Submitted:
04 July 2023
Posted:
19 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
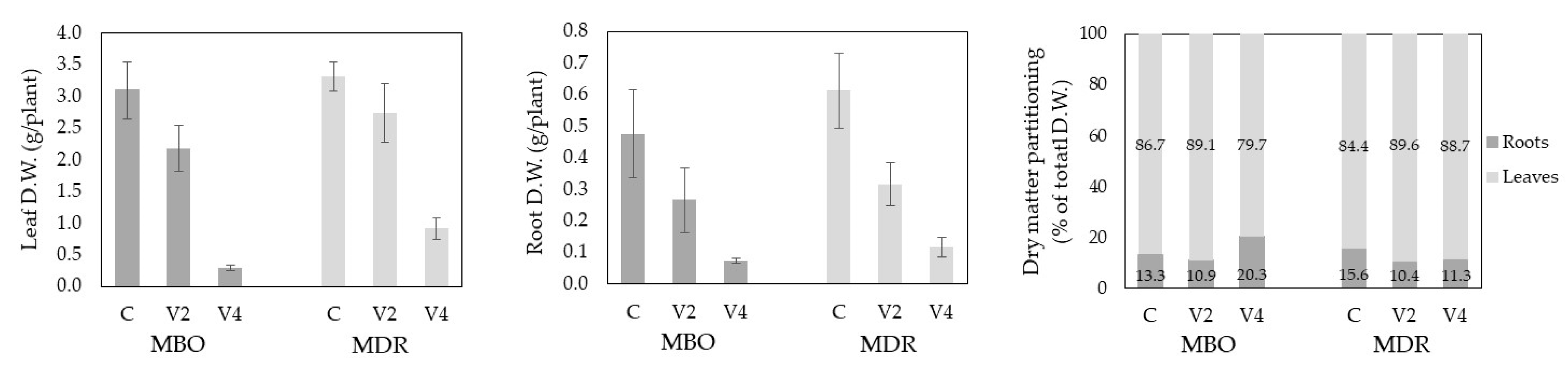
2.1. Plant Growth and Flowering
2.2. Tuberous roots’ Metabolic Profile
2.3. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
- -
- only rehydration: exposure to 12 °C for 24 h in humid chamber (Control, C);
- -
- rehydration followed by vernalization at 3.5 °C for 2 weeks (V2);
- -
- rehydration followed by vernalization at 3.5 °C for 4 weeks (V4).
4.1. Plant Growth and Flowering
4.2. Metabolic Profile
4.2.1. Starch and Soluble Carbohydrate Analysis
4.2.2. Soluble Proteins, Free Amino Acid Analysis
4.2.3. Polyphenols Analysis
5. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Beruto, M.; Rabaglio, M.; Viglione, S.; Van Labeke, M.-C.; Dhooghe, E. Ranunculus. In Ornamental Crops; Van Huylenbroeck, J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 649–671. [Google Scholar] [CrossRef]
- Le Nard, M. and De Hertogh, A. In The physiology of flower bulbs, Chapter 4 in Bulb Growth and Development and Flowering; De Hertogh, A.A.; Le Nard, M., Eds.; Elsevier: Amsterdam, NL, USA, 1993.
- Meynet, J. Ranunculus. In The physiology of Flower Bulbs: A Comprehensive Treatise on the Physiology and Utilization of Ornamental Flowering Bulbous and Tuberous Plants; De Hertogh, A.A., Le Nard, M., Eds.; Elsevier: Amsterdam, NL, USA. 1993; pp. 603–610. [Google Scholar]
- Kamenetsky, R. Production of Flower Bulbs in Regions with Warm Climates; Okubo, H., Miller, W.B., Chastagner, G.A., Eds.; International Society for Horticultural Science: Niigata, Japan, 2005; Volume 2, pp. 59–66. [Google Scholar]
- Beruto, M.; Fibiani, M.; Rinino, S.; Scalzo, R.L.; Curir, P. Plant development of Ranunculus asiaticus L. tuberous roots is affected by different temperature and oxygen conditions during storage period. Isr. J. Plant Sci. 2009, 57, 377–388. [Google Scholar] [CrossRef]
- Glier, J.H.; Caruso, J.L. Low-temperature induction of starch degradation in roots of a biennial weed. Cryobiology 1973, 10, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Su, X.; Tian, Z.; Liu, Y.; Zhou, Y.; He, M. Transcriptome profiling provides insights into dormancy release during cold storage of Lilium pumilum. BMC genomics. 2018, 19(1), 1–17. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, H.; Wu, G.; Sun, J.; Hao, L.; Ge, X.; Yu, J.; Wang, W. Sequence variation and expression analysis of seed dormancy-and germination-associated ABA-and GA-related genes in rice cultivars. Frontiers in plant science. 2011, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.M. A review of plants’ flowering physiology: The control of floral induction by juvenility, temperature and photoperiod in annual and ornamental crops. Asian J. Agric. Food Sci. 2014, 2, 186–195. [Google Scholar]
- Zhao, L.; Li, S.; Yu, Q.; Zhang, C.; Wang, L.; Jiang, Y.; Wu, Z.; Pi, Z. Vernalization Promotes GA-Mediated Bolting Initiation via the Inhibition of ABA and JA Biosynthesis. Agronomy. 2023, 13(5), 1251. [Google Scholar] [CrossRef]
- Carillo, P.; Arena, C.; Modarelli, G.C.; De Pascale, S.; Paradiso, R. Photosynthesis in Ranunculus asiaticus L.: the influence of the hybrid and the preparation procedure of tuberous roots. Front. Plant Sci. 2019, 10, 241. [Google Scholar] [CrossRef]
- Fusco, G.M.; Carillo, P.; Nicastro, R.; Modarelli, G.C.; Arena, C.; De Pascale, S.; Paradiso, R. Vernalization Procedure of Tuberous Roots Affects Growth, Photosynthesis and Metabolic Profile of Ranunculus asiaticus L. Plants. 2023, 12(3), 425. [Google Scholar] [CrossRef]
- Fritz, C.; Palacios-Rojas, N.; Feil, R.; Stitt, M. Regulation of secondary metabolism by the carbon–nitrogen status in tobacco: nitrate inhibits large sectors of phenylpropanoid metabolism. The Plant Journal 2006, 46, 533–548. [Google Scholar] [CrossRef]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D'Amelia, L.; Dell'Aversana, E.; Piccolella, S.; Fuggi, A. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef]
- Carillo, P. GABA shunt in durum wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef]
- Carillo, P.; Dell’Aversana, E.; Modarelli, G.C.; Fusco, G.M.; De Pascale, S.; Paradiso, R. Metabolic profile and performance responses of Ranunculus asiaticus L. hybrids as affected by light quality of photoperiodic lighting. Frontiers in Plant Science. 2020, 11, 597823. [Google Scholar] [CrossRef] [PubMed]
- Ranwala, A.P.; Miller, W.B. Analysis of nonstructural carbohydrates in storage organs of 30 ornamental geophytes by high performance anion exchange chromatography with pulsed amperometric detection. New Phytol. 2008, 180, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Kamenetsky, R. Production of Flower Bulbs in Regions with Warm Climates; International Society for Horticultural Science, Eds.: H. Okubo, W.B. Miller and G.A. Chastagner, Niigata, Japan, 2005; Volume 2, pp. 59–66. [CrossRef]
- Modarelli, G.C.; Arena, C.; Pesce, G.; Dell'Aversana, E.; Fusco, G.M.; Carillo, P.; De Pascale, S.; Paradiso, R. The role of light quality of photoperiodic lighting on photosynthesis, flowering and metabolic profiling in Ranunculus asiaticus L. Physiologia plantarum, 2020, 170(2), 187-201. [CrossRef]
- Carillo, P.; Modarelli, G.C.; Fusco, G.M.; Dell’Aversana, E.; Arena, C.; De Pascale, S.; Paradiso, R. Light spectral composition affects metabolic response and flowering in non-vernalized Ranunculus asiaticus L. Environmental and Experimental Botany. 2021, 192, 104649. [Google Scholar] [CrossRef]
- Ohkawa, K. Growth and Flowering of Ranunculus Asiaticus; Doss, R.P., Byther, R.S., Chastagner, G.A., Eds.; Acta Horticolturae: Seattle, WA, USA, 1986; Volume 1, pp. 165–172. [CrossRef]
- Bouche, N. & Fromm, H. GABA in plants: just a metabolite? Trends in plant science, 2004, 9(3), 110-115. [CrossRef]
- Eom, S.H.; Ahn, M.A.; Kim, E.; Lee, H.J.; Lee, J.H.; Wi, S.H.; Kim, S.K.; Lim, J.H., Hyun, T.K. Plant response to cold Stress: Cold stress changes antioxidant metabolism in heading type Kimchi cabbage (Brassica rapa L. ssp. Pekinensis). Antioxidants, 2022, 11(4), 700. [CrossRef]
- Carillo, P.; Colla, G.; El-Nakhel, C.; Bonini, P.; D’Amelia, L.; Dell’Aversana, E.; Pannico, A.; Giordano, M.; Sifola, M.I.; Kyriacou, M.C.; De Pascale, S.; Rouphael, Y. Biostimulant application with a tropical plant extract enhances Corchorus olitorius adaptation to sub-optimal nutrient regimens by improving physiological parameters. Agronomy, 2019, 9(5), 249. [CrossRef]
- Van Oosten, M.J.; Dell’Aversana, E.; Ruggiero, A.; Cirillo, V.; Gibon, Y.; Woodrow, P.; Maggio, A.; Carillo, P. Omeprazole treatment enhances nitrogen use efficiency through increased nitrogen uptake and assimilation in corn. Frontiers in Plant Science, 2019, 10, 1507. [CrossRef]
- Liu, C.; Zhao, L.; Yu, G. The dominant glutamic acid metabolic flux to produce γ-amino butyric acid over proline in Nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. Journal of Integrative Plant Biology, 2011, 53(8), 608-618. [CrossRef]
- Molina-Rueda, J.J.; Pascual, M.B.; Pissarra, J.; Gallardo, F. A putative role for γ-aminobutyric acid (GABA) in vascular development in pine seedlings. Planta, 2015, 241, 257–267. [Google Scholar] [CrossRef]
- Carillo, P.; Mastrolonardo, G.; Nacca, F.; Parisi, D.; Verlotta, A.; Fuggi, A. Nitrogen metabolism in durum wheat under salinity: accumulation of proline and glycine betaine. Functional Plant Biology, 2008, 35(5), 412-426. [CrossRef]
- Zhou, Z.; Xu, Z.; Feng, Q.; Yao, D.; Yu, J.; Wang, D.; Lv, S.; Liu, Y.; Zhou, N.; Zhong, M.E. Effect of pyrolysis condition on the adsorption mechanism of lead, cadmium and copper on tobacco stem biochar. Journal of Cleaner Production, 2018, 187, 996–1005. [Google Scholar] [CrossRef]
- Pollock, C.J. and Lloyd, E.J. The effect of low temperature upon starch, sucrose and fructan synthesis in leaves. Ann. Bot. 1987, 60: 231-235. [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, M.A.S.; Babar, M. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Jouve, L.; Hoffmann, L.; Hausman, J.F. Polyamine, carbohydrate, and proline content changes during salt stress exposure of aspen (Populus tremula L.): involvement of oxidation and osmoregulation metabolism. Plant Biol. 2004, 6: 74-80. [CrossRef]
- Salbitani, G.; Carillo, P.; Di Martino, C.; Bolinesi, F.; Mangoni, O.; Loreto, F.; Carfagna, S. Microalgae cross-fertilization: Shortterm effects of Galdieria phlegrea extract on growth, photosynthesis and enzyme activity of Chlorella sorokiniana cells. J. Appl. Phycol. 2022, 34, 1957–1966. [Google Scholar] [CrossRef]
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Carillo, P.; Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Dell’Aversana, E.; D’Amelia, L.; Colla, G.; Caruso, G.; De Pascale, S.; Rouphael, Y. Sensory and functional quality characterization of protected designation of origin ‘Piennolo del Vesuvio’ cherry tomato landraces from Campania-Italy. Food Chem. 2019, 292, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; Kyriacaou M.C.; Cardarelli, M.; Rouphael, Y. Morphological and physiological responses induced by protein hydrolysate-based biostimulant and nitrogen rates in greenhouse spinach. Agronomy, 2019, 9. [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R. M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]

| MDR | MBO | H | V | H x V | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | V2 | V4 | Mean | C | V2 | V4 | Mean | ||||
| Pre-Germinative phase | |||||||||||
| Polyphenols | 4.05 ab | 3.30 a | 4.28 b | 3.88 | 3.67 ab | 3.61 a | 3.92 b | 3.73 | ns | * | ns |
| Glucose | 24.96 ab | 24.01 bc | 27.52 a | 25.5 A | 22.95 bd | 21.22 cd | 19.94 d | 21.37 B | *** | ns | ** |
| Fructose | 23.50 | 20.94 | 23.36 | 22.60 | 21.69 | 21.76 | 21.09 | 21.51 | ns | ns | ns |
| Sucrose | 8.78 | 8.35 | 8.76 | 8.63 A | 8.41 | 8.13 | 8.30 | 8.28 B | * | ns | ns |
| Starch | 41.05 a | 38.38 a | 39.21 a | 39.55 B | 54.14 bc | 59.82 b | 44.61 ac | 52.86 A | *** | ns | ** |
| Vegetative phase | |||||||||||
| Polyphenols | 13.97 a | 16.00 b | 7.94 cd | 12.64 A | 7.64 cd | 11.21 c | 5.16 d | 8.01 B | * | ** | ** |
| Glucose | 20.76 a | 21.47 a | 10.25 b | 17.49 | 18.97 a | 8.80 b | 22.47 a | 16.75 | ns | ns | *** |
| Fructose | 7.45 a | 6.17 a | 11.58 b | 8.40 | 6.62 a | 2.27 c | 22.50 d | 10.46 | ns | ** | *** |
| Sucrose | 2.86 a | 2.15 a | 4.43 b | 3.15 | 2.28 a | 0.52 c | 8.46 d | 3.76 | ns | ** | *** |
| Starch | 27.18 ab | 28.64 ab | 89.43 a | 48.41 | 27.42 ab | 20.95 b | 35.46 a | 27.94 | ns | ** | * |
| Flowering phase | |||||||||||
| Polyphenols | 5.85 a | 12.85 b | 11.69 b | 10.13 A | 10.82 b | 4.77 ac | 2.45 c | 6.01 | * | ns | *** |
| Glucose | 35.63 a | 31.04 a | 18.59 bc | 28.42 A | 15.26 c | 23.58 b | 1.08 d | 13.31 B | ** | * | *** |
| Fructose | 13.49 a | 13.89 a | 12.72 a | 13.36 | 10.63 a | 13.13 a | 0.42 b | 8.06 | ns | ns | *** |
| Sucrose | 5.79 a | 6.15 a | 4.78 a | 5.57 A | 2.12 b | 3.07 b | 0.09 c | 1.76 B | *** | ns | *** |
| Starch | 40.24 acd | 49.75 ab | 25.28 c | 38.42 B | 47.61 bd | 60.79 b | 52.83 b | 53.75 A | * | ns | * |
| MDR | MBO | H | V | H x V | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | V2 | V4 | Mean | C | V2 | V4 | Mean | ||||
| Pre-Germinative phase | |||||||||||
| Soluble proteins | 36.49 | 32.6 | 35.78 | 34.96 | 42.33 | 33.45 | 41.56 | 39.11 | ns | ns | ns |
| Alanine | 4.29 a | 20.9 b | 38.87 c | 21.35 | 8.73 a | 29.39 b | 43.99 c | 27.37 | ns | *** | ** |
| Asparagine | 59.72 ac | 65.39 ac | 156.8 b | 83.97 | 32.24 a | 50.37 c | 88.12 c | 56.91 | ns | *** | ** |
| Aspartate | 2.34 ab | 1.64 ac | 5.72 b | 3.23 | 2.39 bc | 0.63 d | 2.22 b | 1.75 | ns | ** | * |
| GABA | 2.42 a | 16.28 b | 32.59 c | 17.10 | 6.63 a | 29.72 c | 65.57 d | 33.97 | ns | *** | *** |
| Glutamine | 67.21 a | 39.05 a | 105.8 b | 70.69 | 51.56 a | 60.59 a | 129.5 b | 80.55 | ns | *** | *** |
| Glutamate | 20.92 a | 31.17 abd | 54.86 c | 35.65 | 23.95 a | 42.79 cd | 46.53 bc | 37.76 | ns | *** | * |
| Glycine | 2.18 a | 3.47 ab | 7.31 c | 4.32 | 1.6 a | 4.8 b | 11.05 d | 5.82 | ns | *** | *** |
| MEA | 3.43 a | 3.07 a | 6.55 b | 4.35 | 2.62 a | 5.76 b | 8.55 c | 5.64 | ns | *** | *** |
| Ornithine | 0.9 a | 1.18 ab | 2.32 c | 1.47 | 0.96 a | 1.67 b | 2.8 c | 1.81 | ns | *** | *** |
| Proline | 5.59 a | 9.97 b | 11.36 b | 8.97 | 6.77 a | 6.83 a | 13.84 d | 9.15 | ns | *** | *** |
| Serine | 5.03 a | 7.23 a | 13.63 b | 8.63 B | 7.44 a | 14.13 b | 19.43 c | 13.67 A | * | *** | *** |
| Threonine | 8.92 a | 7.13 a | 20.44 b | 12.16 | 6.77 a | 9.00 a | 19.46 b | 11.74 | ns | *** | *** |
| Total AA | 228.5 a | 258.2 a | 564 b | 350.20 | 196.4 a | 324.8 a | 598.6 b | 373.30 | ns | *** | *** |
| Minor AA | 45.61 a | 51.75 a | 107.7 b | 68.35 | 44.73 a | 69.14 a | 147.5 c | 87.12 | ns | * | *** |
| BCAAs | 17.68 a | 22.88 a | 53.25 b | 31.27 | 18.19 a | 30.74 ab | 71.18 c | 40.04 | ns | *** | ** |
| Vegetative phase | |||||||||||
| Soluble Proteins | 36.27 a | 38.44 a | 53.03 b | 45.58 A | 20.11 c | 39.21 a | 23.15 c | 27.49 B | * | ns | *** |
| Alanine | 4.81 ab | 5.77 a | 1.53 c | 4.04 | 4.20 bc | 2.20 c | 3.00 c | 3.13 | ns | * | *** |
| Asparagine | 687.1 a | 175.2 bc | 157.8 bc | 340.0 | 221 b | 208.8 b | 69.41 c | 166.4 | ns | ** | *** |
| Aspartate | 5.34 ab | 7.57 a | 5.96 ac | 6.29 | 5.99 ac | 3.28 d | 4.95 bc | 4.74 | ns | ns | * |
| GABA | 5.53 a | 1.81 b | 0.64 c | 2.66 | 2.83 d | 1.86 b | 2.62 d | 2.44 | ns | * | *** |
| Glutamine | 87.61 a | 67.04 ab | 34.04 c | 62.90 | 64.00 b | 56.4 b | 26.26 c | 48.89 | ns | ** | *** |
| Glutamate | 48.11 a | 18.13 bd | 31.23 c | 32.49 A | 24.89 bc | 14.95 d | 29.69 b | 23.18 B | * | * | *** |
| Glycine | 1.25 a | 1.2 a | 0.54 b | 1 B | 2.03 c | 1.95 c | 1.14 a | 1.71 A | ** | * | *** |
| MEA | 16.12 a | 15.9 a | 7.01 b | 13.01 A | 15.67 a | 5.93 b | 4.58 b | 8.73 B | * | * | *** |
| Ornithine | 2.9 a | 2.85 a | 2.01 b | 2.59 | 3.37 a | 1.81 b | 1.65 b | 2.28 | ns | ** | *** |
| Proline | 5.97 a | 5.84 a | 2.53 b | 4.78 | 3.35 b | 3.44 b | 6.04 a | 4.28 | ns | ns | *** |
| Serine | 4.83 a | 2.63 b | 0.93 b | 2.80 | 5.74 a | 2.21 b | 2.67 b | 3.54 | ns | *** | * |
| Threonine | 12.17 a | 2.12 b | 8.64 c | 7.64 | 9.71 ac | 1.66 d | 1.8 bd | 4.39 | ns | ** | *** |
| Total AA | 922.2 a | 325.4 bc | 267.7 cd | 505.10 | 410.6 b | 314.5 c | 158.9 d | 294.70 | ns | ** | *** |
| Minor AA | 40.43 a | 19.35 b | 14.77 bd | 24.85 | 47.77 c | 10.01 d | 15.12 bd | 24.30 | ns | *** | *** |
| BCAAs | 11.63 a | 6.93 b | 4.16 c | 7.57 | 13.04 a | 4.3 c | 6.25 bc | 7.86 | ns | *** | *** |
| Flowering phase | |||||||||||
| Soluble Proteins | 5.85 a | 19.04 b | 31.94 c | 18.94 | 29.51 c | 20.97 b | 14.68 b | 21.72 | ns | ns | *** |
| Alanine | 6.91 a | 3.42 a | 5.06 a | 5.13 | 4.04 a | 12.01 b | 0.51 c | 5.52 | ns | ns | *** |
| Asparagine | 108.3 ab | 80.17 bc | 120.4 a | 103 A | 76.37 bc | 56.57 c | 0.02 d | 44.32 B | ** | ns | *** |
| Aspartate | 6.09 a | 3.64 bc | 5.27 ab | 5.00 | 5.22 ac | 6.33 a | 0.08 d | 3.88 | ns | ns | *** |
| GABA | 8.76 a | 2.31 bc | 3.72 bc | 4.93 | 3.93 b | 12.36 d | 0.26 c | 5.52 | ns | ns | *** |
| Glutamine | 38.63 a | 36.47 a | 37.23 a | 37.44 | 81.61 b | 79.08 b | 0.07 c | 53.59 | ns | * | *** |
| Glutamate | 44.66 a | 17.2 b | 17.79 b | 26.55 | 29.58 c | 20.12 bc | 2.86 d | 17.52 | ns | *** | *** |
| Glycine | 1.79 a | 1.06 b | 2.44 a | 1.76 | 1.94 a | 1.00 b | 0.34 b | 1.09 | ns | ns | *** |
| MEA | 7.66 a | 3.22 b | 0.25 a | 5.63 A | 2.97 b | 3.36 b | 2.27 b | 2.87 B | ** | ns | * |
| Ornithine | 1.06 a | 1.02 a | 0.54 b | 0.87 | 0.87 ab | 1.81 c | 0.44 b | 1.04 | ns | ** | *** |
| Proline | 6.66 a | 3.63 ab | 12.07 c | 7.45 | 3.95 ab | 11.38 c | 2.20 b | 5.84 | ns | ns | *** |
| Serine | 3.44 a | 3.1 a | 3.74 a | 3.43 | 3.45 a | 3.91 a | 0.14 b | 2.50 | ns | ns | *** |
| Threonine | 2.83 a | 2.81 a | 3.97 ab | 3.20 | 2.91 a | 4.40 b | 0.08 b | 2.46 | ns | ns | *** |
| Total AA | 256 a | 173.9 a | 231.1 a | 220.30 | 233.5 a | 231.6 a | 12.03 b | 159 | ns | ns | * |
| Minor AA | 19.18 a | 15.82 ab | 12.9 bc | 15.97 | 16.72 a | 19.27 a | 2 ac | 12.66 | ns | ** | *** |
| BCAAs | 8.22 a | 6.02 a | 7.69 a | 7.31 A | 5.75 a | 6.83 a | 1.27 b | 4.62 B | * | ns | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





