Submitted:
19 July 2023
Posted:
20 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
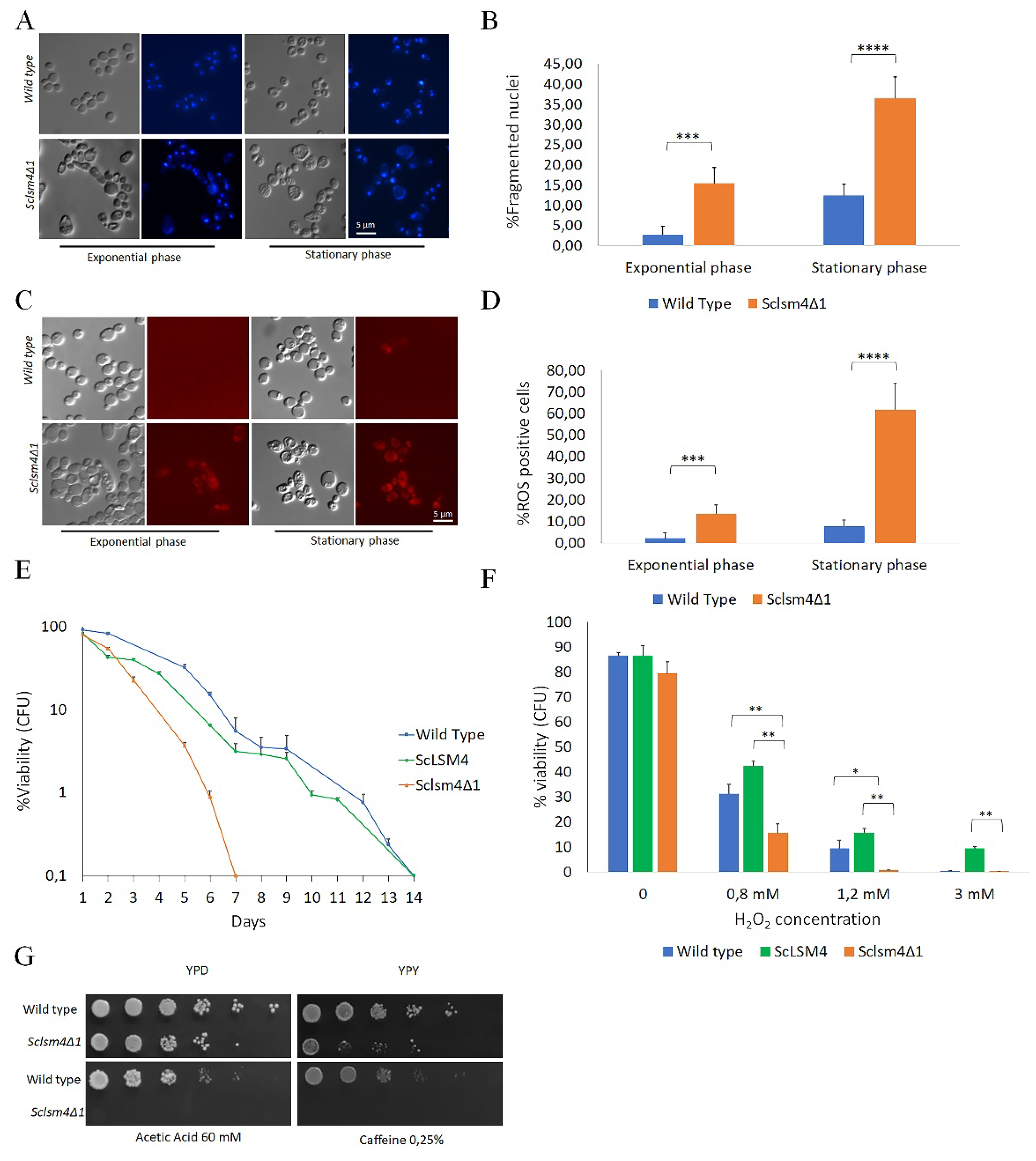
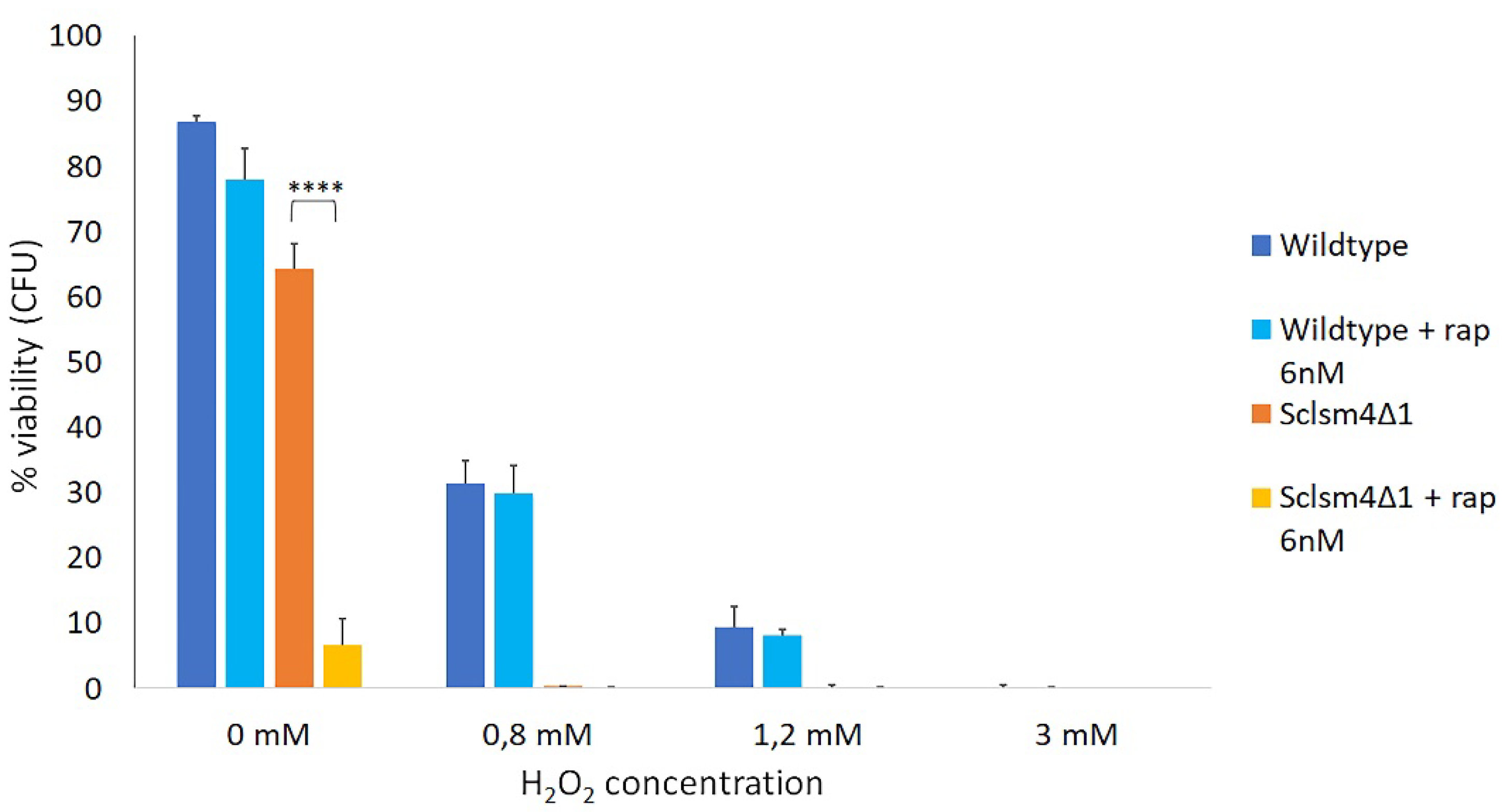
2.1. Sclsm4Δ1 mutant shows regulated cell death markers and premature ageing
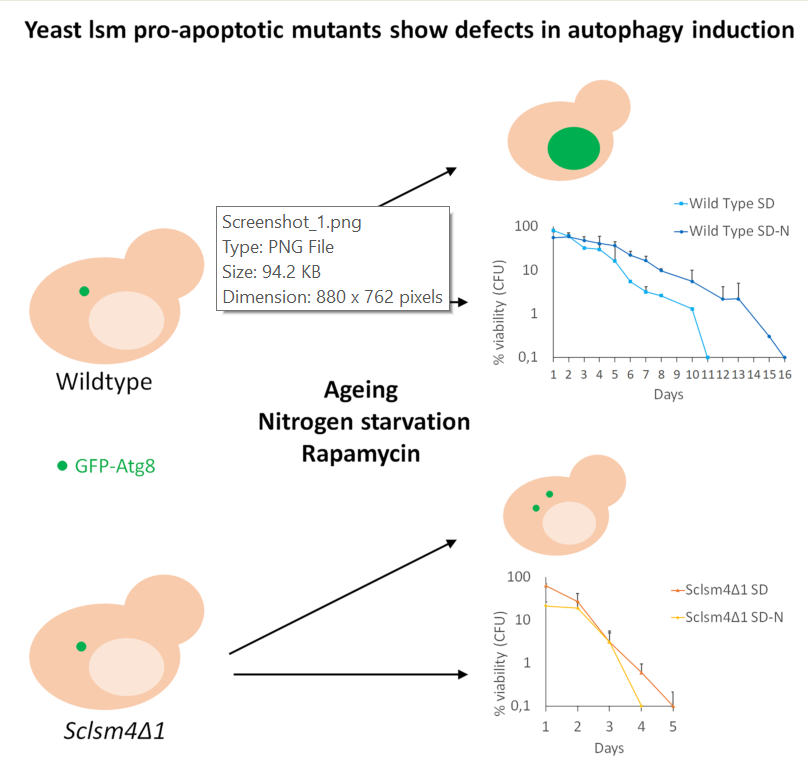
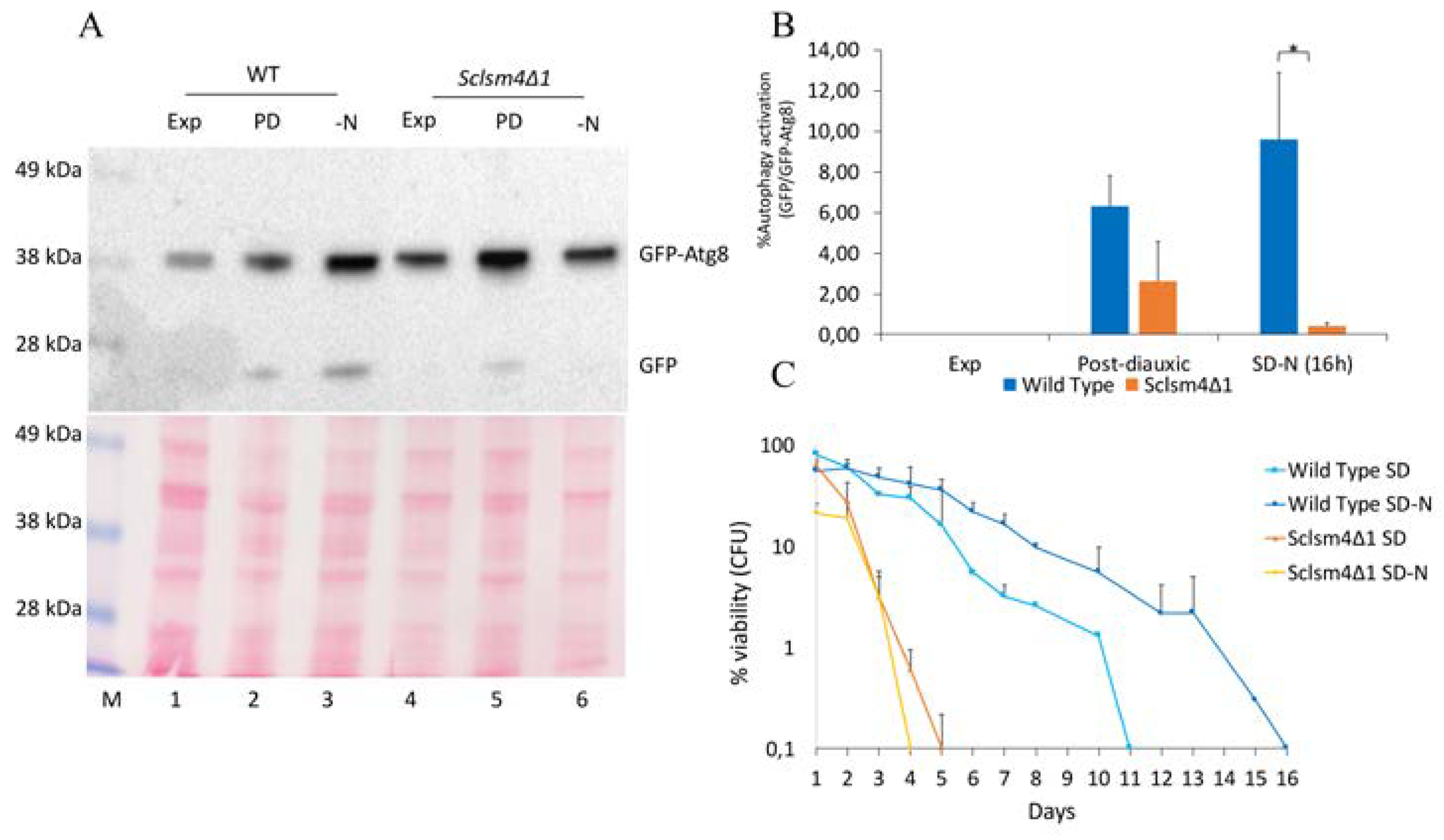
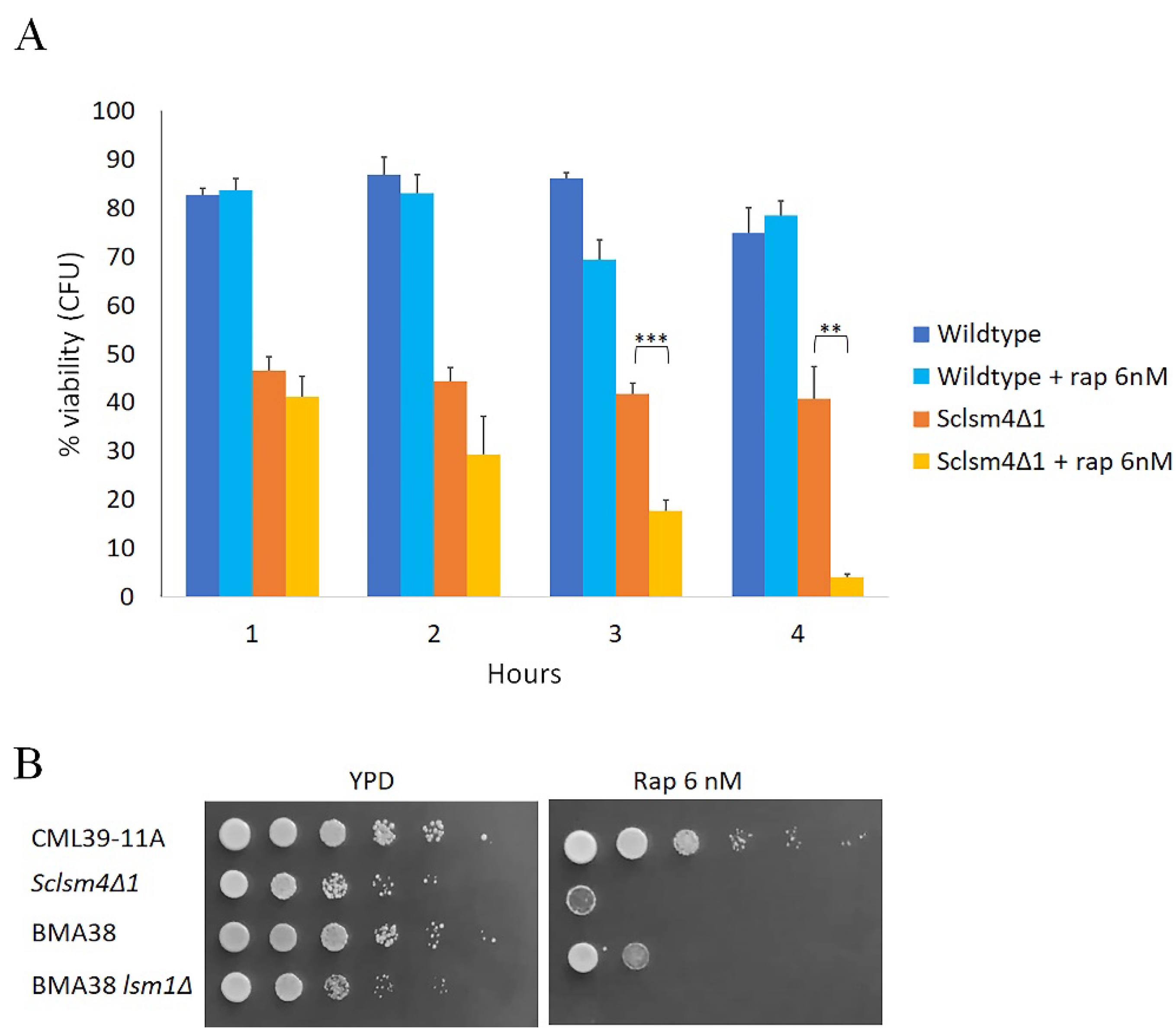
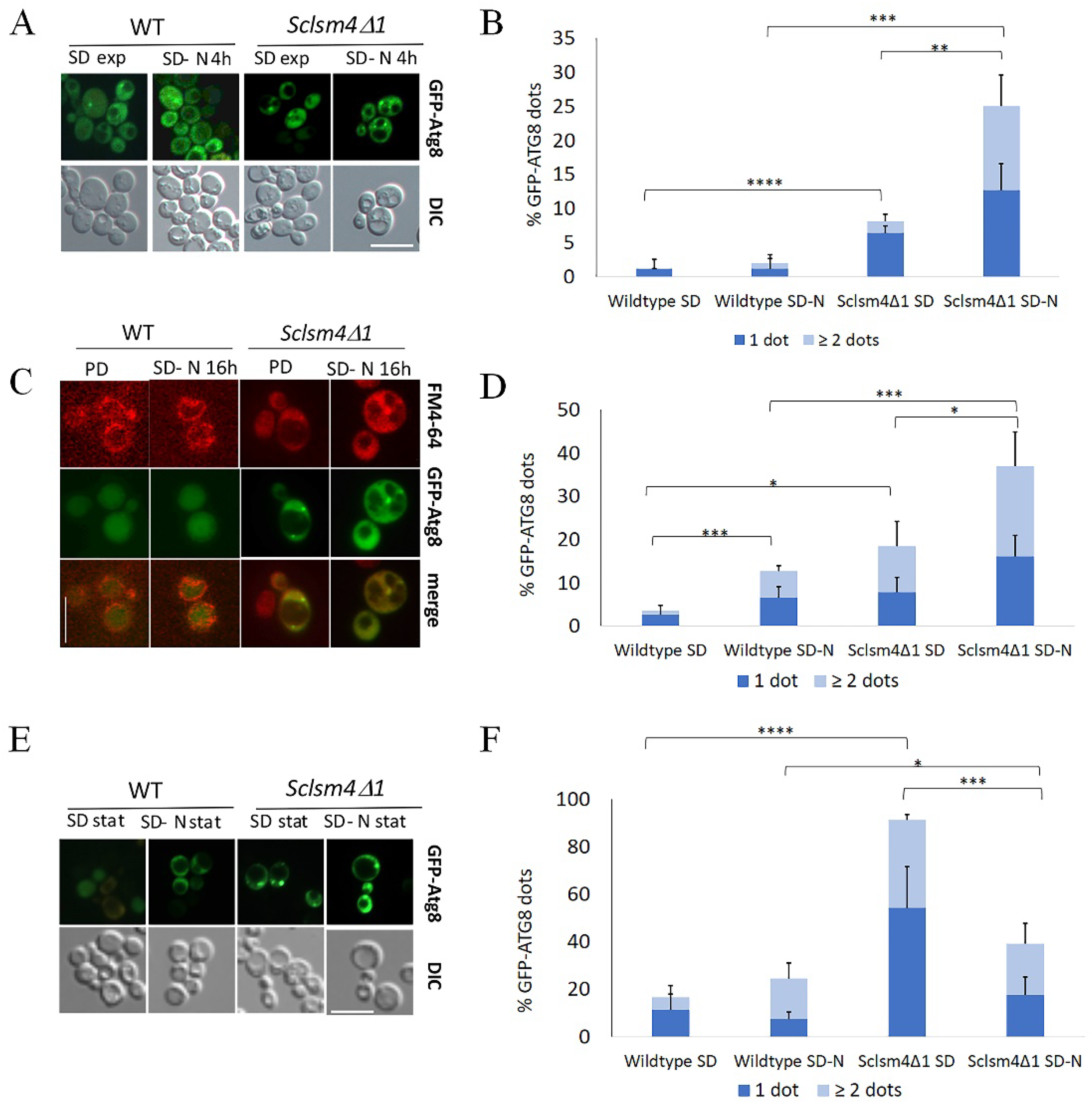
2.2. Sclsm4Δ1 mutant is defective in autophagy induction
3. Discussion
4. Materials and Methods
4.1. Yeast strains, growth conditions and plasmids construction
| Strain | Genotype | Source |
|---|---|---|
| MCY4 | MATα, ade1-101, his3-Δ1, trp1-289, ura3, LEU-GAL1-SDB23 | [56] |
| MCY4/Kllsm4Δ1 | MATα, ade1-101, his3-Δ1, trp1-289, ura3, LEU-GAL1-SDB23 pRS313/Kllsm4Δ1 | [42] |
| MCY4/Sclsm4Δ1 | MATα, ade1-101, his3-Δ1, trp1-289, ura3, LEU-GAL1-SDB23 pRS313/Sclsm4Δ1 | This work |
| CML39-11A | MATa, ade1-101, his3-Δ1, leu2, ura3, trp1-289 | [8] |
| MCY4/ScLSM4 | MATα, ade1-101, his3-Δ1, trp1-289, ura3, LEU-GAL1-SDB23 pRS313/ScLSM4 | This work |
| MCY4/Kllsm4Δ1 pUG36/ATG8 | MATα, ade1-101, his3-Δ1, trp1-289, ura3, LEU-GAL1-SDB23 pRS313/Kllsm4Δ1, pUG36/ATG8 | This work |
| MCY4/Sclsm4Δ1 pUG36/ATG8 | MATα, ade1-101, his3-Δ1, trp1-289, ura3, LEU-GAL1-SDB23 pRS313/Sclsm4Δ1, pUG36/ATG8 | This work |
| CML39-11A pUG36/ATG8 | MATa, ade1-101, his3-Δ1, leu2, ura3, trp1-289 pUG36/ATG8 | This work |
| MCY4/ScLSM4 pUG36/ATG8 | MATα, ade1-101, his3-Δ1, trp1-289, ura3, LEU-GAL1-SDB23 pRS313/ScLSM4, pUG36/ATG8 | This work |
| BMA38 | Matα, ura3-1, leu2-3, -112, ade2-1, can1-100, his3-11, -15, trp1Δ1 | [39] |
| BMA38 lsm1Δ | Matα, ura3-1, leu2-3, -112, ade2-1, can1-100, his3-11, -15, trp1Δ1, lsm1Δ::TRP1 | [39] |
| BY4741 | Mat a, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0 | [57] |
| Lsm1Δ | ||
| Lsm6Δ | ||
| Lsm7Δ | ||
| Atg5Δ | ||
| Dhh1Δ |
| Primer Name | Oligonucleotide Sequence |
|---|---|
| BamH1-ScLSM4/Sclsm4Δ1 Fw | 5’-AAAAAAGGATCCGTACGCAGTCACAATGCGG-3’ |
| SacI-ScLSM4 Rv | 5’-GGGGGGAGCTCACCTGTAAACTAAAGGAAAGCTCG-3’ |
| SacI-Sclsm4Δ1 Rv | 5’-GGGGGGAGCTCTTATCTTGCAATTTGATAAACTTGATAAAAGTCC-3’ |
4.2. Viability assays
4.3. Rapamycin treatment
4.4. H2O2 sensitivity test
4.5. Caffeine, acetic acid and rapamycin sensitivity test
4.6. Fluorescence microscopy
4.7. Protein extraction and Western Blot analysis of autophagy induced cells
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- C.J. Wilusz, J. Wilusz, Lsm proteins and Hfq: Life at the 3′ end, RNA Biol. 10 (2013) 592–601. [CrossRef]
- W. He, R. Parker, Functions of Lsm proteins in mRNA degradation and splicing, Curr. Opin. Cell Biol. 12 (2000) 346–350. [CrossRef]
- Z. Chen, C. Han, X. Zhou, X. Wang, X. Liao, Y. He, S. Mo, X. Li, G. Zhu, X. Ye, T. Peng, Prognostic value and potential molecular mechanism of the like-Sm gene family in early-stage pancreatic ductal adenocarcinoma, Transl. Cancer Res. 10 (2021) 1744–1760. [CrossRef]
- H.D.K. Ta, WotherJ. Wang, N.N. Phan, N.T. An Ton, G. Anuraga, S.-C. Ku, Y.-F. Wu, C.-Y. Wang, K.-H. Lee, Potential Therapeutic and Prognostic Values of LSM Family Genes in Breast Cancer, Cancers. 13 (2021) 4902. [CrossRef]
- Z.-P. Sun, Z.-G. Tan, C. Peng, Long noncoding RNA LINC01419 promotes hepatocellular carcinoma malignancy by mediating miR-485-5p/LSM4 axis, Kaohsiung J. Med. Sci. 38 (2022) 826–838. [CrossRef]
- C. Mazzoni, P. Mancini, F. Madeo, V. Palermo, C. Falcone, A Kluyveromyces lactis mutant in the essential gene KlLSM4 shows phenotypic markers of apoptosis, FEMS Yeast Res. 4 (2003) 29–35. [CrossRef]
- C. Mazzoni, P. Mancini, L. Verdone, F. Madeo, A. Serafini, E. Herker, C. Falcone, A Truncated Form of KlLsm4p and the Absence of Factors Involved in mRNA Decapping Trigger Apoptosis in Yeast, Mol. Biol. Cell. 14 (2003) 721–729. [CrossRef]
- C. Mazzoni, E. Herker, V. Palermo, H. Jungwirth, T. Eisenberg, F. Madeo, C. Falcone, Yeast caspase 1 links messenger RNA stability to apoptosis in yeast, EMBO Rep. 6 (2005) 1076–1081. [CrossRef]
- C. Mazzoni, I. D’Addario, C. Falcone, The C-terminus of the yeast Lsm4p is required for the association to P-bodies, FEBS Lett. 581 (2007) 4836–4840. [CrossRef]
- M.A.M. Reijns, R.D. Alexander, M.P. Spiller, J.D. Beggs, A role for Q/N-rich aggregation-prone regions in P-body localization, J. Cell Sci. 121 (2008) 2463–2472. [CrossRef]
- C.J. Decker, D. Teixeira, R. Parker, Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae, J. Cell Biol. 179 (2007) 437–449. [CrossRef]
- M. Kato, T.W. Han, S. Xie, K. Shi, X. Du, L.C. Wu, H. Mirzaei, E.J. Goldsmith, J. Longgood, J. Pei, N.V. Grishin, D.E. Frantz, J.W. Schneider, S. Chen, L. Li, M.R. Sawaya, D. Eisenberg, R. Tycko, S.L. McKnight, Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels, Cell. 149 (2012) 753–767. [CrossRef]
- M. Arribas-Layton, J. Dennis, E.J. Bennett, C.K. Damgaard, J. Lykke-Andersen, The C-Terminal RGG Domain of Human Lsm4 Promotes Processing Body Formation Stimulated by Arginine Dimethylation, Mol. Cell. Biol. 36 (2016) 2226–2235. [CrossRef]
- V. Palermo, E. Cundari, E. Mangiapelo, C. Falcone, C. Mazzoni, Yeast lsm pro-apoptotic mutants show defects in S-phase entry and progression, Cell Cycle Georget. Tex. 9 (2010) 3991–3996. [CrossRef]
- S. Lyons, A. Ricciardi, A. Guo, C. Kambach, W. Marzluff, The C-terminal extension of Lsm4 interacts directly with the 3′ end of the histone mRNP and is required for efficient histone mRNA degradation, RNA N. Y. N. 20 (2014). [CrossRef]
- M. Costanzo, A. Baryshnikova, J. Bellay, Y. Kim, E.D. Spear, C.S. Sevier, H. Ding, J.L.Y. Koh, K. Toufighi, S. Mostafavi, J. Prinz, R.P. St Onge, B. VanderSluis, T. Makhnevych, F.J. Vizeacoumar, S. Alizadeh, S. Bahr, R.L. Brost, Y. Chen, M. Cokol, R. Deshpande, Z. Li, Z.-Y. Lin, W. Liang, M. Marback, J. Paw, B.-J. San Luis, E. Shuteriqi, A.H.Y. Tong, N. van Dyk, I.M. Wallace, J.A. Whitney, M.T. Weirauch, G. Zhong, H. Zhu, W.A. Houry, M. Brudno, S. Ragibizadeh, B. Papp, C. Pál, F.P. Roth, G. Giaever, C. Nislow, O.G. Troyanskaya, H. Bussey, G.D. Bader, A.-C. Gingras, Q.D. Morris, P.M. Kim, C.A. Kaiser, C.L. Myers, B.J. Andrews, C. Boone, The genetic landscape of a cell, Science. 327 (2010) 425–431. [CrossRef]
- C. Mazzoni, C. Falcone, mRNA stability and control of cell proliferation, Biochem. Soc. Trans. 39 (2011) 1461–1465. [CrossRef]
- S.F. Mitchell, S. Jain, M. She, R. Parker, Global analysis of yeast mRNPs, Nat. Struct. Mol. Biol. 20 (2013) 127–133. [CrossRef]
- E. Delorme-Axford, D.J. Klionsky, On the edge of degradation: Autophagy regulation by RNA decay, Wiley Interdiscip. Rev. RNA. 10 (2019) e1522. [CrossRef]
- G. Hu, T. McQuiston, A. Bernard, Y.-D. Park, J. Qiu, A. Vural, N. Zhang, S.R. Waterman, N.H. Blewett, T.G. Myers, R.J. Maraia, J.H. Kehrl, G. Uzel, D.J. Klionsky, P.R. Williamson, A conserved mechanism of TOR-dependent RCK-mediated mRNA degradation regulates autophagy, Nat. Cell Biol. 17 (2015) 930–942. [CrossRef]
- X. Liu, M. Jin, Z. Yao, A. Bernard, D.J. Klionsky, Bidirectional roles of Dhh1 in regulating autophagy, Autophagy. 15 (2019) 1838–1839. [CrossRef]
- Z. Yin, Z. Zhang, Y. Lei, D.J. Klionsky, Bidirectional roles of the Ccr4-Not complex in regulating autophagy before and after nitrogen starvation, Autophagy. 19 (2023) 415–425. [CrossRef]
- Chowdhury, J. Mukhopadhyay, S. Tharun, The decapping activator Lsm1p-7p–Pat1p complex has the intrinsic ability to distinguish between oligoadenylated and polyadenylated RNAs, RNA. 13 (2007) 998–1016. [CrossRef]
- Chowdhury, S. Kalurupalle, S. Tharun, Pat1 contributes to the RNA binding activity of the Lsm1-7–Pat1 complex, RNA. 20 (2014) 1465–1475. [CrossRef]
- W. He, R. Parker, The yeast cytoplasmic LsmI/Pat1p complex protects mRNA 3′ termini from partial degradation, Genetics. 158 (2001) 1445–1455. [CrossRef]
- S. Tharun, D. Muhlrad, A. Chowdhury, R. Parker, Mutations in the Saccharomyces cerevisiae LSM1 gene that affect mRNA decapping and 3′ end protection, Genetics. 170 (2005) 33–46. [CrossRef]
- Chowdhury, S. Tharun, Activation of decapping involves binding of the mRNA and facilitation of the post-binding steps by the Lsm1-7-Pat1 complex, RNA N. Y. N. 15 (2009) 1837–1848. [CrossRef]
- D. Gatica, G. Hu, N. Zhang, P.R. Williamson, D.J. Klionsky, The Pat1-Lsm complex prevents 3′ to 5′ degradation of a specific subset of ATG mRNAs during nitrogen starvation-induced autophagy, Autophagy. 15 (2019) 750–751. [CrossRef]
- H. Huang, T. Kawamata, T. Horie, H. Tsugawa, Y. Nakayama, Y. Ohsumi, E. Fukusaki, Bulk RNA degradation by nitrogen starvation-induced autophagy in yeast, EMBO J. 34 (2015) 154–168. [CrossRef]
- S. Makino, T. Kawamata, S. Iwasaki, Y. Ohsumi, Selectivity of mRNA degradation by autophagy in yeast, Nat. Commun. 12 (2021) 2316. [CrossRef]
- M. Stirpe, V. Palermo, M. Ferrari, S. Mroczek, J. Kufel, C. Falcone, C. Mazzoni, Increased levels of RNA oxidation enhance the reversion frequency in aging pro-apoptotic yeast mutants, Apoptosis. 22 (2017) 200–206. [CrossRef]
- D. Carmona-Gutierrez, M.A. Bauer, A. Zimmermann, A. Aguilera, N. Austriaco, K. Ayscough, R. Balzan, S. Bar-Nun, A. Barrientos, P. Belenky, M. Blondel, R.J. Braun, M. Breitenbach, W.C. Burhans, S. Büttner, D. Cavalieri, M. Chang, K.F. Cooper, M. Côrte-Real, V. Costa, C. Cullin, I. Dawes, J. Dengjel, M.B. Dickman, T. Eisenberg, B. Fahrenkrog, N. Fasel, K.-U. Fröhlich, A. Gargouri, S. Giannattasio, P. Goffrini, C.W. Gourlay, C.M. Grant, M.T. Greenwood, N. Guaragnella, T. Heger, J. Heinisch, E. Herker, J.M. Herrmann, S. Hofer, A. Jiménez-Ruiz, H. Jungwirth, K. Kainz, D.P. Kontoyiannis, P. Ludovico, S. Manon, E. Martegani, C. Mazzoni, L.A. Megeney, C. Meisinger, J. Nielsen, T. Nyström, H.D. Osiewacz, T.F. Outeiro, H.-O. Park, T. Pendl, D. Petranovic, S. Picot, P. Polčic, T. Powers, M. Ramsdale, M. Rinnerthaler, P. Rockenfeller, C. Ruckenstuhl, R. Schaffrath, M. Segovia, F.F. Severin, A. Sharon, S.J. Sigrist, C. Sommer-Ruck, M.J. Sousa, J.M. Thevelein, K. Thevissen, V. Titorenko, M.B. Toledano, M. Tuite, F.-N. Vögtle, B. Westermann, J. Winderickx, S. Wissing, S. Wölfl, Z.J. Zhang, R.Y. Zhao, B. Zhou, L. Galluzzi, G. Kroemer, F. Madeo, Guidelines and recommendations on yeast cell death nomenclature, Microb. Cell Graz Austria. 5 (2018) 4–31. [CrossRef]
- V. Palermo, M. Stirpe, M. Torella, C. Falcone, C. Mazzoni, NEM1 acts as a suppressor of apoptotic phenotypes in LSM4 yeast mutants, FEMS Yeast Res. 15 (2015). [CrossRef]
- M.A. Rahman, M.G. Mostofa, T. Ushimaru, The Nem1/Spo7-Pah1/lipin axis is required for autophagy induction after TORC1 inactivation, FEBS J. 285 (2018) 1840–1860. [CrossRef]
- D.J. Klionsky, A.K. Abdel-Aziz, S. Abdelfatah, M. Abdellatif, A. Abdoli, S. Abel, H. Abeliovich, M.H. Abildgaard, Y.P. Abudu, A. Acevedo-Arozena, I.E. Adamopoulos, K. Adeli, T.E. Adolph, A. Adornetto, E. Aflaki, G. Agam, A. Agarwal, B.B. Aggarwal, M. Agnello, P. Agostinis, J.N. Agrewala, A. Agrotis, P.V. Aguilar, S.T. Ahmad, Z.M. Ahmed, U. Ahumada-Castro, S. Aits, S. Aizawa, Y. Akkoc, T. Akoumianaki, H.A. Akpinar, A.M. Al-Abd, L. Al-Akra, A. Al-Gharaibeh, M.A. Alaoui-Jamali, S. Alberti, E. Alcocer-Gómez, C. Alessandri, M. Ali, M.A. Alim Al-Bari, S. Aliwaini, J. Alizadeh, E. Almacellas, A. Almasan, A. Alonso, G.D. Alonso, N. Altan-Bonnet, D.C. Altieri, É.M.C. Álvarez, S. Alves, C. Alves da Costa, M.M. Alzaharna, M. Amadio, C. Amantini, C. Amaral, S. Ambrosio, A.O. Amer, V. Ammanathan, Z. An, S.U. Andersen, S.A. Andrabi, M. Andrade-Silva, A.M. Andres, S. Angelini, D. Ann, U.C. Anozie, M.Y. Ansari, P. Antas, A. Antebi, Z. Antón, T. Anwar, L. Apetoh, N. Apostolova, T. Araki, Y. Araki, K. Arasaki, W.L. Araújo, J. Araya, C. Arden, M.-A. Arévalo, S. Arguelles, E. Arias, J. Arikkath, H. Arimoto, A.R. Ariosa, D. Armstrong-James, L. Arnauné-Pelloquin, A. Aroca, D.S. Arroyo, I. Arsov, R. Artero, D.M.L. Asaro, M. Aschner, M. Ashrafizadeh, O. Ashur-Fabian, A.G. Atanasov, A.K. Au, P. Auberger, H.W. Auner, L. Aurelian, R. Autelli, L. Avagliano, Y. Ávalos, S. Aveic, C.A. Aveleira, T. Avin-Wittenberg, Y. Aydin, S. Ayton, S. Ayyadevara, M. Azzopardi, M. Baba, J.M. Backer, S.K. Backues, D.-H. Bae, O.-N. Bae, S.H. Bae, E.H. Baehrecke, A. Baek, S.-H. Baek, S.H. Baek, G. Bagetta, A. Bagniewska-Zadworna, H. Bai, J. Bai, X. Bai, Y. Bai, N. Bairagi, S. Baksi, T. Balbi, C.T. Baldari, W. Balduini, A. Ballabio, M. Ballester, S. Balazadeh, R. Balzan, R. Bandopadhyay, S. Banerjee, S. Banerjee, Á. Bánréti, Y. Bao, M.S. Baptista, A. Baracca, C. Barbati, A. Bargiela, D. Barilà, P.G. Barlow, S.J. Barmada, E. Barreiro, G.E. Barreto, J. Bartek, B. Bartel, A. Bartolome, G.R. Barve, S.H. Basagoudanavar, D.C. Bassham, R.C. Bast, A. Basu, H. Batoko, I. Batten, E.E. Baulieu, B.L. Baumgarner, J. Bayry, R. Beale, I. Beau, F. Beaumatin, L.R.G. Bechara, G.R. Beck, M.F. Beers, J. Begun, C. Behrends, G.M.N. Behrens, R. Bei, E. Bejarano, S. Bel, C. Behl, A. Belaid, N. Belgareh-Touzé, C. Bellarosa, F. Belleudi, M. Belló Pérez, R. Bello-Morales, J.S. de O. Beltran, S. Beltran, D.M. Benbrook, M. Bendorius, B.A. Benitez, I. Benito-Cuesta, J. Bensalem, M.W. Berchtold, S. Berezowska, D. Bergamaschi, M. Bergami, A. Bergmann, L. Berliocchi, C. Berlioz-Torrent, A. Bernard, L. Berthoux, C.G. Besirli, S. Besteiro, V.M. Betin, R. Beyaert, J.S. Bezbradica, K. Bhaskar, I. Bhatia-Kissova, R. Bhattacharya, S. Bhattacharya, S. Bhattacharyya, M.S. Bhuiyan, S.K. Bhutia, L. Bi, X. Bi, T.J. Biden, K. Bijian, V.A. Billes, N. Binart, C. Bincoletto, A.B. Birgisdottir, G. Bjorkoy, G. Blanco, A. Blas-Garcia, J. Blasiak, R. Blomgran, K. Blomgren, J.S. Blum, E. Boada-Romero, M. Boban, K. Boesze-Battaglia, P. Boeuf, B. Boland, P. Bomont, P. Bonaldo, S.R. Bonam, L. Bonfili, J.S. Bonifacino, B.A. Boone, M.D. Bootman, M. Bordi, C. Borner, B.C. Bornhauser, G. Borthakur, J. Bosch, S. Bose, L.M. Botana, J. Botas, C.M. Boulanger, M.E. Boulton, M. Bourdenx, B. Bourgeois, N.M. Bourke, G. Bousquet, P. Boya, P.V. Bozhkov, L.H.M. Bozi, T.O. Bozkurt, D.E. Brackney, C.H. Brandts, R.J. Braun, G.H. Braus, R. Bravo-Sagua, J.M. Bravo-San Pedro, P. Brest, M.-A. Bringer, A. Briones-Herrera, V.C. Broaddus, P. Brodersen, J.L. Brodsky, S.L. Brody, P.G. Bronson, J.M. Bronstein, C.N. Brown, R.E. Brown, P.C. Brum, J.H. Brumell, N. Brunetti-Pierri, D. Bruno, R.J. Bryson-Richardson, C. Bucci, C. Buchrieser, M. Bueno, L.E. Buitrago-Molina, S. Buraschi, S. Buch, J.R. Buchan, E.M. Buckingham, H. Budak, M. Budini, G. Bultynck, F. Burada, J.R. Burgoyne, M.I. Burón, V. Bustos, S. Büttner, E. Butturini, A. Byrd, I. Cabas, S. Cabrera-Benitez, K. Cadwell, J. Cai, L. Cai, Q. Cai, M. Cairó, J.A. Calbet, G.A. Caldwell, K.A. Caldwell, J.A. Call, R. Calvani, A.C. Calvo, M. Calvo-Rubio Barrera, N.O. Camara, J.H. Camonis, N. Camougrand, M. Campanella, E.M. Campbell, F.-X. Campbell-Valois, S. Campello, I. Campesi, J.C. Campos, O. Camuzard, J. Cancino, D. Candido de Almeida, L. Canesi, I. Caniggia, B. Canonico, C. Cantí, B. Cao, M. Caraglia, B. Caramés, E.H. Carchman, E. Cardenal-Muñoz, C. Cardenas, L. Cardenas, S.M. Cardoso, J.S. Carew, G.F. Carle, G. Carleton, S. Carloni, D. Carmona-Gutierrez, L.A. Carneiro, O. Carnevali, J.M. Carosi, S. Carra, A. Carrier, L. Carrier, B. Carroll, A.B. Carter, A.N. Carvalho, M. Casanova, C. Casas, J. Casas, C. Cassioli, E.F. Castillo, K. Castillo, S. Castillo-Lluva, F. Castoldi, M. Castori, A.F. Castro, M. Castro-Caldas, J. Castro-Hernandez, S. Castro-Obregon, S.D. Catz, C. Cavadas, F. Cavaliere, G. Cavallini, M. Cavinato, M.L. Cayuela, P. Cebollada Rica, V. Cecarini, F. Cecconi, M. Cechowska-Pasko, S. Cenci, V. Ceperuelo-Mallafré, J.J. Cerqueira, J.M. Cerutti, D. Cervia, V.B. Cetintas, S. Cetrullo, H.-J. Chae, A.S. Chagin, C.-Y. Chai, G. Chakrabarti, O. Chakrabarti, T. Chakraborty, T. Chakraborty, M. Chami, G. Chamilos, D.W. Chan, E.Y.W. Chan, E.D. Chan, H.Y.E. Chan, H.H. Chan, H. Chan, M.T.V. Chan, Y.S. Chan, P.K. Chandra, C.-P. Chang, C. Chang, H.-C. Chang, K. Chang, J. Chao, T. Chapman, N. Charlet-Berguerand, S. Chatterjee, S.K. Chaube, A. Chaudhary, S. Chauhan, E. Chaum, F. Checler, M.E. Cheetham, C.-S. Chen, G.-C. Chen, J.-F. Chen, L.L. Chen, L. Chen, L. Chen, M. Chen, M.-K. Chen, N. Chen, Q. Chen, R.-H. Chen, S. Chen, W. Chen, W. Chen, X.-M. Chen, X.-W. Chen, X. Chen, Y. Chen, Y.-G. Chen, Y. Chen, Y. Chen, Y.-J. Chen, Y.-Q. Chen, Z.S. Chen, Z. Chen, Z.-H. Chen, Z.J. Chen, Z. Chen, H. Cheng, J. Cheng, S.-Y. Cheng, W. Cheng, X. Cheng, X.-T. Cheng, Y. Cheng, Z. Cheng, Z. Chen, H. Cheong, J.K. Cheong, B.V. Chernyak, S. Cherry, C.F.R. Cheung, C.H.A. Cheung, K.-H. Cheung, E. Chevet, R.J. Chi, A.K.S. Chiang, F. Chiaradonna, R. Chiarelli, M. Chiariello, N. Chica, S. Chiocca, M. Chiong, S.-H. Chiou, A.I. Chiramel, V. Chiurchiù, D.-H. Cho, S.-K. Choe, A.M.K. Choi, M.E. Choi, K.R. Choudhury, N.S. Chow, C.T. Chu, J.P. Chua, J.J.E. Chua, H. Chung, K.P. Chung, S. Chung, S.-H. Chung, Y.-L. Chung, V. Cianfanelli, I.A. Ciechomska, M. Cifuentes, L. Cinque, S. Cirak, M. Cirone, M.J. Clague, R. Clarke, E. Clementi, E.M. Coccia, P. Codogno, E. Cohen, M.M. Cohen, T. Colasanti, F. Colasuonno, R.A. Colbert, A. Colell, M. Čolić, N.S. Coll, M.O. Collins, M.I. Colombo, D.A. Colón-Ramos, L. Combaret, S. Comincini, M.R. Cominetti, A. Consiglio, A. Conte, F. Conti, V.R. Contu, M.R. Cookson, K.M. Coombs, I. Coppens, M.T. Corasaniti, D.P. Corkery, N. Cordes, K. Cortese, M. do C. Costa, S. Costantino, P. Costelli, A. Coto-Montes, P.J. Crack, J.L. Crespo, A. Criollo, V. Crippa, R. Cristofani, T. Csizmadia, A. Cuadrado, B. Cui, J. Cui, Y. Cui, Y. Cui, E. Culetto, A.C. Cumino, A.V. Cybulsky, M.J. Czaja, S.J. Czuczwar, S. D’Adamo, M. D’Amelio, D. D’Arcangelo, A.C. D’Lugos, G. D’Orazi, J.A. da Silva, H.S. Dafsari, R.K. Dagda, Y. Dagdas, M. Daglia, X. Dai, Y. Dai, Y. Dai, J. Dal Col, P. Dalhaimer, L. Dalla Valle, T. Dallenga, G. Dalmasso, M. Damme, I. Dando, N.P. Dantuma, A.L. Darling, H. Das, S. Dasarathy, S.K. Dasari, S. Dash, O. Daumke, A.N. Dauphinee, J.S. Davies, V.A. Dávila, R.J. Davis, T. Davis, S. Dayalan Naidu, F. De Amicis, K. De Bosscher, F. De Felice, L. De Franceschi, C. De Leonibus, M.G. de Mattos Barbosa, G.R.Y. De Meyer, A. De Milito, C. De Nunzio, C. De Palma, M. De Santi, C. De Virgilio, D. De Zio, J. Debnath, B.J. DeBosch, J.-P. Decuypere, M.A. Deehan, G. Deflorian, J. DeGregori, B. Dehay, G. Del Rio, J.R. Delaney, L.M.D. Delbridge, E. Delorme-Axford, M.V. Delpino, F. Demarchi, V. Dembitz, N.D. Demers, H. Deng, Z. Deng, J. Dengjel, P. Dent, D. Denton, M.L. DePamphilis, C.J. Der, V. Deretic, A. Descoteaux, L. Devis, S. Devkota, O. Devuyst, G. Dewson, M. Dharmasivam, R. Dhiman, D. di Bernardo, M. Di Cristina, F. Di Domenico, P. Di Fazio, A. Di Fonzo, G. Di Guardo, G.M. Di Guglielmo, L. Di Leo, C. Di Malta, A. Di Nardo, M. Di Rienzo, F. Di Sano, G. Diallinas, J. Diao, G. Diaz-Araya, I. Díaz-Laviada, J.M. Dickinson, M. Diederich, M. Dieudé, I. Dikic, S. Ding, W.-X. Ding, L. Dini, J. Dinić, M. Dinic, A.T. Dinkova-Kostova, M.S. Dionne, J.H.W. Distler, A. Diwan, I.M.C. Dixon, M. Djavaheri-Mergny, I. Dobrinski, O. Dobrovinskaya, R. Dobrowolski, R.C.J. Dobson, J. Đokić, S. Dokmeci Emre, M. Donadelli, B. Dong, X. Dong, Z. Dong, G.W. Dorn Ii, V. Dotsch, H. Dou, J. Dou, M. Dowaidar, S. Dridi, L. Drucker, A. Du, C. Du, G. Du, H.-N. Du, L.-L. Du, A. du Toit, S.-B. Duan, X. Duan, S.P. Duarte, A. Dubrovska, E.A. Dunlop, N. Dupont, R.V. Durán, B.S. Dwarakanath, S.A. Dyshlovoy, D. Ebrahimi-Fakhari, L. Eckhart, C.L. Edelstein, T. Efferth, E. Eftekharpour, L. Eichinger, N. Eid, T. Eisenberg, N.T. Eissa, S. Eissa, M. Ejarque, A. El Andaloussi, N. El-Hage, S. El-Naggar, A.M. Eleuteri, E.S. El-Shafey, M. Elgendy, A.G. Eliopoulos, M.M. Elizalde, P.M. Elks, H.-P. Elsasser, E.S. Elsherbiny, B.M. Emerling, N.C.T. Emre, C.H. Eng, N. Engedal, A.-M. Engelbrecht, A.S.T. Engelsen, J.M. Enserink, R. Escalante, A. Esclatine, M. Escobar-Henriques, E.-L. Eskelinen, L. Espert, M.-O. Eusebio, G. Fabrias, C. Fabrizi, A. Facchiano, F. Facchiano, B. Fadeel, C. Fader, A.C. Faesen, W.D. Fairlie, A. Falcó, B.H. Falkenburger, D. Fan, J. Fan, Y. Fan, E.F. Fang, Y. Fang, Y. Fang, M. Fanto, T. Farfel-Becker, M. Faure, G. Fazeli, A.O. Fedele, A.M. Feldman, D. Feng, J. Feng, L. Feng, Y. Feng, Y. Feng, W. Feng, T. Fenz Araujo, T.A. Ferguson, Á.F. Fernández, J.C. Fernandez-Checa, S. Fernández-Veledo, A.R. Fernie, A.W. Ferrante, A. Ferraresi, M.F. Ferrari, J.C.B. Ferreira, S. Ferro-Novick, A. Figueras, R. Filadi, N. Filigheddu, E. Filippi-Chiela, G. Filomeni, G.M. Fimia, V. Fineschi, F. Finetti, S. Finkbeiner, E.A. Fisher, P.B. Fisher, F. Flamigni, S.J. Fliesler, T.H. Flo, I. Florance, O. Florey, T. Florio, E. Fodor, C. Follo, E.A. Fon, A. Forlino, F. Fornai, P. Fortini, A. Fracassi, A. Fraldi, B. Franco, R. Franco, F. Franconi, L.B. Frankel, S.L. Friedman, L.F. Fröhlich, G. Frühbeck, J.M. Fuentes, Y. Fujiki, N. Fujita, Y. Fujiwara, M. Fukuda, S. Fulda, L. Furic, N. Furuya, C. Fusco, M.U. Gack, L. Gaffke, S. Galadari, A. Galasso, M.F. Galindo, S. Gallolu Kankanamalage, L. Galluzzi, V. Galy, N. Gammoh, B. Gan, I.G. Ganley, F. Gao, H. Gao, M. Gao, P. Gao, S.-J. Gao, W. Gao, X. Gao, A. Garcera, M.N. Garcia, V.E. Garcia, F. García-Del Portillo, V. Garcia-Escudero, A. Garcia-Garcia, M. Garcia-Macia, D. García-Moreno, C. Garcia-Ruiz, P. García-Sanz, A.D. Garg, R. Gargini, T. Garofalo, R.F. Garry, N.C. Gassen, D. Gatica, L. Ge, W. Ge, R. Geiss-Friedlander, C. Gelfi, P. Genschik, I.E. Gentle, V. Gerbino, C. Gerhardt, K. Germain, M. Germain, D.A. Gewirtz, E. Ghasemipour Afshar, S. Ghavami, A. Ghigo, M. Ghosh, G. Giamas, C. Giampietri, A. Giatromanolaki, G.E. Gibson, S.B. Gibson, V. Ginet, E. Giniger, C. Giorgi, H. Girao, S.E. Girardin, M. Giridharan, S. Giuliano, C. Giulivi, S. Giuriato, J. Giustiniani, A. Gluschko, V. Goder, A. Goginashvili, J. Golab, D.C. Goldstone, A. Golebiewska, L.R. Gomes, R. Gomez, R. Gómez-Sánchez, M.C. Gomez-Puerto, R. Gomez-Sintes, Q. Gong, F.M. Goni, J. González-Gallego, T. Gonzalez-Hernandez, R.A. Gonzalez-Polo, J.A. Gonzalez-Reyes, P. González-Rodríguez, I.S. Goping, M.S. Gorbatyuk, N.V. Gorbunov, K. Görgülü, R.M. Gorojod, S.M. Gorski, S. Goruppi, C. Gotor, R.A. Gottlieb, I. Gozes, D. Gozuacik, M. Graef, M.H. Gräler, V. Granatiero, D. Grasso, J.P. Gray, D.R. Green, A. Greenhough, S.L. Gregory, E.F. Griffin, M.W. Grinstaff, F. Gros, C. Grose, A.S. Gross, F. Gruber, P. Grumati, T. Grune, X. Gu, J.-L. Guan, C.M. Guardia, K. Guda, F. Guerra, C. Guerri, P. Guha, C. Guillén, S. Gujar, A. Gukovskaya, I. Gukovsky, J. Gunst, A. Günther, A.R. Guntur, C. Guo, C. Guo, H. Guo, L.-W. Guo, M. Guo, P. Gupta, S.K. Gupta, S. Gupta, V.B. Gupta, V. Gupta, A.B. Gustafsson, D.D. Gutterman, R. H B, A. Haapasalo, J.E. Haber, A. Hać, S. Hadano, A.J. Hafrén, M. Haidar, B.S. Hall, G. Halldén, A. Hamacher-Brady, A. Hamann, M. Hamasaki, W. Han, M. Hansen, P.I. Hanson, Z. Hao, M. Harada, L. Harhaji-Trajkovic, N. Hariharan, N. Haroon, J. Harris, T. Hasegawa, N. Hasima Nagoor, J.A. Haspel, V. Haucke, W.D. Hawkins, B.A. Hay, C.M. Haynes, S.B. Hayrabedyan, T.S. Hays, C. He, Q. He, R.-R. He, Y.-W. He, Y.-Y. He, Y. Heakal, A.M. Heberle, J.F. Hejtmancik, G.V. Helgason, V. Henkel, M. Herb, A. Hergovich, A. Herman-Antosiewicz, A. Hernández, C. Hernandez, S. Hernandez-Diaz, V. Hernandez-Gea, A. Herpin, J. Herreros, J.H. Hervás, D. Hesselson, C. Hetz, V.T. Heussler, Y. Higuchi, S. Hilfiker, J.A. Hill, W.S. Hlavacek, E.A. Ho, I.H.T. Ho, P.W.-L. Ho, S.-L. Ho, W.Y. Ho, G.A. Hobbs, M. Hochstrasser, P.H.M. Hoet, D. Hofius, P. Hofman, A. Höhn, C.I. Holmberg, J.R. Hombrebueno, C.-W.H. Yi-Ren Hong, L.V. Hooper, T. Hoppe, R. Horos, Y. Hoshida, I.-L. Hsin, H.-Y. Hsu, B. Hu, D. Hu, L.-F. Hu, M.C. Hu, R. Hu, W. Hu, Y.-C. Hu, Z.-W. Hu, F. Hua, J. Hua, Y. Hua, C. Huan, C. Huang, C. Huang, C. Huang, C. Huang, H. Huang, K. Huang, M.L.H. Huang, R. Huang, S. Huang, T. Huang, X. Huang, Y.J. Huang, T.B. Huber, V. Hubert, C.A. Hubner, S.M. Hughes, W.E. Hughes, M. Humbert, G. Hummer, J.H. Hurley, S. Hussain, S. Hussain, P.J. Hussey, M. Hutabarat, H.-Y. Hwang, S. Hwang, A. Ieni, F. Ikeda, Y. Imagawa, Y. Imai, C. Imbriano, M. Imoto, D.M. Inman, K. Inoki, J. Iovanna, R.V. Iozzo, G. Ippolito, J.E. Irazoqui, P. Iribarren, M. Ishaq, M. Ishikawa, N. Ishimwe, C. Isidoro, N. Ismail, S. Issazadeh-Navikas, E. Itakura, D. Ito, D. Ivankovic, S. Ivanova, A.K.V. Iyer, J.M. Izquierdo, M. Izumi, M. Jäättelä, M.S. Jabir, W.T. Jackson, N. Jacobo-Herrera, A.-C. Jacomin, E. Jacquin, P. Jadiya, H. Jaeschke, C. Jagannath, A.J. Jakobi, J. Jakobsson, B. Janji, P. Jansen-Dürr, P.J. Jansson, J. Jantsch, S. Januszewski, A. Jassey, S. Jean, H. Jeltsch-David, P. Jendelova, A. Jenny, T.E. Jensen, N. Jessen, J.L. Jewell, J. Ji, L. Jia, R. Jia, L. Jiang, Q. Jiang, R. Jiang, T. Jiang, X. Jiang, Y. Jiang, M. Jimenez-Sanchez, E.-J. Jin, F. Jin, H. Jin, L. Jin, L. Jin, M. Jin, S. Jin, E.-K. Jo, C. Joffre, T. Johansen, G.V.W. Johnson, S.A. Johnston, E. Jokitalo, M.K. Jolly, L.A.B. Joosten, J. Jordan, B. Joseph, D. Ju, J.-S. Ju, J. Ju, E. Juárez, D. Judith, G. Juhász, Y. Jun, C.H. Jung, S.-C. Jung, Y.K. Jung, H. Jungbluth, J. Jungverdorben, S. Just, K. Kaarniranta, A. Kaasik, T. Kabuta, D. Kaganovich, A. Kahana, R. Kain, S. Kajimura, M. Kalamvoki, M. Kalia, D.S. Kalinowski, N. Kaludercic, I. Kalvari, J. Kaminska, V.O. Kaminskyy, H. Kanamori, K. Kanasaki, C. Kang, R. Kang, S.S. Kang, S. Kaniyappan, T. Kanki, T.-D. Kanneganti, A.G. Kanthasamy, A. Kanthasamy, M. Kantorow, O. Kapuy, M.V. Karamouzis, M.R. Karim, P. Karmakar, R.G. Katare, M. Kato, S.H.E. Kaufmann, A. Kauppinen, G.P. Kaushal, S. Kaushik, K. Kawasaki, K. Kazan, P.-Y. Ke, D.J. Keating, U. Keber, J.H. Kehrl, K.E. Keller, C.W. Keller, J.K. Kemper, C.M. Kenific, O. Kepp, S. Kermorgant, A. Kern, R. Ketteler, T.G. Keulers, B. Khalfin, H. Khalil, B. Khambu, S.Y. Khan, V.K.M. Khandelwal, R. Khandia, W. Kho, N.V. Khobrekar, S. Khuansuwan, M. Khundadze, S.A. Killackey, D. Kim, D.R. Kim, D.-H. Kim, D.-E. Kim, E.Y. Kim, E.-K. Kim, H.-R. Kim, H.-S. Kim, null Hyung-Ryong Kim, J.H. Kim, J.K. Kim, J.-H. Kim, J. Kim, J.H. Kim, K.I. Kim, P.K. Kim, S.-J. Kim, S.R. Kimball, A. Kimchi, A.C. Kimmelman, T. Kimura, M.A. King, K.J. Kinghorn, C.G. Kinsey, V. Kirkin, L.A. Kirshenbaum, S.L. Kiselev, S. Kishi, K. Kitamoto, Y. Kitaoka, K. Kitazato, R.N. Kitsis, J.T. Kittler, O. Kjaerulff, P.S. Klein, T. Klopstock, J. Klucken, H. Knævelsrud, R.L. Knorr, B.C.B. Ko, F. Ko, J.-L. Ko, H. Kobayashi, S. Kobayashi, I. Koch, J.C. Koch, U. Koenig, D. Kögel, Y.H. Koh, M. Koike, S.D. Kohlwein, N.M. Kocaturk, M. Komatsu, J. König, T. Kono, B.T. Kopp, T. Korcsmaros, G. Korkmaz, V.I. Korolchuk, M.S. Korsnes, A. Koskela, J. Kota, Y. Kotake, M.L. Kotler, Y. Kou, M.I. Koukourakis, E. Koustas, A.L. Kovacs, T. Kovács, D. Koya, T. Kozako, C. Kraft, D. Krainc, H. Krämer, A.D. Krasnodembskaya, C. Kretz-Remy, G. Kroemer, N.T. Ktistakis, K. Kuchitsu, S. Kuenen, L. Kuerschner, T. Kukar, A. Kumar, A. Kumar, D. Kumar, D. Kumar, S. Kumar, S. Kume, C. Kumsta, C.N. Kundu, M. Kundu, A.B. Kunnumakkara, L. Kurgan, T.G. Kutateladze, O. Kutlu, S. Kwak, H.J. Kwon, T.K. Kwon, Y.T. Kwon, I. Kyrmizi, A. La Spada, P. Labonté, S. Ladoire, I. Laface, F. Lafont, D.C. Lagace, V. Lahiri, Z. Lai, A.S. Laird, A. Lakkaraju, T. Lamark, S.-H. Lan, A. Landajuela, D.J.R. Lane, J.D. Lane, C.H. Lang, C. Lange, Ü. Langel, R. Langer, P. Lapaquette, J. Laporte, N.F. LaRusso, I. Lastres-Becker, W.C.Y. Lau, G.W. Laurie, S. Lavandero, B.Y.K. Law, H.K.-W. Law, R. Layfield, W. Le, H. Le Stunff, A.Y. Leary, J.-J. Lebrun, L.Y.W. Leck, J.-P. Leduc-Gaudet, C. Lee, C.-P. Lee, D.-H. Lee, E.B. Lee, E.F. Lee, G.M. Lee, H.-J. Lee, H.K. Lee, J.M. Lee, J.S. Lee, J.-A. Lee, J.-Y. Lee, J.H. Lee, M. Lee, M.G. Lee, M.J. Lee, M.-S. Lee, S.Y. Lee, S.-J. Lee, S.Y. Lee, S.B. Lee, W.H. Lee, Y.-R. Lee, Y.-H. Lee, Y. Lee, C. Lefebvre, R. Legouis, Y.L. Lei, Y. Lei, S. Leikin, G. Leitinger, L. Lemus, S. Leng, O. Lenoir, G. Lenz, H.J. Lenz, P. Lenzi, Y. León, A.M. Leopoldino, C. Leschczyk, S. Leskelä, E. Letellier, C.-T. Leung, P.S. Leung, J.S. Leventhal, B. Levine, P.A. Lewis, K. Ley, B. Li, D.-Q. Li, J. Li, J. Li, J. Li, K. Li, L. Li, M. Li, M. Li, M. Li, M. Li, M. Li, P.-L. Li, M.-Q. Li, Q. Li, S. Li, T. Li, W. Li, W. Li, X. Li, Y.-P. Li, Y. Li, Z. Li, Z. Li, Z. Li, J. Lian, C. Liang, Q. Liang, W. Liang, Y. Liang, Y. Liang, G. Liao, L. Liao, M. Liao, Y.-F. Liao, M. Librizzi, P.P.Y. Lie, M.A. Lilly, H.J. Lim, T.R.R. Lima, F. Limana, C. Lin, C.-W. Lin, D.-S. Lin, F.-C. Lin, J.D. Lin, K.M. Lin, K.-H. Lin, L.-T. Lin, P.-H. Lin, Q. Lin, S. Lin, S.-J. Lin, W. Lin, X. Lin, Y.-X. Lin, Y.-S. Lin, R. Linden, P. Lindner, S.-C. Ling, P. Lingor, A.K. Linnemann, Y.-C. Liou, M.M. Lipinski, S. Lipovšek, V.A. Lira, N. Lisiak, P.B. Liton, C. Liu, C.-H. Liu, C.-F. Liu, C.H. Liu, F. Liu, H. Liu, H.-S. Liu, H.-F. Liu, H. Liu, J. Liu, J. Liu, J. Liu, L. Liu, L. Liu, M. Liu, Q. Liu, W. Liu, W. Liu, X.-H. Liu, X. Liu, X. Liu, X. Liu, X. Liu, Y. Liu, Y. Liu, Y. Liu, Y. Liu, Y. Liu, J.A. Livingston, G. Lizard, J.M. Lizcano, S. Ljubojevic-Holzer, M.E. LLeonart, D. Llobet-Navàs, A. Llorente, C.H. Lo, D. Lobato-Márquez, Q. Long, Y.C. Long, B. Loos, J.A. Loos, M.G. López, G. López-Doménech, J.A. López-Guerrero, A.T. López-Jiménez, Ó. López-Pérez, I. López-Valero, M.J. Lorenowicz, M. Lorente, P. Lorincz, L. Lossi, S. Lotersztajn, P.E. Lovat, J.F. Lovell, A. Lovy, P. Lőw, G. Lu, H. Lu, J.-H. Lu, J.-J. Lu, M. Lu, S. Lu, A. Luciani, J.M. Lucocq, P. Ludovico, M.A. Luftig, M. Luhr, D. Luis-Ravelo, J.J. Lum, L. Luna-Dulcey, A.H. Lund, V.K. Lund, J.D. Lünemann, P. Lüningschrör, H. Luo, R. Luo, S. Luo, Z. Luo, C. Luparello, B. Lüscher, L. Luu, A. Lyakhovich, K.G. Lyamzaev, A.H. Lystad, L. Lytvynchuk, A.C. Ma, C. Ma, M. Ma, N.-F. Ma, Q.-H. Ma, X. Ma, Y. Ma, Z. Ma, O.A. MacDougald, F. Macian, G.C. MacIntosh, J.P. MacKeigan, K.F. Macleod, S. Maday, F. Madeo, M. Madesh, T. Madl, J. Madrigal-Matute, A. Maeda, Y. Maejima, M. Magarinos, P. Mahavadi, E. Maiani, K. Maiese, P. Maiti, M.C. Maiuri, B. Majello, M.B. Major, E. Makareeva, F. Malik, K. Mallilankaraman, W. Malorni, A. Maloyan, N. Mammadova, G.C.W. Man, F. Manai, J.D. Mancias, E.-M. Mandelkow, M.A. Mandell, A.A. Manfredi, M.H. Manjili, R. Manjithaya, P. Manque, B.B. Manshian, R. Manzano, C. Manzoni, K. Mao, C. Marchese, S. Marchetti, A.M. Marconi, F. Marcucci, S. Mardente, O.A. Mareninova, M. Margeta, M. Mari, S. Marinelli, O. Marinelli, G. Mariño, S. Mariotto, R.S. Marshall, M.R. Marten, S. Martens, A.P.J. Martin, K.R. Martin, S. Martin, S. Martin, A. Martín-Segura, M.A. Martín-Acebes, I. Martin-Burriel, M. Martin-Rincon, P. Martin-Sanz, J.A. Martina, W. Martinet, A. Martinez, A. Martinez, J. Martinez, M. Martinez Velazquez, N. Martinez-Lopez, M. Martinez-Vicente, D.O. Martins, J.O. Martins, W.K. Martins, T. Martins-Marques, E. Marzetti, S. Masaldan, C. Masclaux-Daubresse, D.G. Mashek, V. Massa, L. Massieu, G.R. Masson, L. Masuelli, A.I. Masyuk, T.V. Masyuk, P. Matarrese, A. Matheu, S. Matoba, S. Matsuzaki, P. Mattar, A. Matte, D. Mattoscio, J.L. Mauriz, M. Mauthe, C. Mauvezin, E. Maverakis, P. Maycotte, J. Mayer, G. Mazzoccoli, C. Mazzoni, J.R. Mazzulli, N. McCarty, C. McDonald, M.R. McGill, S.L. McKenna, B. McLaughlin, F. McLoughlin, M.A. McNiven, T.G. McWilliams, F. Mechta-Grigoriou, T.C. Medeiros, D.L. Medina, L.A. Megeney, K. Megyeri, M. Mehrpour, J.L. Mehta, A.J. Meijer, A.H. Meijer, J. Mejlvang, A. Meléndez, A. Melk, G. Memisoglu, A.F. Mendes, D. Meng, F. Meng, T. Meng, R. Menna-Barreto, M.B. Menon, C. Mercer, A.E. Mercier, J.-L. Mergny, A. Merighi, S.D. Merkley, G. Merla, V. Meske, A.C. Mestre, S.P. Metur, C. Meyer, H. Meyer, W. Mi, J. Mialet-Perez, J. Miao, L. Micale, Y. Miki, E. Milan, M. Milczarek, D.L. Miller, S.I. Miller, S. Miller, S.W. Millward, I. Milosevic, E.A. Minina, H. Mirzaei, H.R. Mirzaei, M. Mirzaei, A. Mishra, N. Mishra, P.K. Mishra, M. Misirkic Marjanovic, R. Misasi, A. Misra, G. Misso, C. Mitchell, G. Mitou, T. Miura, S. Miyamoto, M. Miyazaki, M. Miyazaki, T. Miyazaki, K. Miyazawa, N. Mizushima, T.H. Mogensen, B. Mograbi, R. Mohammadinejad, Y. Mohamud, A. Mohanty, S. Mohapatra, T. Möhlmann, A. Mohmmed, A. Moles, K.H. Moley, M. Molinari, V. Mollace, A.B. Møller, B. Mollereau, F. Mollinedo, C. Montagna, M.J. Monteiro, A. Montella, L.R. Montes, B. Montico, V.K. Mony, G. Monzio Compagnoni, M.N. Moore, M.A. Moosavi, A.L. Mora, M. Mora, D. Morales-Alamo, R. Moratalla, P.I. Moreira, E. Morelli, S. Moreno, D. Moreno-Blas, V. Moresi, B. Morga, A.H. Morgan, F. Morin, H. Morishita, O.L. Moritz, M. Moriyama, Y. Moriyasu, M. Morleo, E. Morselli, J.F. Moruno-Manchon, J. Moscat, S. Mostowy, E. Motori, A.F. Moura, N. Moustaid-Moussa, M. Mrakovcic, G. Muciño-Hernández, A. Mukherjee, S. Mukhopadhyay, J.M. Mulcahy Levy, V. Mulero, S. Muller, C. Münch, A. Munjal, P. Munoz-Canoves, T. Muñoz-Galdeano, C. Münz, T. Murakawa, C. Muratori, B.M. Murphy, J.P. Murphy, A. Murthy, T.T. Myöhänen, I.U. Mysorekar, J. Mytych, S.M. Nabavi, M. Nabissi, P. Nagy, J. Nah, A. Nahimana, I. Nakagawa, K. Nakamura, H. Nakatogawa, S.S. Nandi, M. Nanjundan, M. Nanni, G. Napolitano, R. Nardacci, M. Narita, M. Nassif, I. Nathan, M. Natsumeda, R.J. Naude, C. Naumann, O. Naveiras, F. Navid, S.T. Nawrocki, T.Y. Nazarko, F. Nazio, F. Negoita, T. Neill, A.L. Neisch, L.M. Neri, M.G. Netea, P. Neubert, T.P. Neufeld, D. Neumann, A. Neutzner, P.T. Newton, P.A. Ney, I.P. Nezis, C.C.W. Ng, T.B. Ng, H.T.T. Nguyen, L.T. Nguyen, H.-M. Ni, C. Ní Cheallaigh, Z. Ni, M.C. Nicolao, F. Nicoli, M. Nieto-Diaz, P. Nilsson, S. Ning, R. Niranjan, H. Nishimune, M. Niso-Santano, R.A. Nixon, A. Nobili, C. Nobrega, T. Noda, U. Nogueira-Recalde, T.M. Nolan, I. Nombela, I. Novak, B. Novoa, T. Nozawa, N. Nukina, C. Nussbaum-Krammer, J. Nylandsted, T.R. O’Donovan, S.M. O’Leary, E.J. O’Rourke, M.P. O’Sullivan, T.E. O’Sullivan, S. Oddo, I. Oehme, M. Ogawa, E. Ogier-Denis, M.H. Ogmundsdottir, B. Ogretmen, G.T. Oh, S.-H. Oh, Y.J. Oh, T. Ohama, Y. Ohashi, M. Ohmuraya, V. Oikonomou, R. Ojha, K. Okamoto, H. Okazawa, M. Oku, S. Oliván, J.M.A. Oliveira, M. Ollmann, J.A. Olzmann, S. Omari, M.B. Omary, G. Önal, M. Ondrej, S.-B. Ong, S.-G. Ong, A. Onnis, J.A. Orellana, S. Orellana-Muñoz, M.D.M. Ortega-Villaizan, X.R. Ortiz-Gonzalez, E. Ortona, H.D. Osiewacz, A.-H.K. Osman, R. Osta, M.S. Otegui, K. Otsu, C. Ott, L. Ottobrini, J.-H.J. Ou, T.F. Outeiro, I. Oynebraten, M. Ozturk, G. Pagès, S. Pahari, M. Pajares, U.B. Pajvani, R. Pal, S. Paladino, N. Pallet, M. Palmieri, G. Palmisano, C. Palumbo, F. Pampaloni, L. Pan, Q. Pan, W. Pan, X. Pan, G. Panasyuk, R. Pandey, U.B. Pandey, V. Pandya, F. Paneni, S.Y. Pang, E. Panzarini, D.L. Papademetrio, E. Papaleo, D. Papinski, D. Papp, E.C. Park, H.T. Park, J.-M. Park, J.-I. Park, J.T. Park, J. Park, S.C. Park, S.-Y. Park, A.H. Parola, J.B. Parys, A. Pasquier, B. Pasquier, J.F. Passos, N. Pastore, H.H. Patel, D. Patschan, S. Pattingre, G. Pedraza-Alva, J. Pedraza-Chaverri, Z. Pedrozo, G. Pei, J. Pei, H. Peled-Zehavi, J.M. Pellegrini, J. Pelletier, M.A. Peñalva, D. Peng, Y. Peng, F. Penna, M. Pennuto, F. Pentimalli, C.M. Pereira, G.J.S. Pereira, L.C. Pereira, L. Pereira de Almeida, N.D. Perera, Á. Pérez-Lara, A.B. Perez-Oliva, M.E. Pérez-Pérez, P. Periyasamy, A. Perl, C. Perrotta, I. Perrotta, R.G. Pestell, M. Petersen, I. Petrache, G. Petrovski, T. Pfirrmann, A.S. Pfister, J.A. Philips, H. Pi, A. Picca, A.M. Pickrell, S. Picot, G.M. Pierantoni, M. Pierdominici, P. Pierre, V. Pierrefite-Carle, K. Pierzynowska, F. Pietrocola, M. Pietruczuk, C. Pignata, F.X. Pimentel-Muiños, M. Pinar, R.O. Pinheiro, R. Pinkas-Kramarski, P. Pinton, K. Pircs, S. Piya, P. Pizzo, T.S. Plantinga, H.W. Platta, A. Plaza-Zabala, M. Plomann, E.Y. Plotnikov, H. Plun-Favreau, R. Pluta, R. Pocock, S. Pöggeler, C. Pohl, M. Poirot, A. Poletti, M. Ponpuak, H. Popelka, B. Popova, H. Porta, S. Porte Alcon, E. Portilla-Fernandez, M. Post, M.B. Potts, J. Poulton, T. Powers, V. Prahlad, T.K. Prajsnar, D. Praticò, R. Prencipe, M. Priault, T. Proikas-Cezanne, V.J. Promponas, C.G. Proud, R. Puertollano, L. Puglielli, T. Pulinilkunnil, D. Puri, R. Puri, J. Puyal, X. Qi, Y. Qi, W. Qian, L. Qiang, Y. Qiu, J. Quadrilatero, J. Quarleri, N. Raben, H. Rabinowich, D. Ragona, M.J. Ragusa, N. Rahimi, M. Rahmati, V. Raia, N. Raimundo, N.-S. Rajasekaran, S. Ramachandra Rao, A. Rami, I. Ramírez-Pardo, D.B. Ramsden, F. Randow, P.N. Rangarajan, D. Ranieri, H. Rao, L. Rao, R. Rao, S. Rathore, J.A. Ratnayaka, E.A. Ratovitski, P. Ravanan, G. Ravegnini, S.K. Ray, B. Razani, V. Rebecca, F. Reggiori, A. Régnier-Vigouroux, A.S. Reichert, D. Reigada, J.H. Reiling, T. Rein, S. Reipert, R.S. Rekha, H. Ren, J. Ren, W. Ren, T. Renault, G. Renga, K. Reue, K. Rewitz, B. Ribeiro de Andrade Ramos, S.A. Riazuddin, T.M. Ribeiro-Rodrigues, J.-E. Ricci, R. Ricci, V. Riccio, D.R. Richardson, Y. Rikihisa, M.V. Risbud, R.M. Risueño, K. Ritis, S. Rizza, R. Rizzuto, H.C. Roberts, L.D. Roberts, K.J. Robinson, M.C. Roccheri, S. Rocchi, G.G. Rodney, T. Rodrigues, V.R. Rodrigues Silva, A. Rodriguez, R. Rodriguez-Barrueco, N. Rodriguez-Henche, H. Rodriguez-Rocha, J. Roelofs, R.S. Rogers, V.V. Rogov, A.I. Rojo, K. Rolka, V. Romanello, L. Romani, A. Romano, P.S. Romano, D. Romeo-Guitart, L.C. Romero, M. Romero, J.C. Roney, C. Rongo, S. Roperto, M.T. Rosenfeldt, P. Rosenstiel, A.G. Rosenwald, K.A. Roth, L. Roth, S. Roth, K.M.A. Rouschop, B.D. Roussel, S. Roux, P. Rovere-Querini, A. Roy, A. Rozieres, D. Ruano, D.C. Rubinsztein, M.P. Rubtsova, K. Ruckdeschel, C. Ruckenstuhl, E. Rudolf, R. Rudolf, A. Ruggieri, A.A. Ruparelia, P. Rusmini, R.R. Russell, G.L. Russo, M. Russo, R. Russo, O.O. Ryabaya, K.M. Ryan, K.-Y. Ryu, M. Sabater-Arcis, U. Sachdev, M. Sacher, C. Sachse, A. Sadhu, J. Sadoshima, N. Safren, P. Saftig, A.P. Sagona, G. Sahay, A. Sahebkar, M. Sahin, O. Sahin, S. Sahni, N. Saito, S. Saito, T. Saito, R. Sakai, Y. Sakai, J.-I. Sakamaki, K. Saksela, G. Salazar, A. Salazar-Degracia, G.H. Salekdeh, A.K. Saluja, B. Sampaio-Marques, M.C. Sanchez, J.A. Sanchez-Alcazar, V. Sanchez-Vera, V. Sancho-Shimizu, J.T. Sanderson, M. Sandri, S. Santaguida, L. Santambrogio, M.M. Santana, G. Santoni, A. Sanz, P. Sanz, S. Saran, M. Sardiello, T.J. Sargeant, A. Sarin, C. Sarkar, S. Sarkar, M.-R. Sarrias, S. Sarkar, D.T. Sarmah, J. Sarparanta, A. Sathyanarayan, R. Sathyanarayanan, K.M. Scaglione, F. Scatozza, L. Schaefer, Z.T. Schafer, U.E. Schaible, A.H.V. Schapira, M. Scharl, H.M. Schatzl, C.H. Schein, W. Scheper, D. Scheuring, M.V. Schiaffino, M. Schiappacassi, R. Schindl, U. Schlattner, O. Schmidt, R. Schmitt, S.D. Schmidt, I. Schmitz, E. Schmukler, A. Schneider, B.E. Schneider, R. Schober, A.C. Schoijet, M.B. Schott, M. Schramm, B. Schröder, K. Schuh, C. Schüller, R.J. Schulze, L. Schürmanns, J.C. Schwamborn, M. Schwarten, F. Scialo, S. Sciarretta, M.J. Scott, K.W. Scotto, A.I. Scovassi, A. Scrima, A. Scrivo, D. Sebastian, S. Sebti, S. Sedej, L. Segatori, N. Segev, P.O. Seglen, I. Seiliez, E. Seki, S.B. Selleck, F.W. Sellke, J.T. Selsby, M. Sendtner, S. Senturk, E. Seranova, C. Sergi, R. Serra-Moreno, H. Sesaki, C. Settembre, S.R.G. Setty, G. Sgarbi, O. Sha, J.J. Shacka, J.A. Shah, D. Shang, C. Shao, F. Shao, S. Sharbati, L.M. Sharkey, D. Sharma, G. Sharma, K. Sharma, P. Sharma, S. Sharma, H.-M. Shen, H. Shen, J. Shen, M. Shen, W. Shen, Z. Shen, R. Sheng, Z. Sheng, Z.-H. Sheng, J. Shi, X. Shi, Y.-H. Shi, K. Shiba-Fukushima, J.-J. Shieh, Y. Shimada, S. Shimizu, M. Shimozawa, T. Shintani, C.J. Shoemaker, S. Shojaei, I. Shoji, B.V. Shravage, V. Shridhar, C.-W. Shu, H.-B. Shu, K. Shui, A.K. Shukla, T.E. Shutt, V. Sica, A. Siddiqui, A. Sierra, V. Sierra-Torre, S. Signorelli, P. Sil, B.J. de A. Silva, J.D. Silva, E. Silva-Pavez, S. Silvente-Poirot, R.E. Simmonds, A.K. Simon, H.-U. Simon, M. Simons, A. Singh, L.P. Singh, R. Singh, S.V. Singh, S.K. Singh, S.B. Singh, S. Singh, S.P. Singh, D. Sinha, R.A. Sinha, S. Sinha, A. Sirko, K. Sirohi, E.L. Sivridis, P. Skendros, A. Skirycz, I. Slaninová, S.S. Smaili, A. Smertenko, M.D. Smith, S.J. Soenen, E.J. Sohn, S.P.M. Sok, G. Solaini, T. Soldati, S.A. Soleimanpour, R.M. Soler, A. Solovchenko, J.A. Somarelli, A. Sonawane, F. Song, H.K. Song, J.-X. Song, K. Song, Z. Song, L.R. Soria, M. Sorice, A.A. Soukas, S.-F. Soukup, D. Sousa, N. Sousa, P.A. Spagnuolo, S.A. Spector, M.M. Srinivas Bharath, D. St Clair, V. Stagni, L. Staiano, C.A. Stalnecker, M.V. Stankov, P.B. Stathopulos, K. Stefan, S.M. Stefan, L. Stefanis, J.S. Steffan, A. Steinkasserer, H. Stenmark, J. Sterneckert, C. Stevens, V. Stoka, S. Storch, B. Stork, F. Strappazzon, A.M. Strohecker, D.G. Stupack, H. Su, L.-Y. Su, L. Su, A.M. Suarez-Fontes, C.S. Subauste, S. Subbian, P.V. Subirada, G. Sudhandiran, C.M. Sue, X. Sui, C. Summers, G. Sun, J. Sun, K. Sun, M.-X. Sun, Q. Sun, Y. Sun, Z. Sun, K.K.S. Sunahara, E. Sundberg, K. Susztak, P. Sutovsky, H. Suzuki, G. Sweeney, J.D. Symons, S.C.W. Sze, N.J. Szewczyk, A. Tabęcka-Łonczynska, C. Tabolacci, F. Tacke, H. Taegtmeyer, M. Tafani, M. Tagaya, H. Tai, S.W.G. Tait, Y. Takahashi, S. Takats, P. Talwar, C. Tam, S.Y. Tam, D. Tampellini, A. Tamura, C.T. Tan, E.-K. Tan, Y.-Q. Tan, M. Tanaka, M. Tanaka, D. Tang, J. Tang, T.-S. Tang, I. Tanida, Z. Tao, M. Taouis, L. Tatenhorst, N. Tavernarakis, A. Taylor, G.A. Taylor, J.M. Taylor, E. Tchetina, A.R. Tee, I. Tegeder, D. Teis, N. Teixeira, F. Teixeira-Clerc, K.A. Tekirdag, T. Tencomnao, S. Tenreiro, A.V. Tepikin, P.S. Testillano, G. Tettamanti, P.-L. Tharaux, K. Thedieck, A.A. Thekkinghat, S. Thellung, J.W. Thinwa, V.P. Thirumalaikumar, S.M. Thomas, P.G. Thomes, A. Thorburn, L. Thukral, T. Thum, M. Thumm, L. Tian, A. Tichy, A. Till, V. Timmerman, V.I. Titorenko, S.V. Todi, K. Todorova, J.M. Toivonen, L. Tomaipitinca, D. Tomar, C. Tomas-Zapico, S. Tomić, B.C.-K. Tong, C. Tong, X. Tong, S.A. Tooze, M.L. Torgersen, S. Torii, L. Torres-López, A. Torriglia, C.G. Towers, R. Towns, S. Toyokuni, V. Trajkovic, D. Tramontano, Q.-G. Tran, L.H. Travassos, C.B. Trelford, S. Tremel, I.P. Trougakos, B.P. Tsao, M.P. Tschan, H.-F. Tse, T.F. Tse, H. Tsugawa, A.S. Tsvetkov, D.A. Tumbarello, Y. Tumtas, M.J. Tuñón, S. Turcotte, B. Turk, V. Turk, B.J. Turner, R.I. Tuxworth, J.K. Tyler, E.V. Tyutereva, Y. Uchiyama, A. Ugun-Klusek, H.H. Uhlig, M. Ułamek-Kozioł, I.V. Ulasov, M. Umekawa, C. Ungermann, R. Unno, S. Urbe, E. Uribe-Carretero, S. Üstün, V.N. Uversky, T. Vaccari, M.I. Vaccaro, B.F. Vahsen, H. Vakifahmetoglu-Norberg, R. Valdor, M.J. Valente, A. Valko, R.B. Vallee, A.M. Valverde, G. Van den Berghe, S. van der Veen, L. Van Kaer, J. van Loosdregt, S.J.L. van Wijk, W. Vandenberghe, I. Vanhorebeek, M.A. Vannier-Santos, N. Vannini, M.C. Vanrell, C. Vantaggiato, G. Varano, I. Varela-Nieto, M. Varga, M.H. Vasconcelos, S. Vats, D.G. Vavvas, I. Vega-Naredo, S. Vega-Rubin-de-Celis, G. Velasco, A.P. Velázquez, T. Vellai, E. Vellenga, F. Velotti, M. Verdier, P. Verginis, I. Vergne, P. Verkade, M. Verma, P. Verstreken, T. Vervliet, J. Vervoorts, A.T. Vessoni, V.M. Victor, M. Vidal, C. Vidoni, O.V. Vieira, R.D. Vierstra, S. Viganó, H. Vihinen, V. Vijayan, M. Vila, M. Vilar, J.M. Villalba, A. Villalobo, B. Villarejo-Zori, F. Villarroya, J. Villarroya, O. Vincent, C. Vindis, C. Viret, M.T. Viscomi, D. Visnjic, I. Vitale, D.J. Vocadlo, O.V. Voitsekhovskaja, C. Volonté, M. Volta, M. Vomero, C. Von Haefen, M.A. Vooijs, W. Voos, L. Vucicevic, R. Wade-Martins, S. Waguri, K.A. Waite, S. Wakatsuki, D.W. Walker, M.J. Walker, S.A. Walker, J. Walter, F.G. Wandosell, B. Wang, C.-Y. Wang, C. Wang, C. Wang, C. Wang, C.-Y. Wang, D. Wang, F. Wang, F. Wang, F. Wang, G. Wang, H. Wang, H. Wang, H. Wang, H.-G. Wang, J. Wang, J. Wang, J. Wang, J. Wang, K. Wang, L. Wang, L. Wang, M.H. Wang, M. Wang, N. Wang, P. Wang, P. Wang, P. Wang, P. Wang, Q.J. Wang, Q. Wang, Q.K. Wang, Q.A. Wang, W.-T. Wang, W. Wang, X. Wang, X. Wang, Y. Wang, Y. Wang, Y. Wang, Y.-Y. Wang, Y. Wang, Y. Wang, Y. Wang, Y. Wang, Z. Wang, Z. Wang, Z. Wang, G. Warnes, V. Warnsmann, H. Watada, E. Watanabe, M. Watchon, A. Wawrzyńska, T.E. Weaver, G. Wegrzyn, A.M. Wehman, H. Wei, L. Wei, T. Wei, Y. Wei, O.H. Weiergräber, C.C. Weihl, G. Weindl, R. Weiskirchen, A. Wells, R.H. Wen, X. Wen, A. Werner, B. Weykopf, S.P. Wheatley, J.L. Whitton, A.J. Whitworth, K. Wiktorska, M.E. Wildenberg, T. Wileman, S. Wilkinson, D. Willbold, B. Williams, R.S.B. Williams, R.L. Williams, P.R. Williamson, R.A. Wilson, B. Winner, N.J. Winsor, S.S. Witkin, H. Wodrich, U. Woehlbier, T. Wollert, E. Wong, J.H. Wong, R.W. Wong, V.K.W. Wong, W.W.-L. Wong, A.-G. Wu, C. Wu, J. Wu, J. Wu, K.K. Wu, M. Wu, S.-Y. Wu, S. Wu, S.-Y. Wu, S. Wu, W.K.K. Wu, X. Wu, X. Wu, Y.-W. Wu, Y. Wu, R.J. Xavier, H. Xia, L. Xia, Z. Xia, G. Xiang, J. Xiang, M. Xiang, W. Xiang, B. Xiao, G. Xiao, H. Xiao, H.-T. Xiao, J. Xiao, L. Xiao, S. Xiao, Y. Xiao, B. Xie, C.-M. Xie, M. Xie, Y. Xie, Z. Xie, Z. Xie, M. Xilouri, C. Xu, E. Xu, H. Xu, J. Xu, J. Xu, L. Xu, W.W. Xu, X. Xu, Y. Xue, S.M.S. Yakhine-Diop, M. Yamaguchi, O. Yamaguchi, A. Yamamoto, S. Yamashina, S. Yan, S.-J. Yan, Z. Yan, Y. Yanagi, C. Yang, D.-S. Yang, H. Yang, H.-T. Yang, H. Yang, J.-M. Yang, J. Yang, J. Yang, L. Yang, L. Yang, M. Yang, P.-M. Yang, Q. Yang, S. Yang, S. Yang, S.-F. Yang, W. Yang, W.Y. Yang, X. Yang, X. Yang, Y. Yang, Y. Yang, H. Yao, S. Yao, X. Yao, Y.-G. Yao, Y.-M. Yao, T. Yasui, M. Yazdankhah, P.M. Yen, C. Yi, X.-M. Yin, Y. Yin, Z. Yin, Z. Yin, M. Ying, Z. Ying, C.K. Yip, S.P.T. Yiu, Y.H. Yoo, K. Yoshida, S.R. Yoshii, T. Yoshimori, B. Yousefi, B. Yu, H. Yu, J. Yu, J. Yu, L. Yu, M.-L. Yu, S.-W. Yu, V.C. Yu, W.H. Yu, Z. Yu, Z. Yu, J. Yuan, L.-Q. Yuan, S. Yuan, S.-S.F. Yuan, Y. Yuan, Z. Yuan, J. Yue, Z. Yue, J. Yun, R.L. Yung, D.N. Zacks, G. Zaffagnini, V.O. Zambelli, I. Zanella, Q.S. Zang, S. Zanivan, S. Zappavigna, P. Zaragoza, K.S. Zarbalis, A. Zarebkohan, A. Zarrouk, S.O. Zeitlin, J. Zeng, J.-D. Zeng, E. Žerovnik, L. Zhan, B. Zhang, D.D. Zhang, H. Zhang, H. Zhang, H. Zhang, H. Zhang, H. Zhang, H. Zhang, H. Zhang, H.-L. Zhang, J. Zhang, J. Zhang, J.-P. Zhang, K.Y.B. Zhang, L.W. Zhang, L. Zhang, L. Zhang, L. Zhang, L. Zhang, M. Zhang, P. Zhang, S. Zhang, W. Zhang, X. Zhang, X.-W. Zhang, X. Zhang, X. Zhang, X. Zhang, X. Zhang, X.D. Zhang, Y. Zhang, Y. Zhang, Y. Zhang, Y.-D. Zhang, Y. Zhang, Y.-Y. Zhang, Y. Zhang, Z. Zhang, Z. Zhang, Z. Zhang, Z. Zhang, Z. Zhang, Z. Zhang, H. Zhao, L. Zhao, S. Zhao, T. Zhao, X.-F. Zhao, Y. Zhao, Y. Zhao, Y. Zhao, Y. Zhao, G. Zheng, K. Zheng, L. Zheng, S. Zheng, X.-L. Zheng, Y. Zheng, Z.-G. Zheng, B. Zhivotovsky, Q. Zhong, A. Zhou, B. Zhou, C. Zhou, G. Zhou, H. Zhou, H. Zhou, H. Zhou, J. Zhou, J. Zhou, J. Zhou, J. Zhou, K. Zhou, R. Zhou, X.-J. Zhou, Y. Zhou, Y. Zhou, Y. Zhou, Z.-Y. Zhou, Z. Zhou, B. Zhu, C. Zhu, G.-Q. Zhu, H. Zhu, H. Zhu, H. Zhu, W.-G. Zhu, Y. Zhu, Y. Zhu, H. Zhuang, X. Zhuang, K. Zientara-Rytter, C.M. Zimmermann, E. Ziviani, T. Zoladek, W.-X. Zong, D.B. Zorov, A. Zorzano, W. Zou, Z. Zou, Z. Zou, S. Zuryn, W. Zwerschke, B. Brand-Saberi, X.C. Dong, C.S. Kenchappa, Z. Li, Y. Lin, S. Oshima, Y. Rong, J.C. Sluimer, C.L. Stallings, C.-K. Tong, Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1, Autophagy. 17 (2021) 1–382. [CrossRef]
- R. Iwama, Y. Ohsumi, Analysis of autophagy activated during changes in carbon source availability in yeast cells, J. Biol. Chem. 294 (2019) 5590–5603. [CrossRef]
- M. Tsukada, Y. Ohsumi, Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae, FEBS Lett. 333 (1993) 169–174. [CrossRef]
- J. Onodera, Y. Ohsumi, Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation, J. Biol. Chem. 280 (2005) 31582–31586. [CrossRef]
- A.E. Mayes, L. Verdone, P. Legrain, J.D. Beggs, Characterization of Sm-like proteins in yeast and their association with U6 snRNA, EMBO J. 18 (1999) 4321–4331. [CrossRef]
- Y. Lei, Y. Huang, X. Wen, Z. Yin, Z. Zhang, D.J. Klionsky, How Cells Deal with the Fluctuating Environment: Autophagy Regulation under Stress in Yeast and Mammalian Systems, Antioxidants. 11 (2022) 304. [CrossRef]
- A.K. Singh, S. Singh, V.K. Tripathi, A. Bissoyi, G. Garg, S.I. Rizvi, Rapamycin Confers Neuroprotection Against Aging-Induced Oxidative Stress, Mitochondrial Dysfunction, and Neurodegeneration in Old Rats Through Activation of Autophagy, Rejuvenation Res. 22 (2019) 60–70. [CrossRef]
- Mazzoni, C. Falcone, Isolation and study ofKlLSM4 , aKluyveromyces lactis gene homologous to the essential geneLSM4 ofSaccharomyces cerevisiae, Yeast. 18 (2001) 1249–1256. [CrossRef]
- P. Kumar, D. Kundu, A.K. Mondal, V. Nain, R. Puria, Inhibition of TOR signalling in lea1 mutant induces apoptosis in Saccharomyces cerevisiae, Ann. Microbiol. 69 (2019) 341–352. [CrossRef]
- Z. Zhang, Y. Zhang, W. Mo, The Autophagy Related Gene CHAF1B Is a Relevant Prognostic and Diagnostic Biomarker in Hepatocellular Carcinoma, Front. Oncol. 10 (2021). https://www.frontiersin.org/articles/10.3389/fonc.2020.626175 (accessed June 26, 2023).
- S.W. Suzuki, J. Onodera, Y. Ohsumi, Starvation induced cell death in autophagy-defective yeast mutants is caused by mitochondria dysfunction, PloS One. 6 (2011) e17412. [CrossRef]
- M.E. Pérez-Pérez, M. Zaffagnini, C.H. Marchand, J.L. Crespo, S.D. Lemaire, The yeast autophagy protease Atg4 is regulated by thioredoxin, Autophagy. 10 (2014) 1953–1964. [CrossRef]
- E. Hirata, Y. Ohya, K. Suzuki, Atg4 plays an important role in efficient expansion of autophagic isolation membranes by cleaving lipidated Atg8 in Saccharomyces cerevisiae, PLOS ONE. 12 (2017) e0181047. [CrossRef]
- J. Sánchez-Wandelmer, F. Kriegenburg, S. Rohringer, M. Schuschnig, R. Gómez-Sánchez, B. Zens, S. Abreu, R. Hardenberg, D. Hollenstein, J. Gao, C. Ungermann, S. Martens, C. Kraft, F. Reggiori, Atg4 proteolytic activity can be inhibited by Atg1 phosphorylation, Nat. Commun. 8 (2017) 295. [CrossRef]
- S. Barz, F. Kriegenburg, A. Henning, A. Bhattacharya, H. Mancilla, P. Sánchez-Martín, C. Kraft, Atg1 kinase regulates autophagosome-vacuole fusion by controlling SNARE bundling, EMBO Rep. 21 (2020) e51869. [CrossRef]
- 50. Y. Lee, B. Kim, H.-S. Jang, W.-K. Huh, Atg1-dependent phosphorylation of Vps34 is required for dynamic regulation of the phagophore assembly site and autophagy in Saccharomyces cerevisiae, Autophagy. (2023). [CrossRef]
- J.J. Bearss, S.K. Padi, N. Singh, M. Cardo-Vila, J.H. Song, G. Mouneimne, N. Fernandes, Y. Li, M.R. Harter, J.M. Gard, A.E. Cress, W. Peti, A.D. Nelson, J.R. Buchan, A.S. Kraft, K. Okumura, EDC3 phosphorylation regulates growth and invasion through controlling P-body formation and dynamics, EMBO Rep. 22 (2021) e50835. [CrossRef]
- J. Ptacek, G. Devgan, G. Michaud, H. Zhu, X. Zhu, J. Fasolo, H. Guo, G. Jona, A. Breitkreutz, R. Sopko, R.R. McCartney, M.C. Schmidt, N. Rachidi, S.-J. Lee, A.S. Mah, L. Meng, M.J.R. Stark, D.F. Stern, C. De Virgilio, M. Tyers, B. Andrews, M. Gerstein, B. Schweitzer, P.F. Predki, M. Snyder, Global analysis of protein phosphorylation in yeast, Nature. 438 (2005) 679–684. [CrossRef]
- D.-C. Chen, B.-C. Yang, T.-T. Kuo, One-step transformation of yeast in stationary phase, Curr. Genet. 21 (1992) 83–84. [CrossRef]
- T. Eisenberg, H. Knauer, A. Schauer, S. Büttner, C. Ruckenstuhl, D. Carmona-Gutierrez, J. Ring, S. Schroeder, C. Magnes, L. Antonacci, H. Fussi, L. Deszcz, R. Hartl, E. Schraml, A. Criollo, E. Megalou, D. Weiskopf, P. Laun, G. Heeren, M. Breitenbach, B. Grubeck-Loebenstein, E. Herker, B. Fahrenkrog, K.-U. Fröhlich, F. Sinner, N. Tavernarakis, N. Minois, G. Kroemer, F. Madeo, Induction of autophagy by spermidine promotes longevity, Nat. Cell Biol. 11 (2009) 1305–1314. [CrossRef]
- V. Palermo, C. Falcone, C. Mazzoni, Apoptosis and aging in mitochondrial morphology mutants of S. cerevisiae, Folia Microbiol. (Praha). 52 (2007) 479–483. [CrossRef]
- M. Cooper, L.H. Johnston, J.D. Beggs, Identification and characterization of Uss1p (Sdb23p): a novel U6 snRNA-associated protein with significant similarity to core proteins of small nuclear ribonucleoproteins., EMBO J. 14 (1995) 2066–2075.
- C.B. Brachmann, A. Davies, G.J. Cost, E. Caputo, J. Li, P. Hieter, J.D. Boeke, Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications, Yeast Chichester Engl. 14 (1998) 115–132. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





