Submitted:
17 July 2023
Posted:
18 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study site
2.2. Plant material
2.3. Germination and post-germination experiment
2.4. Growth and physiological experiment
2.4.1. Plant growth and physiological measurements
2.4.2. Stem water potential
2.4.3. Leaf relative water content
2.4.4. Gas exchange measurements
2.5. Statistical analysis
3. Results
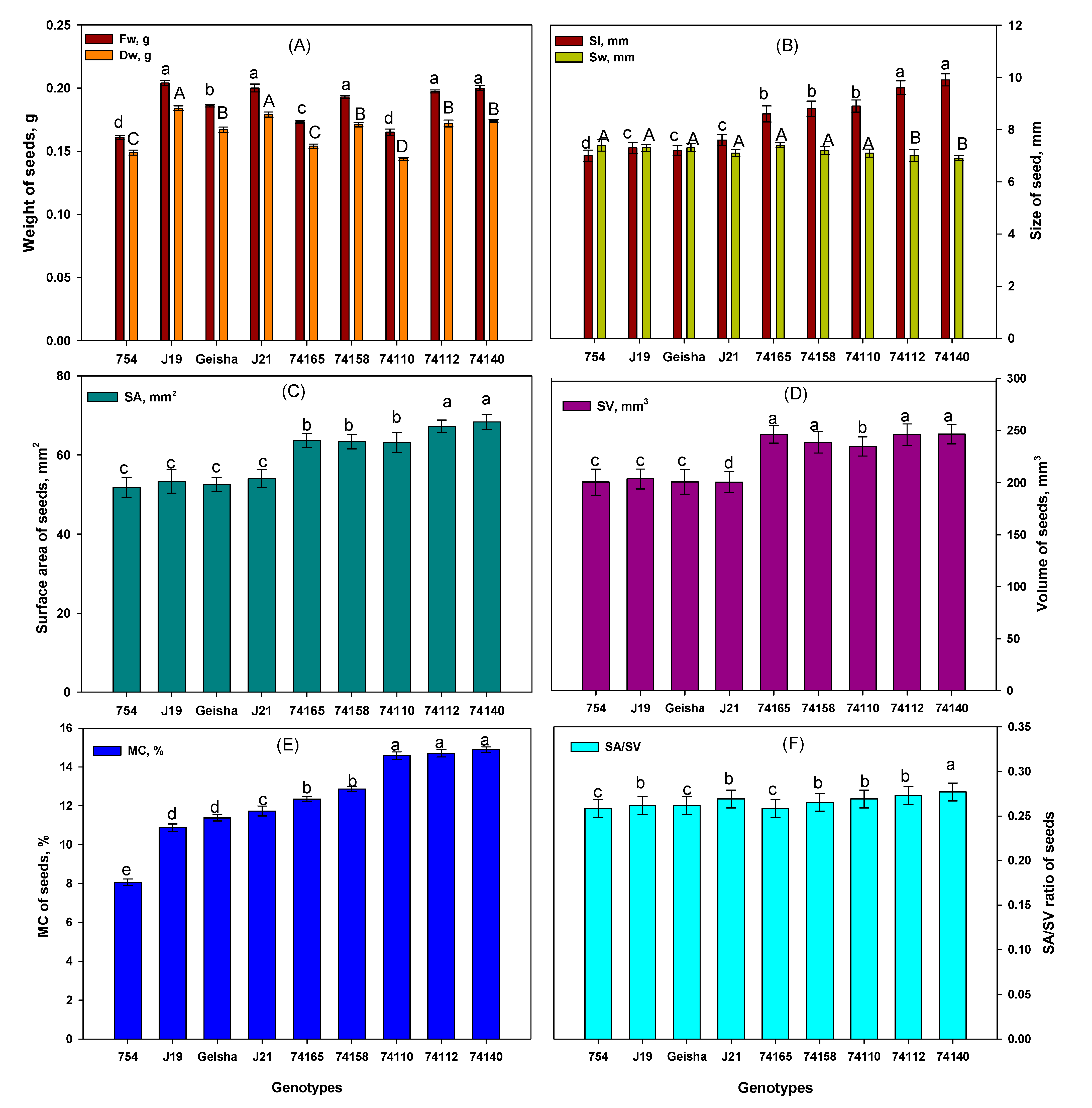
3.1. Assessing C. arabica seed quality traits of the different genotypes
3.2. Assessment of variabilities in germination and post-germination events of C. arabica
3.3. Assessing seed germination potential indicators of the different genotypes
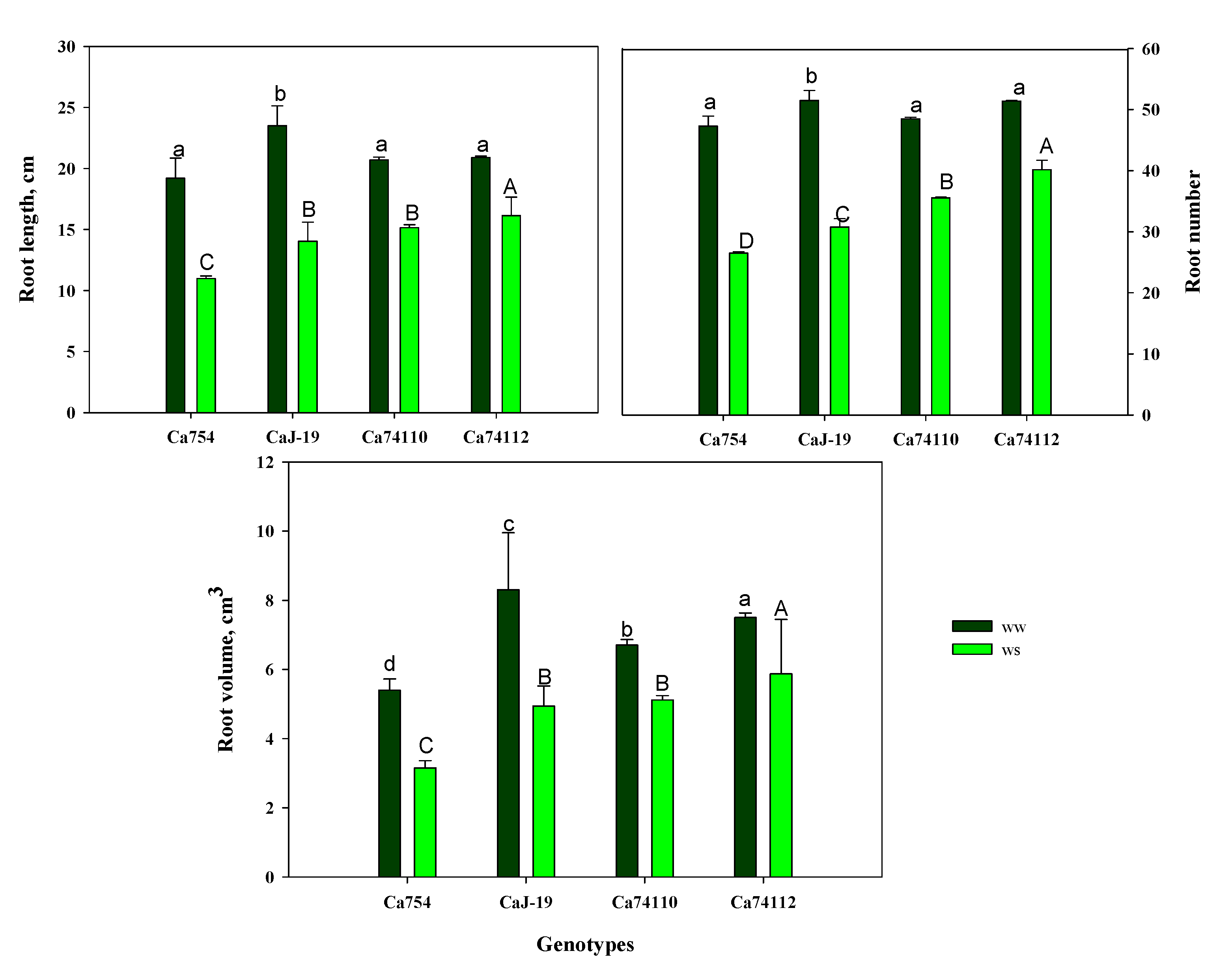
3.4. Growth and development of C. arabica seedlings
3.5. Assessing the effect of drought stress on the growth and physiology ofadult coffee genotypes
3.5.1. Shoot growth of coffee plants in control and drought stress conditions
3.5.2. Root growth of coffee plants in control and drought stress conditions
3.5.3. Biomass of coffee plants in control and drought stress conditions
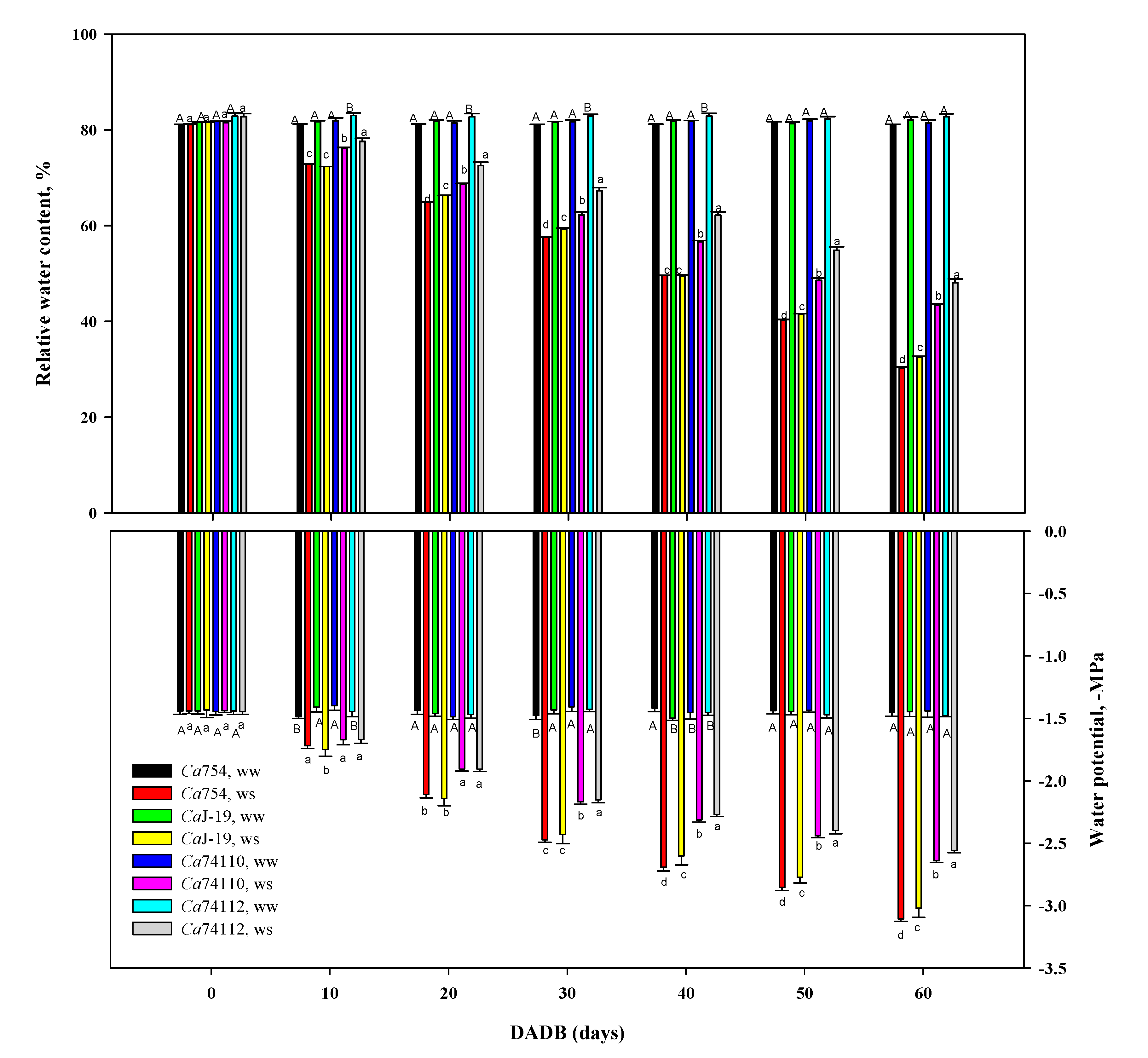
3.5.4. Relative water content and stem water potential
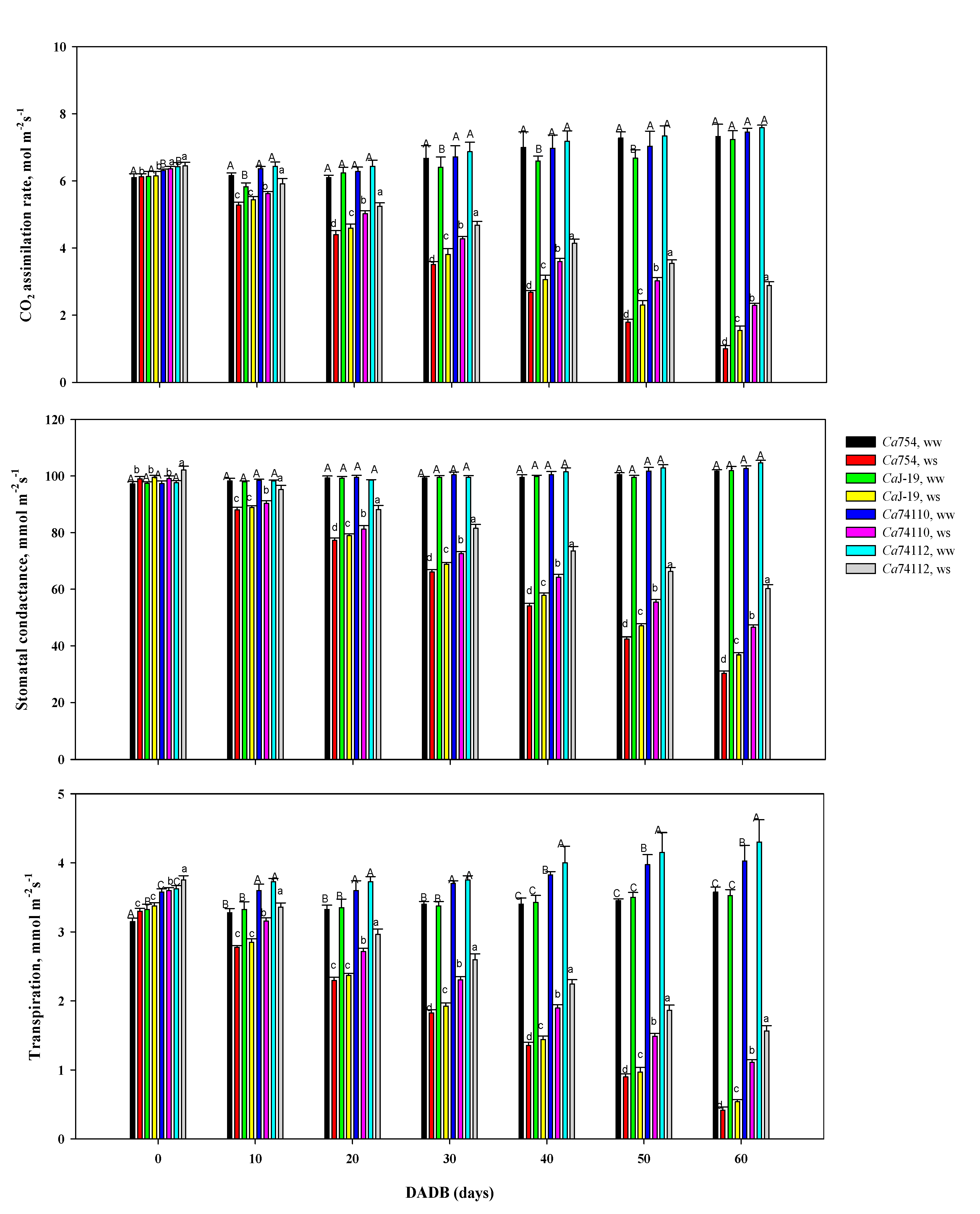
3.5.5. Photosynthesis assimilation rate, stomatal conductance, and transpiration rate
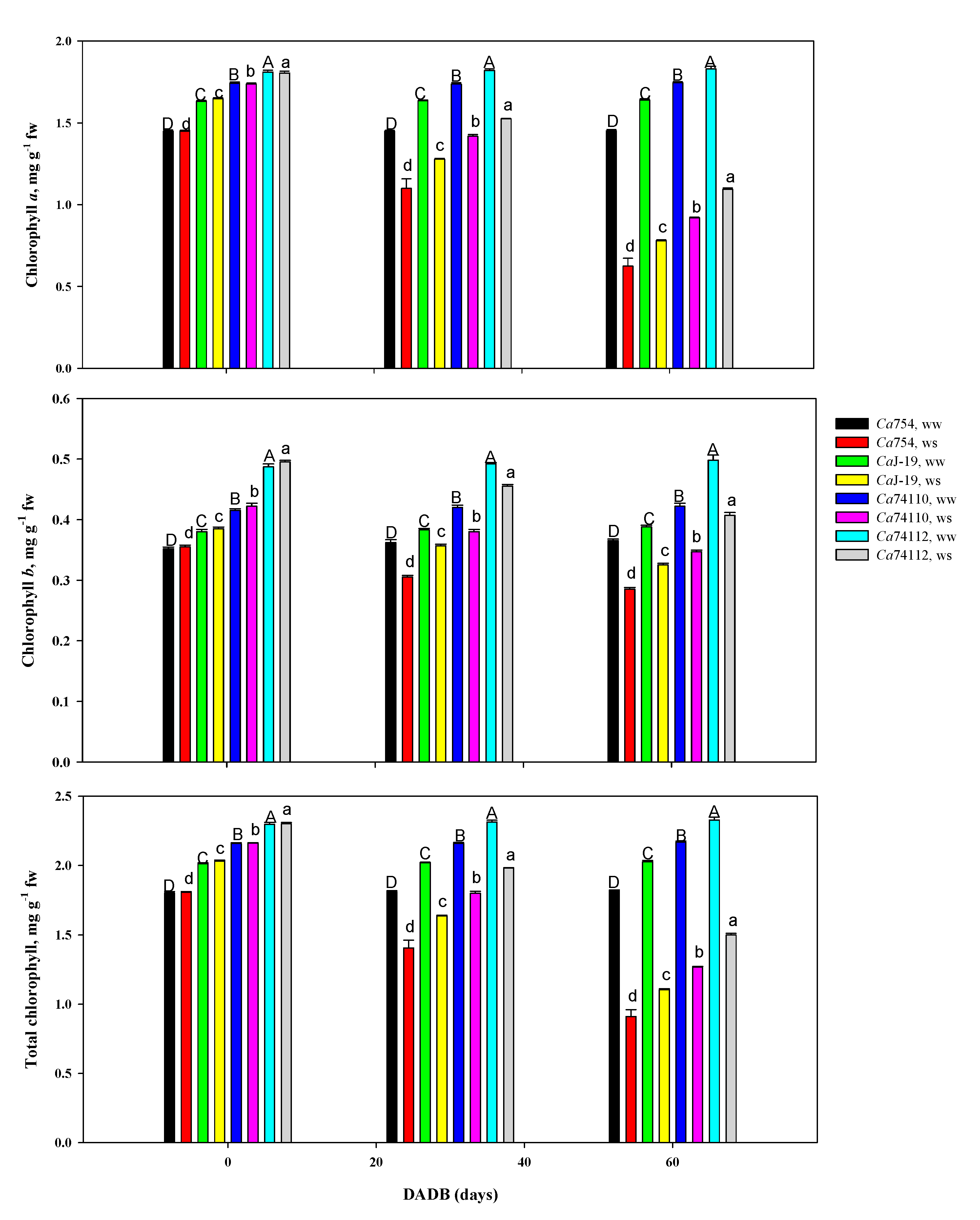
3.5.6. Photosynthetic pigments
3.5.7. Cell membrane stability and relative cell injury
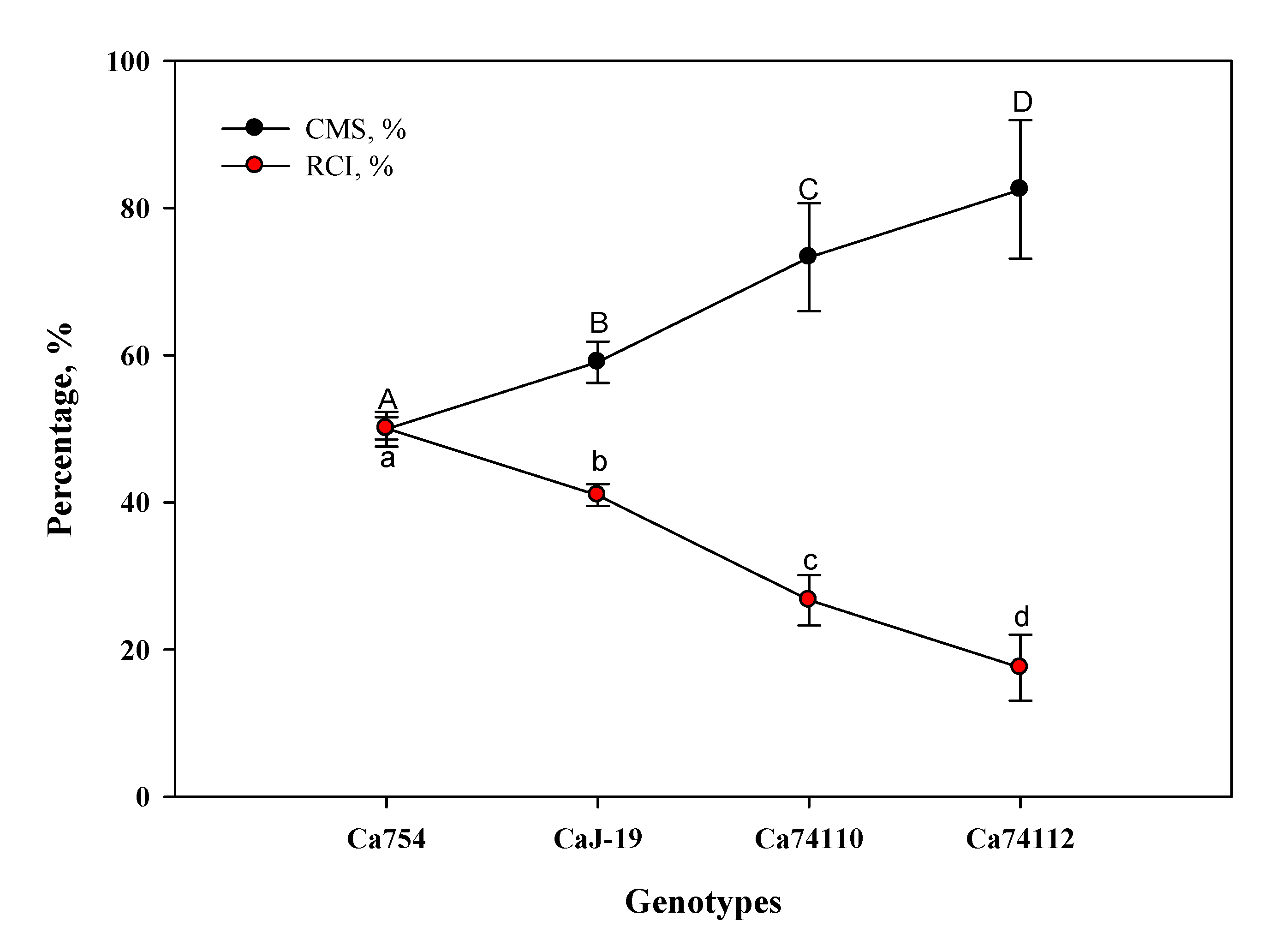
3.6. Pearson correlation among tested parameters
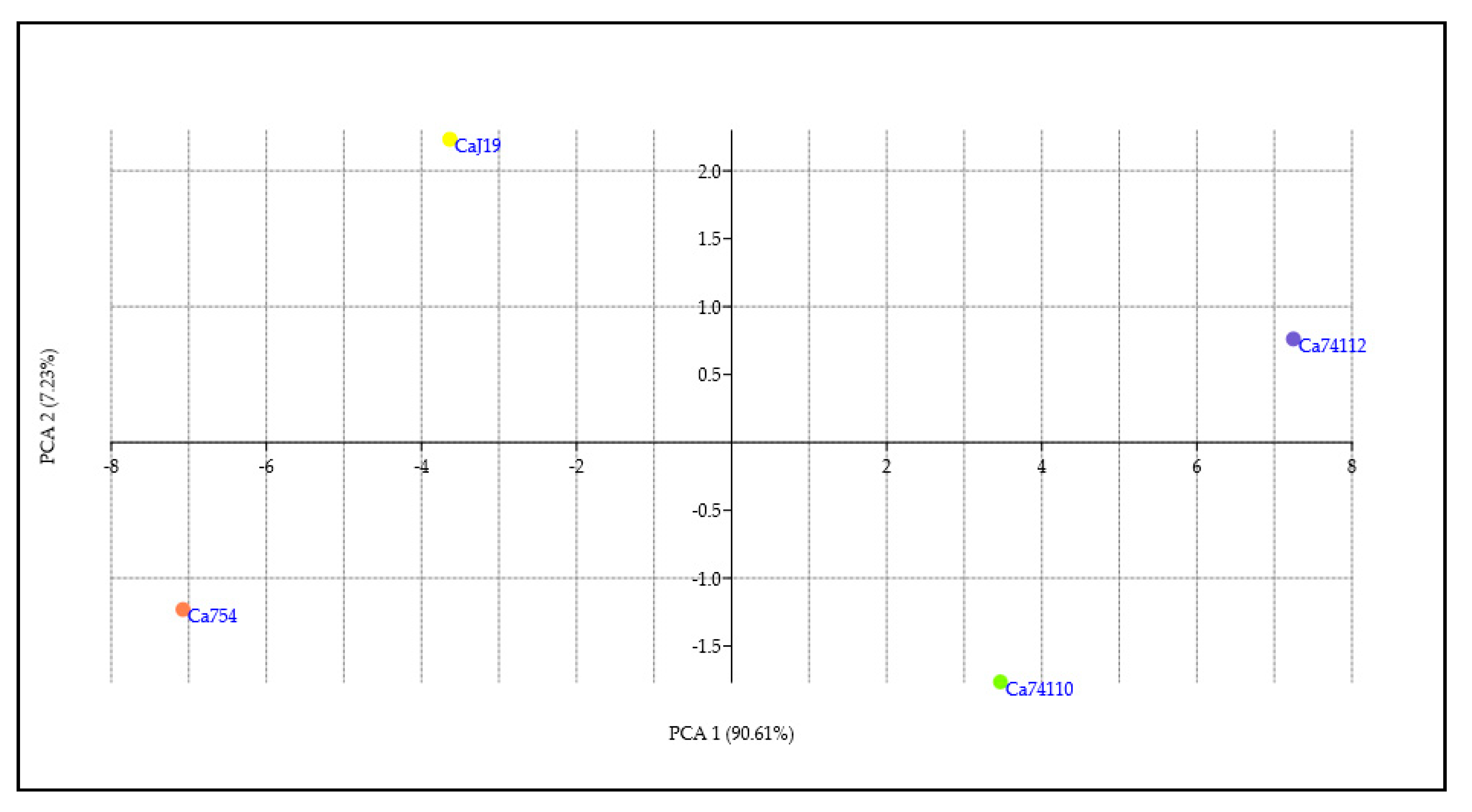
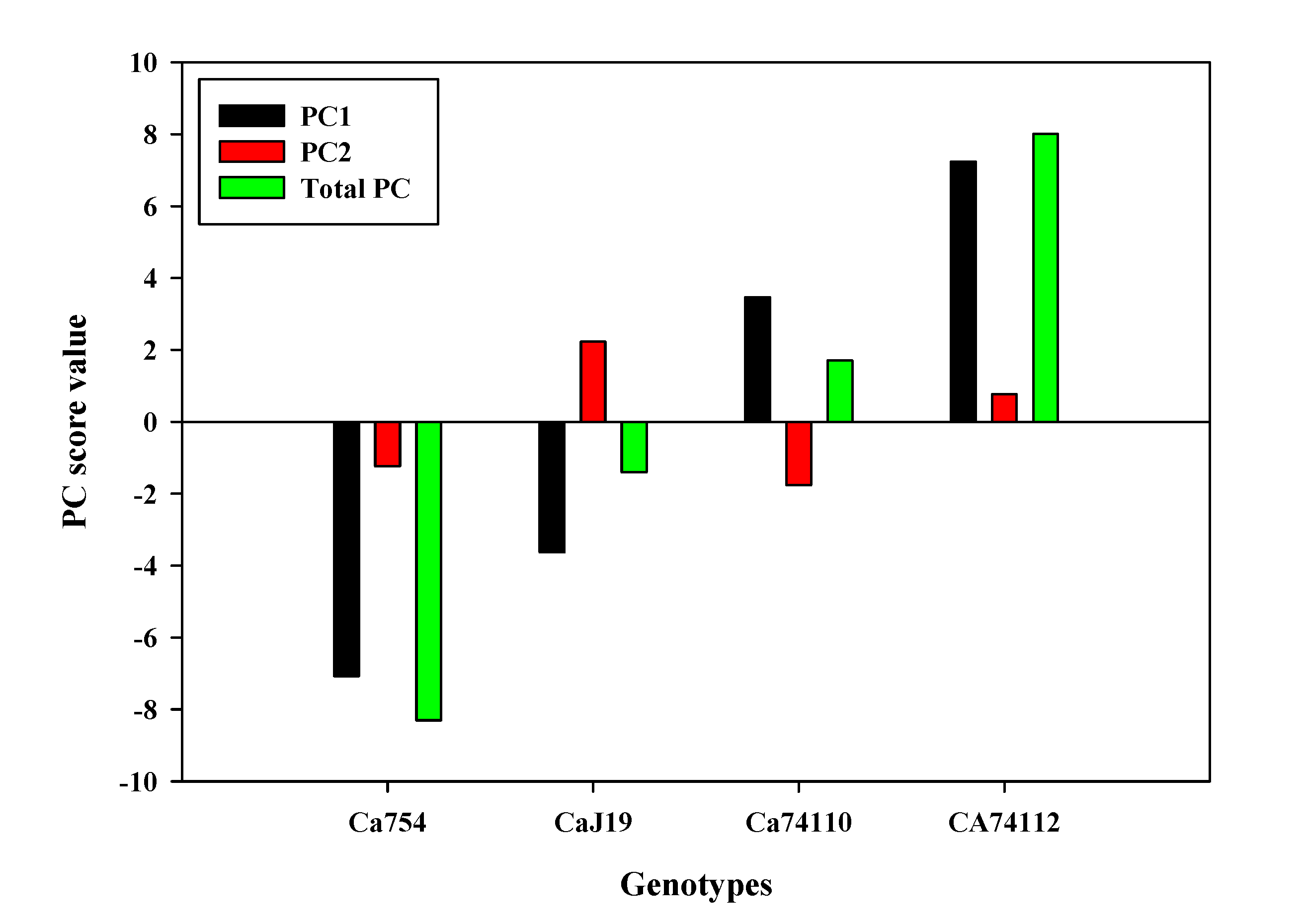
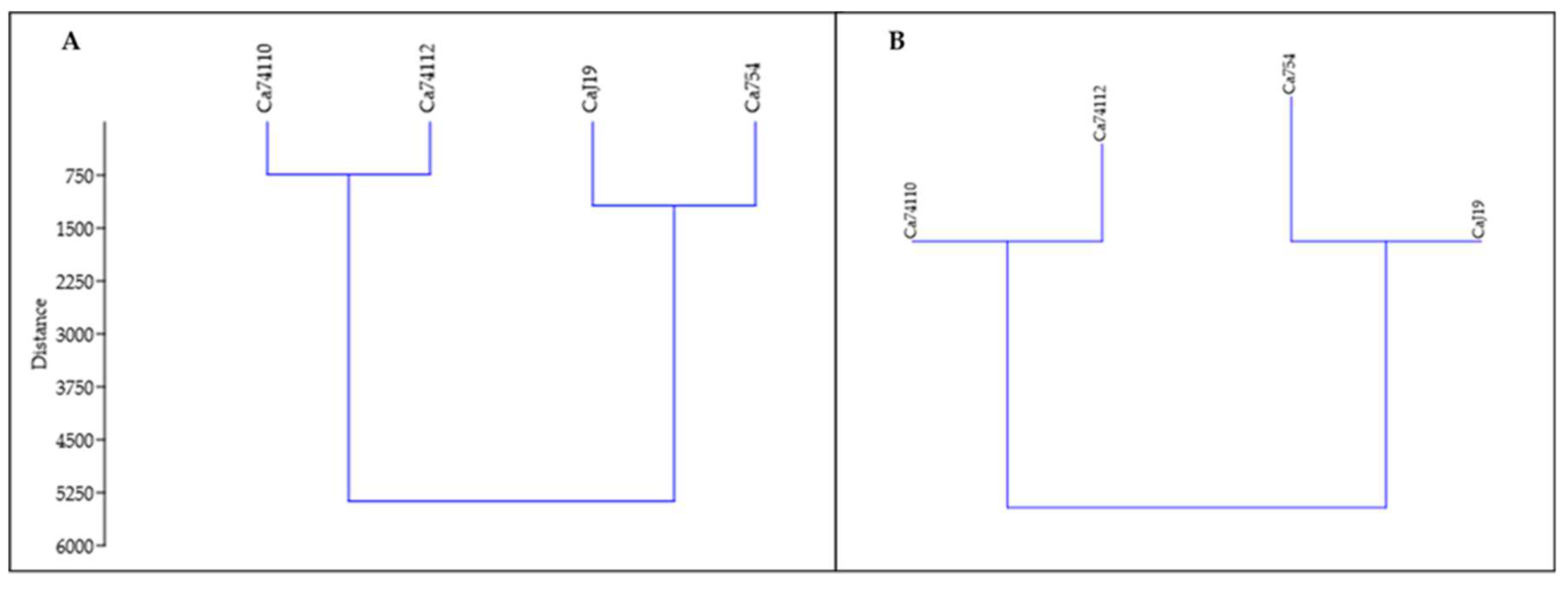
3.7. PCA and cluster analysis
4. Discussion
4.1. Seed trait variation associated with germination potential
4.2. The speed of morphological changes during germination is highly associated with genotypes
4.3. Germination performance variability is highly related to genotype
4.4. The extent of drought stress impact on growth vary among coffee genotypes
4.5. Relative water contents and water potential affected differently among genotypes under drought stress
4.6. Drought stress affected the gas exchange differently in coffee genotypes
4.7. Impact of drought stress on photosynthetic pigments concentration
4.8. The magnitude of cell membrane stability and relative cell injury differ among genotypes under drought stress
4.9. Multivariate analysis of parameters analysed in this study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Competing Interests
References
- Aga, E.; Tomas, B.; Endashaw, B.; Bjorn, S. Genetic diversity of forest Arabica coffee (Coffea arabica L.) in Ethiopia as revealed by random amplified polymorphic DNA (RAPD) analysis. Hereditas 2003, 138, 36–46. [Google Scholar] [CrossRef]
- WCR (World Coffee Research). Good Practice Guide Coffee Nursery Management; WCR publishing: Portland, OR 9 7225, USA, 2019; pp. 1–80. [Google Scholar]
- Smith, R.F. A history of coffee. In: Coffee Botany, Biochemistry and Production of Beans and Beverage, 2nd ed.; Clifford, M.N., Willson, K.C., eds.; Croom Helm, New York, USA, 1985; pp. 1–12.
- Shimber, T. Variations among coffee (Coffea arabica L.) genotypes for sensitivity to drought induced by soil drying at early stages of growth in Ethiopia. Int. J. Plant Bre. Crop Sci. 2018, 5, 453–462. [Google Scholar]
- Kufa, T. Biomass production and distribution in seedlings of Coffea arabica genotypes under contrasting nursery environments in south-western Ethiopia. Agri. Sci. 2012, 3, 835–843. [Google Scholar]
- ECX (Ethiopian Commodity Exchange). The Ethiopian Annual Export Products, Annual Reports; ECX publishing: Addis Ababa, Ethiopia, 2019; pp. 1–130. [Google Scholar]
- Gebresselasei, S.; Eva, L. (2008). Agricultural Commercialization in Coffee Growing Areas of Ethiopia; Protected Livelihoods and Agricultural Growth Programme, Overseas Development Institute (ODI): London, 2008; pp. 45–57. [Google Scholar]
- CSA (Central Statistical Agency). Agricultural Sample Survey: Area and production of crops; Central Statistical Agency of Ethiopia: Addis Ababa, Ethiopia, 2019; pp. 1–450. [Google Scholar]
- Karssen, C.M.; Zagorski, S.; Kepczynski, J.; Groot, S.C. A key role for endogenous gibberellins in the control of seed germination. Ann. of Bot. 1989; 63, 71–80. [Google Scholar]
- Rosa, S.F.; McDonald, M.B.; Veiga, A.D.; Vilela, F.L.; Ferreira, I.A. Staging coffee seedling growth: a rationale for shortenning the coffee seed germination test. Seed Sci. Tech. 2010, 38, 421–431. [Google Scholar] [CrossRef]
- Giorgini, J.F.; Campos, C. Changes in the content of soluble sugars and starch synthesis and degradation during germination and seedling growth of Coffea arabica L. Bra. J. Plant Physio. 1992, 4, 11–15. [Google Scholar]
- Linkies, A.; Graeber, K.; Knight, C.; Leubner-Metzger, G. The evolution of seeds. New Phyt. 2010, 186, 817–831. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ronchi, C.P.; Maestri, M.; Barros, R.S. . Ecophysiology of coffee growth and production. Bra. J. Plant Physio. 2007, 19, 485–510. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, 2014; pp. 150–162. [Google Scholar]
- da Silva, E.A.; Toorop, P.E.; Van Lammeren, A.M.; Hilhorst, H.M. ABA inhibits embryo cell expansion and early cell division events during coffee (Coffea arabica ‘Rubi’) seed germination. Ann. of Bot. 2008, 102, 425–433. [Google Scholar] [CrossRef]
- Takaki, M.; Dietrich, S.C. Effect of GA3 and light on polysaccharide levels and metabolism in germinating coffee seeds. J. Exp. Bot. 1980, 31, 1643–1649. [Google Scholar] [CrossRef]
- Steinbrecher, T.; Leubner-Metzger, L. The biomechanics of seed germination. J. Exp. Bot. 2017, 68, 765–783. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phyt. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Schopfer, P. Biomechanics of plant growth. Ame. J. Bot. 2006, 93, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Weitbrecht, K.; Muller, K.; Leubner-Metzger, G. First of the mark: early seed germination. J. Exp.Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef] [PubMed]
- Mirian, E.S.; da Silva, E.A.; de Castro, R.D.; Dussert, S.; Walters, C.; Bewley, J.D.; Hilhorst, H.M. Coffee seed physiology. Bra. J. Plant Physio. 2006, 18, 149–163. [Google Scholar]
- Voegele, A.; Graeber, K.; Oracz, K.; Tarkowska, D.; Jacquemoud, D.; Tureekova, V.; Urbanova, T.; Strnad, M.; Leubner-Metzger, G. Embryo growth, testa permeability, and endosperm weakening are major targets for the environmentally regulated inhibition of Lepidium sativum seed germination by myrigalone A. J. Exp. Bot. 2012, 63, 5337–5350. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.A.; Toorop, P.E.; Jaap, N.J.; Bewley, J.D.; Hilhorst, H.M. Exogenous gibberellins inhibit coffee (Coffea arabica cv. Rubi) seed germination and cause cell death in the embryo. J. Exp. Bot. 2005, 56, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Pour-Aboughadareh, A.; Omidi, M.; Naghavi, M.R.; Etminan, A.; Mehrabi, A.A.; Poczai, P.; Bayat, H. Effect of water deficit stress on seedling biomass and physio-chemical characteristics in different species of wheat possessing the D genome. Agronomy, 2019; 9, 522. [Google Scholar]
- Xiong, S.; Wang, Y.; Chen, Y.; Gao, M.; Zhao, Y.; Wu, L. Effects of drought stress and rehydration on physiological and biochemical properties of four oak species in China. Plants 2022, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Taiz, L.; E. Zeiger. Plant Physiology. Massachusetts, Sinauer Associates, 2010, pp782.
- Queiroz, M.S.; Oliveira, C.S.; Steiner, F.; Zuffo, A.M.; Zoz, T.; Vendruscolo, E.P.; Silva, M.V.; Mello, B.R.; Cabral, R.C.; Menis, F.T. Drought Stresses on Seed Germination and Early Growth of Maize and Sorghum. J. Agri. Sci. 2019, 11, 310–318. [Google Scholar] [CrossRef]
- Abreha, K.B.; Enyew, M.; Carlsson, A.S.; Vetukuri, R.R.; Feyissa, T.; Motlhaodi, T.; Ng’uni, D.; Geleta, M. Sorghum in Dryland: Morphological, Physiological, and Molecular Responses of Sorghum under Drought Stress. Planta 2022, 255, 1–23. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Muller, B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fountain, J.C.; Ji, P.; Ni, X.; Chen, S.; Lee, R.D.; Guo, B. Deciphering drought-induced metabolic responses and regulation in developing maize kernels. Plant biotechnology journal 2018, 16, 1616–1628. [Google Scholar] [CrossRef]
- Visentin, I.; Vitali, M.; Ferrero, M.; Zhang, Y.; Ruyter-Spira, C.; Novak, O.; Strnad, M.; Lovisolo, C.; Schubert, A.; Cardinale, F. Low levels of Strigolactones in roots as a component of the systemic signal of drought stress in Tomato. New Phytol. 2016, 212, 954–963. [Google Scholar] [CrossRef]
- DaMatta, F.M. ; Ramalho. J. Impacts of drought and temperature stress on coffee physiology and production: a review. Brazilian Journal of Plant Physiology, 2006; 18, 55–81. [Google Scholar]
- Negash, L. A Selection of Ethiopia's Indigenous Trees: Biology, Uses and Propagation Techniques; Addis Ababa University Press: Addis Ababa, Ethiopia, 2010; pp. 1–386. ISBN 978-99944-52-27-9. [Google Scholar]
- Etienne, H.; Benoit, B.; Frederic, G.; Marc, L.; Fabienne, M.; Eveline, D.; Jean-Luc, V.; Dominique, B. Development of coffee somatic and zygotic embryos to plants differs in the morphological, histochemical and hydration aspects. Tree Physio. 2013, 33, 640–653. [Google Scholar] [CrossRef]
- Michiel, K.; Don, M.J.; Nguyen, V.T. Manual for Arabica Coffee Cultivation; Tan Lam Agricultural Product Joint Stock Company: Cam Lo, Quang Tri, 2004; pp. 1–219. [Google Scholar]
- ISTA (International Seed Testing Association). International Rules for Seed Testing; International Seed Testing Association, Bassersdorf, Switzerland, 2005; pp 1-4.
- Scott, S.; Jones, R.; Williams, W. Review of data analysis methods for seed germination. Crop Science 1984, 24, 1192–1199. [Google Scholar] [CrossRef]
- Ellis, R.A.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Tech. 1981, 9, 373–409. [Google Scholar]
- Ranal, M.A.; Santana, D.G. How and why to measure the germination process? Bra. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Ranal, M.A.; Santana, D.G.; Ferreira, W.R.; Rodrigues, C.M. Calculating germination measurements and organizing spreadsheets. Bra. J. Bot. 2009, 32, 849–855. [Google Scholar] [CrossRef]
- Jones, K.; Sanders, D. The influence of soaking pepper seed in water or potassium salt solutions on germination at three temperatures. J. Seed Tech. 1987, 11, 97–102. [Google Scholar]
- Bench, A.R.; Fenner, M.; Edwards, P. Changes in germinability, ABA content and ABA embryonic sensitivity in developing seeds of Sorghum bicolor (L.) Moench induced by water stress during grain filling. New Phytologist, 1991; 118, 339–347. [Google Scholar]
- Esechie, H. Interaction of salinity and temperature on the germination of sorghum. J. Agr. Crop Sci. 1994, 172, 194–199. [Google Scholar] [CrossRef]
- Kader, M. Notes on various parameters recording the speed of seed germination. J. Agr. Tropics and Subtro. 1998, 99, 147–154. [Google Scholar]
- Labouriau, L.G.; Valadares, M.B. On the Germination of Seeds of Calotropis procera (Ait. ) Ait. f. Annals Bra. Aca. Sci. 1976, 48, 263–284. [Google Scholar]
- Labouriau, L.G. On the frequency of isothermal germination in seeds of Dolichos biflorus L. Plant and Cell Physio. 1978, 19, 507–512. [Google Scholar] [CrossRef]
- Adams, J.C.; Farrish, K.W. Seed coat Removal Increases Speed and Completeness of Germination of Water Oak. Tree Planters’ 1992, 43, 1–2. [Google Scholar]
- Czabator, F.J. Germination value: An index combining speed and completeness of pine seed germination. Forest Science 1962, 8, 386–396. [Google Scholar]
- Allan, R.E.; Vogel, O.A.; Peterson, C.J. Seedling emergence rate of fall-sown wheat and its association with plant height and coleoptile length. Agr. J. 1962, 54, 347–351. [Google Scholar] [CrossRef]
- Tavares-Junior, J.E.; Favarin, J.L.; Dourado-Neto, D.; Maia, A.H.; Fazuoli, L.C.; Bernardes, M.S. Comparative analysis among methods of estimating coffee-tree leaf area. Bragantia 2002, 61, 199–203. [Google Scholar]
- Taye, K. Biomass production and distribution in seedlings of Coffea arabica genotypes under contrasting nursery environments in south-western Ethiopia. Agricultural Sciences 2012, 3, 835–843. [Google Scholar]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidit estimating water deficits in leaves. Aust. J. Boil. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. , Chlorophylls and carotenoids, the pigments of photosynthetic biomembranes. Methods Enzymology 1987, 148, 350–382. [Google Scholar]
- Nijabat, A.; Adam, B.; Muhammad, M.; Adeel, I.S.; Rameez, H.; Naima, H.N.; Aamir, A.; Philipp, S. Cell membrane stability and relative cell injury in response to heat stress during early and late seedling stages of diverse carrot (Daucus carota L.) Germplasm. Hortscience, 2020; 55, 1446–1452. [Google Scholar]
- Hammer, Ø.; David, A.; Harper, T.; Paul, D.R. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 2001, 4, 9. [Google Scholar]
- Shimber, T. Some physiological aspects of drought tolerance in selected arabica coffee (Coffea arabica L.) genotypes as evaluated by early-stage response to soil drying. Int. J. Agri. Biosci. 2018, 7, 81–90. [Google Scholar]
- Wang, X.; Alvarez, M.; Donohue, K.; Ge, W.; Cao, Y.; Liu, K.; Du, G.; Bu, H. Elevation filters seed traits and germination strategies in the eastern Tibetan Plateau. Ecography 2021, 44, 242–254. [Google Scholar] [CrossRef]
- Harper, J.L.; Lovell, P.H.; Moore, K.G. The shapes and size of seeds. Annual Rev. Ecology and Syste. 1970, 1, 327–356. [Google Scholar] [CrossRef]
- da Silva, E.A. Coffee (Coffea arabica cv. Rubi) Seed Germination: Mechanism and Regulation. PhD thesis, Wageningen Agricultural University, Wageningen, The Netherlands, 2002.
- Rosa, S.F.; Carvalho, A.M.; McDonald, M.B.; Von-Pinho, E.V.; Silva, A.P.; Veiga, A.D. The effect of storage conditions on coffee seed and seedling quality. Seed Sci. Tech. 2011, 39, 151–164. [Google Scholar] [CrossRef]
- Linkies, A.; Leubner-Metzger, G. Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Reports 2012, 31, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Currey, J.D. Materials science. Hierarchies in biomineral structures. Science 2005, 309, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Black, M. Seeds - Physiology of Development and Germination; Plenum Press: New York, London, 1994; pp. 1–445. [Google Scholar]
- Barry-Etienne, D.; Bertrand, B.; Vasquez, N.; Etienne, H. Comparison of somatic embryogenesis-derived coffee (Coffea arabica L.) plantlets regenerated in vitro or ex vitro conditions: morphological, mineral and water characteristics. Ann. Bot. 2002, 90, 77–85. [Google Scholar] [CrossRef]
- Ronchi, C.P.; DaMatta, F.M.; Batista, K.D.; Moraes, G.A.; Loureiro, M.E.; Ducatti, C. Growth and photosynthetic down-regulation in Coffea arabica in response to restricting root volume. Func. Plant Biol. 2006, 33, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Huxley, P.A. Some factors which can regulate germination and influence viability of coffee seeds. Proc. Int. Seed Test Asso. 1964, 29, 33–60. [Google Scholar]
- Hong, T.D.; Ellis, R.H. Optimum moisture status for the exceptional survival of seeds of arabica coffee (Coffea arabica L.) in medium-term storage at -20oC. Seed Sci. Tech, 2002; 30, 131–136. [Google Scholar]
- da Silva, E.A.; Toorop, P.E.; van Aelst, A.C.; Hilhorst, H.M. Abscisic acid controls embryo growth potential and endosperm cap weakening during coffee (Coffea arabica cv. Rubi) seed germination. Planta, 2004; 220, 251–261. [Google Scholar]
- Kader, M.A. A Comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. Royal Soci. New South Wales 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Brown, R.F.; Mayer, D.G. Representing cumulative germination. A critical analysis of single-value germination indices. Ann. Bot. 1988, 61, 117–125. [Google Scholar] [CrossRef]
- Giorgini, J.F.; Comoli, E. Effect of embryo and exogenous GA3 on endospermic endo-β-mannanase activity of Coffea arabica L. during germination and early seedling growth. Bra. J. Plant Physio. 1996, 8, 43–49. [Google Scholar]
- Dias, P.C.; Araujo, W.L.; Moraes, G.A.; Barros, R.S.; DaMatta, F.M. Morphological and physiological responses of two coffee progenies to soil water availability. Journal of Plant Physiology 2007, 164, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Leon-Burgos, A.F.; Carlos, U.; Helber, E.B. Can prolonged conditions of water deficit alter photosynthetic performance and water relations of coffee plants in central-west Colombia? South African Journal of Botany 2022, 149, 366–375. [Google Scholar] [CrossRef]
- Shao, H.B.; Li-Ye, C.; Abdul, J.; Manivannan, P.; Panneerselvam, R.; Ming-An, S. Understanding water deficit stress-induced changes in the basic metabolism of higher plants - biotechnologically and sustainably improving agriculture and the ecoenvironment in arid regions of the globe. Crit Rev Biotechnol 2009, 29, 131–151. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought stress tolerance in plants: interplay of molecular, biochemical and physiological responses in important development stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Cai, C.T.; Cai, Z.Q.; Yao, T.Q.; Qi, X. Vegetative growth and photosynthesis in coffee plants under different watering and fertilization managements in Yunnan, SW China. Photosynthetica 2007, 45, 455–461. [Google Scholar] [CrossRef]
- Tounekti, T.; Mahdhi, M.; Al-Turki, T.A.; Khemira, H. Water relations and photo-protection mechanisms during drought stress in four coffee (Coffea arabica) cultivars from southwestern Saudi Arabia. South African Journal of Botany 2018, 117, 17–25. [Google Scholar] [CrossRef]
- Gheidary, S.; Davoud, A.; Mohammad, P. Effects of salinity, drought, and priming treatments on seed germination and growth parameters of Lathyrus sativus L. Journal of Plant Nutrition 2017, 40, 1507–1514. [Google Scholar] [CrossRef]
- Razmjoo, K.; Heydarizadeh, P.; Sabzalian, M.R. Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomila. Int. J. Agri. Biol. 2008, 10, 451–454. [Google Scholar]
- Kapoor, D.; Savita, B.; Marco, L. , Arti, S.; Muthusamy, R.; Anket, S. The impact of drought in plant metabolism: how to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Bhargavi, B.; Kalpana, K.; Reddy, J.K. , Influence of water stress on morphological and physiological changes in Andrographis paniculata. Int. J. Pure App. Biosci. 2017, 5, 1550–1556. [Google Scholar]
- Srivastava, S.; Srivastava, M. Morphological Changes and Antioxidant Activity of Steviarebaudiana under Water Stress. American Journal of Plant Sciences 2014, 5, 3417–3422. [Google Scholar] [CrossRef]
- Smika, D.E.; Klute, A. Surface Area Measurement of Corn Root Systems. Agronomy Journal 1982, 74, 1091–1093. [Google Scholar] [CrossRef]
- Wright, S.R.; Michael, W.J.; Harold, D.C.; Rufty, T.W. Root Morphology of Young Glycine max, Senna obtusifolia, and Amaranthus palmeri. Weed Science 1999, 47, 706–711. [Google Scholar] [CrossRef]
- Tagliavini, M.; Veto, L.J.; Looney, N.E. Measuring root surface area and mean root diameter of peach seedlings by digital image analysis. Hort. Sci. 1993, 28, 1129–1130. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Zhang, Q.; Ashraf, U.; Anjum, S.A.; Ali, I.; Wang, L. Maize tolerance against drought and chilling stresses varied with root morphology and antioxidative defense system. Plants 2020, 9, 720. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L. Response of plants to water stress. Frontiers in Plant Science 2014, 5. [Google Scholar] [CrossRef]
- Smith, S.; De Smet, I. Root system architecture: insights from Arabidopsis and cereal crops. Biological sciences 2012, 367, 1441–1452. [Google Scholar] [CrossRef]
- Ronchi, C.P.; Terra, A.A.; Silva, A.A. Growth and nutrient concentration in coffee root system under weed species competition. Planta Daninha 2007, 26, 679–687. [Google Scholar] [CrossRef]
- Mateva, K.I.; Chai, H.H.; Mayes, S.; Massawe, F. Natural Genotypic Variation Underpins Root System Response to Drought Stress in Bambara Groundnut [Vigna subterranea (L.) Verdc.]. Frontiers in Plant Science 2022, 13, 760879. [Google Scholar] [CrossRef]
- Vurayai, R.; Emongor, V.; Moseki, B. Effect of water stress imposed at different growth and development stages on morphological traits and yield of bambara groundnuts (Vigna Subterranea L. Verdc). Am. J. Plant Physiol. 2011, 6, 17–27. [Google Scholar] [CrossRef]
- Poorter, H.; Karl, J.; Niklas, P.B.; Reich, J.O.; Pieter, P.; Liesje, M. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist 2011, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.M. Drought as a multidimensional stress affecting photosynthesis in tropical tree crops. In: Hemantaranjan A, ed. In Advances in Plant Physiology. Scientific Publishers, Jodhpur, 2003; 227–265. [Google Scholar]
- Soizig, L.S.; Catherine, R.; Giselda, D.; Leonardo, C.; Alessandra, F. Variation in biomass allocation and root functional parameters in response to fire history in Brazilian savannas. Journal of Ecology 2021, 109(12), 4143–4157. [Google Scholar]
- Pirzad, A.; Shakiba, M.R.; Zehtab-Salmasi, S.; Mohammadi, S.A. Effect of water stress on leaf relative water content, chlorophyll, proline and soluble carbohydrates in Matricaria chamomilla L. J. Med. Plant Res. 2011, 5, 2483–2488. [Google Scholar]
- Hayatu, M.; Muhammad, S.Y.; Habibu, U.A. Effect of water stress on the leaf relative water content and yield of some cowpea (Vigna unguiculata (L) Walp.) genotype. I. J. S. T. R. 2014, 3, 148–152. [Google Scholar]
- Silva, P.C.; Junior, W.R.; Ramos, M.G.; Rocha, O.C.; Veiga, A.D.; Silva, N.H.; Brasileiro, L.O.; Santana, C.C.; Soares, G.F.; Malaquias, J.V. Physiological Changes of Arabica Coffee under Different Intensities and Durations of Water Stress in the Brazilian Cerrado. Plants 2022, 11, 2198. [Google Scholar] [CrossRef]
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Żyta, D.; Śliwka, J.; Marczewski, W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of 'Katahdin'-derived potato cultivars. Breeding science 2016, 66, 328–331. [Google Scholar] [CrossRef]
- Siddique, M.B.; Hamid, A.; Islam, M.S. Drought stress effects on water relations of wheat. Bot. Bull. Acad. Sin. 2000, 41, 35–39. [Google Scholar]
- Bayoumi, T.Y.; Eid, M.H.; Metwali, E.M. Application of physiological and biochemical indices as a screening technique for drought tolerance in wheat genotypes. Afr. J. Biotech. 2008, 7, 2341–2352. [Google Scholar]
- Lugojan, C.; Ciulca, S. Evaluation of relative water content in winter wheat. J. Hortic. Fores. Biotechnol. 2011, 15, 173–177. [Google Scholar]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.W. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Blackman, C.J.; Brodribb, T.J.; Jordan, G.J. Leaf hydraulics and drought stress: response, recovery and survivorship in four woody temperate plant species. Plant, Cell & Environment 2009, 32, 1584–1595. [Google Scholar]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Science of the Total Environment 2021, 779, 146466. [Google Scholar] [CrossRef]
- Pinheiro, H.A.; DaMatta, F.M.; Chaves, A.R.; Loureiro, M.E.; Ducatti, C. Drought tolerance is associated with rooting depth and stomatal control of water use in clones of Coffea canephora. Annals of botany 2005, 96, 101–108. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Scientific reports 2015, 5, 12449. [Google Scholar] [CrossRef]
- Barros, R.S.; Maestri, M.; Rena, A.B. Physiology of growth and production of the coffee tree. J. Coffee Res. 1999, 27, 1–54. [Google Scholar]
- Catalina, J.B.; Ricardo, H.S.; Herminia, E.P.; Paulo, R.C.; Merci, P.F. Production and vegetative growth of coffee trees under fertilization and shade levels. Sci. Agric. 2010, 67, 639–645. [Google Scholar]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biology open 2018, 7, bio035279. [Google Scholar] [CrossRef]
- Nikolaeva, M.K.; Maevskaya, S.N.; Shugaev, A.G. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russ. J. Plant Physiol. 2010, 57, 87–95. [Google Scholar] [CrossRef]
- Zargar, S.M.; Nancy, G.; Muslima, N.; Reetika, M.; Firdose, A.M.; Nageebul, R.S.; Asif, B.S.; Salgotra, R.K. Impact of drought on photosynthesis: Molecular perspective. Plant Gene 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, M.; Gu, W. Effect of drought on photosynthesis, total antioxidant capacity, bioactive component accumulation, and the transcriptome of Atractylodes lancea. BMC Plant Biol. 2021, 21, 293. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Cunha, R.L.; Antunes, W.C.; Martins, S.V.; Araujo, W.L.; Fernie, A.R.; Moraes, G. In field grown coffee trees source-sink manipulation alters photosynthetic rates, independently of carbon metabolism, via alterations in stomatal function. New Phytol. 2008, 178, 348–357. [Google Scholar] [CrossRef]
- Herbinger, K.; Tausz, M.; Wonisch, A.; Soja, G.; Sorger, A.; Grill, D. Complex interactive effects of drought and ozone stress on the antioxidant defence systems of two wheat cultivars. Plant Physiology and Biochemistry, 2002; 40(6-8), 691–696. [Google Scholar]
- Maxiselly, Y.; Anusornwanit, P.; Rugkong, A.; Chiarawipa, R.; Chanjula, P. Morpho-Physiological traits, phytochemical composition, and antioxidant activity of Canephora coffee leaves at various stages. Int. J. Plant Biol. 2022, 13, 106–114. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.A.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.A.; Panneerselvam, R. Drought stress in plants: a review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 1, 100–105. [Google Scholar]
- Kirnak, H.; Kaya, C.; Tas, I.; Higgs, D. The influence of water deficit on vegetative growth, physiology, fruit yield and quality in eggplants. Bulg. J. Plant Physiol, 2001; 27(3-4), 34–46. [Google Scholar]
- Manivannan, P.; Jaleel, C.A.; Sankar, B.; Kishorekumar, A.; Somasundaram, R.; Lakshmanan, G.A.; Panneerselvam, R. Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Biointerfaces 2007, 59, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Mafakheri, A.; Siosemardeh, A.F.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Australian journal of crop science 2010, 4, 580–585. [Google Scholar]
- Rehman, S.U.; Bilal, M.; Rana, R.M.; Tahir, M.N.; Shah, M.N.; Ayalew, H.; Yan, G. Cell membrane stability and chlorophyll content variation in wheat (Triticum aestivum) genotypes under conditions of heat and drought. Crop Pasture Sci. 2016, 67, 712–718. [Google Scholar] [CrossRef]
- Kumar, R.R.; Goswami, S.; Sharma, S.K.; Singh, K.; Gadpayle, K.A.; Kumar, N.; Rai, R.D. Protection against heat stress in wheat involves change in cell membrane stability, antioxidant enzymes, osmolyte, H2O2 and transcript of heat shock protein. Int. J. Plant Physiol. Biochem. 2012, 4, 83–91. [Google Scholar]
- Quilambo, O.A. Proline content, water retention capability and cell membrane integrity as parameters for drought tolerance in two peanut cultivars. South African Journal of Botany 2004, 70, 227–234. [Google Scholar] [CrossRef]
- ElBasyoni, I.; Saadalla, M.; Baenziger, S.; Bockelman, H.; Morsy, S. Cell membrane stability and association mapping for drought and heat tolerance in a worldwide wheat collection. Sustainability 2017, 9, 1606. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Ramegowda, V.; Senthil-Kumar, M. Shared and unique responses of plants to multiple individual stresses and stress combinations: physiological and molecular mechanisms. Frontiers in Plant Science 2015, 6, 723. [Google Scholar] [CrossRef]
- Prasch, C.M.; Sonnewald, U. Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant physiology 2013, 162, 1849–1866. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biology 2017, 17, 24. [Google Scholar] [CrossRef]
- de Oliveira Sousa, A.R.; de Andrade Silva, E.M.; Coelho Filho, M.A.; Costa, M.C.; dos Santos Soares Filho, W.; Micheli, F.; da Silva Gesteira, A. Metabolic responses to drought stress and rehydration in leaves and roots of three Citrus scion/rootstock combinations. Scientia Horticulturae 2022, 292, 110490. [Google Scholar] [CrossRef]
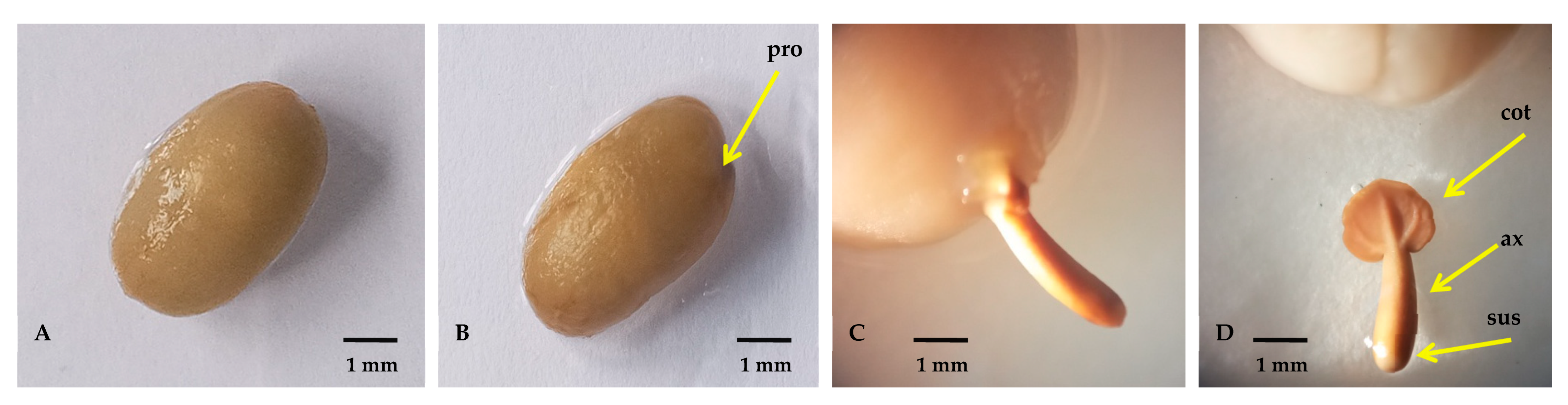
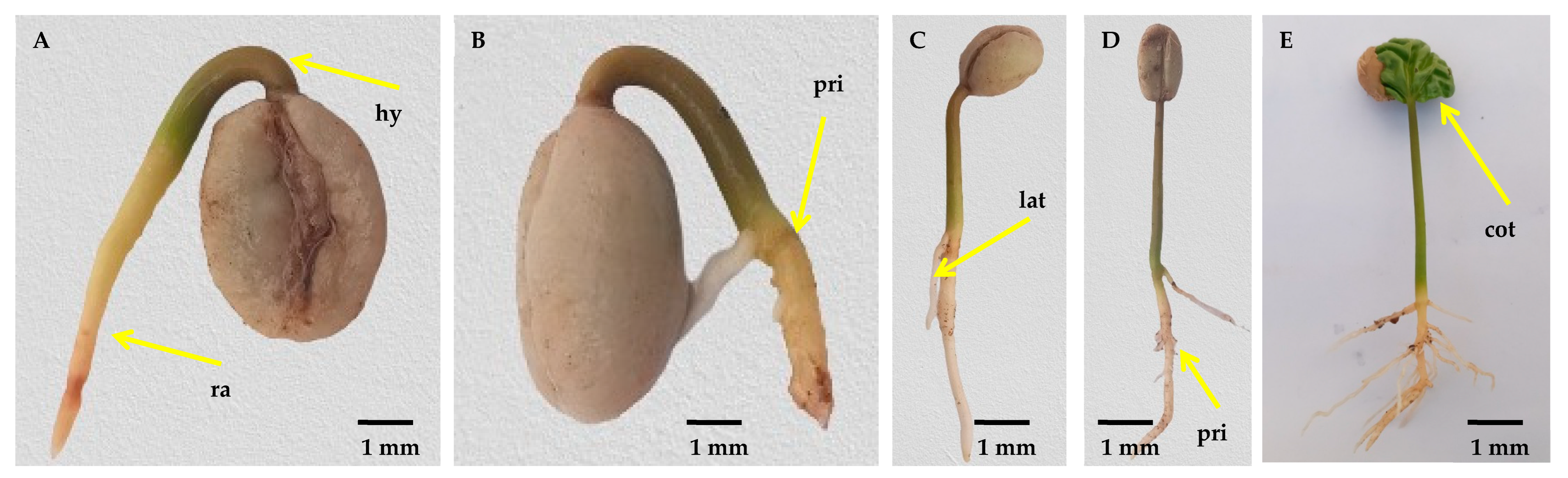
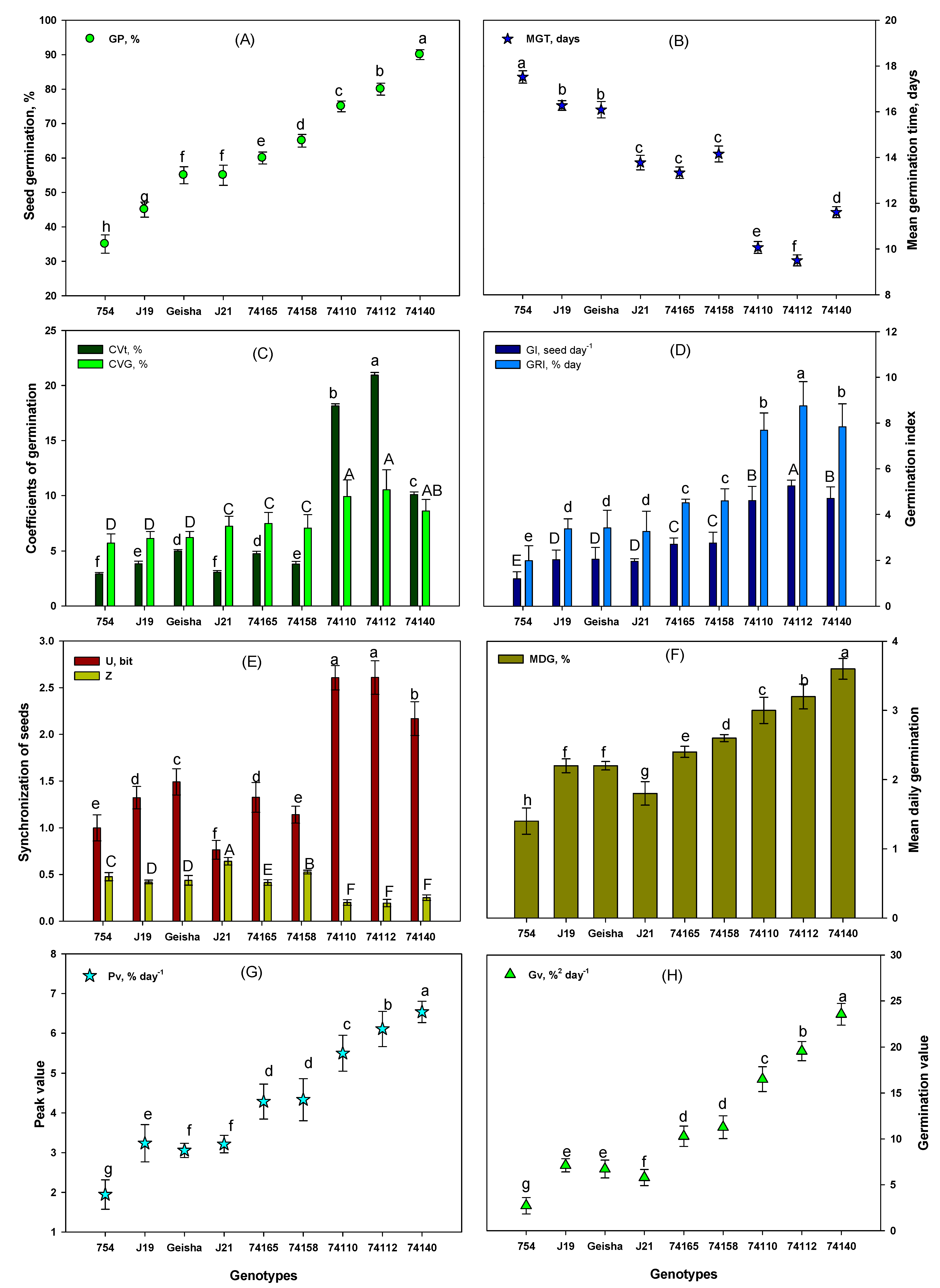
| Stage index | Stage name | Average period (days) of coffee seed developmental stages | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ca754 | CaJ19 | CaGeisha | CaJ21 | Ca74165 | Ca74158 | Ca74110 | Ca74112 | Ca74140 | ||
| bg-1 | Imbibition 1-primary imbibed seed (bg-1) | 5.94±0.83b | 5.70±0.61b | 6.01±0.47c | 5.3±0.21b | 5.2±0.28b | 5.3±0.27b | 4.01±0.22a | 3.2±0.28a | 4.2±0.23a |
| bg-2 | Imbibition 2- visible protuberance (bg-2) | 11.1±0.71d | 11.02±0.63d | 10.0±0.52c | 8.2±0.37c | 7.25±0.32b | 9.3±0.44c | 7.0±0.29b | 5.13±0.47a | 7.0±0.34b |
| g | Germinated seed (g) | 17.52±0.27c | 16.27±0.21c | 16.09±0.36c | 13.78±0.32b | 13.33±0.25b | 14.15±0.35b | 10.07±0.26a | 9.5±0.24a | 11.61±0.24a |
| sd-1 | Seedling 1-arrow-shaped radicle (sd-1) | 20±2.16c | 19.3±2.86c | 19.1±2.51c | 16±1.98b | 16±1.64b | 17.2±1.87b | 13.5±1.92a | 12.6±1.98a | 14.1±1.61a |
| sd-2 | Seedling 2-root primordia emergence (sd-2) | 23.0±2.92c | 22.0±1.78c | 22.0±2.82c | 19.11±2.31b | 19.13±1.35b | 20.2±2.04b | 16.53±2.42a | 15.49±1.26a | 17.36±1.24a |
| sd-3 | Seedling 3-lateral roots emergence (sd-3) | 26.0±2.31d | 25.0±1.98d | 25.26±1.78d | 22.7±2.34c | 22.2±2.74c | 23.1±1.56c | 19.2±1.36b | 17.3±1.21a | 20.14±2.04b |
| sd-4 | Seedling 4-lateral roots development (sd-4) | 32.0±2.09d | 31.1±2.11d | 30.21±2.05d | 28.2±1.28c | 27.05±1.37c | 28.1±2.27c | 24.6±2.42b | 22.3±2.39a | 25.0±2.75b |
| sd-5 | Seedling 5-photosynthetic cotyledons appear (sd-5) | 51.2±3.07c | 53.2±3.86c | 50.02±3.54c | 47.31±3.22b | 49.27±2.84b | 49.3±3.05b | 46.0±3.01a | 44.26±3.21a | 46.0±2.23a |
| Genotypes | RL(mm) | SdL(mm) | RN | R/Sr | VI |
|---|---|---|---|---|---|
| Ca754 | 45 ± 0.41c | 42.75 ± 0.1b | 13.5 ± 1.19c | 1.05 | 3071.25d |
| CaJ19 | 51.75 ± 0.21b | 42.75 ± 0.25c | 14 ± 2.46b | 1.21 | 4252.5c |
| CaGeisha | 52.5 ± 0.51c | 43.5 ± 0.23b | 15 ± 1.47c | 1.21 | 5280c |
| CaJ21 | 59 ± 0.21c | 46.5 ± 0.25b | 19.25 ± 2.46c | 1.27 | 5802.5c |
| Ca74165 | 60.75 ± 0.41b | 47.75 ± 0.23c | 21.75 ± 2.75b | 1.27 | 6510b |
| Ca74158 | 53.75 ± 0.53c | 44.25 ± 0.21b | 16 ± 1.58b | 1.21 | 6370b |
| Ca74110 | 65.75 ± 0.31a | 49.75 ± 0.09a | 22.25 ± 2.66a | 1.32 | 8662.5a |
| Ca74112 | 67.5 ± 0.30a | 50 ± 0.21a | 22.5 ± 3.08a | 1.35 | 9400a |
| Ca74140 | 62.75 ± 0.90a | 48 ± 0.20a | 22.75 ± 1.75a | 1.31 | 9967.5a |
| Variable | Time (DADB) | Ca754 | CaJ-19 | Ca74110 | Ca74112 | ||||
|---|---|---|---|---|---|---|---|---|---|
| ww | ws | ww | ws | ww | ws | ww | ws | ||
| Stem height (cm) | 0 | 3±0.20a | 3±0.24a | 4.9±0.09b | 4.88±0.21b | 7.1±0.2c | 7±0.13c | 9.28±0.08d | 9.75±0.05d |
| 10 | 4.25±0.13a | 4.2±0.31a | 6.13±0.05b | 6.12±0.2b | 8.3±0.21c | 8.22±0.11c | 10.49±0.08d | 10.96±0.06d | |
| 20 | 7.68±0.14a | 7.57±0.28a | 9.53±0.06b | 9.45±0.23b | 11.7±0.2c | 11.54±0.08c | 13.85±0.09d | 14.32±0.04d | |
| 30 | 11.61±0.15a | 8.59±0.28e | 13.48±0.05b | 10.45±0.2a | 15.65±0.18c | 12.55±0.11b | 17.8±0.06d | 15.31±0.04c | |
| 40 | 15.7±0.15a | 9.88±0.26c | 17.58±0.07a | 11.75±0.21c | 19.76±0.2b | 13.85±0.11d | 21.92±0.08b | 16.6±0.03e | |
| 50 | 18.08±0.17a | 10.91±0.29c | 19.99±0.07a | 12.76±0.21c | 22.15±0.2b | 14.86±0.12d | 24.3±0.06b | 17.6±0.04e | |
| 60 | 23.15±0.20a | 11.38±0.3c | 25.08±0.09a | 13.25±0.19c | 27.21±0.18b | 15.35±0.09d | 29.41±0.07b | 18.14±0.04e | |
| Stem diameter (mm) | 0 | 1.96±0.05a | 1.9±0.11a | 1.57±0.06a | 1.61±0.08a | 2.05±0.09a | 2.04±0.21a | 2.23±0.16a | 2.08±0.05a |
| 10 | 2.43±0.03a | 2.41±0.13a | 1.98±0.09a | 2.03±0.12a | 2.58±0.08b | 2.52±0.23b | 2.71±0.19b | 2.56±0.05b | |
| 20 | 2.9±0.06a | 2.8±0.11a | 2.48±0.06b | 2.53±0.09b | 3±0.09c | 2.97±0.2c | 3.18±0.2c | 2.9±0.09c | |
| 30 | 3.44±0.05c | 3.03±0.12a | 3.0±0.07a | 2.74±0.07a | 3.51±0.05d | 3.17±0.22a | 3.69±0.19e | 3.26±0.07b | |
| 40 | 3.88±0.05a | 3.07±0.11c | 3.47±0.06a | 2.76±0.08c | 3.98±0.09b | 3.20±0.22d | 4.16±0.2b | 3.29±0.07d | |
| 50 | 4.39±0.03a | 3.12±0.11c | 3.98±0.07a | 2.82±0.09c | 4.49±0.05b | 3.24±0.22d | 4.69±0.18b | 3.31±0.07d | |
| 60 | 4.88±0.05a | 3.11±0.12b | 4.49±0.06a | 2.84±0.08b | 5.02±0.05a | 3.27±0.21c | 5.19±0.18a | 3.34±0.08d | |
| Leaf number | 0 | 6±0.0a | 6±0.0a | 6.5±0.5a | 6.5±0.4a | 7±0.51b | 7±0.54b | 7.5±0.5c | 7.5±0.3c |
| 10 | 8±0.0a | 8±0.0a | 8.5±0.4b | 8.5±0.5b | 9±0.53c | 9±0.58c | 9.5±0.5d | 9.5±0.3d | |
| 20 | 8±0.0a | 8±0.0a | 8.5±0.5b | 8.5±0.3b | 9±0.58c | 9±0.58c | 9.5±0.6d | 9.5±0.7d | |
| 30 | 8±0.0a | 8±0.0a | 8.5±0.3b | 8.5±0.5b | 9±0.55c | 9±0.55c | 9.5±0.5d | 9.5±0.5d | |
| 40 | 10±0.0a | 8±0.0c | 10.5±0.5a | 8.5±0.4c | 11±0.56b | 9±0.56d | 11.5±0.4b | 9.5±0.5d | |
| 50 | 12±0.0a | 8±0.0c | 12.5±0.6a | 8.5±0.5c | 13±0.50b | 9±0.56d | 13.5±0.5b | 9.5±0.4d | |
| 60 | 16±0.0a | 8±0.0c | 16.5±0.2a | 8.5±0.4c | 17±0.58b | 9±0.52d | 17.5±0.7b | 9.5±0.4e | |
| Leaf area (cm2) | 0 | 7.75±0.73a | 7.68±0.18a | 8.35±1.53a | 8.24±0.68a | 11.16±1.63b | 10.83±0.72b | 14.79±0.75c | 15.42±0.38c |
| 10 | 9.14±0.74a | 9.01±0.18a | 9.74±1.56b | 9.63±0.71b | 12.52±1.64c | 12.16±0.73c | 16.16±0.75d | 16.75±0.38d | |
| 20 | 10.73±0.73a | 10.31±0.2a | 11.33±1.53b | 10.95±0.74b | 14.11±1.62c | 13.48±0.73c | 17.75±0.77d | 18.05±0.4d | |
| 30 | 12.59±0.73a | 10.58±0.18b | 13.19±1.53a | 11.22±0.71b | 15.97±1.61c | 13.75±0.73a | 19.66±0.74d | 18.36±0.39d | |
| 40 | 14.51±0.73a | 10.67±0.18d | 15.11±1.53a | 11.32±0.72d | 17.89±1.62b | 13.86±0.72a | 21.53±0.76c | 18.45±0.38e | |
| 50 | 17.07±0.74a | 10.7±0.18c | 17.68±1.53a | 11.34±0.72c | 20.45±1.62b | 13.88±0.72d | 24.08±0.75b | 18.47±0.38e | |
| 60 | 19.85±0.7a | 10.72±0.18d | 20.44±1.57a | 11.36±0.72d | 23.23±1.62b | 13.9±0.72d | 26.87±0.77c | 18.49±0.38e | |
| Variable | Ca754 | CaJ-19 | Ca74110 | Ca74112 | ||||
|---|---|---|---|---|---|---|---|---|
| ww | ws | ww | ws | ww | ws | ww | ws | |
| RFM, g | 4.12±0.26a | 1.51±0.11A | 4.65±0.2b | 1.85±0.06A | 4.15±0.26a | 2.21±0.06B | 4.23±0.07a | 3.03±0.48C |
| LFM, g | 4.38±0.03a | 1.63±0.26A | 5.45±0.17b | 2.13±0.06B | 4.67±0.03a | 2.75±0.06C | 5.25±0.06b | 3.81±0.75D |
| SFM, g | 3.2±0.18a | 1.28±0.13A | 4.3±0.18b | 1.76±0.06A | 3.3±0.18a | 2.16±0.06B | 4±0.05b | 2.46±1.12B |
| TFM, g | 11.7±0.77a | 4.43±0.08A | 14.4±0.18b | 5.73±0.07B | 12.12±0.77a | 7.11±0.07C | 13.48±0.07b | 9.3±0.1D |
| RDM, g | 1.09±0.06a | 0.4±0.03A | 1.28±0.18a | 0.51±0.06B | 1.17±0.03a | 0.62±0.07C | 1.23±0.01a | 0.88±0.03D |
| LDM, g | 1.16±0.04a | 0.43±0.01A | 1.49±0.18b | 0.58±0.06B | 1.32±0.01b | 0.78±0.06C | 1.53±0.02b | 1.11±0.04D |
| SDM, g | 0.84±0.04a | 0.34±0.06A | 1.18±0.06b | 0.48±0.06A | 0.93±0.03a | 0.61±0.06B | 1.16±0.04b | 0.72±0.01C |
| TDM, g | 3.09±0.03a | 1.17±0.06A | 3.95±0.06a | 1.57±0.07B | 3.42±0.04a | 2.01±0.05C | 3.92±0.02a | 2.71±0.03D |
| -1 | 0 | 1 | ||||||||||||||||||
| Variable | SA | SV | GP | GI | SdL | Rn | VI | LN | LA | RV | LFM | LDM | RWC | Ψw | A | Gs | E | Chl-a | Chl-b | CMS |
| Mc | 0.94 | 0.94 | 0.98 | 0.98 | 0.93 | 0.95 | 0.97 | 0.95 | 0.82 | 0.91 | 0.89 | 0.89 | 0.95 | 0.97 | 0.96 | 0.90 | 0.91 | 0.94 | 0.88 | 0.97 |
| SA | 1.00 | 0.99 | 0.99 | 0.98 | 0.98 | 0.99 | 0.96 | 0.94 | 0.81 | 0.95 | 0.96 | 1.00 | 0.99 | 0.98 | 0.97 | 0.99 | 0.96 | 0.93 | 0.98 | |
| SV | 0.99 | 0.99 | 0.98 | 0.98 | 0.99 | 0.96 | 0.94 | 0.80 | 0.95 | 0.95 | 1.00 | 0.99 | 0.97 | 0.96 | 0.99 | 0.95 | 0.91 | 0.98 | ||
| GP | 1.00 | 0.98 | 0.99 | 1.00 | 0.96 | 0.89 | 0.85 | 0.92 | 0.93 | 0.99 | 1.00 | 0.98 | 0.94 | 0.96 | 0.95 | 0.90 | 0.98 | |||
| GI | 0.98 | 0.99 | 1.00 | 0.97 | 0.91 | 0.85 | 0.94 | 0.94 | 0.99 | 1.00 | 0.98 | 0.95 | 0.97 | 0.96 | 0.92 | 0.99 | ||||
| SdL | 1.00 | 0.99 | 0.90 | 0.86 | 0.73 | 0.87 | 0.88 | 0.97 | 0.99 | 0.93 | 0.89 | 0.94 | 0.89 | 0.83 | 0.94 | |||||
| Rn | 0.99 | 0.91 | 0.86 | 0.76 | 0.88 | 0.88 | 0.98 | 0.99 | 0.93 | 0.90 | 0.94 | 0.90 | 0.84 | 0.95 | ||||||
| VI | 0.96 | 0.89 | 0.84 | 0.92 | 0.93 | 0.99 | 1.00 | 0.97 | 0.94 | 0.97 | 0.95 | 0.90 | 0.98 | |||||||
| LN | 0.95 | 0.93 | 0.98 | 0.98 | 0.97 | 0.96 | 1.00 | 0.99 | 0.97 | 1.00 | 0.98 | 1.00 | ||||||||
| LA | 0.80 | 0.99 | 0.99 | 0.94 | 0.91 | 0.95 | 0.99 | 0.98 | 0.95 | 0.97 | 0.94 | |||||||||
| RV | 0.89 | 0.88 | 0.83 | 0.83 | 0.91 | 0.88 | 0.83 | 0.93 | 0.92 | 0.91 | ||||||||||
| LFM | 1.00 | 0.96 | 0.93 | 0.98 | 1.00 | 0.98 | 0.99 | 0.99 | 0.98 | |||||||||||
| LDM | 0.96 | 0.93 | 0.98 | 1.00 | 0.99 | 0.99 | 0.99 | 0.98 | ||||||||||||
| RWC | 1.00 | 0.98 | 0.97 | 0.99 | 0.97 | 0.93 | 0.99 | |||||||||||||
| Ψw | 0.97 | 0.94 | 0.97 | 0.95 | 0.90 | 0.98 | ||||||||||||||
| A | 0.99 | 0.98 | 1.00 | 0.98 | 1.00 | |||||||||||||||
| Gs | 0.99 | 0.99 | 0.99 | 0.98 | ||||||||||||||||
| E | 0.97 | 0.96 | 0.98 | |||||||||||||||||
| Chl-a | 0.99 | 0.99 | ||||||||||||||||||
| Chl-b | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





