2.1. Chemical composition of Euterpe oleracea Martius oils; Abelmoschus esculentus L. Moench.
The ingestion of lipids in the human diet triggers an increase in free fatty acids that can raise cholesterol levels, which contributes to the process of dyslipidemia, being one of the main factors in the occurrence of cardiovascular diseases. The high concentration of saturated fatty acids is among the main lipids that favor hyperlipidemia, raise serum levels of triglycerides (TG), total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-c), being responsible for the development of atherosclerosis and chronic inflammation in blood vessels [
1,
29].
The oils obtained from plant species present variations in their chemical composition according to the degree of unsaturation of each species, they are rich in essential fatty acids free of trans fats or cholesterol and fat-soluble vitamins. The chemical composition of vegetable oils in relation to the presence of fatty acids can determine the use of these oils for various purposes such as food and/or therapy [
20].
Saturated fatty acids (SFA) are obtained in the diet, mainly from fats of animal and vegetable origin, and only have single bonds between the carbons. In turn, unsaturated fatty acids (UFA) can be obtained in the diet through vegetable and fish oils, they are classified as monounsaturated (MUFAs), with only one double bond, and polyunsaturated (PUFAs), containing two or more double bonds and belong to different series, being classified into ω-3, ω-6 and ω-9 [
30].
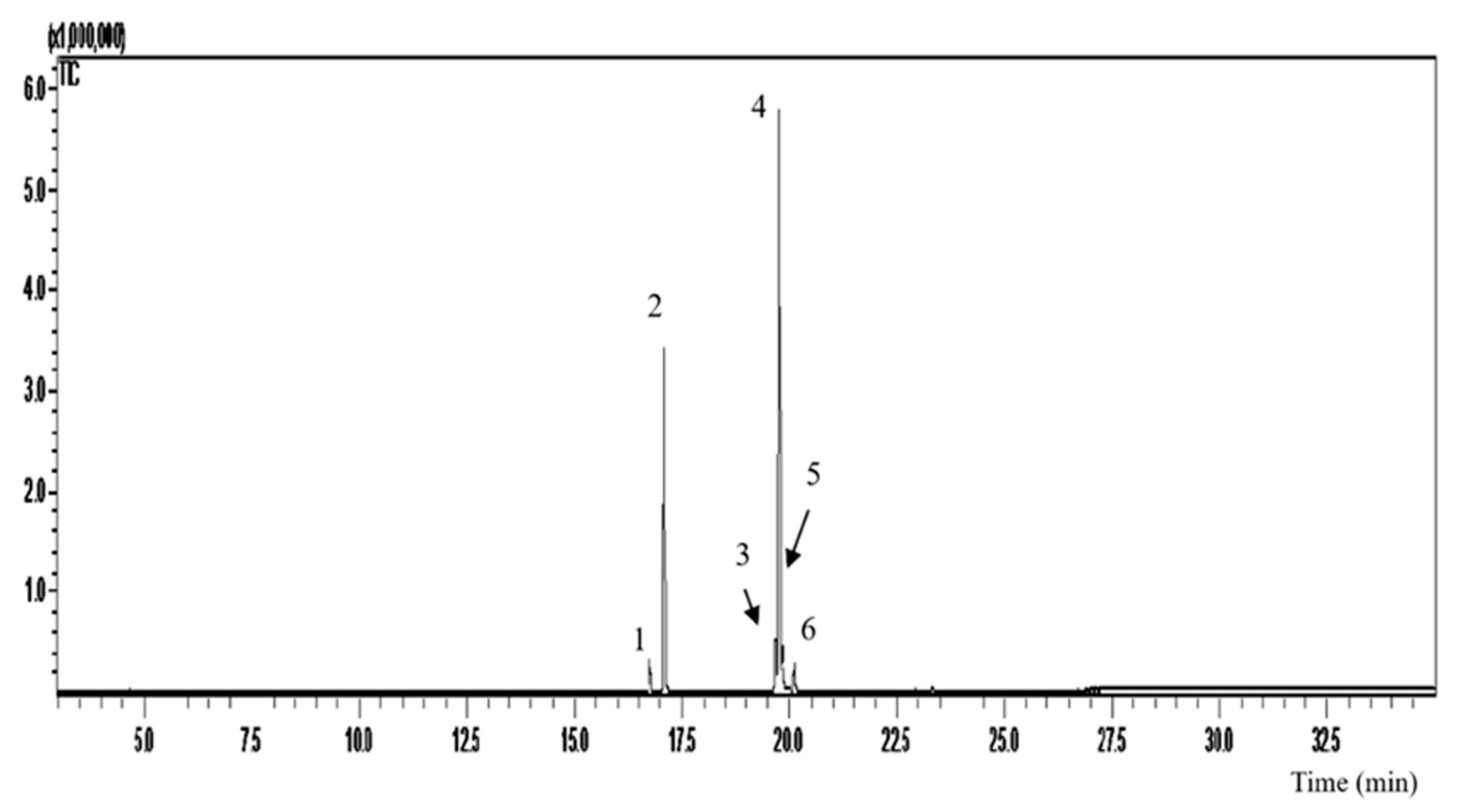
In the analyzes by gas chromatography coupled to mass spectrometry (GC-MS) we can identify six compound retention peaks (
Figure 1). According to the OFEO chromatographic data (
Table 1), we observed a higher percentage of unsaturated fatty acids (67.83%), with polyunsaturated (5.9%) and monounsaturated (61.27%). Mostly we can find in its composition oleic acid (54.32%), palmitic acid (30.0%), linoleic acid (5.9%), followed by elaidic acid (4.29%), palmitoleic acid (2.62%) and stearic acid (2.29%). %). The data presented are in accordance with the literature, according to [
31] Nascimento et al., (2008) 71% of unsaturated fatty acids are present, of which 60.81% are monounsaturated and 10.36% are polyunsaturated. Predominant in its composition oleic acid (56.2%) and palmitic acid (24.1%) [
19].
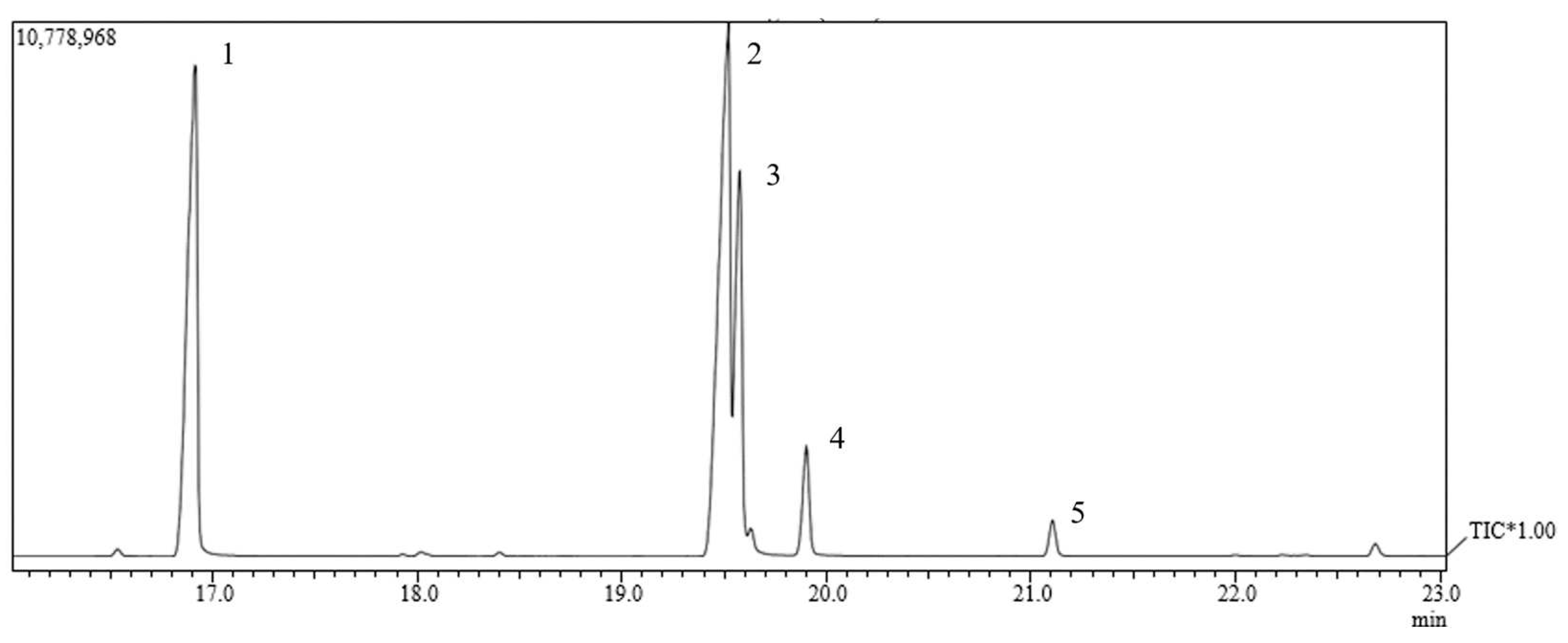
Through chromatographic analysis of the fatty acids present in OFAE, it was possible to identify five compound retention peaks (
Figure 2). We can observe a higher percentage of unsaturated fatty acids (61.05%), polyunsaturated (42.12%), monounsaturated (19.38%) (
Table 2). This result is in line with other authors, since large amounts of unsaturated lipids (66.32%), especially oleic (20.38%) and linoleic (44.48%) acids, were found in okra grown in northeastern Brazil. Brazil [
32], variations of linoleic acid (23.6 to 50.6%) and palmitic acid (10.03 to 36.03%) were also observed [
33]. However, we observed in this work the presence mostly of linoleic acid (42.12%) and palmitic acid (33.55%), followed by oleic acid (17.85%), stearic acid (4.96%), gondoic acid (1.53%) in the OFAE, thus presenting form, similarity with those described in the literature.
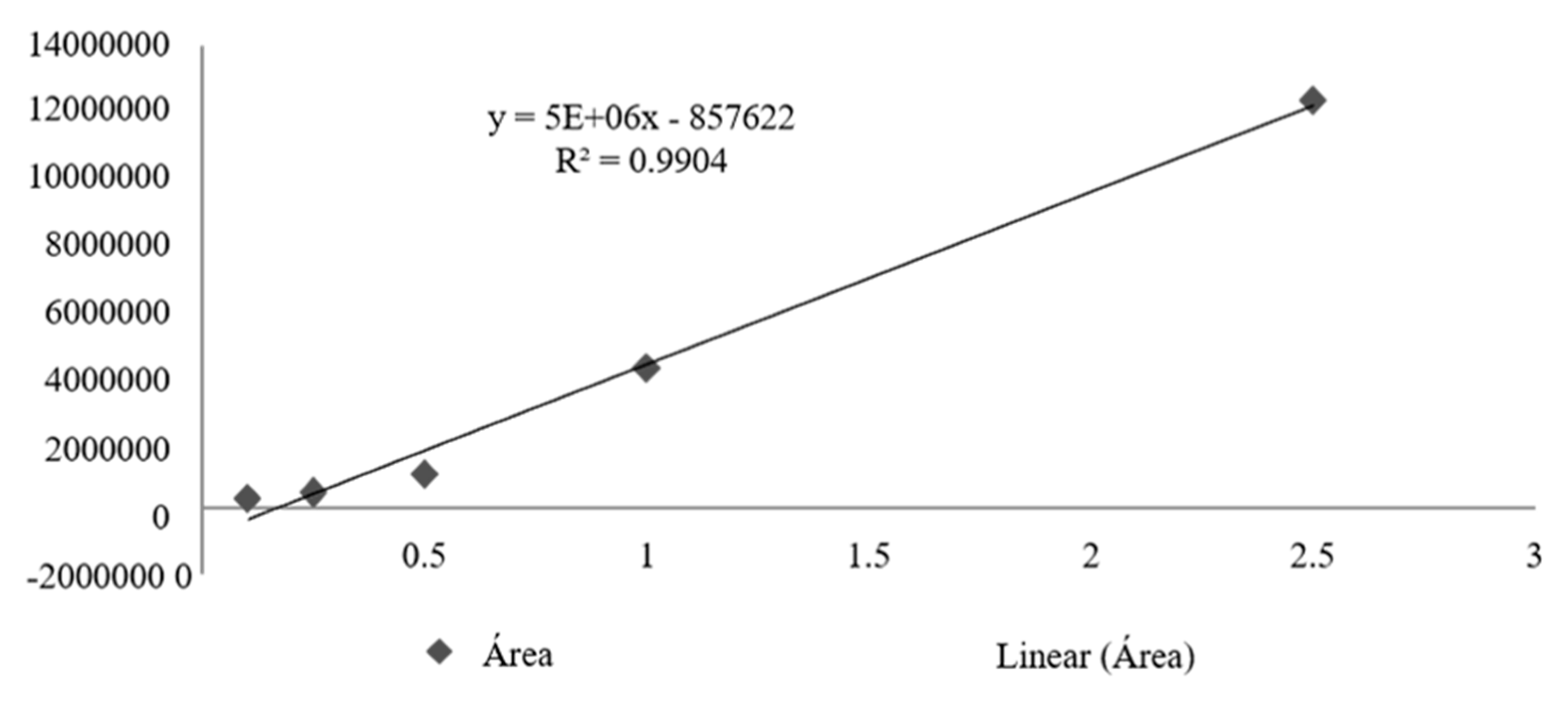
The calibration curve obtained for total tocotrienols based on delta-tocotrienol presented R2= 0.9904, obtaining the following straight line equation: Y=5000000x-857622 (
Figure 3). From the quantification of total tocotrienols it was possible to obtain an average concentration of 617.4 mg/mL, with an average content of 61.7% for OFBO and for CHR it was possible to obtain 46.7 mg/mL with an average content of 48, 7% of total tocotrienols (
Table 3).
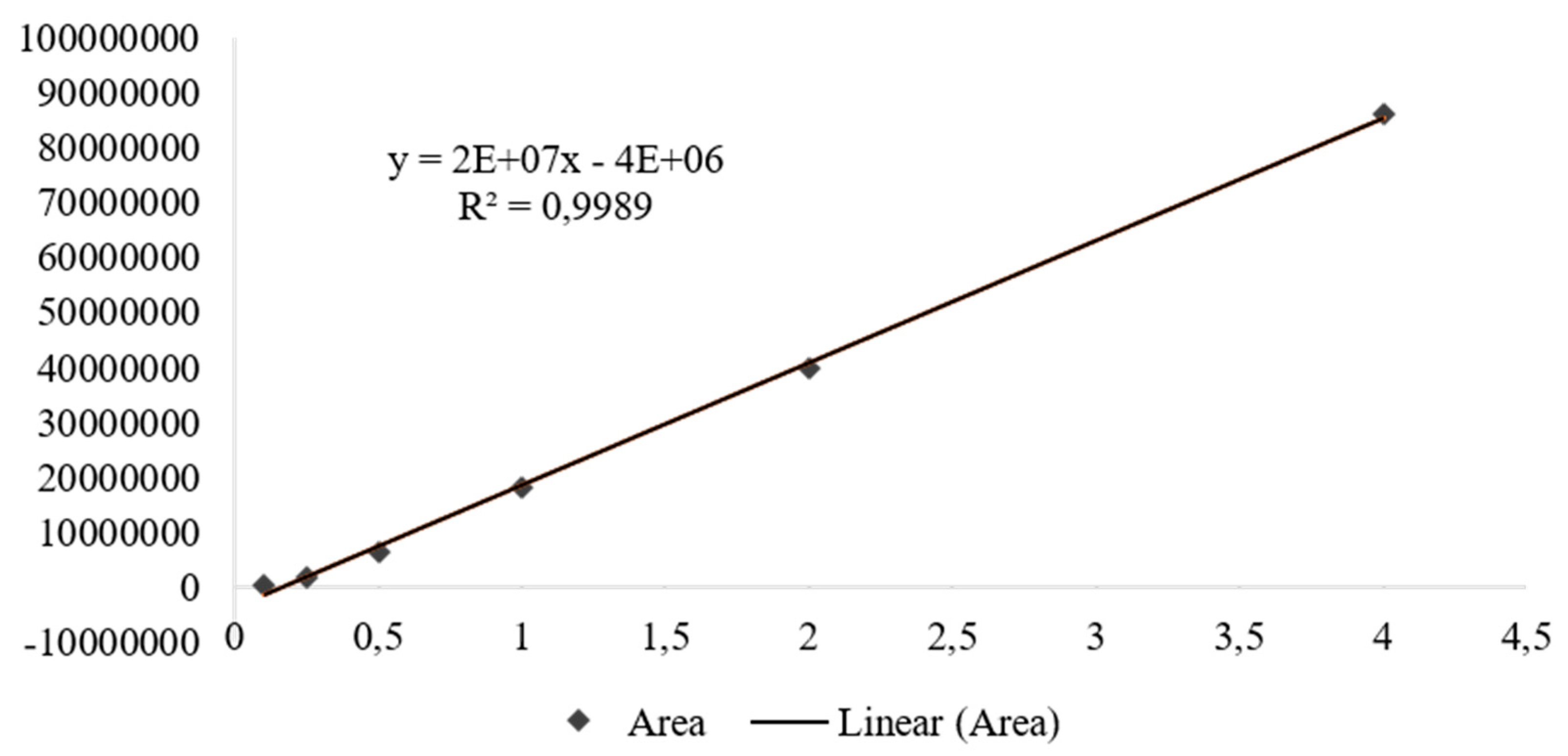
The calibration curve obtained for geranylgeraniol showed R2= 0.9989, obtaining the following straight line equation: Y=20000000x-400000 (
Figure 4). From the quantification of geranylgeraniol present in the samples, it was possible to obtain an average concentration of 872.1 mg/mL for OFBO and an average content of 87.21% of geranylgeraniol, for CHR it was possible to obtain 141.6 mg/mL and an average content 14.16% of geranylgeraniol (
Table 4).
2.2. Analysis of clinical and biochemical parameters
Diets rich in saturated fatty acids (SFA) increase insulin resistance and the incidence of cardiovascular disease. While those rich in mono- and polyunsaturated fatty acids play important roles in the adjuvant treatment of heart disease, coronary disease, hypertension, dyslipidemia, type 2 diabetes mellitus and insulin resistance [
40,
41,
42].
MUFAs such as PUFAs act in the modulation of the lipid profile, especially the fatty acids of the ω-3, ω-6 and ω-9 series that are present in the composition of oils, these fatty acids are regulators of the expression of genes involved in lipid metabolism and glucose and adipogenesis, and may act via mediators, such as the peroxisome proliferator activated receptor (PPAR) (α, β and γ), hepatic X receptors (LXRs) (α and β), hepatic nuclear factor receptors (α and β), 4 (HNF4-α) and sterol regulatory element binding proteins (SREBPs) 1 and 2, being important factors in the hepatic metabolism of carbohydrates, fatty acids, triglycerides, cholesterol and bile acids [
43,
44,
45].
Activation of the PPARγ transcription factor is essential in adipogenesis. Certain fatty acids of the ω-3 and ω-6 series can act as PPARγ ligands, and thus control adipogenesis, such as reducing cholesterol and triglyceride levels, in addition to contributing to the reduction of adipose tissue formation [
46]. Thus, we can observe in our data, reduction of the body weight of the treatment groups CHR, OFEO, OFAE and OFBO. The reduction in body weight may be associated with the high levels of AGIs present in these oils and in the granules. The CHR (259.57±4.00) and OFBO (243.52±4.25) groups, both with p>0.001, had lower body weight and daily feed intake when compared to the GSC group (
Table 5).
The supplementation of UFAs content is also associated with the reduction of hyperplasia and hypertrophy of adipocytes, in the modulation of metabolism through the stimulation of mitochondrial biogenesis and β-oxidation, which can lead to an induction of PPAR-α, consequently the control of differentiation and proliferation of adipose cells [
47]. The VEI, SIN, OFEO, OFAE groups also showed significance of p>0.001 when comparing with GSC. The increase in body weight observed in the GSC group is related to the high daily calorie consumption of these animals in a diet composed of SFA, which contributes to the increase in adipose tissue [
48].
Regarding the investigated biochemical markers of liver function (AST and ALT) and kidney function (urea and creatinine), it was possible to observe that supplementation with a GSC-induced diet caused a significant increase in the levels of these biochemical markers, both in the liver and in the kidneys (
Table 6). However, the treatments positively influenced these results, especially the CHR and OFBO groups demonstrating statistically significant results.
The increase in AST and ALT levels may be due to the administration of saturated fat, associated with increased serum levels of blood cholesterol, as well as the accumulation of fat in the liver, the SFAs generate suppression of the activity of hepatic LDL receptors in messenger RNA (rLDL), blocking intracellular and extracellular cholesterol transport, since as a result of cholesterol reduction, cells via SREBP-2 exhibit increased expression of genes involved in cholesterol synthesis [
49,
50].
2.3. Analysis of lipid profile and atherogenic index
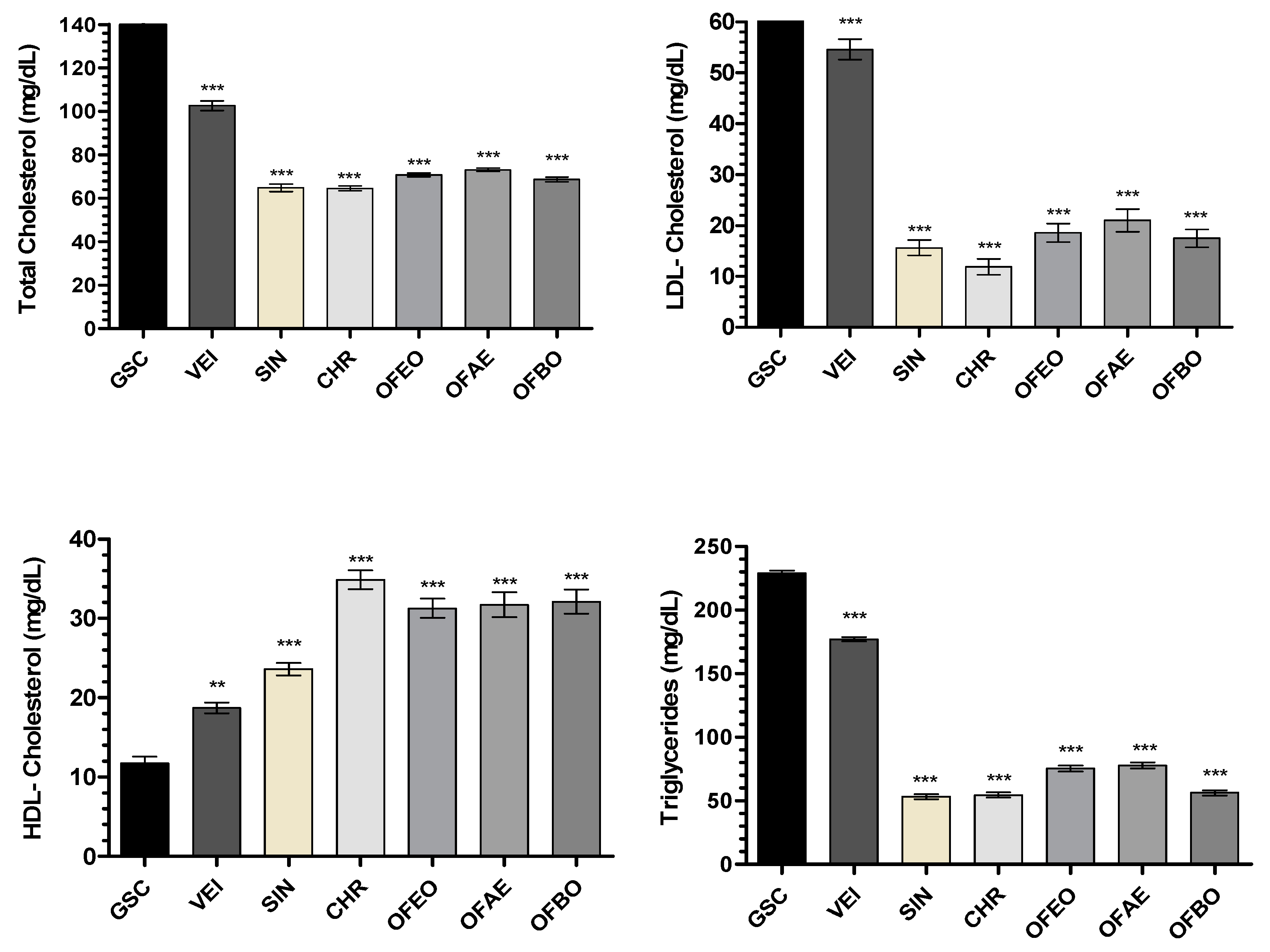
The lipid profile of the SIN, CHR, OFEO, OFAE and OFBO treatment groups showed significant results, both with ***p>0.001, when compared with the GSC group, which showed high levels of TC, LDL-c and TG (
Figure 5). Coconut oil is a rich source of saturated fatty acids and its excess administration triggers the biosynthesis of cholesterol in the liver, causing hypercholesterolemia [
51].
A study conducted in Wistar rats submitted to a treatment with coconut oil showed high levels of CT, TG, as well as an increase in LDL-c and a decrease in HDL-c [
51]. According to the study [
52], it was demonstrated that GSC has a satisfactory effect on the induction of dyslipidemia because it contains 92.64% of saturated fat, which may increase the rate of cholesterol production, as well as triglyceride levels, thus being a well-suited model. described for the induction of hyperlipidemia by GSC in animals.
In the present study, the data demonstrate that the CHR, OFEO, OFAE and OFBO groups act in the prevention of hyperlipidemia induced by GSC. This effect may be associated with the presence of unsaturated, monounsaturated (MUFAs) and polyunsaturated (PUFAs) fatty acids in the composition of the oils tested, as in the granules, it is known that these fatty acids act in the modulation of lipid metabolism at levels of transcription of receptors such as PPARα, present mainly in the liver, essential in the capture and transport of fatty acids and β-oxidation, inducing levels of lipoprotein lipase (LPL), which has the function of hydrolyzing lipoprotein triglycerides into circulating fatty acids [
53].
In addition to the AGIs providing great benefits in the lipid profile, the saturated fatty acid stearic (18:0), present in the chemical composition of the species
A. esculentus,
E. oleracea,
B. orellana does not promote hypercholesterolemia, since the dehydrogenation of this fatty acid is faster than chain elongation, causing it to be converted to oleic acid (monounsaturated) in the liver, thus promoting the previous peroxisomal β-oxidation [
54].
Another possible response pathway in metabolic homeostasis is through the antioxidant activity indirectly attributed to polyunsaturated fatty acids, which are present in the chemical composition of the studied species. These fatty acids act on vascular endothelial cells, decreasing inflammation and, in turn, the risk of atherosclerosis and cardiovascular disease [
55]. They act by preventing lipid peroxidation, eliminating reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are responsible for the oxidation of LDL, a lipoprotein attributed to the formation of foam cells, consequently in the development of atherosclerosis [
55,
56]. ROS include superoxide anion, hydroxyl radical and hydrogen peroxide. RNS are mainly nitric oxide and peroxynitrite [
57,
58].
In a study carried out by Dias et al. (2014) [
59] the polyphenols present in
E. oleracea oil are agents capable of neutralizing reactive oxygen species, thus presenting antioxidant activity. Dong et al. (2014) [
60] when obtaining the oil from the seeds of
A. esculentus, demonstrated antioxidant activity by the DPPH radical scavenging activity test. The tocotrienols present in the
B. orellana species also showed antioxidant properties, improving oxidative stress in metabolic disorders and protecting cellular functions [
61], contributing to the decrease of antiproliferative effects, immunoprotection, reducing the risk of cancer and especially cholesterol [
62,
63]. Tocotrienols are present in CHR and OFBO.
Simvastatin was the drug chosen as the standard drug; it acts as a widely used hypocholesterolemic agent. It is an inactive lactone which is converted into its corresponding β,δ-hydroxy acid in its active form, acting on the pathway of hepatic metabolism by cytochrome P450 after oral administration [
64].
Simvastatin known as statin, is a class of more effective drugs for the treatment of lipid alterations, it is a potent inhibitor of the enzyme 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMGCoA) reductase, preventing the formation of mevalonate, which leads to a reduction in the hepatic synthesis of cholesterol, consequently acting in the increase of LDL-c receptors in hepatocytes, thus increasing its uptake from the circulation to restore cholesterol [
65,
66].
Regarding HDL-c, the results of this study showed a significant increase in the CHR, OFEO, OFAE and OFBO groups when compared with the GSC groups. The CHR group had the highest levels of HDL-c (34.87±3.4), followed by the OFBO group (32.13±4.36), OFAE (31.71±4.15), OFEO (31. 29±3.15), thus indicating that the treatments were able to prevent the increase in LDL-c in this experimental model. These results are possibly related to the lipid composition of the species under study, which present in their composition high levels of monounsaturated fatty acids such as oleic acid and eicosenoic acid (ω-9), and polyunsaturated fatty acids such as α-linoleic acid (ω- 3) and linoleic (ω-6), which can act in the treatment of cardiovascular diseases, as well as in dyslipidemia [
30,
67,
68].
These unsaturated fatty acids are natural ligands of PPARα activation, they can increase the transport of fatty acids, increase the expression of lipoprotein lipase receptors (LPLr), suppress the levels of SREBP-1c, responsible for the regulation of enzymes involved in the synthesis of fatty acids, in addition to increasing the transcription of the main apolipoproteins, such as ApoAI and ApoAII, present in HDL. Unsaturated fatty acids can also inhibit HNF-4α, resulting in reduced expression of genes involved in cholesterol biosynthesis, resulting in addition to increasing the transcription of major apolipoproteins, such as ApoAI and ApoAII, present in HDL [
69].
As for the glycemic levels, we can observe significant satisfactory results, the lowest glycemia values were presented in the CHR, OFEO, OFAE and OFBO groups, both with p>0.001 when compared to the GSC (
Table 7). The increase in glycemia in the GSC group may be related to the ingestion of SFAs, corroborating the accumulation of body fat, since the excess consumption of carbohydrates and lipids contributes to resistance to insulin action, consequently leading to a chronic increase and gradual increase in glycemia [
70].
In addition to its role in lipid homeostasis, PPARα can influence glucose homeostasis, acting by directly regulating gluconeogenesis via stimulation of pyruvate dehydrogenase kinase 4 (PDK4) expression, which favors the use of pyruvate for gluconeogenesis to the detriment of fatty acid synthesis [
71]. In animal models of insulin resistance, PPARα agonists increased insulin sensitivity, consequently increasing fatty acid oxidation in the liver, skeletal muscle, and pancreas by endogenous glucose depletion [
72].
Glucose homeostasis can also be attributed to the direct action of PPARγ on insulin-stimulated glucose disposal. Activation of PPARγ can increase the expression and translocation to the cell surface of the glucose transporters GLUT1 and GLUT4, thereby increasing glucose uptake into adipocytes and muscle cells, as well as reducing plasma glucose levels [
73].
2.4. Formation of atherogenesis
Elevated levels of LDL-c in the blood are recognized as one of the risk factors for cardiovascular disease and are a consequence of the atherosclerosis process [
60]. Atherosclerosis is a chronic inflammatory disorder that occurs in response to vascular endothelial aggression caused by an increased concentration of serum LDL in the arteries. After LDL-lipoprotein enters the intima of arteries, they undergo oxidation in the pro-oxidant environment, favored by an increase in reactive oxygen species (ROS) that oxidize LDL-cholesterol into oxidized LDL (oxLDL), thus triggering the process of inflammation and the recruitment of immune cells, especially monocytes, where they differentiate into macrophages and phagocytose the oxLDL, presenting lipids in their interior, and are now called foam cells, thus forming the atherosclerotic plaque [
52,
74,
75].
The consumption of large amounts of saturated fat triggers the atherogenic process, especially in the abdominal aorta region, which is prone to plaque formation. The thoracic and abdominal arteries are the most affected during the process of atherosclerotic plaque formation [
52,
76].
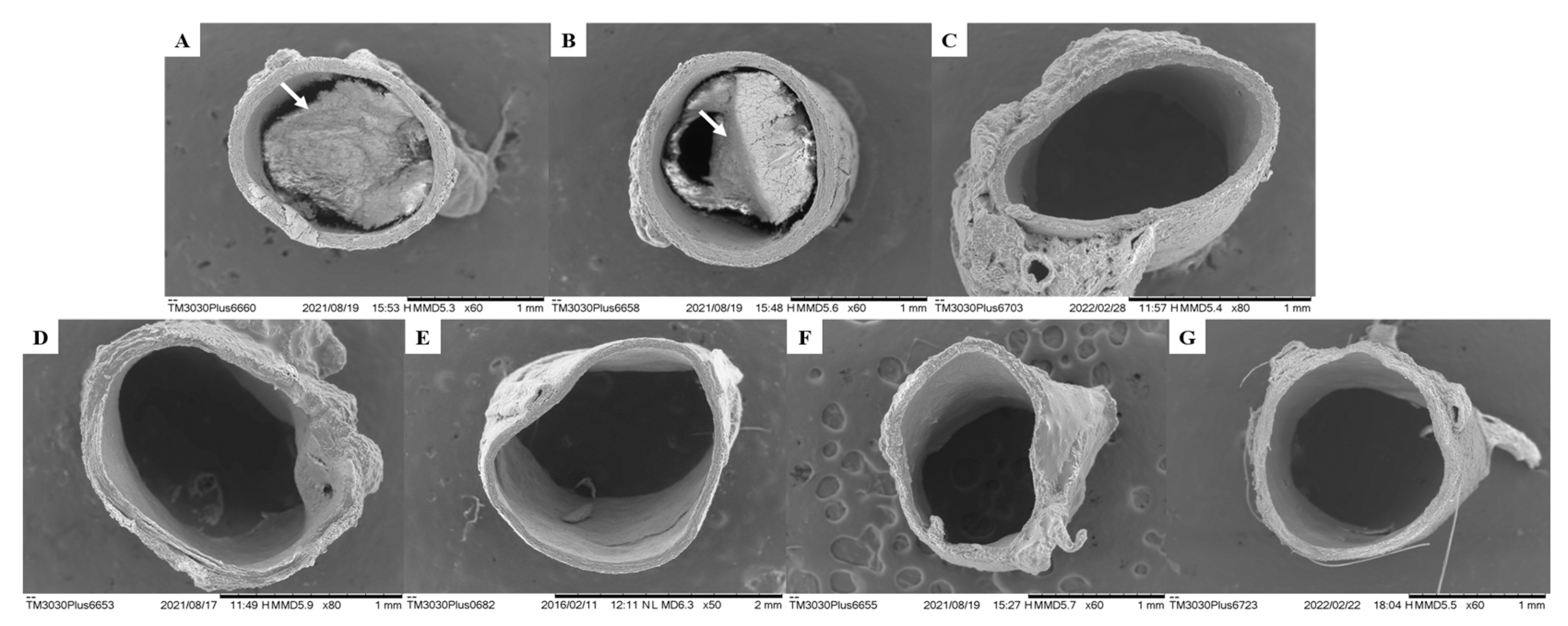
The data presented in this study demonstrate compliance with the formation of atherosclerotic plaques in the vascular endothelium, as well as the Plasma Atherogenic Index (AI) presented by the GSC group (1.30±0.07) (
Figure 6 and
Table 7). However, when evaluating aortic atherogenesis, it was possible to demonstrate a great antiatherogenic potential of the CHR, OFEO, OFAE and OFBO treatment groups, due to the absence of plaque formation in the vascular endothelium, corroborating the data obtained from the AI of the treated groups.
This action can be explained by the capacity of the treatment groups (CHR, OFEO, OFAE and OFBO) to present unsaturated fatty acids in their chemical composition, especially of the ω-3 and ω-6 series, as natural ligands of gene targets, which act in biological processes mainly related to lipid metabolism and the inflammatory process, thus playing a key role in several cardiovascular diseases. Contributing effectively to the preservation of endothelial function, reduction of plasma cholesterol, reduction of LDL-c, which is an important lipoprotein in the process of early atherogenesis, and in the stability of the atheroma plaque [
77].
The SIN group did not form atherosclerotic plaque. This response can be explained, as simvastatin acts to decrease the levels of oxLDL and macrophages, actions that stabilize the atheromatous plaque, in addition to inhibiting low molecular weight G proteins of the Ras superfamily (Ras and Rho), key proteins involved in cell proliferation, differentiation, apoptosis, migration, contraction and regulation of gene transcription, therefore improving vascular function. Simvastatins reduce the activity of NF-κB in inflammatory and vascular cells, as well as inflammatory biomarkers C-reactive protein (CRP), however they increase the endothelial expression of NO synthase (eNOS), increasing blood flow in the vessels, therefore improving the hemostasis and recovery of endothelium-dependent vasoreactivity [
78].
Lecithin, the surfactant used, is described as a complex mixture derived from crude soybean oil, consisting mainly of phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine and phosphatidylinositol, including other lipophilic substances such as glycolipids, triglycerides or fatty acids, in addition to a hydrophilic fraction of phosphoric acid, glycerol, choline and inositol [
79,
80]. Soy lecithin is used as a dispersant, emulsifier, stabilizer, marketed in different solid pharmaceutical forms, such as capsules, tablets and granules, in the category of dietary supplements [
81].
Due to their high lipophilicity, lecithins promote the physical coating of particles, especially those containing fat in their composition, in such a way that the oil, when in an aqueous medium, promotes a reduction in the surface tension between the solid and liquid phases, making these phases mix forming only one, having, therefore, important properties in the drug delivery system [
82].
According to the literature, lecithins form fat transport lipoproteins, allowing to reduce blood cholesterol levels. The phospholipids present play an important role during the intestinal absorption of lipids, thus facilitating the formation of micelles, increasing absorption, in addition to having an activating action on the circulation, thus reducing the risk of cardiovascular diseases due to their emulsifying action, which does not allow the deposit of lipids to occur. fat in blood vessels [
83,
84].
The lecithin group (VEI) used in this study was able to reduce the lipid profile of TC, LDL and TG, and increase HDL showing significant values when compared to the GSC, but when comparing with the treatments (CHR, OFEO, OFAE and OFBO), it was not possible to obtain significant results. In addition to observing increased AI values, as well as the presence of atheroma plaque when analyzed in SEM, presenting, therefore, adverse results in the literature. Thus, we can infer that although lecithin presents responses in lipid levels, it did not demonstrate action in the reduction of atheromatous plaque, however, the treatment groups were more effective in modulating lipid metabolism, as well as antiatherogenic activity obtained from the species under study, this response may be related to the attributed bioavailability, especially to the studied granules (CHR).
AGIs can modulate the inflammatory response by several mechanisms, such as via PPARα which acts by inhibiting the expression of monocyte chemoattractant protein-1 (MCP-1) induced by C-reactive protein (CRP) inhibition of endothelin 1 (ET-1) expression, inhibition of interleukin-6 (IL-6) release induced by interleukin-1 (IL-1) and inhibition of expression of vascular cell adhesion molecule 1 (VCAM-1) induced by liposaccharides (LPS) [
85,
86,
87].
PPARα and γ can also attenuate levels of interferon-gamma (IFN-γ) released by T lymphocytes; it also stimulates expression of the cholesterol efflux regulator protein ABCA1 in foam cells in a liver X receptor (LXR)-dependent manner, promoting ApoA-I-mediated cholesterol efflux; regulates class B type 1 scavenger receptors (SRBI), which plays a role in esterified HDL uptake by the liver and cholesterol efflux from macrophages; as well as induces the secretion of lipoprotein lipase (LPL) and decreases the uptake of glycated LDL by macrophages [
87,
88,
89].
In turn, the PPARγ in macrophages increases the expression of the scavenger receptor CD36/FAT, responsible for the uptake of oxLDL, being of fundamental importance in the differentiation of macrophages with characteristics of foam cells [
90]. Later stages of atherosclerosis are also regulated by PPARα, inhibiting the activation of smooth muscle cell (SMC) proliferation, which is a key event in the development of atherosclerosis and its complications such as tissue factor, an important procoagulant, hence PPARα may block atherothrombosis [
91,
92].
In addition to the AGIs promoting activity in the inflammatory response, compounds such as tocotrienols are important inflammatory modulators, as they are negative regulators of PPARγ receptors, and can act by suppressing the formation of nitric oxide inducible by an inflammatory signal in macrophages, tumor necrosis factor alpha (TNF-α), as well as inhibition of nuclear factor-κB (NF-κB) activation, thereby stopping tissue inflammation [
34,
93,
94]. In addition to these compounds, geranylgeraniol, present in the chemical composition of CHR and OFBO, can also promote the modulation of PPARγ receptors and can act as an agonist, exert negative regulation via HMG-Coa-reductase and enzymes involved in cholesterol biosynthesis, playing a key role in the development of atherosclerosis [
34,
35,
95].
Inflammation is recognized as a key regulatory process in the face of several risk factors for atherosclerosis. C-reactive protein (CRP) is the inflammatory marker, generated from the liver, it is an acute phase reactant protein, responds immediately after the increase of nonspecific inflammation in the blood vessel, which may reflect in the process of atherosclerotic development, therefore being, recommended as a cardiovascular risk marker [
36,
37,
96]. In view of the results obtained in this study, we can observe increased CRP values in the GSC group (0.84±0.06), which corroborates the results by SEM in the appearance of atheromatous plaque, thus suggesting chronic inflammation in the aortic artery by atherogenic processes. However, we can observe decreased values of the CRP biomarker in the treatment groups CHR (0.41±0.04), OFEO (0.49±0.03), OFAE (0.50±0.03) and OFBO (0 .47±0.04), as well as in the positive control group SIN (0.39±0.04), demonstrating, therefore, a reduction in the inflammatory process and, consequently, antiatherogenic activity, observed in the images obtained from the aortas by SEM.