Submitted:
17 July 2023
Posted:
18 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Perioperative and Postoperative Outcomes
2.3. Angiographic and Surgical Procedure
2.4. Follow-up
2.5. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. Operative Outcomes
3.3. Postoperative Outcomes
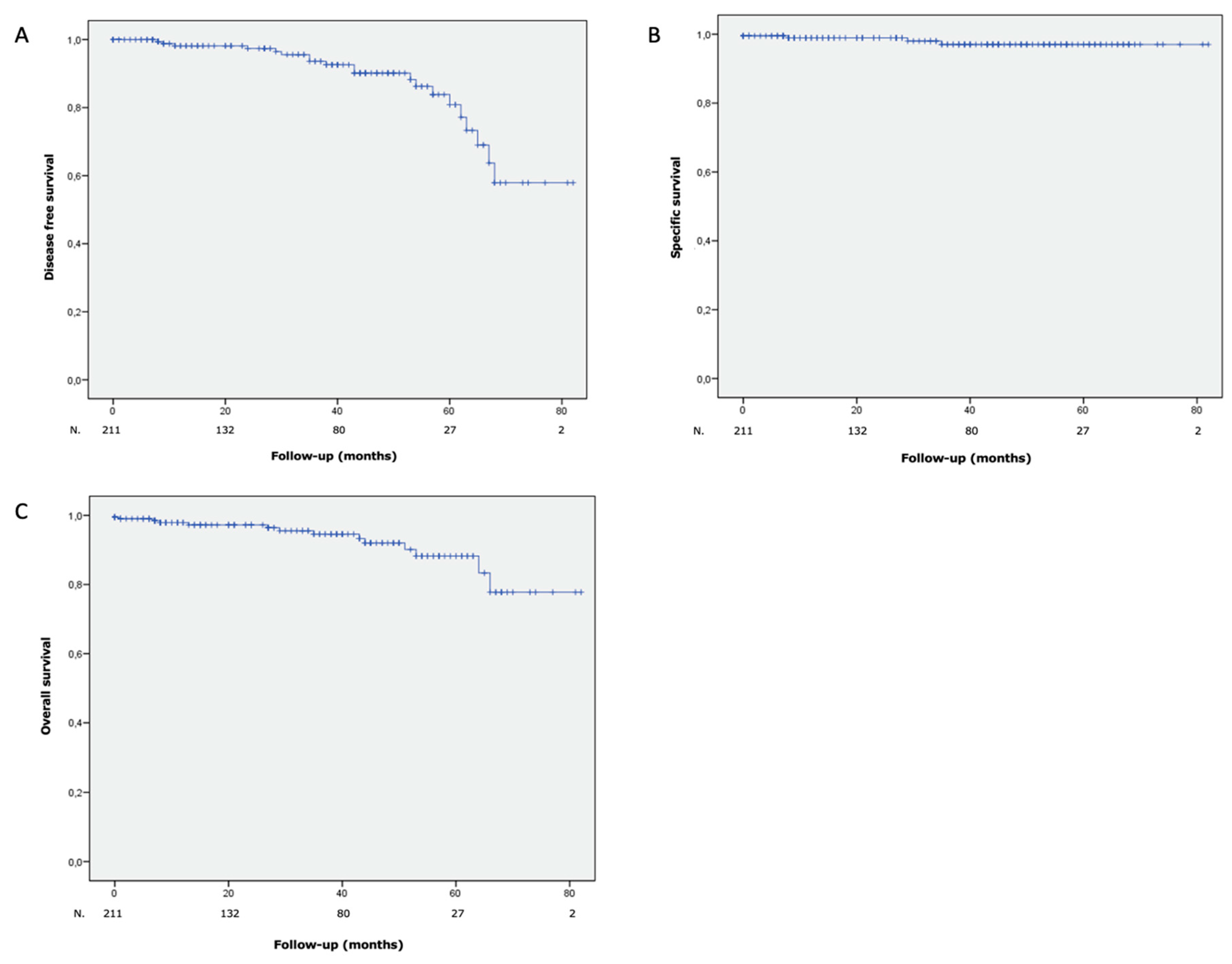
3.4. Oncological Outcomes
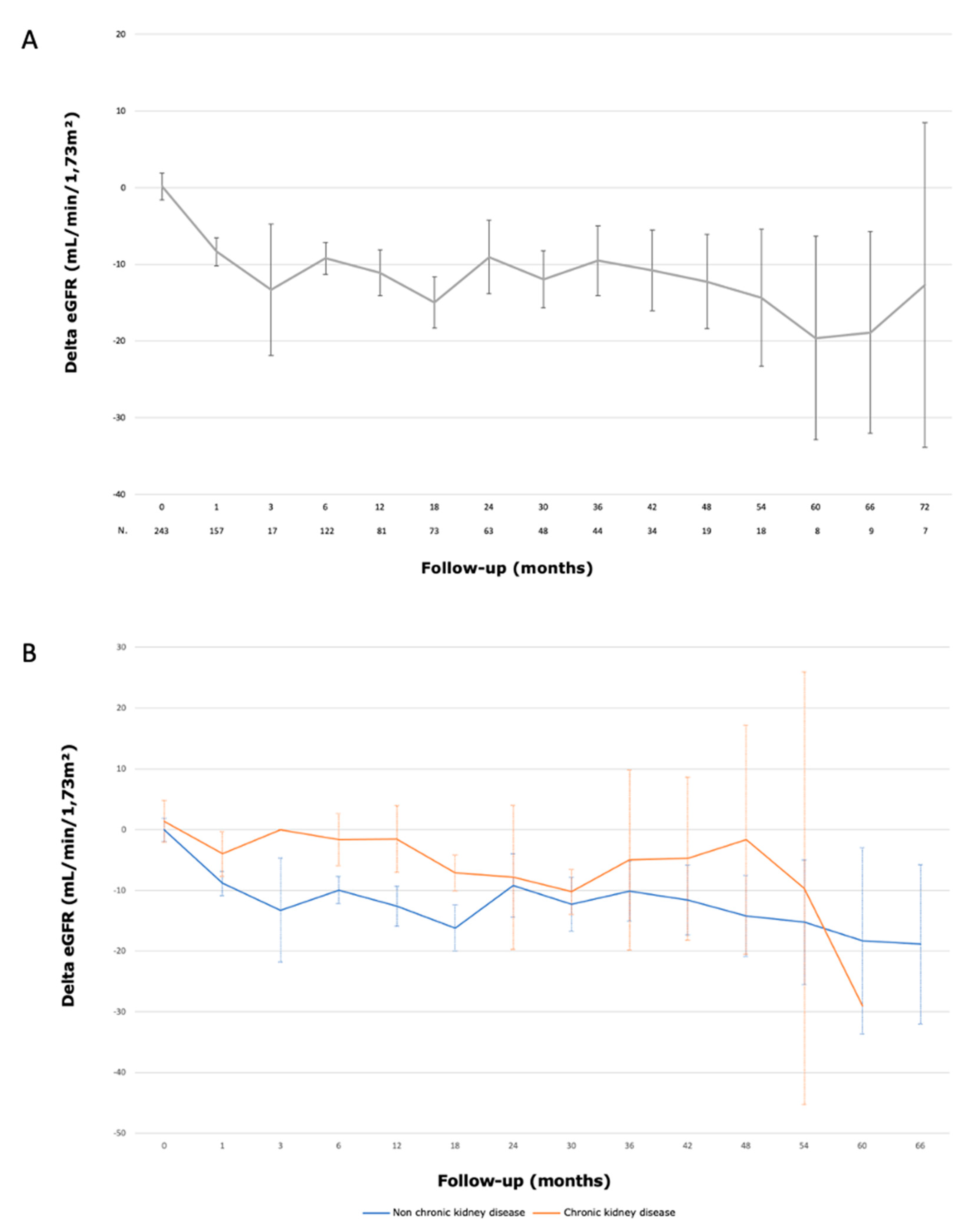
3.5. Functional Outcomes
4. Discussion
5. Conclusions
Author Contributions
Informed Consent Statement
References
- The Global Cancer Observatory.
- Bigot, P.; Barthelemy, P.; Boissier, R.; et al. French AFU Cancer Committee Guidelines - Update 2022-2024: management of kidney cancer. Prog En Urol. 2022, 32, 1195–1274. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- French Comittee of Urologic Oncology (CCAFU); Peyronnet, B.; Seisen, T.; et al. Comparison of 1800 Robotic and Open Partial Nephrectomies for Renal Tumors. Ann Surg Oncol. 2016, 23, 4277–4283. [Google Scholar] [CrossRef]
- Ingels, A.; Bensalah, K.; Beauval, J.B.; et al. Comparison of open and robotic-assisted partial nephrectomy approaches using multicentric data (UroCCR-47 study). Sci Rep. 2022, 12, 18981. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; You, J.H.; Kim, D.K.; Rha, K.H.; Lee, S.H. Comparison of Perioperative Outcomes Between Robotic and Laparoscopic Partial Nephrectomy: A Systematic Review and Meta-analysis. Eur Urol. 2015, 67, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Bertolo, R.; Amparore, D.; Fiori, C. Margins, ischaemia and complications rate after laparoscopic partial nephrectomy: impact of learning curve and tumour anatomical characteristics: MIC rate after LPN. BJU Int. 2013, 112, 1125–1132. [Google Scholar] [CrossRef]
- Simone, G.; Papalia, R.; Guaglianone, S.; Carpanese, L.; Gallucci, M. Zero Ischemia Laparoscopic Partial Nephrectomy After Superselective Transarterial Tumor Embolization for Tumors with Moderate Nephrometry Score: Long-Term Results of a Single-Center Experience. J Endourol. 2011, 25, 1443–1446. [Google Scholar] [CrossRef]
- D’Urso, L.; Simone, G.; Rosso, R.; et al. Benefits and shortcomings of superselective transarterial embolization of renal tumors before zero ischemia laparoscopic partial nephrectomy. Eur J Surg Oncol EJSO. 2014, 40, 1731–1737. [Google Scholar] [CrossRef]
- Benoit, M.; Bouvier, A.; Bigot, P. Salle hybride : pour quoi faire ? Prog En Urol. 2017, 27, 841–844. [Google Scholar] [CrossRef]
- Bigot, P.; Bouvier, A.; Panayotopoulos, P.; Aubé, C.; Azzouzi, A.R. Partial nephrectomy after selective embolization of tumor vessels in a hybrid operating room: A new approach of zero ischemia in renal surgery: Hybrid Operating Room for Kidney Surgery. J Surg Oncol. 2016, 113, 135–137. [Google Scholar] [CrossRef]
- Panayotopoulos, P.; Bouvier, A.; Besnier, L.; et al. Laparoscopic partial nephrectomy following tumor embolization in a hybrid room. Feasibility and clinical outcomes. Surg Oncol. 2017, 26, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Aubert, C.; Rolley, C.; Mauny, M.; et al. Évaluation de l’utilisation de la salle hybride sur l’activité de chirurgie en cancérologie rénale. Prog En Urol. 2020, 30, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Bréhier, G.; Bouvier, A.; Besnier, L.; et al. Renal function after partial nephrectomy following intra-arterial embolization of renal tumors. Sci Rep. 2020, 10, 21352. [Google Scholar] [CrossRef] [PubMed]
- Delahunt, B.; Srigley, J.R.; Egevad, L.; Montironi, R. International Society of Urological Pathology Grading and Other Prognostic Factors for Renal Neoplasia. Eur Urol. 2014, 66, 795–798. [Google Scholar] [CrossRef]
- Kutikov, A.; Uzzo, R.G. The R.E.N.A.L. Nephrometry Score: A Comprehensive Standardized System for Quantitating Renal Tumor Size, Location and Depth. J Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Bouvier, A.; Besnier, L.; Paisant, A.; et al. Blue Dye Embolization of Renal Tumor: A New Technique to Improve Tumor Localization During Laparoscopic Partial Nephrectomy. J Laparoendosc Adv Surg Tech. 2020, 30, 299–303. [Google Scholar] [CrossRef]
- The CCAFU members Pignot, G.; Méjean, A.; et al. The use of partial nephrectomy: results from a contemporary national prospective multicenter study. World J Urol. 2015, 33, 33–40. [Google Scholar] [CrossRef]
- Gill, I.S.; Kavoussi, L.R.; Lane, B.R.; et al. Comparison of 1,800 Laparoscopic and Open Partial Nephrectomies for Single Renal Tumors. J Urol. 2007, 178, 41–46. [Google Scholar] [CrossRef]
- Simmons, M.N.; Chung, B.I.; Gill, I.S. Perioperative Efficacy of Laparoscopic Partial Nephrectomy for Tumors Larger than 4cm. Eur Urol. 2009, 55, 199–208. [Google Scholar] [CrossRef]
- Masson-Lecomte, A.; Bensalah, K.; Seringe, E.; et al. A prospective comparison of surgical and pathological outcomes obtained after robot-assisted or pure laparoscopic partial nephrectomy in moderate to complex renal tumours: results from a French multicentre collaborative study: Outcomes after robot-assisted or pure laparoscopic partial nephrectomy. BJU Int. 2013, 111, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Bravi, C.A. The IRON Study: Investigation of Robot-assisted Versus Open Nephron-sparing Surgery. Published online 2023. [CrossRef] [PubMed]
- Hidas, G.; Lupinsky, L.; Kastin, A.; Moskovitz, B.; Groshar, D.; Nativ, O. Functional Significance of Using Tissue Adhesive Substance in Nephron-Sparing Surgery: Assessment by Quantitative SPECT of 99m Tc-Dimercaptosuccinic Acid Scintigraphy. Eur Urol. 2007, 52, 785–790. [Google Scholar] [CrossRef] [PubMed]
- George, A.K.; Herati, A.S.; Srinivasan, A.K.; et al. Perioperative outcomes of off-clamp vs complete hilar control laparoscopic partial nephrectomy: Off-clamp vs complete hilar control LPN. BJU Int 2013, 111, E235–E241. [Google Scholar] [CrossRef]
- Mir, M.C.; Ercole, C.; Takagi, T.; et al. Decline in Renal Function after Partial Nephrectomy: Etiology and Prevention. J Urol. 2015, 193, 1889–1898. [Google Scholar] [CrossRef]
- Khalifeh, A.; Autorino, R.; Eyraud, R.; et al. Three-year Oncologic and Renal Functional Outcomes After Robot-assisted Partial Nephrectomy. Eur Urol. 2013, 64, 744–750. [Google Scholar] [CrossRef]
- Thompson, R.H.; Lane, B.R.; Lohse, C.M.; et al. Every Minute Counts When the Renal Hilum Is Clamped During Partial Nephrectomy. Eur Urol. 2010, 58, 340–345. [Google Scholar] [CrossRef]
- Branger, N.; Bigot, P.; Pignot, G.; et al. Oncocytoma on renal mass biopsy: is it still the same histology when surgery is performed? Results from UroCCR-104 study. World J Urol. 2023, 41, 483–489. [Google Scholar] [CrossRef]
- Baboudjian, M.; Moser, D.; Yanagisawa, T.; et al. Benefit and Harm of Active Surveillance for Biopsy-proven Renal Oncocytoma: A Systematic Review and Pooled Analysis. Eur Urol Open Sci. 2022, 41, 8–15. [Google Scholar] [CrossRef]
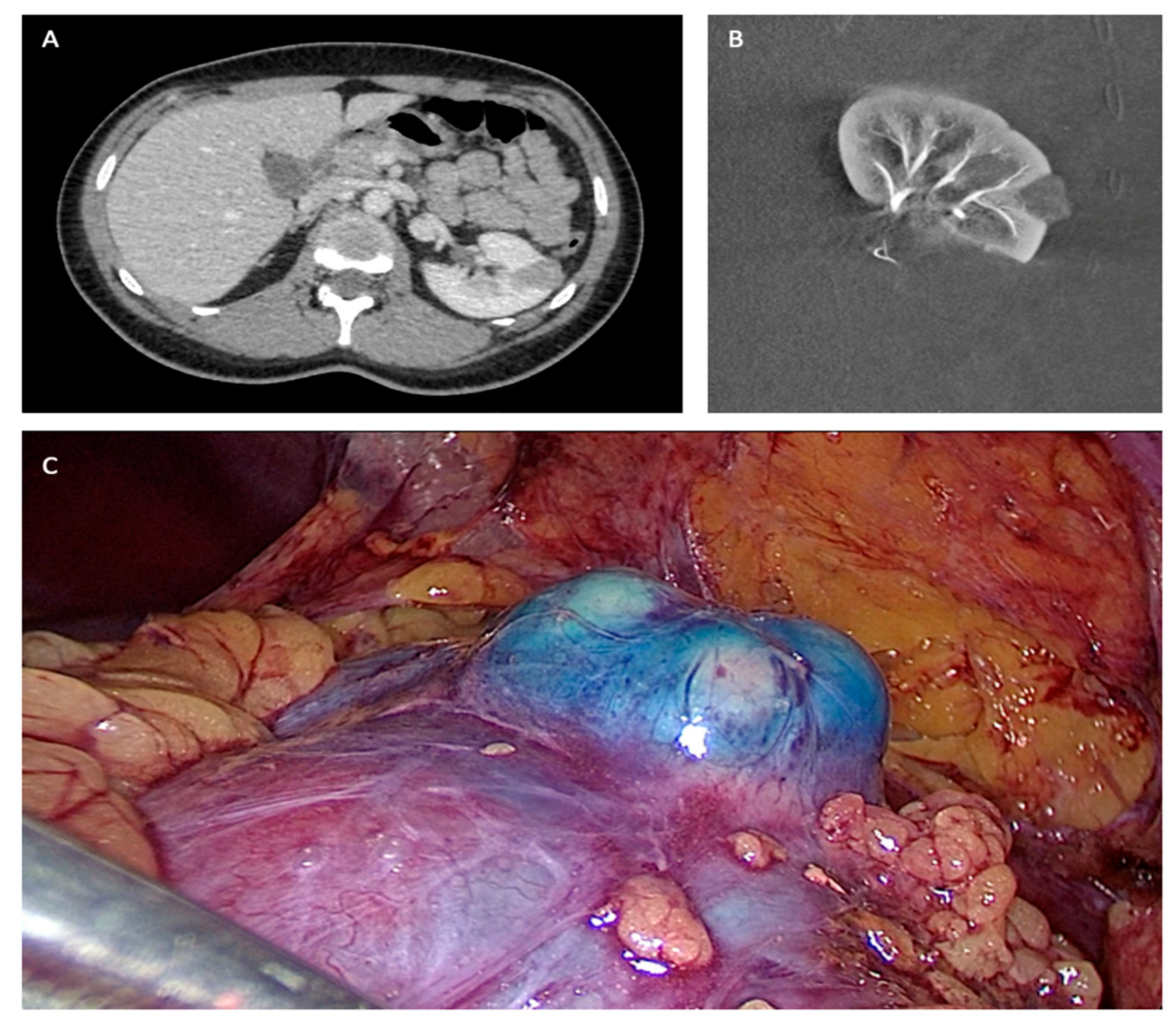
| Laparoscopic Partial Nephrectomy After Selective Embolization of Tumor Vessels (n = 245) | |
|---|---|
| Median age, years [IQR] | 64 [52 - 72] |
| Gender, n (%) | |
| Male | 163 (66.5) |
| Female | 82 (33.5) |
| Median BMI, kg/m2 [IQR] | 27.3 [24.5 - 30.8] |
| Solitary kidney, n (%) | 2 (0.8) |
| ASA score, n (%) | |
| ASA 1 | 43 (17.6) |
| ASA 2 | 145 (59.4) |
| ASA 3 | 55 (22.5) |
| ASA 4 | 1 (0.5) |
| Side, n (%) | |
| Right | 127 (51.8) |
| Left | 118 (48.2) |
| Median tumor size, cm [IQR] | 3.2 [2.5 - 4.4] |
| R.E.N.A.L. complexity, n (%) | |
| Low | 104 (43.5) |
| Moderate | 109 (45.6) |
| High | 26 (10.9) |
| Indication, n (%) | |
| Elective | 215 (87.8) |
| Imperative | 30 (12.2) |
| Median preoperative eGFR CKD-EPI, mL/min/1,73m2 [IQR] | 90.5 [77 - 101.8] |
| Median follow-up, month [IQR] | 27 [8 - 49] |
| BMI = Body Mass Index; IQR = Inter-Quartile Range; eGFR = estimated Glomerular Filtration Rate | |
| Laparoscopic Partial Nephrectomy After Selective Embolization of Tumor Vessels (n = 245) | |
|---|---|
| Median total operative time, min [IQR] | 168 [145 - 199] |
| Median laparoscopic time, min [IQR] | 75 [60 - 100] |
| Median blood loss, mL [IQR] | 100 [50 - 300] |
| Number of arterial branches embolized, n (%) | |
| 1 | 88 (36.1) |
| 2 | 99 (40.6) |
| 3 | 41 (16.8) |
| 4 | 15 (6.1) |
| 5 | 1 (0.4) |
| Perioperative complications, n (%) | 10 (4.1) |
| Perioperative transfusions, n (%) | 4 (1.6) |
| Postoperative complications CLAVIEN, n (%) | |
| I | 32 (13.1) |
| II | 7 (2.9) |
| IIIa | 3 (1.2) |
| IIIb | 12 (4.9) |
| IVa | 1 (0.4) |
| V | 1 (0.4) |
| Median hospital stay, days [IQR] | 4 [3 - 4] |
| IQR: Inter-Quartile Range | |
| All tumors (n=245) | Low complexity group1 (n=104) | Moderate Complexity group2 (n=109) | High Complexity group3 (n=26) | p Value | |
|---|---|---|---|---|---|
| All complications, n (%) | 56 (22.9) | 24 (23) | 23 (21) | 7 (26) | 0.8 |
| Major complications (Clavien > 2), n(%) | 17 (6.9) | 4 (3.8) | 7 (6.4) | 4 (15) | 0.095 |
|
1 Renal Score 4-6: Low complexity 2 Renal Score 7-9: Moderate complexity 3 Renal Score 10-12: High complexity | |||||
| Laparoscopic Partial Nephrectomy After Selective Embolization of Tumor Vessels (n = 245) | |
|---|---|
| Histology, n (%) | |
| Benign | 34 (13.9) |
| Angiomyolipoma | 5 (2) |
| Renal cyst | 5 (2) |
| Oncocytoma | 22 (9) |
| Hemangioma | 1 (0.4) |
| Metanephric adenoma | 1 (0.4) |
| Malignant | 211 (86.1) |
| Clear cell renal cell carcinoma | 158 (64.5) |
| Collecting duct / Bellini duct carcinoma | 1 (0.4) |
| Chromophobe renal cell carcinoma | 16 (6.5) |
| Papillary renal cell carcinoma | 34 (13.9) |
| Eosinophilic renal cell carcinoma | 1 (0.4) |
| Pulmonary metastasis | 1 (0.4) |
| pT stage, n (%) | |
| pT1a | 142 (67.3) |
| pT1b | 42 (19.9) |
| pT2a | 9 (4.3) |
| pT2b | 1 (0.5) |
| pT3a | 17 (8.1) |
| ISUP grade, n (%) | |
| 1 | 22 (10.5) |
| 2 | 125 (59.2) |
| 3 | 40 (19) |
| 4 | 6 (2.8) |
| NA | 18 (8.5) |
| Surgical margins, n (%) | |
| Negative | 233 (95.1) |
| Positive | 12 (4.9) |
| Recurrences, n (%) | |
| All recurrences | 20 (9.5) |
| Local recurrences | 17 (8.1) |
| Metastatic progression | 7 (3.3) |
| Surgical reoperation, n (%) | 17 (8.1) |
| Totalisation | 8 (3.8) |
| Partial nephrectomy | 1 (0.5) |
| Radiofrequency ablation | 6 (2.8) |
| Extra peritoneal nodule excision | 2 (1) |
| Deaths, n (%) | 14 (5.7) |
| Specific deaths, n (%) | 4 (1.6) |
| Hazard Ratio (CI 95%) | p Value | |
|---|---|---|
| Age, (continuous) | 1.02 (0.977 ; 1.07) | 0.340 |
| Tumor size, (continuous) | 1.15 (0.843 ; 1.55) | 0.386 |
| Indication NSS | ||
| Elective | Reference | — |
| Imperative | 1.00 (0.289 ; 3.47) | 0.997 |
| Histology | ||
| Other | Reference | — |
| Clear cell renal cell carcinoma | 1.77 (0.496 ; 6.33) | 0.378 |
| ISUP grade | ||
| 1 / 2 | Reference | — |
| 3 / 4 | 1.71 (0.618 ; 4.75) | 0.300 |
| T Stade | ||
| T1 & T2 | Reference | — |
| ≥ T3 | 4.04 (1.18 ; 13.91) | 0.027 |
| Surgical margins | ||
| Negative | Reference | — |
| Positive | 4.29 (1.17 ; 15.82) | 0.029 |
| Multivariate analysis with Cox-proportion hazard regression | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




