Introduction
During pregnancy, major physiological changes occur in every system. Changes occur in the cardiovascular, renal, respiratory, endocrine, metabolic and immune systems to accommodate the developing fetus and to prepare the mother for delivery.1,2 As a result of pregnancy, maternal physiological profiles undergo dynamic and temporal changes.3 For instance, the cardiovascular system changes during pregnancy are profound and begin early in pregnancy, so that cardiac output has increased by 20% by 8 weeks of gestation. The maximum cardiac output occurs around 20-28 weeks of gestation. Blood pressure decreases in early and mid-gestation, but increases to non-pregnancy levels in late gestation.4 Changes in the physiology of pregnancy may also make diagnosis difficult. Understanding the physiological changes during each gestation period can help monitor the occurrence of physiological abnormalities.
Blood pressure measurement and arterial pressure waveform analysis have been used to monitor and study cardiovascular diseases for more than a hundred years.5 In the last two decades, an emerging research method is to measure the arterial pressure waveform at one to two locations and extract the characteristics through the turning point in the time domain. In recent years, augmentation index has also been used in studies of pregnancy and related disorders.6,7 However, the arterial pressure waveform characteristics extracted using time domain analysis are not sufficient to provide the complete information of the arterial pressure waveform. To fully characterize the waveform of arterial pressure waves, harmonic analysis is a feasible method5. The pulse waveform of the radial pressure wave describes the change of arterial pressure with time. Changes in blood flow and organ function can alter the radial pulse waveform.8,9 Harmonic analysis converts waveforms into harmonic indices and provides hemodynamic information about the state of the ventricular-arterial system.10,11 Many studies have shown that radial pulse waveforms provide an independent predictor of cardiovascular variability.12 In recent years, we have also found in clinical studies that the harmonic component is associated with the onset of menopause, pregnancy13 and diabetes14 in women.
In addition, several studies have found that the harmonic component of radial pulse or photoplethysmography (PPG) harmonic analysis is highly correlated with disease or treatment [
15]. the PPG signal is generated by changes in blood volume as blood moves in and out of tissues, whereas the pulse signal is generated by changes in pressure as blood passes through the radial artery.
15 Both signals are products of blood circulation and both have similar waveforms and waveform periods. PPG measurements have also been used in the assessment of maternal hemodynamics.
16 In previous clinical studies, PPG harmonic analysis was found to be useful in predicting the success of implantable reproduction.
17 In this study, the PPG signal will be used for harmonic analysis.
Understanding the hemodynamic changes in the different stages of pregnancy can help to distinguish abnormal states during pregnancy. Harmonic analysis may be a useful tool in informing the state of the ventricular-arterial system during pregnancy. The aim of this study was to investigate the specific statistical relationship between changes in harmonic indices during different stages of pregnancy. Simple PPG measurements with harmonic frequency analysis may provide additional insights in terms of blood circulation during these periods of transition.
Materials and Methods
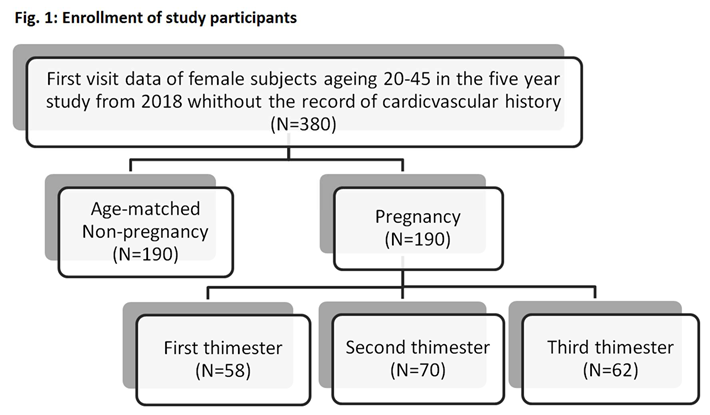
A total of 380 women (190 pregnant women and 190 age-matched non-pregnant women) between the ages of 20 and 45 and without cardiovascular history were participated in this non-invasive observational study at the Department of Gynecology and Obstetrics of the Renai Branch of Taipei City Hospital and Keelung Hospital of the Ministry of Health and Welfare from 2018 May to 2022 March. All participants were provided with written informed consent. The individual information of participants is protected in accordance with the provisions of the Personal Information Protection Act. The study protocol followed good clinical and scientific practice. and was approved by the Institutional Review Board of Taipei City Hospital (IRB number: TCHIRB-10703121-E). As Figure 1 showed, the study size was recorded and all population were separated to the dataset of non-pregnancy group and dataset of pregnancy group, according to pregnancy status. To address the potential bias caused by age, subjects who were age-matched and not pregnant were selected as the control group. In this cross-sectional study, enrolled participants underwent blood pressure and finger photoplethysmography measurements. In order to compare the hemodynamic status of different subjects, we recorded the systolic and diastolic blood pressure and calculated the first five harmonic amplitudes components (C1~C5) and phases (P1~P5) of PPG signals using Fourier transform method.
Each subject was asked to rest for five minutes before the measurements. The PPG signal was recorded on the index finger of both hands with a PPG sensor (AFE4400SPO2EVM, TI, USA). After completing the PPG measurement, the blood pressure and heart rate of each subject were measured using an automatic blood pressure monitor (HBP-1300, OmRon, Japan). A trained operator conducted blood pressure measurements and instructions were followed to avoid the white coat effect. The continuous pulse data was collected in 12 seconds with a sampling rate of 200 data points per second. These data were then converted into harmonic amplitude components (Cn) and harmonic phases (Pn) using Fourier series calculation.
18 Cn and Pn are defined by the following equation:
where 1≦n≦5, and A
n,m and θ
n,m are the amplitude and phase of the nth harmonic of the mth pulse within one measurement. A
0,m is the mean value of the mth pulse. P(t) is a wave function with a period T.
18 In addition to the harmonic indices (Cn, Pn, n=1~5), other demographic characteristics and hemodynamic variables were also recorded, including age, body mass index (BMI), waist Circumference, diastolic blood pressure (DBP), systolic blood pressure (SBP) and heart rate (HR).
The pregnancy status was diagnosed by a specialist doctor and confirmed from the ICD-10 codes18, O, Z32.1 and Z34, on the medical record. The dataset coded with O00 were from those subjects who experienced complications of pregnancy, such as an ectopic pregnancy, and these data were excluded in the analysis. Pregnancy is divided into three trimesters, each lasting for approximately three months. The first trimester is defined as the first 13 weeks of pregnancy, the second trimester is defined as 14-26 weeks of pregnancy, and the third trimester is after 27 weeks of pregnancy.
Clinical characteristics are expressed as mean ± standard deviation. Student’s t-test was used to evaluate the difference of basic demographic information and hemodynamic variables based on the status. The logistic regression analysis was used to determine the associations between pregnancy and harmonics indexes and the odds ratios. Age, BMI, waist circumference, HR, SBP and DBP were controlled by acting as the covariates in the logistic regression model. p-value smaller than 0.05 was accepted as statistically significant. Missing data is addressed by removing the missing data. All statistical analyses were performed using R version 4.0.3
Results
Age also changes specific harmonic components of radial pressure wave.
19 To eliminate the confounding effect of age in pregnancy, we randomly selected and matched pregnancy and non-pregnancy woman by age. During the pregnancy, the subject’s hemodynamic data were measured at the first visit during the study and the subjects can be at different stage of the pregnancy. As
Table 1 showed, a series of changes in clinical characteristics could be observed during the pregnancy, including lowering DBP, increasing heart rate and waist circumference. SBP and DBP have their lowest values during the second trimester and return to their original values towards the end of pregnancy. HR and waist circumference continue to increase until the end of pregnancy.
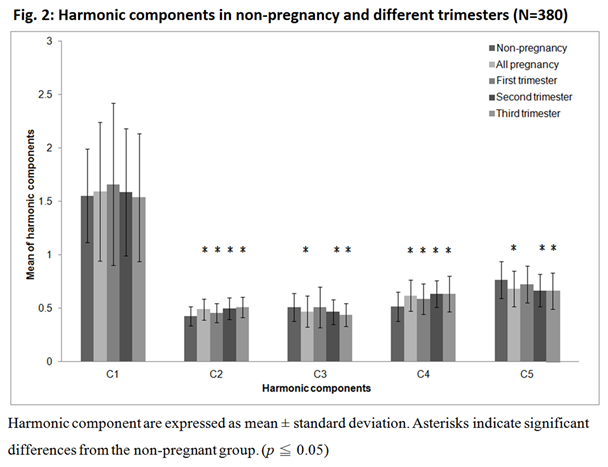
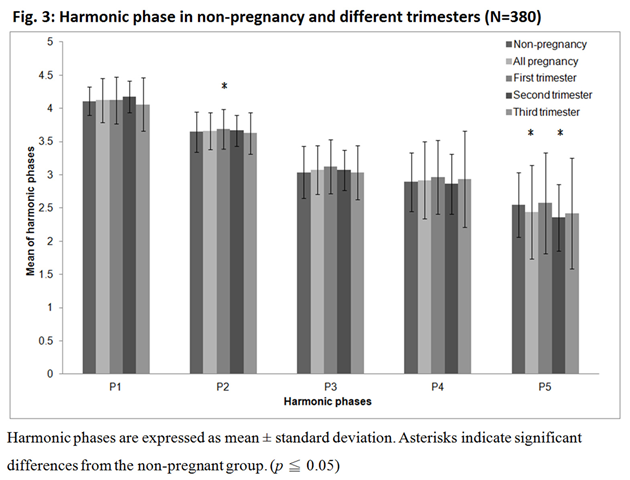
Figure 2 showed that C2 and C4 increase significantly during pregnancy and increase with the stage of gestation period. Compared to non-pregnancy (C2=0.429, C4=0.517), from the first to the third trimester, C2 increased from 0.459 to 0.510 and C4 from 0.587 to 0.634. In contrast, C3 and C5 decreased significantly during pregnancy, especially in the second and third trimesters (C3 from 0.512 to 0.439 and C5 from 0.724 to 0.664). On the other hand, Figure 3 showed the variation of the harmonic phase at different trimesters. P2 increased significantly in the first trimester, while P5 decreased significantly in the first and third trimesters.
To investigate the association between pregnancy and the harmonic indices, we apply the logistic regression of the generalized linear model. As
Table 2 showed that after controlling age, BMI, waist circumference, HR, SBP and DBP, C2, C4 and P3 were positively and P1 and P4 were negatively associated with pregnancy. Odds ratios obtained from logistic regression describe positively association of waist circumference (OR = 1.075, CI = 1.034 - 1.121, p < 0.001), HR (OR = 1.031, CI = 1.007 - 1.058, p = 0.013), C2 (OR = 1.083, CI = 1.026 - 1.150, p = 0.006), C4 (OR = 1.054, CI = 1.021 - 1.088, p = 0.001) and P3 (OR = 1.351, CI = 1.066 - 1.758, p = 0.016) with pregnancy, and negatively association of BMI (OR = 0.851, CI = 0.762 - 0.948, p = 0.004), DBP (OR = 0.960, CI = 0.919 - 0.998, p = 0.049), P1 (OR = 0.696, CI = 0.501 - 0.914, p = 0.016) and P4 (OR = 0.784, CI = 0.639 - 0.955, p = 0.016) with pregnancy.
Discussion
As a complex physiological condition, pregnancy involves the integration of multiple regulatory and organ systems.1 In this study, it was found that HR continued to increase until the end of pregnancy, while SBP and DBP decreased and then recovered. It was consistent with the findings of other studies.6,20 The gradual increased heart rate is mainly to maintain the increased cardiac output during the third trimester. Cardiac output gradually increased by 50% during pregnancy.20 Cardiac stroke volume increased and reached a peak in the second trimester. The continuous increased heart rate was mainly to maintain the increased cardiac output in the third trimester.20 There were many mechanisms that caused a decrease in systemic vascular resistance during pregnancy. In addition to the effects of various female hormones such as estrogen, progesterone, prostaglandins and prolactin, nitric oxide and relaxin also had an impact.1 Relaxin reached peak levels during early pregnancy and delivery and worked on the heart, smooth muscle and connective tissue to reduce systemic vascular resistance, which also helped in delivery at full term.21
The results of this study found an increase in C2 and C4 with increasing gestation. C2 has been previously found to be associated with renal circulation,8 which is consistent with some clinical findings that renal blood flow increases by more than 50% during pregnancy.1 The hormonal effects of progesterone and relaxin during pregnancy cause systemic vasodilation22 and concomitant renal vasodilatation, resulting in a compensatory increase in glomerular filtration rate (GFR) and effective renal plasma flow (RPF).2 Although the renin-angiotensin-aldosterone (RAA) system is activated in early gestation, it also produces a relative resistance to angiotensin II, counteracting the vasoconstrictor effect and enabling profound vasodilation.2 The vasoreactivity of angiotensin II may also be the same as that of other vasoconstrictors such as adrenergic agonists and arginine vasopressin (AVP).2 This is consistent with previous studies of the effects of vasoconstrictor drugs on harmonic components.23 C4 has been found to be associated with pulmonary circulation in previous studies,24 and associated with peripheral vascular relaxation in studies of hypertensive drugs.25 These are consistent with clinical findings of increased pulmonary circulation26 and decreased vascular resistance21 during pregnancy. Oxygen consumption increases by 30% to 60% (30-40 mL/min) during pregnancy due to increased metabolic demands of maternal organs, placenta and fetus.26,27 Elevated serum progesterone levels in early pregnancy stimulate the medullary respiratory center in the brain and provide increased respiratory depth, thus increasing alveolar ventilation.27 The physiological adaptations of the cardiovascular vessels permit optimal oxygen delivery to maternal and fetal tissues. In our previous animal studies, we found that C3 and C5 are associated with the digestive system.8 C3 has also been shown to be associated with diabetes mellitus.13 During pregnancy, the adaptation of glucose metabolism maintains adequate maternal nutrition while allowing glucose shunting to the fetus to facilitate development.28 The decrease in C3 and C5 may provide another hemodynamic explanation for the high risk of diabetes mellitus and morning sickness during pregnancy. In addition, an increase in C2 and C4 coinciding with a decrease in C3 and C5 is a characteristic of hemodynamics during pregnancy and may serve as a predictor of pregnancy.
The harmonic phase corresponds physiologically to the same organ or tissue as the harmonic component. The harmonic component describes the changes in the distribution of blood flow to specific organs and tissues in the body. The harmonic phase describes changes in the structure of specific organs and tissues. Both P2 and C2 are associated with the kidneys, and the significant increase in P2 at the onset of pregnancy corroborates the observations of other studies in clinical settings that kidney physiology may be the first to change during pregnancy.22 P5 is significantly reduced in the second trimester, showing that the growing uterus displaces the stomach and intestines upward as pregnancy progresses. The mechanical changes in the digestive tract that occur as pregnancy progresses are due to the growth of the uterus. Increasing upward displacement of the stomach results in axial changes and increased intra-gastric pressure. There is also a decrease in esophageal sphincter tension, which may lead to reflux symptoms as well as nausea and vomiting.29
Radial pulse measurement with harmonic analysis has been proven to be useful in the hemodynamic description of many physiological phenomena and in the prediction of disease risk.10,12 The PPG signal is a signal that detects the change of blood flow pulsation in the blood vessel due to heartbeat by using the principle of light sensor to absorb light energy. The PPG signal has been shown to be highly correlated with the radial arterial pressure signal in many studies.15 In this study, the PPG signal was measured from the index finger and further harmonics analysis was performed. The changes in the harmonic components are consistent with the observations of clinical physiological phenomena1,2 and also correspond to the results of radial pulse harmonic analysis.14 PPG measurement has the advantage of being portable and easy to operate, so that users can take measurements at home at any time to understand the state of the body. When the indicators are abnormal, relevant examination and treatment can be carried out immediately. However, there are still some parts of this study that could be enhanced. The PPG measurements are subject to external interference, such as power interference, skin or finger tremors. The signal interference may also be caused by the different force of the sensor connection point during the measurement.30 In addition, the results of this study were conducted on Taiwanese females, and it is worthwhile to further investigate whether there are differences among different ethnic groups.
In conclusions, pregnancy is characterized by major maternal hemodynamic changes. The results of this study provide information on the changes in the harmonic frequency components during the various stages of pregnancy. Understanding the baseline values of physiological changes in pregnancy can help diagnose other disorders during pregnancy. The harmonic component of the PPG signal has independent predictive value for changes in a woman's physiology during pregnancy. Non-invasive PPG measurements can be performed more frequently and easily. In the future , the hemodynamic effects of more pregnancy-related diseases such as gestational diabetes mellitus, gestational hypertension, toxemia of pregnancy (preeclampsia), eclampsia, etc., will be investigated by harmonic analysis.
Funding/Support
This research was supported by Keelung Hospital of the Ministry of Health and Welfar (111-01).
Acknowledgments
The authors acknowledge that the Mii-Ann Medical Research Center provided Photoplethysmography measurement equipment in this collaborative project and both Taipei City Hospital Renai Branch and Keelung Hospital of the Ministry of Health and Welfare supported professional medical staff and venue.
Financial Disclosure/Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin. 2012; 30: 317–329.
- Soma-Pillay P, Nelson-Piercy C, Tolppanen H, Mebazaa A. Physiological changes in pregnancy. Cardiovasc J Afr. 2016; 27: 89–94.
- Granger, JP. Maternal and fetal adaptations during pregnancy: Lessons in regulatory and integrative physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:1289–1292.
- Mabie WC, DiSessa TG, Crocker LG, Sibai BM, Arheart KL. A longitudinal study of cardiac output in normal human pregnancy. Am J Obstet Gynecol 1994;170:849–856.
- Milnor, WR. Hemodynamics. Baltimore; Williams & Wilkins, 1982. [Google Scholar]
- van der Graaf AM, Zeeman GG, Groen H, Roberts C, Dekker GA. Non-invasive assessment of maternal hemodynamics in early pregnancy. Pregnancy Hypertens. 2013; 3(4):261-269.
- Khalil AA, Cooper DJ, Harrington KF, Pulse wave analysis: a preliminary study of a novel technique for the prediction of pre-eclampsia. BJOG 2009; 116(2):268-276.
- Young ST, Wang WK, Chang L, Kuo TS. The filter properties of the arterial beds of organs in rats. Acta Physiol Scand. 1992;145(4):401-406.
- Lin Wang YY, Chang SL, Wu YE, Hsu TL, Wang WK. Resonance - the missing phenomenon in hemodynamics. Circ Res. 1991;69(1):246-249.
- Wang YL, Wang WK. Did you know how the cardiovascular system achieves its high efficiency as a compound irrigation device and why this is relevant to future cardiovascular studies. Acta Physiol (Oxf). 2019;226(4):e13206.
- Lin Wang YY, Wang WK. A hemodynamics model to study the collective behavior of the ventricular-arterial system. Journal of Applied Physics. 2013;113(2):024702.
- Liao KM, Chang CW, Wang SH, Chang YT, Chen YC, Wang GC, Risk assessment of macrovascular and microvascular events in patients with type 2 diabetes by analyzing the amplitude variation of the fourth harmonic component of radial pulse wave. Physiol Rep. 2019;7(19):e14252.
- Chen CY, Liao KM, Wang SH, Chen SC, Chang CJ, Wang TC, Wang GC. Non-Invasive radial pressure wave analysis may digitally predict women’s risks of type 2 diabetes (T2DM): A case and control group study. PLoS One. 2021; 6(10):e0259269.
- Chen CY, Wang SH, Chen SC, Chang CJ, Wang TC, Wang GC. Noninvasively measured radial pressure wave analysis provides insight into cardiovascular changes during pregnancy and menopause. Taiwan J Obstet Gynecol. 2021;60(5):888-893.
- Hsiu H, Hsu CL, Chen CT, Hsu WC, Hu HF, Chen FC, Correlation of harmonic components between the blood pressure and photoplethysmography waveforms following local-heating stimulation. Int. J Biosci Biochem Bioinform. 2012; 2(4):248-253.
- von Wowern E, Källén K, Olofsson P. Arterial stiffness in normal pregnancy as assessed by digital pulse wave analysis by photoplethysmography - A longitudinal study. Pregnancy Hypertens. 2019;15:51-56.
- Chang CW, Xie DN, Wang SJ, et al. Hemodynamic status from photoplethysmography, signs of successful pregnancy. Circulation Research. 2019;125:A177.
- Katznelson Y, An Introduction to Harmonic Analysis. Cambridge, U.K.: Cambridge Univ. Press, 2004.
- Wang SH, Hsu TL, Jan MY, Lin Wang YY, Wang WK. Age-related changes in specific harmonic indices of pressure pulse waveform. ICBME Proceedings, 2009;23:183-185.
- Robson SC, Hunter S, Boys RJ, et. al. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 1989; 256:H1060-H1065.
- Bryant-Greenwood GD Schwabe C, Human relaxins: chemistry and biology. Endocr Rev 1994;15:5-26.
- Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis. 2013;20(3):209–214.
- Hsu TL, Chao PT, Hsiu H, Wang WK, Li SP, Wang YY. Organ-specific ligation-induced changes in harmonic components of the pulse spectrum and regional vasoconstrictor selectivity in Wistar rats. Exp Physiol. 2006;91(1):163-170.
- Wu TJ, Chen JJ, Shiah DC, Lee SM, Lee KC, Lee CY, Wang WK. Pulse analysis for different lungs. Journal of Chinese Medical Sciences. 2000.1(1):1-7.
- Wang SH, Jan MY, Wang WK, Lin Wang YY. Effects of antihypertensive drugs on specific harmonic indices of the pulse waveform in normotensive Wistar Kyoto rats. Clin Exp Hypertens, 2012;34(1):74-78.
- Rees GB, Broughton Pipkin F, Symonds EM, Patrick JM. A longitudinal study of respiratory changes in normal human pregnancy with cross-sectional data on subjects with pregnancy-induced hypertension. Am J Obstet Gynecol. 1990 Mar;162(3):826-30.
- Crapo, RO. Normal cardiopulmonary physiology during pregnancy. Clin Obstet Gynecol. 1996 Mar;39(1):3-16.
- Angueira AR, Ludvik AE, Reddy TE, Wicksteed B. et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327–334.
- Koch, KL. Gastrointestinal factors in nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2002;186(5 Suppl Understanding):S198–203.
- Teng XF, Zhang YT, “The effect of contacting force on photoplethysmographic signals”, Physiol Meas, 2004; 1323-1335.
Table 1.
Clinical characteristics from pregnancy and non-pregnancy woman.
Table 1.
Clinical characteristics from pregnancy and non-pregnancy woman.
| Clinical characteristics |
Non-pregnancy |
Pregnancy |
| All pregnancy |
First trimester |
Second trimester |
Third trimester |
| N |
190 |
190 |
58 |
70 |
62 |
| Age (year) |
32.8±4.8 |
32.8±4.8 |
34.2±4.5 * |
32.2±4.6 |
32.3±5.1 |
| BMI (kg/m2) |
23.1±4.7 |
23.0±3.8 |
22.8±3.9 |
22.9±3.7 |
23.4±4.0 |
| Waist Circumference (cm) |
76.6±11.7 |
80.7±10.7 * |
76.8±9.5 |
81.3±10.4 * |
83.7±11.3 * |
| Heart rate (Beat/minute) |
78.5±12.4 |
85.7±13.9 * |
84.0±16.1 * |
85.7±13.0 * |
87.2±12.8 * |
| SBP (mm-Hg) |
112.5±16.3 |
111.1±12.0 |
109.8±11.7 |
108.1±10.6 * |
115.2±12.7 |
| DBP (mm-Hg) |
70.3±11.6 |
67.0±9.1 * |
66.5±9.4 * |
65.6±7.5 * |
69.1±10.1 |
Table 2.
Logistic regression analysis for pregnancy.
Table 2.
Logistic regression analysis for pregnancy.
| |
estimate |
StandardError (SE) |
P-value |
Odds Ratio(OR) |
Confidence Interval of OR |
| (Intercept) |
2.593 |
4.978 |
0.602 |
|
|
| Age (year) |
0.040 |
0.031 |
0.194 |
1.041 |
0.980-1.106 |
| BMI (kg/m2) |
-0.161 |
0.056 |
0.004 * |
0.851 |
0.762-0.948 |
| Waist Circumference (cm) |
0.030 |
0.012 |
< 0.001 * |
1.075 |
1.034-1.121 |
| Heart rate (Beat/minute) |
1.806 |
0.728 |
0.013 * |
1.031 |
1.007-1.058 |
| SBP (mmHg) |
0.001 |
0.015 |
0.930 |
1.001 |
0.973-1.032 |
| DBP (mmHg) |
-0.041 |
0.021 |
0.049 * |
0.960 |
0.919-0.998 |
| C1 |
0.005 |
0.005 |
0.284 |
1.005 |
0.996-1.015 |
| C2 |
0.080 |
0.029 |
0.006 * |
1.083 |
1.026-1.150 |
| C3 |
-0.019 |
0.026 |
0.458 |
0.981 |
0.932-1.034 |
| C4 |
0.052 |
0.016 |
0.001 * |
1.054 |
1.021-1.088 |
| C5 |
0.021 |
0.013 |
0.112 |
1.021 |
0.995-1.048 |
| P1 |
-0.363 |
0.151 |
0.016 * |
0.696 |
0.501-0.914 |
| P2 |
-0.063 |
0.159 |
0.694 |
0.939 |
0.691-1.296 |
| P3 |
0.301 |
0.125 |
0.016 * |
1.351 |
1.066-1.758 |
| P4 |
-0.244 |
0.102 |
0.016 * |
0.784 |
0.639-0.955 |
| P5 |
0.063 |
0.059 |
0.286 |
1.065 |
0.939-1.196 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).








