Submitted:
14 July 2023
Posted:
17 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
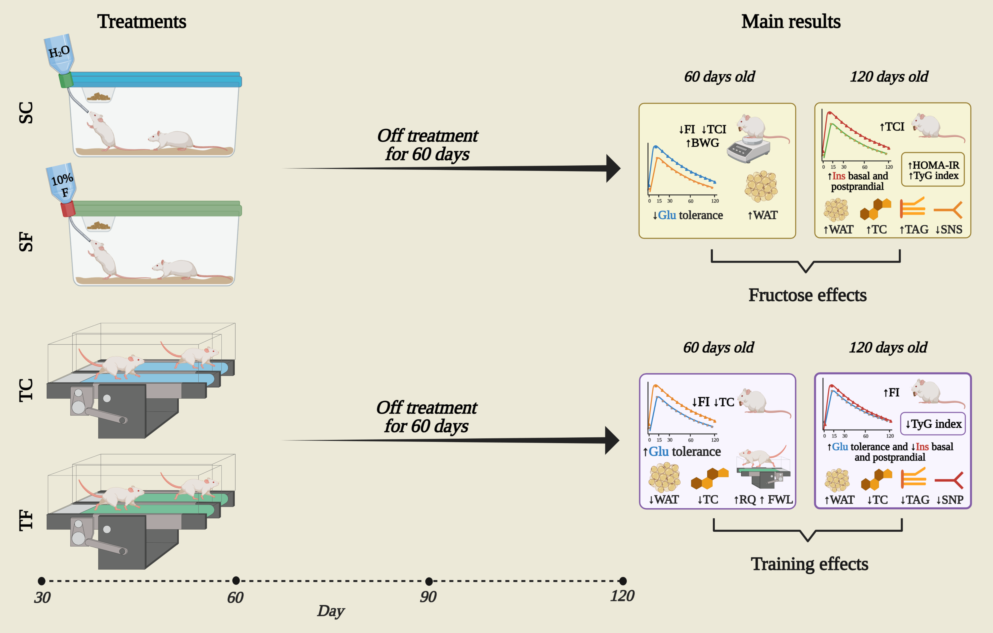
2. Results
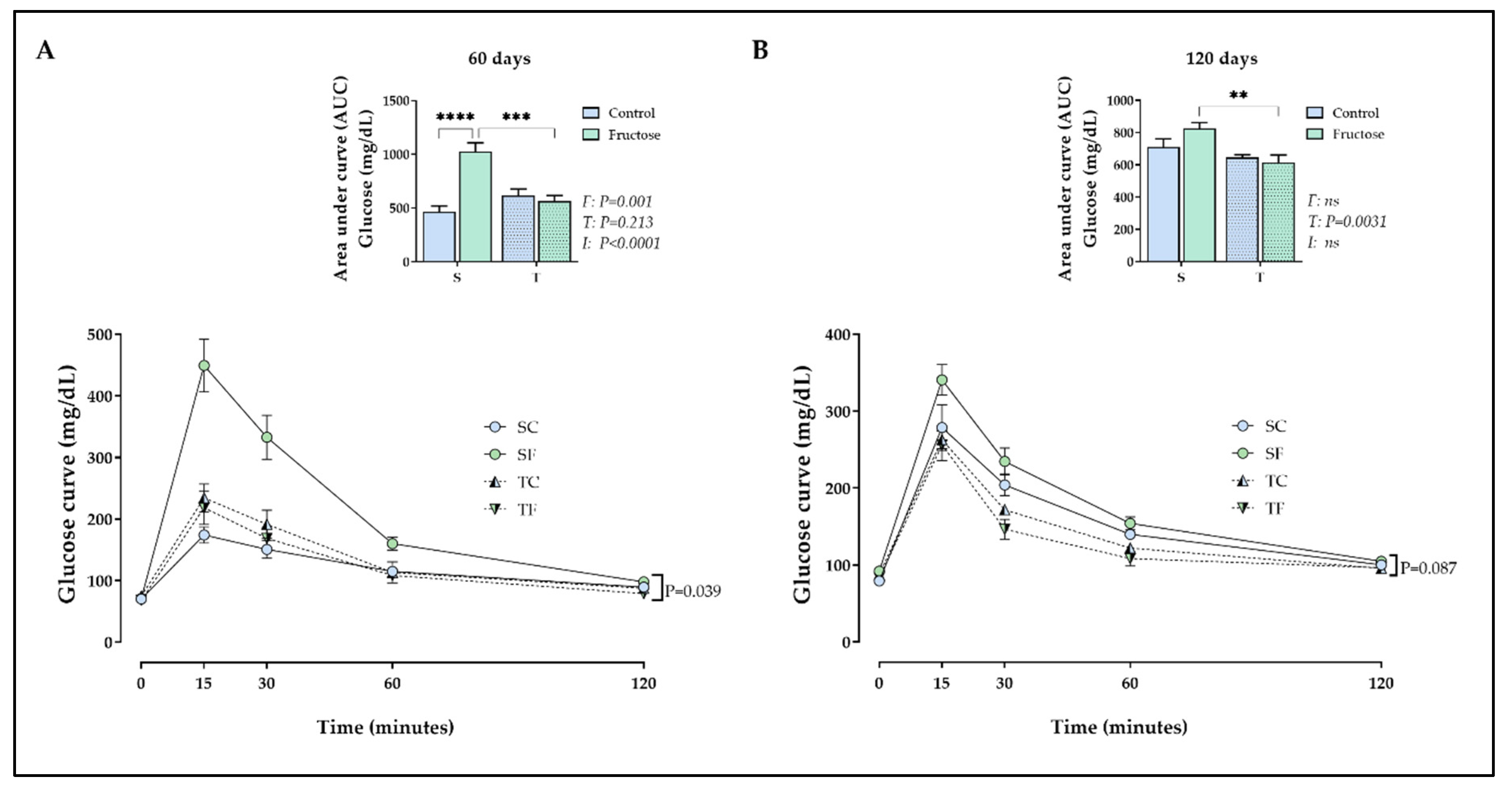
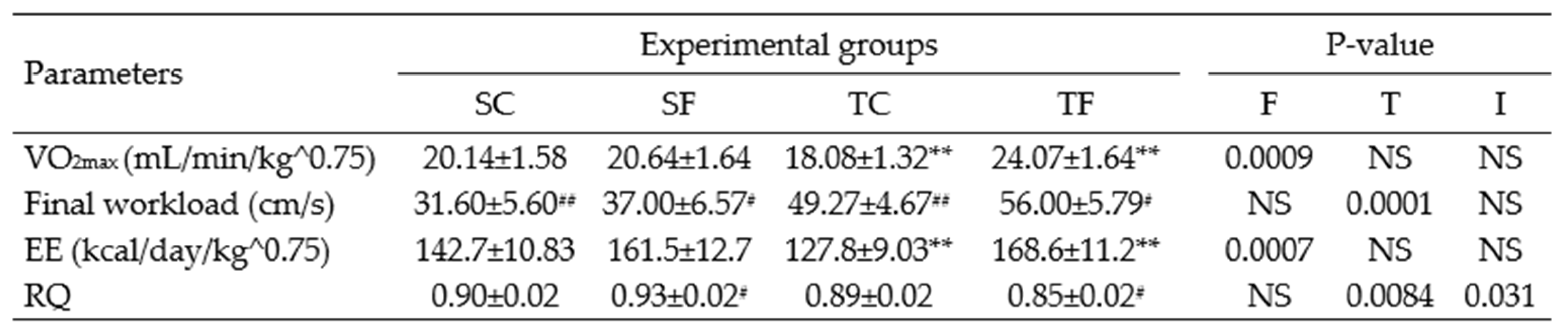
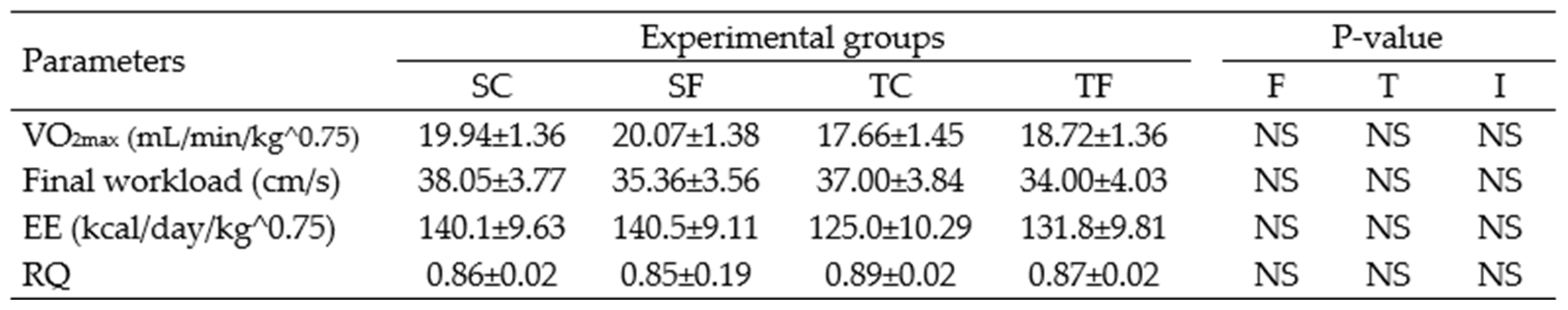
2.1. Oximetry and treadmill parameters
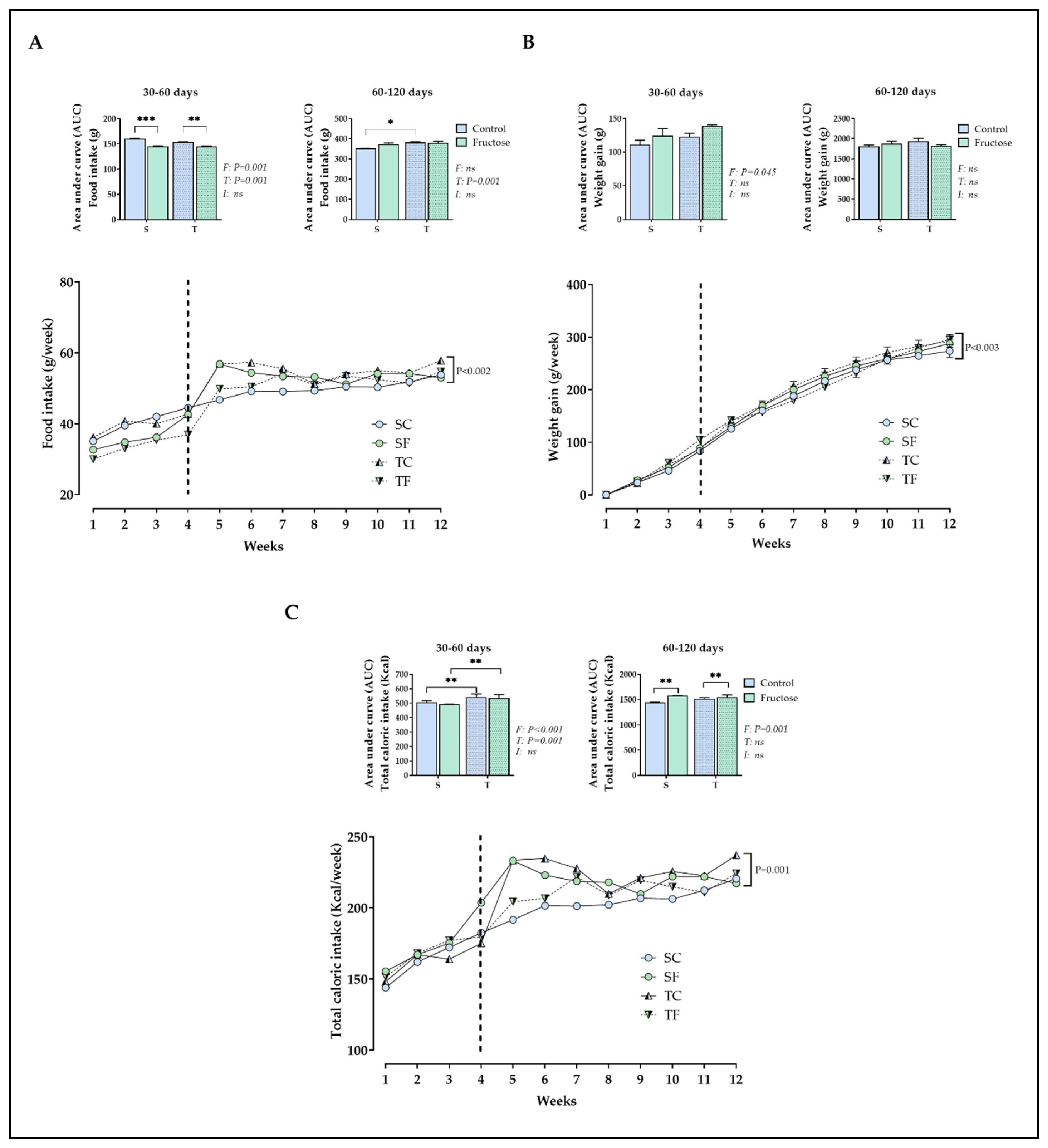
2.2. Biometric parameters
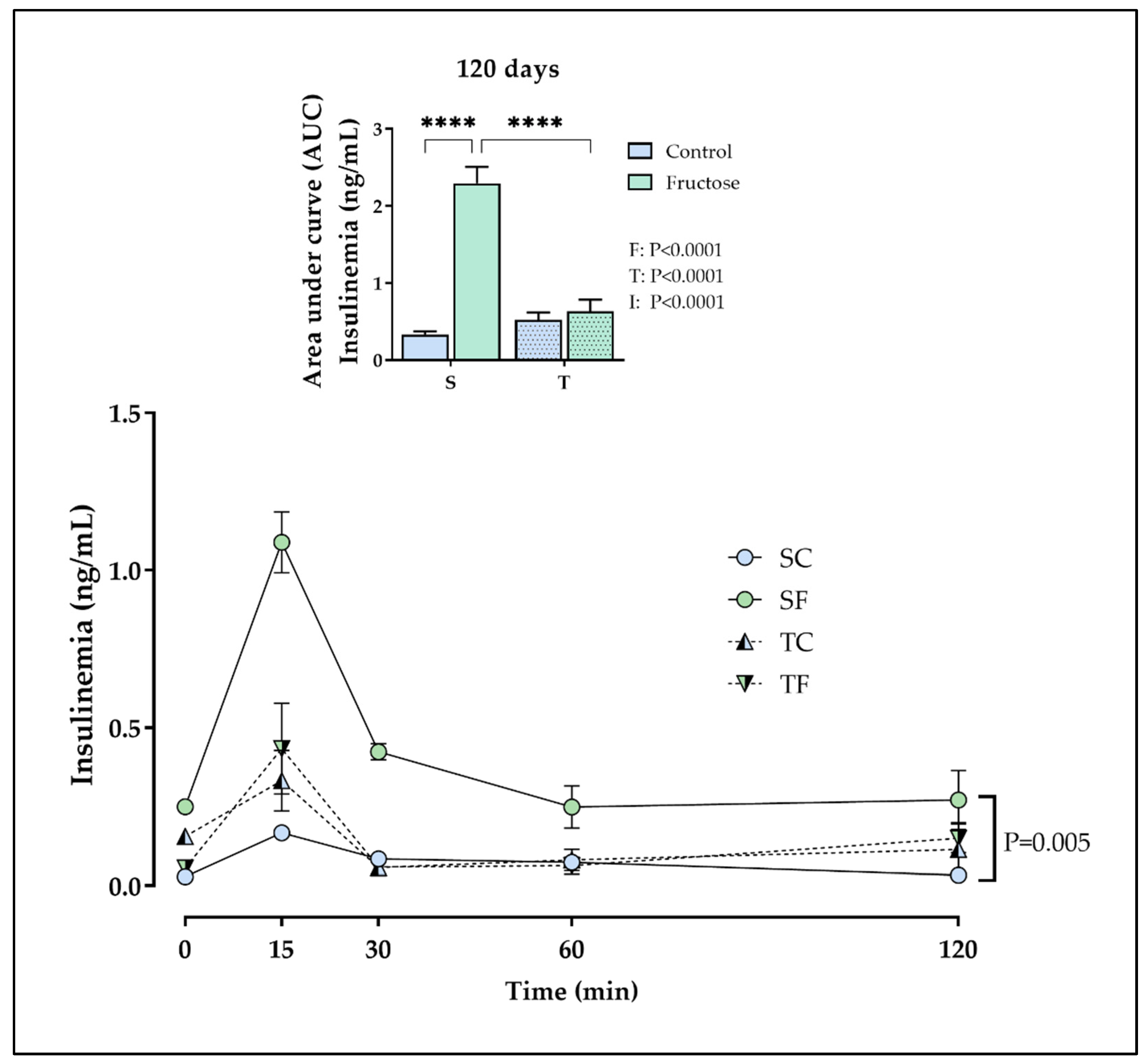
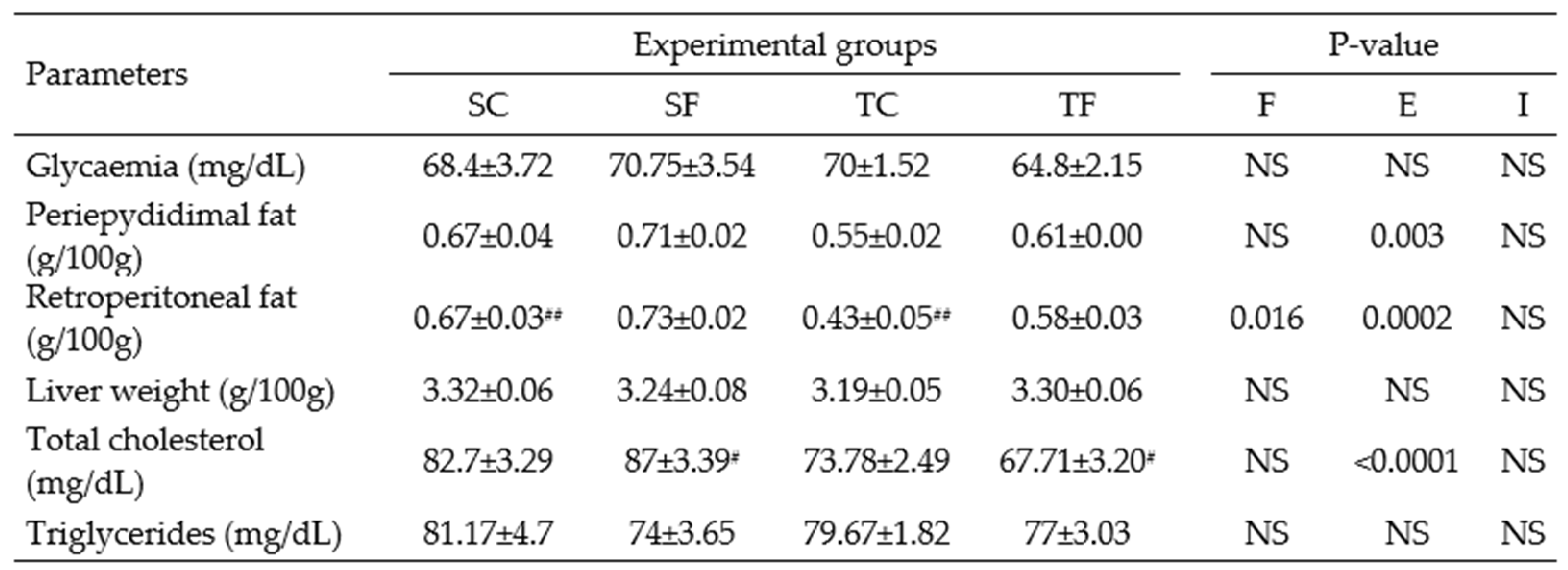
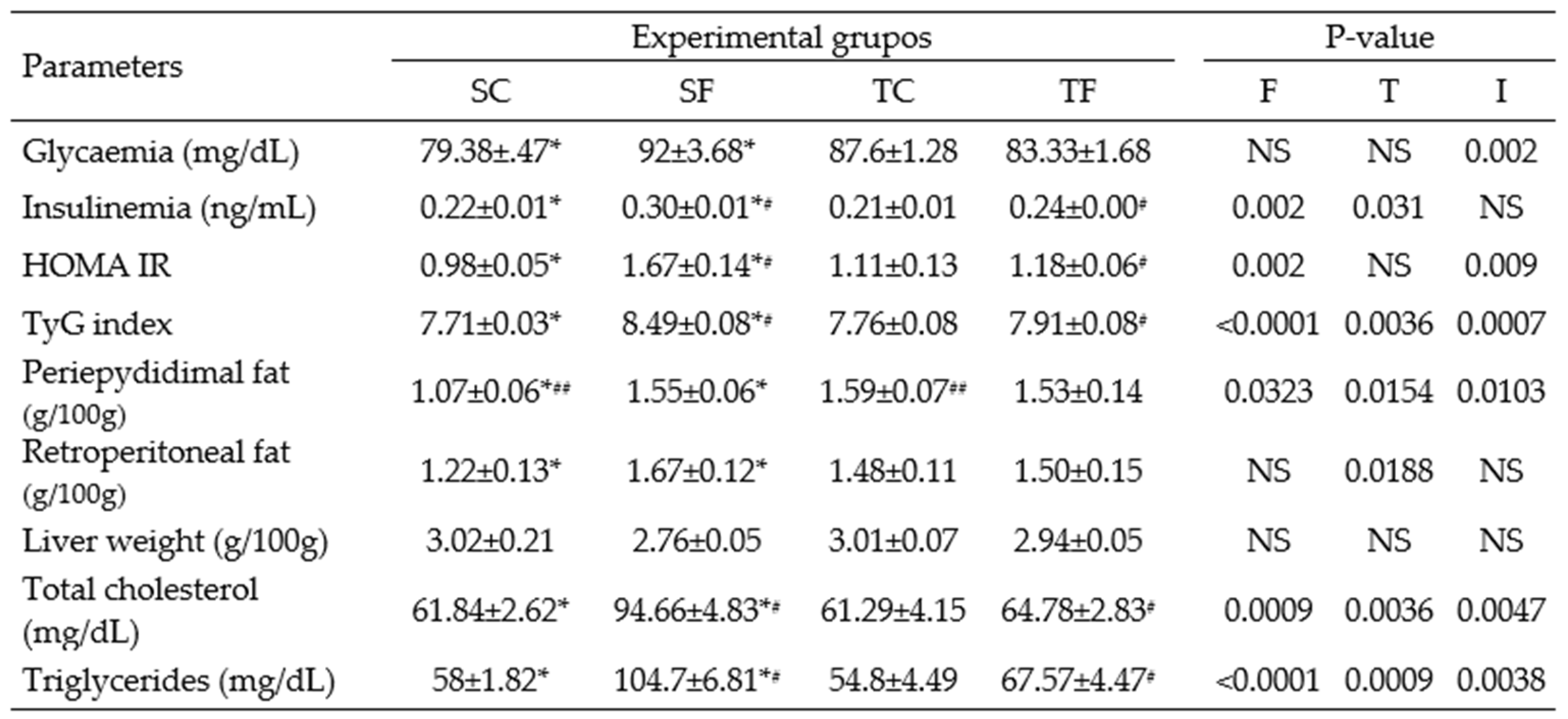
2.3. Tissues and plasma parameters
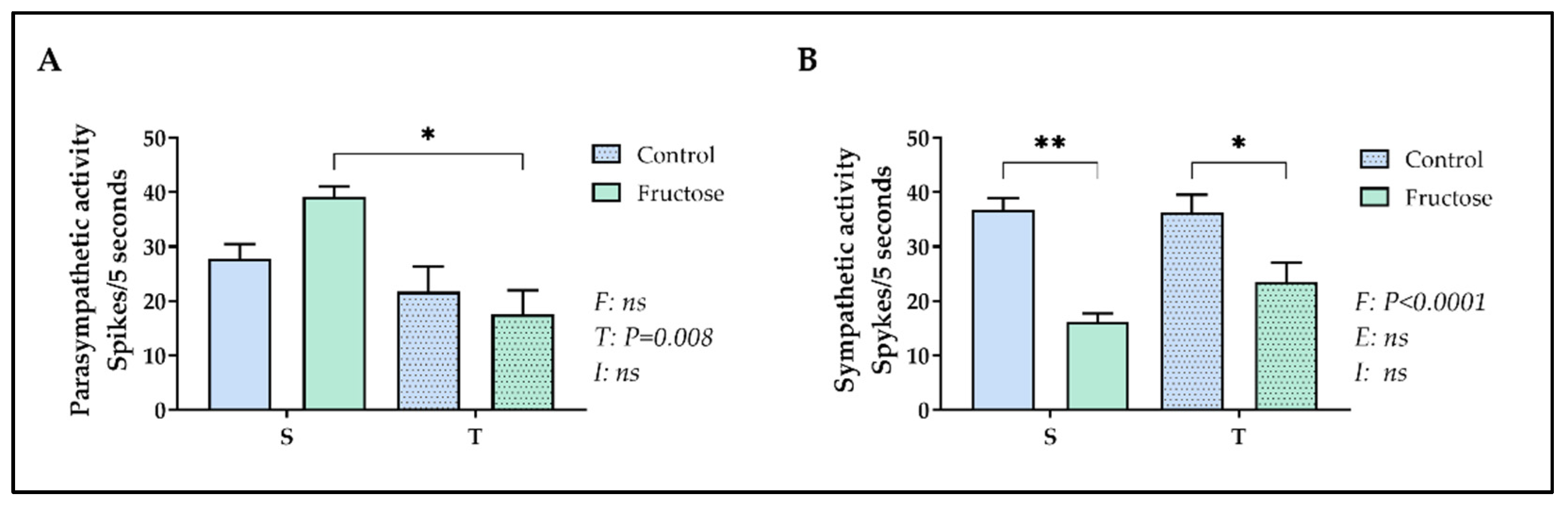
2.4. Parasympathetic and sympathetic electrical nerve activity
3. Discussion
4. Materials and Methods
4.1. Animals and experimental design
4.2. Preparation of fructose drinking water
4.3. Effort test and physical training
4.4. Biometric parameters
4.5. Tissues and plasma parameters
4.6. Intraperitoneal glucose tolerance test (ipGTT)
4.7. Parasympathetic and sympathetic electrical nerve activity
4.8. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amaral, F.; Lima, N.E.A.; Ornelas, E.; Simardi, L.; Fonseca, F.L.A.; Maifrino, L.B.M. Effect of Different Exercise Intensities on the Pancreas of Animals with Metabolic Syndrome. Diabetes, Metab. Syndr. Obes. Targets Ther. 2015, 8, 115–120. [Google Scholar] [CrossRef]
- Frantz, E.D.C.; Prodel, E.; Braz, I.D.; Giori, I.G.; Bargut, T.C.L.; Magliano, D.C.; da Nobrega, A.C.L. Modulation of the Renin-Angiotensin System in White Adipose Tissue and Skeletal Muscle: Focus on Exercise Training. Clin. Sci. 2018, 132, 1487–1507. [Google Scholar] [CrossRef] [PubMed]
- Bargut, T.C.L.; Santos, L.P.; Machado, D.G.L.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Eicosapentaenoic Acid (EPA) vs. Docosahexaenoic Acid (DHA): Effects in Epididymal White Adipose Tissue of Mice Fed a High-Fructose Diet. Prostaglandins Leukot. Essent. Fat. Acids 2017, 123, 14–24. [Google Scholar] [CrossRef]
- Mamikutty, N.; Thent, Z.C.; Sapri, S.R.; Sahruddin, N.N.; Mohd Yusof, M.R.; Haji Suhaimi, F. The Establishment of Metabolic Syndrome Model by Induction of Fructose Drinking Water in Male Wistar Rats. Biomed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lebda, M.A.; Tohamy, H.G.; El-Sayed, Y.S. Long-Term Soft Drink and Aspartame Intake Induces Hepatic Damage via Dysregulation of Adipocytokines and Alteration of the Lipid Profile and Antioxidant Status. Nutr. Res. 2017, 41, 47–55. [Google Scholar] [CrossRef] [PubMed]
- White, A.H.; James, S.A.; Paulson, S.W.; Beebe, L.A. Sugar Sweetened Beverage Consumption among Adults with Children in the Home. Front. Nutr. 2018, 5, 1–7. [Google Scholar] [CrossRef]
- de Oliveira, J.C.; Lisboa, P.C.; de Moura, E.G.; Barella, L.F.; Miranda, R.A.; Malta, A.; da Silva Franco, C.C.; da Silva Ribeiro, T.A.; Torrezan, R.; Gravena, C.; et al. Poor Pubertal Protein Nutrition Disturbs Glucose-Induced Insulin Secretion Process in Pancreatic Islets and Programs Rats in Adulthood to Increase Fat Accumulation. J. Endocrinol. 2013, 216, 195–206. [Google Scholar] [CrossRef]
- Trombini, A.B.; Franco, C.C.S.; Miranda, R.A.; de Oliveira, J.C.; Barella, L.F.; Prates, K. V.; de Souza, A.A.; Pavanello, A.; Malta, A.; Almeida, D.L.; et al. Early Treatment with Metformin Induces Resistance against Tumor Growth in Adult Rats. Cancer Biol. Ther. 2015, 16, 958–964. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Beedle, A.S. Early Life Events and Their Consequences for Later Disease: A Life History and Evolutionary Perspective. Am. J. Hum. Biol. 2007, 19, 1–19. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in Utero and Early-Life Conditions on Adult Health and Disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef]
- Barker, D.J.P. The Developmental Origins of Chronic Adult Disease. Acta Paediatr. 2007, 93, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, C.A.; Erthal, R.P.; Ogo, F.M.; Peres, M.N.C.; Vieira, H.R.; Conejo, C.; Tófolo, L.P.; Francisco, F.A.; Silveira, S. da S.; Malta, A.; et al. A High Fat Diet during Adolescence in Male Rats Negatively Programs Reproductive and Metabolic Function Which Is Partially Ameliorated by Exercise. Front. Physiol. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.R.O.; Ribeiro, M.V.G.; Peres, M.N.C.; Piovan, S.; Gonçalves, G.D.; Saavedra, L.P.J.; Martins, J.N. de L.; Junior, M.D.F.; Cavalcante, K.V.N.; Lopes, G. kian G.; et al. Protein Restriction in the Peri-Pubertal Period Induces Autonomic Dysfunction and Cardiac and Vascular Structural Changes in Adult Rats. Front. Physiol. 2022, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; McNeill, J.H. Fructose-Induced Hypertension in Rats Is Concentration- and Duration-Dependent. J. Pharmacol. Toxicol. Methods 1995, 33, 101–107. [Google Scholar] [CrossRef]
- Maiztegui, B.; Morelli, M.I.; Raschia, M.A.; Del Zotto, H.; Gagliardino, J.J. Islet Adaptive Changes to Fructose-Induced Insulin Resistance: β-Cell Mass, Glucokinase, Glucose Metabolism, and Insulin Secretion. J. Endocrinol. 2009, 200, 139–149. [Google Scholar] [CrossRef]
- de Moura, R.F.; Ribeiro, C.; de Oliveira, J.A.; Stevanato, E.; de Mello, M.A.R. Metabolic Syndrome Signs in Wistar Rats Submitted to Different High-Fructose Ingestion Protocols. Br. J. Nutr. 2009, 101, 1178–1184. [Google Scholar] [CrossRef]
- Sadowska, J.; Bruszkowska, M. Comparing the Effects of Sucrose and High-Fructose Corn Syrup on Lipid Metabolism and the Risk of Cardiovascular Disease in Male Rats. Acta Sci. Pol. Technol. Aliment. 2017, 16, 231–240. [Google Scholar] [CrossRef]
- Castro, M.C.; Francini, F.; Schinella, G.; Caldiz, C.I.; Zubiría, M.G.; Gagliardino, J.J.; Massa, M.L. Apocynin Administration Prevents the Changes Induced by a Fructose-Rich Diet on Rat Liver Metabolism and the Antioxidant System. Clin. Sci. 2012, 123, 681–692. [Google Scholar] [CrossRef]
- Francini, F.; Castro, M.C.; Gagliardino, J.J.; Massa, M.L. Regulation of Liver Glucokinase Activity in Rats with Fructose-Induced Insulin Resistance and Impaired Glucose and Lipid Metabolism. Can. J. Physiol. Pharmacol. 2009, 87, 702–710. [Google Scholar] [CrossRef]
- Kindlovits, R.; Bertoldi, J.M.C.R.J.; Rocha, H.N.M.; Bento-Bernardes, T.; Gomes, J.L.P.; de Oliveira, E.M.; Muniz, I.C.; Pereira, J.F.; Fernandes-Santos, C.; Rocha, N.G.; et al. Molecular Mechanisms Underlying Fructose-Induced Cardiovascular Disease: Exercise, Metabolic Pathways and MicroRNAs. Exp. Physiol. 2021, 106, 1224–1234. [Google Scholar] [CrossRef]
- Steenson, S.; Umpleby, A.M.; Lovegrove, J.A.; Jackson, K.G.; Fielding, B.A. Role of the Enterocyte in Fructose-induced Hypertriglyceridaemia. Nutrients 2017, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Frantz, E.D.C.; Medeiros, R.F.; Giori, I.G.; Lima, J.B.S.; Bento-Bernardes, T.; Gaique, T.G.; Fernandes-Santos, C.; Fernandes, T.; Oliveira, E.M.; Vieira, C.P.; et al. Exercise Training Modulates the Hepatic Renin–Angiotensin System in Fructose-Fed Rats. Exp. Physiol. 2017, 102, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Soares de Alencar Mota, C.; Ribeiro, C.; de Araújo, G.G.; de Araújo, M.B.; de Barros Manchado-Gobatto, F.; Voltarelli, F.A.; de Oliveira, C.A.M.; Luciano, E.; de Mello, M.A.R. Exercise Training in the Aerobic/Anaerobic Metabolic Transition Prevents Glucose Intolerance in Alloxan-Treated Rats. BMC Endocr. Disord. 2008, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mostarda, C.; Rogow, A.; Moraes Silva, I.C.; De La Fuente, R.N.; Jorge, L.; Rodrigues, B.; Heeren, M. V.; Caldini, E.G.; De Angelis, K.; Irigoyen, M.C. Benefits of Exercise Training in Diabetic Rats Persist after Three Weeks of Detraining. Auton. Neurosci. Basic Clin. 2009, 145, 11–16. [Google Scholar] [CrossRef]
- Tófolo, L.P.; da Silva Ribeiro, T.A.; Malta, A.; Miranda, R.A.; Gomes, R.M.; de Oliveira, J.C.; Abdennebi-Najar, L.; de Almeida, D.L.; Trombini, A.B.; da Silva Franco, C.C.; et al. Short-Term Moderate Exercise Provides Long-Lasting Protective Effects against Metabolic Dysfunction in Rats Fed a High-Fat Diet. Eur. J. Nutr. 2014, 54, 1353–1362. [Google Scholar] [CrossRef]
- Lehnen, A.M.; Leguisamo, N.M.; Pinto, G.H.; Markoski, M.M.; de Angelis, K.; Machado, U.F.; Schaan, B. The Beneficial Effects of Exercise in Rodents Are Preserved after Detraining: A Phenomenon Unrelated to GLUT4 Expression. Cardiovasc. Diabetol. 2010, 9, 1–8. [Google Scholar] [CrossRef]
- Hötting, K.; Röder, B. Beneficial Effects of Physical Exercise on Neuroplasticity and Cognition. Neurosci. Biobehav. Rev. 2013, 37, 2243–2257. [Google Scholar] [CrossRef]
- Moreira, V.M.; Franco, C.C. da S.; Prates, K.V.; Gomes, R.M.; de Moraes, A.M.P.; Ribeiro, T.A.; Martins, I.P.; Previate, C.; Pavanello, A.; Matiusso, C.C.I.; et al. Aerobic Exercise Training Attenuates Tumor Growth and Reduces Insulin Secretion in Walker 256 Tumor-Bearing Rats. Front. Physiol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Moreira, V.M.; Almeida, D.; da Silva Franco, C.C.; Gomes, R.M.; Palma-Rigo, K.; Prates, K.V.; Tófolo, L.P.; Malta, A.; Francisco, F.A.; Pavanello, A.; et al. Moderate Exercise Training since Adolescence Reduces Walker 256 Tumour Growth in Adult Rats. J. Physiol. 2019, 597, 3905–3925. [Google Scholar] [CrossRef]
- Sulis, P.M.; Motta, K.; Barbosa, A.M.; Besen, M.H.; Da Silva, J.S.; Nunes, E.A.; Rafacho, A. Impact of Fish Oil Supplementation and Interruption of Fructose Ingestion on Glucose and Lipid Homeostasis of Rats Drinking Different Concentrations of Fructose. Biomed Res. Int. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Vasiljević, A.; Bursać, B.; Djordjevic, A.; Milutinović, D.V.; Nikolić, M.; Matić, G.; Veličković, N. Hepatic Inflammation Induced by High-Fructose Diet Is Associated with Altered 11βHSD1 Expression in the Liver of Wistar Rats. Eur. J. Nutr. 2014, 53, 1393–1402. [Google Scholar] [CrossRef]
- Ghezzi, A.C.; Cambri, L.T.; Ribeiro, C.; Botezelli, J.D.; Mello, M.A. Impact of Early Fructose Intake on Metabolic Profile and Aerobic Capacity of Rats. Lipids Health Dis. 2011, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.J.; Gonzalez, J.T.; van Loon, L.J.C. Fructose Co-Ingestion to Increase Carbohydrate Availability in Athletes. J. Physiol. 2019, 597, 3549–3560. [Google Scholar] [CrossRef] [PubMed]
- Moraes-Silva, I.C.; Mostarda, C.; Dias Moreira, E.; Silva, K.A.S.; dos Santos, F.; de Angelis, K.; de Moura Azevedo Farah, V.; Irigoyen, M.C. Preventive Role of Exercise Training in Autonomic, Hemodynamic, and Metabolic Parameters in Rats under High Risk of Metabolic Syndrome Development. J. Appl. Physiol. 2013, 114, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Vettorazzi, J.F.; Kurauti, M.A.; Soares, G.M.; Borck, P.C.; Ferreira, S.M.; Branco, R.C.S.; Michelone, L.D.S.L.; Boschero, A.C.; Junior, J.M.C.; Carneiro, E.M. Bile Acid TUDCA Improves Insulin Clearance by Increasing the Expression of Insulin-Degrading Enzyme in the Liver of Obese Mice. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Dupas, J.; Feray, A.; Goanvec, C.; Guernec, A.; Samson, N.; Bougaran, P.; Guerrero, F.; Mansourati, J. Metabolic Syndrome and Hypertension Resulting from Fructose Enriched Diet in Wistar Rats. Biomed Res. Int. 2017. [Google Scholar] [CrossRef]
- Dupas, J.; Feray, A.; Guernec, A.; Pengam, M.; Inizan, M.; Guerrero, F.; Mansourati, J.; Goanvec, C. Effect of Personalized Moderate Exercise Training on Wistar Rats Fed with a Fructose Enriched Water. Nutr. Metab. (Lond). 2018, 15, 1–12. [Google Scholar] [CrossRef]
- Dengel, D.R.; Pratley, R.E.; Hagberg, J.M.; Rogus, E.M.; Goldberg, A.P. Distinct Effects of Aerobic Exercise Training and Weight Loss on Glucose Homeostasis in Obese Sedentary Men. J. Appl. Physiol. 1996, 81, 318–325. [Google Scholar] [CrossRef]
- Vasques, A.C.J.; Novaes, F.S.; de Oliveira, M. da S.; Matos Souza, J.R.; Yamanaka, A.; Pareja, J.C.; Tambascia, M.A.; Saad, M.J.A.; Geloneze, B. TyG Index Performs Better than HOMA in a Brazilian Population: A Hyperglycemic Clamp Validated Study. Diabetes Res. Clin. Pract. 2011, 93, e98–e100. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Yoffe, P.; Hills, N.; Lustig, R.H. The Relationship of Sugar to Population-Level Diabetes Prevalence: An Econometric Analysis of Repeated Cross-Sectional Data. PLoS One 2013, 8, e57873. [Google Scholar] [CrossRef]
- Page, K.A.; Chan, O.; Arora, J.; Belfort-Deaguiar, R.; Dzuira, J.; Roehmholdt, B.; Cline, G.W.; Naik, S.; Sinha, R.; Constable, R.T.; et al. Effects of Fructose vs Glucose on Regional Cerebral Blood Flow in Brain Regions Involved with Appetite and Reward Pathways. JAMA - J. Am. Med. Assoc. 2013, 309, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming Fructose-Sweetened, Not Glucose-Sweetened, Beverages Increases Visceral Adiposity and Lipids and Decreases Insulin Sensitivity in Overweight/Obese Humans. J. Clin. Invest. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tian, Y.; Zuo, Y.; Zhang, X.; Gao, Y.; Wang, P.; Sun, L.; Zhang, H.; Liang, H. Nicotinamide Riboside Ameliorates High-Fructose-Induced Lipid Metabolism Disorder in Mice via Improving FGF21 Resistance in the Liver and White Adipose Tissue. Food Funct. 2022, 13, 12400–12411. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Díazcouder, A.; Romero-Nava, R.; Carbó, R.; Sánchez-Lozada, L.G.; Sánchez-Muñoz, F. High Fructose Intake and Adipogenesis. Int. J. Mol. Sci. 2019, 20, 2787. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.S.; Burgado, J.; Kelly, S.D.; Johnson, Z.P.; Neigh, G.N. High-Fructose Diet during Periadolescent Development Increases Depressive-like Behavior and Remodels the Hypothalamic Transcriptome in Male Rats. Psychoneuroendocrinology 2015, 62, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H. Fructose and Nonalcoholic Fatty Liver Disease. J Calif Dent Assoc 2016, 44, 613–617. [Google Scholar] [CrossRef]
- Munetsuna, E.; Yamada, H.; Yamazaki, M.; Ando, Y.; Mizuno, G.; Ota, T.; Hattori, Y.; Sadamoto, N.; Suzuki, K.; Ishikawa, H.; et al. Maternal Fructose Intake Disturbs Ovarian Estradiol Synthesis in Rats. Life Sci. 2018, 202, 117–123. [Google Scholar] [CrossRef]
- Almeida, D.L.; Moreira, V.M.; Cardoso, L.E.; Junior, M.D.F.; Pavanelo, A.; Ribeiro, T.A.; da Silva Franco, C.C.; Tófolo, L.P.; Peres, M.N.C.; Ribeiro, M.V.G.; et al. Lean in One Way, in Obesity Another: Effects of Moderate Exercise in Brown Adipose Tissue of Early Overfed Male Wistar Rats. Int. J. Obes. 2022, 46, 137–143. [Google Scholar] [CrossRef]
- Almeida, D.L.; Fabricio, G.S.; Tófolo, L.P.; Ribeiro, T.A.; Matiusso, C.C.I.; Ribeiro, M.V.G.; Oliveira Ferreira, A.R.; Pavanello, A.; Malta, A.; Palma-Rigo, K.; et al. Early Postnatal Overnutrition Impairs VO2max Gains with Moderate Exercise and Increase Post-Exercise Muscle Damage in Adult Male Rats. J. Dev. Orig. Health Dis. 2022, 13, 406–410. [Google Scholar] [CrossRef]
- Ribeiro, T.A.; Tófolo, L.P.; Martins, I.P.; Pavanello, A.; De Oliveira, J.C.; Prates, K.V.; Miranda, R.A.; Da Silva Franco, C.C.; Gomes, R.M.; Francisco, F.A.; et al. Maternal Low Intensity Physical Exercise Prevents Obesity in Offspring Rats Exposed to Early Overnutrition. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Barella, L.F.; de Oliveira, J.C.; Branco, R.C.S.; Camargo, R.L.; Gomes, R.M.; Mendes, F.C.V.; Miranda, R.A.; Gravena, C.; Torrezan, R.; Grassiolli, S.; et al. Early Exposure to a High-Fat Diet Has More Drastic Consequences on Metabolism Compared with Exposure during Adulthood in Rats. Horm. Metab. Res. 2012, 44, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; York, D.A. The MONA LISA Hypothesis in the Time of Leptin. Recent Prog. Horm. Res. 1998, 53, 95–117. [Google Scholar]
- Messina, G.; de Luca, V.; Viggiano, A.; Ascione, A.; Iannaccone, T.; Chieffi, S.; Monda, M. Autonomic Nervous System in the Control of Energy Balance and Body Weight: Personal Contributions. Neurol. Res. Int. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Barateiro, A.; Mahú, I.; Domingos, A.I. Leptin Resistance and the Neuro-Adipose Connection. Front. Endocrinol. (Lausanne). 2017, 8, 1–4. [Google Scholar] [CrossRef]
- Bray, G.A. Obesity, a Disorder of Nutrient Partitioning: The MONA LISA Hypothesis. J. Nutr. 1991, 121, 1146–1162. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.; Dudfield, M. What Is the Relationship between Exercise and Metabolic Abnormalities? A Review of the Metabolic Syndrome. Sport. Med. 2004, 34, 371–418. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Ellacott, K.L.J.; King, V.L.; Hasty, A.H. Mouse Models of the Metabolic Syndrome. Dis. Model. Mech. 2010, 3, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Arias-Mutis, O.J.; Marrachelli, V.G.; Ruiz-Saurí, A.; Alberola, A.; Morales, J.M.; Such-Miquel, L.; Monleon, D.; Chorro, F.J.; Such, L.; Zarzoso, M. Development and Characterization of an Experimental Model of Diet-Induced Metabolic Syndrome in Rabbit. PLoS One 2017, 12, e0178315. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Rosset, R.; Petersen, O.; Gonzalez, J. Health Outcomes of a High Fructose Intake: The Importance of Physical Activity. J. Physiol. 2019, 597, 3561–3571. [Google Scholar] [CrossRef] [PubMed]
- Denke, M.A.; Pasternak, R.C. Defining and Treating the Metabolic Syndrome: A Primer from the Adult Treatment Panel III. Curr. Treat. Options Cardiovasc. Med. 2001, 3, 251–253. [Google Scholar] [CrossRef]
- Ross, R.; Dagnone, D.; Jones, P.J.H.; Smith, H.; Paddags, A.; Hudson, R.; Janssen, I. Reduction in Obesity and Related Comorbid Conditions after Diet-Induced Weight Loss or Exercise-Induced Weight Loss in Men: A Randomized, Controlled Trial. Ann. Intern. Med. 2000, 133, 92–103. [Google Scholar] [CrossRef]
- Gomes, R.M.; Tófolo, L.P.; Rinaldi, W.; Scomparin, D.X.; Grassiolli, S.; Barella, L.F.; de Oliveira, J.C.; Branco, R.C.S.; Agostinho, A.R.; da Silva Ribeiro, T.A.; et al. Moderate Exercise Restores Pancreatic Beta-Cell Function and Autonomic Nervous System Activity in Obese Rats Induced by High-Fat Diet. Cell. Physiol. Biochem. 2013, 32, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Dias, D.; Bernardes, N.; Stoyell-Conti, F.F.; Dos Santos, C.P.; De Araujo, A.A.; Llesuy, S.; Irigoyen, M.C.; De Angelis, K. Impact of Combined Exercise Training on the Development of Cardiometabolic and Neuroimmune Complications Induced by Fructose Consumption in Hypertensive Rats. PLoS One 2020, 15, e0233785. [Google Scholar] [CrossRef]
- du Sert, N.P.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting Animal Research: Explanation and Elaboration for the ARRIVE Guidelines 2.0. PLOS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Wisløff, U.; Helgerud, J.; Kemi, O.J.; Ellingsen, Ø. Intensity-Controlled Treadmill Running in Rats: VO2 Max and Cardiac Hypertrophy. Am. J. Physiol. - Hear. Circ. Physiol. 2001, 280, H1301–H131. [Google Scholar] [CrossRef]
- Jones, J.H. Resource Book for the Design of Animal Exercise Protocols. Am. J. Vet. Res. 2007, 68, 1–137. [Google Scholar] [CrossRef]
- Rodrigues, B.; Figueroa, D.M.; Mostarda, C.T.; Heeren, M. V.; Irigoyen, M.C.; De Angelis, K. Maximal Exercise Test Is a Useful Method for Physical Capacity and Oxygen Consumption Determination in Streptozotocin-Diabetic Rats. Cardiovasc. Diabetol. 2007, 6, 1–7. [Google Scholar] [CrossRef]
- Caponi, P.W.; Lehnen, A.M.; Pinto, G.H.; Borges, J.; Markoski, M.; Machado, U.F.; Schaan, B.D. Aerobic Exercise Training Induces Metabolic Benefits in Rats with Metabolic Syndrome Independent of Dietary Changes. Clinics 2013, 68, 1010–1017. [Google Scholar] [CrossRef]
- Abdulla, M.H.; Sattar, M.A.; Abdullah, N.A.; Khan, M.A.H.; Anand Swarup, K.R.L.; Johns, E.J. The Contribution of A1B-Adrenoceptor Subtype in the Renal Vasculature of Fructose-Fed Sprague-Dawley Rats. Eur. J. Nutr. 2011, 50, 251–260. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Scott, M.L.; Thornley, M.J.; Coombs, R.R.A. Comparison of Red-Cell Linked Anti-IgE and 125I-Labelled Anti-IgE in a Solid-Phase System for the Measurement of IgE Specific for Castor Bean Allergen. Int. Arch. Allergy Immunol. 1981, 64, 230–235. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





