Submitted:
17 July 2023
Posted:
18 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
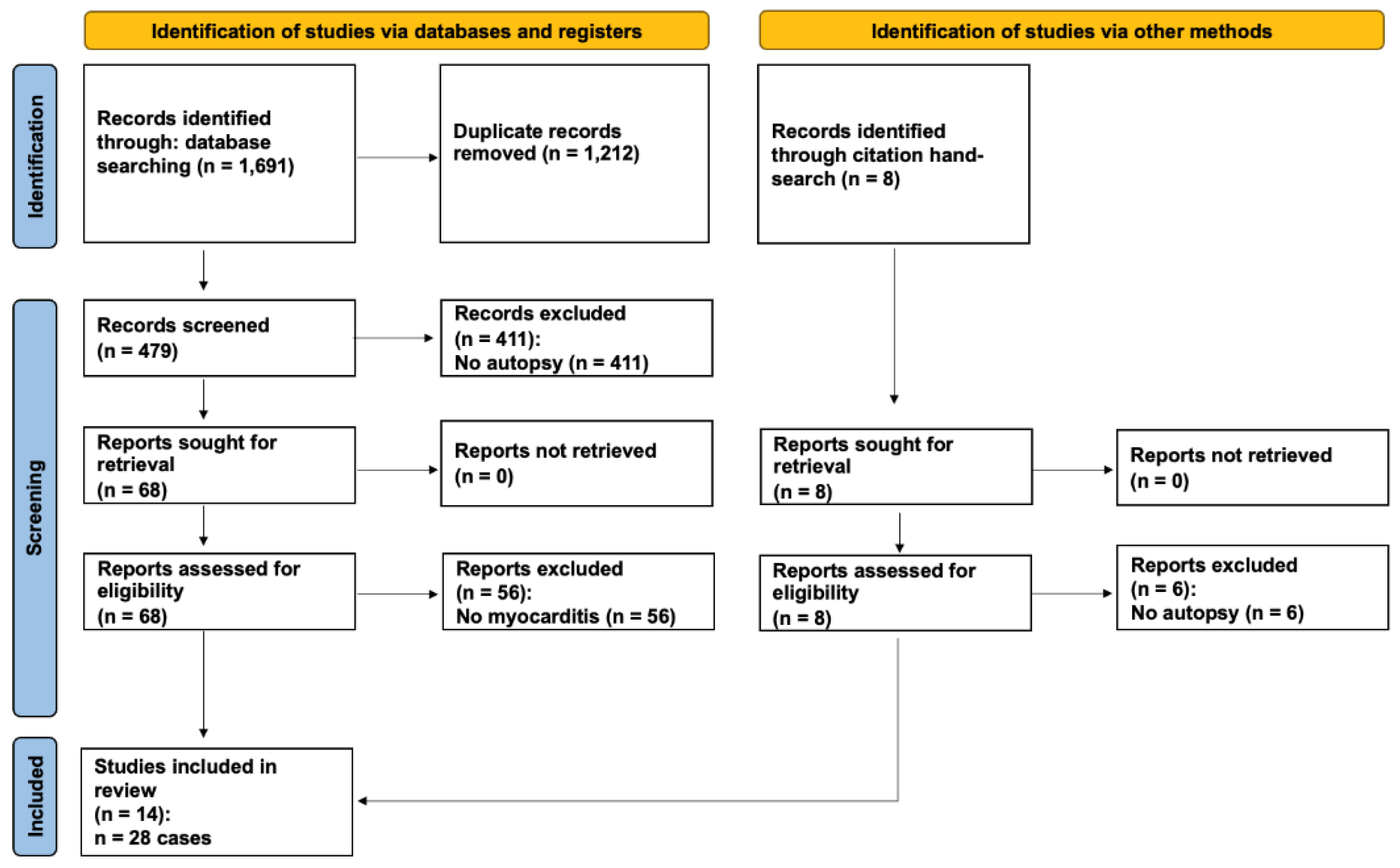
Methods
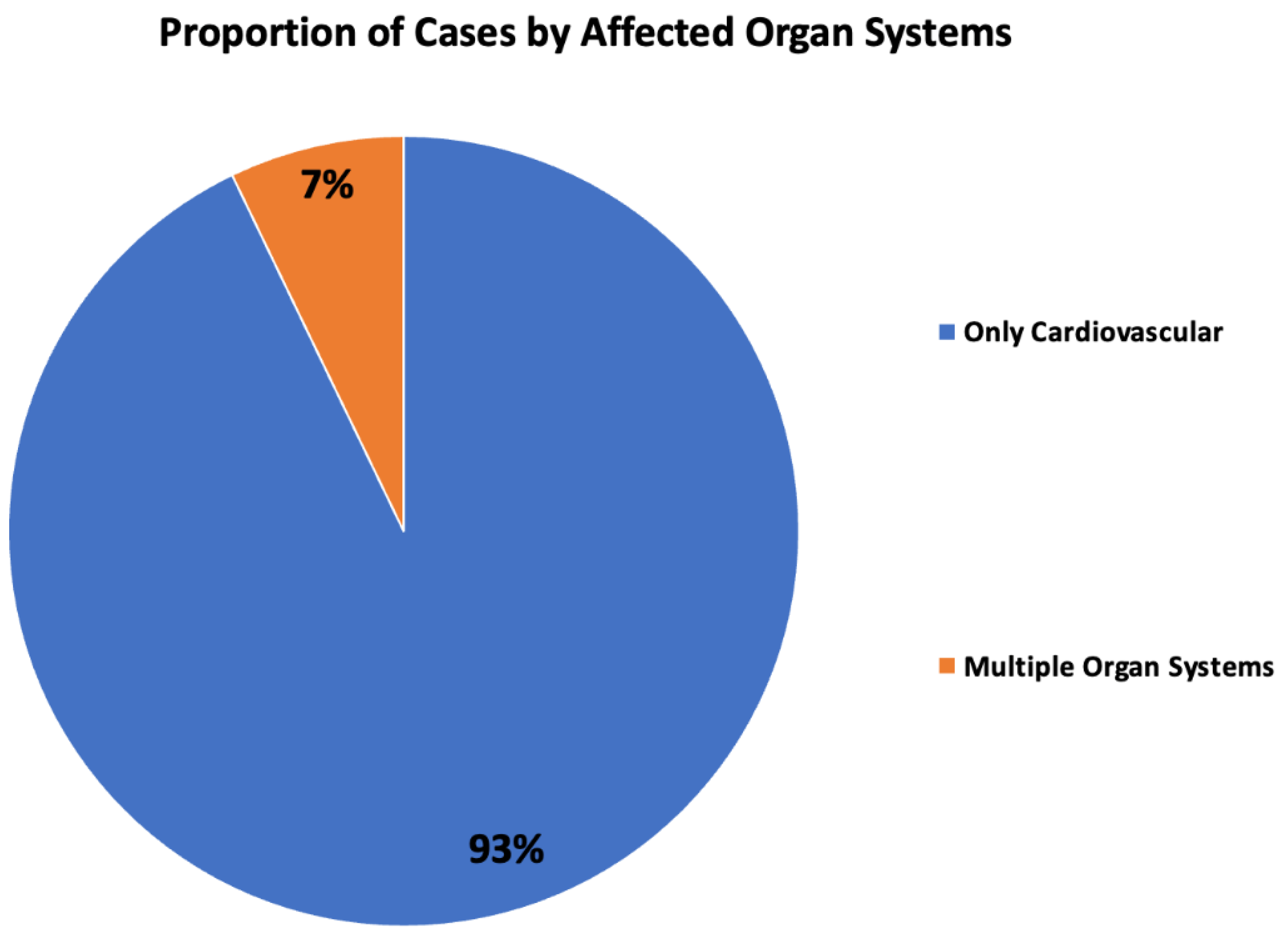
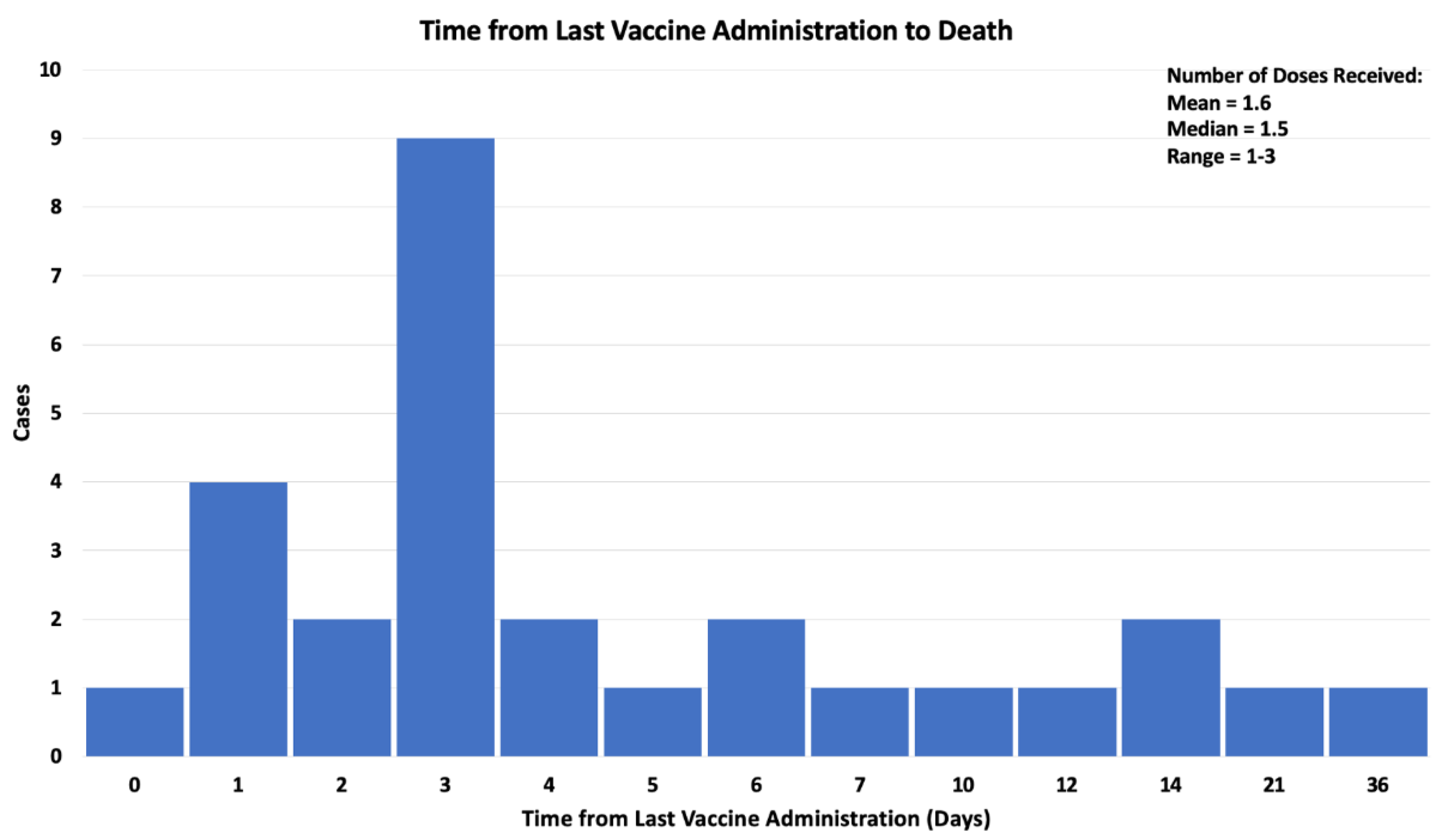
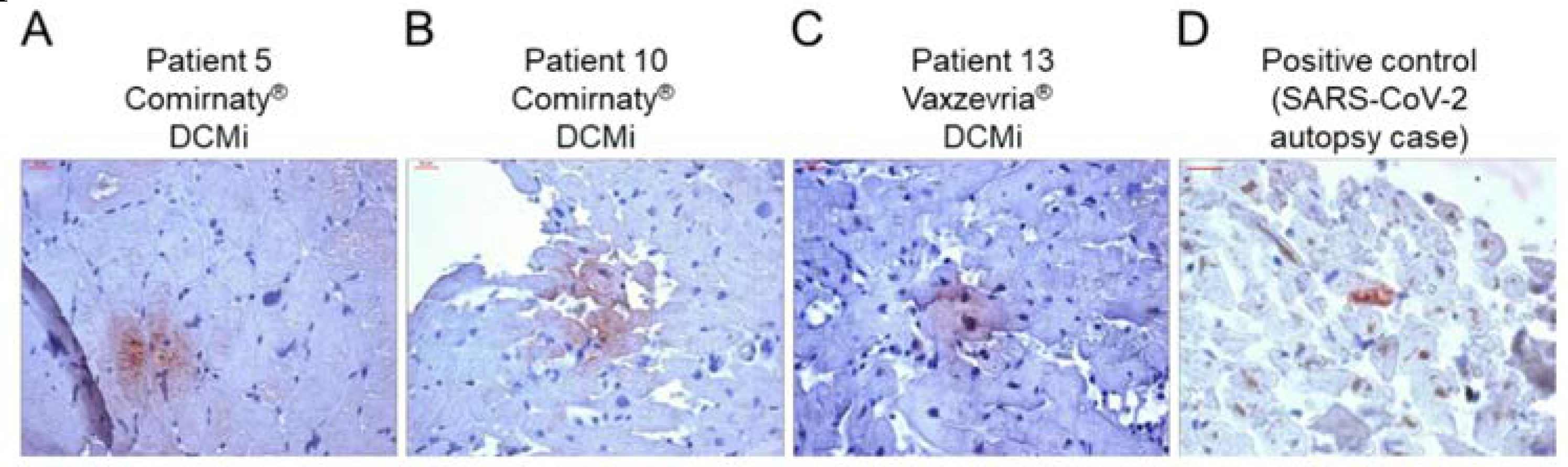
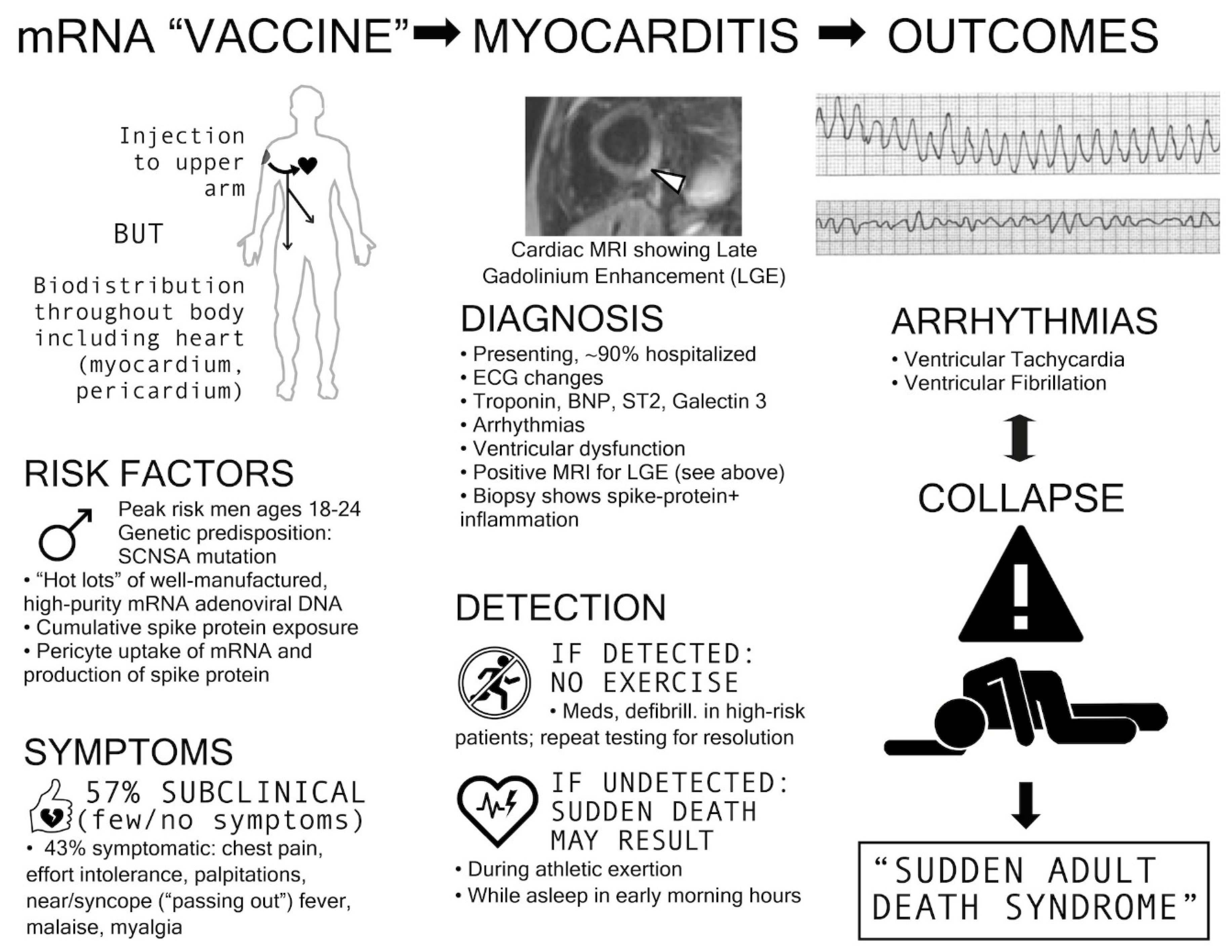
Results
Discussion
Funding
Acknowledgements
Disclosures
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 25 February 2023).
- Kuter, B.J.; Offit, P.A.; Poland, G.A. The development of COVID-19 vaccines in the United States: Why and how so fast? Vaccine 2021, 39, 2491–2495. [Google Scholar] [CrossRef] [PubMed]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 2023, CD015477. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem. Toxicol. 2022, 164, 113008–113008. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, A.B. S2 subunit of SARS-nCoV-2 interacts with tumor suppressor protein p53 and BRCA: an in silico study. Transl. Oncol. 2020, 13, 100814. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef]
- Theoharides, T.C. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol. Neurobiol. 2022, 59, 1850–1861. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Conti, P. Be aware of SARS-CoV-2 spike protein: There is more than meets the eye. J Biol Regul Homeost Agents 2021, 35, 833–838. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post–COVID-19 mRNA Vaccine Myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef]
- Castruita, J.A.S.; Vest Schneider, U.; Mollerup, S.; Leineweber, T.D.; Weis, N.; Bukh, J.; Pedersen, M.S.; Westh, H. SARS-CoV -2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID -19 vaccination. APMIS 2023, 131, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Avolio, E.; Carrabba, M.; Milligan, R.; Williamson, M.K.; Beltrami, A.P.; Gupta, K.; Elvers, K.T.; Gamez, M.; Foster, R.R.; Gillespie, K.; et al. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease. Clin. Sci. 2021, 135, 2667–2689. [Google Scholar] [CrossRef]
- Coronavirus (COVID-19) Update: June 25, 2021 [Internet]. U.S. Food and Drug Administration. 2021. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-june-25-2021.
- Rose, Jessica, McCullough, Peter. Determinants of COVID-19 Vaccine-Induced Myocarditis Requiring Hospitalization 2022. [CrossRef]
- Nonclinical Evaluation of BNT162b2 [mRNA] COVID-19 vaccine (COMIRNATY) [Internet]. Australian Government Department of Health - Therapeutic Goods Administration; 2021 [cited 2023 May 23]. Available online: https://www.tga.gov.au/sites/default/files/foi-2389-06.pdf.
- Baumeier, C.; Aleshcheva, G.; Harms, D.; Gross, U.; Hamm, C.; Assmus, B.; Westenfeld, R.; Kelm, M.; Rammos, S.; Wenzel, P.; et al. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. Int. J. Mol. Sci. 2022, 23, 6940. [Google Scholar] [CrossRef] [PubMed]
- Mansanguan, S.; Charunwatthana, P.; Piyaphanee, W.; Dechkhajorn, W.; Poolcharoen, A.; Mansanguan, C. Cardiovascular Manifestation of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents. Trop. Med. Infect. Dis. 2022, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Le Pessec G. Significant incidence of myocarditis after 3rd dose of anti-COVID 19 messenger RNA vaccine [Internet]. 2022 [cited 2023 Jul 6]. Available online: https://www.cardio-online.fr/Actualites/A-la-une/ESC-2022/Incidence-non-negligeable-myocardites-apres-3-dose-vaccin-ARN messager-anti-COVID-19.
- Myocarditis and pericarditis after COVID-19 vaccination: clinical management guidance for healthcare professionals Jan 9, 2023. Available online: https://www.gov.uk/government/publications/myocarditis-and-pericarditis-after-covid-19-vaccination/myocarditis-and-pericarditis-after-covid-19-vaccination-guidance-for-healthcare-professionals.
- Guidance on Myocarditis and Pericarditis after COVID-19 vaccines Sept 23, 2022. Available online: https://www.health.gov.au/sites/default/files/documents/2022/11/covid-19-vaccination-guidance-on-myocarditis-and-pericarditis-after-covid-19-vaccines.pdf.
- Vaccine Adverse Event Reporting System (VAERS) [online]. Available online: https://vaers.hhs.gov.
- Meissner CH. Vaccine Adverse Event Reporting System plays vital role in safety [Internet]. 2016 [cited 2023 Jul 6]. Available online: https://publications.aap.org/aapnews/news/14631.
- Scarl, R.; Parkinson, B.; Arole, V.; Hardy, T.; Allenby, P. The hospital autopsy: the importance in keeping autopsy an option. Autops. Case Rep. 2022, 12, e2021333. [Google Scholar] [CrossRef]
- Nushida, H.; Ito, A.; Kurata, H.; Umemoto, H.; Tokunaga, I.; Iseki, H.; Nishimura, A. A case of fatal multi-organ inflammation following COVID-19 vaccination. Leg. Med. 2023, 63, 102244–102244. [Google Scholar] [CrossRef] [PubMed]
- Mörz, M. A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19. Vaccines 2022, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kondo, T.; Yamasaki, G.; Sugimoto, M.; Asano, M.; Ueno, Y.; Nagasaki, Y. An autopsy case report of aortic dissection complicated with histiolymphocytic pericarditis and aortic inflammation after mRNA COVID-19 vaccination. Leg. Med. 2022, 59, 102154. [Google Scholar] [CrossRef]
- Satomi, H.; Katano, H.; Kanno, H.; Kobayashi, M.; Ohkuma, Y.; Hashidume, N.; Usui, T.; Tsukada, S.; Ito, I. An autopsy case of fulminant myocarditis after severe acute respiratory syndrome coronavirus 2 vaccine inoculation. Pathol. Int. 2022, 72, 519–524. [Google Scholar] [CrossRef]
- Suzuki, H.; Ro, A.; Takada, A.; Saito, K.; Hayashi, K. Autopsy findings of post-COVID-19 vaccination deaths in Tokyo Metropolis, Japan, 2021. Leg. Med. 2022, 59, 102134. [Google Scholar] [CrossRef]
- Gill, J.R.; Tashjian, R.; Duncanson, E. Autopsy Histopathologic Cardiac Findings in 2 Adolescents Following the Second COVID-19 Vaccine Dose. Arch. Pathol. Lab. Med. 2022, 146, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Ameratunga, R.; Woon, S.-T.; Sheppard, M.N.; Garland, J.; Ondruschka, B.; Wong, C.X.; Stewart, R.A.H.; Tatley, M.; Stables, S.R.; Tse, R.D. First Identified Case of Fatal Fulminant Necrotizing Eosinophilic Myocarditis Following the Initial Dose of the Pfizer-BioNTech mRNA COVID-19 Vaccine (BNT162b2, Comirnaty): an Extremely Rare Idiosyncratic Hypersensitivity Reaction. J. Clin. Immunol. 2022, 42, 441–447. [Google Scholar] [CrossRef]
- Choi, S.; Lee, S.; Seo, J.-W.; Kim, M.-J.; Jeon, Y.H.; Park, J.H.; Lee, J.K.; Yeo, N.S. Myocarditis-induced Sudden Death after BNT162b2 mRNA COVID-19 Vaccination in Korea: Case Report Focusing on Histopathological Findings. J. Korean Med Sci. 2021, 36, e286. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Sottmann, L.; Greinacher, A.; Hagen, M.; Kasper, H.-U.; Kuhnen, C.; Schlepper, S.; Schmidt, S.; Schulz, R.; Thiele, T.; et al. Postmortem investigation of fatalities following vaccination with COVID-19 vaccines. Int. J. Leg. Med. 2021, 135, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Lavine, K.J.; Lin, C.-Y. Myocarditis after Covid-19 mRNA Vaccination. New Engl. J. Med. 2021, 385, 1332–1334. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; Domke, L.M.; Hartmann, L.; Stenzinger, A.; Longerich, T.; Schirmacher, P. Autopsy-based histopathological characterization of myocarditis after anti-SARS-CoV-2-vaccination. Clin Res Cardiol. 2022, 112, 431–440. [Google Scholar] [CrossRef]
- Hoshino, N.; Yanase, M.; Ichiyasu, T.; Kuwahara, K.; Kawai, H.; Muramatsu, T.; Ishii, H.; Tsukamoto, T.; Morimoto, S.-I.; Izawa, H. An autopsy case report of fulminant myocarditis: Following mRNA COVID-19 vaccination. J. Cardiol. Cases 2022, 26, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-M.; Liu, X.; Yang, C.-T.; Qi, Q.; Shi, W.-B.; Li, Y.-M.; Zuo, M.; Wang, S.-J.; Bi, H.-T.; Ma, R.-F.; et al. Case report: Myocarditis following COVID-19 protein subunit vaccination. Front. Cardiovasc. Med. 2022, 9, 970045. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Kim, K.H.; Lee, N.; Cho, S.H.; Kim, S.Y.; Kim, E.K.; Park, J.-H.; Choi, E.-Y.; Choi, J.-O.; Park, H.; et al. COVID-19 vaccination-related myocarditis: a Korean nationwide study. Eur. Hear. J. 2023, 44, 2234–2243. [Google Scholar] [CrossRef]
- Fedak, K.M.; Bernal, A.; Capshaw, Z.A.; Gross, S. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg. Themes Epidemiology 2015, 12, 14. [Google Scholar] [CrossRef]
- Polykretis, P.; McCullough, P.A. Rational harm-benefit assessments by age group are required for continued COVID-19 vaccination. Scand. J. Immunol. 2022, 98, e13242. [Google Scholar] [CrossRef]
- Cadegiani, F.A. Catecholamines Are the Key Trigger of COVID-19 mRNA Vaccine-Induced Myocarditis: A Compelling Hypothesis Supported by Epidemiological, Anatomopathological, Molecular, and Physiological Findings. Cureus 2022, 14, e27883. [Google Scholar] [CrossRef] [PubMed]
- Dodt, C.; Breckling, U.; Derad, I.; Fehm, H.L.; Born, J. Plasma Epinephrine and Norepinephrine Concentrations of Healthy Humans Associated With Nighttime Sleep and Morning Arousal. Hypertension 1997, 30, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wangu, Z.; Swartz, H.; Doherty, M. Multisystem inflammatory syndrome in children (MIS-C) possibly secondary to COVID-19 mRNA vaccination. BMJ Case Rep. 2022, 15, e247176. [Google Scholar] [CrossRef] [PubMed]
- Ehikhametalor, K.; Deans-Minott, J.; Duncan, J.P. Multisystem Inflammatory Syndrome in Adults (MIS-A) After COVID-19 Infection and Recent Vaccination with Recombinant Adenoviral Vector Encoding the Spike Protein Antigen of SARS-CoV-2 (ChAdOx1 nCoV-19, Vaxzevria). J. Intensiv. Care Med. 2022, 38, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Ittiwut, C.; Mahasirimongkol, S.; Srisont, S.; Ittiwut, R.; Chockjamsai, M.; Durongkadech, P.; Sawaengdee, W.; Khunphon, A.; Larpadisorn, K.; Wattanapokayakit, S.; et al. Genetic basis of sudden death after COVID-19 vaccination in Thailand. Hear. Rhythm. 2022, 19, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Engler, R.J.M.; Nelson, M.R.; Jr. , L.C.C.; Spooner, C.; Hemann, B.A.; Gibbs, B.T.; Atwood, J.E.; Howard, R.S.; Chang, A.S.; Cruser, D.L.; et al. A Prospective Study of the Incidence of Myocarditis/Pericarditis and New Onset Cardiac Symptoms following Smallpox and Influenza Vaccination. PLOS ONE 2015, 10, e0118283. [Google Scholar] [CrossRef]
- Schmeling, M.; Manniche, V.; Hansen, P.R. Batch-dependent safety of the BNT162b2 mRNA COVID-19 vaccine. Eur. J. Clin. Investig. 2023, 53, e13998. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.F.; Cheng, C.-A.; Desjardins, M.; Senussi, Y.; Sherman, A.C.; Powell, M.; Novack, L.; Von, S.; Li, X.; Baden, L.R.; et al. Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients. Clin. Infect. Dis. 2021, 74, 715–718. [Google Scholar] [CrossRef]
- Keshavarz, P.; Yazdanpanah, F.; Emad, M.; Hajati, A.; Nejati, S.F.; Sadabad, F.E.; Azrumelashvili, T.; Mizandari, M.; Raman, S.S. Myocarditis Following COVID-19 Vaccination: Cardiac Imaging Findings in 118 Studies. Tomography 2022, 8, 1959–1973. [Google Scholar] [CrossRef]
- Pantazatos, S.; Seligmann, H. COVID vaccination and age-stratified all-cause mortality risk. Research Gate 2021. [CrossRef]
- Skidmore, M. The role of social circle COVID-19 illness and vaccination experiences in COVID-19 vaccination decisions: an online survey of the United States population. BMC Infect. Dis. 2023, 23, 51. [Google Scholar] [CrossRef]
- Aarstad, J.; Kvitastein, O.A. Is there a Link between the 2021 COVID-19 Vaccination Uptake in Europe and 2022 Excess All-Cause Mortality? org 2023, 2023020350. [Google Scholar] [CrossRef]
- Beesoon, S.; Bakal, J.A.; Youngson, E.; Williams, K.P.; Berzins, S.A.; Brindle, M.E.; Joffe, A.M. Excess deaths during the COVID-19 pandemic in Alberta, Canada. IJID Reg. 2022, 5, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Todd, M.; Scheeres, A. Excess Mortality From Non–COVID-19 Causes During the COVID-19 Pandemic in Philadelphia, Pennsylvania, 2020–2021. Am J Public Health 2022, 112, 1800–1803. [Google Scholar] [CrossRef] [PubMed]
- Karlinsky, A.; Kobak, D. The World Mortality Dataset: Tracking excess mortality across countries during the COVID-19 pandemic. medRxiv 2021. [CrossRef]
- Wang, H.; Paulson, K.R.; A Pease, S.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T.; et al. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Msemburi, W.; Karlinsky, A.; Knutson, V.; Aleshin-Guendel, S.; Chatterji, S.; Wakefield, J. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature 2022, 613, 130–137. [Google Scholar] [CrossRef]
- Shang, W.; Wang, Y.; Yuan, J.; Guo, Z.; Liu, J.; Liu, M. Global Excess Mortality during COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1702. [Google Scholar] [CrossRef]
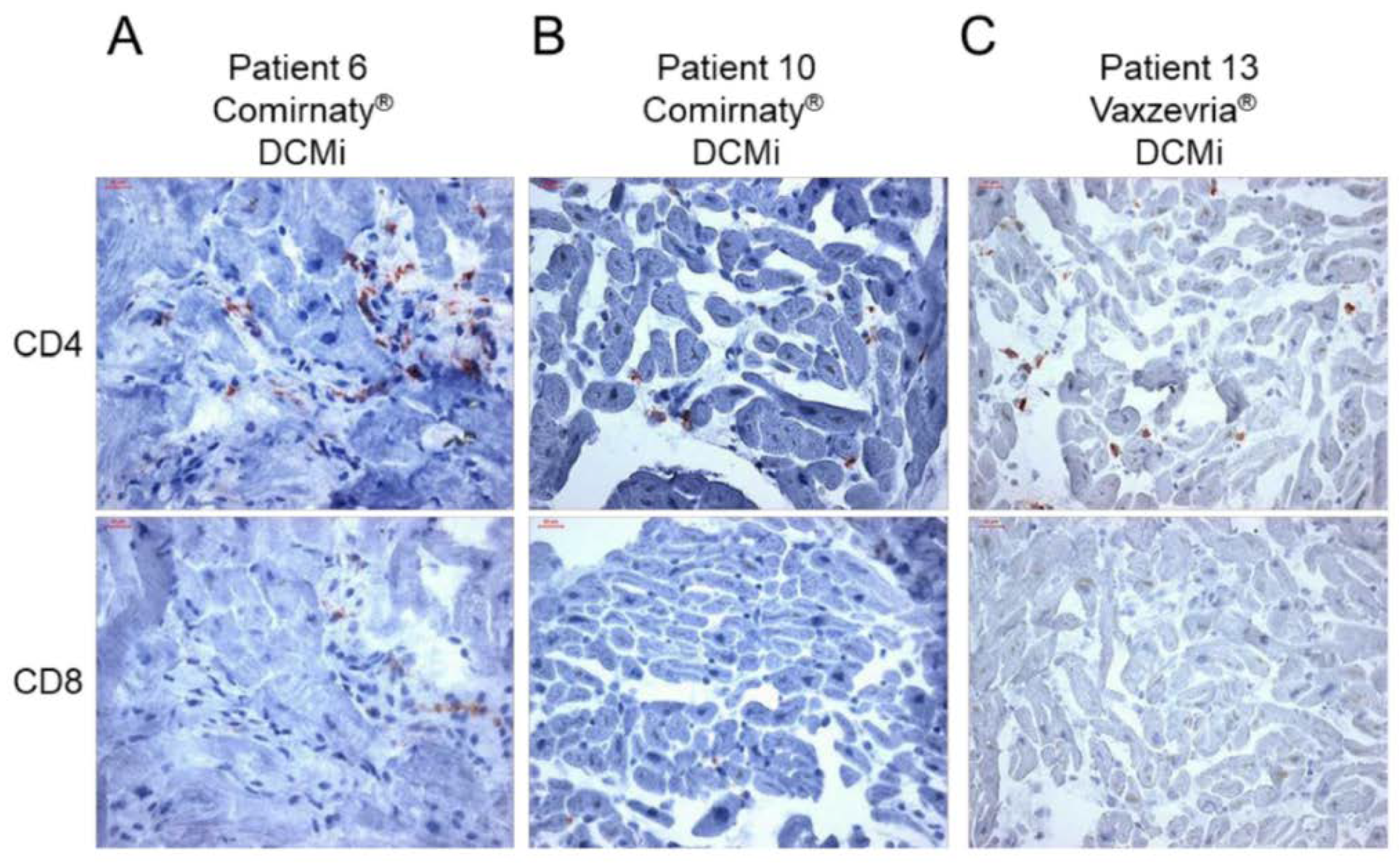
| AUthor | Case | AGE | Sex | Vaccine | Dose* | disease | period ** | Post-Mortem Findings |
|---|---|---|---|---|---|---|---|---|
| Nushida, 2023 (Japan) [25] | 1 | 14 | F | Pfizer | 3 | MIS, Myopericarditis | 2 days | Congestive edema of the lungs, T-cell lymphocytic and macrophage infiltration in the lungs, pericardium, and myocardium of the left atria and left ventricle, liver, kidneys, stomach, duodenum, bladder, and diaphragm.The presence of foci centered on the atria and breathlessness are the findings that led to the diagnosis that the cause of death was vaccine-related myopericarditis, which led to severe arrhythmias and progressive heart failure. |
| Morz, 2022 (germany) [26] | 1 | 76 | M | Pfizer | 2 | encephalitis, myocarditis | 21 days | Signs of aspiration pneumonia and systemic arteriosclerosis were evident. Brain examination uncovered acute vasculitis (predominantly lymphocytic) as well as multifocal necrotizing encephalitis of unknown etiology with pronounced inflammation including glial and lymphocytic reaction. In the heart, signs of chronic cardiomyopathy as well as mild acute lympho-histiocytic myocarditis and vasculitis were present. Only spike protein but no nucleocapsid protein could be detected within the foci of inflammation in both the brain and the heart. Also, mild acute splenitis, gastric mucosal bleeding, liver lipofuscinosis, and mild active nephritis were found. |
| Takahashi, 2022 (Japan)[27] | 1 | ‘90s’ | M | Pfizer | 3 | Pericarditis | 14 days | Dissection of the ascending aorta and pericardial hemotamponade. The heart showed a white villous surface, and the pericardium was fibrously thick. Microscopic examination revealed pericarditis with predominantly macrophage and lymphocyte infiltration. |
| Satomi, 2022 (japan) [28] | 1 | 61 | F | Pfizer | 1 | Myocarditis | 10 days | The heart showed moderate dilatation of both ventricles, and the myocardium showed an uneven color change and decreased elasticity. Histologically, severe myocarditis with extensive myocytolysis was observed. The myocarditis showed severe inflammatory cell infiltration with T-lymphocyte and macrophage predominance, and vast nuclear dust accompanying neutrophilic infiltration was observed. In the bone marrow and lymph nodes, hemophagocytosis was observed. SARS-CoV-2 nucleic acids were not detected using multivirus real-time PCR system. |
| Suzuki, 2021 (japan) [29] | 1 | 91 | M | Moderna | 1 | Ischemic heart disease, myocarditis | 6 days | Old myocardial infarction in the post lateral wall, severe coronary artery sclerosis, leukocyte and lymphocyte infiltration in the left anterior wall, diabetic nephropathy, aortic sclerosis. |
| 2 | 24 | M | Moderna | 2 | Myocarditis | 3 days | Scattered necrosis and fibrosis of cardiomyocytes with a perivascular pattern of inflammatory cell infiltration (consisting of predominantly lymphocytes). | |
| 3 | 39 | M | Moderna | 2 | Myocarditis | 3 days | Scattered inflammatory cell infiltration (consisting of predominantly monocytes) in the interstitial space of cardiomyocytes/around the coronary arteries, interstitial edema, eosinophilic and wavy change of cardiomyocytes, Lung edema, coronary sclerosis. | |
| Gill, 2022 (usa) [30] | 1 | ‘teenage’ | M | Pfizer | 2 | Myocarditis | 3 days | No molecular evidence of SARS-CoV-2 infection. Global myocardial injury with areas of coagulative myocytolysis and contractions bands, with a perivascular pattern of inflammation consisting of mainly neutrophils and histocytes, scant lymphocytes, and occasional eosinophils. No acute or organizing thrombi were detected. Pattern of injury is consistent with stress cardiomyopathy. |
| 2 | ‘teenage’ | M | Pfizer | 2 | Myocarditis | 4 days | No molecular evidence of SARS-CoV-2 infection. As with the previous case, global myocardial injury was found but with more widespread transmural ischemic changes and more interstitial inflammation. Subepicardial distribution of injury was not seen. No acute or organizing thrombi were detected. | |
| Ameratunga, 2022 (new zealand) [31] | 1 | 57 | F | Pfizer | 1 | Myocarditis | 3 days | Left pleural mass originating from the mediastinum was found. Multifocal inflammatory cell infiltration in the myocardium and areas of eosinophil-rich inflammatory aggregates with myocyte necrosis were found. An abundant eosinophilic infiltrate with myocyte necrosis was observed. Antibodies to SARS-CoV-2 were not detected. |
| Choi, 2021 (korea) [32] | 1 | 22 | M | Pfizer | 1 | Myocarditis | 5 days | Histological examination of the heart showed isolated atrial myocarditis, with neutrophil and histiocyte predominance. Immunohistochemical C4d staining showed scattered single-cell necrosis of myocytes which was not accompanied by inflammatory infiltrates. Extensive contraction band necrosis was seen in the atria and ventricles. There was no evidence of microthrombosis or infection in the heart and other organs. |
| Schneider, 2021 (germany) [33] | 1 | 65 | M | Pfizer | 1 | Myocarditis | 1 day | Severe coronary sclerosis, massive cardiac hypertrophy, myocardial infarction scars, myocarditis, anaphylaxis diagnostics negative. |
| Verma, 2021 (usa) [34] | 1 | 42 | M | Moderna | 2 | Myocarditis | ~14 days | Autopsy revealed biventricular myocarditis. An inflammatory infiltrate admixed with macrophages, T-cells, eosinophils, and B cells was also observed. |
| Schwab, 2023 (germany) [35] | 1 | 46 | M | Pfizer | 1 | Myocarditis | 0 days | Histological examination showed inflammatory infiltration of the myocardium. The infiltrate was focal and interstitial. It was predominantly detected in sections taken from the right ventricular wall and interventricular septum. The histological and immunohistochemical characterization revealed that the inflammatory infiltrate was predominantly composed of lymphocytes. Micro focal myocyte injury was demonstrable. Lacked pre-existing, clinically relevant heart disease. |
| 2 | 50 | F | Moderna | 1 | Myocarditis | 1 day | Histological examination showed inflammatory infiltration of the myocardium. The infiltrate was focal and interstitial. It was predominantly detected in sections taken from the right ventricular wall and interventricular septum. The histological and immunohistochemical characterization revealed that the inflammatory infiltrate was predominantly composed of lymphocytes. Micro focal myocyte injury was demonstrable. A n inflammatory infiltration of the epicardium and the subepicardial fat tissue was concomitantly found. L acked pre-existing, clinically relevant heart disease. | |
| 3 | 62 | F | Pfizer | 1 | Myocarditis | 7 days | Histological examination showed inflammatory infiltration of the myocardium. The infiltrate was focal and interstitial. It was predominantly detected in sections taken from the right ventricular wall and interventricular septum. The histological and immunohistochemical characterization revealed that the inflammatory infiltrate was predominantly composed of lymphocytes. Micro focal myocyte injury was demonstrable. An inflammatory infiltration of the epicardium and the subepicardial fat tissue was concomitantly found. Lacked pre-existing, clinically relevant heart disease. | |
| 4 | 55 | M | Pfizer | 2 | Myocarditis | 4 days | Histological examination showed inflammatory infiltration of the myocardium. The infiltrate was focal and interstitial. It was predominantly detected in sections taken from the right ventricular wall and interventricular septum. The histological and immunohistochemical characterization revealed that the inflammatory infiltrate was predominantly composed of lymphocytes. A n inflammatory infiltration of the epicardium and the subepicardial fat tissue was concomitantly found. L acked pre-existing, clinically relevant heart disease. | |
| 5 | 75 | F | Pfizer | 1 | Myocarditis | 1 days | Histological examination showed inflammatory infiltration of the myocardium. The infiltrate was focal and interstitial. It was predominantly detected in sections taken from the right ventricular wall and interventricular septum. The histological and immunohistochemical characterization revealed that the inflammatory infiltrate was predominantly composed of lymphocytes. An inflammatory infiltration of the epicardium and the subepicardial fat tissue was concomitantly found. Lacked pre-existing, clinically relevant heart disease. Analysis for potential infectious agents causing a myocarditis revealed low viral copy numbers of human herpes virus 6. | |
| Hoshino, 2022 (japan) [36] | 1 | 27 | M | Moderna | 1 | Myocarditis | 36 days | An autopsy revealed asymmetric left ventricular hypertrophy, thickening of the right ventricular wall (550 g; LV wall, 11–16 mm; RV wall, 5–7 mm), myxomatous degeneration of the posterior leaflet of the mitral valve, and hypertrophy of the posteromedial papillary muscle. Microscopic findings revealed that cardiac myocytolysis and widespread fibrosis were observed, and significant mixed inflammatory infiltration (T cells, macrophages, and eosinophils) was observed in the left ventricular free wall and the anterior potion of the ventricular septum. |
| DONG, 2022 (china) [37] | 1 | 34 | F | Zifivax | 1 | Myocarditis | 12 days | Autopsy showed severe interstitial myocarditis, including multiple patchy infiltrations of lymphocytes and monocytes in the myocardium of the left and right ventricular walls associated with myocyte degeneration and necrosis. |
| Cho, 2023 (korea) [38] | 1 | 22 | M | Pfizer | 1 | SCD from Myocarditis | 6 days | Diffuse inflammatory infiltration, with neutrophil and histiocyte predominance in both atria and near AV node and SA node. Free of inflammatory infiltrates in ventricular myocardium. |
| 2 | 30 | F | Pfizer | 1 | SCD from Myocarditis | 3 days | Diffuse inflammatory cell infiltration, myocardial fiber disarray, interstitial fibrosis, and localized necrosis of myocyte. | |
| 3 | 45 | M | Pfizer | 2 | SCD from Myocarditis | 3 days | Localized infiltration of neutrophils, lymphocytes, histocyte, and a few eosinophils was noted. A small number of cardiomyocyte necrosis were also seen. | |
| 4 | 25 | M | Pfizer | 2 | SCD from Myocarditis | 3 days | Autopsy revealed myocarditis. | |
| 5 | 45 | M | Pfizer | 2 | SCD from Myocarditis | 3 days | Interstitial infiltration of various inflammatory cells including lymphocyte, neutrophil, eosinophil, and focal necrosis suggesting the diagnosis of myocarditis. | |
| 6 | 36 | F | Moderna | 1 | SCD from Myocarditis | 2 days | Neutrophil, eosinophil, and histiocyte infiltration in the myocardium suggesting acute myocarditis. | |
| 7 | 33 | M | Moderna | 2 | SCD from Myocarditis | 1 day | Multiple focal infiltrations of acute inflammatory cells and chronic inflammatory cells in the myocardium. | |
| 8 | 33 | M | Moderna | 2 | SCD from Myocarditis | 3 days | Various inflammatory cells such as neutrophils, eosinophils, lymphocytes, macrophages, and cardiomyocyte necrosis in the myocardial interstitium and epicardium suggested myocarditis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





