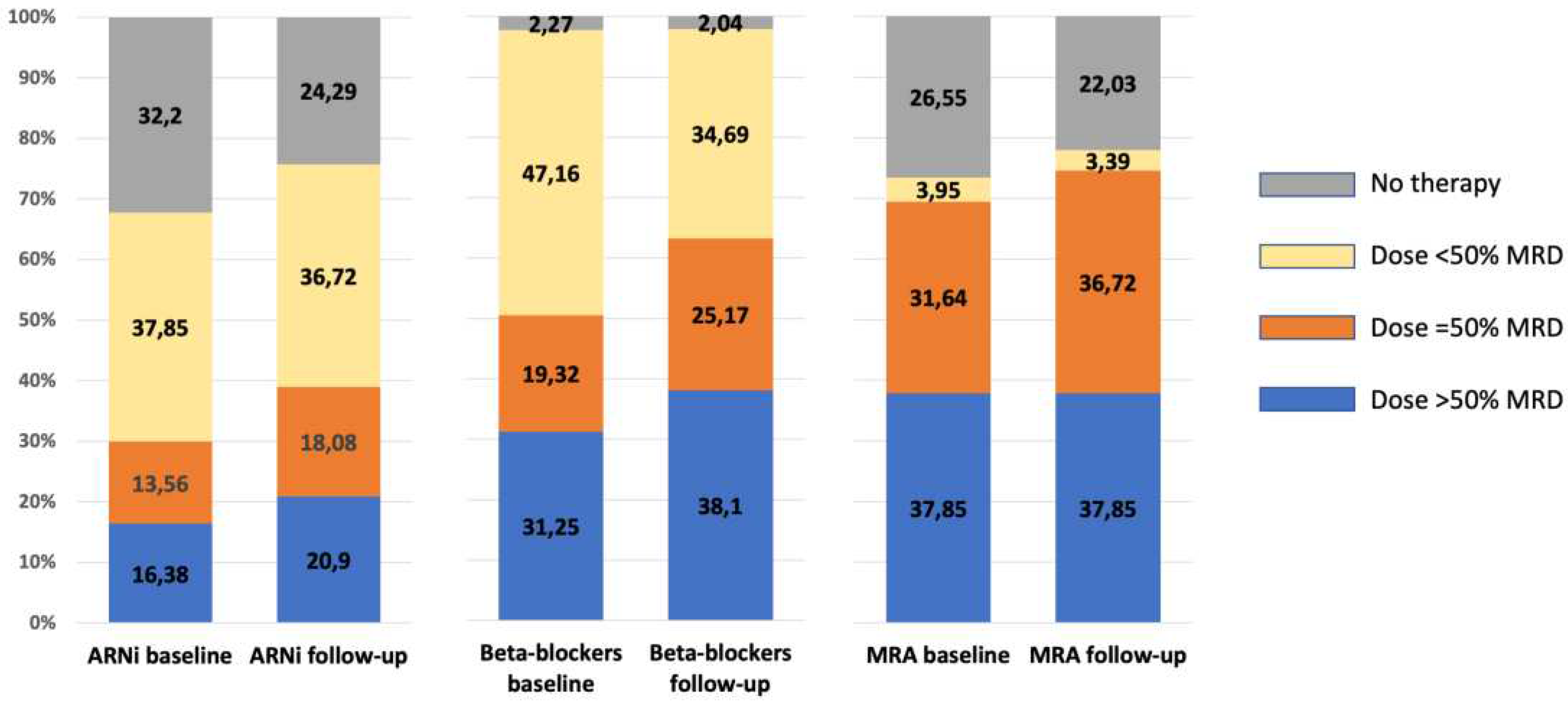
Figure 2 the changes in the proportion of patients no taking or taking low, mean and high dose of ARNi, beta-blockers and MRA is shown. The proportion of patients taking the higher doses was stable or a trend toward an increase was observed.
Table 2.
Changes of studied parameters in patients treated with SGLT2i.
Table 2.
Changes of studied parameters in patients treated with SGLT2i.
| All patients with SGLT2i (n: 220) |
Baseline |
After |
p |
| |
|
|
|
| Weight (kg)
|
80.7±16.7 |
79.6±16.4 |
0.002 |
| NYHA class
|
2.4±0.6 |
2.3±0.6 |
0.008 |
| SAP (mmHg)
|
122±18 |
118±18 |
0.005 |
| Heart Rate (bpm)
|
68±11 |
67±10 |
0.463 |
| LVEF (%)
|
35±8 |
37±9 |
<0.001 |
| Creatinine (mg/dl)
|
1.26±0.37 |
1.30±0.45 |
0.044 |
| GFR-EPI (ml/min/1.73 m²)
|
61±20 |
61±21 |
0.469 |
| Concomitant therapy |
|
|
|
| ARNi, n (%)
|
61 |
68 |
<0.001 |
| Sacubitril/Valsartan dose (mg/die)
|
178±133 |
207±130 |
<0001 |
| ACE-I %
|
14 |
11 |
0.070 |
| Enalapril equivalent dose (mg/die)
|
9.3±7.5 |
8.4±6.6 |
0.213 |
| ARB %
|
14 |
12 |
0.343 |
| Valsartan equivalent dose (mg/die)
|
72±72 |
72±76 |
1.00 |
| Beta-blockers (%)
|
97 |
97 |
1.00 |
| Bisoprolol equivalent dose (mg/die)
|
5.1±3.3 |
5.3±3.3 |
0.094 |
| MRA %
|
77 |
79 |
0.522 |
| MRA dose (mg/die)
|
43±27 |
43±25 |
0.826 |
| Loop diuretics %
|
75 |
69 |
0.014 |
| Furosemide equivalent dose (mg/die)
|
69±79 |
63±106 |
0.309
|
| Patients with HFrEF and SGLT2i (n: 178) |
Baseline |
After |
|
| |
|
|
|
| Weight (kg)
|
79.5±16.8 |
78.4±16.4 |
0.007 |
| NYHA class
|
2.3±0.6 |
2.2±0.6 |
0.004 |
| SAP (mmHg)
|
119±16 |
116±16 |
0.019 |
| Heart Rate (bpm)
|
67±11 |
67±10 |
0.117 |
| LVEF (%)
|
32±6 |
35±8 |
<0.001 |
| Creatinine (mg/dl)
|
1.28±0.35 |
1.30±0.43 |
0.183 |
| GFR-EPI (ml/min/1.73 m²)
|
60±18 |
60±21 |
0.798 |
| Concomitant therapy |
|
|
|
| ARNi, n (%)
|
68 |
77 |
<0.001 |
| Sacubitril/Valsartan dose (mg/die)
|
175±131 |
206±128 |
<0001 |
| ACE-I %
|
14 |
9 |
0.027 |
| Enalapril equivalent dose (mg/die)
|
7.7±6.4 |
6.7±5.4 |
0.189 |
| ARB %
|
10 |
8 |
0.289 |
| Valsartan equivalent dose (mg/die)
|
70±35 |
67±20 |
0.674 |
| Beta-blockers (%)
|
98 |
98 |
1.00 |
| Bisoprolol equivalent dose (mg/die)
|
5.1±3.3 |
5.2±3.2 |
0.240 |
| MRA %
|
76 |
79 |
0.359 |
| MRA dose (mg/die)
|
42±28 |
41±24 |
0.651 |
| Loop diuretics %
|
76 |
69 |
0.009 |
| Furosemide equivalent dose (mg/die)
|
63±73 |
59±102 |
0.342
|
Figure 2.
Presence and dose at baseline and during follow-up of ARNi, beta-blockers and MRA in HFrEF patients with SGLT2i therapy. When the class of drugs is prescribed, the dose is expressed as below, equal or above 50% of the current recommended maximum dose. ARNi: angiotensin II receptor and neprylisin inibithor; MRA: mineralcorticoid receptor antagonists; SGLT2i: inhibitors of type 2 sodium-glucose cotransporter.
Figure 2.
Presence and dose at baseline and during follow-up of ARNi, beta-blockers and MRA in HFrEF patients with SGLT2i therapy. When the class of drugs is prescribed, the dose is expressed as below, equal or above 50% of the current recommended maximum dose. ARNi: angiotensin II receptor and neprylisin inibithor; MRA: mineralcorticoid receptor antagonists; SGLT2i: inhibitors of type 2 sodium-glucose cotransporter.
4. Discussion
In our single-center "real-world" study, SGLT2i therapy was prescribed in a high percentage of patients with HFrEF. The introduction of this therapy was associated with an improvement in the use of the other disease modifiers’ drugs currently recommended in HFrEF [
4]. These results are significant for several reasons. SGLT2is, first dapagliflozin [
1] and later empagliflozin [
2], have demonstrated to reduce the combined endpoint of hospitalization for heart failure and cardiovascular death in HFrEF. Furthermore, in the case of dapagliflozin, a significant reduction in cardiovascular mortality and all-cause mortality was observed [
1]. For this evidence the recent European [
4] and American [
12] guidelines recommend the use of this class of drugs with a Class I recommendation with Level of Evidence A. Moreover, differently from previous stepwise approach, the use of the four classes of drugs capable of modifying the natural history of HFrEF, i.e. ARNi preferred over ACEi/ARBs, MRAs, beta-blockers, and SGLT2i, is recommended as early as possible [
4,
12]. This novel approach is supported by recent data further demonstrating its beneficial effects [13-14]. However, in the randomized controlled trials [1-3] as well as in the real world data studies evaluating SGLT2i, the percentage of patients taking ARNi (sacubitril/valsartan) was low. In this context, it is relevant to observe that, in our study, not only SGLT2i can be introduced in a very large part of patients but its introduction is also associated with a high percentage of sacubitril/valsartan treatment. Moreover, during follow-up, the prescription rate of sacubitril/valsartan was even improved in HFrEF patients.
The optimization of therapy in HFrEF patients, made possible by the introduction of SGLT2i as well as the optimization of the remaining therapy, can explain two other interesting results of our study. The first is related to the improvement in NYHA class and LVEF. This is certainly an expected outcome considering that, through an effective neurohormonal modulation and the still not completely understood effects of SGLT2is [
15], there is a high likelihood of improving both left ventricular systolic function and functional capacity. The second aspect concerns the use of loop diuretics after SGLT2is. During the follow-up, we observed a significant reduction in the percentage of patients receiving diuretic treatment. This could be a consequence previously described improvements as well as of a mild diuretic effect of both SGLT2is and sacubitril/valsartan [
16,
17]. The reduced need of diuretics could also carry a beneficial pathophysiological effect because allowing to avoid the undesirable consequence related to the loop diuretic use [
18].
The final aspect for HFrEF patients concerns the therapy with beta-blockers and MRAs. For these two classes, a significant change in the prescription rate was not observed, but, as shown in
Figure 2, a trend towards the use of higher doses, as for sacubitril/valsartan, was observed. The ability to introduce the four classes and titrate their doses offers a clinically significant outcome for clinical practice as recently demonstrated in the recent STRONG-HF trial [
8].
In our study, in patients with HFmrEF and HFpEF, the prescription rate of SGLT2 inhibitors was much lower than expected. This was due to the fact that, until June 2022, the end of our follow-up period, in Italy, the prescription of SGLT2 inhibitors was reimbursed only for patients with type 2 diabetes mellitus (T2DM). Consequently, we could put every effort to start SGLT2i only in T2DM patients with HFmrEF and HFpEF as evident by the rate of prescription and the prevalence of diabetic patients. Despite this effort, the low percentage of HFmrEF/HFpEF patients receiving prescription of SGLT2i make very relevant in negative terms the delay of Italian National Health System in achieving the evidence of the trials and the recommendations of guidelines [
4,
12] and in allowing the reimbursement of the new effective drugs. This delay will be responsible of an increased risk of heart failure progression, considering the ability of SGLT2i of early improving prognosis regardless of LVEF [
10,
12] and also in patients with improved ejection fraction (HFimpEF) [19-20].
5. Conclusions
In conclusion, our results provide real-world data indicating that SGLT2 inhibitor therapy can be prescribed in a significant proportion of patients with HFrEF. The introduction of this therapy does not hinder the optimization of therapy with ARNi, beta-blockers, and MRAs, and is associated with a reduction in the use of diuretics. Future studies should also clarify the clinical aspects related to the use of SGLT2 inhibitors in patients with HFmrEF, HFpEF, and HFimpEF. Our data, in fact, were limited by the Italian reimbursement regulations.
Author Contributions
Conceptualization, E.T. and M.I.; methodology, M.C., M.I.; formal analysis, M.I.; investigation, E.T., M.C., G.A., R.P., S.I., M.R., M.I.; data curation, E.T., G.A., R.P., S.I., M.R., M.I.; writing—original draft preparation, E.T., M.I.; writing—review and editing, M.C., M.D.B, N.D.B.; supervision, M.C., M.D.B, N.D.B..
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of the Polyclinic University Hospital of Foggia, Foggia, Italy (protocol code 68/CE/20, date of approval 26th may 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data could be available on request
Conflicts of Interest
The authors declare no conflict of interest.
References
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; et al. EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Iacoviello, M.; Palazzuoli, A.; Gronda, E. Recent advances in pharmacological treatment of heart failure. Eur J Clin Invest 2021, 51, e13624. [Google Scholar] [CrossRef] [PubMed]
- De Marzo, V.; Savarese, G.; Tricarico, L.; et al. Network meta-analysis of medical therapy efficacy in more than 90,000 patients with heart failure and reduced ejection fraction. J Intern Med 2022, 292, 333–349. [Google Scholar] [CrossRef]
- Stolfo, D.; Lund, L.H.; Benson, L.; et al. Real-world use of sodium-glucose co-transporter 2 inhibitors in patients with heart failure and reduced ejection fraction: Data from the Swedish Heart Failure Registry. Eur J Heart Fail 2023. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Davison, B.; Chioncel, O.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; et al. EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; et al. DELIVER Trial Committees and Investigators. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; et al. . 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022, 79, 1757–1780. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Claggett, B.L.; Jhund, P.S.; et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet 2020, 396, 121–128. [Google Scholar] [CrossRef]
- D'Amario, D.; Rodolico, D.; Rosano, G.M.C.; et al. Association between dosing and combination use of medications and outcomes in heart failure with reduced ejection fraction: data from the Swedish Heart Failure Registry. Eur J Heart Fail 2022, 24, 871–884. [Google Scholar] [CrossRef]
- Gronda, E.; Vanoli, E.; Iacoviello, M.; et al. The Benefit of Sodium-Glucose Co-Transporter Inhibition in Heart Failure: The Role of the Kidney. Int J Mol Sci 2022, 23, 11987. [Google Scholar] [CrossRef]
- Vardeny, O.; Claggett, B.; Kachadourian, J.; et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM-HF trial. Eur J Heart Fail 2019, 21, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Scheen, A.J. The diuretic effects of SGLT2 inhibitors: A comprehensive review of their specificities and their role in renal protection. Diabetes Metab 2021, 47, 101285. [Google Scholar] [CrossRef] [PubMed]
- Ter Maaten, J.M. Unravelling the effect of sacubitril/valsartan on loop diuretic dosing. Eur J Heart Fail 2019, 21, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail 2021, S1071-9164(21)00050-6. [Google Scholar]
- Vardeny, O.; Fang, J.C.; Desai, A.S.; et al. Dapagliflozin in heart failure with improved ejection fraction: a prespecified analysis of the DELIVER trial. Nat Med 2022, 28, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).