Submitted:
18 July 2023
Posted:
19 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Initiation and Stock
2.1.1. Proliferation of Citrus Rootstocks
2.1.2. Mass-Production of Citrus Rootstocks
2.2. Stress Treatments
2.2.1. Data Collection and Morphological and Biochemical Characteristics
2.2.1.1. Determination of Morphological Characteristics
2.2.1.2. Determination of Photosynthetic Pigments
2.2.1.3. Determination Proline Content
2.2.1.4. Leaf Relative Water Content
2.2.1.5. Ions Accumulation
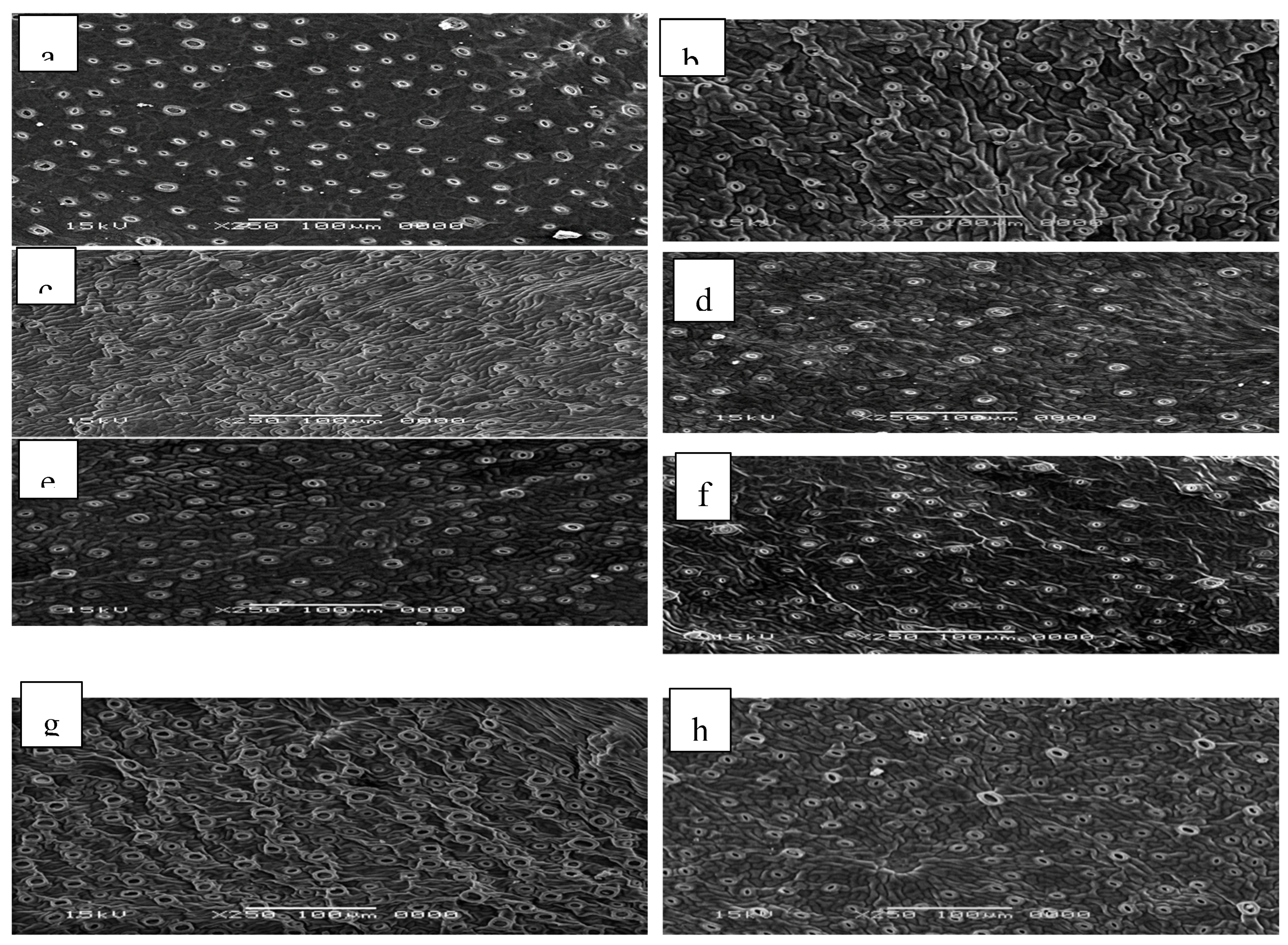
2.2.2. Stomata Behavior
2.3. Statistical Procedure
3. Results
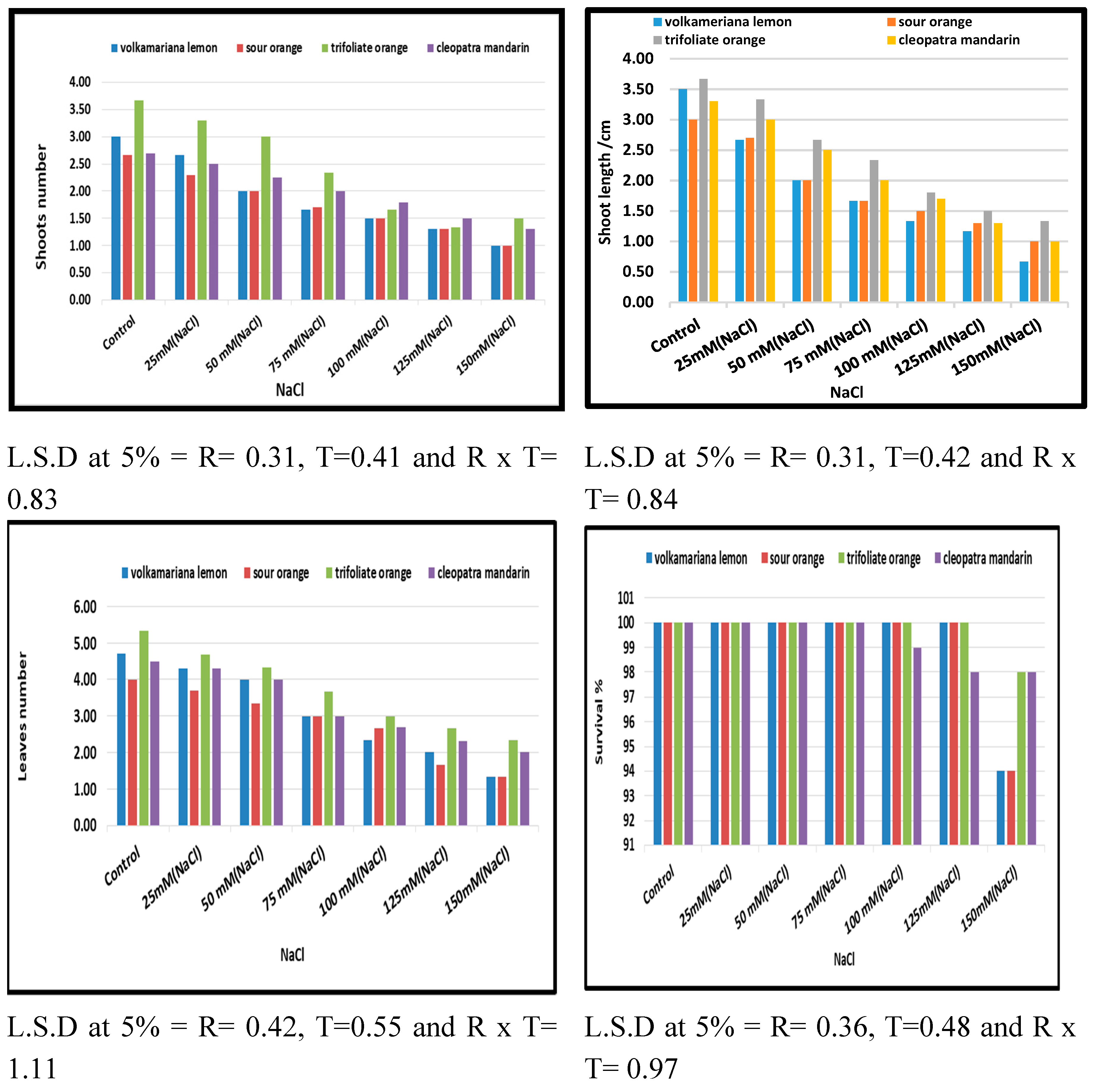
3.1. Evaluation of Growth Characteristics
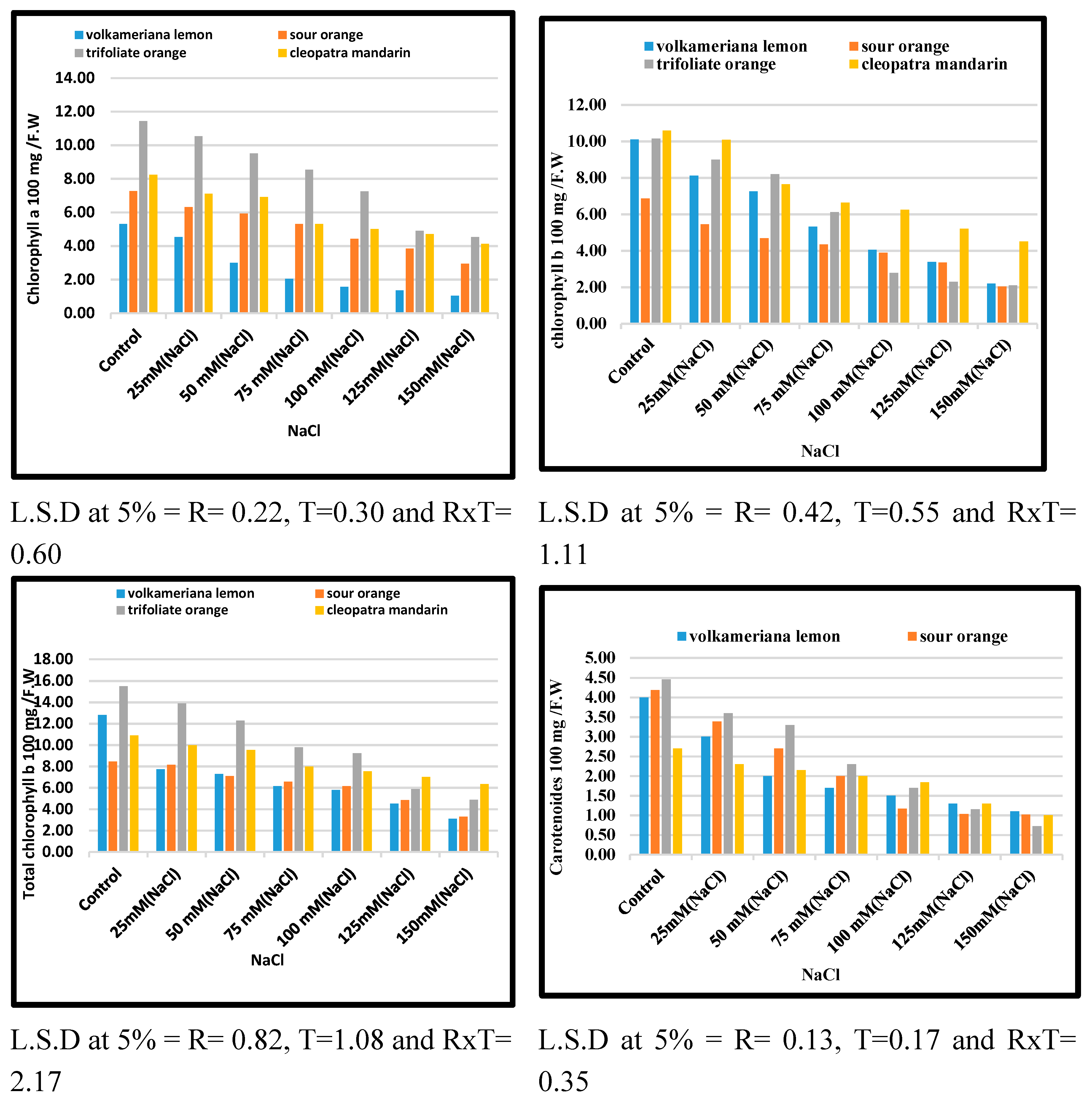
3.2. Evaluation of Photosynthesis Pigment
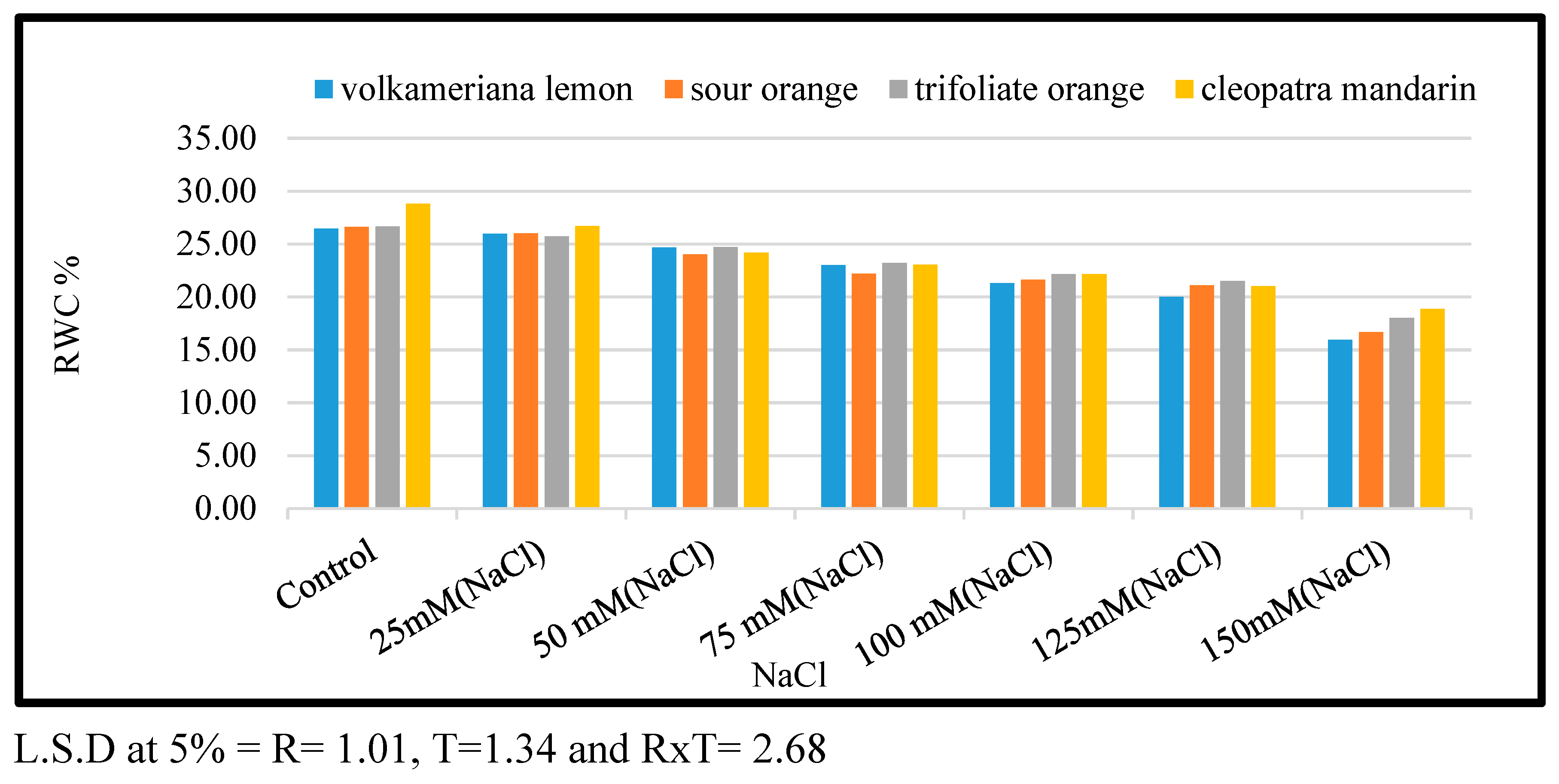
3.3. Effect of NaCl on RWC
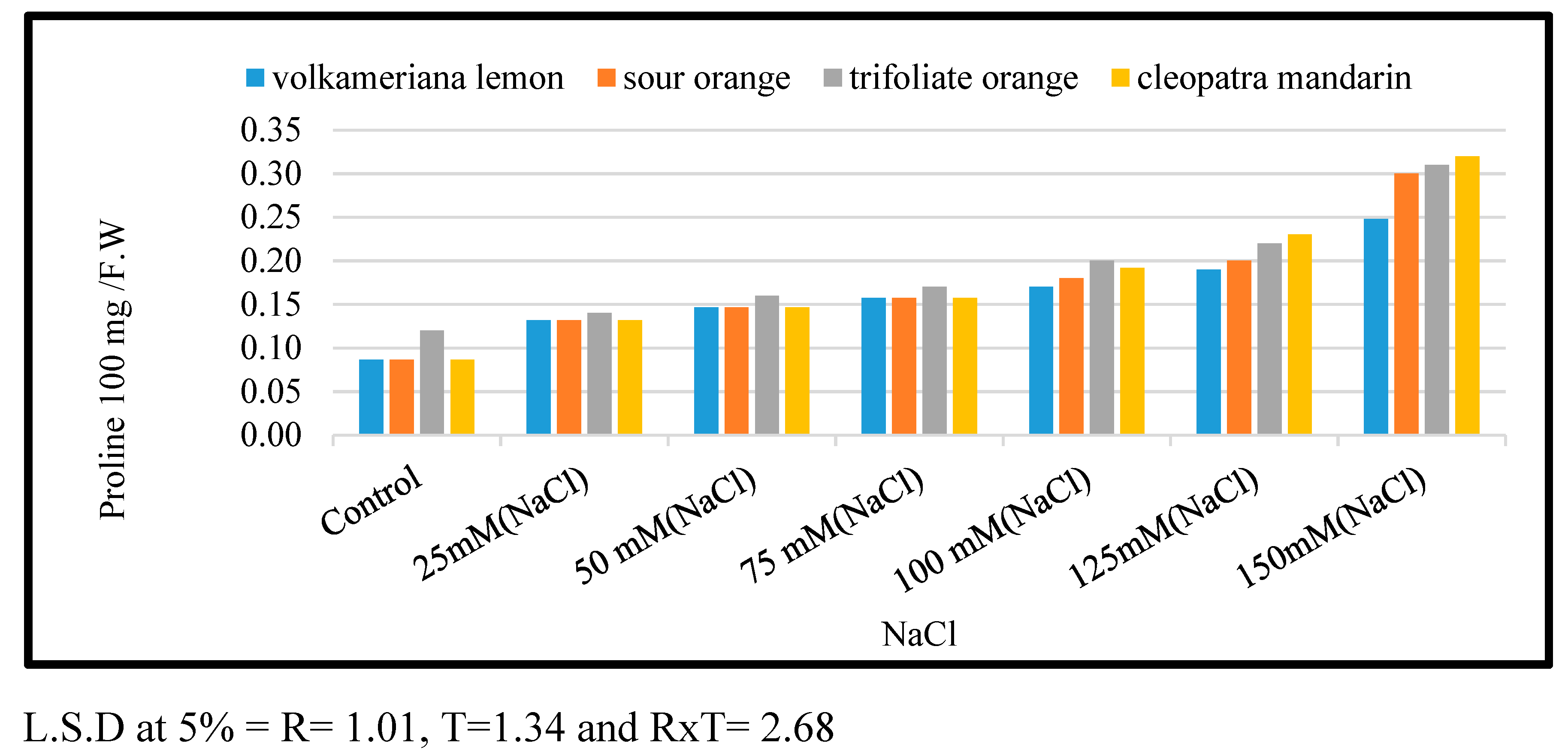
3.4. Effect of NaCl on Proline Content
3.5. Effect of NaCl on Ion Content
3.6. Effect of NaCl on the Stomata Status
3.7. Principal Components Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Vinocur, B.; Altman, A. Plant Responses to Drought, Salinity and Extreme Temperatures: Towards Genetic Engineering for Stress Tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Ahmad, K.; Akhtar, M.A.; Maqbool, M.A. Salinity Stress in Crop Plants: Effects of Stress, Tolerance Mechanisms and Breeding Strategies for Improvement. 2017, 02, 17. [Google Scholar]
- Hamouda, M.M.; Badr, A.; Ahmed, H.I.S.; Saad-Allah, K.M. Growth, Physiological, and Molecular Responses of Three Phaeophyte Extracts on Salt-Stressed Pea (Pisum sativum L.) Seedlings. In Review. 2022. [Google Scholar]
- Galvan-Ampudia, C.S.; Testerink, C. Salt Stress Signals Shape the Plant Root. Current Opinion in Plant Biology 2011, 14, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Plant Salt Tolerance: Adaptations in Halophytes. Annals of Botany 2015, 115, 327–331. [Google Scholar] [CrossRef]
- Kubitzki, K. (Ed.) Flowering Plants, Eudicots: Sapindales, Cucurbitales, Myrtaceae; The Families and Genera of Vascular, Plants; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-14396-0. [Google Scholar]
- Guerra, M.; Pedrosa, A.; Silva, A.E.B.e; Cornélio, M.T.M.; Santos, K.; Soares Filho, W.d.S. Chromosome Number and Secondary Constriction Variation in 51 Accessions of a Citrus Germplasm Bank. Braz. J. Genet. 1997, 20, 489–496. [Google Scholar] [CrossRef]
- Cai, X.; Fu, J.; Guo, W. Mitochondrial Genome of Callus Protoplast Has a Role in Mesophyll Protoplast Regeneration in Citrus : Evidence From Transgenic GFP Somatic Homo-Fusion. Horticultural Plant Journal 2017, 3, 177–182. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Citrus Plants Exude Proline and Phytohormones under Abiotic Stress Conditions. Plant Cell Rep 2017, 36, 1971–1984. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Elsevier, 1987; Vol. 148, pp. 350–382. ISBN 978-0-12-182048-0. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Garcı́a-Mata, C.; Lamattina, L. Nitric Oxide Induces Stomatal Closure and Enhances the Adaptive Plant Responses against Drought Stress. Plant Physiol. 2001, 126, 1196–1204. [Google Scholar] [CrossRef]
- Chen, S.; Theodorou, I.G.; Goode, A.E.; Gow, A.; Schwander, S.; Zhang, J. (Jim); Chung, K.F.; Tetley, T.D.; Shaffer, M.S.; Ryan, M.P.; et al. High-Resolution Analytical Electron Microscopy Reveals Cell Culture Media-Induced Changes to the Chemistry of Silver Nanowires. Environ. Sci. Technol. 2013, 47, 13813–13821. [Google Scholar] [CrossRef]
- Stern, R.D. CoStat-Statutical Software. California: CoHort Software (1989), Pp. 302, $76.00. Ex. Agric. 1991, 27, 87. [Google Scholar] [CrossRef]
- Ghaleb, W.S.; Sawwan, J.S.; Akash, M.W.; Al-Abdallat, A.M. In Vitro Response of Two Citrus Rootstocks to Salt Stress. International Journal of Fruit Science 2010, 10, 40–53. [Google Scholar] [CrossRef]
- Rostamian, L.; Chalavi, V.; Sadeghi, H. Effect of salt stress on physiological responses of four citrus rootstock plantlets under in vitro condition. Journal of Crops Improvement. 2019, 21, 447–458. [Google Scholar] [CrossRef]
- Prior, L.; Grieve, A.; Cullis, B. Sodium Chloride and Soil Texture Interactions in Irrigated Field Grown Sultana Grapevines. II. Plant Mineral Content, Growth and Physiology. Aust. J. Agric. Res. 1992, 43, 1067. [Google Scholar] [CrossRef]
- Mulholland, B.J.; Taylor, I.B.; Jackson, A.C.; Thompson, A.J. Can ABA Mediate Responses of Salinity Stressed Tomato. Environmental and Experimental Botany 2003, 50, 17–28. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. IJMS 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Munns, R. Comparative Physiology of Salt and Water Stress: Comparative Physiology of Salt and Water Stress. Plant, Cell & Environment 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Loreto, F.; Centritto, M.; Chartzoulakis, K. Photosynthetic Limitations in Olive Cultivars with Different Sensitivity to Salt Stress: Photosynthetic Limitations and Salt Stress in Olive Cultivars. Plant, Cell & Environment 2003, 26, 595–601. [Google Scholar] [CrossRef]
- Ndayiragije, A.; Lutts, S. Do Exogenous Polyamines Have an Impact on the Response of a Salt-Sensitive Rice Cultivar to NaCl? Journal of Plant Physiology 2006, 163, 506–516. [Google Scholar] [CrossRef]
- Craine, J.M. Reconciling Plant Strategy Theories of Grime and Tilman: Reconciling Plant Strategy Theories. Journal of Ecology 2005, 93, 1041–1052. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to Increasing the Salt Tolerance of Wheat and Other Cereals. Journal of Experimental Botany 2006, 57, 1025–1043. [Google Scholar] [CrossRef]
- Shah, S.H. EFFECTS OF SALT STRESS ON MUSTARD AS AFFECTED BY GIBBERELLIC ACID APPLICATION. 10.
- Gu, R.; Fonseca, S.; Puskas, L.G.; Hackler, L.; Zvara, A.; Dudits, D.; Pais, M.S. Transcript Identification and Profiling during Salt Stress and Recovery of Populus Euphratica. Tree Physiology 2004, 24, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Kreps, J.A.; Wu, Y.; Chang, H.-S.; Zhu, T.; Wang, X.; Harper, J.F. Transcriptome Changes for Arabidopsis in Response to Salt, Osmotic, and Cold Stress. Plant Physiology 2002, 130, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Keutgen, A.J.; Pawelzik, E. Modifications of Strawberry Fruit Antioxidant Pools and Fruit Quality under NaCl Stress. J. Agric. Food Chem. 2007, 55, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, I.E.; Jeffrey, O. Does Financial Risks Has Effects on The Performance of Deposit Money Banks in Nigeria? Saudi J. Bus. Manag. Stud 2021, 6, 71–85. [Google Scholar] [CrossRef]
- Molazem, D.; Qurbanov, E.M.; Dunyamaliyev, S.A. Role of Proline, Na and Chlorophyll Content in Salt Tolerance of Corn (Zea Mays L.). Environ. Sci. 2010, 6. [Google Scholar]
- Garcia, M.; Charbaji, T. Effect of Sodium Chloride Salinity on Cation Equilibria in Grapevine. Journal of Plant Nutrition 1993, 16, 2225–2237. [Google Scholar] [CrossRef]
- Pascale, S.D.; Maggio, A.; Fogliano, V.; Ambrosino, P.; Ritieni, A. Irrigation with Saline Water Improves Carotenoids Content and Antioxidant Activity of Tomato. The Journal of Horticultural Science and Biotechnology 2001, 76, 447–453. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B.; Mittra, B. Effects of Salt on Growth, Ion Accumulation, Photosynthesis and Leaf Anatomy of the Mangrove, Bruguiera Parviflora. Trees - Structure and Function 2004, 18, 167–174. [Google Scholar] [CrossRef]
- Chutipaijit, S.; Cha-um, S.; Sompornpailin, K. Modulation of Proline and Anthocyanin Levels Improves Salt Tolerant in Indica Rice Seedlings. Journal of Biotechnology 2008, 136, S152. [Google Scholar] [CrossRef]
- Hatami, E.; Esna-Ashari, M.; Javadi, T. Effect of Salinity on Some Growth Characteristics and Concentration of Elements in Two Grape (Vitis Vinifera L.) Cultivars, ‘Rishbaba’ and ‘Sahebi.’ 4.
- Damani, Z.; Karimi, H.R.; Mirik, A.A.M.; Esmaelizadeh, M. Effect of Salinity and Drought Stresses on Growth and Eco Physiological Parameters in Carob Seedlings (Ceratonia Siliqua L.). 1.
- Yang, F.; Xiao, X.; Zhang, S.; Korpelainen, H.; Li, C. Salt Stress Responses in Populus Cathayana Rehder. Plant Science 2009, 176, 669–677. [Google Scholar] [CrossRef]
- Jiang, X.; Qi, W.; Xu, X.; Li, Y.; Liao, Y.; Wang, B. Higher Soil Salinity Causes More Physiological Stress in Female of Populus Cathayana Cuttings. Acta Ecologica Sinica 2014, 34, 225–231. [Google Scholar] [CrossRef]
- Azimzadeh, Z.; Hassani, A.; Abdollahi Mandoulakani, B.; Sepehr, E. Effects of salinity stress on some morpho-physiological characteristics, essential oil content, and ion relations of two oregano subspecies (Origanum vulgare ssp. vulgare & ssp. gracile). Iranian Journal of Medicinal and Aromatic Plants Research 2021, 37, 658–674. [Google Scholar]
- Emami, S.; Asghari, A.; Mohammaddoust Chamanabad, H.; Rasoulzadeh, A.; Ramzi, E. Evaluation of osmotic stress tolerance in durum wheat (Triticum durum L.) advanced lines. Environmental Stresses in Crop Sciences 2019, 12, 697–707. [Google Scholar] [CrossRef]
- Walker, R.R.; Blackmore, D.H.; Clingeleffer, P.R.; Tarr, C.R. Rootstock Effects on Salt Tolerance of Irrigated Field-Grown Grapevines (Vitis Vinifera L. Cv. Sultana). 3. Fresh Fruit Composition and Dried Grape Quality. Aust J Grape Wine Res 2007, 13, 130–141. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A Key Player in Plant Abiotic Stress Tolerance. Biologia plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Azza, C.-C.; Mosbah, A.B.; Maalej, M.; Gargouri, K.; Gargouri-Bouzid, R.; Drira, N. In Vitro Salinity Tolerance of Two Pistachio Rootstocks: Pistacia Vera L. and P. Atlantica Desf. Environmental and Experimental Botany 2010, 69, 302–312. [Google Scholar] [CrossRef]
- Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- El-Habashy, S. In Vitro Evaluation and Selection for Salinity Tolerance in Some Citrus Rootstock Seedlings. 2018, 11. [Google Scholar]
- Gutierrez-Partida, E.; Hempel, H.; Caicedo-Dávila, S.; Raoufi, M.; Peña-Camargo, F.; Grischek, M.; Gunder, R.; Diekmann, J.; Caprioglio, P.; Brinkmann, K.O.; et al. Large-Grain Double Cation Perovskites with 18 Μs Lifetime and High Luminescence Yield for Efficient Inverted Perovskite Solar Cells. ACS Energy Lett. 2021, 6, 1045–1054. [Google Scholar] [CrossRef]
- Murillo-Amador, B.; Yamada, S.; Yamaguchi, T.; Rueda-Puente, E.; Ávila-Serrano, N.; García-Hernández, J.L.; López-Aguilar, R.; Troyo-Diéguez, E.; Nieto-Garibay, A. Influence of Calcium Silicate on Growth, Physiological Parameters and Mineral Nutrition in Two Legume Species Under Salt Stress. J Agron Crop Sci 2007, 193, 413–421. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Enhancement of Tolerance of Abiotic Stress by Metabolic Engineering of Betaines and Other Compatible Solutes. Current Opinion in Plant Biology 2002, 5, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Javaid, T.; Farooq, M.A.; Akhtar, J.; Saqib, Z.A.; Anwar-ul-Haq, M. Silicon Nutrition Improves Growth of Salt-Stressed Wheat by Modulating Flows and Partitioning of Na+, Cl− and Mineral Ions. Plant Physiology and Biochemistry 2019, 141, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Rasel, M.; Tahjib-Ul-Arif, M.; Hossain, M.A.; Hassan, L.; Farzana, S.; Brestic, M. Screening of Salt-Tolerant Rice Landraces by Seedling Stage Phenotyping and Dissecting Biochemical Determinants of Tolerance Mechanism. J Plant Growth Regul 2021, 40, 1853–1868. [Google Scholar] [CrossRef]
- Mahmood-ur-Rahman; Munazza, I.; Qamar, S.; Bukhari, S.A.; Malik, K. Abiotic Stress Signaling in Rice Crop. In Advances in Rice Research for Abiotic Stress Tolerance. Elsevier, 2019; pp. 551–569. ISBN 978-0-12-814332-2. [Google Scholar]
- Wenji, L.; Ma, X.; Wan, P.; Liu, L. Plant Salt-Tolerance Mechanism: A Review. Biochemical and Biophysical Research Communications 2018, 495, 286–291. [Google Scholar] [CrossRef]
- El-Moneim, A.; Eman, A.A.; Abd-Allah AS, E.; Ebeed, S.S. EFFECT OF SOME ORGANIC AND BIOFERTILIZER TREATMENTS ON MINIMIZING MINERAL NITROGEN FERTILIZATION OF WASHINGTON NAVEL ORANGE TREES. Arab Universities Journal of Agricultural Sciences 2008, 16, 451–457. [Google Scholar] [CrossRef]
- Sharma, L.K.; Kaushal, M.; Bali, S.K.; Choudhary, O.P. Evaluation of Rough Lemon (Citrus Jambhiri Lush.) as Rootstock for Salinity Tolerance at Seedling Stage under in Vitro Conditions. Afr. J. Biotechnol. 2013, 12, 6267–6275. [Google Scholar] [CrossRef]
- Pérez-Jiménez, M.; Pérez-Tornero, O. In Vitro Plant Evaluation Trial: Reliability Test of Salinity Assays in Citrus Plants. Plants 2020, 9, 1352. [Google Scholar] [CrossRef]
- Showkat Ahmad, G.; Molla, K.A.; Henry, R.J.; Bhat, K.V.; Mondal, T.K. Advances in Understanding Salt Tolerance in Rice. Theor Appl Genet 2019, 132, 851–870. [Google Scholar] [CrossRef]
- Davis, L.; Sumner, M.; Stasolla, C.; Renault, S. Salinity-Induced Changes in the Root Development of a Northern Woody Species, Cornus Sericea. Botany 2014, 92, 597–606. [Google Scholar] [CrossRef]
- Weise, A.; Barker, L.; Kühn, C.; Lalonde, S.; Buschmann, H.; Frommer, W.B.; Ward, J.M. A New Subfamily of Sucrose Transporters, SUT4, with Low Affinity/High Capacity Localized in Enucleate Sieve Elements of Plants. Plant Cell 2000, 12, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Fariba, A.; Ehsanpour, A.A. Soluble Proteins, Proline, Carbohydrates and Na+/K+ Changes in Two Tomato (Lycopersicon Esculentum Mill.) Cultivars under in Vitro Salt Stress. American J. of Biochemistry and Biotechnology 2005, 1, 212–216. [Google Scholar] [CrossRef]
- Tester, M. Na+ Tolerance and Na+ Transport in Higher Plants. Annals of Botany 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Ashraf, M.; Khanum, A. Relationship Between Ion Accumulation and Growth in Two Spring Wheat Lines Differing in Salt Tolerance at Different Growth Stages. J Agron Crop Sci 1997, 178, 39–51. [Google Scholar] [CrossRef]
- Cram, W.J. Chloride Accumulation as a Homeostatic System: Set Points and Perturbations: THE PHYSIOLOGICAL SIGNIFICANCE OF INFLUX ISOTHERMS, TEMPERATURE EFFECTS AND THE INFLUENCE OF PLANT GROWTH SUBSTANCES. J Exp Bot 1983, 34, 1484–1502. [Google Scholar] [CrossRef]
- Edward, P.G.; Nelson, S.G.; Ambrose, B.; Martinez, R.; Soliz, D.; Pabendinskas, V.; Hultine, K. Comparison of Salinity Tolerance of Three Atriplex Spp. in Well-Watered and Drying Soils. Environmental and Experimental Botany 2012, 83, 62–72. [Google Scholar] [CrossRef]
- Haffter, P.; Granato, M.; Brand, M.; Mullins, M.C.; Hammerschmidt, M.; Kane, D.A.; Odenthal, J.; van Eeden, F.J.; Jiang, Y.J.; Heisenberg, C.P.; et al. The Identification of Genes with Unique and Essential Functions in the Development of the Zebrafish, Danio Rerio. Development 1996, 123, 1–36. [Google Scholar] [CrossRef]
- Ceusters, N.; Valcke, R.; Frans, M.; Claes, J.E.; Van den Ende, W.; Ceusters, J. Performance Index and PSII Connectivity Under Drought and Contrasting Light Regimes in the CAM Orchid Phalaenopsis. Front. Plant Sci. 2019, 10, 1012. [Google Scholar] [CrossRef]
- Stirbet, A.; Riznichenko, G.Y.; Rubin, A.B.; Govindjee. Modeling Chlorophyll a Fluorescence Transient: Relation to Photosynthesis. Biochemistry Moscow 2014, 79, 291–323. [Google Scholar] [CrossRef]
- Salim Akhter, M.; Noreen, S.; Mahmood, S.; Athar, H.-R.; Ashraf, M.; Abdullah Alsahli, A.; Ahmad, P. Influence of Salinity Stress on PSII in Barley (Hordeum Vulgare L.) Genotypes, Probed by Chlorophyll-a Fluorescence. Journal of King Saud University - Science 2021, 33, 101239. [Google Scholar] [CrossRef]
- El-Baz, E.; EL-Dengawy, E.; El-Shahat, S.; El-Hassan, E. STUDIES ON SOME MORPHOLOGICAL ASPECTS OF JOJOBA [Simmondsia Chinensis (LINK) SCHNEIDER] UNDER EGYPTION CONDITIONS. Journal of Plant Production 2009, 34, 10575–10568. [Google Scholar] [CrossRef]
- Adhikari, T.; Kumar, A. Phytoaccumulation and Tolerance of Riccinus Communis L. to Nickel. International Journal of Phytoremediation 2012, 14, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Amit Kumar, G.; Kumar, M. Prediction and Analysis of Quorum Sensing Peptides Based on Sequence Features. PLoS ONE 2015, 10, e0120066. [Google Scholar] [CrossRef]
- Sharam, S.; Abbasnia Zare, S.K. Interactive Effects of Salinity and Drought Stresses on the Growth Parameters and Nitrogen Content of Three Hedge Shrubs. Cogent Environmental Science 2019, 5, 1682106. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of Decreased Photosynthetic Rate and Metabolic Capacity in Water-Deficient Leaf Cells: A Critical Evaluation of Mechanisms and Integration of Processes. Annals of Botany 2009, 103, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, H. Influence of Salt Stress on Growth, Pigments, Soluble Sugars and Ion Accumulation in Three Pistachio Cultivars. J. Med. Plants Res. 2012, 6, 2468–2473. [Google Scholar] [CrossRef]
- Morales, C.G.; Pino, M.T.; del Pozo, A. Phenological and Physiological Responses to Drought Stress and Subsequent Rehydration Cycles in Two Raspberry Cultivars. Scientia Horticulturae 2013, 162, 234–241. [Google Scholar] [CrossRef]
- Tardieu, F.; Parent, B.; Caldeira, C.F.; Welcker, C. Genetic and Physiological Controls of Growth under Water Deficit. Plant Physiology 2014, 164, 1628–1635. [Google Scholar] [CrossRef]
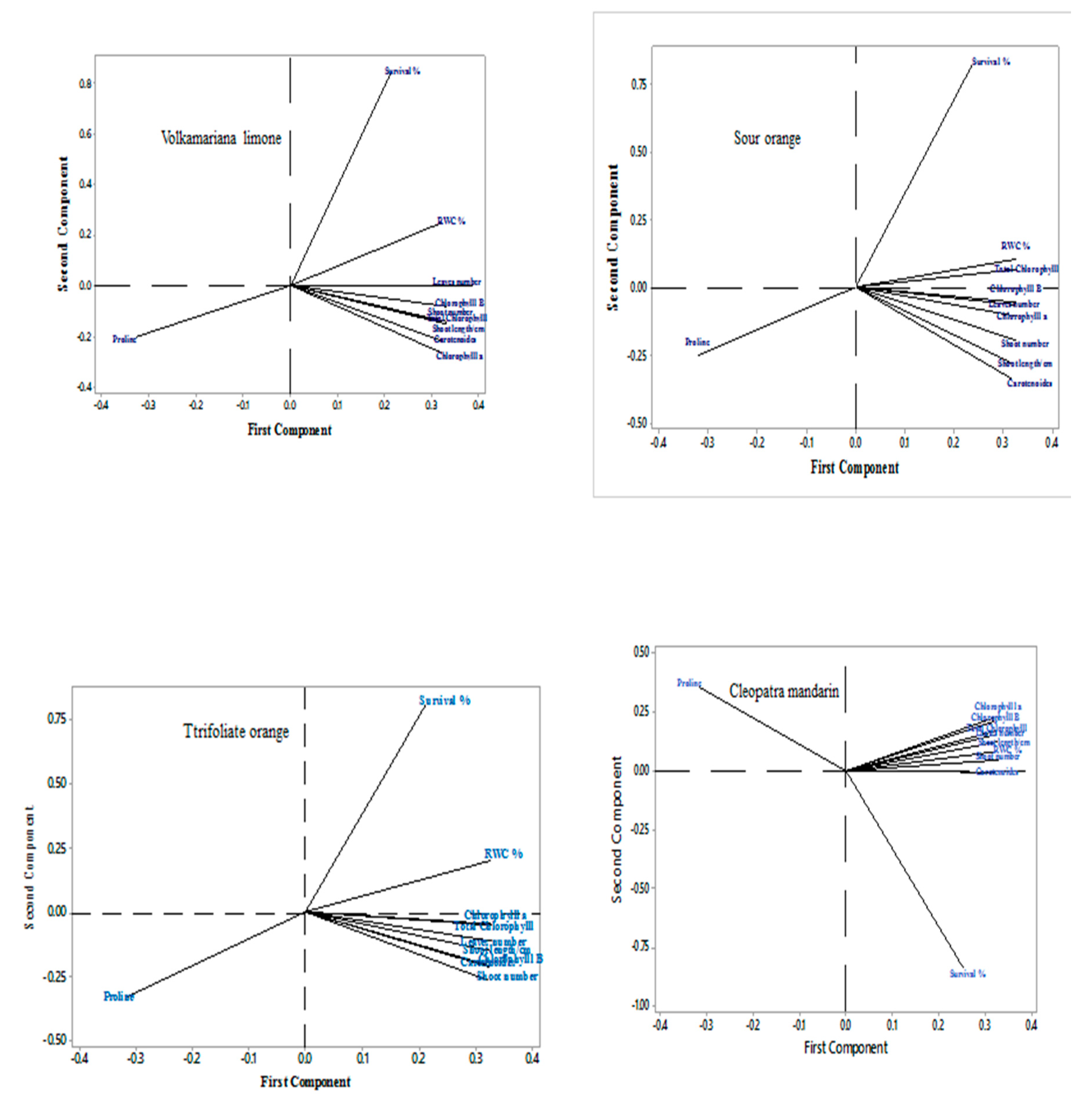
| Cleopatra Mandarin | Sour Orange | ||||||
|---|---|---|---|---|---|---|---|
| Variable | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |
| Eigenvalue | 9.2714 | 0.5646 | 0.075 | 9.1224 | 0.7171 | 0.0892 | |
| Variance | 92.7 | 5.6 | 0.007 | 91. 2 | 7.2 | 0.009 | |
| Cumulative | 92.7 | 98.4 | 0.991 | 91. 2 | 98.4 | 0.993 | |
| Components loadings | |||||||
| Shoot number | 0.328 | 0.046 | -0.100 | 0.326 | -0.195 | 0.112 | |
| Shoot length/cm | 0.327 | 0.125 | -0.017 | 0.318 | -0.279 | 0.34 | |
| Leaves number | 0.322 | 0.154 | 0.455 | 0.322 | -0.063 | -0.74 | |
| Survival % | 0.253 | -0.839 | 0.230 | 0.237 | 0.822 | 0.131 | |
| RWC % | 0.324 | 0.080 | -0.294 | 0.327 | 0.106 | 0.248 | |
| Chlorophyll a | 0.320 | 0.225 | 0.416 | 0.328 | -0.106 | -0.17 | |
| Chlorophyll B | 0.320 | 0.212 | -0.266 | 0.327 | -0.056 | 0.244 | |
| Total Chlorophyll | 0.324 | 0.168 | 0.314 | 0.328 | 0.065 | -0.351 | |
| Carotenoids | 0.321 | -0.005 | -0.521 | 0.316 | -0.335 | 0.175 | |
| Proline | -0.316 | 0.352 | 0.172 | 0.322 | -0.25 | -0.054 | |
| Trifoliate orange | volkamariana lemon | ||||||
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | ||
| Eigenvalue | 8.9933 | 0.9101 | 0.0494 | 8.9435 | 0.8147 | 0.1682 | |
| Variance | 89.9 | 9.1 | 0.005 | 89. 4 | 8. 1 | 0.017 | |
| Cumulative | 89.9 | 99 | 0.995 | 89. 4 | 97. 6 | 0.993 | |
| Components loadings | |||||||
| Shoot number | 0.322 | -0.264 | 0.18 | 0.329 | -0.153 | 0.106 | |
| Shoot length/cm | 0.328 | -0.152 | 0.014 | 0.331 | -0.142 | -0.13 | |
| Leaves number | 0.331 | -0.114 | 0.168 | 0.326 | 0.002 | 0.491 | |
| Survival % | 0.212 | 0.807 | 0.126 | 0.215 | 0.842 | -0.16 | |
| RWC % | 0.327 | 0.201 | 0.144 | 0.321 | 0.246 | 0.38 | |
| Chlorophyll a | 0.329 | -0.052 | -0.54 | 0.322 | -0.267 | 0.173 | |
| Chlorophyll B | 0.323 | -0.213 | 0.517 | 0.331 | -0.081 | 0.187 | |
| Total Chlorophyll | 0.33 | -4.60% | -0.586 | 0.318 | -0.142 | -0.604 | |
| Carotenoids | 0.326 | -0.208 | 0.035 | 0.324 | -0.22 | -0.274 | |
| Proline | -0.316 | -0.33 | -0.002 | -0.327 | -0.201 | 0.234 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





