Submitted:
14 July 2023
Posted:
17 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
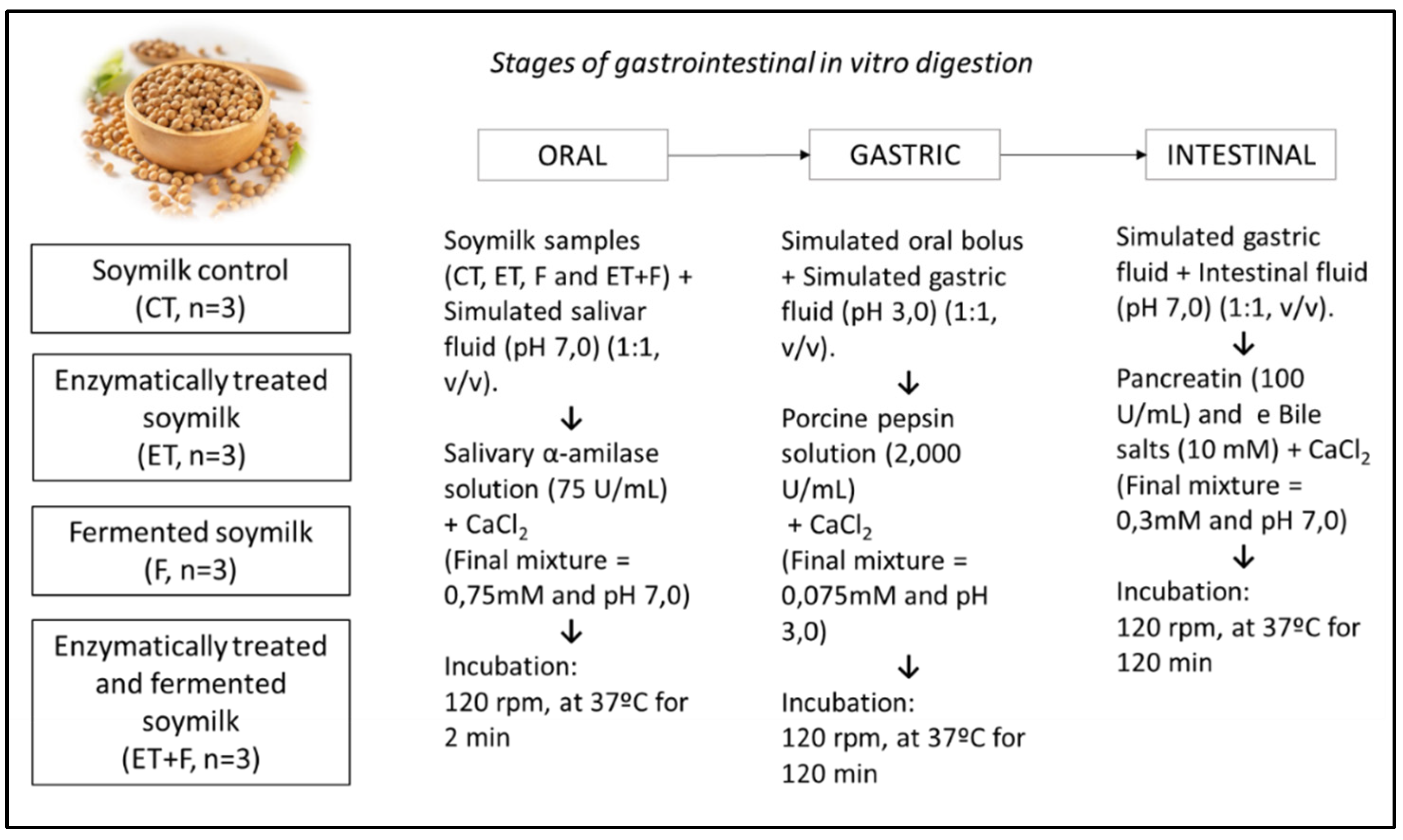
2.2.1. Processing of soymilk extracts
2.2.2. In vitro digestion assay
2.2.3. Analysis of isoflavone metabolites
2.2.4. Analysis of Total Phenolics
2.2.5. Analysis of Antioxidant Capacity
2.2.6. Statistical analysis
3. Results and Discussion
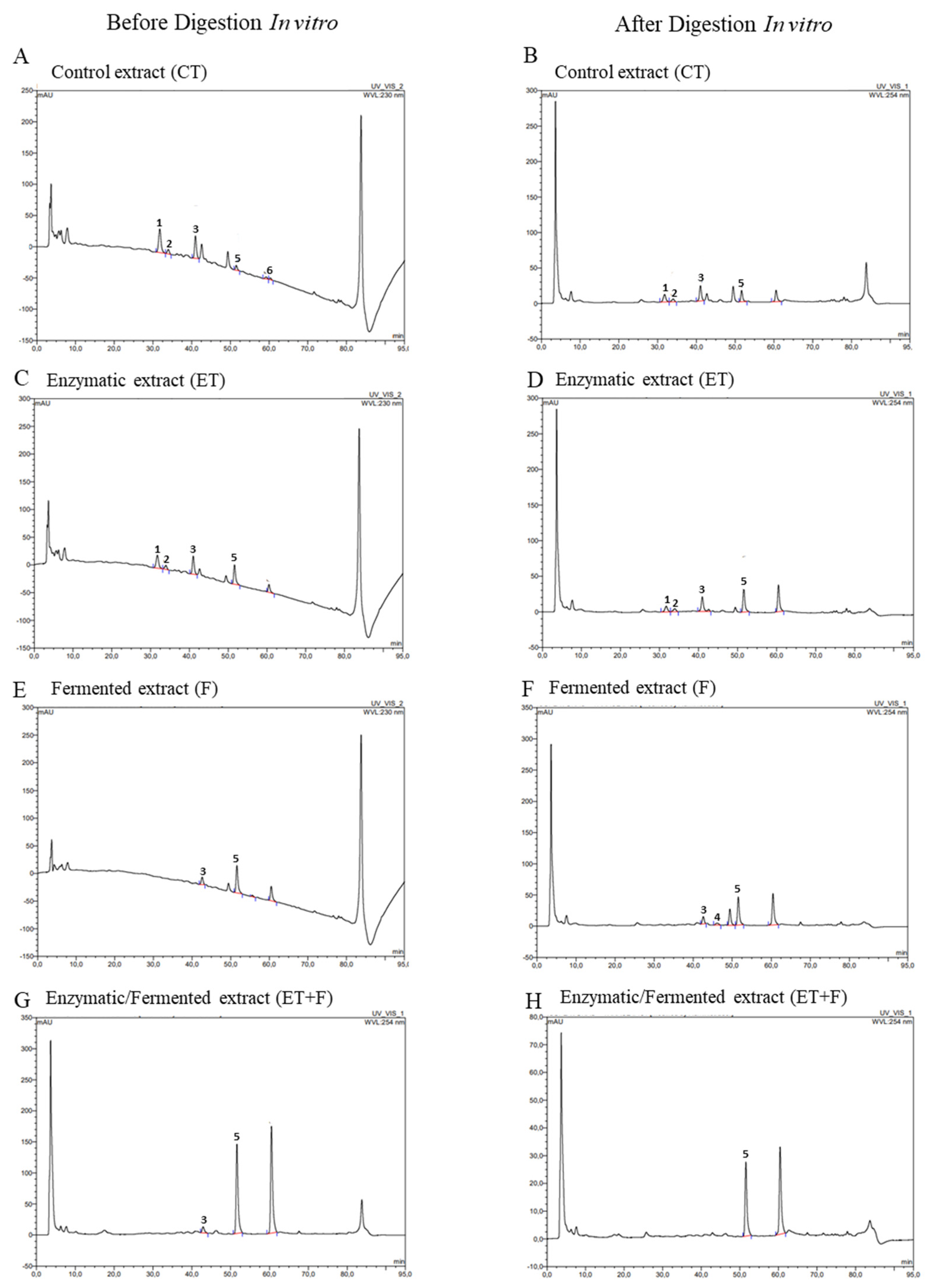
3.1. Profiling of bioactive isoflavones
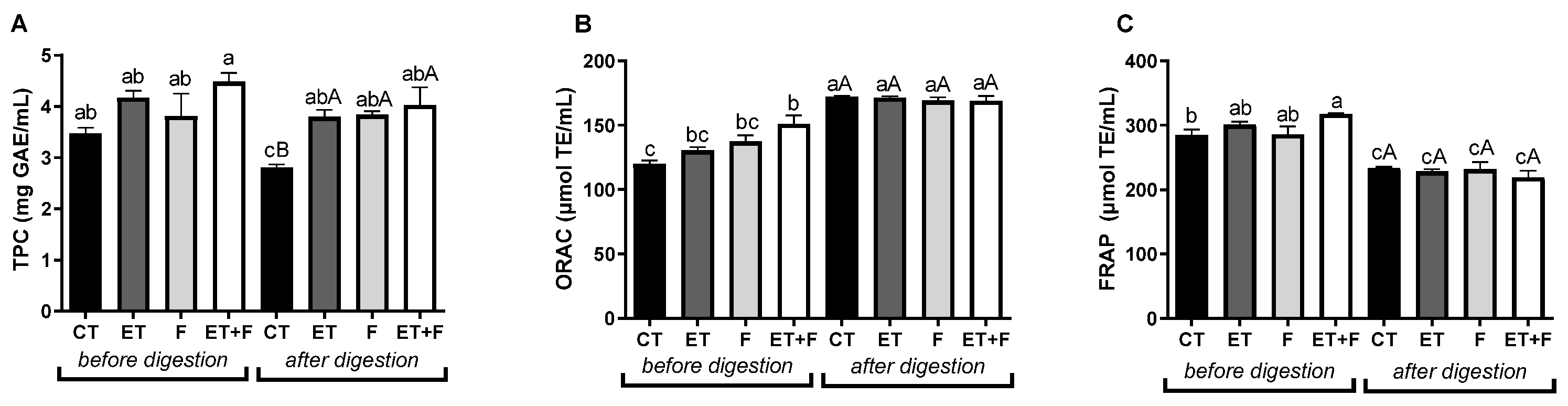
3.2. Phenolic compounds and antioxidant activity of soymilk extracts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ningtyas, D.W.; Hati, S.; Prakash, S. Bioconversion and Bioaccessibility of Isoflavones from Sogurt during in Vitro Digestion. Food Chem. 2021, 343, 128553. [CrossRef]
- Leonard, L.M.; Choi, M.S.; Cross, T.W.L. Maximizing the Estrogenic Potential of Soy Isoflavones Through the Gut Microbiome: Implication for Cardiometabolic Health in Postmenopausal Women. Nutrients 2022, 14. [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [CrossRef]
- Gaya, P.; Peirotén, Á.; Medina, M.; Landete, J.M. Isoflavone Metabolism by a Collection of Lactic Acid Bacteria and Bifidobacteria with Biotechnological Interest. Int. J. Food Sci. Nutr. 2016, 67, 117–124. [CrossRef]
- Decroos, K.; Vanhemmens, S.; Cattoir, S.; Boon, N.; Verstraete, W. Isolation and Characterisation of an Equol-Producing Mixed Microbial Culture from a Human Faecal Sample and Its Activity under Gastrointestinal Conditions. Arch. Microbiol. 2005, 183, 45–55. [CrossRef]
- de Queirós, L.D.; Macedo, J.A.; Macedo, G.A. A New Biotechnological Process to Enhance the Soymilk Bioactivity. Food Sci. Biotechnol. 2016, 25, 763–770. [CrossRef]
- Chun, J.; Jeong, W.J.; Kim, J.-S.; Lim, J.; Park, C.-S.; Kwon, D.Y.; Choi, I.; Kim, J.H. Hydrolysis of Isoflavone Glucosides in Soymilk Fermented with Single or Mixed Cultures of Lactobacillus Paraplantarum KM, Weissella Sp. 33, and Enterococcus Faecium 35 Isolated from Humans. J. Microbiol. Biotechnol. 2008, 18, 573–578.
- Fujita, A.; Alencar, S.M.; Park, Y.K. Conversion of Isoflavone Glucosides to Aglycones by Partially Purified β-Glucosidases from Microbial and Vegetable Sources. Appl. Biochem. Biotechnol. 2015, 176, 1659–1672. [CrossRef]
- Ribeiro, A.E.; Monteiro, N.E.S.; Moraes, A.V.G. De; Costa-Paiva, L.H.; Pedro, A.O. Can the Use of Probiotics in Association with Isoflavone Improve the Symptoms of Genitourinary Syndrome of Menopause? Results from a Randomized Controlled Trial. Menopause 2019, 26, 643–652. [CrossRef]
- Genova, V.M.; Fernandes, A.C.F.; Hiramatsu, É.; Queirós, L.D.; Macedo, J.A.; Macedo, G.A. Biotransformed Antioxidant Isoflavone Extracts Present High-Capacity to Attenuate the in Vitro Formation of Advanced Glycation End Products. Food Biotechnol. 2021, 35, 50–66. [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food-an International Consensus. Food Funct. 2014, 5, 1113–1124. [CrossRef]
- de Ávila, A.R.A.; de Queirós, L.D.; Ueta, T.M.; Macedo, G.A.; Macedo, J.A. Exploring in Vitro Effects of Biotransformed Isoflavones Extracts: Antioxidant, Antiinflammatory, and Antilipogenic. J. Food Biochem. 2019, 43, 1–9. [CrossRef]
- de Ávila, A.R.A.; de Queirós, L.D.; Lopes, D.B.; Barin, C.G.; Ueta, T.M.; Ruiz, A.L.T.G.; Macedo, G.A.; Macedo, J.A. Enhanced Estrogenic Effects of Biotransformed Soy Extracts. J. Funct. Foods 2018, 48, 117–124. [CrossRef]
- Williams, C.F.; Walton, G.E.; Jiang, L.; Plummer, S.; Garaiova, I.; Gibson, G.R. Comparative Analysis of Intestinal Tract Models. Annu. Rev. Food Sci. Technol. 2015, 6, 329–350. [CrossRef]
- de Queirós, L.D.; Dias, F.F.G.; de Ávila, A.R.A.; Macedo, J.A.; Macedo, G.A.; Leite Nobrega de Moura Bell, J.M. Effects of Enzyme-Assisted Extraction on the Profile and Bioaccessibility of Isoflavones from Soybean Flour. Food Res. Int. 2021, 147. [CrossRef]
- da Silva Fernandes, M.; Sanches Lima, F.; Rodrigues, D.; Handa, C.; Guelfi, M.; Garcia, S.; Ida, E.I. Evaluation of the Isoflavone and Total Phenolic Contents of Kefir-Fermented Soymilk Storage and after the in Vitro Digestive System Simulation. Food Chem. 2017, 229, 373–380. [CrossRef]
- Baú, T.R.; Ida, E.I. Soymilk Processing with Higher Isoflavone Aglycone Content. Food Chem. 2015, 183, 161–168. [CrossRef]
- Nakai, S.; Fujita, M.; Kamei, Y. Health Promotion Effects of Soy Isoflavones. J. Nutr. Sci. Vitaminol. (Tokyo). 2020, 66, 502–507. [CrossRef]
- de Queirós, L.D.; de Ávila, A.R.A.; Botaro, A.V.; Chirotto, D.B.L.; Macedo, J.A.; Macedo, G.A. Combined Isoflavones Biotransformation Increases the Bioactive and Antioxidant Capacity of Soymilk. Appl. Microbiol. Biotechnol. 2020, 104, 10019–10031. [CrossRef]
- Tsangalis, D.; Ashton, J.F.; Mcgill, A.E.J.; Shah, N.P. Enzymic Transformation of Isoflavone Phytoestrogens in Soymilk by β-Glucosidase-Producing Bifidobacteria. J. Food Sci. 2002, 67, 3104–3113. [CrossRef]
- Donkor, O.N.; Shah, N.P. Production of β-Glucosidase and Hydrolysis of Isoflavone Phytoestrogens by Lactobacillus Acidophilus, Bifidobacterium Lactis, and Lactobacillus Casei in Soymilk. J. Food Sci. 2008, 73, 15–20. [CrossRef]
- Rekha, C.R.; Vijayalakshmi, G. Isoflavone Phytoestrogens in Soymilk Fermented with β-Glucosidase Producing Probiotic Lactic Acid Bacteria. Int. J. Food Sci. Nutr. 2011, 62, 111–120. [CrossRef]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and Free Radical Scavenging Activity of Spondias Pinnata. BMC Complement. Altern. Med. 2008, 8, 1–10. [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22. [CrossRef]
- Yu, X.; Meenu, M.; Xu, B.; Yu, H. Impact of Processing Technologies on Isoflavones, Phenolic Acids, and Antioxidant Capacities of Soymilk Prepared from 15 Soybean Varieties. Food Chem. 2021, 345, 128612. [CrossRef]
- Niyibituronsa, M.; Onyango, A.N.; Gaidashova, S.; Imathiu, S.; Boevre, M. De; Leenknecht, D.; Neirnck, E.; Saeger, S. De; Vermeir, P.; Raes, K. The Growth of Different Probiotic Microorganisms in Soymilk from Different Soybean Varieties and Their Effects on Anti-Oxidant Activity and Oligosaccharide Content. J. Food Res. 2019, 8, 41. [CrossRef]
- Król-Grzymała, A.; Amarowicz, R. Phenolic Compounds of Soybean Seeds from Two European Countries and Their Antioxidant Properties. Molecules 2020, 25, 1–11. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



