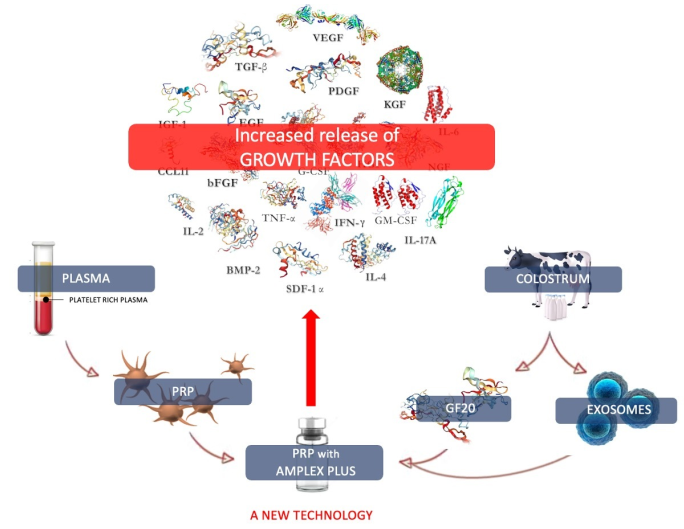
1. Introduction
Tissue regeneration is a complex process for restoring the normal structure and functionality of damaged tissue, which initiates immediately after a traumatic injury and is mediated by a wide range of bioactive molecules such as cytokines and growth factors released from platelets upon activation [
1,
2]. Several soluble factors are released by the platelets, including platelet – derived growth factor (PDGF), epidermal growth factor (EGF), insulin like growth factor (IGF-1), transforming growth factor (TGF-β) and fibroblast growth factor (FGF), that are responsible for initiating the healing process. Platelet-Rich Plasma (PRP) has a high concentration of some growth factors and cytokines that enhance tissue repair mechanisms [
3]. These biologically active peptides sequentially promote angiogenesis of healing wounds which relies on the proliferation, differentiation and migration of endothelial cells forming new blood vessels; restoration of damaged connective tissue through the proliferation and activation of fibroblasts which synthesize new collagen, fibronectin and proteoglycan molecules that form the initial scar; recruitment of neutrophils and macrophages to the injured area that, along their role in defense, induce the formation of granulation tissue, and proliferation and differentiation of cells of mesenchymal origin into tissue specific cell types [
4,
5,
6,
7,
8,
9]. Unsurprisingly, platelet derivatives are related to wound healing and the delivering of PRP has been investigated to facilitate wound resolution. For this reason, the use of PRP has been very successful. It consists of a standardized technique that exploits the properties of growth factors, proteins normally present in our blood capable of stimulating and promoting cell differentiation [
8,
9]. Today PRP is an Autologous Platelet Concentrate or APCs obtained by centrifugation of blood [
10,
11].
In addition to platelet derivates, there are other natural sources of bioactive factors such as biological fluid, including colostrum [
12]. Colostrum is a biological liquid produced by female mammals shortly after giving birth, compositionally similar to mature milk. It contains active factors that are essential to enhance the growth and repair of several tissues, to promote development and to provide immune support of the new-born [
12].
From colostrum it is possible to isolate a mixture of growth factors, which contains 20 different biologically active factors essential for specific function (GF20). The main bioactive components of GF20 that influence cell survival, differentiation, proliferation, and control over migration, needed to provide a local environment for tissue regeneration, especially includes growth factors and cytokines [
13]. GF20 contains different growth factors that show multiple and interrelated functions. They modulate the growth, the maturation, the maintenance and repair of bone, muscle, nervous, cartilage, skin tissues, control blood glucose levels and regulate protein and fat metabolism.
GF20 contains numerous cytokines, proteins involved in immune activation and recruitment but become relevant at times of inflammation or injury stimulating differentiation, chemotaxis and protein synthesis [
14], including TNFα, GM-CSF and interleukins (IL)1, IL-4, IL-6, IL-9 and IL-17. Among the cytokines present in the mixture, IL-17 plays an important role against bacterial and fungal infections and has a regulatory activity in wound healing by stimulating macrophages and neutrophils to produce lactoferrin (an antimicrobial glycoprotein which has multiple effects, including enhancing iron uptake as well as possessing antibacterial and antiviral activity, and stimulating growth of fibroblasts and intestinal epithelial cells), chemokines and remodeling proteins [
13]. Cytokines and growth factors have overlapping activities: cells are exposed to multiple factors at any one time and may result in additive or even synergistic responses.
From colostrum it is also possible to extract and concentrate exosomes which they play a fundamental role in cellular repair processes (14, 15), but also in cellular immunological response (16). The latter put in contact with GF20 are passively loaded with further growth factors and cytokines, favoring their diffusion in damaged tissues and allowing their regeneration. This patented technology is called AMPLEX PLUS technology (D-biotech, Catania, Italy).
It is well known that platelets react first to injury: they clump together to form a platelet plug, releasing their contents; growth factors released by platelets in the damaged area essentially promote tissue repair, influencing the behavior of the cells of the injured tissue. Factors present in colostrum directly stimulate development and differentiation of several subsets of cell types, to guide the formation of mature healthy tissues. These functional evidences have highlighted the possible combined use of platelets concentrates and colostrum derivates to improve tissue repair mechanism.
Despite the efforts of both
in vitro and animal studies to demonstrate the positive influence of PRP on tissue regeneration, the results have not yet been satisfied. Developments in research of the regenerative effects of GF20 in a range of tissue types, particularly in the context of traumatic injury, have attracted great interest. Colostrum isolate mixture may provide insights into the prevention and treatment of several clinical conditions including burns, bone diseases, muscle injuries, ulcers, skin diseases such as acne, alopecia and vitiligo. However, most research should focus on the synergistic effects yielded by the combination of GF20 and PRP to improve the efficacy of regenerative treatments, as already demonstrated in several research [
17,
18].
The objective of this research is to evaluate through enzyme-linked immunosorbent assay (ELISA) the concentration of 20 between growth factors and cytokines in plasma, in PRP and in the PRP with AMPLEX PLUS technology. The analysis was conducted to demonstrate that by combining the factors extracted from colostrum in active form (GF20 and exosomes) with platelet concentrates, the activity of the latter is considerably enhanced. The effectiveness of this combination is closely linked to the synergy of the two components and their functional interdependence. The bioactive substances extracted from the colostrum, in fact, bring nourishment to the cells in the growth and proliferation phase and supply further bioactive factors capable of modulating the action of the growth factors already present in the PRP.
2. Materials and Methods
2.1. PRP preparation:
Blood samples were collected from 10 healthy volunteers without any comorbid disease or history of any medications in the past 2 months (they signed informed consent for participation to study). PRP was prepared by using the DPGPRP Regenerative Therapy kit (D-biotech, Catania, Italy) which allows PRP preparation through centrifugation of a fraction of autologous blood. After blood collection, the platelet preparation tube was placed in the centrifuge (Heraeus, Megafuge 16R, Thermo Scientific) with the counterbalance tube (with water) with the same weight on the opposite side and was centrifuged for 5 min at 4000 rpm. After centrifugation, the tube should contain upper clear yellowish fraction (Platelet Poor Plasma, PPP), separation gel barrier based on an inert polymer and an anticoagulant, and a lower red colored fraction of blood cells (clot). PRP fraction enriched in growth factors that resides on the top of the separation gel was collected while the others were discarded. PRP thus obtained contained a platelet concentration four times higher than the basal count.
2.2. Bovine colostrum derivative preparation (GF20)
In this study, the colostrum was obtained from healthy Holstein cows. The sample was processed according to the procedure described by Sacerdote et al. (2013) [
13]. As a first step, a 100 kg amount of colostrum was diluted 1:10 in deionized water added with NaCl to reach the concentration of 0,9%. In the second step of skimming, to remove the fat part, the suspension was centrifuged at 12,400 x g at 20 to 25 °C. The cream layer was discarded while the supernatant was ultrafiltered through a membrane with a 300 kDa cutoff (SEPRA srl, Via Como 69/A, 20811 Cesano Maderno – MB, Italy) at the same temperature of 20-25°C to eliminate large proteins, including casein, and pathogenetic microorganism. In the following phase of dialysis, the resultant product underwent a series of sterilizing crossflow filtration passages with 0,2 µm membranes (SEPRA srl, Via Como 69/A, 20811 Cesano Maderno – MB, Italy) and then frozen at -20°C. In the last step, after removing the immunoglobulins, the concentrated product was lyophilized (Lyo Quest, Telstar).
The colostrum purification from IgM, with an average molecular weight of 800-900 kDa, was performed by tangential filtration using cartridge filters type Savana plus cartridge filters (Entegris) of 750 kDa/0.02 micron with high flow rate performance and good contaminant retention capabilities. The purification phase also included depletion of IgG and IgA, with a molecular weight of 150 and 380 kDa, respectively, which was carried out through affinity chromatography. The system CaptureSelect IgG-Fc Affinity Matrix (Thermofisher) was used to purify IgG through high-affinity binding of the IgG Fc region, instead for IgA depletion affinity chromatography was used by means of CaptureSelect Bovine IgA Affinity Matrix (Thermofisher). This affinity matrix contains ligands of 13 kDa, comprising the three complementarity determining regions (CDRs) that form the antigen-binding domain. Last step was the removal of endotoxins by affinity chromatography using Bio-Rad Proteus Endotoxin Removal kit.
2.3. Isolation of exosome from colostrum
Exosomes from colostrum were concentrated through ultracentrifuge (Sorvall WX Ultra 100, Thermo Scientific) with successive centrifuges at ultrahigh speeds up to 100,000 x g. All centrifuges were performed at 4 °C.
Briefly, colostrum was centrifuged for 10 min at 2,000 x g. The upper layer of fat globules and the pellet of dead cells were discarded, only the supernatant was collected. Thereafter, the supernatant was ultracentrifuged at 10,000 x g for 30 min to remove cell debris, and as in the previous step, pellet (cell debris) was discarded while the supernatant was collected and used for the following step. The supernatant was then subjected to two consecutive ultracentrifugations at 100,000 x g for 70 min to purify exosomes. After the first of the two 100,000 x g ultracentrifugations, pellet containing exosomes and contaminating proteins was collected, and the supernatant was discarded. The resultant pellet was resuspended in a large volume of filtered PBS to thrown away the contaminating proteins and centrifuged at least for 70 min at the same high speed to wash the exosome pellet.
2.4. Exosomes characterization
Light scattering measurements were performed by a homemade apparatus using a quartz scattering cell, confocal collecting optics, a Hamamatzu photomultiplier mounted on a rotating arm, a BI-9100AT hardware correlator (Brookhaven Instruments Corporation) and illuminating the sample with a 660 nm laser. The power ranged between 5 and 15mW. Low power intensity was used to avoid convective motions due to local heating. Because of the Brownian motion of the particles in solution, the light scattered by particles suspended in solution fluctuates in time. The intensity auto-correlation function g2 provided by the hardware correlator operating in “single photon counting regime” is a decreasing function with a well-defined baseline. The field autocorrelation function g1 is, thus, calculated by the g2 using the Siegert relation g2=|g1|2. For polydisperse noninteracting particles in Brownian motion, the g1 shows several exponential decay components which are analyzed by the cumulants method, thus, following the standard theory of DLS, it is possible to calculate the hydrodynamic diameter [
20,
21].
2.5. AMPLEX PLUS composition
In 5 mL of AMPLEX PLUS are contained 20 billion exosomes and 200 mg of GF20. The exosomes contained in AMPLEX PLUS thanks to new technology, carry more growth factors as they are transferred from GF20 into the nanovesicles by passive transport trough the co-incubation.
2.6. PRP with AMPLEX PLUS technology
For enzyme-linked immunosorbent assay 1mL of high concentration PRP (1x106/μL) was added to 1mL of AMPLEX PLUS.
2.7. Detection of growth factors by ELISA and test validation
The concentrations of 20 growth factors in plasma, PRP and PRP with AMPLEX PLUS technology were determined using commercially available ELISA tests specific for human molecules (Quantikine, RD Systems Inc., Minneapolis, MN, USA). All procedures were performed according to the instructions of the manufacturer.
After identifying two factors significantly involved in the regenerative process, i.e. IGF-1 and TGF-β, and we validated from the analytical point of view since the matrices of the samples used are different (human and bovine). In particular, we evaluated the use of the commercial immunometric methods QUANTIKINE human IGF-1 Immunoassay (DG100B) and QUANTIKINE human TGF-β1 Immunoassay (DB100B) for the quantitative determination of the cytokine IGF-1 and of TGF-β in samples of bovine colostrum or its derivatives (S1, S2). This validation is possible due to the sufficient degree of analogy sequential between the human-derived molecule and the bovine-derived molecule (Identity Factor is 95% for IGF-1 and 94% for TGF- β).
2.8. Statistical analysis
The analyzed parameters were represented by descriptive statistics, indicating continuous variables as mean± standard deviation. Statistical comparison of the data was performed by t-test considering p value less than 0.05 as significant.
3. Results
The distribution and comparison of the growth factors concentrations in plasma, PRP and PRP with AMPLEX PLUS technology are shown using box-plots, in order to demonstrate the shape of the distribution, the central value, and the variability. The analyzes performed highlighted a homogeneous distribution of the data and the absence of outliers in all samples (Figs 1-5). All the mean values of the growth factors obtained from the three matrices were also compared (
Figure 6) demonstrating a statistically significant difference (p<0.00001). In particular, in the PRP with AMPLEX PLUS technology the concentration of the 20 characterized factors was always higher (
Figure 6).
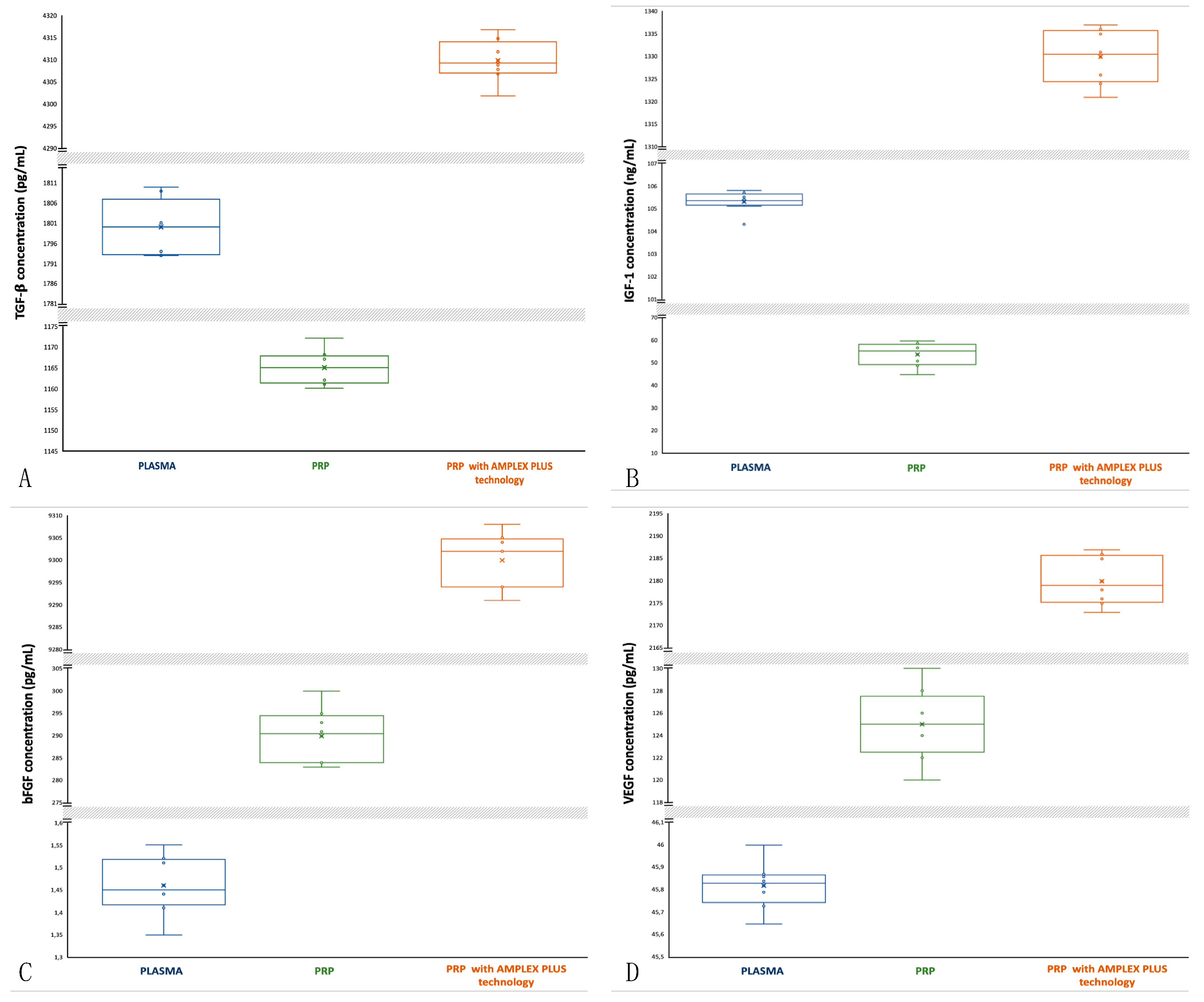
3.1. Transforming Growth Factors β (TGF-β): activity and concentrations
TGF-
β stimulates the growth of cells, especially in connective tissue. It has an important role in embryogenesis, tissue repair, formation of bone and cartilage and in the control of the immune system. TGF-
β acts as a chemoattractant for neutrophils, increases epithelial cell migration at the edges of wounds and stimulates the increase in the synthesis of collagen and fibronectin [
22,
23]. As far as the plasma samples are concerned, the median is central, but the data distribution is asymmetric with a tendency of the data to disperse towards higher values (
Figure 1A). TGF-
β concentrations in plasma are between 1793 pg/mL (min) and 1810 pg/mL (max); mean is 1800±6.284 pg/mL. For PRP the median is closer to the 75th percentile and data distribution is asymmetric with a tendency of the data to disperse towards higher values (
Figure 1A). In PRP samples TGF-
β concentration decreases (1160 and 1172 pg/mL, respectively min and max value; mean is 1165±3.742 pg/mL). For PRP with AMPLEX PLUS technology the median is closer to the 25th percentile and data distribution is asymmetric with a tendency of the data to disperse towards lower values (
Figure 1A). In PRP+GF20 samples TGF-
β concentration significantly increases (4302 and 4317 pg/mL, respectively min and max value; mean is 4310±4.415 pg/mL). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology, as well as among the other mean compared.
3.2. Insulin-Like Growth Factor 1 (IGF-1): activity and concentrations
IGF-1 stimulates the proliferation of many cell types. Is a stronger mitogen which stimulates primarily cells of fetal origin and regulates cell proliferation and differentiation, especially in cartilage and muscle tissue. Furthermore, IGF-1 is an anabolic factor promoting protein accumulation and is involved in mediating the growth promoting actions of growth hormone (GH) [
24,
25]. In plasma samples the median is central, and the data distribution is symmetric (
Figure 1B). IGF-1 concentration in plasma samples is between 104.3 ng/mL (min) and 105.8 ng/mL (max); mean is 105.3±0.433 ng/mL. For PRP the median is closer to the 75th percentile and data distribution is asymmetric with a tendency of the data to disperse towards lower values (
Figure 1B). In PRP, IGF-1 concentration decreases (min 45 ng/mL, max 60 ng/mL; mean is 54±4.934 ng/mL). For PRP with AMPLEX PLUS technology the median is central, but data distribution is asymmetric with a tendency of the data to disperse towards lower values (more extended lower whisker) (
Figure 1B). IGF-1 concentration significantly increases (min 1321 ng/mL, max 1337 ng/mL; mean is 1330±5.522 ng/mL). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) for all mean compared.
3.3. Basic Fibroblast Growth Factor (bFGF): activity and concentrations
bFGF promotes proliferation, differentiation and survival of fibroblasts, keratinocytes, smooth muscle cells, stem cells, osseous healing and chondrogenesis. It is involved in angiogenesis and hematopoiesis, stimulates the migration of epithelial cells in wound healing process and the synthesis of the extracellular matrix, it also improves myogenesis (26, 27). As far as plasma samples are concerned, the median is closer to the 25th percentile and data distribution is asymmetric with a tendency of the data to disperse towards lower values (
Figure 1C). bFGF concentrations in plasma are between 1.35 pg/mL (min) and 1.36 pg/mL (max); mean is 1.46+0.060 pg/mL. In PRP samples the median is closer to the 75th percentile and the data distribution is asymmetric with a tendency of the data to disperse towards lower values (
Figure 1C). bFGF concentration is between 283 pg/mL (min) and 300 pg/mL (max); mean is 290±5.565 pg/mL. In PRP with AMPLEX PLUS technology samples the median is closer to the 75th percentile and data distribution is symmetric (
Figure 1C). bFGF concentration on PRP with AMPLEX PLUS technology is significantly increases (min 9291 pg/mL, max 9308 pg/mL; mean is 9300±5.766 pg/mL). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) for all mean compared.
3.4. Vascular Endothelial Growth Factor (VEGF); activity and concentrations
VEGF increases proliferation and migration of endothelial cells and stimulates the growth of new blood vessels after injury and enhances vascular permeability. VEGF restores the blood supply to cells and tissues when they are deprived of oxygenated blood due to compromised blood circulation. Furthermore, VEGF promotes collagen deposition, improves re-epithelialization associated with enhanced vessel formation and it plays a role in lymphangiogenesis [
28,
29]. VEGF concentrations in plasma the median is closer to the 75
th percentile and data distribution is asymmetric with a tendency of the data to disperse towards lower values (
Figure 1D). VEGF concentrations in plasma are between 45.65 pg/mL (min) and 46 pg/mL (max); mean is 45.82±0.096 pg/mL. In PRP samples the median is central and data distribution is symmetric (
Figure 1D). VEGF concentration is between 120 pg/mL (min) and 130 pg/mL (max); mean is 125±2.958 pg/mL. In PRP with AMPLEX PLUS technology samples the median is closer to the 25
th percentile and data distribution is asymmetric (
Figure 1D). VEGF concentrations on PRP with AMPLEX PLUS technology are increased (min 2173 pg/mL, max 2187 pg/mL; mean is 2180±5.049 pg/mL). The t-test done to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology, as well as among the other mean compared.
Figure 1.
Comparative box-plot analysis of growth factors concentrations isolated from plasma, PRP and PRP with AMPLEX PLUS technology. A: TGF-β; B; IGF-1; C: bFGF; D: VEGF.
Figure 1.
Comparative box-plot analysis of growth factors concentrations isolated from plasma, PRP and PRP with AMPLEX PLUS technology. A: TGF-β; B; IGF-1; C: bFGF; D: VEGF.
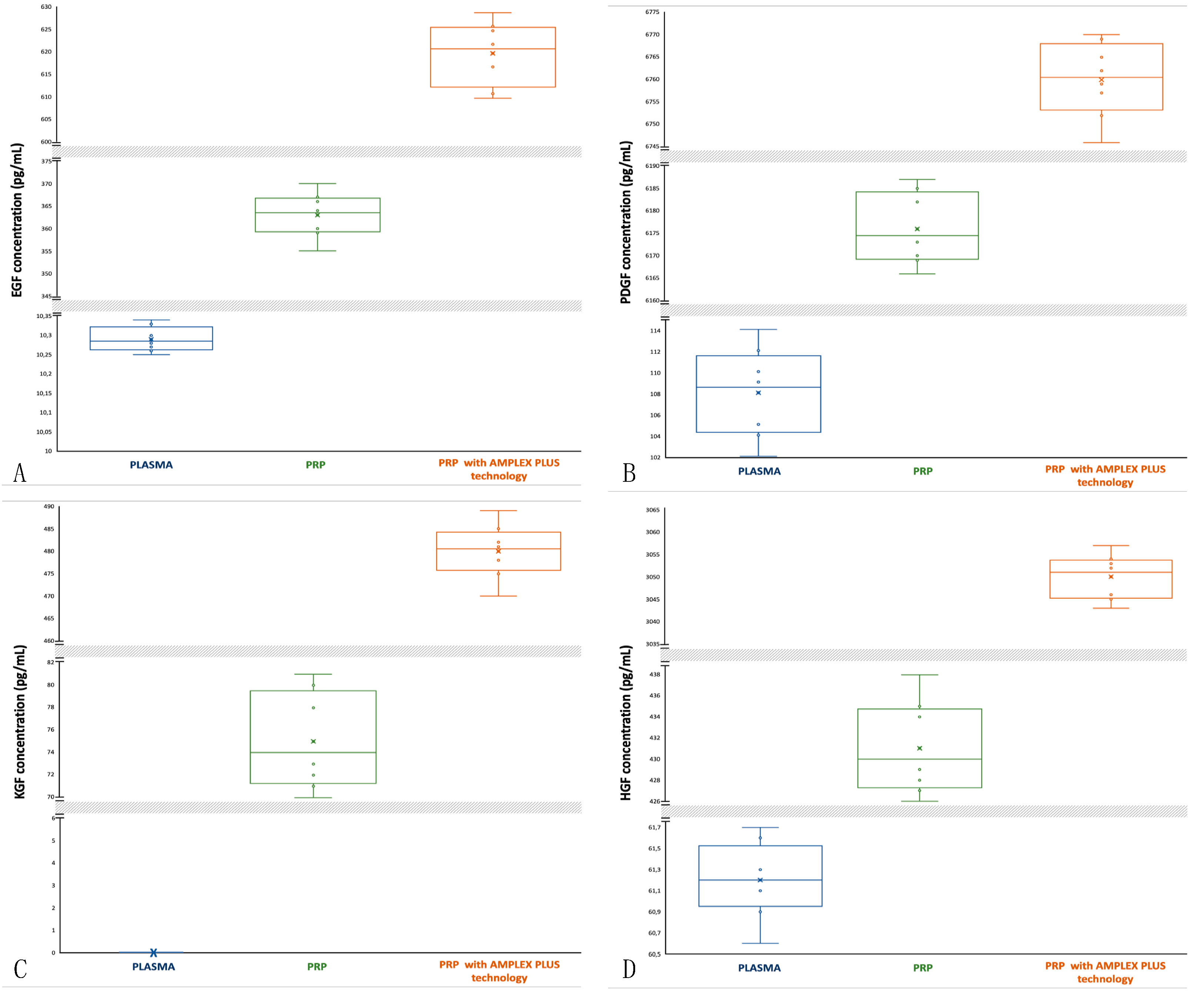
3.5. Epidermal Growth Factor (EGF): activity and concentrations
EGF stimulates the proliferation of epidermal, epithelial and embryonic cells and promotes wound healing. EGF is involved in angiogenesis and re-epithelialization; it acts as an angiogenic factor and as differentiation factor for some cell types [
30,
31]. For EGF plasma samples, the median is closer to the 25th percentile and data distribution is almost symmetric with a low tendency of the data to disperse towards lower values (
Figure 2A). EGF concentrations in plasma samples are between 10.25 pg/mL (min) and 10.34 pg/mL (max); mean is 10.29±0.030 pg/mL. For PRP the median is central and data distribution is symmetric (
Figure 2A). In PRP, EGF concentration increases (min 355 pg/mL, max 370 pg/mL; mean is 363±4.527 pg/mL). For PRP with AMPLEX PLUS technology the median is closer to the 75th percentile and data distribution is slightly asymmetric with a tendency of the data to disperse towards upper values (more extended upper whisker) (
Figure 2A). EGF concentration significantly increases (min 610 pg/mL, max 629 pg/mL; mean is 620±6.480 pg/mL). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) for all mean compared.
3.6. Platelet-Derived Growth Factor (PDGF): activity and concentrations
PDGF plays an important role during embryogenesis, in particular for the development of the kidneys, blood vessels, lungs and central nervous system. PDGF has a growth-promoting activity on mesangial cells, pericytes, alveolar fibroblast and glial cells. It is involved also in angiogenesis and plays a role in re-epithelialization by up-regulating the production of IGF-1. During tissue remodeling, PDGF helps to break down old collagen [
32,
33]. The median of PDGF concentrations in plasma is closer to the 75th percentile and data distribution is symmetric (
Figure 2B). PDGF concentrations in plasma are between 102 pg/mL (min) and 114 pg/mL (max); mean is 108±3.840 pg/mL. In PRP samples the median is closer to the 25th percentile and data distribution is asymmetric (
Figure 2B). PDGF concentration are increases (min 6166 pg/mL; max 6187 pg/mL; mean is 6176±7.348 pg/mL). In PRP with AMPLEX PLUS technology samples the median is central and data distribution is asymmetric with a tendency of the data to disperse towards lower values (
Figure 2B). PDGF concentration on AMPLEX PLUS is between 6746 pg/mL (min) and 6770 pg/mL (max); mean is 6760±7.745 pg/mL. The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology, as well as among the other mean compared.
3.7. Keratinocyte Growth Factor (KGF): activity and concentrations
KGF stimulates proliferation and differentiation of keratinocytes and it is a potent mitogen for vascular endothelial cells. KGF helps in the up-regulation of VEGF and increases transcription of factors involved in the detoxification of ROS preserving keratinocytes for re-epithelialization. It accelerates also tissue repair upon skin injury due to the controlled released of bFGF [
34,
35]. KGF is undetectable in blood (
Figure 2C) and it has a low concentration in PRP (min 70 pg/mL; max 81 pg/mL; mean 75±3.937 pg/mL) compared to PRP with AMPLEX PLUS technology (min 470 pg/mL; max 489 pg/mL; mean 480±5.477 pg/mL). The median of KGF concentrations in PRP is closer to the 25th percentile and data distribution is symmetric (
Figure 2C). In PRP+GF20 samples the median is central and data distribution is symmetric (
Figure 2C). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology.
3.8. Hepatocyte Growth Factor (HGF): activity and concentrations
HGF regulates epithelial and endothelial cells growth. It is a potent mitogen for hepatocytes and plays important roles in the embryonic development of the liver and the placenta and in the migration of myogenic precursor cells. HGF increases angiogenesis and re-epithelialization by promoting migration of endothelial cells to the injured area [
36,
37]. HGF has a low concentration in plasma (min 60.6 pg/mL; max 61.7 pg/mL; mean 61.2±0.331 pg/mL) compared to PRP (min 426 pg/mL; max 438 pg/mL; mean 431±4 pg/mL) and especially to PRP with AMPLEX PLUS technology (min 3043 pg/mL; max 3057 pg/mL; mean 3050±4.582 pg/mL). The median of HGF concentrations in plasma is closer to the 25th percentile and data distribution is slightly asymmetric (more extended lower whisker) (
Figure 2D). The median of HGF in PRP is also closer to the 25th percentile but data distribution is slightly asymmetric with more extended upper whisker (
Figure 2D). In PRP with AMPLEX PLUS technology samples the median is central and data distribution is asymmetric with more extended lower whisker (
Figure 2D). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology, as well as among the other mean compared.
Figure 1.
Comparative box-plot analysis of growth factors concentrations isolated from plasma, PRP and PRP with AMPLEX PLUS technology. A: EGF; B: PDGF; C: KGF; D: HGF.
Figure 1.
Comparative box-plot analysis of growth factors concentrations isolated from plasma, PRP and PRP with AMPLEX PLUS technology. A: EGF; B: PDGF; C: KGF; D: HGF.
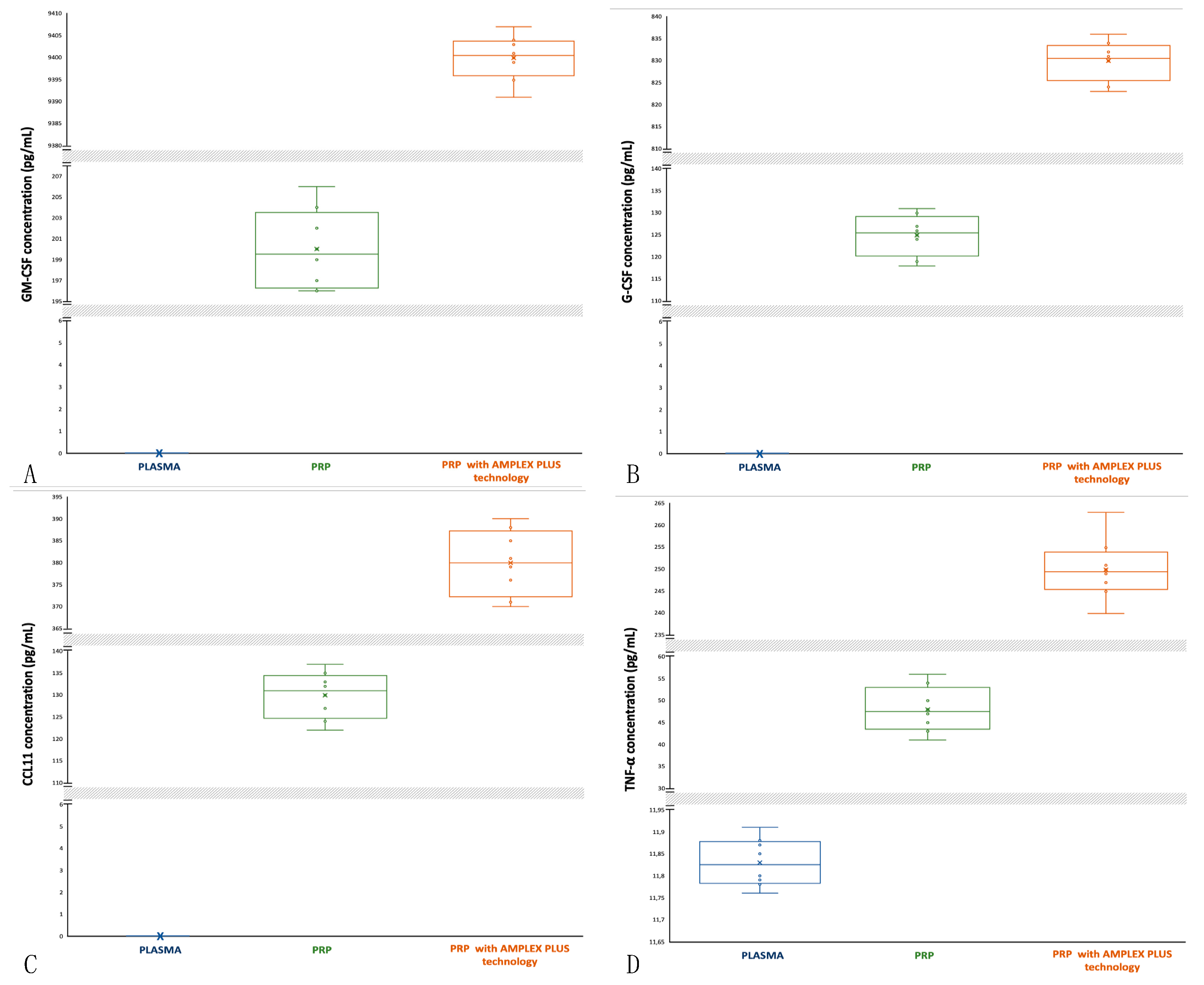
3.9. Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF): activity and concentrations
GM-CSF affects wound healing by inducing keratinocyte and endothelial cells proliferation and promoting migration of epithelial cells. It increases re-epithelialization, recruiting neutrophils, monocytes and lymphocytes and it stimulates phagocytosis in matured leucocytes. GM-CSF increases angiogenesis and lymphangiogenesis [
38,
39]. GM-CSF is undetectable in blood (
Figure 3A) and it has a low concentration in PRP (min 196 pg/mL; max 206 pg/mL; mean 200±3.5 pg/mL) compared to PRP with AMPLEX PLUS technology (min 9391 pg/mL; max 9407 pg/mL; mean 9400±4.769 pg/mL). The median of GM-CSF concentrations in PRP is central and data distribution is asymmetric with more extended upper whisker (
Figure 3A). In PRP with AMPLEX PLUS technology samples the median is closer to the 75
th percentile and data distribution is slightly asymmetric (
Figure 3A). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology.
3.10. Granulocyte-Colony Stimulating Factor (G-CSF): activity and concentrations
G-CSF influences the proliferation, differentiation, and survival of haemopoietic stem cells and mature neutrophils and induces the proliferation of endothelial cells and their migration to the damaged area, postinjury accelerating would healing. G-CSF stimulates also angiogenesis and neurogenesis [
40,
41]. G-CSF is undetectable in blood (
Figure 3B) and it has a low concentration in PRP (min 118 pg/mL; max 131 pg/mL; mean 125±4.358 pg/mL) compared to PRP with AMPLEX PLUS technology (min 823 pg/mL; max 836 pg/mL; mean 830±4.213 pg/mL). The median of G-CSF concentrations in PRP is slightly closer to the 75th percentile and data distribution is symmetric (
Figure 3B), as well as in the PRP with AMPLEX PLUS technology samples (
Figure 3B). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology.
3.11. EOTAXIN-CCL11: activity and concentrations
CCL11 is a chemokine that stimulates eosinophil chemotaxis and enhances their adhesion to endothelial cells and contributes to the activation of eosinophils recruited into the damage tissue playing roles in epithelial remodeling. It modulates fibroblast activities by increasing their proliferation and collagen synthesis and promotes angiogenesis [
42,
43]. CCL11 is undetectable in blood (
Figure 3C),and it has a low concentration in PRP (min 122 pg/mL; max 137 pg/mL; mean 130±4.949 pg/mL) compared to PRP with AMPLEX PLUS technology (min 370 pg/mL; max 390 pg/mL; mean 380±6.964 pg/mL). The median of CCL11 concentrations in PRP is closer to the 75
th percentile and data distribution is symmetric (
Figure 3C). In PRP with AMPLEX PLUS technology samples median is central and data distribution is symmetric (
Figure 3C). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology.
3.12. Tumor Necrosis Factor α (TNF-α): activity and concentrations
TNF-α regulates the activity of fibroblasts, vascular endothelial cells and keratinocytes and promotes synthesis of extracellular matrix proteins, which are closely involved in the healing of injured tissues. TNF-α induces angiogenesis and shows anti-malignant cell cytotoxicity, especially in combination with Interferon. It contributes to bone remodeling by activating osteoclasts and plays an important role in controlling infection [
44,
45]. The median of TNF-α concentrations in plasma is central and data distribution is asymmetric with a tendency of the data to disperse towards upper values (
Figure 3D). TGF-α concentrations in plasma are between 11.76 pg/mL (min) and 11.91 pg/mL (max); mean is 11.83±0.050 pg/mL. In PRP samples the median is closer to the 25
th percentile and data distribution is symmetric (
Figure 3D). TNF-α concentration is between 41 pg/mL (min) and 56 pg/mL (max); mean is 48±4.847 pg/mL. In PRP+G PRP with AMPLEX PLUS technology samples the median is central and data distribution is asymmetric with more extended upper whisker (
Figure 3D). TNF-α concentration un PRP with AMPLEX PLUS technology are increased (min 240 pg/mL, max 263 pg/mL; mean is 250±6.422 pg/mL). The t-test done to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology, as well as among the other mean compared.
Figure 2.
Comparative box-plot analysis of growth factors concentrations isolated from plasma, PRP and PRP with AMPLEX PLUS technology. A: GM-CSF; B: G-CSF; C: CCL11; D: TNF-α.
Figure 2.
Comparative box-plot analysis of growth factors concentrations isolated from plasma, PRP and PRP with AMPLEX PLUS technology. A: GM-CSF; B: G-CSF; C: CCL11; D: TNF-α.
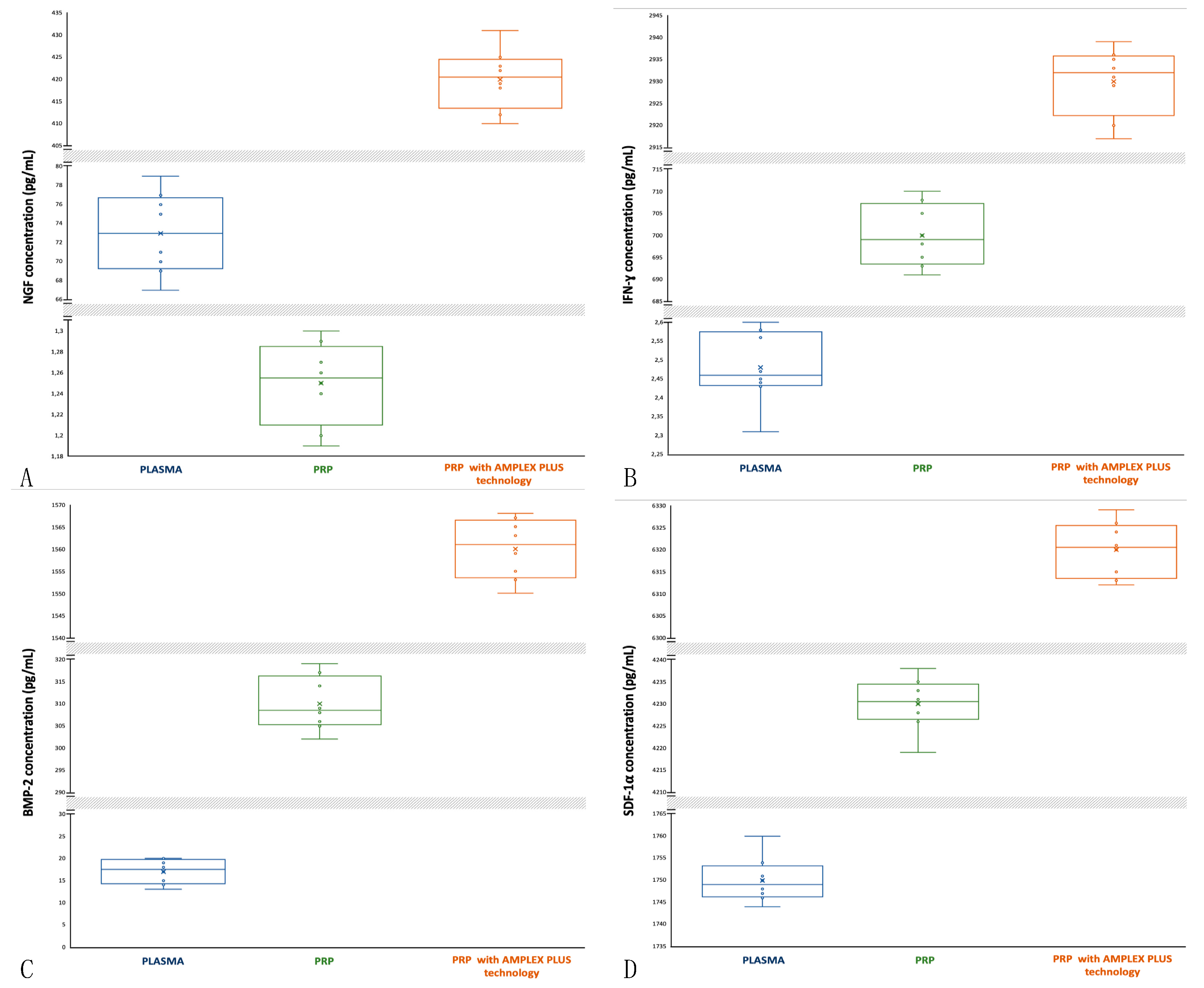
3.13. Nerve Growth Factor (NGF): activity and concentrations
NGF promotes fibroblast and keratinocyte proliferation, extracellular matrix component expression and secretion and it stimulates the proliferation and differentiation of local immune cells, blood vessels and even neurite outgrowth. NGF contributes also to the reestablishment of a normal sensory and sympathetic innervation of the damaged skin [
46,
47]. NGF has a high concentration in plasma (min 67 pg/mL; max 79 pg/mL; mean 73±4.031 pg/mL) compared to PRP (min 1.19 pg/mL; max 1.30 pg/mL; mean 1.25±0.036 pg/mL) and a low concentration compared to PRP with AMPLEX PLUS technology (min 410 pg/mL; max 431 pg/mL; mean 420±6.403 pg/mL). The median of NGF concentrations in plasma is central and data distribution is symmetric (Figure 4A). The median of NGF in PRP is also closer to the 75th percentile and data distribution is slightly asymmetric with more extended lower whisker (Figure 4A), also in PRP with AMPLEX PLUS technology samples the median is closer to the 75th percentile but data distribution is asymmetric with more extended upper whisker (Figure 4A). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology, as well as among the other mean compared.
3.14. Gamma Interferon (INF-γ); activity and concentrations
INF-γ promotes macrophage activation and mediates antiviral and antibacterial immunity. During cutaneous wound healing INF-γ contributes to angiogenesis and collagen deposition and encourages CD4+ T helper polarization, which contributes to the initial wound microenvironment. INF-γ controls cellular proliferation and apoptosis and it also coordinates lymphocyte-endothelium interaction [
48,
49]. INF-γ has a low concentration in plasma (min 2.31 pg/mL; max 2.60 pg/mL; mean 2.48±0.089 pg/mL) compared to PRP (min 691 pg/mL; max 710 pg/mL; mean 700±6.595 pg/mL) and especially to PRP with AMPLEX PLUS (min 2917 pg/mL; max 2939 pg/mL; mean 2930+7.262 pg/mL). The median of INF-γ concentrations in plasma is closer to the 25
th percentile and data distribution is asymmetric with more extended lower whisker (Figure 4B). The median of INF-γ in PRP is slightly closer to the 25
th percentile but data distribution is symmetric (Figure 4B). In PRP with AMPLEX PLUS technology samples the median is closer to the 75
th percentile and data distribution is slightly asymmetric with more extended lower whisker (Figure 4B). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology, as well as among the other mean compared.
3.15. Bone Morphogenetic Protein 2 (BMP-2): activity and concentrations
BMP-2 induces the formation of both cartilage and bone. BMP-2 directs the development of neural crests cells into neural phenotypes and induces chemotaxis, mesenchymal cell proliferation and differentiation. BMP-2 enhances angiogenesis and regulates the healing process by promoting dermal and epidermal growth, leading to keratinized and thickened skin [
50,
51]. BMP-2 has a low concentration in plasma (min 13 pg/mL; max 20 pg/mL; mean 17±2.549 pg/mL) compared to PRP (min 302 pg/mL; max 319 pg/mL; mean 310± 5.656 pg/mL) and especially to PRP with AMPLEX PLUS technology (min 1550 pg/mL; max 1568 pg/mL; mean 1560±6.344 pg/mL). The median of BMP-2 concentrations in plasma is slightly closer to the 75
th percentile and data distribution is marginally asymmetric (Figure 4C). The median of BMP-2 in PRP is slightly closer to the 25
th percentile but data distribution is symmetric (Figure 4C). In PRP with AMPLEX PLUS technology samples the median is closer to the 75
th percentile and data distribution is slightly asymmetric with more extended lower whisker (Figure 4C). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology, as well as among the other mean compared.
3.16. Stromal Cell-Derived Factor 1α (SDF1-α): activity and concentrations
SDF1-α is one of the most potent chemokines for stem cells recruitment. SDF1-α can regulate multiple physiological processes such as organogenesis, regeneration and angiogenesis and administered at the site of ischemia, is associated with ischemic neovascularization. SDF1-α has an important role during bone fracture healing and play a central role during the process of angiogenesis, such as chemotaxis, cell proliferation, migration and the secretion of angiopoietin factors [
52,
53]. Plasma concentration of SDF1-α are 1744 pg/mL (min) and 1760 pg/mL (max); mean is 1750±4.769 pg/mL. PRP concentration is 4219 pg/mL (min), 4238 pg/mL (max) and 4230±5.477 pg/mL (mean); these values are lower than the PRP with AMPLEX PLUS technology (min 6312 pg/mL; max 6329 pg/mL; mean 6320±5.830 pg/mL). The median of SDF1-α concentrations in plasma is slightly closer to the 25
th percentile and data distribution is asymmetric with more extended upper whisker (Figure 4D). The median of SDF1-α in PRP is central and data distribution is asymmetric with more extended lower whisker (Figure 4D). In PRP with AMPLEX PLUS technology samples the median is closer to the 75
th percentile and data distribution is slightly asymmetric (Figure 4D). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology, as well as among the other mean compared.
Figure 3.
Comparative box-plot analysis of growth factors concentrations isolated from plasma, PRP and PRP with AMPLEX PLUS technology. A: NGF; B: INF-γ; C: BMP-2; D: SDF1-α.
Figure 3.
Comparative box-plot analysis of growth factors concentrations isolated from plasma, PRP and PRP with AMPLEX PLUS technology. A: NGF; B: INF-γ; C: BMP-2; D: SDF1-α.
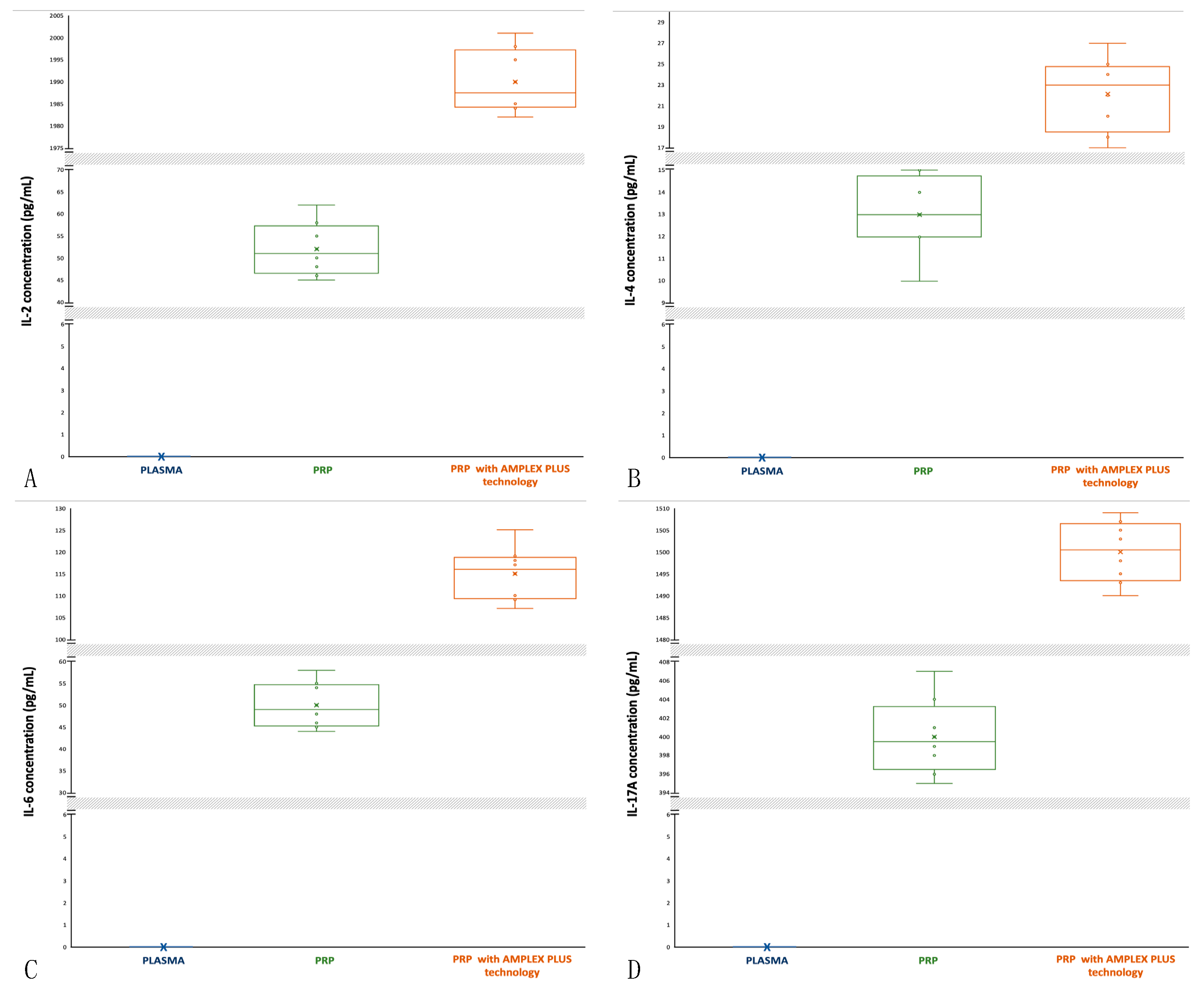
3.17. Interleukin-2 (IL-2): activity and concentrations
IL-2 stimulates proliferation and enhances function of T-cells, NK cells and B-cells and induces B-cells to generate secretory IgM. IL-2 stimulates macrophages to gain maturity and elaborate TGF-β.
In synergy with bFGF, IL-2 can impact the rate and quality of the closure at the wound site by promoting skin cell proliferation. Furthermore, it can promote angiogenesis [
54,
55]. IL-2 is undetectable in blood (
Figure 5A), and it has a low concentration in PRP (min 45 pg/mL; max 62 pg/mL; mean 52±5.590 pg/mL) compared to PRP with PRP with AMPLEX PLUS technology (min 1982 pg/mL; max 2001 pg/mL; mean 1990±6.708 pg/mL). The median of IL-2 concentrations in PRP is slightly closer to the 25
th percentile and data distribution is asymmetric with more extended upper whisker (
Figure 5A). In PRP with AMPLEX PLUS technology samples the median is closer to the 25
th percentile and data distribution is slightly asymmetric with more extended upper whisker (
Figure 5A). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology.
3.18. Interleukin-4 (IL-4): activity and concentrations
IL-4 has an important role in regulating antibody production, hematopoiesis, inflammation, and T cells development and can rescue B-cells from apoptosis, enhancing their survival and promoting their secretion of IgE and IgG. It is almost twice as potent as TGF- β at stimulating collagen synthesis by fibroblasts and is a potent mitogen for microvascular endothelial cells [
56,
57]. IL-4 is undetectable in blood (
Figure 5B) and it has a slightly lower concentration in PRP (min 10 pg/mL; max 15 pg/mL; mean 13±1.581 pg/mL) compared to PRP with AMPLEX PLUS technology (min 16 pg/mL; max 27 pg/mL; mean 22±3.5 pg/mL). The median of IL-4 concentrations in PRP is slightly closer to the 25
th percentile and data distribution is asymmetric with more extended upper whisker (
Figure 5B). In PRP with AMPLEX PLUS technology samples the median is closer to the 25
th percentile and data distribution is asymmetric with more extended upper whisker (
Figure 5B). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology.
3.19. Interleukin-6 (IL-6): activity and concentrations
IL-6 stimulates the activation of osteoclasts for the continual physiological process of bone remodeling as well as for the repair process during bone healing. IL-6 induces excess production of VEGF, leading to enhanced angiogenesis and increased vascular permeability and can aid keratinocyte proliferation and the generation of collagen by dermal fibroblasts [
58,
59]. IL-6 is undetectable in blood (
Figure 5C), and it has a lower concentration in PRP (min 44 pg/mL; max 58 pg/mL; mean 50±4.821 pg/mL) compared to PRP with AMPLEX PLUS technology (min 107 pg/mL; max 125 pg/mL; mean 115±5.634 pg/mL). The median of IL-6 concentrations in PRP is slightly closer to the 25
th percentile and data distribution is asymmetric with more extended lower whisker (
Figure 5C). In PRP with AMPLEX PLUS technology samples the median is closer to the 75
th percentile and data distribution is symmetric (
Figure 5C). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology.
3.20. Interleukin-17A (IL-17A): activity and concentrations
IL-17A promotes the production of G-CSF which act in synergy with TNF-α to induce neutrophil recruitment. It can stimulate macrophages and neutrophils to produce lactoferrins and regenerating proteins, helping to kill bacteria. IL-17A maintains integrity of the mucosal tissues and promotes wound closure, myofibroblast differentiation and collagen deposition [
60,
61]. IL-17A is undetectable in blood (
Figure 5D) and it has a lower concentration in PRP (min 395 pg/mL; max 407 pg/mL; mean 400±3.741 pg/mL) compared to PRP with AMPLEX PLUS technology (min 1490 pg/mL; max 1509 pg/mL; mean 1500±6.538 pg/mL). The median of IL-17A concentrations in PRP is almost central and data distribution is asymmetric with more extended upper whisker (
Figure 5D). In PRP with AMPLEX PLUS technology samples the median is central and data distribution is asymmetric (
Figure 5D). The t-test performed to compare the mean values showed a highly significant difference (p<0.00001) between PRP and PRP with AMPLEX PLUS technology.
Figure 5.
Comparative box-plot analysis of growth factors concentrations isolated from plasma, PRP and PRP with AMPLEX PLUS technology. A: IL-2; B: IL-4; C: IL-6; D: IL-17A.
Figure 5.
Comparative box-plot analysis of growth factors concentrations isolated from plasma, PRP and PRP with AMPLEX PLUS technology. A: IL-2; B: IL-4; C: IL-6; D: IL-17A.
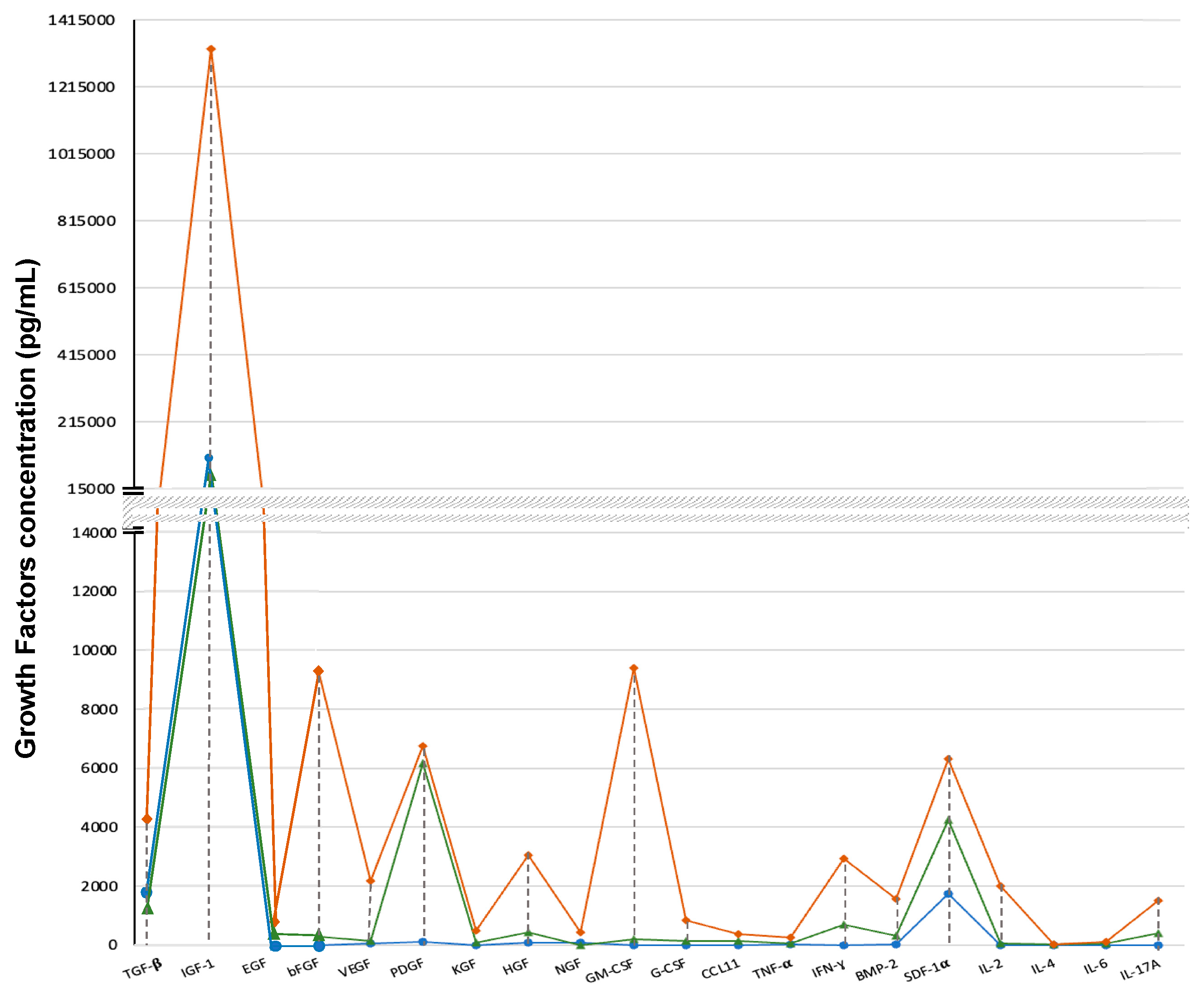
Figure 6.
Comparative analysis of concentrations (pg/mL) of the 20 bioactive compounds in plasma, PRP and PRP with AMPLEX PLUS technology.
Figure 6.
Comparative analysis of concentrations (pg/mL) of the 20 bioactive compounds in plasma, PRP and PRP with AMPLEX PLUS technology.
4. Discussion
In recent years there has been an increasing interest in the study of growth factors extracted from different matrices especially for their role in tissue repair [
62]. Tissue injury healing is a complex process involving a cascade of cellular and molecular events mostly shared by the different tissues of the body [
63]. Interestingly, the tissue repair process begins immediately after a traumatic injury and is mediated and controlled by a diverse range of cytokines, proteins, and growth factors, some of which are released by platelets after activation [
64].
Particularly relevant for tissue healing are the factors TGF-β1, TGF-β2 (transforming growth factor-β), PDGF-AA, PDGF-BB, PDGF-AB (platelet-derived growth factors), IGF-I insulin-like growth factor), EGF 4 (epidermal growth factor), HGF (hepatocyte growth factor), which are found inside the α granules of platelets and which, following platelet activation, are released into the circulation blood. These and other growth factors act synergistically by increasing neutrophil and macrophage infiltration thereby promoting angiogenesis, fibroplasia, matrix deposition and re-epithelialization [
65,
66,
67]. For this reason, the use of Platelet-Rich Plasma or PRP has been very successful, also as Autologous Platelet Concentrate or APCs. As can be seen from numerous studies [
68,
69], for many applications of PRP the results obtained have not been satisfactory, and also for those applications for which the effects of treatment with PRP have been judged positively, the results obtained are clearly susceptible to further notable improvements. An attempt has therefore been made to enhance the effect of PRP by using them in combination with adult stem cells taken from the same patient, antibiotics, anti-inflammatories, vitamins, etc., however the increase in efficacy obtained with these combinations, compared to the use of PRP from alone, it was quite modest [
70,
71]. It was therefore assumed that the limited effectiveness of the various preparations was due to a low platelet concentration and, consequently, to a low concentration of the factors contained in the platelet granules.
In nearly all published studies, a platelet concentration ranging from 1.0 – 1.5 × 10
6 platelet /µL is used. This platelet concentration is scientifically supported by evidence that lower concentrations are not effective enough in promoting healing and regeneration of bone and soft tissue. It has also been seen that higher concentrations are often not applicable for legislative reasons, but even in cases where these higher concentrations have been used in experimental studies, they have shown substantially the same efficacy as the standard concentrations [
72].
There is therefore the need to have preparations based on natural substances, at low cost and without side effects, which do not alter the action of autologous platelet concentrates, but which, on the contrary, amplify their effectiveness, such as GF20.
GF20 is a mixture, isolate from colostrum bovine, which contains different biologically active factors. These components are activators of numerous regeneration and growth processes, they activate and improve the body's natural defense systems. The growth factors contained in it have an action aimed at protecting the integrity of the tissues. In contact with damaged or injured epithelial or mucous membrane structures, they carry out a contrasting action against pathogenic microorganisms, promoting tissue repair and the reconstruction of damaged, contaminated, and inflamed epithelia.
Our research shown that the combination of PRP, GF20 and exosomes (PRP with AMPLEX PLUS technology) could improve the efficacy of regenerative treatments, due to the high presence of bioactive factors. It is known that colostrum-derived exosomes are highly biocompatible and therefore safe nanovesicles capable of transferring and transporting different molecules, including growth factors, then released in a specific and targeted manner [
14].
In particular, the efficacy of this combination is closely linked to the synergy of the two components (PRP and AMPLEX PLUS technology) and their functional interdependence. In fact, the bioactive substances present in AMPLEX PLUS provide nourishment to the cells in the growth and proliferation phase and supply further bioactive factors, (present in very low concentrations in PRP) capable of modulating the action of the growth factors. As has been highlighted by our analyses, the mean values of the all factors were much higher in PRP with AMPLEX PLUS technology (TGF-β: 4310±4.415 pg/mL; IGF-1: 1330±5.522 ng/mL; bFGF: 9300±5.766 pg/mL; VEGF: 2180±5.049 pg/mL; EGF: 620±6.480 pg/mL; PDGF: 6760±7.745 pg/mL; KGF: 480±5.477 pg/mL; HGF: 3050±4.582 pg/mL; GM-CSF: 9400±4.769 pg/mL; G-CSF: 830±4.213 pg/mL; CCL11: 380±6.964 pg/mL; TGF-α: 250±6.422 pg/mL; NGF: 420±6.403 pg/mL; INF-γ: 930±7.262 pg/mL; BMP-2: 1560±6.344 pg/mL; SDF1-α: 6320±5.830 pg/mL; IL-2: 1990±6.708 pg/mL; IL-4: 22±3.5 pg/mL; IL-6: 115±5.634 pg/mL; IL-17A:1500±6.638 pg/mL) compared to PRP alone (TGF-β: 1165±3.742 pg/mL; IGF-1: 54±4.924 ng/mL; bFGF: 290±5.565 pg/mL; VEGF: 125±2.958 pg/mL; EGF: 363±4.527 pg/mL; PDGF: 6176±7.348 pg/mL; KGF: 75±3.937 pg/mL; HGF: 431±4 pg/mL; GM-CSF: 200±3.5 pg/mL; G-CSF: 125±4.358 pg/mL; CCL11: 130±4.949 pg/mL; TNF-α: 48±4.847 pg/mL; NGF: 1.25±0.036 pg/mL; INF-γ: 700±6.595 pg/mL; BMP-2: 310±5.656 pg/mL; SDF1-α: 4230±5.477 pg/mL; IL-2: 52±5.590 pg/mL; IL-4; 13±1.581 pg/mL; IL-6: 50±4.821 pg/mL; IL-17A: 400±3.741 pg/mL).
Through the combination of PRP and AMPLEX PLUS technology, a vertiginous increase was found for the concentrations of NGF, GM-CSF, IL-2, bFGF, and IGF-1 (in order of percentage increase over PRP). More moderate, but still greater than 100% increases were obtained instead for most of the compounds analyzed (VEGF, HGF, G-CSF, KGF, TNF-α, BMP-2, INF-γ, IL-17A, TGF-β, CCL11, and IL-6, in order of percentage increase over PRP). The concentration of EGF increased by 70% while of SDF1-α and of IL-4 by 49.4% and 40%, respectively. Less significant increases are evident for PDGF whose concentration increased by only 9% following the addition of AMPLEX PLUS technology to PRP.
Interestingly, the concentrations obtained with the addition of AMPLEX PLUS technology have never been achieved before. During the past few years, in fact, PRP has been enriched with different matrices to enhance its action. For example, the combination of PRP and autologous conditioned serum (ACS) resulted in increased concentrations of PDGF, IGF-1, and TNF-α. However, not equaling those achieved by the addition of AMPLEX PLUS. Enrichment with leukocytes [L-PRP] also did not have the desired effects [
73]. These attempts, however, did not result in substantial improvement in its efficacy, probably due to the missing supply of some growth factors not present or present in trace amounts in the matrices used. Colostrum appears, therefore, to be one of the richest and most complete natural matrices in growth factors and bioactive compounds.
In addition, the presence in optimal amounts of all growth factors results in a synergistic action that promotes the repair process; this occurs by stimulating neoangiogenesis, even in poorly vascularized areas, and the regeneration of damaged tissues which are replaced with cells of the same type, thus avoiding their replacement with connective tissue (fibrosis) [
74]. Furthermore, the possible use of PRP with AMPLEX PLUS technology could prevent the establishment of hypertrophic or keloid scars, which represent one of the most common wound healing disorders.
In this context, the effective concentration for each bioactive compound, which results in the performance of its biological activity in a physiological and optimal environment, becomes of primary importance, since the excess or the lack of some factors could be the cause of adverse and toxic events for biological systems in vitro and in vivo. For most of the compounds, there are no concentration data in the literature so remains a need to investigate their concentration-dependent behavior.
5. Conclusions
Healthy regeneration of damaged tissues relies on a well-orchestrated release of growth factors. The results of the present study show that the concentrations of growth factors involved in regenerative and reparative pathways is increased significantly following the combination of PRP with the new AMPLEX PLUS formulation. Therefore, given the considerable increase in growth factors concentration, the action and the powerful influence on tissue healing of PRP, combined with the properties of AMPLEX PLUS, is enhanced. In addition, the findings in this study show that AMPLEX PLUS contains a high level of some factors, which are not present in PRP or the relative concentration of each respective growth factor in PRP is very low. Each of these factors possesses numerous activities that make them attractive agent for stimulating important biological processes. IGF-1, VEGF, bFGF and NGF are growth factors almost absent in PRP. These molecules remain essential to the field of regenerative medicine, showing additional functions that could be further investigated by in vitro and in vivo methods. IGF-1 and VEGF have been shown to affect hair growth: IGF-1 stimulates follicular proliferation and tissue remodeling, as well as follicular differentiation, identifying IGF-1 signaling as an important mitogenic and morphogenetic regulator in hair follicle biology. VEGF controls follicle cycling mediating angiogenesis, identifying VEGF as a major mediator of hair follicle growth.
Besides its role as a proangiogenic factor, VEGF has a variety of functions: it also stimulates mitogenesis and migration of macrophage lineage and endothelial cells and increases vascular permeability during the wound healing process, so trough these mechanisms the amount of VEGF present in a wound can significantly impact healing.
IGF-1 also plays a central role in muscle development and growth by regulating protein turnover and muscle function, and previous in vitro studies suggest that IGF-1 and bFGF regulate muscle regeneration, by acting on the proliferation and the differentiation of myogenic precursor cells.
NGF has an essential role in proliferation, differentiation, and survival of sensory and sympathetic sensory of the damaged skin. NGF also promotes the proliferation of local immune cells in wounds, blood vessel and even neurite outgrowth, thereby accelerating the rate of wound healing.
These results strongly suggest that AMPLEX PLUS could enhance PRP, in addition to its already well-known functions, also by making it a more valuable tool for future therapies of common disorders such as hair diseases (scarring alopecia, baldness), muscular diseases (hypotrophy and muscle hypotonia), and skin diseases (different types of wounds, including ulcers).
Author Contributions
Conceptualization, M.V.B.; methodology, M.V.B.; software, G.F., M.C. and M.Z.; validation, G.F. and M.C.; formal analysis, G.F., M.C. and M.Z.; investigation, G.F. and M.C.; resources, G.F. and M.C.; data curation, G.F. and M.C.; writing—original draft preparation, M.V.B.; writing—review and editing, G.F. and M.C.; visualization, G.F. and M.C.; supervision, M.V.B.; funding acquisition, M.V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was performed in line with the principles of the Declaration of Helsinki and does not require approval by the Ethics Committee of University of Catania.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
G.F. thanks PhD program FSE Notice 1/2021. Authors acknowledge the PON project Bio-nanotech Research and Innovation Tower (BRIT), financed by the Italian Ministry for Education, University and Research (MIUR) (Grant no. PONa3_00136).
Conflicts of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing - A literature review. An Bras Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Platelet-derived growth factor. Ann. Rev. Med. 1987, 38, 71–9. [Google Scholar] [CrossRef] [PubMed]
- Misso, S.; D’Onofrio, M.; Paesano, L. Our experience in the treatment of refractory ulcers with platelet gel. Blood Transfus. 2006, 4, 196–205. [Google Scholar]
- Uhl, E.; Barker, J.H.; Bondar, I. Basic fibroblast growth factor accelerates wound healing in chronically ischaemic tissue. Br J. Surg. 1993, 80, 977–80. [Google Scholar] [CrossRef] [PubMed]
- Bhora, F.Y.; Dunkin, B.J.; Batzri, S. Effect of growth factors on cell proliferation and epithelialization in human skin. J. Surg. Res. 1995, 59, 236–44. [Google Scholar] [CrossRef]
- Grimaud, E.; Heymann, D.; Redini, F. Recent advances in TGF-beta effects on chondrocytemetabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev. 2002, 13, 241–57. [Google Scholar] [CrossRef]
- Katsura, T.; Tohyama, H.; Kondo, E. Effects of administration of transforming growth factor (TGF)-beta1 and anti-TGF-beta1 antibody on the mechanical properties of the stressshielded patellar tendon. J Biomech. 2006, 39, 2566–72. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Tan, Y.; Peng, Q.; Zuo, J.; Li, N. Novel applications of platelet concentrates in tissue regeneration (Review). Exp Ther Med. 2021, 21, 226. [Google Scholar] [CrossRef]
- Anitua, E.; Sanchez, M.; Nurden, A.T. Autologous fibrin matrices: a potential source of biological mediators that modulate tendon cell activities. J Biomed Mater Res A. 2006, 77, 285–93. [Google Scholar] [CrossRef]
- Yuan, T.; Zhang, C.Q.; Tang, M.J.; Guo, S.C.; Zeng, B.F. Autologous Platelet-rich Plasma Enhances Healing of Chronic Wounds. Wounds. 2009, 21, 280–5. [Google Scholar] [PubMed]
- Tripathi, V.; Vashishtha, B. Bioactive compounds of colostrum and its application. Food Rev. Int. 2006, 22(3), 225–244. [Google Scholar] [CrossRef]
- Sacerdote, P.; Mussano, F.; Franchi, S.; Panerai, A.E.; Bussolati, G.; Carossa, S.; Bartorelli, A.; Bussolati, B. Biological components in a standardized derivative of bovine colostrum. J. Dairy Sci. 2013, 96(3), 1745–54. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Kim, H.; Kim, D.E.; Ahn, Y.; Kim, J.; Jang, Y.J.; Kim, K.; Yang, Y.; Kim, S.H. The Potential of Bovine Colostrum-Derived Exosomes to Repair Aged and Damaged Skin Cells. Pharmaceutics. 2022, 14(2), 307. [Google Scholar] [CrossRef]
- Samuel, M.; Chisanga, D.; Liem, M.; Keerthikumar, S.; Anand, S.; Ang, C.S.; Adda, C.G.; Versteegen, E.; Jois, M.; Mathivanan, S. Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci. Rep. 2017, 7, 5933. [Google Scholar] [CrossRef]
- Ross, M.; Atalla, H.; Karrow, N.; Mallard, B.A. The bioactivity of colostrum and milk exosomes of high, average, and low immune responder cows on human intestinal epithelial cells. J. Dairy Sci. 2021, 104(3), 2499–2510. [Google Scholar] [CrossRef]
- Hiraki, Y.; Inoue, H.; Kato, Y. Combined effects of somatomedin-like growth factors with fibroblast growth factor or epidermal growth factor in DNA synthesis in rabbit chondrocytes. Mol Cell Biochem 1987, 76, 185–193. [Google Scholar] [CrossRef]
- Imler, S.M.; Doshi, A.N.; Levenston, M.E. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarth. Cartil 2004, 12, 736–44. [Google Scholar] [CrossRef]
- Sathish, M.; Anand, K.; Karthik, R.R. Standardization and validation of a conventional high yield platelet-rich plasma preparation protocol. Ann. Med. Surg. 2022, 82, 104593. [Google Scholar] [CrossRef]
- Zimbone, M.; Musumeci, P.; Baeri, P.; Messina, E.; Boninelli, S.; Compagnini, G.; Calcagno, L. Rotational dynamics of gold nanoparticle chains in water solution. J. Nanopart. Res. 2012, 14(12), 1308. [Google Scholar] [CrossRef]
- Zimbone, M.; Baeri, P.; Calcagno, L.; Musumeci, P.; Contino, A.; Barcellona, M.L.; Bonaventura, G. Dynamic light scattering on bioconjugated laser generated gold nanoparticles. PLoS One 2014, 9(3), e89048. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjuna, P.; Zhou, Y.; Landström, M. The Synergistic Cooperation between TGF-β and Hypoxia in Cancer and Fibrosis. Biomolecules 2022, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Troncone, E.; Marafini, I.; Monteleone, G. Role of TGF-Beta and Smad7 in Gut Inflammation, Fibrosis and Cancer. Biomolecules 2021, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Biello, F.; Platini, F.; D’Avanzo, F.; Cattrini, C.; Mennitto, A.; Genestroni, S.; Martini, V.; Marzullo, P.; Aimaretti, G.; Gennari, A. Insulin/IGF Axis in Breast Cancer: Clinical Evidence and Translational Insights. Biomolecules 2021, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Garoufalia, Z.; Papadopetraki, A.; Karatza, E.; Vardakostas, D.; Philippou, A.; Kouraklis, G.; Mantas, D. Insulin-like growth factor-I and wound healing, a potential answer to non-healing wounds: A systematic review of the literature and future perspectives. Biomed Rep 2021, 15, 1–5. [Google Scholar] [CrossRef]
- Erel-Akbaba, G.; Akbaba, H.; Keselik, E.; Bahceci, S.A.; Senyigit, Z.; Temiz, T.K. Octaarginine functionalized nanoencapsulated system: In vitro and in vivo evaluation of bFGF loaded formulation for wound healing. J. Drug Deliv. Sci. Technol. 2022, 71, 103343. [Google Scholar] [CrossRef]
- Bian, D.; Wu, Y.; Song, G. bFGF combined with extracellular matrix-inspired mimetic systems for effective skin regeneration and wound healing. Mater. Today Commun. 2023, 105876. [Google Scholar] [CrossRef]
- Wiszniak, S.; Schwarz, Q. Exploring the Intracrine Functions of VEGF-A. Biomolecules 2021, 11, 128. [Google Scholar] [CrossRef]
- White, A.L.; Bix, G.J. VEGFA Isoforms as Pro-Angiogenic Therapeutics for Cerebrovascular Diseases. Biomolecules 2023, 13, 702. [Google Scholar] [CrossRef]
- Shakhakarmi, K.; Seo, J.E.; Lamichhane, S.; Thapa, C.; Lee, S. EGF, a veteran of wound healing: highlights on its mode of action, clinical applications with focus on wound treatment, and recent drug delivery strategies. Arch. Pharm. Res. 2023, 46, 299–322. [Google Scholar] [CrossRef]
- Sotoyama, H.; Namba, H.; Tohmi, M.; Nawa, H. Schizophrenia Animal Modeling with Epidermal Growth Factor and Its Homologs: Their Connections to the Inflammatory Pathway and the Dopamine System. Biomolecules 2023, 13, 372. [Google Scholar] [CrossRef] [PubMed]
- Deptuła, M.; Karpowicz, P.; Wardowska, A.; Sass, P.; Sosnowski, P.; Mieczkowska, A.; Pikuła, M. Development of a peptide derived from platelet-derived growth factor (PDGF-BB) into a potential drug candidate for the treatment of wounds. Adv. in Wound Care 2020, 9, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, D.; Qin, X.; Mi, L.Z. PDGF-D Prodomain Differentially Inhibits the Biological Activities of PDGF-D and PDGF-B. J. Mol. Biol. 2022, 434, 167709. [Google Scholar] [CrossRef] [PubMed]
- Xiaojie, W.; Banda, J.; Qi, H.; Chang, A.K.; Bwalya, C.; Chao, L.; Li, X. Scarless wound healing: Current insights from the perspectives of TGF-β, KGF-1, and KGF-2. Cytokine & growth factor Rev. 2022, 66, 26–37. [Google Scholar]
- Seitz, T.; Hellerbrand, C. Role of fibroblast growth factor signalling in hepatic fibrosis. Liver Int. 2021, 41, 1201–1215. [Google Scholar] [CrossRef]
- Grundy, M.; Narendran, A. The hepatocyte growth factor/mesenchymal epithelial transition factor axis in high-risk pediatric solid tumors and the anti-tumor activity of targeted therapeutic agents. Front Pediatr 2022, 10. [Google Scholar] [CrossRef]
- Sanada, F.; Fujikawa, T.; Shibata, K.; Taniyama, Y.; Rakugi, H.; Morishita, R. Therapeutic angiogenesis using HGF plasmid. Ann. Vasc. Dis. 2020, 13, 109–115. [Google Scholar] [CrossRef]
- van Riet, S.; van Schadewijk, A.; De Vos, S.; Vandeghinste, N.; Rottier, R. J.; Stolk, J.; Khedoe, P. Modulation of airway epithelial innate immunity and wound repair by M (GM-CSF) and M (M-CSF) macrophages. J. Innate Immun. 2020, 12, 410–421. [Google Scholar] [CrossRef]
- Ead, J.K.; Armstrong, D.G. Granulocyte-macrophage colony-stimulating factor: Conductor of the wound healing orchestra? Int Wound J 2023, 20, 1229–1234. [Google Scholar] [CrossRef]
- Mehta, H.M.; Corey, S.J. G-CSF, the guardian of granulopoiesis. Semin. Immunol. 2021, 54, 101515. [Google Scholar] [CrossRef]
- Link, H. Current state and future opportunities in granulocyte colony-stimulating factor (G-CSF). Support. Care Cancer 2022, 30, 7067–7077. [Google Scholar] [CrossRef] [PubMed]
- Bacci, S. Fine regulation during wound healing by mast cells, a physiological role not yet clarified. Int. J. Mol. Sci. 2022, 23, 1820. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Saligan, L.N. Wound pain and wound healing biomarkers from wound exudate: a scoping review. J. Wound Ostomy Cont. Nurs. 2020, 47, 559–568. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Dong, M.; Li, Y.; Zhou, Q.; Yang, L. The proinflammatory cytokines IL-1β and TNF-α modulate corneal epithelial wound healing through p16Ink4a suppressing STAT3 activity. J. Cell. Physiol. 2020, 235, 10081–10093. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, T.; Wang, S.; He, W.; Qian, W.; Luo, G. Effects of IL-1β and TNF-α on the Expression of P311 in Vascular Endothelial Cells and Wound Healing in Mice. Front. Physiol. 2020, 11, 545008. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, H.; Huang, S. Role of NGF and its receptors in wound healing. Exp. Ther. Med. 2021, 21, 1–9. [Google Scholar] [CrossRef]
- El Baassiri, M.; Dosh, L.; Haidar, H.; Gerges, A.; Baassiri, S.; Leone, A.; Jurjus, A. Nerve growth factor and burn wound healing: Update of molecular interactions with skin cells. Burns 2022. [Google Scholar] [CrossRef]
- Aoyama, S.; Nakagawa, R.; Nemoto, S.; Villarroel, P.P.; Mulé, J.; Mailloux, A. 226 Checkpoint blockade hastens a switch from an NKT dominant, TNF-alpha-driven to a CD4+/CD8+ IFN-gamma-driven immune response within MC-38 tumor-infiltrating lymphocytes. J. ImmunoTher. Cancer 2020, 8. [Google Scholar]
- Tran, T.D.X.; Pham, V.Q.; Tran, N.N.T.; Dang, H.C.N.; Tran, N.T.A.; Vu, N.B.; Van Pham, P. Stromal Vascular Fraction and Mesenchymal Stem Cells from Human Adipose Tissue: A Comparison of Immune Modulation and Angiogenic Potential. In Advances in Experimental Medicine and Biology; Springer: Cham, 2022. [Google Scholar]
- Wytrwal, M.; Sekuła-Stryjewska, M.; Pomorska, A.; Oclon, E.; Zuba-Surma, E.; Zapotoczny, S.; Szczubiałka, K. Cellular Response to Bone Morphogenetic Proteins-2 and -7 Covalently Bound to Photocrosslinked Heparin–Diazoresin Multilayer. Biomolecules 2023, 13, 842. [Google Scholar] [CrossRef]
- Shen, M.; Wang, L.; Feng, L.; Xu, C.; Gao, Y.; Li, S.; Pei, G. Cefazolin/BMP-2-Loaded Mesoporous Silica Nanoparticles for the Repair of Open Fractures with Bone Defects. Oxid. Med. Cel. Longev. 2022. [Google Scholar] [CrossRef]
- Yeboah, A.; Cohen, R.I.; Faulknor, R.; Schloss, R.; Yarmush, M.L.; Berthiaume, F. The development and characterization of SDF1α-elastin-like-peptide nanoparticles for wound healing. J Control release 2016, 232, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.H.; Chen, K.Y.; Cheng, M.H.; Chen, Y.S.; Huang, C.H. Effect of genipin crosslinked chitosan scaffolds containing SDF-1 on wound healing in a rat model. Mater. Sci. Eng. C 2020, 109, 110368. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Trotta, E.; R. Simeonov, D.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3, 1482. [Google Scholar] [CrossRef]
- Damoiseaux, J. The IL-2–IL-2 receptor pathway in health and disease: The role of the soluble IL-2 receptor. Clini. Immunol. 2020, 218, 108515. [Google Scholar] [CrossRef]
- Woytschak, J.; Keller, N.; Krieg, C.; Impellizzieri, D.; Thompson, R.W.; Wynn, T.A.; Boyman, O. Type 2 interleukin-4 receptor signaling in neutrophils antagonizes their expansion and migration during infection and inflammation. Immunity 2016, 45, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhang, Z.; Yan, J.; Wang, Y.; Hu, Z.; Mitch, W.E.; Wang, Y. The IL-4 receptor α has a critical role in bone marrow–derived fibroblast activation and renal fibrosis. Kidney Int. 2017, 92, 1433–1443. [Google Scholar] [CrossRef]
- Forcina, L.; Miano, C.; Scicchitano, B.M.; Musarò, A. Signals from the Niche: Insights into the Role of IGF-1 and IL-6 in Modulating Skeletal Muscle Fibrosis. Cells 2019, 8, 232. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Y.; Lu, Y.; Du, P.; Li, X.; Wang, C.; Lu, G. Mesenchymal stromal cells pretreated with proinflammatory cytokines enhance skin wound healing via IL-6-dependent M2 polarization. Stem Cell Res. Ther. 2022, 13, 414. [Google Scholar] [CrossRef]
- Foti, M.; Locati, M. Cytokine Effector Functions in Tissues, 1st ed.; Academic Press: London, United Kingdom, 2017; pp. 4–21. [Google Scholar]
- Tang, M.; Lingyun, L.; Xijie, Y. Interleukin-17A interweaves the skeletal and immune systems. Front. Immunol. 2021, 11, 625034. [Google Scholar] [CrossRef]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E. M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Goldman, R. Growth factors and chronic wound healing: past, present, and future. Adv skin wound care 2004, 17, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Grazul-Bilska, A.T.; Johnson, M.L.; Bilski, J.J.; Redmer, D.A.; Reynolds, L.P.; Abdullah, A.; Abdullah, K.M. Wound healing: the role of growth factors. Drugs Today (Barc) 2003, 39, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Benoy, I.; Bogers, J.; Weytjens, R.; Vermeulen, P.; Dirix, L.; Van Marck, E. Platelets and vascular endothelial growth factor (VEGF): a morphological and functional study. Angiogenesis 2001, 4, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Woodell, J.E.; Higgins, J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast. Reconst. Surg. 2004, 114, 1502–1508. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; Lana, J.F. The regenerative mechanisms of platelet-rich plasma: A review. Cytokine 2021, 144, 155560. [Google Scholar] [CrossRef]
- Margolis, D.J.; Kantor, K.; Santanna, J.; Strom, B.L.; Berlin, J.A. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care. 2001, 3, 483–488. [Google Scholar] [CrossRef]
- Krupski, W.C.; Reilly, L.M.; Perez, S.; Moss, K.M.; Crombleholme, P.A.; Rapp, J.H. A prospective randomized trial of autologous platelet-derived wound healing factors for treatment of chronic nonhealing wounds: a preliminary report. J Vasc Surg. 1991, 14, 526–532. [Google Scholar] [CrossRef]
- Tatsis, D.; Vasalou, V.; Kotidis, E.; Anestiadou, E.; Grivas, I.; Cheva, A.; Koliakos, G.; Venetis, G.; Pramateftakis, M.G.; Ouzounidis, N.; Angelopoulos, S. The Combined Use of Platelet-Rich Plasma and Adipose-Derived Mesenchymal Stem Cells Promotes Healing. A Review of Experimental Models and Future Perspectives. Biomolecules 2021, 11, 1403. [Google Scholar] [CrossRef]
- Chen, J.; Wan, Y.; Lin, Y.; Jiang, H. Current art of combination therapy with autologous platelet-rich plasma for stable vitiligo: A meta-analysis. Int Wound J. 2021, 18, 251–260. [Google Scholar] [CrossRef]
- Straum, O.K. The optimal platelet concentration in platelet-rich plasma for proliferation of human cells in vitro-diversity, biases, and possible basic experimental principles for further research in the field: A review. PeerJ 2020, 8, 10303. [Google Scholar] [CrossRef]
- Cheng, P.G.; Yang, K.D.; Huang, L.G.; Wang, C.H.; Ko, W.S. Comparisons of Cytokines, Growth Factors and Clinical Efficacy between Platelet-Rich Plasma and Autologous Conditioned Serum for Knee Osteoarthritis Management. Biomolecules 2023, 13, 555. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Pino, A.; Azkargorta, M.; Elortza, F.; Prado, R. High-Throughput Proteomic Analysis of Human Dermal Fibroblast Response to Different Blood Derivatives: Autologous Topical Serum Derived from Plasma Rich in Growth Factors (PRGF) versus Leukocyte- and Platelet-Rich Plasma (L-PRP). Biomolecules 2022, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).