Submitted:
14 July 2023
Posted:
18 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
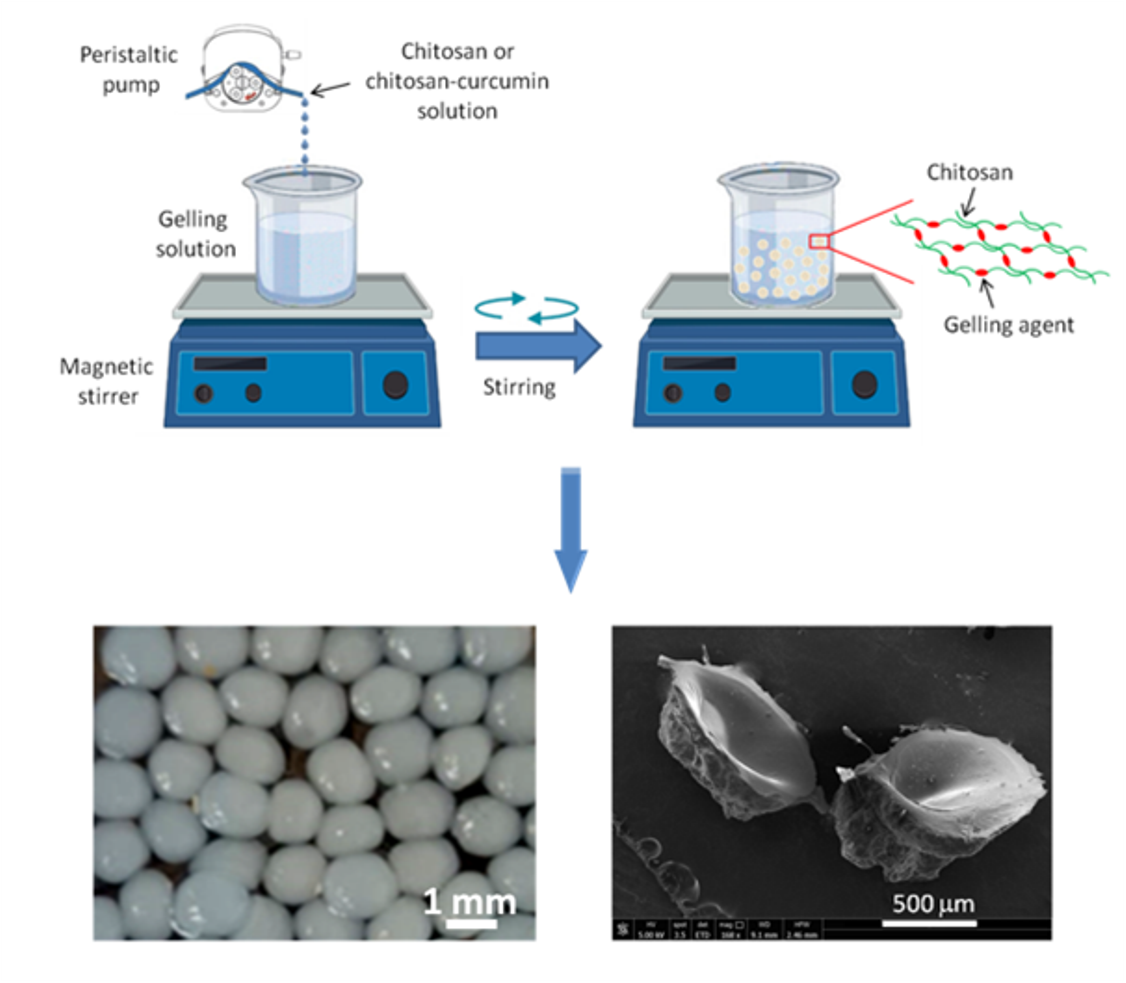
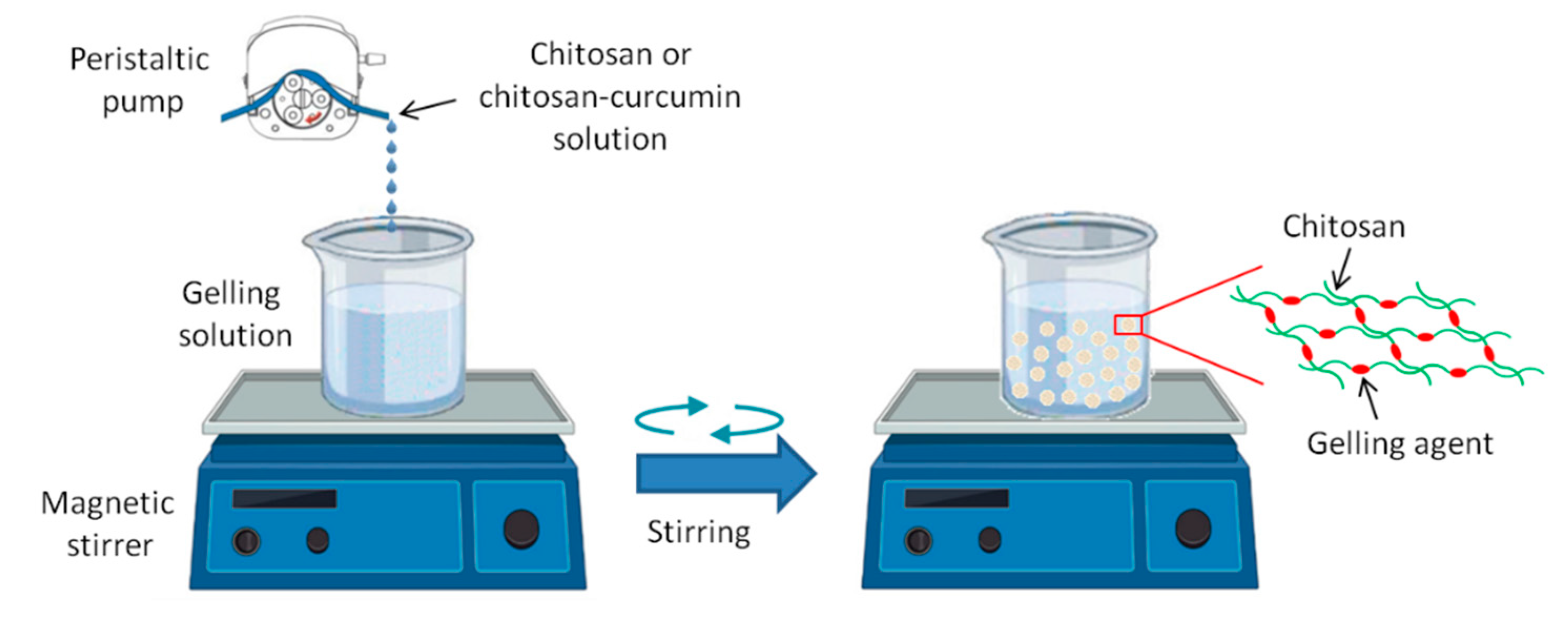
2.2. Preparation of the CS- and CS- CUR- based macrobeads
2.3. Characterization of the CS- and CS- CUR macrobeads
3. Results
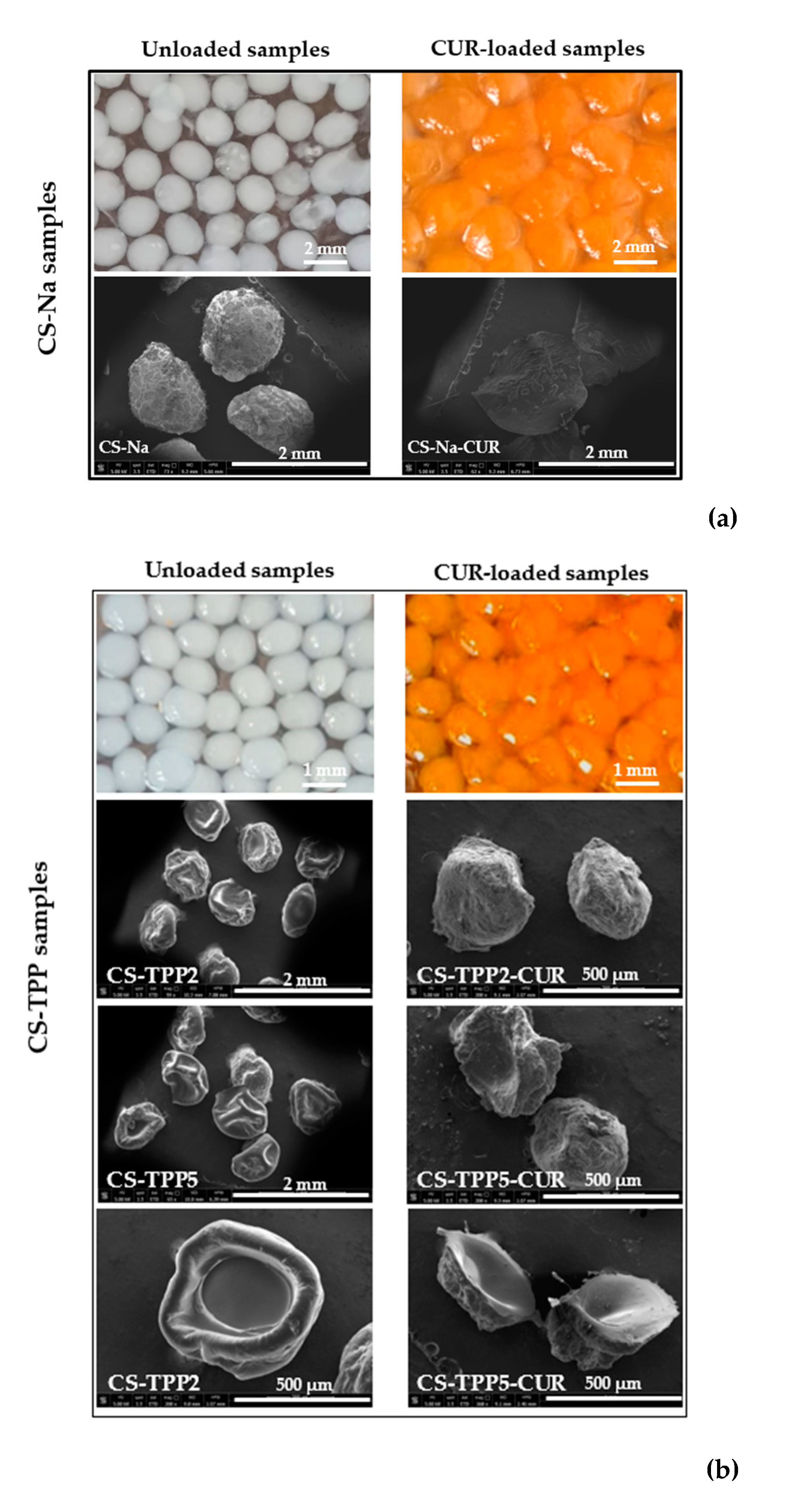
3.1. Morphological Characterization of the macrobeads
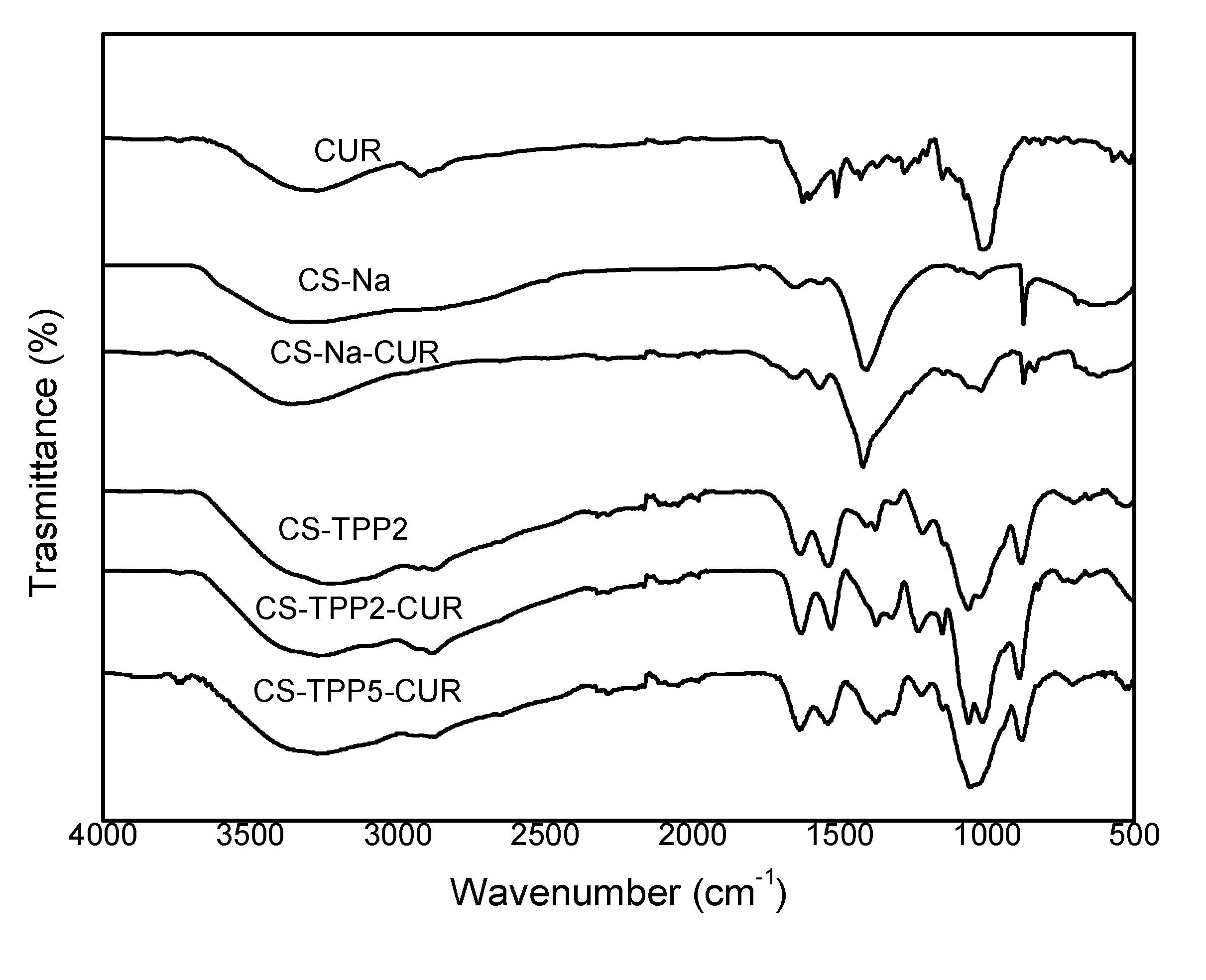
3.2. FTIR analyses
3.3. TGA and DSC analyses
3.4. Evaluation of encapsulation and swelling degree of CUR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shariatinia, Z. , Pharmaceutical applications of chitosan. Advances in colloid and interface science 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, I.; Kertmen, A. , Methods of Chitosan Identification: History and Trends. Letters in Applied NanoBioScience 2023, 12, 94. [Google Scholar]
- Madian, N.G.; El-Ashmanty, B.A.; Abdel-Rahim, H.K. , Improvement of Chitosan Films Properties by Blending with Cellulose, Honey and Curcumin. Polymers 2023, 15, 2587. [Google Scholar] [CrossRef]
- Dhanavel, S.; Nivethaa, E.A.K.; Narayanan, V.; Stephen, A. , In vitro cytotoxicity study of dual drug loaded chitosan/palladium nanocomposite towards HT-29 cancer cells. Materials Science and Engineering: C 2017, 75, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Lal, J.; Gupta, S.K.; Agarwal, D.D. , Chitosan: An efficient biodegradable and recyclable green catalyst for one-pot synthesis of 3,4-dihydropyrimidinones of curcumin in aqueous media. Catalysis Communications 2012, 27, 38–43. [Google Scholar] [CrossRef]
- Perez, J.J.; Francois, N.J.; Maroniche, G.A.; Borrajo, M.P.; Pereyra, M.A.; Creus, C.M. , A novel, green, low-cost chitosan-starch hydrogel as potential delivery system for plant growth-promoting bacteria. Carbohydrate Polymers 2018, 202, 409–417. [Google Scholar] [CrossRef]
- Dhanavel, S.; Praveena, P.; Narayanan, V.; Stephen, A. , Chitosan/reduced graphene oxide/Pd nanocomposites for co-delivery of 5-fluorouracil and curcumin towards HT-29 colon cancer cells. Polymer Bulletin 2020, 77, 5681–5696. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-C.; Qian, L.-H.; Zhang, Y.-H.; Gong, P.-X.; Liu, W.; Li, H.-J. , Fabrication of foxtail millet prolamin/caseinate/chitosan hydrochloride composite nanoparticles using antisolvent and pH-driven methods for curcumin delivery. Food chemistry 2023, 404, 134604. [Google Scholar] [CrossRef]
- Chen, W.; Shen, X.; Hu, Y.; Xu, K.; Ran, Q.; Yu, Y.; Dai, L.; Yuan, Z.; Huang, L.; Shen, T.; Cai, K. , Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ROS-induced cells damage and improvement of osteogenesis. Biomaterials 2017, 114, 82–96. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Li, M.; Wu, H.; Zhen, T.; Xiong, L.; Sun, Q. , pH-Sensitive Chitosan-Sodium Phytate Core-Shell Hollow Beads and Nanocapsules for the Encapsulation of Active Ingredients. Journal of agricultural and food chemistry 2019, 67, 2894–2905. [Google Scholar] [CrossRef]
- Soliman, G.M.; Zhang, Y.L.; Merle, G.; Cerruti, M.; Barralet, J. , Hydrocaffeic acid-chitosan nanoparticles with enhanced stability, mucoadhesion and permeation properties. European Journal of Pharmaceutics and Biopharmaceutics 2014, 88, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, Y.; Dong, L.; Chen, P.; Liu, W.; Yang, L. , Cartilage-targeting and inflammatory-responsive nanocarriers for effective osteoarthritis treatment via reactive oxygen species scavenging and anti-angiogenesis. Journal of Materials Science & Technology 2023, 143, 30–42. [Google Scholar]
- Jagtap, S.; Thakre, D.; Wanjari, S.; Kamble, S.; Labhsetwar, N.; Rayalu, S. , New modified chitosan-based adsorbent for defluoridation of water. Journal of colloid and interface science 2009, 332, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. , Insight on the formation of chitosan nanoparticles through ionotropic gelation with tripolyphosphate. Molecular pharmaceutics 2012, 9, 2856–2862. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. , Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids and surfaces. B, Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, P.; Zeng, H.; Rui, Z. , Construction of porous chitosan macrospheres via dual pore-forming strategy as host for alkaline protease immobilization with high activity and stability. Carbohydrate Polymers 2023, 305, 120476. [Google Scholar] [CrossRef]
- Fajardo, H.V.; Martins, A.O.; de Almeida, R.M.; Noda, L.K.; Probst, L.F.D.; Carreño, N.L.V.; Valentini, A. , Synthesis of mesoporous Al2O3 macrospheres using the biopolymer chitosan as a template: A novel active catalyst system for CO2 reforming of methane. Materials Letters 2005, 59, 3963–3967. [Google Scholar] [CrossRef]
- Muresan, E.I.; Drobota, M.; Bargan, A.; Dumitriu, C.A.M. , Hard porous chromium containing macrospheres as new catalysts for the esterification reaction of acetic acid with epichlorohydrin. Central European Journal of Chemistry 2014, 12, 528–536. [Google Scholar] [CrossRef]
- Luan, Q.; Zhang, H.; Wang, J.; Li, Y.; Gan, M.; Deng, Q.; Cai, L.; Tang, H.; Huang, F. , Electrostatically reinforced and sealed nanocellulose-based macrosphere by alginate/chitosan multi-layer coatings for delivery of probiotics. Food Hydrocolloids 2023, 142, 108804. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Z.; Ma, M.; Sheng, L.; Huang, X. , Effect of eggshell membrane as porogen on the physicochemical structure and protease immobilization of chitosan-based macroparticles. Carbohydrate Polymers 2020, 242, 116387. [Google Scholar] [CrossRef]
- Behbahani, E.; Ghaedi, M.; Abbaspour, M.; Rostamizadeh, K.; Dashtian, K. , Curcumin loaded nanostructured lipid carriers: In vitro digestion and release studies. Polyhedron 2019, 164, 113–122. [Google Scholar] [CrossRef]
- Mandal, D.; Sarkar, T.; Chakraborty, R. , Critical Review on Nutritional, Bioactive, and Medicinal Potential of Spices and Herbs and Their Application in Food Fortification and Nanotechnology. Applied biochemistry and biotechnology 2023, 195, 1319–1513. [Google Scholar] [CrossRef]
- Fitriani, L.; Azizah, H.; Hasanah, U.; Zaini, E. , ENHANCEMENT OF CURCUMIN SOLUBILITY AND DISSOLUTION BY ADSORPTION IN MESOPOROUS SBA-15. International Journal of Applied Pharmaceutics 2023, 15, 61–67. [Google Scholar] [CrossRef]
- Krishnan, V.; Venkatasubbu, G.D.; Kalaivani, T. , Investigation of hemolysis and antibacterial analysis of curcumin-loaded mesoporous SiO2 nanoparticles. Applied Nanoscience 2023, 13, 811–818. [Google Scholar] [CrossRef]
- Liang, F.; Wang, M.; Hu, Y.; Guo, Z.; Yang, W. , Cetyltrimethylammonium bromide promoted dispersing and incorporation of curcumin into silica particles in alkaline ethanol/water mixture. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 624, 126789. [Google Scholar] [CrossRef]
- Saputra, O.A.; Wibowo, F.R.; Lestari, W.W. , High storage capacity of curcumin loaded onto hollow mesoporous silica nanoparticles prepared via improved hard-templating method optimized by Taguchi DoE. Engineering Science and Technology, an International Journal 2022, 33, 101070. [Google Scholar] [CrossRef]
- Meng, W.; Sun, H.; Mu, T.; Garcia-Vaquero, M. , Chitosan-based Pickering emulsion: A comprehensive review on their stabilizers, bioavailability, applications and regulations. Carbohydrate Polymers 2023, 304, 120491. [Google Scholar] [CrossRef]
- El-Sherbiny, M.; Elekhtiar, R.; El-Hefnawy, M.; Mahrous, H.; Alhayyani, S.; Al-Goul, S.; Orif, M.; Tayel, A. , Fabrication and assessment of potent anticancer nanoconjugates from chitosan nanoparticles, curcumin, and eugenol. Frontiers in Bioengineering and Biotechnology 2022, 10, 1030936. [Google Scholar] [CrossRef]
- Ishak, N.A.; Hamidon, T.S.; Zi-Hui, T.; Hussin, M.H. , Extracts of curcumin-incorporated hybrid sol–gel coatings for the corrosion mitigation of mild steel in 0.5 M HCl. Journal of Coatings Technology and Research 2020, 17, 1515–1535. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. , beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids and surfaces. B, Biointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. , Curcumin: the story so far. European journal of cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, Y. , Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. International journal of pharmaceutics 2003, 250, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Laus, R.; Laranjeira, M.C.M.; Martins, A.O.; Fávere, V.T.; Pedrosa, R.C.; Benassi, J.C.; Geremias, R. , MICROESFERAS DE QUITOSANA RETICULADAS COM TRIPOLIFOSFATO UTILIZADAS PARA REMOÇÃO DA ACIDEZ, FERRO(III) E MANGANÊS(II) DE ÁGUAS CONTAMINADAS PELA MINERAÇÃO DE CARVÃO. Quimica Nova 2006, 29, 34–39. [Google Scholar] [CrossRef]
- Lee, S.-T.; Mi, F.-L.; Shen, Y.-J.; Shyu, S.-S. , Equilibrium and kinetic studies of copper(II) ion uptake by Chitosan-tripolyphosphate chelating resin. Polymer 2001, 42, 1879–1892. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. , In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors. European Journal of Pharmaceutical Sciences 2008, 35, 404–416. [Google Scholar] [CrossRef]
- Parize, A.; Stulzer, H.; Laranjeira, M.; Brighente, I.I.; Souza, T. , Evaluation of chitosan microparticles containing curcumin and crosslinked with sodium tripolyphosphate produced by spray drying. Química Nova 2012, 35, 1127–1132. [Google Scholar] [CrossRef]
- Desai, K.G.; Park, H. , Preparation and characterization of drug-loaded chitosan-tripolyphosphate microspheres by spray drying. Drug Development Research 2005, 64, 114–128. [Google Scholar] [CrossRef]
- Desai, K.G.; Park, H.J. , Encapsulation of vitamin C in tripolyphosphate cross-linked chitosan microspheres by spray drying. Journal of microencapsulation 2005, 22, 179–192. [Google Scholar] [CrossRef]
- Anal, A.K.; Stevens, W.F.; Remuñán-López, C. , Ionotropic cross-linked chitosan microspheres for controlled release of ampicillin. International journal of pharmaceutics 2006, 312, 166–173. [Google Scholar] [CrossRef]
- Liu, C.; Desai, K.G.; Tang, X.; Chen, X. , Drug Release Kinetics of Spray-Dried Chitosan Microspheres. Drying Technology - DRY TECHNOL 2006, 24, 769–776. [Google Scholar] [CrossRef]
- RemunanLopez, C.; Bodmeier, R. , Mechanical, water uptake and permeability properties of crosslinked chitosan glutamate and alginate films. Journal of Controlled Release 1997, 44, 215–225. [Google Scholar] [CrossRef]
- Katas, H.; Hussain, Z.; Ling, T.C. , Chitosan Nanoparticles as a Percutaneous Drug Delivery System for Hydrocortisone. Journal of Nanomaterials 2012, 2012, 372725. [Google Scholar] [CrossRef]
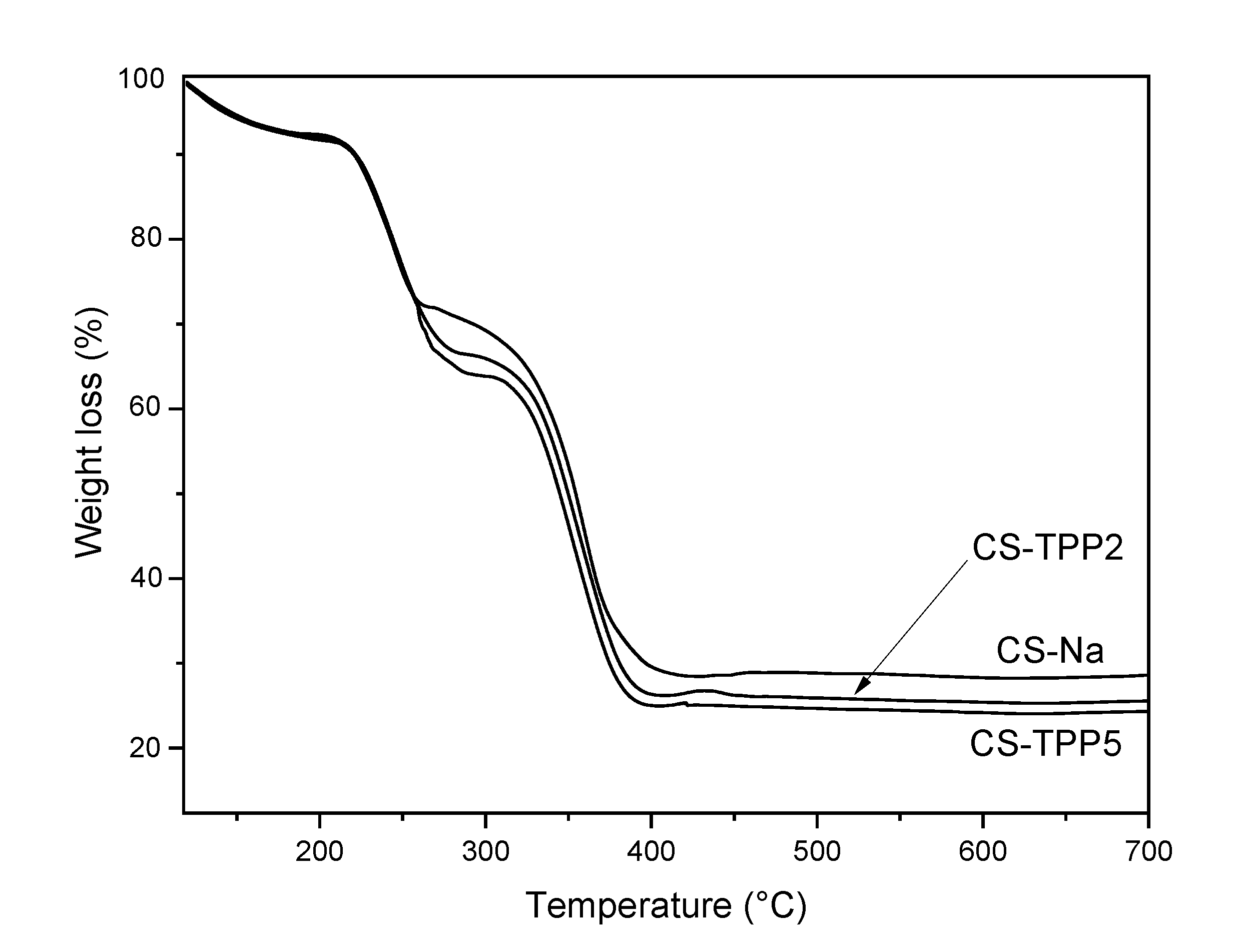
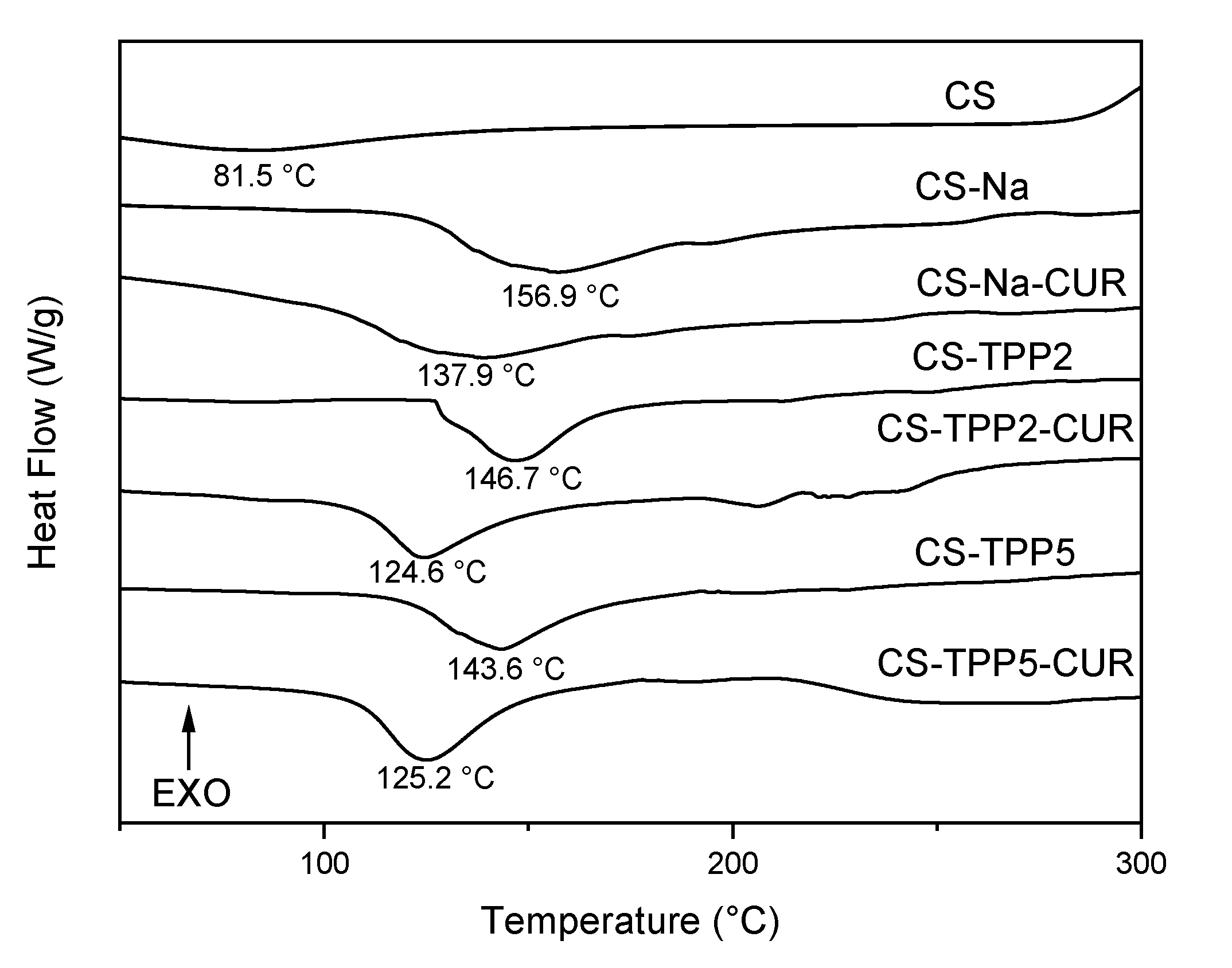
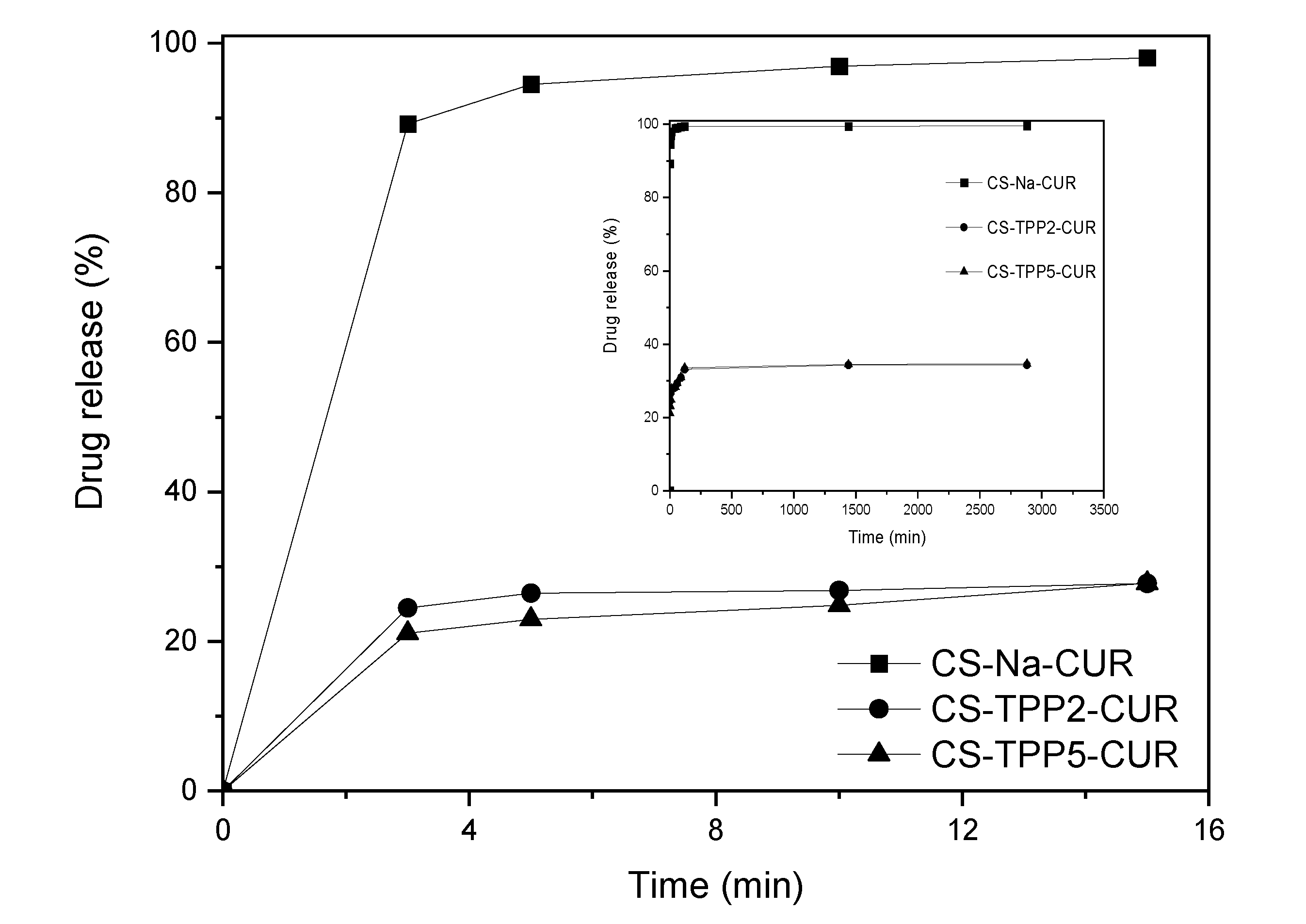
| Sample code | CS (g) | Cross-linking agent | CUR (g) |
|---|---|---|---|
| CS-Na | 0.5 | NaOH 4 wt% | - |
| CS-Na-CUR | 0.5 | NaOH 4 wt% | 0.1 |
| CS-TPP2 | 0.5 | TPP 2 wt% | - |
| CS-TPP2-CUR | 0.5 | TPP 2 wt% | 0.1 |
| CS-TPP5 | 0.5 | TPP 5 wt% | - |
| CS-TPP5-CUR | 0.5 | TPP 5 wt% | 0.1 |
| Sample code | Encapsulation efficiency (wt%) | Swelling degree (wt%) |
|---|---|---|
| CS-Na-CUR | 99.8 | n.d. |
| CS-TPP2-CUR | 95.9 | 120 |
| CS-TPP5-CUR | 91.4 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




