Submitted:
18 July 2023
Posted:
19 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
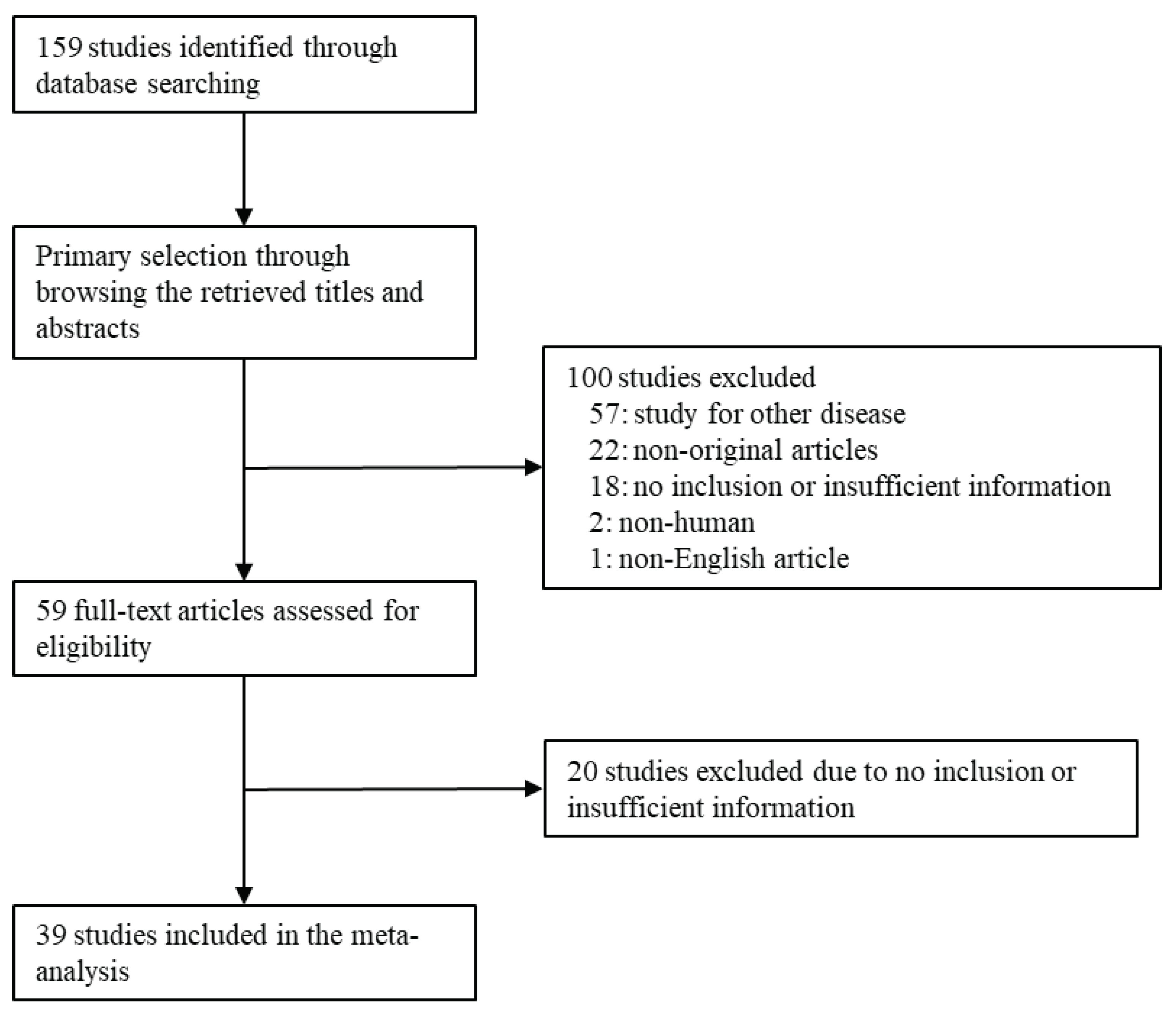
2.1. Literature search and selection criteria
2.2. Data extraction
2.3. Statistical analyses
3. Results
3.1. Selection and characteristics of studies
3.2. Meta-analysis for GATA3 IHC expression in urothelial carcinoma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-R. Urothelial tumorigenesis: a tale of divergent pathways. Nat. Rev. Cancer 2005, 5, 713–725. [Google Scholar] [CrossRef]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2014, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Labastie, M.-C.; Catala, M.; Gregoire, J.-M.; Peault, B. The GATA-3 gene is expressed during human kidney embryogenesis. Kidney Int. 1995, 47, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Debacker, C.; Catala, M.; Labastie, M.-C. Embryonic expression of the human GATA-3 gene. Mech. Dev. 1999, 85, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Yagi, R.; Zhu, J.; Paul, W.E. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int. Immunol. 2011, 23, 415–420. [Google Scholar] [CrossRef]
- Oosterwegel, M.; Timmerman, J.; Leiden, J.; Clevers, H. Expression of GATA-3 During Lymphocyte Differentiation and Mouse Embryogenesis. Dev. Immunol. 1992, 3, 1–11. [Google Scholar] [CrossRef]
- Kaufman, C.K.; Zhou, P.; Pasolli, H.A.; Rendl, M.; Bolotin, D.; Lim, K.-C.; Dai, X.; Alegre, M.-L.; Fuchs, E. GATA-3: an unexpected regulator of cell lineage determination in skin. Genes Dev. 2003, 17, 2108–2122. [Google Scholar] [CrossRef]
- Sellheyer, K.; Krahl, D. Expression pattern of GATA-3 in embryonic and fetal human skin suggests a role in epidermal and follicular morphogenesis. J. Cutan. Pathol. 2010, 37, 357–361. [Google Scholar] [CrossRef]
- Song, H.; Suehiro, J.-I.; Kanki, Y.; Kawai, Y.; Inoue, K.; Daida, H.; Yano, K.; Ohhashi, T.; Oettgen, P.; Aird, W.C.; et al. Critical Role for GATA3 in Mediating Tie2 Expression and Function in Large Vessel Endothelial Cells. PEDIATRICS 2009, 284, 29109–29124. [Google Scholar] [CrossRef]
- Ordóñez, N.G. Value of GATA3 immunostaining in tumor diagnosis: a review. Adv Anat Pathol 2013, 20, 352–360. [Google Scholar] [CrossRef]
- Mehra, R.; Varambally, S.; Ding, L.; Shen, R.; Sabel, M.S.; Ghosh, D.; Chinnaiyan, A.M.; Kleer, C.G. Identification of GATA3 as a Breast Cancer Prognostic Marker by Global Gene Expression Meta-analysis. Cancer Res 2005, 65, 11259–11264. [Google Scholar] [CrossRef]
- Li, Y.; Ishiguro, H.; Kawahara, T.; Kashiwagi, E.; Izumi, K.; Miyamoto, H. Loss of GATA3 in bladder cancer promotes cell migration and invasion. Cancer Biol. Ther. 2014, 15, 428–435. [Google Scholar] [CrossRef]
- Mohammed, K.H.; Siddiqui, M.T.; Cohen, C. GATA3 immunohistochemical expression in invasive urothelial carcinoma. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 432–e9. [Google Scholar] [CrossRef]
- Plage, H.; Samtleben, H.; Hofbauer, S.; Kornienko, K.; Weinberger, S.; Bruch, P.G.; Elezkurtaj, S.; Roßner, F.; Schallenberg, S.; Kluth, M.; et al. GATA3 expression loss is linked to stage progression but is unrelated to prognosis in muscle-invasive urothelial carcinoma of the bladder. Hum. Pathol. 2022, 130, 10–17. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, Y.; Wang, S.; Zhang, Y.; Miao, Q.; Gao, F.; He, H. Expression status of GATA3 and mismatch repair proteins in upper tract urothelial carcinoma. Front. Med. 2019, 13, 730–740. [Google Scholar] [CrossRef]
- Agaimy, A.; Bertz, S.; Cheng, L.; Hes, O.; Junker, K.; Keck, B.; Lopez-Beltran, A.; Stöckle, M.; Wullich, B.; Hartmann, A. Loss of expression of the SWI/SNF complex is a frequent event in undifferentiated/dedifferentiated urothelial carcinoma of the urinary tract. Virchows Arch. 2016, 469, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Rana, C.; Agarwal, H.; Babu, S.; Kumar, M.; Singhai, A.; Shankhwar, S.N.; Singh, V.; Sinha, R.J. Diagnostic utility of GATA3 immunohistochemical expression in urothelial carcinoma. Indian J. Pathol. Microbiol. 2019, 62, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Barth, I.; Schneider, U.; Grimm, T.; Karl, A.; Horst, D.; Gaisa, N.T.; Knüchel, R.; Garczyk, S. Progression of urothelial carcinoma in situ of the urinary bladder: a switch from luminal to basal phenotype and related therapeutic implications. Virchows Arch. 2018, 472, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Bertz, S.; Stöhr, R.; Gaisa, N.T.; Wullich, B.; Hartmann, A.; Agaimy, A. TERT promoter mutation analysis as a surrogate to morphology and immunohistochemistry in problematic spindle cell lesions of the urinary bladder. Histopathology 2020, 77, 949–962. [Google Scholar] [CrossRef]
- Bontoux, C.; Rialland, T.; Cussenot, O.; Compérat, E. A four-antibody immunohistochemical panel can distinguish clinico-pathological clusters of urothelial carcinoma and reveals high concordance between primary tumor and lymph node metastases. Virchows Arch. 2020, 478, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Borhan, W.M.; Cimino-Mathews, A.M.; Montgomery, E.A.; Epstein, J.I. Immunohistochemical Differentiation of Plasmacytoid Urothelial Carcinoma From Secondary Carcinoma Involvement of the Bladder. Am. J. Surg. Pathol. 2017, 41, 1570–1575. [Google Scholar] [CrossRef]
- Bruch, P.G.; Plage, H.; Hofbauer, S.; Kornienko, K.; Weinberger, S.; Roßner, F.; Schallenberg, S.; Kluth, M.; Lennartz, M.; Blessin, N.C.; et al. Cytokeratin 20 expression is linked to stage progression and to poor prognosis in advanced (pT4) urothelial carcinoma of the bladder. Exp. Mol. Pathol. 2023, 131, 104860. [Google Scholar] [CrossRef]
- Brunelli, M.; Tafuri, A.; Cima, L.; Cerruto, M.A.; Milella, M.; Zivi, A.; Buti, S.; Bersanelli, M.; Fornarini, G.; Vellone, V.G.; et al. MDM2 gene amplification as selection tool for innovative targeted approaches in PD-L1 positive or negative muscle-invasive urothelial bladder carcinoma. J. Clin. Pathol. 2020. [Google Scholar] [CrossRef]
- Budina, A.; Farahani, S.J.; Lal, P.; Nayak, A. Subcategorization of T1 Bladder Cancer on Biopsy and Transurethral Resection Specimens for Predicting Progression. Arch. Pathol. Lab. Med. 2021, 146, 1131–1139. [Google Scholar] [CrossRef]
- Chang, A.; Brimo, F.; Montgomery, E.A.; Epstein, J.I. Use of PAX8 and GATA3 in diagnosing sarcomatoid renal cell carcinoma and sarcomatoid urothelial carcinoma. Hum. Pathol. 2013, 44, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.L.; Chang, A.G.; Cimino-Mathews, A.; Argani, P.; Youssef, R.F.; Kapur, P.; Montgomery, E.A.; Epstein, J.I. GATA-3 Immunohistochemistry in the Differential Diagnosis of Adenocarcinoma of the Urinary Bladder. Am. J. Surg. Pathol. 2013, 37, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Roibon, N.; Albadine, R.; Sharma, R.; Faraj, S.F.; Illei, P.B.; Argani, P.; Ertoy, D.; Allaf, M.E.; Netto, G.J. The role of GATA binding protein 3 in the differential diagnosis of collecting duct and upper tract urothelial carcinomas. Hum. Pathol. 2013, 44, 2651–2657. [Google Scholar] [CrossRef]
- Gulmann, C.; Paner, G.P.; Parakh, R.S.; Hansel, D.E.; Shen, S.S.; Ro, J.Y.; Annaiah, C.; Lopez-Beltran, A.; Rao, P.; Arora, K.; et al. Immunohistochemical profile to distinguish urothelial from squamous differentiation in carcinomas of urothelial tract. Hum. Pathol. 2012, 44, 164–172. [Google Scholar] [CrossRef]
- Guo, C.C.; Bondaruk, J.; Yao, H.; Wang, Z.; Zhang, L.; Lee, S.; Lee, J.-G.; Cogdell, D.; Zhang, M.; Yang, G.; et al. Assessment of Luminal and Basal Phenotypes in Bladder Cancer. Sci. Rep. 2020, 10, 9743. [Google Scholar] [CrossRef]
- Haghayeghi, K.; Lu, S.; Matoso, A.; Schiff, S.F.; Mueller-Leonhard, C.; Amin, A. Association of current molecular subtypes in urothelial carcinoma with patterns of muscularis propria invasion. Virchows Arch. 2021, 479, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Mizushima, T.; Fujita, K.; Meliti, A.; Ide, H.; Yamaguchi, S.; Fushimi, H.; Netto, G.J.; Nonomura, N.; Miyamoto, H. GATA3 immunohistochemistry in urothelial carcinoma of the upper urinary tract as a urothelial marker and a prognosticator. Hum. Pathol. 2017, 64, 83–90. [Google Scholar] [CrossRef] [PubMed]
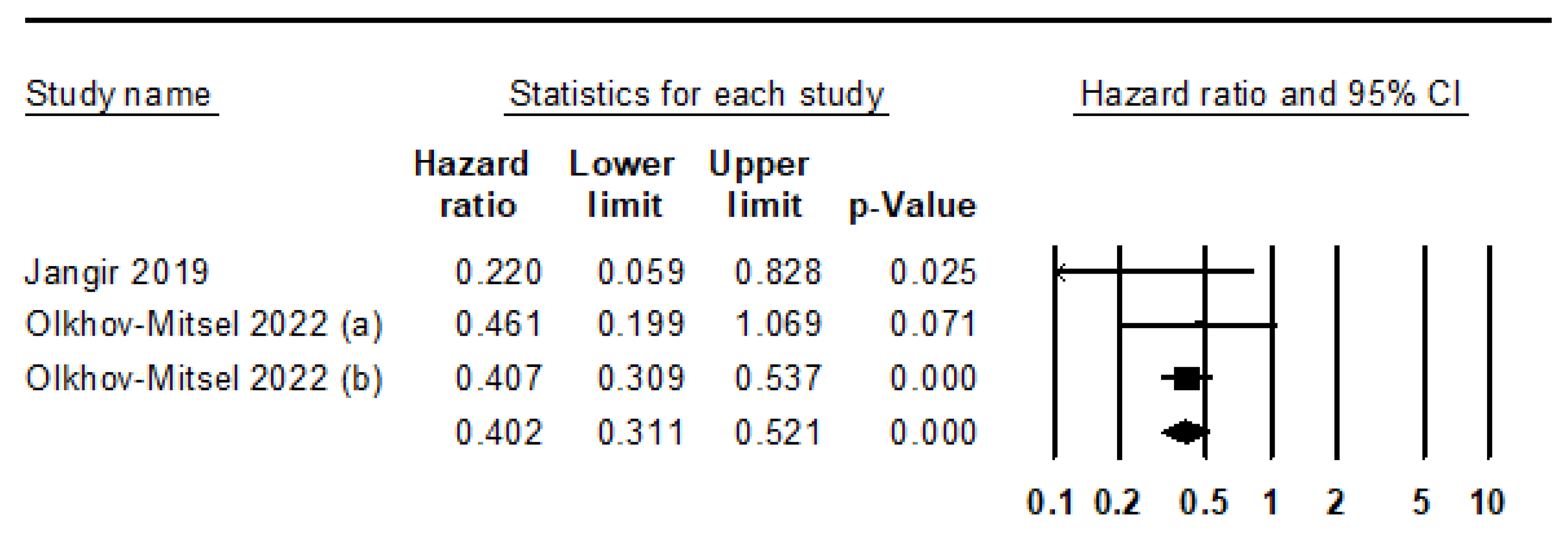
- Jangir, H.; Nambirajan, A.; Seth, A.; Sahoo, R.K.; Dinda, A.K.; Nayak, B.; Kaushal, S. Prognostic stratification of muscle invasive urothelial carcinomas using limited immunohistochemical panel of Gata3 and cytokeratins 5/6, 14 and 20. Ann Diagn Pathol 2019, 43, 151397. [Google Scholar] [CrossRef]
- Kim, B.; Lee, C.; Kim, Y.A.; Moon, K.C. PD-L1 Expression in Muscle-Invasive Urinary Bladder Urothelial Carcinoma According to Basal/Squamous-Like Phenotype. Front. Oncol. 2020, 10, 527385. [Google Scholar] [CrossRef]
- Leite, K.R.M.; Borges, L.L.; Filho, L.R.; Chade, D.; Coelho, R.F.; Cordeiro, M.; Srougi, M.; Nahas, W.C. Histological Variants of Urothelial Carcinoma Predict No Response to Neoadjuvant Chemotherapy. Clin. Genitourin. Cancer 2021, 20, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Leivo, M.Z.; Elson, P.J.; Tacha, D.E.; Delahunt, B.; Hansel, D.E. A combination of p40, GATA-3 and uroplakin II shows utility in the diagnosis and prognosis of muscle-invasive urothelial carcinoma. Pathology 2016, 48, 543–549. [Google Scholar] [CrossRef]
- Liang, Y.; Heitzman, J.; Kamat, A.M.; Dinney, C.P.; Czerniak, B.; Guo, C.C. Differential expression of GATA-3 in urothelial carcinoma variants. Hum. Pathol. 2014, 45, 1466–1472. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, B.; Villa, C.; Zhong, M.; Kundu, S.; Rohan, S.M.; Yang, X.J. The utility of p63, p40, and GATA-binding protein 3 immunohistochemistry in diagnosing micropapillary urothelial carcinoma. Hum. Pathol. 2014, 45, 1824–1829. [Google Scholar] [CrossRef]
- Liu, H.; Shi, J.; Wilkerson, M.L.; Lin, F. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol 2012, 138, 57–64. [Google Scholar] [CrossRef]
- Beltran, A.L.; Montironi, R.; Cheng, L. Microcystic urothelial carcinoma: morphology, immunohistochemistry and clinical behaviour. Histopathology 2013, 64, 872–879. [Google Scholar] [CrossRef]
- Naik, M.; Rao, B.V.; Fonseca, D.; Murthy, S.S.; Giridhar, A.; Sharma, R.; Raju, K.; Rao, T.S.; Challa, S. GATA-3 Expression in all Grades and Different Variants of Primary and Metastatic Urothelial Carcinoma. Indian J. Surg. Oncol. 2020, 12, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.J.; Chung, A.M.; Kim, J.S.; Han, J.H.; Hong, S.H.; Lee, J.Y.; Choi, Y.J. Differential Immunohistochemical Profiles for Distinguishing Prostate Carcinoma and Urothelial Carcinoma. J. Pathol. Transl. Med. 2016, 50, 345–354. [Google Scholar] [CrossRef]
- Olkhov-Mitsel, E.; Hodgson, A.; Liu, S.K.; Vesprini, D.; Xu, B.; Downes, M.R. Three-antibody classifier for muscle invasive urothelial carcinoma and its correlation with p53 expression. J. Clin. Pathol. 2021, 75, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Paner, G.P.; Annaiah, C.; Gulmann, C.; Rao, P.; Ro, J.Y.; Hansel, D.E.; Shen, S.S.; Lopez-Beltran, A.; Aron, M.; Luthringer, D.J.; et al. Immunohistochemical evaluation of novel and traditional markers associated with urothelial differentiation in a spectrum of variants of urothelial carcinoma of the urinary bladder. Hum. Pathol. 2014, 45, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Perrino, C.M.; Eble, J.; Kao, C.-S.; Whaley, R.D.; Cheng, L.; Idrees, M.; Hashemi-Sadraei, N.; Monn, M.F.; Kaimakliotis, H.Z.; Bandali, E.; et al. Plasmacytoid/diffuse urothelial carcinoma: a single-institution immunohistochemical and molecular study of 69 patients. Hum. Pathol. 2019, 90, 27–36. [Google Scholar] [CrossRef]
- Reiswich, V.; Schmidt, C.E.; Lennartz, M.; Höflmayer, D.; Hube-Magg, C.; Weidemann, S.; Fraune, C.; Büscheck, F.; Möller, K.; Bernreuther, C.; et al. GATA3 Expression in Human Tumors: A Tissue Microarray Study on 16,557 Tumors. Pathobiology 2023, 90, 219–232. [Google Scholar] [CrossRef]
- Samaratunga, H.; Delahunt, B.; Egevad, L.; Adamson, M.; Hussey, D.; Malone, G.; Hoyle, K.; Nathan, T.; Kerle, D.; Ferguson, P.; et al. Pleomorphic giant cell carcinoma of the urinary bladder: an extreme form of tumour de-differentiation. Histopathology 2015, 68, 533–540. [Google Scholar] [CrossRef]
- Sanfrancesco, J.; McKenney, J.K.; Leivo, M.Z.; Gupta, S.; Elson, P.; Hansel, D.E. Sarcomatoid Urothelial Carcinoma of the Bladder: Analysis of 28 Cases With Emphasis on Clinicopathologic Features and Markers of Epithelial-to-Mesenchymal Transition. Arch. Pathol. Lab. Med. 2016, 140, 543–551. [CrossRef]
- Tian, W.; Guner, G.; Miyamoto, H.; Cimino-Mathews, A.; Gonzalez-Roibon, N.; Argani, P.; Li, X.; Sharma, R.; Subhawong, A.P.; Rezaei, K.; et al. Utility of uroplakin II expression as a marker of urothelial carcinoma. Hum. Pathol. 2014, 46, 58–64. [Google Scholar] [CrossRef]
- Verduin, L.; Mentrikoski, M.J.; Heitz, C.T.; Wick, M.R. The Utility of GATA3 in the Diagnosis of Urothelial Carcinomas With Variant Morphologic Patterns. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 509–513. [Google Scholar] [CrossRef]
- Wang, X.; Lopez-Beltran, A.; O Osunkoya, A.; Wang, M.; Zhang, S.; Davidson, D.D.; E Emerson, R.; Williamson, S.R.; Tan, P.-H.; Kaimakliotis, H.Z.; et al. TERT promoter mutation status in sarcomatoid urothelial carcinomas of the upper urinary tract. Futur. Oncol. 2017, 13, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Weyerer, V.; Weisser, R.; A Moskalev, E.; Haller, F.; Stoehr, R.; Eckstein, M.; Zinnall, U.; Gaisa, N.T.; Compérat, E.; Perren, A.; et al. Distinct genetic alterations and luminal molecular subtype in nested variant of urothelial carcinoma. Histopathology 2019, 75, 865–875. [Google Scholar] [CrossRef]
- Zhao, L.; Antic, T.; Witten, D.; Paner, G.P.; Taxy, J.B.; Husain, A.; Gwin, K.; Mirza, M.K.; Lingen, M.W.; Tretiakova, M.S. Is GATA3 expression maintained in regional metastases? : a study of paired primary and metastatic urothelial carcinomas. Am J Surg Pathol 2013, 37, 1876–1881. [Google Scholar] [CrossRef] [PubMed]
- Zinnall, U.; Weyerer, V.; Compérat, E.; Camparo, P.; Gaisa, N.T.; Knuechel-Clarke. R.; Perren, A.; Lugli, A.; Toma, M.; Baretton, G.; Kristiansen, G.; Wirtz, R.M.; Cheng, L.; Wullich, B.; Stoehr, R.; Hartmann, A.; Bertz, S. Micropapillary urotheliall carcinoma: evaluation of HER2 status and immunohistochemical characterization of the molecular subtype. Hum Pathol 2018, 80, 55-64. [CrossRef]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Peto, R. Experimental Survival Curves for Interval-Censored Data. J. R. Stat. Soc. Ser. C (Applied Stat. 1973, 22, 86. [Google Scholar] [CrossRef]
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Rys, J.; Czapiewski, P.; Wazny, K.; Langfort, R.; Waloszczyk, P.; Biernat, W.; Lasota, J.; Wang, Z. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelialtumors. Am J Surg Pathol 2014, 38, 13–22. [Google Scholar] [CrossRef]
- Burch, J.B. Regulation of GATA gene expression during vertebrate development. Semin. Cell Dev. Biol. 2005, 16, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Asselin-Labat, M.-L.; Sutherland, K.D.; Barker, H.; Thomas, R.; Shackleton, M.; Forrest, N.C.; Hartley, L.; Robb, L.; Grosveld, F.G.; Van Der Wees, J.; et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol. 2007, 9, 201–209. [Google Scholar] [CrossRef]
- Davis, D.G.; Siddiqui, M.T.; Oprea-Ilies, G.; Stevens, K.; Osunkoya, A.O.; Cohen, C.; Li, X. (. GATA-3 and FOXA1 expression is useful to differentiate breast carcinoma from other carcinomas. Hum. Pathol. 2016, 47, 26–31. [Google Scholar] [CrossRef]
- Kouros-Mehr, H.; Bechis, S.K.; Slorach, E.M.; Littlepage, L.E.; Egeblad, M.; Ewald, A.J.; Pai, S.-Y.; Ho, I.-C.; Werb, Z. GATA-3 Links Tumor Differentiation and Dissemination in a Luminal Breast Cancer Model. Cancer Cell 2008, 13, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Provot, S.; Werb, Z. GATA3 in development and cancer differentiation: cells GATA have it! J Cell Physiol 2010, 222, 42–49. [Google Scholar] [CrossRef] [PubMed]
| First Author | Location | Organ | No of patients |
First Author | Location | Organ | No of patients |
|
|---|---|---|---|---|---|---|---|---|
| Agaimy 2016 [17] | NA | UB, UT | 14 | Liang 2014 [37] | USA | UB | 127 | |
| Agarwal 2019 [18] | India | UB | 74 | Lin 2014 [38] | USA | UB | 98 | |
| Barth 2018 [19] | Germany | UB | 156 | Liu 2012 [39] | USA | UB | 72 | |
| Bertz 2020 [20] | Germany | UB | 18 | Lopez Beltran 2014 [40] | NA | UB | 20 | |
| Bontoux 2021 [21] | France | UB | 184 | Naik 2021 [41] | India | UB, UT | 122 | |
| Borhan 2017 [22] | USA | UB | 45 | Oh 2016 [42] | Korea | UB | 138 | |
| Bruch 2023 [23] | Germany | UB | 2406 | Olkhov-Mitsel 2022 [43] | Canada | UB | 243 | |
| Brunelli 2022 [24] | Italy | UB | 117 | Paner 2014 [44] | Various | UB | 111 | |
| Budina 2022 [25] | USA | UB | 67 | Perrino 2019 [45] | USA | UT | 26 | |
| Chang 2013 [26] | USA | UT | 56 | Plage 2022 [15] | Germany/Poland | UB | 2636 | |
| Ellis 2013 [27] | USA | UB | 49 | Reiswich 2023 [46] | Germany | NA | 1066 | |
| Gonzalez-Roibon 2013 [28] | USA | UT | 25 | Samaratunga 2016 [47] | New Zealand | UB | 11 | |
| Gulmann 2013 [29] | USA | UT | 85 | Sanfrancesco 2016 [48] | USA | UB | 26 | |
| Guo 2020 [30] | USA | UB | 72 | Tian 2015 [49] | USA | UB | 278 | |
| Haghayeghi 2021 [31] | USA | UB | 42 | Verduin 2016 [50] | USA | UB | 86 | |
| Inoue 2017 [32] | USA | UT | 48 | Wang 2017 [51] | USA | UT | 17 | |
| Jangir 2019 [33] | India | UB | 40 | Weyerer 2019 [52] | Germany | UB | 55 | |
| Kim 2020 [34] | Korea | UB | 166 | Zhao 2013 [53] | USA | UB | 69 | |
| Leite 2022 [35] | Brazil | UB | 25 | Zinnall 2018 [54] | Germany | UB | 91 | |
| Leivo 2016 [36] | USA | UB | 89 | |||||
| UB, urinary bladder; UT, urinary tract | ||||||||
| Number of subsets |
Fixed effect [95% CI] |
Heterogeneity test [P-value] |
Random effect [95% CI] |
Egger’s test [P-value] |
|
|---|---|---|---|---|---|
| Overall | 38 | 0.726 [0.716, 0.735] | < 0.001 | 0.748 [0.704, 0.787] | 0.694 |
| Tumor site | |||||
| Urinary bladder* | 29 | 0.731 [0.720, 0.741] | < 0.001 | 0.775 [0.727, 0.818] | 0.395 |
| Urinary tract | 6 | 0.538 [0.477, 0.599] | < 0.001 | 0.614 [0.426, 0.774] | 0.391 |
| Study location | |||||
| America | 20 | 0.670 [0.641, 0.697] | < 0.001 | 0.741 [0.627, 0.829] | 0.215 |
| Asia | 4 | 0.707 [0.663, 0.747] | < 0.001 | 0.748 [0.590, 0.859] | 0.130 |
| Europe | 9 | 0.738 [0.727, 0.749] | < 0.001 | 0.775 [0.723, 0.819] | 0.416 |
| Oceania | 1 | 0.909 [0.561, 0.987] | < 0.001 | 0.909 [0.561, 0.987] | - |
| CI, Confidence interval. *, p = 0.011 in the meta-regression test between urinary bladder and urinary tract subgroups | |||||
| Number of subsets |
Fixed effect [95% CI] |
Heterogeneity test [P-value] |
Random effect [95% CI] |
Egger’s test [P-value] |
||
|---|---|---|---|---|---|---|
| Non-invasive UC* | 11 | 0.969 [0.958, 0.977] | 0.007 | 0.965 [0.938, 0.980] | 0.586 | |
| Carcinoma in situ | 2 | 0.961 [0.916, 0.982] | 0.250 | 0.956 [0.878, 0.985] | - | |
| Invasive UC | 55 | 0.626 [0.608, 0.643] | < 0.001 | 0.644 [0.581, 0.702] | 0.474 | |
| Non-muscular invasion# | 6 | 0.941 [0.902, 0.965] | 0.259 | 0.937 [0.883, 0.967] | 0.419 | |
| Muscular invasion | 13 | 0.720 [0.685, 0.752] | < 0.001 | 0.753 [0.645, 0.836] | 0.400 | |
| Histologic subtypes | ||||||
| Adenocarcinoma | 3 | 0.190 [0.093, 0.350] | 0.647 | 0.190 [0.093, 0.350] | 0.154 | |
| Clear cell | 1 | 0.929 [0.423, 0.996] | 1.000 | 0.929 [0.423, 0.996] | - | |
| Glandular differentiation | 2 | 0.474 [0.268, 0.689] | 0.809 | 0.474 [0.268, 0.689] | - | |
| Lymphoepithelioma-like | 1 | 0.300 [0.100, 0.624] | 1.000 | 0.300 [0.100, 0.624] | - | |
| Microcystic | 2 | 0.952 [0.724, 0.993] | 0.463 | 0.952 [0.724, 0.993] | - | |
| Micropapillary | 5 | 0.773 [0.697, 0.834] | < 0.001 | 0.862 [0.661, 0.952] | 0.264 | |
| Nested | 1 | 0.700 [0.376, 0.900] | 1.000 | 0.700 [0.376, 0.900] | - | |
| Plasmacytoid | 4 | 0.756 [0.637, 0.845] | 0.003 | 0.825 [0.517, 0.954] | 0.544 | |
| Pleomorphic giant cell | 1 | 0.909 [0.561, 0.987] | 1.000 | 0.909 [0.561, 0.987] | - | |
| Sarcomatoid | 8 | 0.385 [0.316, 0.459] | 0.003 | 0.407 [0.282, 0.545] | 0.370 | |
| Small cell neuroendocrine carcinoma | 4 | 0.132 [0.059, 0.267] | 0.310 | 0.125 [0.051, 0.276] | 0.231 | |
| Squamous cell carcinoma | 2 | 0.172 [0.069, 0.367] | 0.220 | 0.141 [0.031, 0.454] | - | |
| Squamous differentiation | 3 | 0.281 [0.159, 0.447] | 0.284 | 0.258 [0.122, 0.467] | 0.003 | |
| Signet ring cell carcinoma | 1 | 0.409 [0.228, 0.618] | 1.000 | 0.409 [0.228, 0.618] | - | |
| Undifferentiated | 1 | 0.643 [0.376, 0.843] | 1.000 | 0.643 [0.376, 0.843] | - | |
| CI, Confidence interval; UC, urothelial carcinoma. *, p < 0.001 in the meta-regression test between non-invasive and invasive urothelial carcinoma subgroups. #, p = 0.041 in the meta-regression test between non-muscular and muscular invasion subgroups | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





