Submitted:
18 July 2023
Posted:
19 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animal husbandry
2.2. Paint Marking, Behavioral Monitoring, and Collections
2.3. Electroantennography
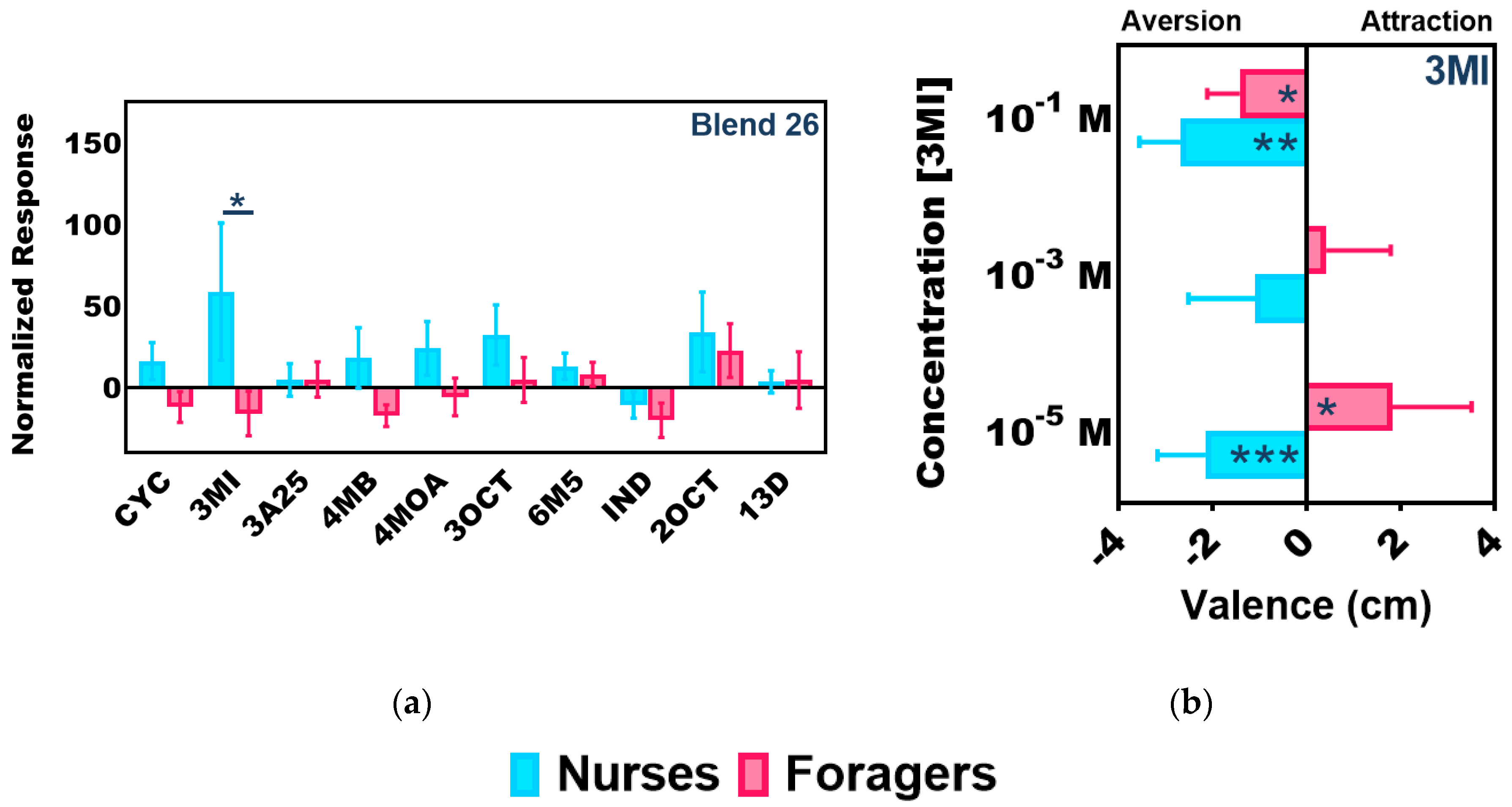
2.4. Colony-Level Responses to 3-Methylindole
2.5. Individual Responses to 3-Methylindole
2.6. Statistical Data Analysis
3. Results
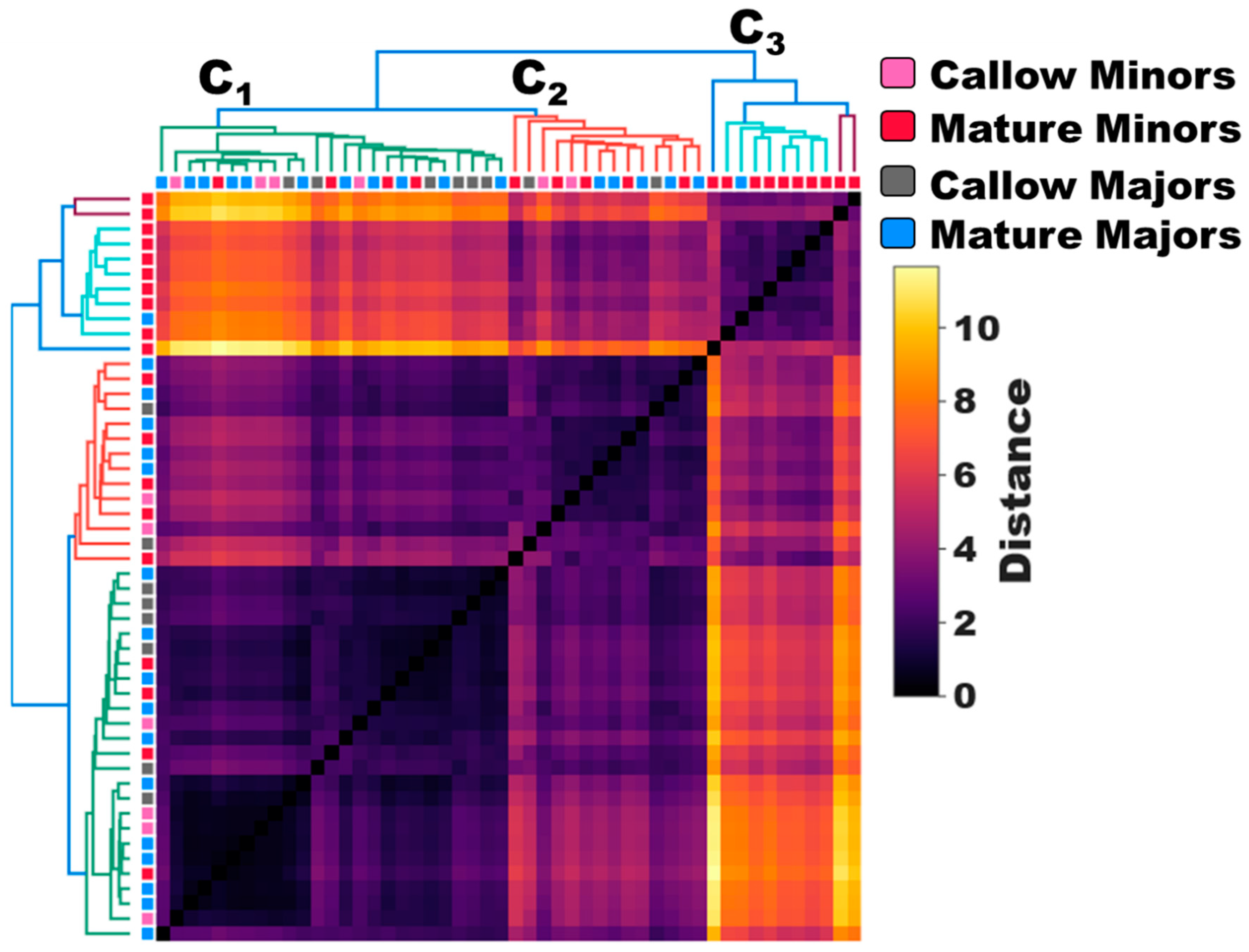
3.1. Morphological Castes Perform Different Tasks
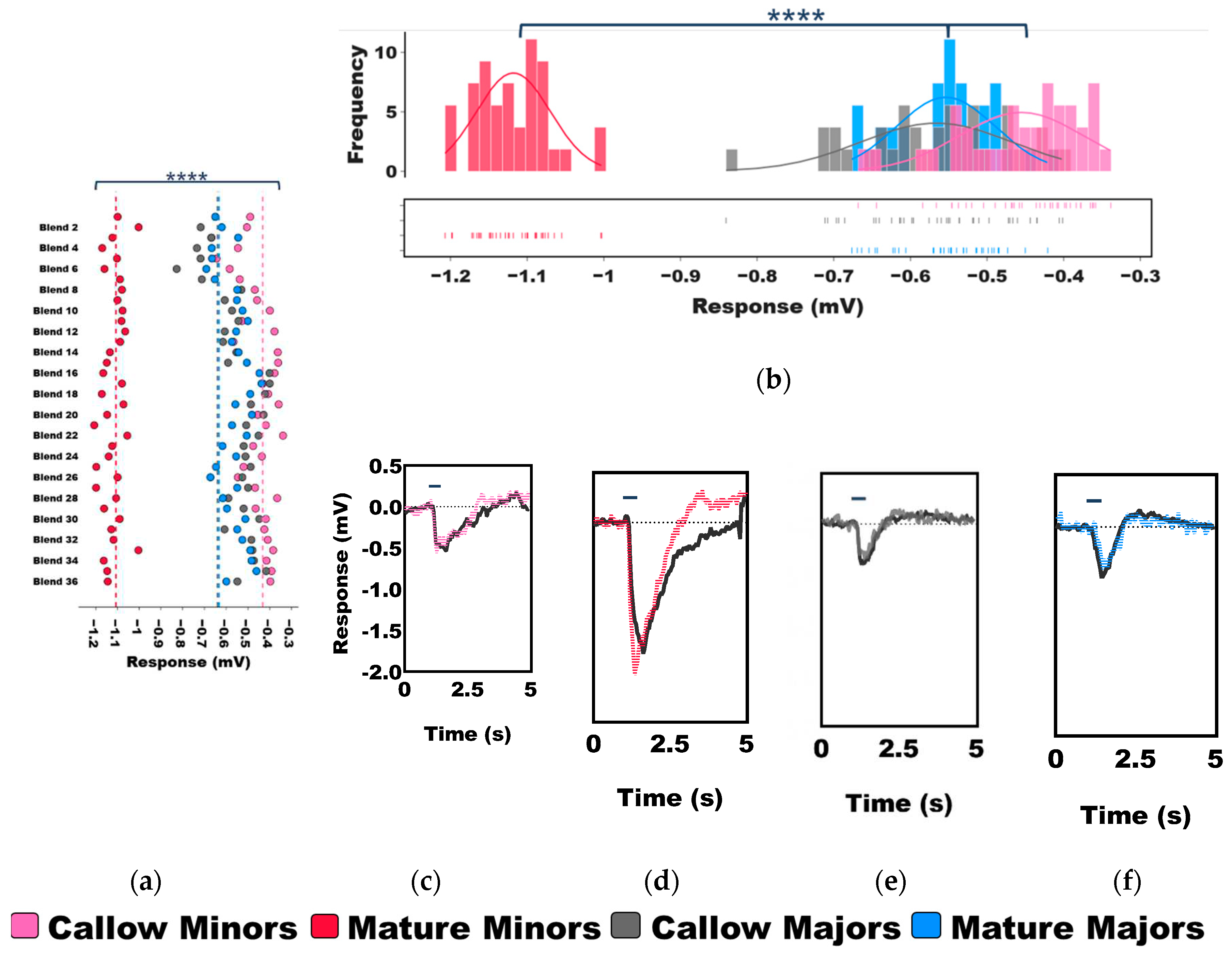
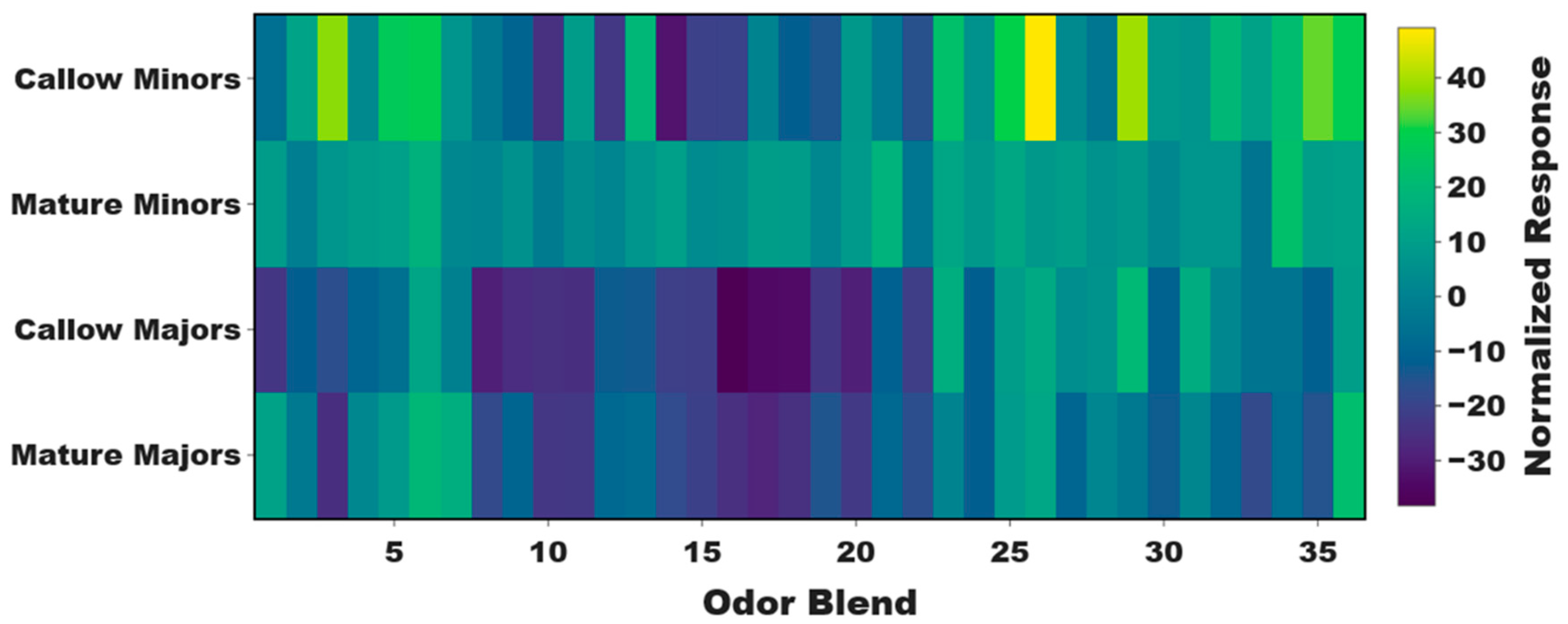
3.2. Age-Associated Shifts in Odor Coding Observed in Minors but Not Majors
3.3. Task-Associated Shifts in Olfactory Sensitivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernadou, A.; Busch, J.; Heinze, J. Diversity in identity: behavioral flexibility, dominance, and age polyethism in a clonal ant. Behavioral Ecology and Sociobiology 2015, 69, 1365–1375. [Google Scholar] [CrossRef]
- Tripet, F.; Nonacs, P. Foraging for Work and Age-Based Polyethism: The Roles of Age and Previous Experience on Task Choice in Ants. Ethology 2004, 110. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The ants; Belknap Press of Harvard University Press: Cambridge, Mass, 1990; p. 732. [Google Scholar]
- Schultner, E.; Pulliainen, U. Brood recognition and discrimination in ants. Insectes Sociaux 2020, 67, 11–34. [Google Scholar] [CrossRef]
- Haak, U.H. , B; Bestman, J; Kern, F. Species-specificity in trail pheromones and dufour's gland contents of Camponotus atriceps and C. floridanus (Hymenoptera: Formicidae). Chemoecology 1996, 7, 8. [Google Scholar] [CrossRef]
- Greene, M.J.; Pinter-Wollman, N.; Gordon, D.M. Interactions with combined chemical cues inform harvester ant foragers' decisions to leave the nest in search of food. PLoS One 2013, 8, e52219. [Google Scholar] [CrossRef]
- Sturgis, S.J.; Gordon, D.M. Nestmate recognition in ants (Hymenoptera: Formicidae): a review. Myrmecological News 2012, 16, 101–110. [Google Scholar]
- Ferguson, S.T.; Park, K.Y.; Ruff, A.A.; Bakis, I.; Zwiebel, L.J. Odor coding of nestmate recognition in the eusocial ant Camponotus floridanus. The Journal of Experimental Biology 2020, 223, jeb215400. [Google Scholar] [CrossRef] [PubMed]
- Simola, D.F.; Graham, R.J.; Brady, C.M.; Enzmann, B.L.; Desplan, C.; Ray, A.; Zwiebel, L.J.; Bonasio, R.; Reinberg, D.; Liebig, J. , et al. Epigenetic (re)programming of caste-specific behavior in the ant Camponotus floridanus. Science 2016, 351, aac6633. [Google Scholar] [CrossRef] [PubMed]
- Glastad, K.M.; Graham, R.J.; Ju, L.; Roessler, J.; Brady, C.M.; Berger, S.L. Epigenetic Regulator CoREST Controls Social Behavior in Ants. Mol Cell 2020, 77, 338–351.e336. [Google Scholar] [CrossRef]
- Ferguson, S.T.; Bakis, I.; Edwards, N.D.; Zwiebel, L.J. Olfactory sensitivity differentiates morphologically distinct worker castes in Camponotus floridanus. BMC Biology 2023, 21, 3. [Google Scholar] [CrossRef]
- Bubak, A.N.; Yaeger, J.D.; Renner, K.J.; Swallow, J.G.; Greene, M.J. Neuromodulation of Nestmate Recognition Decisions by Pavement Ants. PLoS One 2016, 11, e0166417. [Google Scholar] [CrossRef] [PubMed]
- Brandstaetter AS, R.W. , Kleineidam CJ Friends and Foes from an Ant Brain’s Point of View – Neuronal Correlates of Colony Odors in a Social Insect. PLoS ONE 2011, 6, e21383-21392. [Google Scholar] [CrossRef]
- Gordon, D.M. The organization of work in social insect colonies. Nature 1996, 380, 121–124. [Google Scholar] [CrossRef]
- Wilson, E.O. Behavioral discretization and the number of castes in an ant species. Behavioral Ecology and Sociobiology 1976, 1, 141–154. [Google Scholar] [CrossRef]
- Tripet, F.; Nonacs, P. Foraging for Work and Age-Based Polyethism: The Roles of Age and Previous Experience on Task Choice in Ants. Ethology 2004, 110, 863–877. [Google Scholar] [CrossRef]
- Mikheyev, A.S.; Linksvayer, T.A. Genes associated with ant social behavior show distinct transcriptional and evolutionary patterns. eLife 2015, 4, e04775. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D. Adaptive significance of the age polyethism schedule in honeybee colonies. Behavioral Ecology and Sociobiology 1982, 11, 287–293. [Google Scholar] [CrossRef]
- Kolmes, S.A.; Sommeijer, M.J. Distribution of labour among workers of Melipona favosa F.: Age-polyethism and worker oviposition. Insectes Sociaux 1984, 31, 171–184. [Google Scholar] [CrossRef]
- Gordon, D.M. Dynamics of Task Switching in Harvester Ants. Animal Behaviour 1989, 38, 194–204. [Google Scholar] [CrossRef]
- Ben-Shahar, Y.; Robichon, A.; Sokolowski, M.B.; Robinson, G.E. Influence of gene action across different time scales on behavior. Science 2002, 296, 741–744. [Google Scholar] [CrossRef]
- Heylen, K.; Gobin, B.; Billen, J.; Hu, T.T.; Arckens, L.; Huybrechts, R. Amfor expression in the honeybee brain: a trigger mechanism for nurse-forager transition. J Insect Physiol 2008, 54, 1400–1403. [Google Scholar] [CrossRef]
- Ma, W.; Jiang, Y.; Meng, J.; Zhao, H.; Song, H.; Shen, J. Expression Characterization and Localization of the foraging Gene in the Chinese Bee, Apis cerana cerana (Hymenoptera: Apidae). J Insect Sci 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Ingram, K.K.; Oefner, P.; Gordon, D.M. Task-specific expression of the foraging gene in harvester ants. Mol Ecol 2005, 14, 813–818. [Google Scholar] [CrossRef]
- Lucas, C.; Nicolas, M.; Keller, L. Expression of foraging and Gp-9 are associated with social organization in the fire ant Solenopsis invicta. Insect Mol Biol 2015, 24, 93–104. [Google Scholar] [CrossRef]
- Oettler, J.; Nachtigal, A.L.; Schrader, L. Expression of the Foraging Gene Is Associated with Age Polyethism, Not Task Preference, in the Ant Cardiocondyla obscurior. PLoS One 2015, 10, e0144699. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, A.G.; Brent, C.S.; Hölldobler, B.; Amdam, G.V. Worker division of labor and endocrine physiology are associated in the harvester ant, Pogonomyrmex californicus. J Exp Biol 2012, 215, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Bonabeau, E. Social insect colonies as complex adaptive systems. Ecosystems 1998, 1, 437–443. [Google Scholar] [CrossRef]
- Baltiansky, L.; Frankel, G.; Feinerman, O. Emergent regulation of ant foraging frequency through a computationally inexpensive forager movement rule. eLife 2023, 12, e77659. [Google Scholar] [CrossRef]
- Ferguson, S.T.; Bakis, I.; Zwiebel, L.J. Advances in the Study of Olfaction in Eusocial Ants. Insects 2021, 12, 252. [Google Scholar] [CrossRef]
- Suh, E.; Bohbot, J.; Zwiebel, L.J. Peripheral olfactory signaling in insects. Curr Opin Insect Sci 2014, 6, 86–92. [Google Scholar] [CrossRef]
- Pask, G.; Ray, A. Insect Olfactory Receptors: An Interface between Chemistry and Biology. In Chemosensory Transduction: The Detection of Odors, Tastes, and Other Chemostimuli, 1st Ed. ed; Zufall, F., Munger, S.D., Eds. Academic Press: Cambridge, MA, 2016; pp. 101–122. [Google Scholar]
- Galizia, C.G.; Sachse, S. Odor Coding in Insects. In The Neurobiology of Olfaction, Menini, A., Ed. Boca Raton (FL), 2010.
- Zube, C.; Kleineidam, C.J.; Kirschner, S.; Neef, J.; Rossler, W. Organization of the olfactory pathway and odor processing in the antennal lobe of the ant Camponotus floridanus. J Comp Neurol 2008, 506, 425–441. [Google Scholar] [CrossRef]
- Kirschner, S.; Kleineidam, C.J.; Zube, C.; Rybak, J.; Grünewald, B.; Rössler, W. Dual olfactory pathway in the honeybee, Apis mellifera. J Comp Neurol 2006, 499, 933–952. [Google Scholar] [CrossRef]
- Zhou, X.; Slone, J.D.; Rokas, A.; Berger, S.L.; Liebig, J.; Ray, A.; Reinberg, D.; Zwiebel, L.J. Phylogenetic and transcriptomic analysis of chemosensory receptors in a pair of divergent ant species reveals sex-specific signatures of odor coding. PLoS Genet 2012, 8, e1002930. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Rokas, A.; Berger, S.L.; Liebig, J.; Ray, A.; Zwiebel, L.J. Chemoreceptor Evolution in Hymenoptera and Its Implications for the Evolution of Eusociality. Genome Biol Evol 2015, 7, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Smith, C.D.; Robertson, H.M.; Helmkampf, M.; Zimin, A.; Yandell, M.; Holt, C.; Hu, H.; Abouheif, E.; Benton, R., et al. Draft genome of the red harvester ant Pogonomyrmex barbatus. Proceedings of the National Academy of Sciences 2011, 10.1073/pnas.1007901108, 1-6. 1073, -6. [CrossRef]
- Bonasio, R.; Zhang, G.; Ye, C.; Mutti, N.S.; Fang, X.; Qin, N.; Donahue, G.; Yang, P.; Li, Q.; Li, C. , et al. Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator. Science 2010, 329, 1068–1071. [Google Scholar] [CrossRef]
- Oxley, P.R.; Ji, L.; Fetter-Pruneda, I.; McKenzie, S.K.; Li, C.; Hu, H.; Zhang, G.; Kronauer, D.J. The genome of the clonal raider ant Cerapachys biroi. Curr Biol 2014, 24, 451–458. [Google Scholar] [CrossRef]
- Smith, C.D.; Zimin, A.; Holt, C.; Abouheif, E.; Benton, R.; Cash, E.; Croset, V.; Currie, C.R.; Elhaik, E.; Elsik, C.G. , et al. Draft genome of the globally widespread and invasive Argentine ant (Linepithema humile). Proc Natl Acad Sci U S A 2011, 108, 5673–5678. [Google Scholar] [CrossRef] [PubMed]
- Pask, G.M.; Slone, J.D.; Millar, J.G.; Das, P.; Moreira, J.A.; Zhou, X.; Bello, J.; Berger, S.L.; Bonasio, R.; Desplan, C. , et al. Specialized odorant receptors in social insects that detect cuticular hydrocarbon cues and candidate pheromones. Nat Commun 2017, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Slone, J.D.; Pask, G.M.; Ferguson, S.T.; Millar, J.G.; Berger, S.L.; Reinberg, D.; Liebig, J.; Ray, A.; Zwiebel, L.J. Functional characterization of odorant receptors in the ponerine ant, Harpegnathos saltator. Proc Natl Acad Sci U S A 2017, 114, 8586–8591. [Google Scholar] [CrossRef]
- Bos, N.; d'Ettorre, P.; Guerrieri, F.J. Chemical structure of odorants and perceptual similarity in ants. Journal of Experimental Biology 2013, 216, 3314–3320. [Google Scholar] [CrossRef]
- Hughes, W.O.; Howse, P.E.; Goulson, D. Mandibular gland chemistry of grass-cutting ants: species, caste, and colony variation. J Chem Ecol 2001, 27, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.C.; Brownlee, R.C.; Silverstein, R. Alarm pheromones of the ant atta texana. J Insect Physiol 1968, 14, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Duffield, R.M.; Blum, M.S. Identification, role and systematic significance of 3-octanone in the carpenter ant, Camponotus schaefferi Whr. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 1975, 51, 281–282. [Google Scholar] [CrossRef]
- Crewe, R.M.; Blum, M.S. 6-Methyl-5-hepten-2-one. Chemotaxonomic Significance in an Iridomyrmex sp. (Hymenoptera: Formicidae). Annals of the Entomological Society of America 1971, 64, 1007–1010. [Google Scholar] [CrossRef]
- Tomalski, M.D.; Blum, M.S.; Jones, T.H.; Fales, H.M.; Howard, D.F.; Passera, L. Chemistry and functions of exocrine secretions of the antsTapinoma melanocephalum and T. erraticum. Journal of Chemical Ecology 1987, 13, 253–263. [Google Scholar] [CrossRef]
- Sharma, K.R.; Enzmann, B.L.; Schmidt, Y.; Moore, D.; Jones, G.R.; Parker, J.; Berger, S.L.; Reinberg, D.; Zwiebel, L.J.; Breit, B. , et al. Cuticular Hydrocarbon Pheromones for Social Behavior and Their Coding in the Ant Antenna. Cell Rep 2015, 12, 1261–1271. [Google Scholar] [CrossRef]
- Brown, C.A.; Watkins, J.F.; Eldridge, D.W. Repression of Bacteria and Fungi by the Army Ant Secretion: Skatole. Journal of the Kansas Entomological Society 1979, 52, 119–122. [Google Scholar]
- Schiestl, F.P.; Roubik, D.W. Odor Compound Detection in Male Euglossine Bees. Journal of Chemical Ecology 2003, 29, 253–257. [Google Scholar] [CrossRef]
- Leal, W.S.; Barbosa, R.M.; Xu, W.; Ishida, Y.; Syed, Z.; Latte, N.; Chen, A.M.; Morgan, T.I.; Cornel, A.J.; Furtado, A. Reverse and conventional chemical ecology approaches for the development of oviposition attractants for Culex mosquitoes. PLoS ONE 2008, 3, e3045. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




