Submitted:
14 July 2023
Posted:
18 July 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. The chemistry of lipid peroxidation
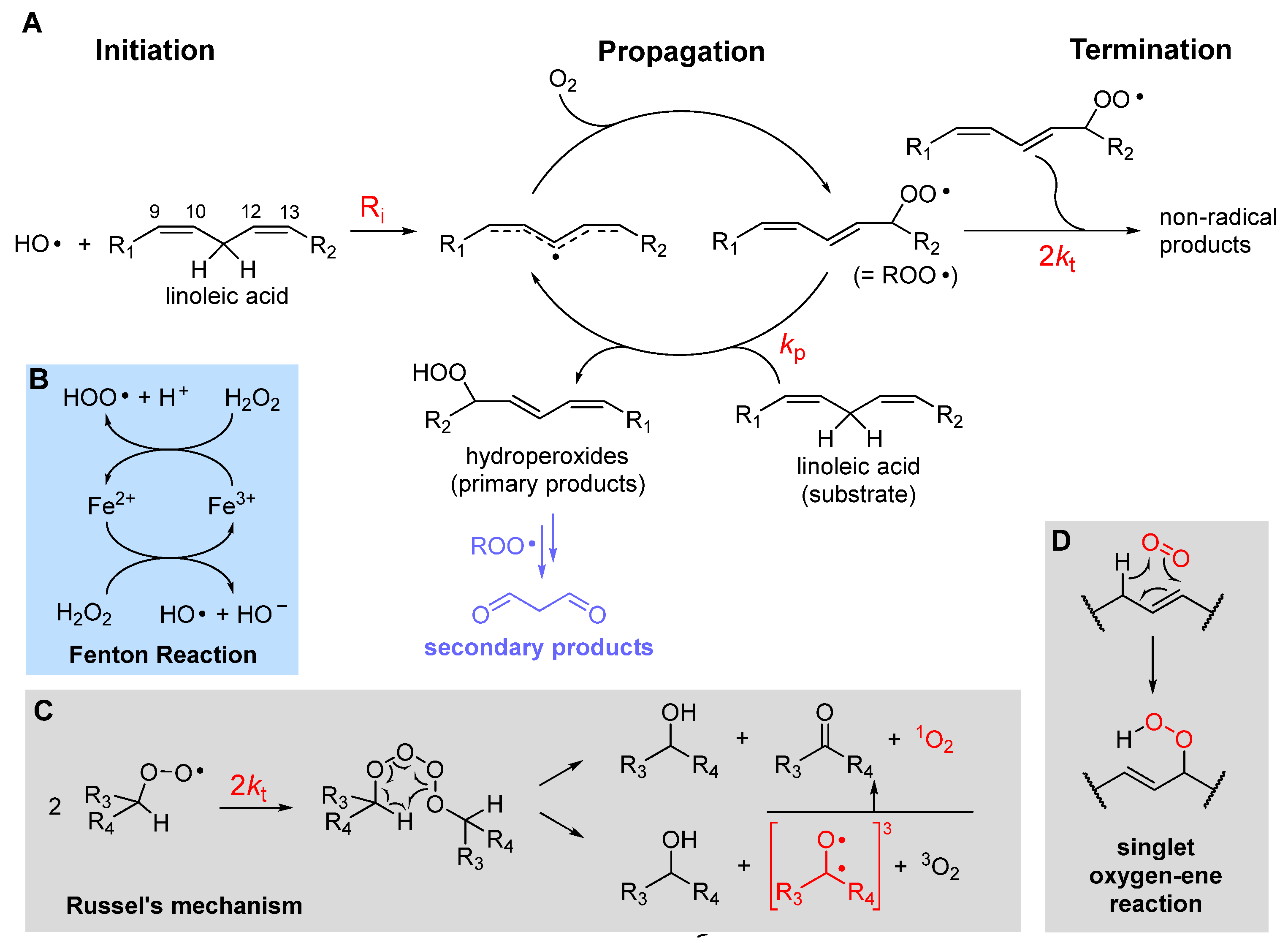
2.1. The three stages of lipid peroxidation
2.2. The rate of lipid peroxidation
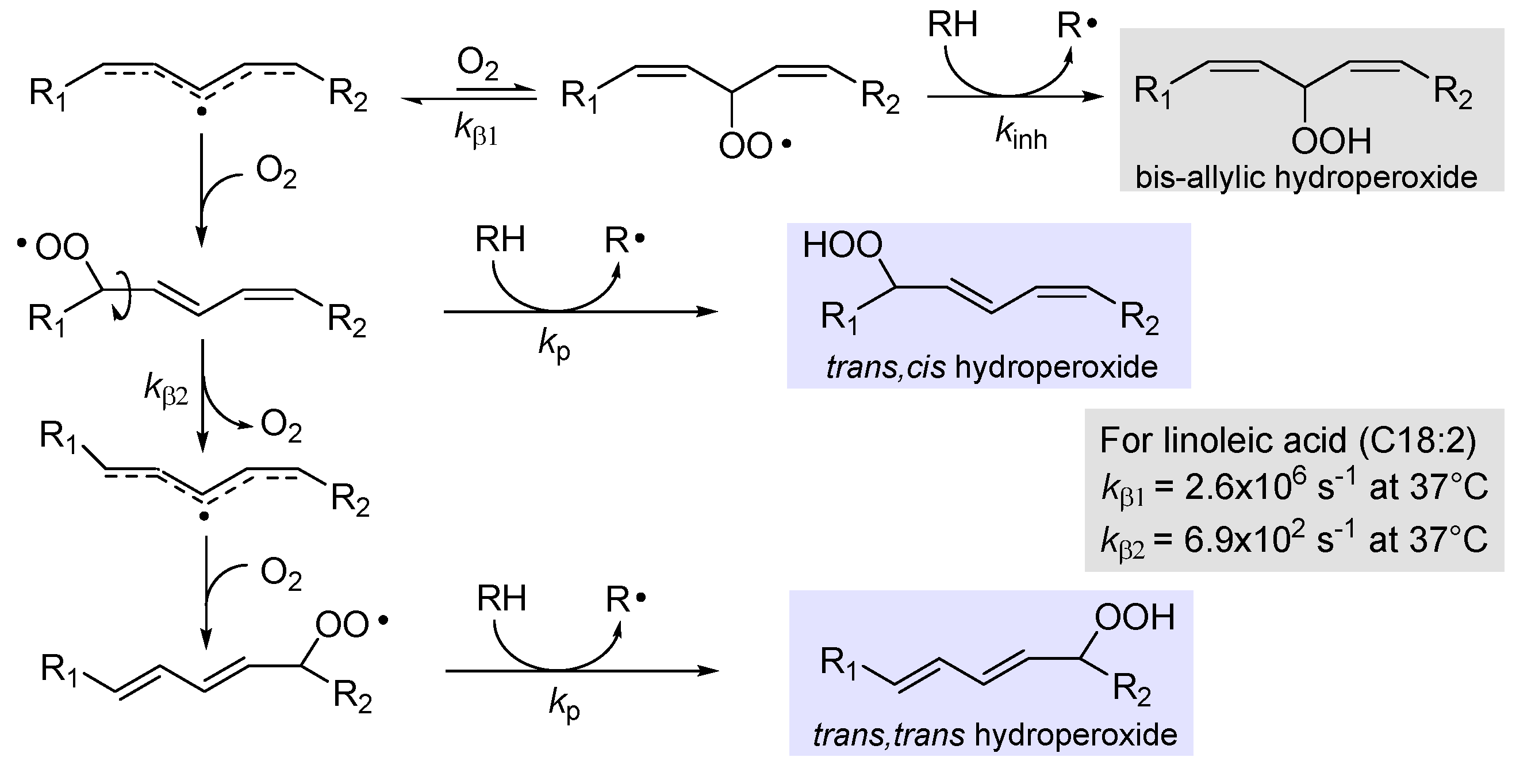
2.3. Further insights into chain-propagation reactions
2.3.1. β-Fragmentation of the peroxyl radical
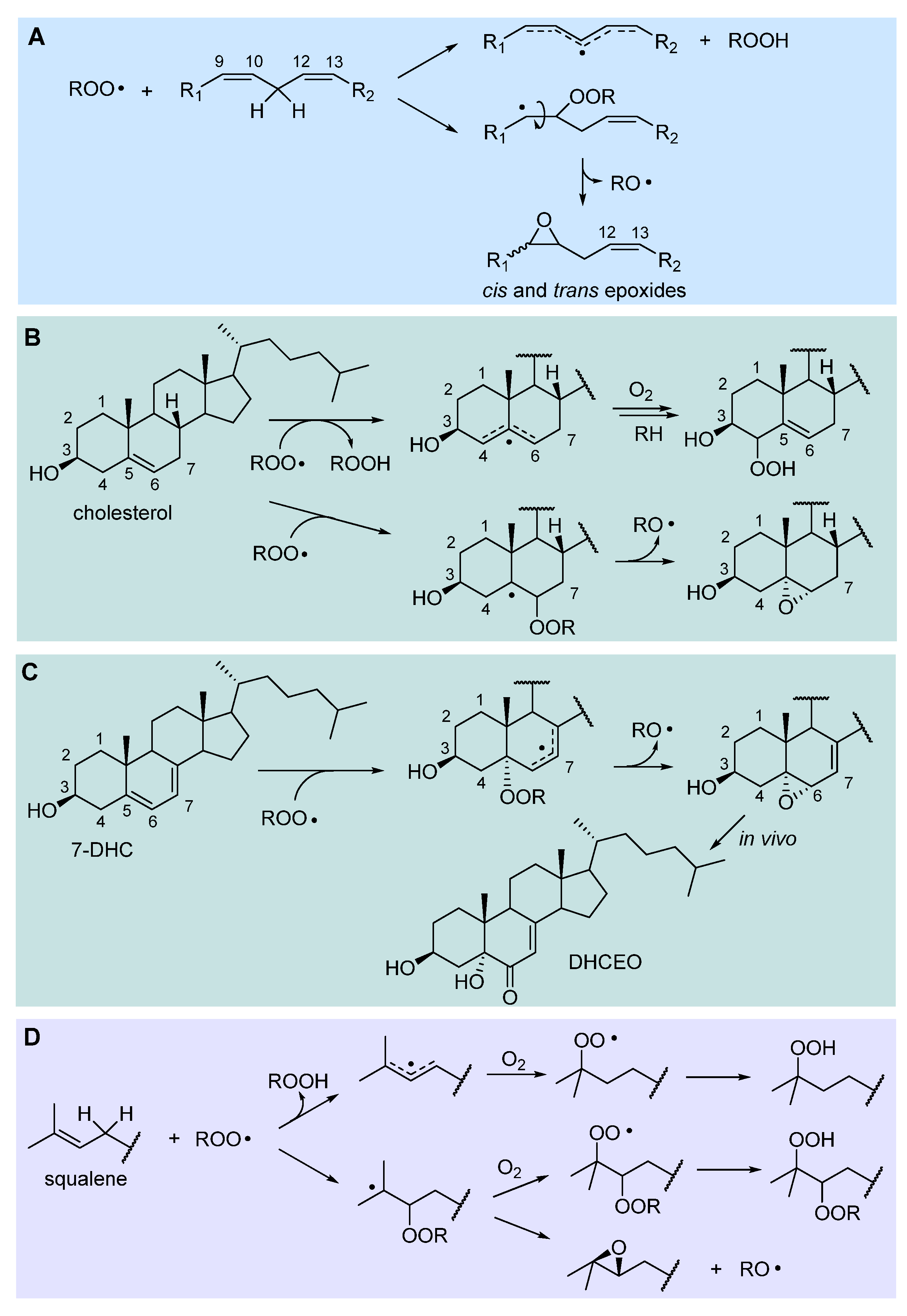
2.3.2. Hydrogen-atom abstraction vs radical addition: formation of primary epoxides
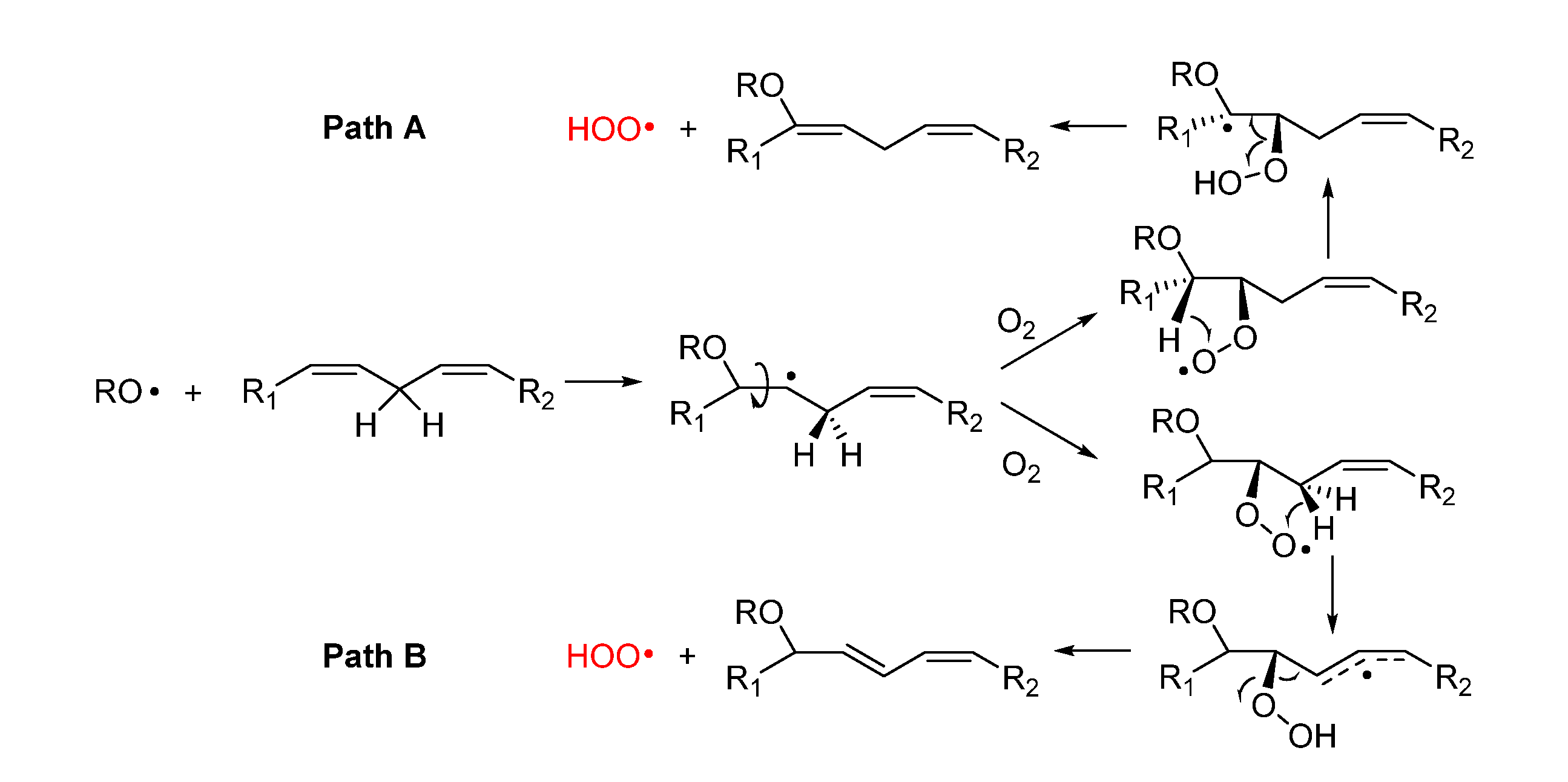
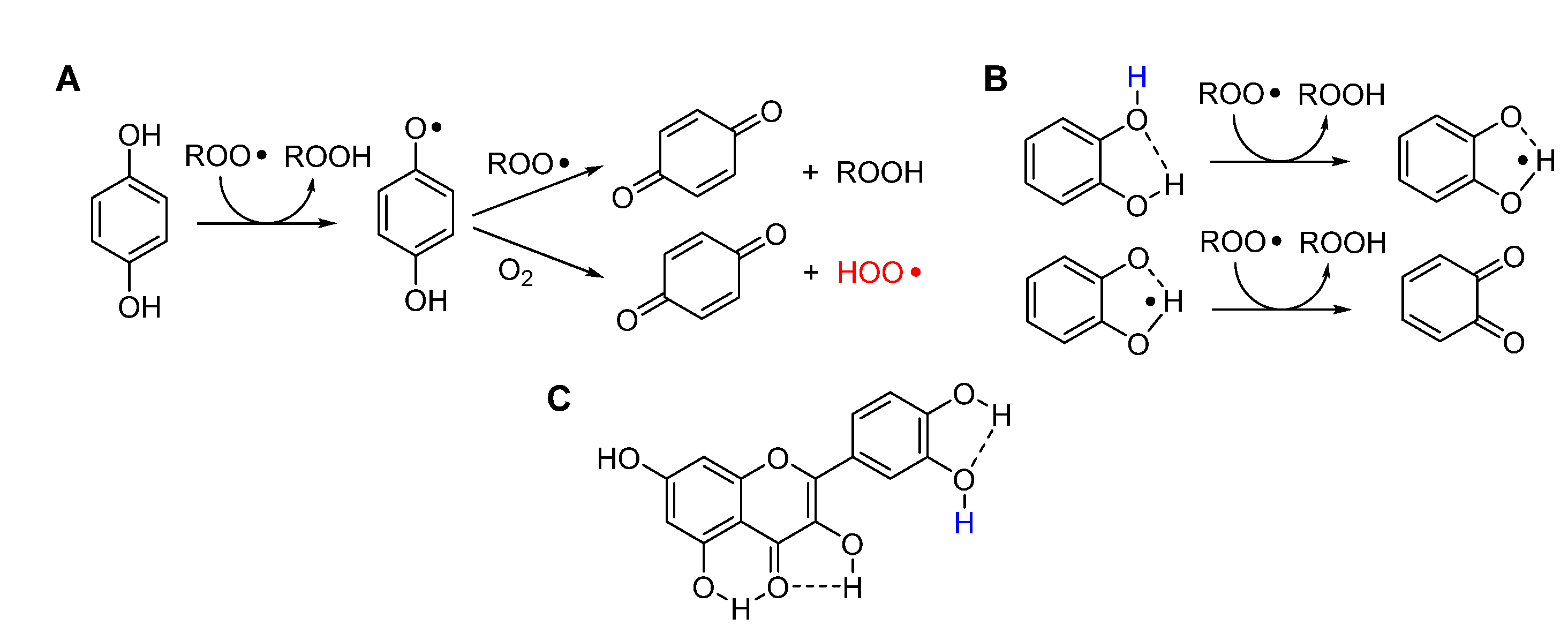
2.3.3. Release of HOO• and chain-transfer processes
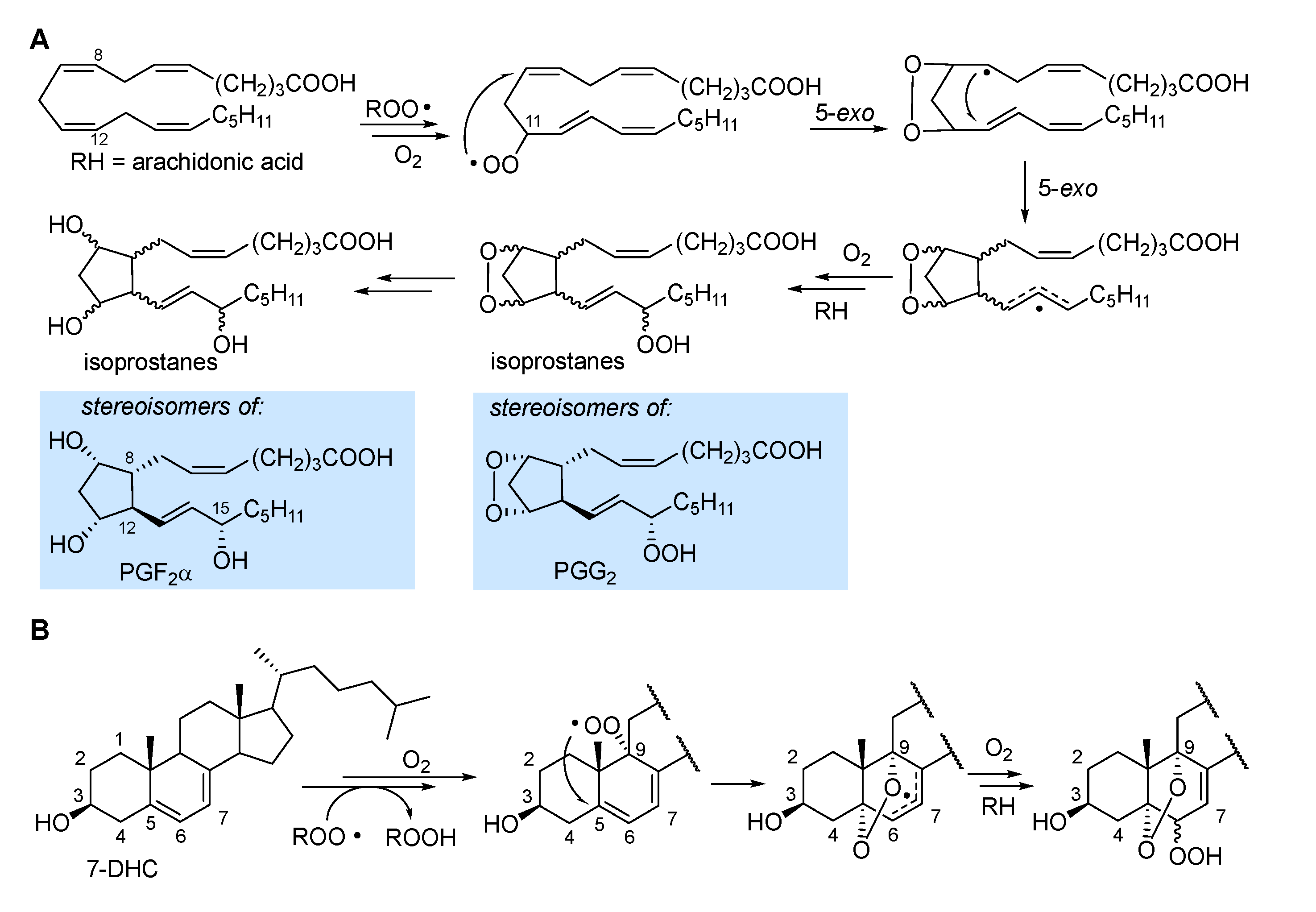
2.3.4. Formation of endoperoxides
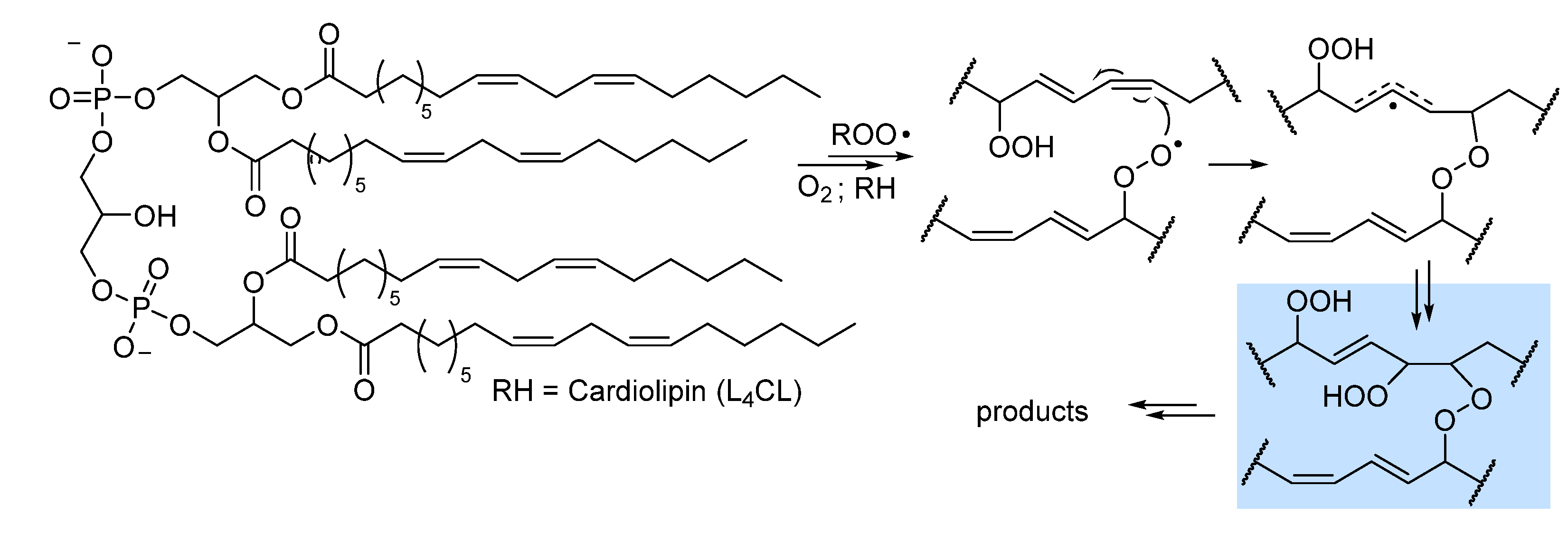
2.3. Peroxidation of intact triglycerides and phospholipids
3. Secondary and late products of lipid peroxidation
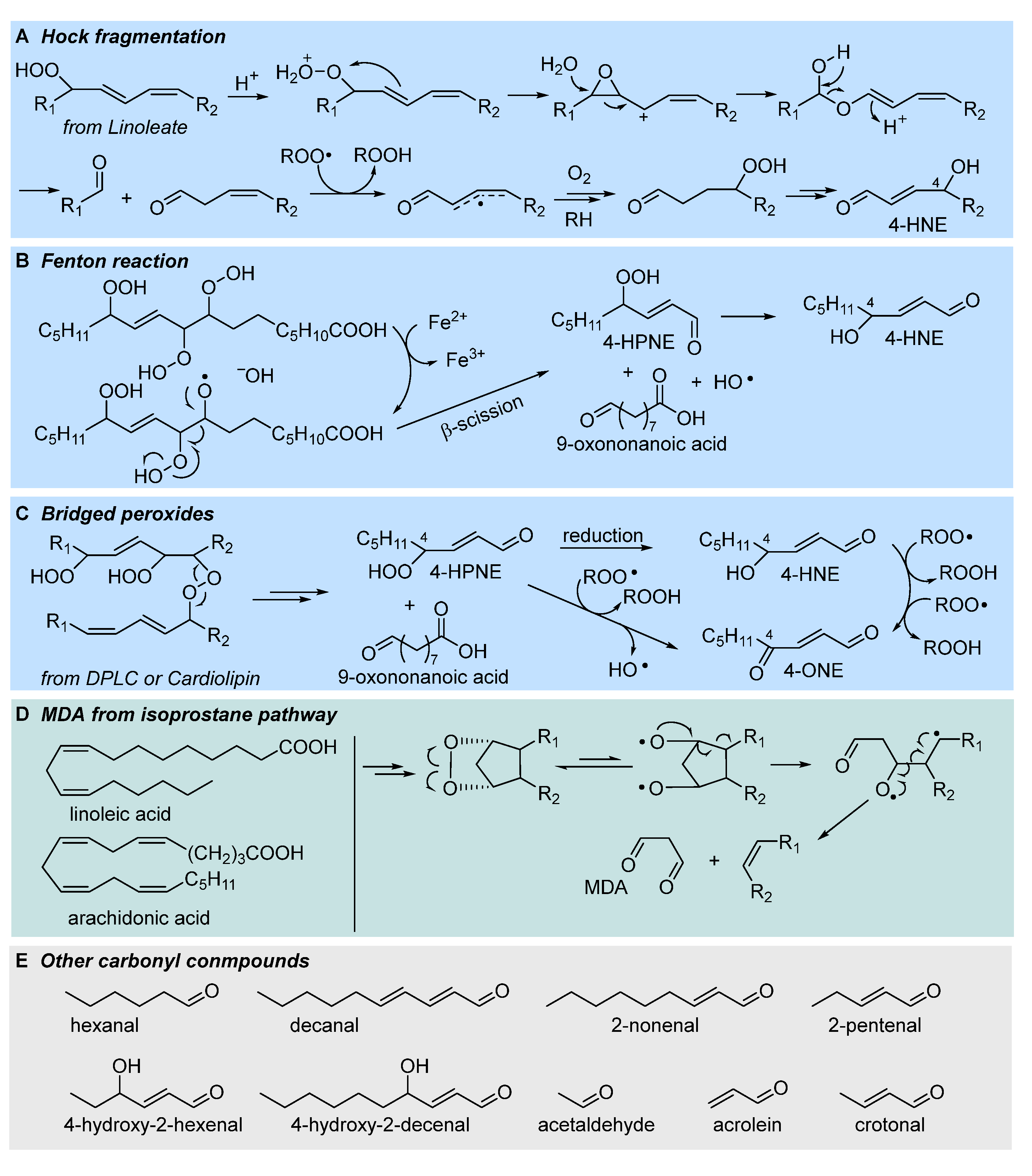
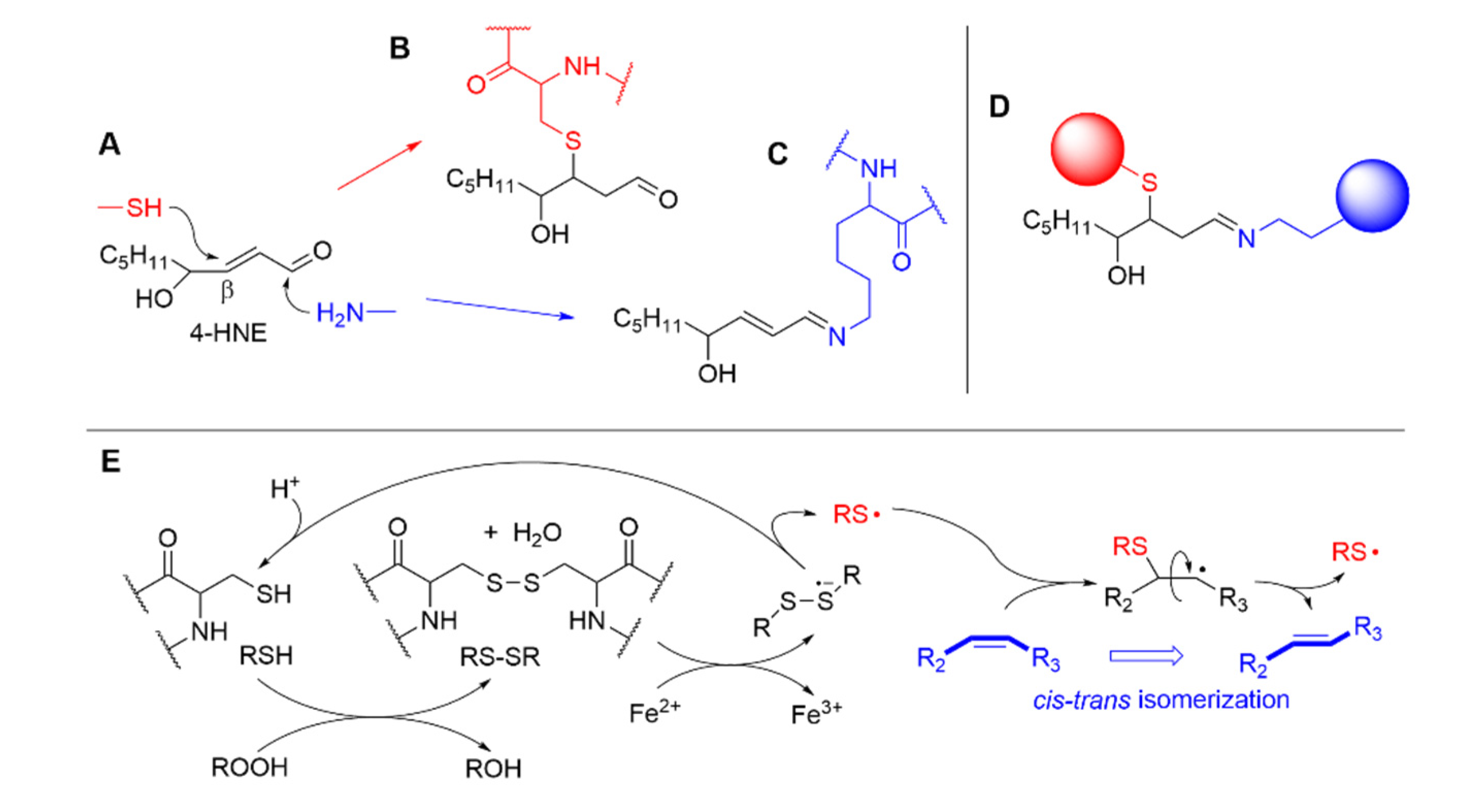
3.1. Formation of electrophilic carbonyl compounds
3.2. Formation of isoprostanes
3.1. Interaction of LP products with aminoacids and proteins
4. Biological consequences of lipid peroxidation
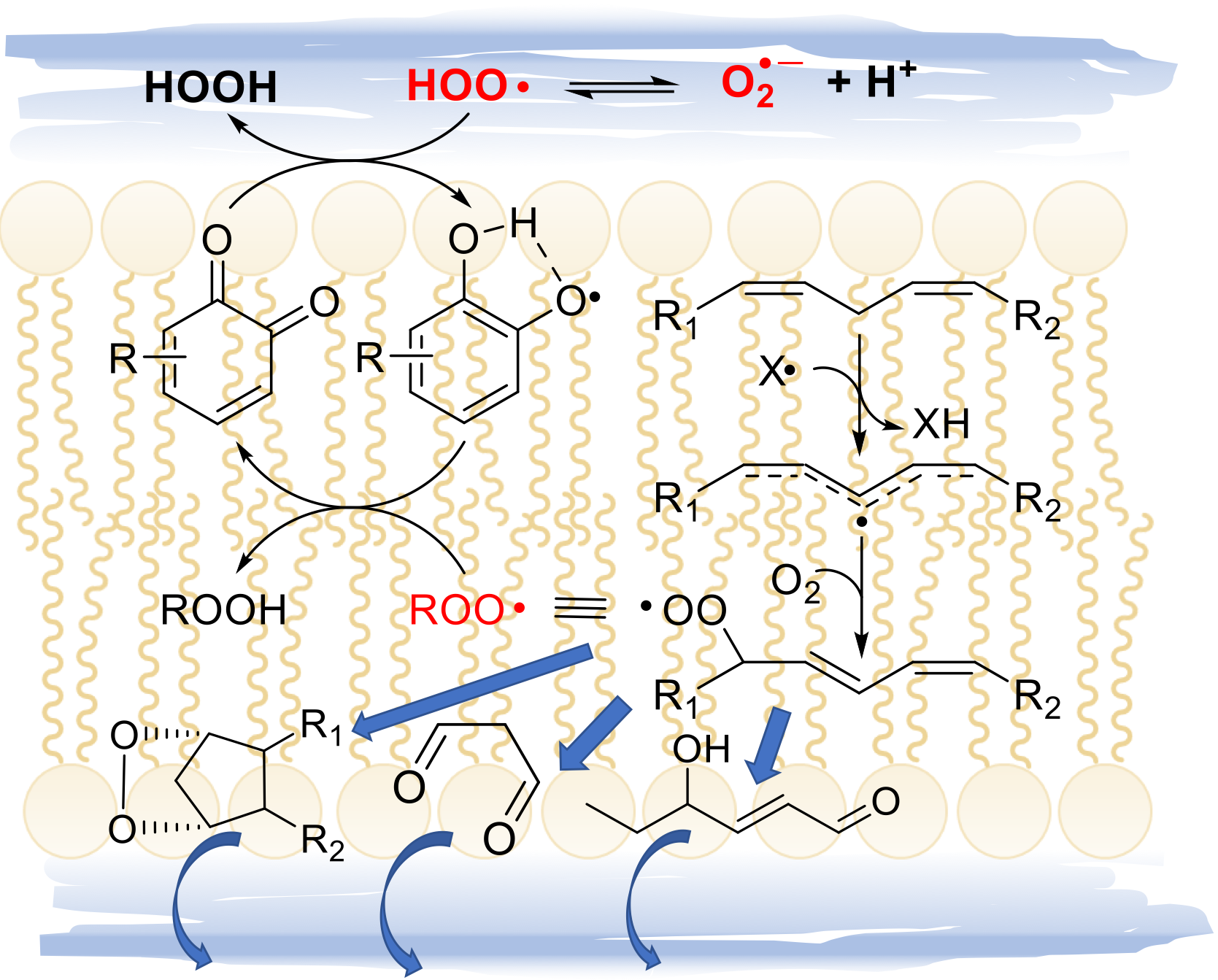
4.1. LP and membranes integrity and functions
4.2. LP and cell signaling
4.3. LP association with cancer and apoptosis
4.4. LP and neurological disorders
4.5. LP and ferroptosis
5. Antioxidants
5.1. Preventive antioxidants
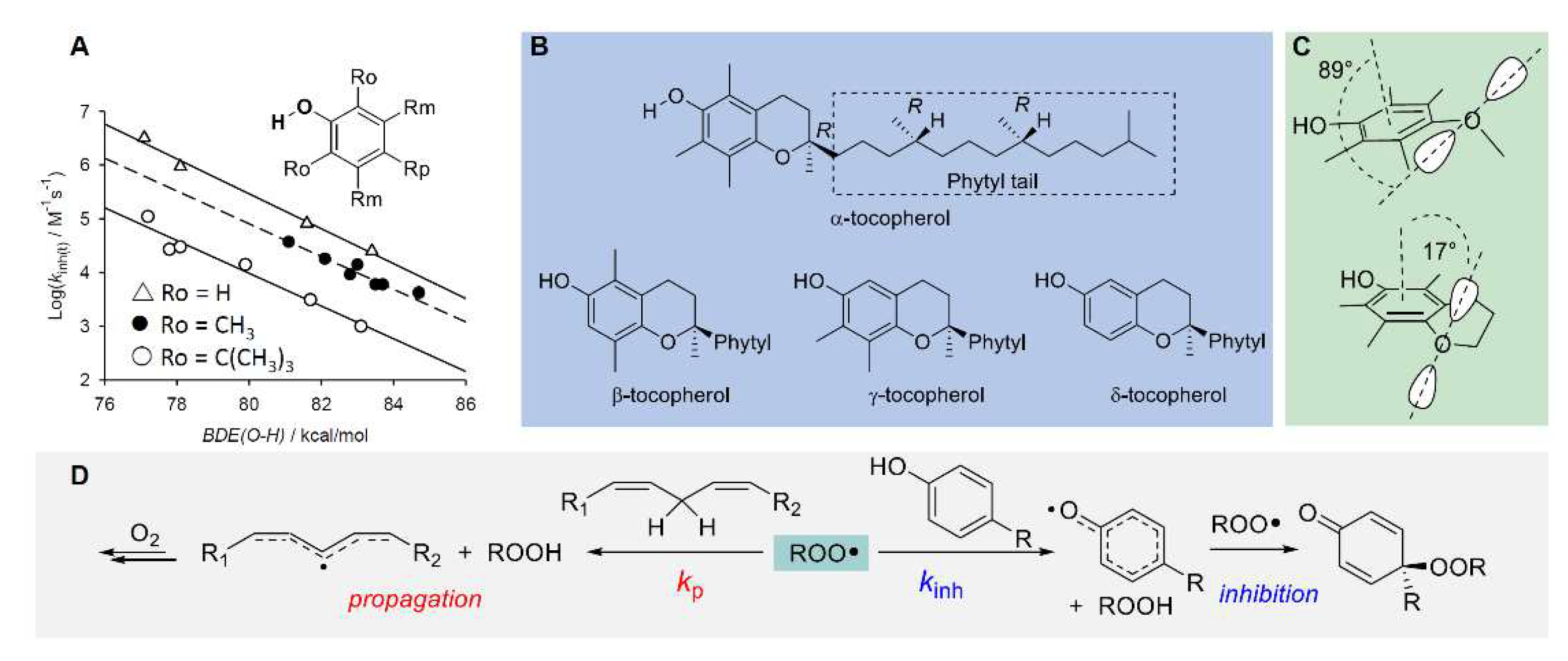
5.2. Chain-breaking antioxidants
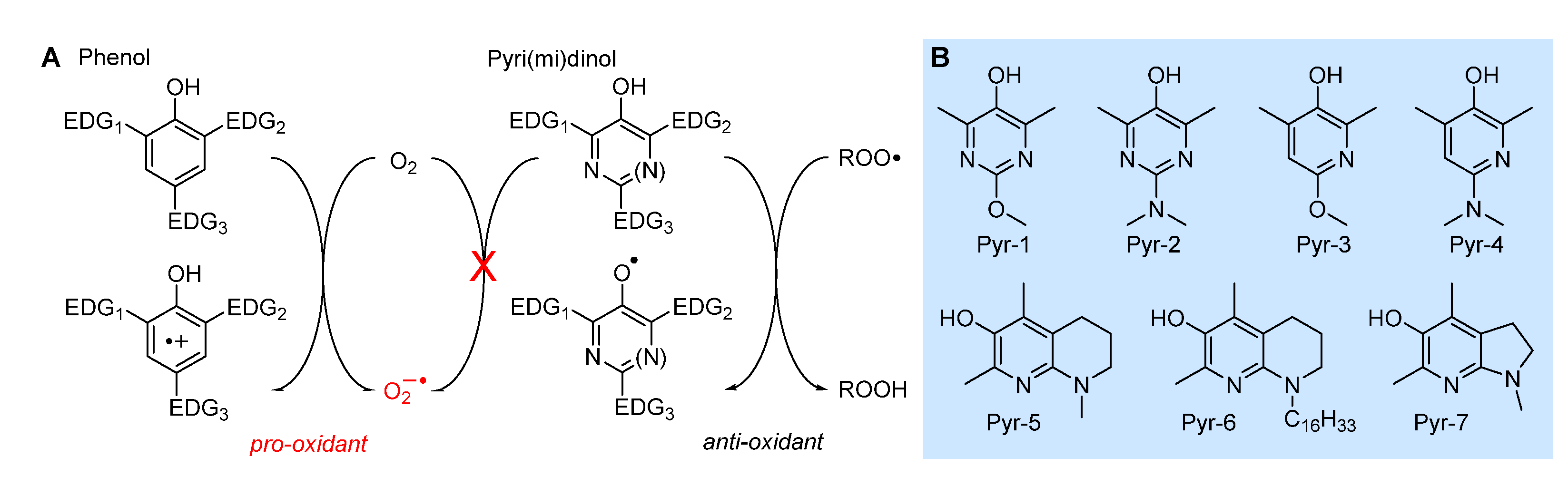
5.2.1. Insertion of N(s) in the phenolic ring: 3-pyridinols and 5-pyrimidinols
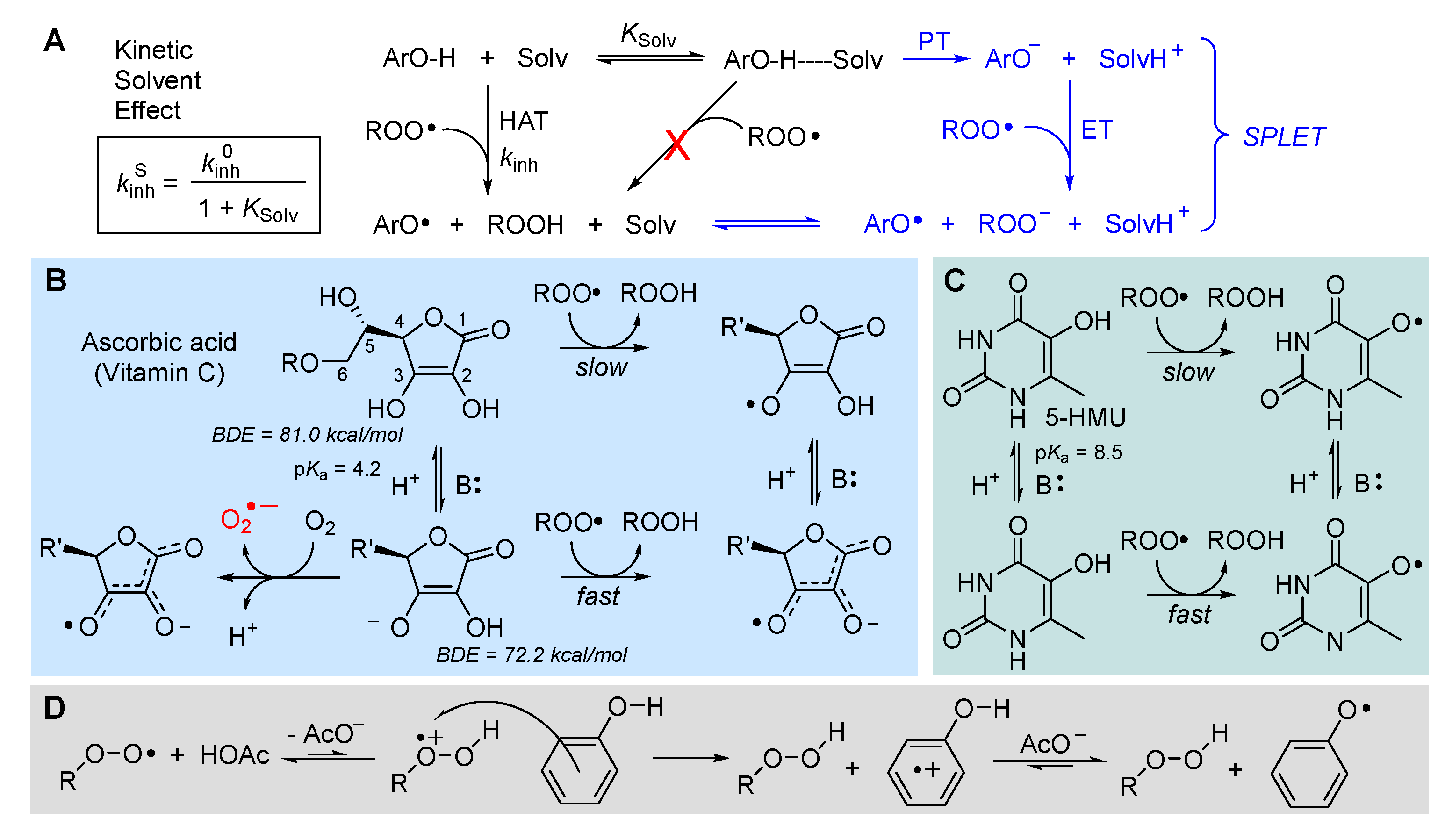
5.2.2. Solvent and medium effects in chain-breaking antioxidant activity
5.2.3. Polyphenols and flavonoids
5.2.4. Synergy among antioxidants and tocopherol-mediated-peroxidation (TMP)
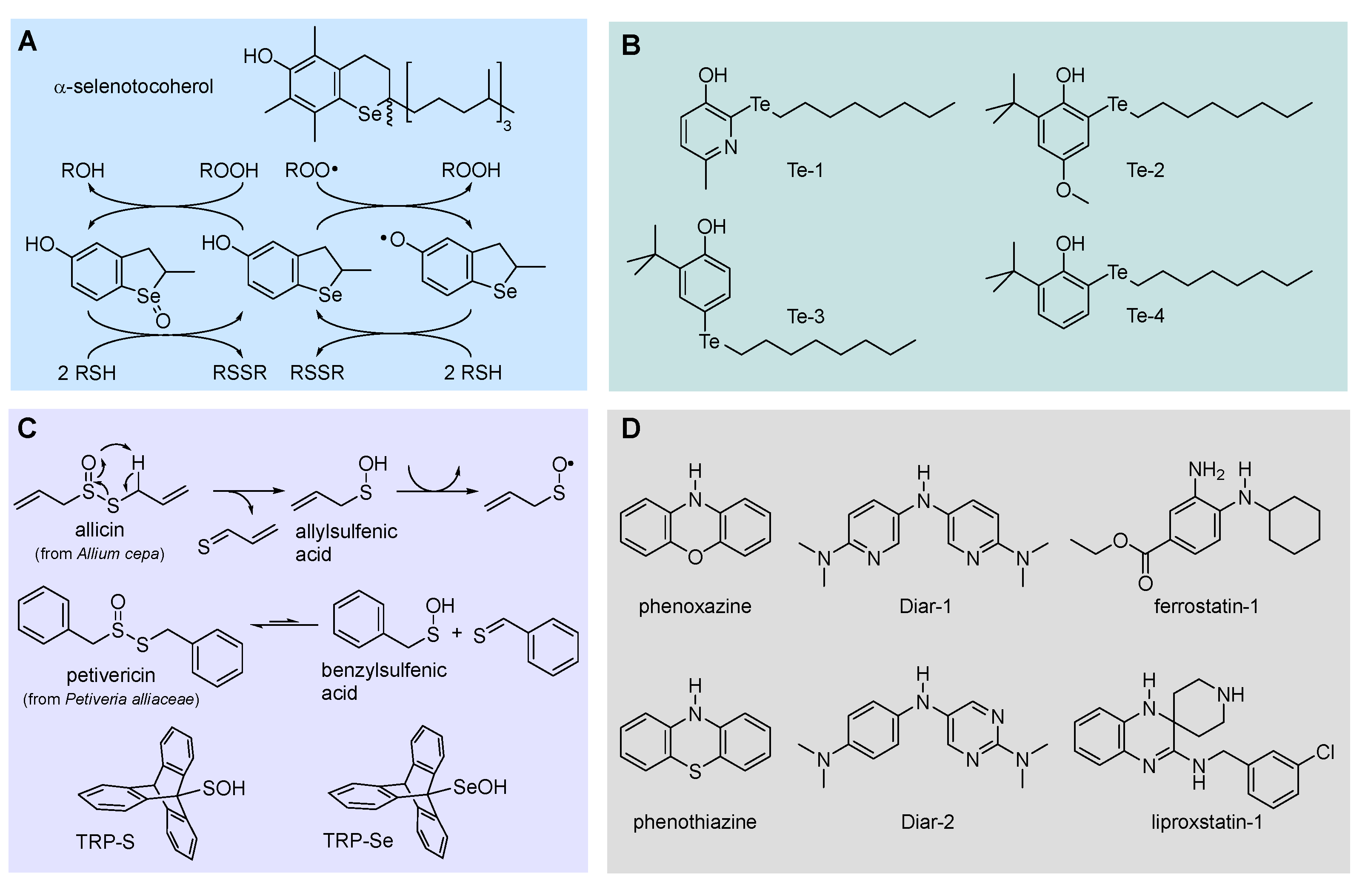
5.2.5. Phenols bearing organochalcogen substituents
5.2.6. Sulfenic and selenenic acids
5.2.7. Aromatic amines and diarylamines as RTAs
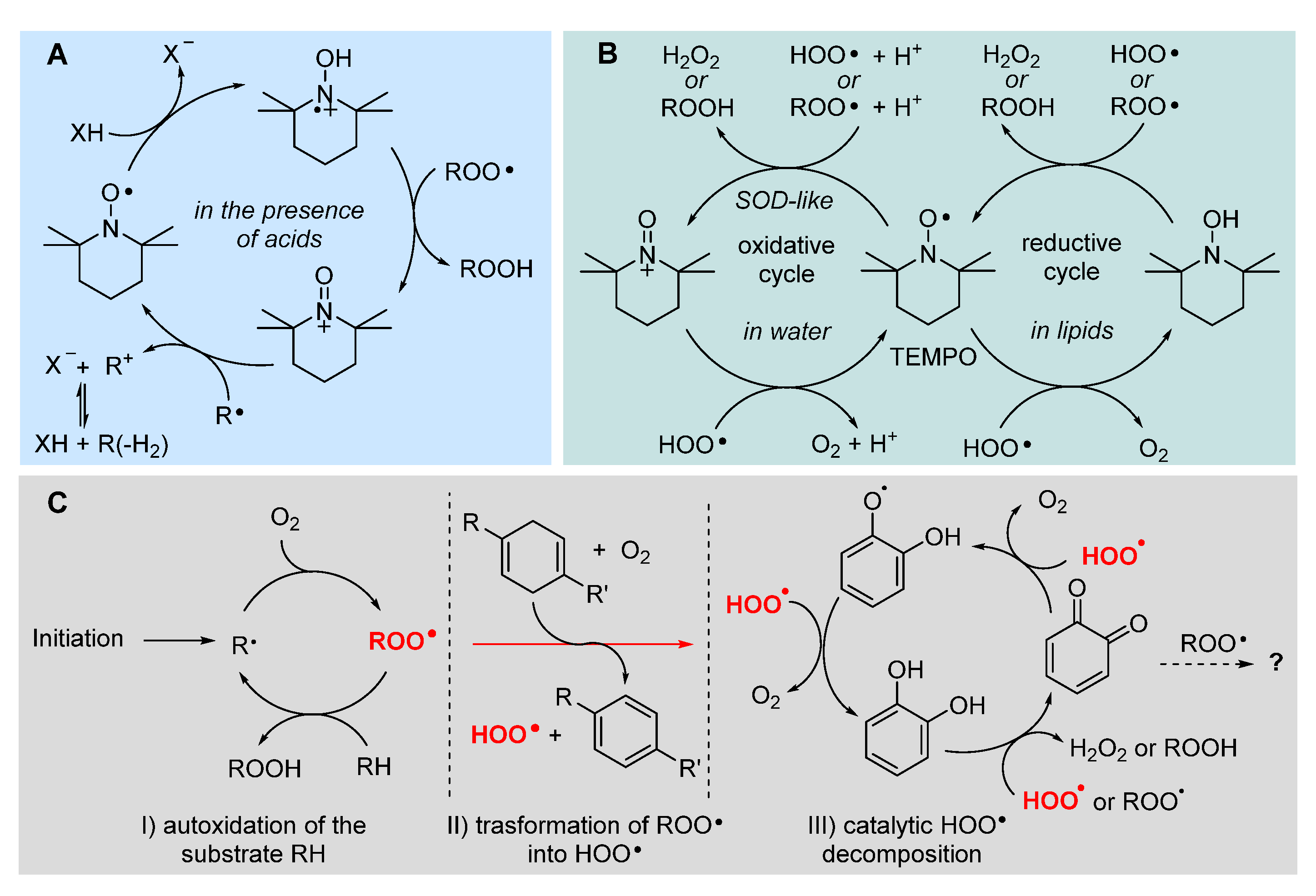
5.2.8. Unconventional antioxidant mechanisms and HOO• as co-antioxidant
5.3. Termination-enhancing antioxidants
5.4. Indirect antioxidants
6. Conclusive remarks and future perspective
Funding
Conflicts of Interest
References
- Porter, N.A. Mechanism for the Autoxidation of Polyunsaturated Lipids. Acc. Chem. Res. 1986, 19, 262–268. [Google Scholar] [CrossRef]
- Ingold, K.U. Peroxyl radicals. Acc. Chem. Res. 1969, 2, 1–9. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Vitamin E: Application of the Principles of Physical Organic Chemistry to the Exploration of Its Structure and Function. Acc. Chem. Res. 1986, 19, 194–201. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Bartolini, M.; Piazzi, L.; Valgimigli, L.; Amorati, R.; Bolondi, C.; Djemil, A.; Mancini, F.; Andrisano, V.; Angela Rampa, A. From the dual function lead AP2238 to AP2469, a multi-target-directed ligand for the treatment of Alzheimer’s disease. Pharma. Res. Per., 2014, 2, e00023. [Google Scholar] [CrossRef]
- Foret, M.K; Lincoln, R.; Do Cormo, S.; Cuello, A.C.; Cosa, G. Connecting the “Dots”: From Free Radical Lipid Autoxidation to Cell Pathology and Disease. Chem. Rev. 2020, 120, 12757–12787. [Google Scholar] [CrossRef] [PubMed]
- Canistro, D.; Boccia, C.; Falconi, R.; Bonamassa, B.; Valgimigli, L.; Vivarelli, F.; Soleti, A.; Genova, M.L.; Lenaz, G.; Sapone, A.; Zaccanti, F.; Abdel-Rahman, S.Z.; Paolini, M. Redox-Based Flagging of the Global Network of Oxidative Stress Greatly Promotes Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 936–943. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; Morrison, B.; Stockwell, B.R. Ferroptosis: An Iron Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- von Krusenstiern, A.N.; Robson, R.N.; Qian, N.; Qiu, B.; Hu, F.; Reznik, E.; Smith, N.; Zandkarimi, F.; Estes, V.E.; Dupont, M.; Hirschhorn, T.; Shchepinov, M.S.; Min, W.; Woerpel, K.A.; Stockwell, B.R. Identification of essential sites of lipid peroxidation in ferroptosis. Nature Chem. Biol. 2023, 19, 719–730. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, R.; Li, H.; Yu, H.; Ren, K.; Jia, S.; Li, Y.; Wang, Q. Insight into the Double-Edged Role of Ferroptosis in Disease. Biomolecules 2021, 11, 1790. [Google Scholar] [CrossRef]
- Shah, R.; Margison, K.; Pratt, D. A. The Potency of Diarylamine Radical-Trapping Antioxidants as Inhibitors of Ferroptosis Underscores the Role of Autoxidation in the Mechanism of Cell Death. ACS Chem. Biol. 2017, 12, 2538–2545. [Google Scholar] [CrossRef]
- Poon, J.-F.; Zilka, O.; Pratt, D. A. Potent Ferroptosis Inhibitors Can Catalyze the Cross-Dismutation of Phospholipid-Derived Peroxyl Radicals and Hydroperoxyl Radicals. J. Am. Chem. Soc. 2020, 142, 14331–14342. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Shah, R.; Pratt, D.A.; Conrad, M. Ferroptosis Inhibition: Mechanisms and Opportunities. Trends Pharmacol. Sci., 2017, 38, 489–498. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Esteras, N.; Abramov, A.Y. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: Finding ways for prevention. Med. Res. Rev. 2021, 41, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Gabbanini, S.; Matera, R.; Valvassori, A.; Valgimigli, L. Rapid liquid chromatography–tandem mass spectrometry analysis of 4-hydroxynonenal for the assessment of oxidative degradation and safety of vegetable oils. Anal. Chim. Acta, 2015, 869, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Takeuchi, M.; Friedrich, H.; van Duynhoven, J.P.M.; Hohlbein, J. Unravelling mechanisms of protein and lipid oxidation in mayonnaise at multiple length scales. Food Chem. 2023, 402, 134417. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, A. Biological effect of protein modifications by lipid peroxidation products. Chem. Phys. Lip. 2019, 221, 46–52. [Google Scholar] [CrossRef]
- Christensen, H.; Sehested, K. HO2 and O2 Radicals at Elevated Temperatures. J. Phys. Chem. 1988, 92, 3007–3011. [Google Scholar] [CrossRef]
- Maillard, B.; Ingold, K.U.; Scaiano, J.C. Rate constants for the reactions of free radicals with oxygen in solution. J. Am. Chem. Soc. 1983, 105, 5095–5099. [Google Scholar] [CrossRef]
- Valgimigli, L.; Pratt, D.A. Antioxidants in Chemistry and Biology. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C.; Studer, A., Eds.; John Wiley & Sons, Ltd., Chirchester, UK, 2012; Volume 3, pp. 1623–1678. [CrossRef]
- Howard, J.A.; Ingold, K.U. Absolute rate constants for hydrocarbon autoxidation. VI. Alkyl aromatic and olefinic hydrocarbons. Can. J. Chem. 1967, 45, 793–802. [Google Scholar] [CrossRef]
- Xu, L.; Davis, T.A.; Porter, N.A. Rate Constants for Peroxidation of Polyunsaturated Fatty Acids and Sterols in Solution and in Liposomes. J. Am. Chem. Soc. 2009, 131, 13037–13044. [Google Scholar] [CrossRef] [PubMed]
- Russell, G. A. Deuterium-Isotope Effects in the Autoxidation of Aralkyl Hydrocarbons. Mechanism of the Interaction of Peroxy Radicals1. J. Am. Chem. Soc. 1957, 79, 3871–3877. [Google Scholar] [CrossRef]
- Miyamoto, S.; Martinez, G. R.; Medeiros, M. H.; Di Mascio, P. Singlet Molecular Oxygen Generated by Biological Hydroperoxides. J. Photochem. Photobiol., B 2014, 139, 24–33. [Google Scholar] [CrossRef]
- Miyamoto, S.; Nantes, I.L.; Faria, P.A.; Cunha, D.; Ronsein, G.E.; Medeiros, M.H.G.; Di Mascio, P. Cytochrome c-promoted cardiolipin oxidation generates singlet molecular oxygen. Photochem. Photobiol. Sci. 2012, 11, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, Z. A. M.; Pratt, D. A. Lipid Peroxidation: Kinetics, Mechanisms, and Products. J. Org. Chem. 2017, 82, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Pizzol, R.; Guo, Y.; Amorati, R.; Valgimigli, L. Calibration of Squalene, p-Cymene, and Sunflower Oil as Standard Oxidizable Substrates for Quantitative Antioxidant Testing. J. Agric. Food Chem. 2019, 67, 6902–6910. [Google Scholar] [CrossRef]
- Porter, N. A. A Perspective on Free Radical Autoxidation: The Physical Organic Chemistry of Polyunsaturated Fatty Acid and Sterol Peroxidation. J. Org. Chem. 2013, 78, 3511–3524. [Google Scholar] [CrossRef]
- Pratt, D.A. Tallman, K.A.; Porter, N.A. Free Radical Oxidation of Polyunsaturated Lipids: New Mechanistic Insights and the Development of Peroxyl Radical Clocks. Acc. Chem. Res. 2011, 44, 458–467. [Google Scholar] [CrossRef]
- Howard, JA, Ingold, KU. Absolute rate constants for hydrocarbon oxidation. XII. Rate constants for secondary peroxy radicals. Can. J. Chem. 1968, 46, 2661–2666. [Google Scholar] [CrossRef]
- Maerker, G, Haeberer, E. T., Ault, W.C. Epoxidation of methyl linoleate. I. The question of positional selectivity in monoepoxidation. J. Am. Oil Chem. Soc. 1966, 43, 100–104. [Google Scholar] [CrossRef]
- Aliwarga, T.; Raccor, B. S.; Lemaitre, R. N.; Sotoodehnia, N.; Gharib, S. A.; Xu, L.; Totah, R. A. Enzymatic and free radical formation of cis- and trans-epoxyeicosatrienoic acids in vitro and in vivo. Free Radical Biol. Med. 2017, 112, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, Z.A.M.; Pratt, D.A. H-Atom Abstraction vs Addition: Accounting for the Diverse Product Distribution in the Autoxidation of Cholesterol and Its Esters. J. Am. Chem. Soc. 2019, 141, 3037–3051. [Google Scholar] [CrossRef] [PubMed]
- Do, Q.; Lee, D.D.; Dinh, A.N.; Seguin, R.P.; Zhang, R.; Xu, L. Development and Application of a Peroxyl Radical Clock Approach for Measuring Both Hydrogen-Atom Transfer and Peroxyl Radical Addition Rate Constants. J. Org. Chem. 2021, 86, 153–168. [Google Scholar] [CrossRef]
- Denisov, E.T. The role of triplet repulsion in alkyl radical addition to a 7π-C-O bond and alkoxy radical addition to a π-C-C bond. Kinet. Catal. 2000, 41, 293–297. [Google Scholar] [CrossRef]
- Degirmenci, I.; Coote, M.L. Comparison of Thiyl, Alkoxyl, and Alkyl Radical Addition to Double Bonds: The Unusual Contrasting Behavior of Sulfur and Oxygen Radical Chemistry. J. Phys. Chem. A 2016, 120, 1750–1755. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Baschieri, A.; Mollica, F.; Valgimigli, L.; Cedrowski, J.; Litwinienko, G.; Amorati, R. Hydrogen Atom Transfer from HOO• to ortho-Quinones Explains the Antioxidant Activity of Polydopamine. Angew. Chem. Int. Ed. 2021, 60, 15220–15224. [Google Scholar] [CrossRef]
- Foti, M.C.; Ingold, K.U. Mechanism of Inhibition of lipid peroxidation by γ-terpinene, an unusual and potentially useful hydrocarbon antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. [Google Scholar] [CrossRef]
- Harrison, K.A.; Haidasz, E.A.; Griesser, M.; Pratt, D.A. Inhibition of hydrocarbon autoxidation by nitroxide-catalyzed cross-dismutation of hydroperoxyl and alkylperoxyl radicals. Chem. Sci. 2018, 9, 6068–6079. [Google Scholar] [CrossRef]
- Antunes, F.; Barclay, L. R. C.; Vinqvist, M. R.; Pinto, R. E. Int. J. Chem. Kinet. 1998, 30, 753–767. [Google Scholar] [CrossRef]
- Liu, W.; Porter, N. A.; Schneider, C.; Brash, A. R.; Yin, H. Formation of 4-Hydroxynonenal from Cardiolipin Oxidation: Intramolecular Peroxyl Radical Addition and Decomposition. Free Radical Biol. Med. 2011, 50, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Bowry, V.W. Arm-to-Arm Autoxidation in a Triglyceride: Remote Group Reaction Kinetics. J. Org. Chem. 1994, 59, 2250–2252. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R. J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Ullery, J. C.; Marnett, L. J. Protein modification by oxidized phospholipids and hydrolytically released lipid electrophiles: Investigating cellular responses. Biochim. Biophys. Acta, Biomembr. 2012, 1818, 2424–2435. [Google Scholar] [CrossRef] [PubMed]
- Codreanu, S. G.; Ullery, J. C.; Zhu, J.; Tallman, K. A.; Beavers, W. N.; Porter, N. A.; Marnett, L. J.; Zhang, B.; Liebler, D. C. Alkylation Damage by Lipid Electrophiles Targets Functional Protein Systems. Mol. Cell. Proteomics 2014, 13, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Spickett, C.M. The lipidperoxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis. Redox Biol., 2013, 2013, 145–152. [Google Scholar] [CrossRef]
- Liang, X.; Qian, R.; Ou, Y.; Wang, D.; Lin, X.; Sun, C. Lipid peroxide-derived short-chain aldehydes promote programmed cell death in wheat roots under aluminum stress. J. Hazard. Mater. 2023, 443, 130142. [Google Scholar] [CrossRef]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox Signaling by Reactive Electrophiles and Oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef]
- Schopfer, F. J.; Cipollina, C.; Freeman, B. A. Formation and Signaling Actions of Electrophilic Lipids. Chem. Rev. 2011, 111, 5997–6021. [Google Scholar] [CrossRef]
- Yang, J.; Tallman, K. A.; Porter, N. A.; Liebler, D. C. Quantitative Chemoproteomics for Site-Specific Analysis of Protein Alkylation by 4-Hydroxy-2-Nonenal in Cells. Anal. Chem. 2015, 87, 2535–2541. [Google Scholar] [CrossRef]
- Perkovic, M.N.; Jaganjac, M.; Milkovic, L.; Horvat, T.; Rojo, D.; Zarkovic, K.; C´ oric´, M.; Hudolin, T.; Waeg, G.; Orehovec, B.; et al. Relationship between 4-Hydroxynonenal (4-HNE) as Systemic Biomarker of Lipid Peroxidation and Metabolomic Profiling of Patients with Prostate Cancer. Biomolecules 2023, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Y.; Cohen, G.; Shamni, O.; Sasson, S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E879–E886. [Google Scholar] [CrossRef] [PubMed]
- Onyango, A.N.; Baba, N. New hypotheses on the pathways of formation of malondialdehyde and isofurans. Free Radical Biol. Med. 2010, 49, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, C.; Galvé, R.; Nieva, J.; Witter, D. P.; Wentworth, A.D.; Troseth, R. P.; Lerner, R. A.; Wentworth, P. Proatherogenic Effects of the Cholesterol Ozonolysis Products, Atheronal-A and Atheronal-B. Biochemistry, 2006; 45, 7162–7170. [Google Scholar] [CrossRef]
- Nieva, J.; Song, B.-D.; Rogel, J. K.; Kujawara, D.; Altobel, L., III; Izharrudin, A.; Boldt, G. E.; Grover, R. K.; Wentworth, A. D.; Wentworth, P., Jr. Cholesterol Secosterol Aldehydes Induce Amyloidogenesis and Dysfunction of Wild-Type Tumor Protein p53. Chem. Biol. 2011, 18, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Hulleman, J. D.; Paulsson, J. F.; Siegel, S. J.; Powers, E.T.; Kelly, J. W. Site-specific modification of Alzheimer's peptides by cholesterol oxidation products enhances aggregation energetics and neurotoxicity. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 18563–18568. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, P.; Žarkovi´, N.; Gegotek, A.; Skrzydlewska, E. Involvement of Metabolic Lipid Mediators in the Regulation of Apoptosis. Biomolecules 2020, 10, 402. [Google Scholar] [CrossRef]
- Graille, M.; Wild, P.; Sauvain, J.-J.; Hemmendinger, M.; Guseva Canu, I.; Hopf, N.B. Urinary 8-isoprostane as a biomarker for oxidative stress. A systematic review and meta-analysis. Toxicol. Lett. 2020; 328, 19–27. [Google Scholar] [CrossRef]
- Gao, X.; Brenner, H.; Holleczek, B.; Cuk, K.; Zhang, Y.; Anusruti, A.; Xuan, Y.; Xu, Y.; Schottker, B. Urinary 8-isoprostane levels and occurrence of lung, colorectal, prostate, breast and overall cancer: Results from a large, population-based cohort study with 14 years of follow-up. Free Radic. Biol. Med., 2018, 123, 20–26. [Google Scholar] [CrossRef]
- Yang, J.; Tallman, K. A.; Porter, N. A.; Liebler, D. C. Quantitative Chemoproteomics for Site-Specific Analysis of Protein Alkylation by 4-Hydroxy-2-Nonenal in Cells. Anal. Chem. 2015, 87, 2535–2541. [Google Scholar] [CrossRef]
- Ferreri, C.; Ferocino, A.; Batani, G.; Chatgilialoglu, C.; Randi, V.; Riontino, M.V.; Vetica, F.; Sansone, A. Plasmalogens: Free Radical Reactivity and Identification of Trans Isomers Relevant to Biological Membranes. Biomolecules 2023, 13, 730. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Melchiorre, M.; Sansone, A.; Torreggiani, A. Lipid geometrical isomerism: From chemistry to biology and diagnostics. Chem. Rev. 2014, 114, 255–284. [Google Scholar] [CrossRef]
- Torreggiani, A.; Tinti, A.; Jurasekova, Z.; Capdevila, M.; Saracino, M.; Foggia, M.D. Structural Lesions of Proteins Connected to Lipid Membrane Damages Caused by Radical Stress: Assessment by Biomimetic Systems and Raman Spectroscopy. Biomolecules 2019, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Ferreri, C.; Yamada, Y.; Inoue, A.; Sansone, A.; Vetica, F.; Suzuki, W.; Takano, S.; Noguchi, T.; Matsuzawa, A.; Chatgilialoglu, C. Geometrical isomerization of arachidonic acid during lipid peroxidation interferes with ferroptosis. Free Radical Biol. Med. 2023, 204, 374–384. [Google Scholar] [CrossRef]
- Itri, R.; Junqueira, H. C.; Mertins, O.; Baptista, M. S. Membrane Changes under Oxidative Stress: The Impact of Oxidized Lipids. Biophys. Rev. 2014, 6, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.; Charitat, T.; Baptista, M. S.; Uchoa, A. F.; Pavani, C.; Junqueira, H. C.; Guo, Y.; Baulin, V. A.; Itri, R.; Marques, C. M.; Schroder, A.P. Lipid Oxidation Induces Structural Changes in Biomimetic Membranes. Soft Matter 2014, 10, 4241–4247. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. , Wang, J., Fan, X., Ansari, G.A.S., Khan, M.F. Protein adducts of malondialdehyde and 4-hydroxynonenal contribute to trichloroethene-mediated autoimmunity via activating Th17 cells: dose–and time–response studies in female MRL +/+ mice. Toxicology, 2012, 292, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T. , Place, N., Kosterina, N., Östberg, T., Zhang, S.J., Grundtman, C., Erlandsson-Harris, H.; Lundberg, I.E.; Glenmark, B.; Bruton, J.D.; Westerblad, H.. Impaired myofibrillar function in the soleus muscle of mice with collagen-induced arthritis. Arthritis Rheumat. 2009, 60, 3280–3289. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Vento, M.; Baquero, M.; Cháfer-Pericás, C. Lipid peroxidation in neurodegeneration. Clin. Chim. Acta, 2019, 497, 178–188. [Google Scholar] [CrossRef]
- da Santana, L.N.; Bittencourt, L.O.; Nascimento, P.C.; Fernandes, R.M.; Teixeira, F.B.; Fernandes, L.M.P.; Freitas Silva, M.C.; Nogueira, L.S.; Amado, L.L.; Crespo-Lopez, M.E.; do Maia, C.S.F.; Lima, R.R. , J. Trace Elem. Med. Biol. 2019, 51, 19–27. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Free radicals in Alzheimer's disease: Lipid peroxidation biomarkers. Clin. Chim. Acta, 2019, 491, 85–90. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; Basavarajappa, D. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nature Cell. Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Pratt, D.A. The chemical basis of ferroptosis. Nature Chem. Biol. 2019, 15, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; Henkelmann, B.; Yamada, K.; Wanninger, J.; Zilka, O.; Sato, E.; Feederle, R.; Hass, D.; Adriano Maida, A.; Mourão, A.S.D.; Linkermann, A.; Geissler, E. K.; Nakagawa, K.; Abe, T.; Fedorova, M.; Proneth, B.; Pratt, D.A.; Conrad. M. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature, 2022, 608, 778–783. [Google Scholar] [CrossRef]
- Soula, M.; Weber, R.A.; Zilka, O.Z.; Alwaseem, H.; La, K.; Yen, F.; Molina, H.; Garcia-Bermudez, J.; Pratt, D.A.; Birsoy, K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nature Chem. Biol. 2020, 16, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Shah, R.; Derek, A. Pratt, D.A.; Conrad, M. Ferroptosis Inhibition: Mechanisms and Opportunities. Trends Pharmacol. Sci. 2017, 38, 489–498. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, R.; Li, H.; Yu, H.; Ren, K.; Jia, S.; Li, Y.; Wang, Q. Insight into the Double-Edged Role of Ferroptosis in Disease. Biomolecules 2021, 11, 1790. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Methods to measure the antioxidant activity of phytochemicals and plant extracts. J. Agric. Food Chem., 2018, 66, 3324–3329. [Google Scholar] [CrossRef]
- Baschieri, A.; Ajvazi, M. D.; Tonfack, J. L. F.; Valgimigli, L.; Amorati, R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017, 232, 656–663. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedulli, G.F. Free radical intermediates in the inhibition of the autoxidation reaction. Chem. Soc. Rev., 2010, 39, 2106–2119. [Google Scholar] [CrossRef]
- Cariola, A.; El Chami, M.; Granatieri, J.; Valgimigli, L. Anti-tyrosinase and antioxidant activity of meroterpene bakuchiol from Psoralea corylifolia (L.). Food Chem. 2023, 405, 134953. [Google Scholar] [CrossRef]
- Helberg, J.; Pratt, D.A. Autoxidation vs. antioxidants – the fight for Forever. Chem. Soc. Rev., 2021, 50, 7343–7358. [Google Scholar] [CrossRef] [PubMed]
- Ingold, K.U.; Pratt, D.A. Advances in Radical-Trapping Antioxidant Chemistry in the 21st Century: A Kinetics and Mechanisms Perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Pratt, D.A. Maximizing the Reactivity of Phenolic and Aminic Radical-Trapping Antioxidants: Just Add Nitrogen! Acc. Chem. Res. 2015, 48, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D. A.; DiLabio, G. A.; Brigati, G.; Pedulli, G. F.; Valgimigli, L. 5-Pyrimidinols: Novel Chain-Breaking Antioxidants More Effective Than Phenols. J. Am. Chem. Soc. 2001, 123, 4625–4626. [Google Scholar] [CrossRef]
- Valgimigli, L.; Brigati, G.; Pedulli, G. F.; DiLabio, G. A.; Mastragostino, M.; Arbizzani, C.; Pratt, D. A. The Effect of Ring Nitrogen Atoms on the Homolytic Reactivity of Phenolic Compounds: Understanding the Radical-Scavenging Ability of 5-Pyrimidinols. Chem. Eur. J. 2003, 9, 4997–5010. [Google Scholar] [CrossRef]
- Wijtmans, M.; Pratt, D. A.; Valgimigli, L.; DiLabio, G. A.; Pedulli, G. F.; Porter, N. A. 6-Amino-3-Pyridinols: Towards Diffusion-Controlled Chain-Breaking Antioxidants. Angew. Chem., Int. Ed. 2003, 42, 4370–4373. [Google Scholar] [CrossRef]
- Wijtmans, M.; Pratt, D. A.; Brinkhorst, J.; Serwa, R.; Valgimigli, L.; Pedulli, G. F.; Porter, N. A. Synthesis and Reactivity of Some 6-Substituted-2,4-Dimethyl-3-Pyridinols, a Novel Class of Chain-Breaking Antioxidants. J. Org. Chem. 2004, 69, 9215–9223. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Pratt, D. A.; Seal, J. R.; Wijtmans, M.; Porter, N. A. Lipid-Soluble 3-Pyridinol Antioxidants Spare α-Tocopherol and Do Not Efficiently Mediate Peroxidation of Cholesterol Esters in Human Low-Density Lipoprotein. J. Med. Chem. 2005, 48, 6787–6789. [Google Scholar] [CrossRef]
- Nam, T.-G.; Nara, S. J.; Zagol-Ikapitte, I.; Cooper, T.; Valgimigli, L.; Oates, J. A.; Porter, N. A.; Boutaud, O.; Pratt, D. A. Pyridine and Pyrimidine Analogs of Acetaminophen as Inhibitors of Lipid Peroxidation and Cyclooxygenase and Lipoxygenase Catalysis. Org. Biomol. Chem. 2009, 7, 5103–5112. [Google Scholar] [CrossRef]
- Serwa, R.; Nam, T.-g.; Valgimigli, L.; Culbertson, S.; Rector, C.L.; Jeong, B.-S.; Pratt, D.A.; Porter, N.A. Preparation and Investigation of Vitamin B 6-Derived Aminopyridinol Antioxidants. Chem. Eur. J. 2010, 16, 14106–14114. [Google Scholar] [CrossRef]
- Valgimigli, L.; Bartolomei, D.; Amorati, R.; Haidasz, E.; Hanthorn, J. J.; Nara, S. J.; Brinkhorst, J.; Pratt, D. A. 3-Pyridinols and 5-Pyrimidinols: Tailor-Made for Use in Synergistic Radical-Trapping Co-antioxidant Systems. Beilstein J. Org. Chem. 2013, 9, 2781–2792. [Google Scholar] [CrossRef]
- Valgimigli, L.; Ingold, K.U.; Lusztyk, J. Antioxidant activities of vitamin E analogues in water and a Kamlet - Taft β-value for water. J. Am. Chem. Soc. 1996, 118, 3545–3549. [Google Scholar] [CrossRef]
- Valgimigli, L.; Ingold, K.U.; Lusztyk, J. Solvent effects on the reactivity and free spin distribution of 2,2-diphenyl-1-picrylhydrazyl radicals. J. Org. Chem. 1996, 61, 7947–7950. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, M.; Pedulli, G.F.; Valgimigli, L. Do Peroxyl Radicals Obey the Principle That Kinetic Solvent Effects on H-Atom Abstraction Are Independent of the Nature of the Abstracting Radical? J. Org. Chem. 1988, 63, 4497–449926. [Google Scholar] [CrossRef]
- Abraham, M. H.; Grellier, P. L.; Prior, D. V.; Taft, R. W.; Morris, J. J.; Taylor, P. J.; Laurence, C.; Berthelot, M.; Doherty, R. M.; Kamlet, M. J.; Abboud, J. L. M.; Sraidi, K.; Guiheneuf, G. A general treatment of hydrogen bond complexation constants in tetrachloromethane. J. Am. Chem. Soc. 1988, 110, 8534–8536. [Google Scholar] [CrossRef]
- Abraham, M. H.; Grellier, P. L.; Prior, D. V.; Morris, J. J.; Taylor, P. J. Hydrogen bonding. Part 10. A scale of solute hydrogen-bond basicity using log K values for complexation in tetrachloromethane. J. Chem. Soc., Perkin Trans. 2 1990, 521–529. [CrossRef]
- Snelgrove, D.W.; Lusztyk, J.; Banks, J.T.; Mulder, P.; Ingold, K.U. Kinetic Solvent Effects on Hydrogen-Atom Abstractions: Reliable, Quantitative Predictions via a Single Empirical Equation. J. Am. Chem. Soc. 2001, 123, 469–477. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Morroni, G.; Gambino, R.; Valgimigli, L. Peroxyl Radical Reactions in Water Solution: A Gym for Proton-Coupled Electron-Transfer Theories. Chem. Eur. J. 2016, 22, 7924–7934. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L.; Pedulli, G.F.; Grabovskiy, S.A.; Kabalnova, N.N.; Chatgilialoglu, C. Base-promoted reaction of 5-hydroxyuracil derivatives with peroxyl radicals. Org. Lett. 2010, 12, 4130–4133. [Google Scholar] [CrossRef]
- Amorati, R. , Pedulli, G.F., Valgimigli, L. Kinetic and thermodynamic aspects of the chain-breaking antioxidant activity of ascorbic acid derivatives in non-aqueous media. Org. Biomol. Chem. 2011, 9, 3792–3800. [Google Scholar] [CrossRef]
- Baschieri, A.; Amorati, R.; Benelli, T.; Mazzocchetti, L.; D’Angelo, E.; Valgimigli, L. Enhanced antioxidant activity under biomimetic settings of ascorbic acid included in halloysite nanotubes. Antioxidants 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Amorati, R.; Petrucci, S.; Pedulli, G.F.; Hu, D.; Hanthorn, J.J.; Pratt, D.A. Unexpected acid catalysis in reactions of peroxyl radicals with phenols. Angew. Chem. Int. Ed. 2009, 48, 8348–8351. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Amorati, R.; Fumo, M.G.; DiLabio, G.A.; Pedulli, G.F.; Ingold, K.U.; Pratt, D.A. The unusual reaction of semiquinone radicals with molecular oxygen. J. Org. Chem. 2008, 73, 1830–1841. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L.; Panzella, L.; Napolitano, A.; D'Ischia, M. 5-S-lipoylhydroxytyrosol, a multidefense antioxidant featuring a solvent-tunable peroxyl radical-scavenging 3-thio-1,2-dihydroxybenzene motif. J. Org. Chem., 2013, 78, 9857–9864. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Cowden, A.; Valgimigli, L. The Antioxidant Activity of Quercetin in Water Solution. Biomimetics 2017, 2, 9. [Google Scholar] [CrossRef]
- Lucarini, M.; Pedulli, G.F.; Valgimigli, L.; Amorati,R. ; Minisci, F. Thermochemical and kinetic studies of a bisphenol antioxidant. J. Org. Chem. 2001, 66, 5456–546210. [Google Scholar] [CrossRef]
- Matera, R.; Gabbanini, S.; Berretti, S.; Amorati, R.; De Nicola, G.R.; Iori, R.; Valgimigli, L. Acylated anthocyanins from sprouts of Raphanus sativus cv. Sango: Isolation, structure elucidation and antioxidant activity. Food Chem. 2015, 166, 397–406. [Google Scholar] [CrossRef]
- Niki, E.; Saito, T.; Kawakami, A.; Kamiya, Y. Inhibition of oxidation of methyl linoleate in solution by vitamin E and vitamin C. J. Biol. Chem. 1984, 259, 4177–4182. [Google Scholar] [CrossRef]
- Bowry, V.W.; Ingold, K.U. The Unexpected Role of Vitamin E (α-Tocopherol) in the Peroxidation of Human Low-Density Lipoprotein. Acc. Chem. Res. 1999, 32, 27–34. [Google Scholar] [CrossRef]
- Valgimigli, L.; Lucarini, M.; Pedulli, G.F.; Ingold, K.U. Does β-carotene really protect vitamin E from oxidation? J. Am. Chem. Soc. 1997, 119, 8095–8096. [Google Scholar] [CrossRef]
- Alfieri, M.L.; Panzella, L.; Amorati, R.; Cariola, A.; Valgimigli, L.; Napolitano, A. Role of Sulphur and Heavier Chalcogens on the Antioxidant Power and Bioactivity of Natural Phenolic Compounds. Biomolecules 2022, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Pedulli, G.F.; Valgimigli, L.; Johansson, H.; Engman, L. Organochalcogen substituents in phenolic antioxidants. Org. Lett. 2010, 12, 2326–2329. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Johansson, H.; Engman, L.; Valgimigli, L.; Amorati, R. , Fumo, M.G.; Pedulli, G.F. Regenerable chain-breaking 2,3-dihydrobenzo[b]selenophene-5-ol antioxidants. J. Org. Chem. 2007, 72, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Johansson, H.; Kanda, T.; Engman, L.; Muller, T.; Jonsson, M.; Pedulli, G.F.; Petrucci, S.; Valgimigli, L. Catalytic chain-breaking pyridinol antioxidants. Org. Lett., 2008, 10, 4895–4898. [Google Scholar] [CrossRef]
- Johansson, H.; Shanks, D.; Engman, L.; Amorati, R.; Pedulli, G.F.; Valgimigli, L. Long-lasting antioxidant protection: A regenerable BHA analogue. J. Org. Chem. 2010, 75, 7535–7541. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Valgimigli, L.; Dinér, P.; Bakhtiari, K.; Saeedi, M.; Engman, L. Multi-faceted reactivity of alkyltellurophenols towards peroxyl radicals: Catalytic antioxidant versus thiol-depletion effect. Chem. Eur. J. 2013, 19, 7510–7522. [Google Scholar] [CrossRef]
- McGrath, A.J.; Garrett, G.E.; Valgimigli, L.; Pratt, D.A. The redox chemistry of sulfenic acids. J. Am. Chem. Soc. 2010, 132, 16759–16761. [Google Scholar] [CrossRef]
- Amorati, R. , Lynett, P.T., Valgimigli, L., Pratt, D.A. The reaction of sulfenic acids with peroxyl radicals: Insights into the radical-trapping antioxidant activity of plant-derived thiosulfinates. Chem. Eur. J. 2012, 18, 6370–6379. [Google Scholar] [CrossRef]
- Zielinski, Z.; Presseau, N.; Amorati, R.; Valgimigli, L.; Pratt, D.A. Redox chemistry of selenenic acids and the insight it brings on transition state geometry in the reactions of peroxyl radicals. J. Am. Chem. Soc. 2014, 136, 1570–1578. [Google Scholar] [CrossRef]
- Hanthorn, J.J.; Valgimigli, L.; Pratt, D.A. Preparation of highly reactive pyridine- and pyrimidine-containing diarylamine antioxidants. J. Org. Chem. 2012, 77, 6908–6916. [Google Scholar] [CrossRef]
- Hanthorn, J.J.; Amorati, R.; Valgimigli, L.; Pratt, D.A. The reactivity of air-stable pyridine- and pyrimidine-containing diarylamine antioxidants. J. Org. Chem. 2012, 77, 6895–6907. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Haidasz, E.A.; Valgimigli, L.; Pratt, D.A. Unprecedented inhibition of hydrocarbon autoxidation by diarylamine radical-trapping antioxidants. J. Am. Chem. Soc. 2015, 137, 2440–2443. [Google Scholar] [CrossRef] [PubMed]
- Vaz, S. M.; Augusto, O. Reactive Oxygen Species Special Feature: Inhibition of myeloperoxidase-mediated protein nitration by tempol: Kinetics, mechanism, and implications. Proc. Natl. Acad. Sci. U.S.A., 2008, 105, 8191–8196. [Google Scholar] [CrossRef]
- Bi, W.; Bi, Y.; XGao, X.; Li, P.; Hou, S.; Zhang, Y.; Bammert, C.; Jockusch, S.; Legalley, T.D.; Gibson, K.M.; Bi, L. Indole-TEMPO conjugates alleviate ischemia-reperfusion injury via attenuation of oxidative stress and preservation of mitochondrial function. Bioorg. Med. Chem. 2017, 5, 2545–2568. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Merenyi, G.; Russo, A.; Samuni, A. The Role of Oxoammonium Cation in the SOD-Mimic Activity of Cyclic Nitroxides. J. Am. Chem. Soc. 2003, 125, 789–795. [Google Scholar] [CrossRef]
- Goldstein, S.; Samuni, A. Kinetics and Mechanism of Peroxyl Radical Reactions with Nitroxides. J. Phys. Chem. A 2007, 111, 1066–1072. [Google Scholar] [CrossRef]
- Amorati, R.; Pedulli, G. F.; Pratt, D. A.; Valgimigli, L. TEMPO reacts with oxygen-centered radicals under acidic conditions. Chem. Commun. 2010, 46, 5139–5141. [Google Scholar] [CrossRef]
- Haidasz, E. A.; Meng, D.; Amorati, R.; Baschieri, A.; Ingold, K. U.; Valgimigli, L.; Pratt, D. A. Acid Is Key to the Radical-Trapping Antioxidant Activity of Nitroxides. J. Am. Chem. Soc. 2016, 138, 5290–5298. [Google Scholar] [CrossRef]
- Baschieri, A.; Valgimigli, L.; Gabbanini, S.; DiLabio, G.A.; Romero-Montalvo, E.; Amorati, R. Extremely Fast Hydrogen Atom Transfer between Nitroxides and HOO· Radicals and Implication for Catalytic Coantioxidant Systems J. Am. Chem. Soc. 2018, 140, 10354–10362. [Google Scholar] [CrossRef]
- Guo, Y.; Baschieri, A.; Amorati, R.; Valgimigli, L. Synergic antioxidant activity of γ-terpinene with phenols and polyphenols enabled by hydroperoxyl radicals. Food Chem. 2021, 345, 128468. [Google Scholar] [CrossRef]
- Baschieri, A.; Daci Ajvazi, M.; Folifack Tonfack, J.L.; Valgimigli, L.; Amorati, R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017, 232, 656–663. [Google Scholar] [CrossRef]
- Guo, Y.; Pizzol, R.; Gabbanini, S.; Baschieri, A.; Amorati, R.; Valgimigli, L. Absolute Antioxidant Activity of Five Phenol-Rich Essential Oils. Molecules 2021, 26, 5237. [Google Scholar] [CrossRef] [PubMed]
- Khoobchandani, M. , Ganesh, N., Gabbanini, S., Valgimigli, L., Srivastava, M.M. Phytochemical potential of Eruca sativa for inhibition of melanoma tumor growth. Fitoterapia, 2011; 82, 647–653. [Google Scholar] [CrossRef]
- Cores, Á.; Piquero, M.; Villacampa, M.; León, R.; Menéndez, J.C. NRF2 Regulation Processes as a Source of Potential Drug Targets against Neurodegenerative Diseases. Biomolecules 2020, 10, 904. [Google Scholar] [CrossRef] [PubMed]
- Matera, R.; Lucchi, E.; Valgimigli, L. Plant Essential Oils as Healthy Functional Ingredients of Nutraceuticals and Diet Supplements: A Review. Molecules 2023, 28, 901. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, M.Y.; Soltani, A.; Khodarahmi, Z.; Alameri, A.A.; Alwan, A.M.R.; Ramírez-Coronel, A.A.; Obaid, R.F.; Abosaooda, M.; Heidari, M.; Golmohammadi, M.; Anoush, M. Targeting Nrf2 signaling pathway by quercetin in the prevention and treatment of neurological disorders: An overview and update on new developments. Fundam. Clin. Pharmacol. 2023, 1–15. [Google Scholar] [CrossRef]
| Lipid |
kp/(2kt)1/2 10-5 M-1/2s-1/2 |
kp M-1s-1 |
2kt 105 M-1s-1 |
Ref. |
|---|---|---|---|---|
| Methyl Stearate (18:0) 1 | ~0.8 | ~0.01 | 15 | [21] |
| Methyl Oleate (18:1) | 89.0 | 0.89 | 10 | [22] |
| Methyl Linoleate (18:2) | 2100 | 62.0 | 88 | [22] |
| Methyl linolenate (18:3) | 3900 | 236.0 | 360 | [22] |
| Linoleic acid (18:2) | - | 62 | - | [23] |
| Arachidonic acid (20:4) | - | 197 | - | [23] |
| Eicosapentaenoic ac. (20:5) | - | 249 | - | [23] |
| Docosahexaenoic ac. (22:6) | - | 334 | - | [23] |
| Cholesterol | - | 11 | - | [23] |
| 7-Dehydrocholesterol | - | 2260 | - | [23] |
| Squalene | 2500 | 68.0 | 74.0 | [28] |
| Sunflower oil (60% of 18:2) | 3600 | 66.9 | 34.5 | [28] |
| PLPC 2 | - | 16.6 | 1.27 | [41] |
| DLPC 3 | - | 13.6 4 | 1.02 | [41] |
| Entry | Compound | BDE kcal/mol |
kinh M-1s-1 |
n | Ref. |
|---|---|---|---|---|---|
| 1 | α-Tocopherol | 77.1 | 3.2×106 | 2.0 | [3,21] |
| 2 | β-Tocopherol | - | 1.3×106 | 2.0 | [3,21] |
| 3 | γ-Tocopherol | - | 1.4×106 | 2.0 | [3,21] |
| 4 | δ-Tocopherol | - | 4.4×105 | 2.0 | [3,21] |
| 5 | 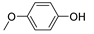 |
81.7 | 2.7×105 | 2.0 | [21,82] |
| 6 | 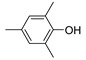 |
81.6 | 8.5×104 | 2.0 | [21,82] |
| 7 | 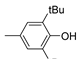 |
79.9 | 1.4×104 | 2.0 | [21,82] |
| 8 | 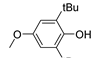 |
77.2 | 1.1×105 | 2.0 | [21,82] |
| 9 | 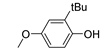 |
80.3 | 6.4×105 | 1.8 | [21,82] |
| 10 | 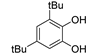 |
78.2 | 1.1×106 | 2.0 | [82,108] |
| 11 | 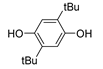 |
79.2 | 1.6×106 1 | 0.3 | [107] |
| 12 | Quercetin | - | 5.5×105 | 2.1 | [108] |
| 13 | Pyr-1 2 | 81.4 | 2.1×105 | 2.0 | [88] |
| 14 | Pyr-2 2 | 77.1 | 8.6×106 | 2.0 | [88] |
| 15 | Pyr-3 | 78.9 | 4.4×105 | 2.1 | [94] |
| 16 | Pyr-4 | 75.9 | 1.6×107 | 2.0 | [89] |
| 17 | Pyr-5 | 75.2 | 8.8×107 | 1.3-2.0 | [89] |
| 18 | Pyr-6 | 75.2 | 8.8×107 | ~2 | [91] |
| 19 | Pyr-7 | 74.3 | 2.8×108 | ~2 | [89] |
| 20 | α-Selenotocopherol | 78.1 | 1.2×106 | 1.9 | [115] |
| 21 | 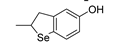 |
81,6 | 3.8×105 | 2.0 | [117] |
| 22 | Te-1 | - | 9.2×106 | 0.4 6 | [118] |
| 23 | Te-2 | 78.9 | 1.0×107 | 0.4 6 | [119] |
| 24 | Te-3 | - | 1.6×106 | 0.3 6 | [120] |
| 25 | Te-4 | - | 1.0×107 | 0.4 6 | [120] |
| 26 | Phenoxazine 2,3 | 76.1 | 2.9×107 | 5 | [82] |
| 27 | Phenothiazine 2,4 | 78.2 | 8.8×106 | 1.8 | [82] |
| 28 | Dia-1 5 | 78.8 | 3.4×107 | >2 | [125] |
| 29 | Dia-2 5 | 79.0 | 3.7×107 | >2 | [125] |
| 30 | Ferrostatin-1 5 | - | 3.5×105 | 2.0 | [11] |
| 31 | Liproxstatin-1 5 | - | 2.4×105 | 1.9 | [11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





