1. Introduction
Water and environmental pollution are producing by
human activities. Nowadays water is a global problem. Contamination of harmful
heavy metals, organic and inorganic pollutants effect the ecosystem. Fresh and
clean water is the basic need of all living things wather it is use for
drinking purposes or commercial use. Contaminated and poor cleanliness of water
are the major source different of diseases like cholera, typhoid, diarrhea, and
hepatitis. It has reported that about 6 million children die daily due to
waterborne disease and most of the population of Asia and Africa doesn’t have
clean water for drinking [1,2].
Currently this wastewater mixed with ground water
and polluted it. The discharge of lead wastewater in rivers, canals effect our
food things. The presence of small amount of lead in water produce skin,
hearth, and kidney etc. According to world health organization the amount of
lead is 0.1 mg/l [3].
Many technologies are being used for water treatment
like adsorption, photo-catalysis, filtration and ion exchange process,
electro-dialysis, chemical precipitation. Each of these technologies have its
own advantages and disadvantages [4,5].
Recent studies show that the adsorption is one of
the best and well-known equilibrium separation process for water treatment.
Adsorption has also been found preferable to other methods because it’s easy
and simple operation, low cost, flexible and simplicity in design and
insensitivity to noxious pollutants [6]. Adsorption
not only eliminate but it can also recover and recycle them to the industrial
processes [7,8].
2. Material and Method
2.1. Materials
Large pieces of kaolin clay obtained from Nagar
parker region of Thar parker district of Sindh province in Pakistan. These
pieces are sent to crusher and converted into powder form. Take 50gm clay
powder and mixed in 500 ml deionized water. The mixture is stirred for 2 hr at
25 0C. Obtained mixture product is placed for sedimentation for 2 hr
and then filter the product. The obtained kaolin gel like material is dried at
100 0C for 2 hr in drying oven. The obtained material is finally
grinded and passed through 200 mesh size screens to obtained fine powder. This
clay powder is stored in airtight bags to use further [9].
The elemental properties of kaolin are tested
before and After washing kaolin. PW 2400 x-ray spectrophotometer is used to
analyze the samples.
Table 4.
composition of kaolin clay.
Table 4.
composition of kaolin clay.
| Oxides |
Raw Kaolin (wt%) |
Washed Kaolin (wt%) |
| SiO2
|
47.691 |
44.958 |
| Al2O3
|
26.529 |
34.559 |
| Na2O |
1.302 |
1.241 |
| TiO2
|
0.502 |
0.610 |
| Fe2O3
|
1.052 |
1.057 |
| MgO |
0.596 |
0.573 |
| CaO |
5.833 |
2.356 |
| K2O |
0.591 |
0.590 |
| Cl2
|
0.752 |
0 |
| LOI |
15.152 |
14.056 |
Sigma Aldrich laboratory grade lead Nitrate (99%)
is used. Take 1.6gm lead nitrate salt and mixed in deionized water to prepared
stock solution. In the whole process deionized water is used because tap or
other source of water contain other metal ions in it and compete with lead in
adsorption process so the correct percentage of lead removal would not be
determined [10].
2.2. Method
Kaolin is added in the solution about 1g per 250ml
of solution which is than stirred for 3hr using magnetic stirrer after stirring
the slurry is equilibrated for 24 hrs. It will be than filtered and water
sample will be tested for lead removal by Parkin Almer UV-spectrophotometer.
Different concentrations and adsorbent dosage are changed and repeat same
procedure to study the effect.
Results and Discussions
4.2.1. Effect of Concentration
Concentration effect is checked by preparing
different concentration solutions. Adding 1g kaolin in 250 ml of different
concentration solution of lead nitrate. The prepared samples are mixed using
magnetic stirrer for 2 hr at 5.5 pH of solution and room temperature to
obtained equilibrium in solution.
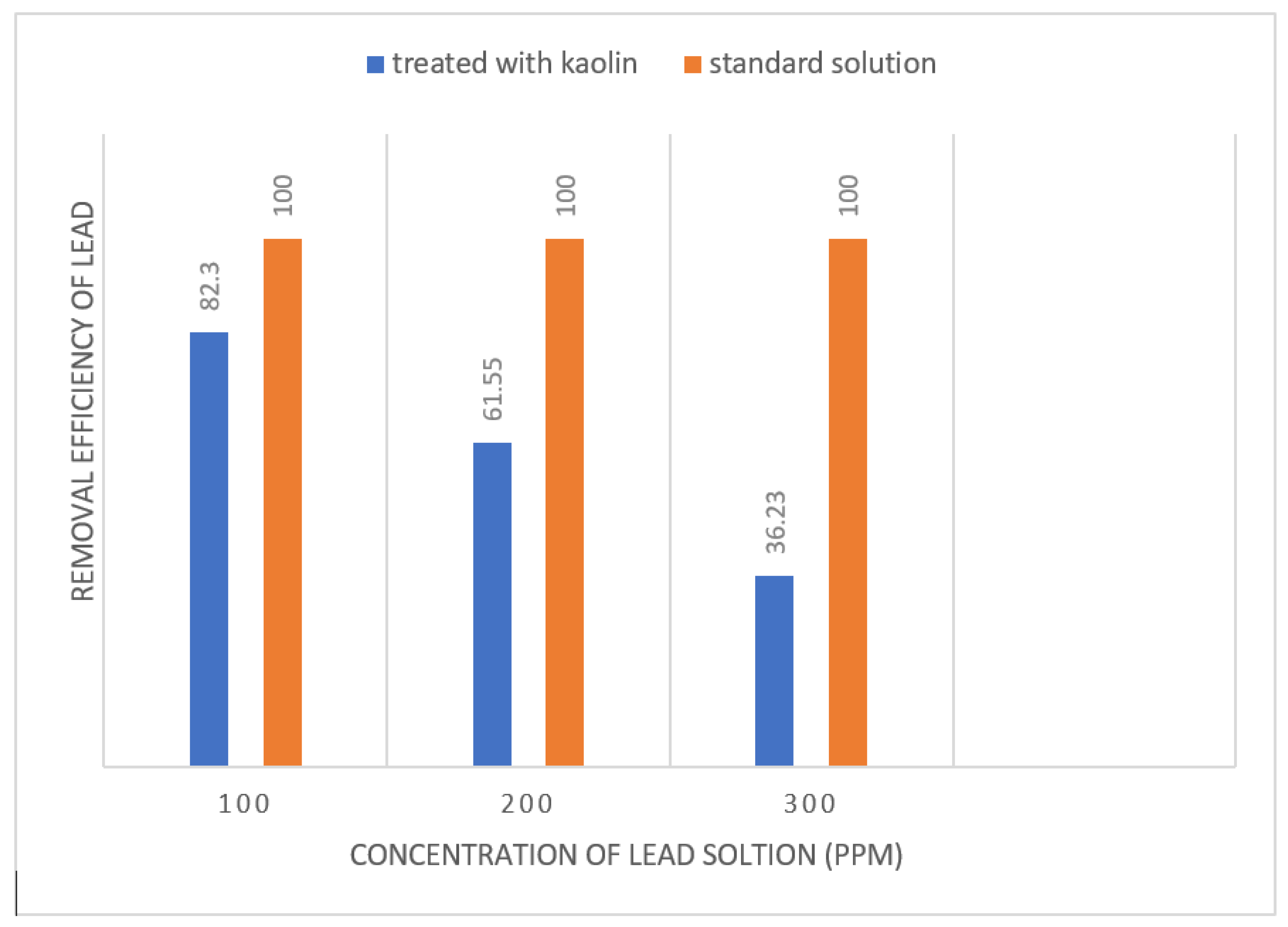
Figure 14.
Effect of kaolin on different concentration of Lead solution.
Figure 14.
Effect of kaolin on different concentration of Lead solution.
The equilibrium time reported in the literature for
the adsorption of kaolin is 60 min. There is only 10% increase in lead removal
by varying the time from 40min to 160 min [45].
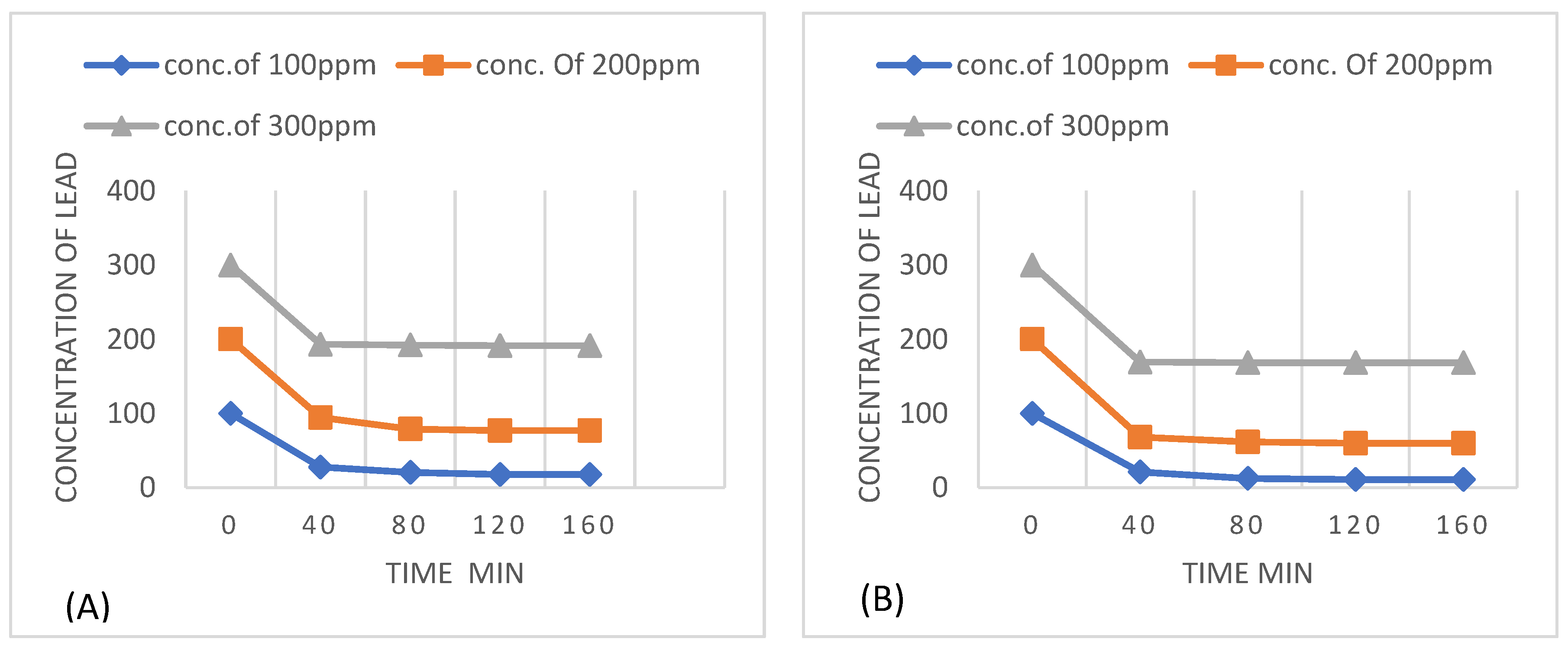
Figure 15.
Effect of concentration of lead with respect to time with (A) 1g kaolin (B) 2g kaolin.
Figure 15.
Effect of concentration of lead with respect to time with (A) 1g kaolin (B) 2g kaolin.
Using the above formula calculate the efficiency of different solutions and draw graph and maximum lead is removed from solution using kaolin adsorbent.
It is observed from the chart that by decreasing concentration from 300ppm to 100ppm lead removal is increased from 36% to 82%. Different adsorbents are used to remove lead from water. Researches that used “bone powder, active carbon, plant powder and commercial carbon.” Bone power has removed lead capacity of 100%, active carbon has 90%, plant powder has 80% and commercial carbon has 50%.
4.2.2. Effect of Dosage
Effect of 1g of kaolin clay is calculated. Now to study the effect of dosage the quantity of kaolin is increased. Take 250 ml solutions of lead with different concentration and add 2g kaolin in it. Same conditions are applied like 1g kaolin dosage experiment and results are calculated.
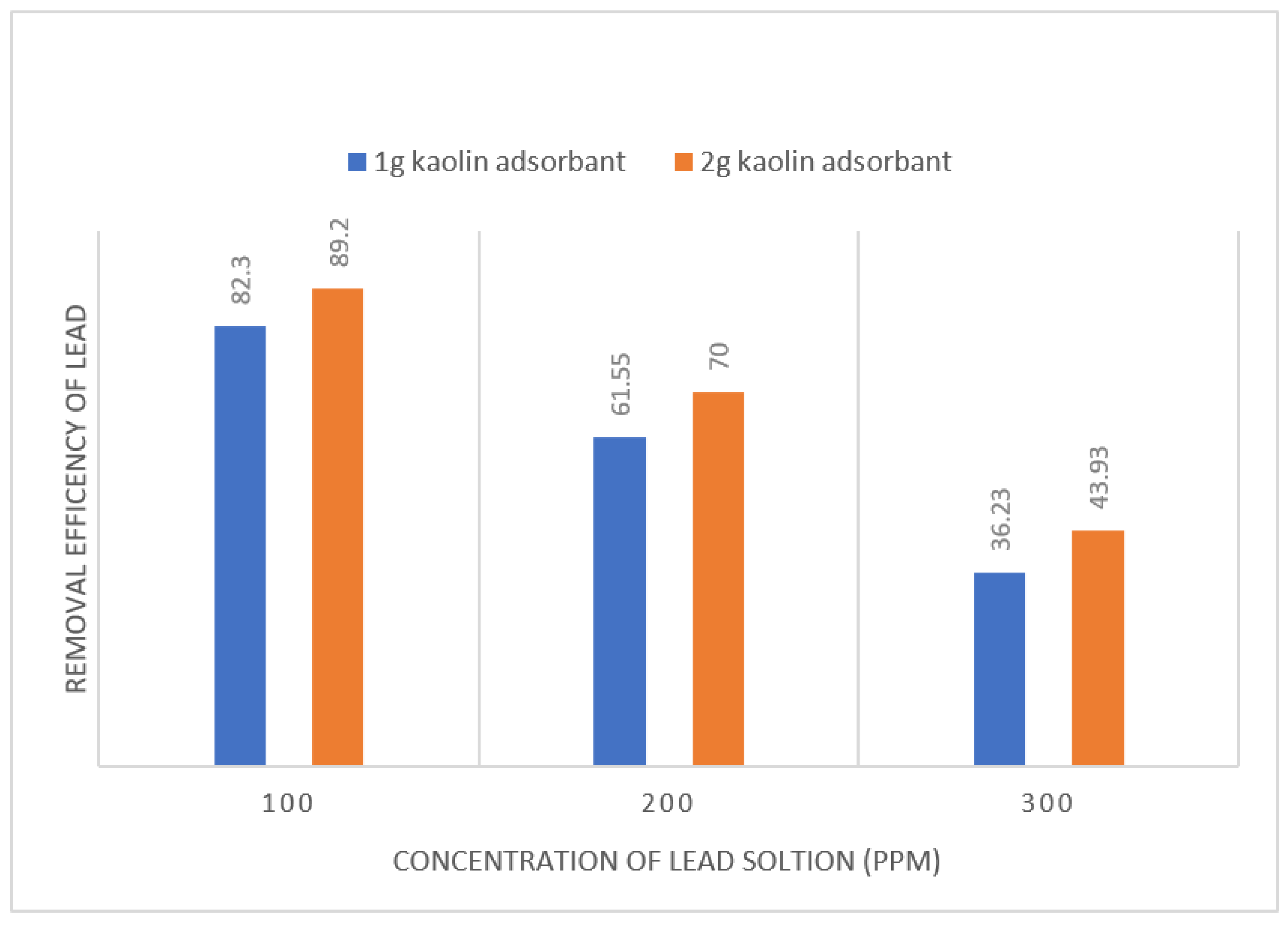
Figure 16.
Effect of kaolin dosage on removal of lead at same concentration.
Figure 16.
Effect of kaolin dosage on removal of lead at same concentration.
The removal efficiency of kaolin is improved by increasing dosage of kaolin. The removal efficiency of 100, 200 and 300 ppm solutions for 1g and 2g kaolin dosage are 82 to 89%, 62 to 70% and 36 to 43% respectively.
The purpose of varying the quantity of adsorbent is to be checked how much increase in lead removal form water that is 7%, 8% and 7% for 100, 200 and 300ppm solution.
4.2.3. Effect of pH
Prepare solutions to check pH effect of solution on adsorption was determined by taking 250 ml lead solution add 1g kaolin in solution containing lead concentration of 100 ppm and adjust pH values ranging from 3.0 to 9 using 0.1M HCl and NaOH solutions. The mixture was shaken for 60 minutes at normal room temperature and then the filtered and examined the solution. The pH is an important controlling parameter in the adsorption process.
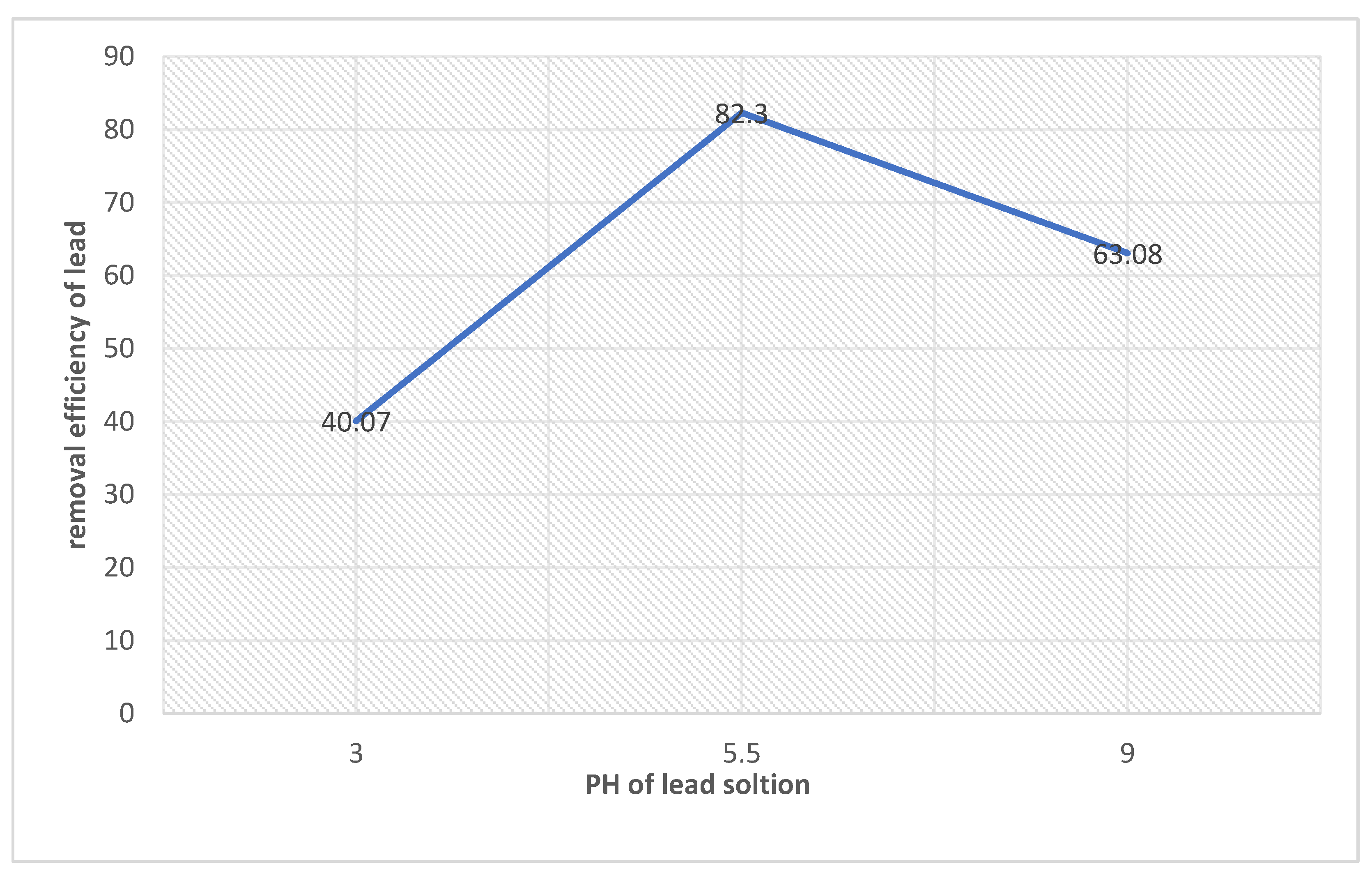
Figure 17.
Effect of pH with respect to removal efficiency of lead.
Figure 17.
Effect of pH with respect to removal efficiency of lead.
Figure shows the effect of pH on amount of metal ion adsorbed; removal efficiency is calculated using formula. Figure also shows that Pb+2 adsorption shows the maximum efficiency at pH of 5.5. It means that the removal of lead ions increases when the solution pH increased from 3.0 to 6.0. “The pH range was chosen as 3 to 6 in order to avoid metal hydroxides, which has been estimated to occur at pH> 6.5 [30].” when pH increases, there is a decrease in positive surface charge.
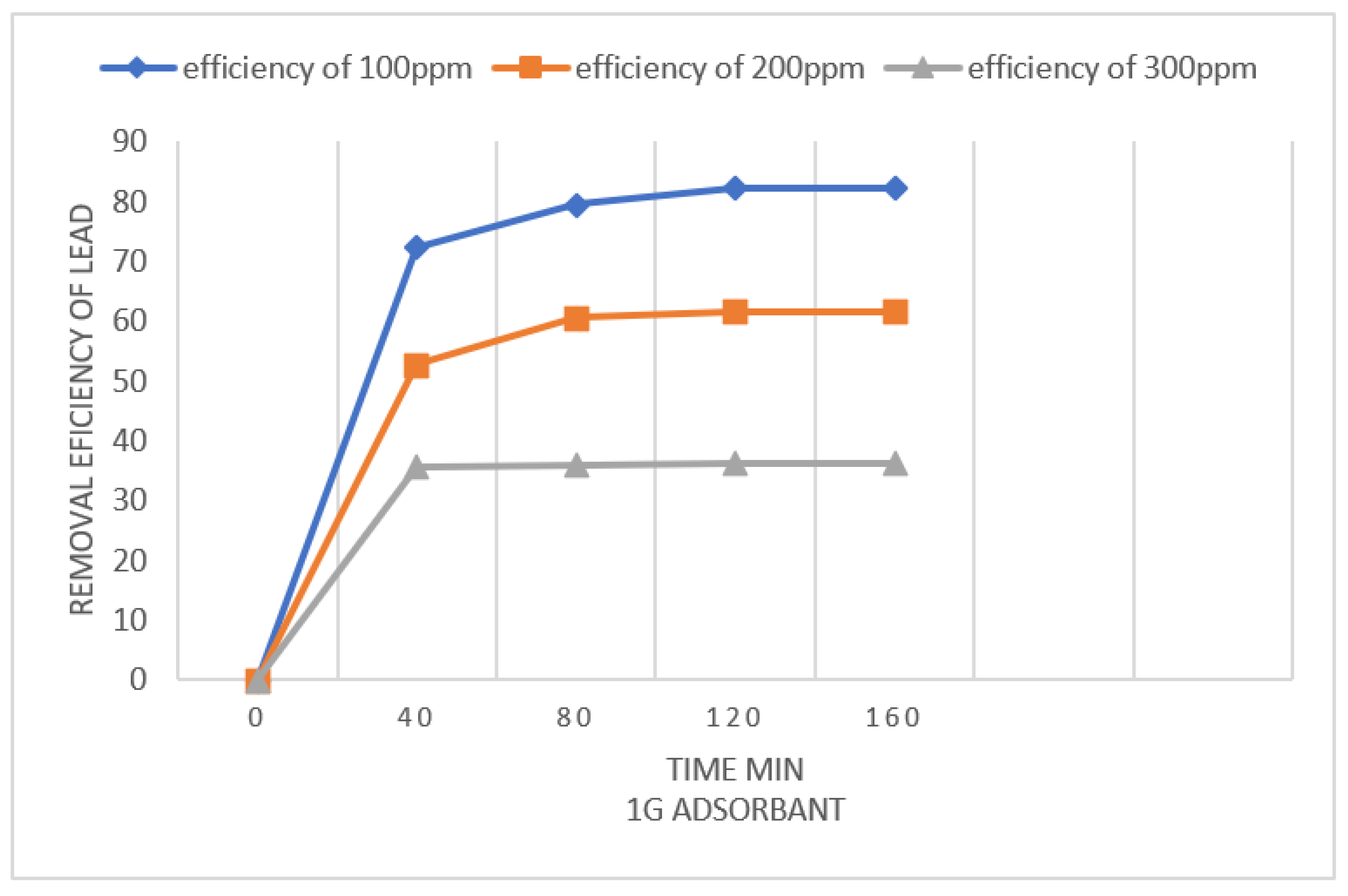
4.2.4. Effect of time
The effect of time of Pb(II) ions onto kaolin clay was done by using 100, 200,300ppm concentration of Pb(II) at initial pH 5.5 onto(1.0 and 2.0 g/250 mL) of adsorbent dose.
Figure 18.
Effect of time on removal efficiency of lead with 1g adsorbent dosage.
Figure 18.
Effect of time on removal efficiency of lead with 1g adsorbent dosage.
Figure 19.
Effect of time on removal efficiency of lead with 2g adsorbent dosage.
Figure 19.
Effect of time on removal efficiency of lead with 2g adsorbent dosage.
The lead adsorption is maximum at the start because adsorbent places are free and lead ions easily attached with these places. A larger amount of Pb(II) was removed in first 60 mins of time content, and after that Pb(II) remove slowly till equilibrium time (2 h).
4.5. Adsorption Isotherms
The adsorption isotherms are important to study mechanism of the adsorption. Adsorption isotherm have different equations and two isotherms are selected to study, which are Langmuir and Freundlich isotherms.
4.5.1. Langmuir Isotherm
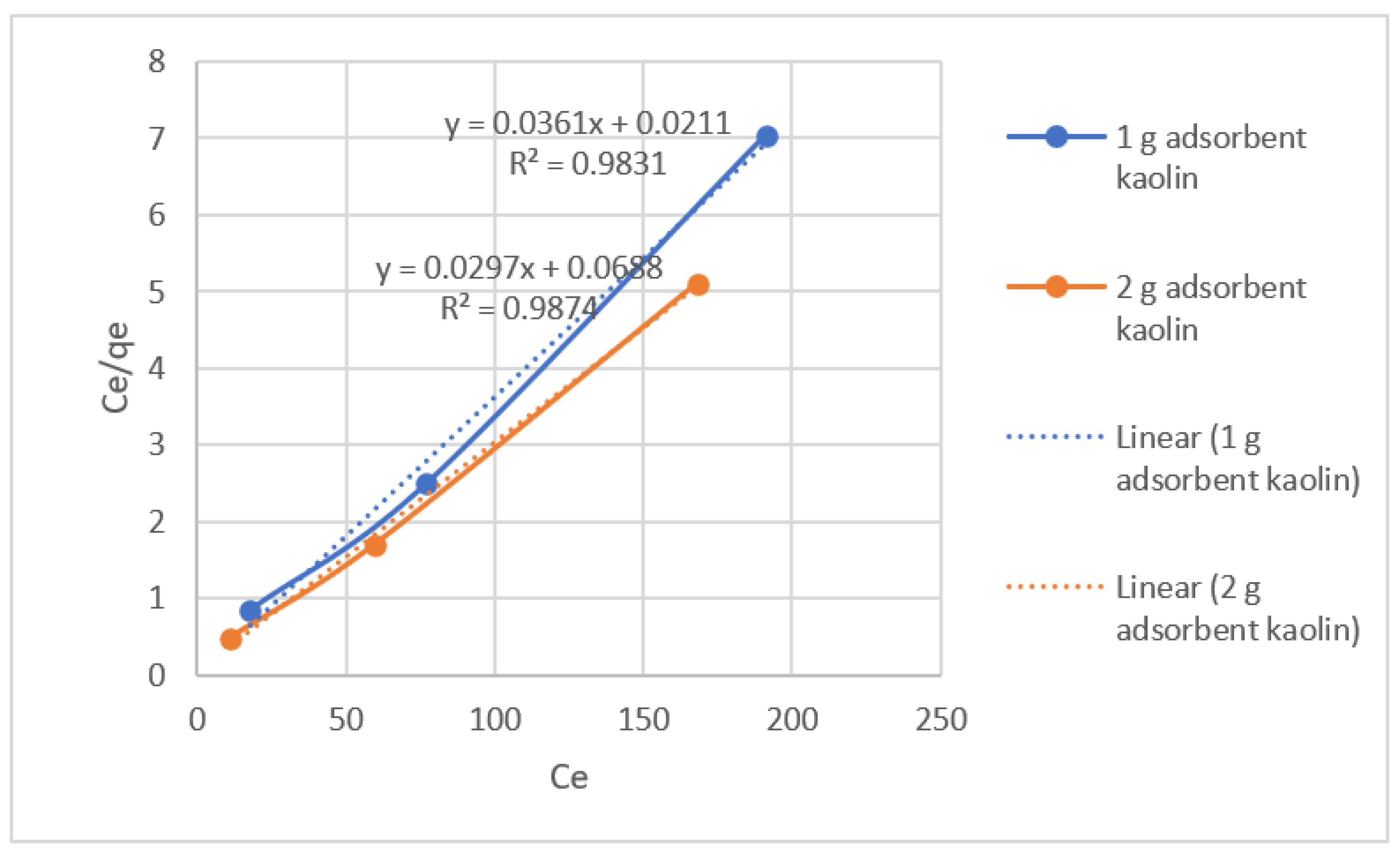
In Langmuir adsorption isotherm adsorption takes place at specific homogeneous sites within the adsorbent and has found successful applications in many adsorption processes of monolayer adsorption. The Langmuir isotherm equation is represented by the following:
Ce/qe = 1/(b.qmax) + (1/qmax) Ce
Where
qmax = Maximum adsorption capacity (mg/g)
b = Langmuir constant (L/mg)
Plot a graph between Ce/qe and Ce for the adsorption of lead(II) ions onto kaolin clay.
1/qmax = slop of the straight line and
1/(qmax b) = intercept of line.
Figure 20.
Langmuir adsorption of lead ions onto different adsorbent amount at room temperature.
Figure 20.
Langmuir adsorption of lead ions onto different adsorbent amount at room temperature.
| Adsorbent Amount |
Langmuir Isotherm Constants |
| Adsorbent /250ml |
qmax (mg/g) |
b (L/mg) |
R2 |
| 1-gram adsorbent |
27.70 |
1.71 |
0.9831 |
| 2-gram adsorbent |
33.67 |
0.432 |
0.9874 |
4.5.2. Freundlich Isotherm:
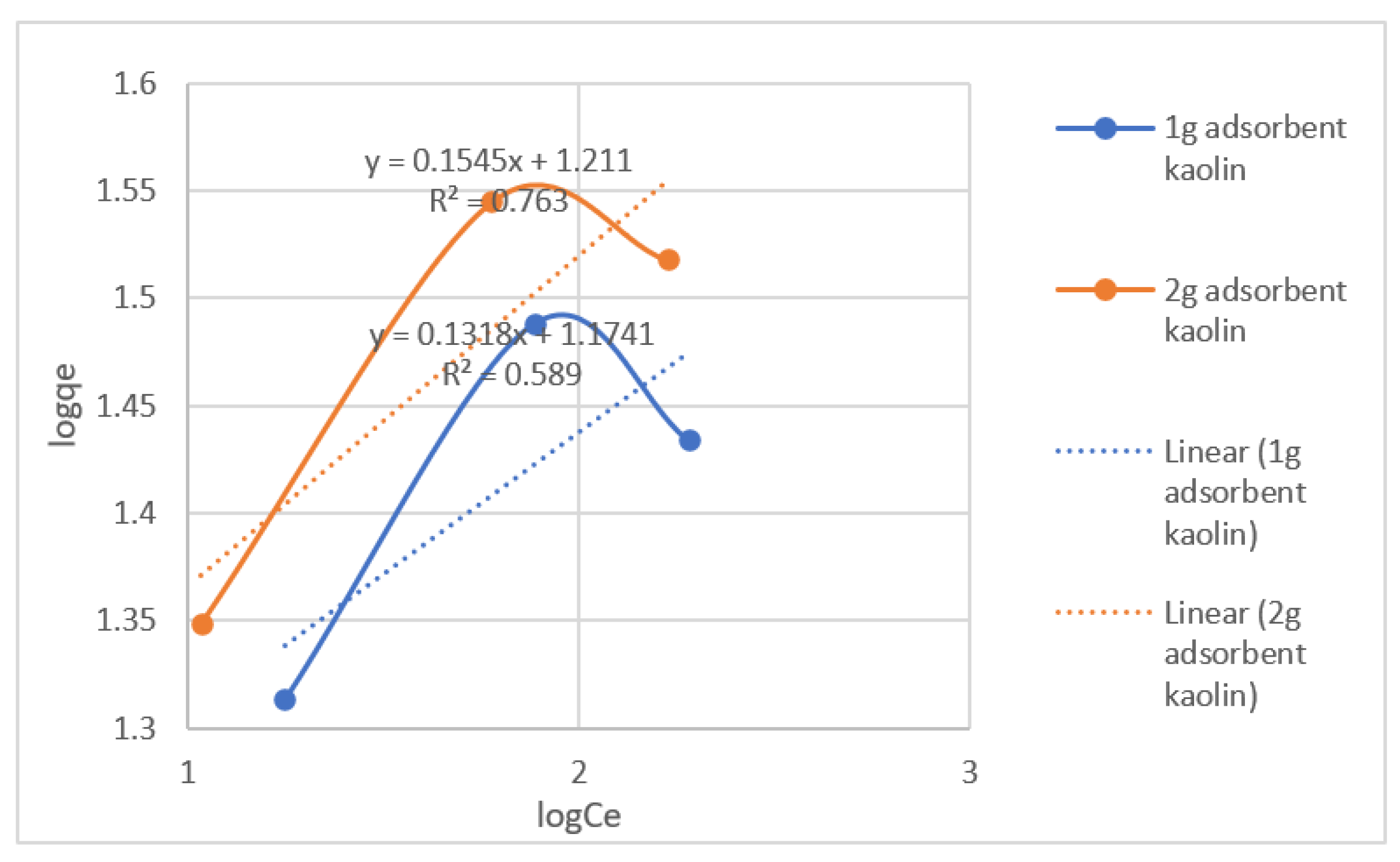
The Freundlich isotherm is an empirical equation used to explain heterogeneous systems. The Freundlich isotherm equation is:
lnqe = lnKF + 1/n lnCe
where
KF and n = Freundlich constants
KF and n respectively indicating the adsorption capacity and the adsorption intensity. They were calculated from the intercept and slope of the plot between lnqe and lnCe for the adsorption of lead(II) ions onto kaolin.
Figure 21.
Freundlich adsorption onto different amount of adsorbent at room temperature.
Figure 21.
Freundlich adsorption onto different amount of adsorbent at room temperature.
| Adsorbent Amount |
Freundlich Isotherm Constants |
| Adsorbent/250mL |
KF (L/mg) |
n |
R2 |
| 1-gram adsorbent |
14.93 |
7.587 |
0.589 |
| 2-gram adsorbent |
16.25 |
6.472 |
0.763 |
Results shows that the value of R2 is equals to 1 in Langmuir adsorption which means the process lies in Langmuir isotherm and show physical adsorption and process is mono-layer.
4.6. Adsorption Kinetics
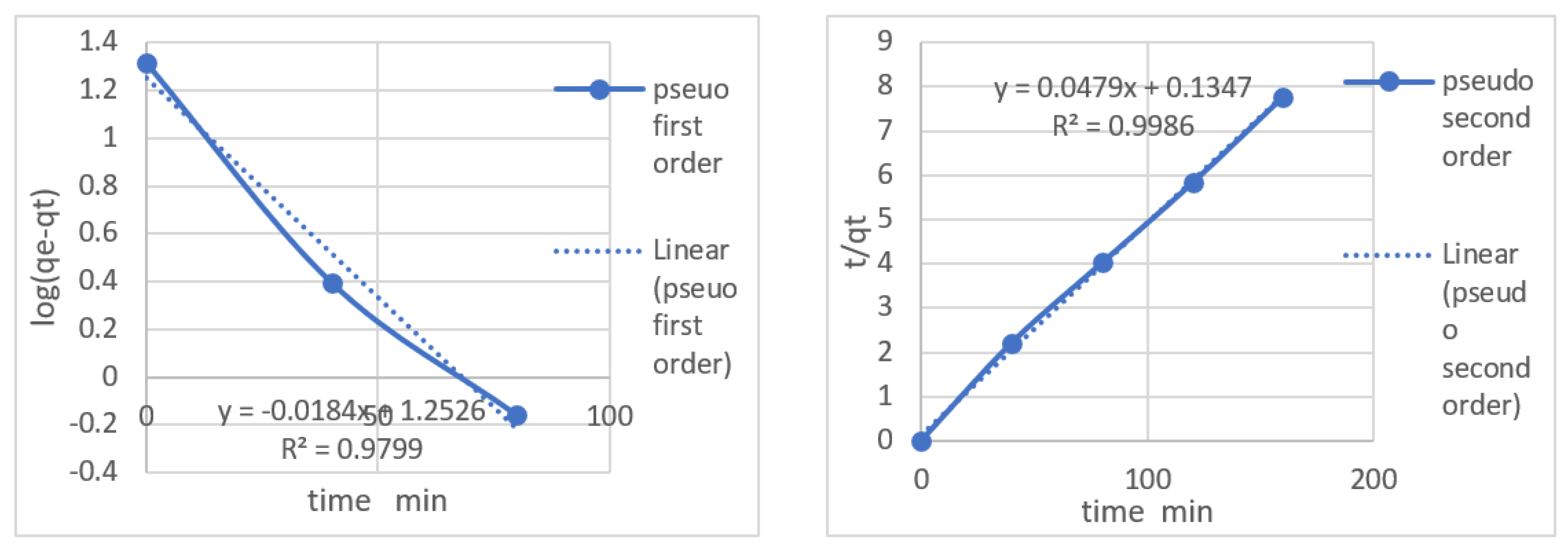
Using these equations calculate the values of K1 and K2
| Pseudo First Order Constants |
| Metal Ion |
qe (mg/g) |
K1 |
R2 |
| Pb2+
|
20.575 |
0.0527 |
0.979 |
| Pseudo second order constants |
| Metal ion |
qe (mg/g) |
K2 |
R2
|
| Pb2+ |
20.575 |
0.0088 |
0.998 |
The pseudo first and second order values shows that the system lies in pseudo second order and showing higher adherence rate then pseudo first order.
Figure 22.
Adsorption kinetics models of lead.
Figure 22.
Adsorption kinetics models of lead.
Conclusions
Kaolin composition obtained from Nagar Parker has same to ideal kaolin. The clay has strong potential to remove lead ions from aqueous solutions. The results show that Nagar parker kaolin is a good low-cost adsorbent. The adsorption studies show that metal ion concentration, contact time, PH and adsorbent dosage intensely affected the lead ion adsorption. PH is one of the parameters that strongly effect the metal ion removal.
Kaolin can be regenerated by reacting with NaNO3 and again it can be used for the remove lead from water. Furthermore, lead removal capacity of kaolin can be enhanced by activation of cation. Cation can be activated through thermal treatment and by reacting with Na, K and calcium chlorides.
References
- Kim, B.C.; Lee, J.; Um, W.; Kim, J.; Joo, J.; Lee, J.H.; Kwak, J.H.; Kim, J.H.; Lee, C.; Lee, H. Magnetic mesoporous materials for removal of environmental wastes. Journal of hazardous materials. 2011, 192, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, A. Effects of industrial effluent on quality of well water within Asa Dam industrial estate, Ilorin, Nigeria. Nature and Science 2009, 7, 39–43. [Google Scholar]
- Afatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: a review. Journal of hazardous materials 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Choi, S.-P.; Thiruvenkatachari, R.; Shim, W.-G.; Moon, H. Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes and pigments 2006, 69, 196–203. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: a review. Bioresource technology 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arabian journal of chemistry 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Wang, P.; Lo, I.M. Synthesis of mesoporous magnetic γ-Fe2O3 and its application to Cr (VI) removal from contaminated water. Water research 2009, 43, 3727–3734. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Tiwari, P.N. Removal and recovery of chromium (VI) from industrial waste water. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental AND Clean Technology 1997, 69, 376–382. [Google Scholar]
- Waid, O.; Hossam, A.-I. Removal of Pb+2 Ions from Aqueous Solutions by Adsorption on Kaolinite Clay. American Journal of Applied Sciences 2007, 4, 502–507. [Google Scholar]
- Farooq, A.; Elsayed, F.; Naveed, A. Removal of lead from wastewater by adsorption Using Saudi Arabian Clay. International Journal of Chemical and Environmental Engineering. April 2014, Volume 5, No.2. 20 April.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).