Submitted:
17 July 2023
Posted:
20 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant material
2.2. Whole plant morphology analysis
2.3. Pollen grains size and shape
2.4. Meiosis analysis
2.5. Pollen fertility assessment
2.6. Plant extract preparation
2.7. Determination of antioxidant activity
2.7.1. Total phenolic content
2.7.2. DPPH free radical scavenging assay
2.7.3. Reducing power assay
2.8. Screening of the antibacterial activity
2.8.1. Bacterial strains
2.8.2. Antibacterial test
2.9. Statistical evaluation
3. Results
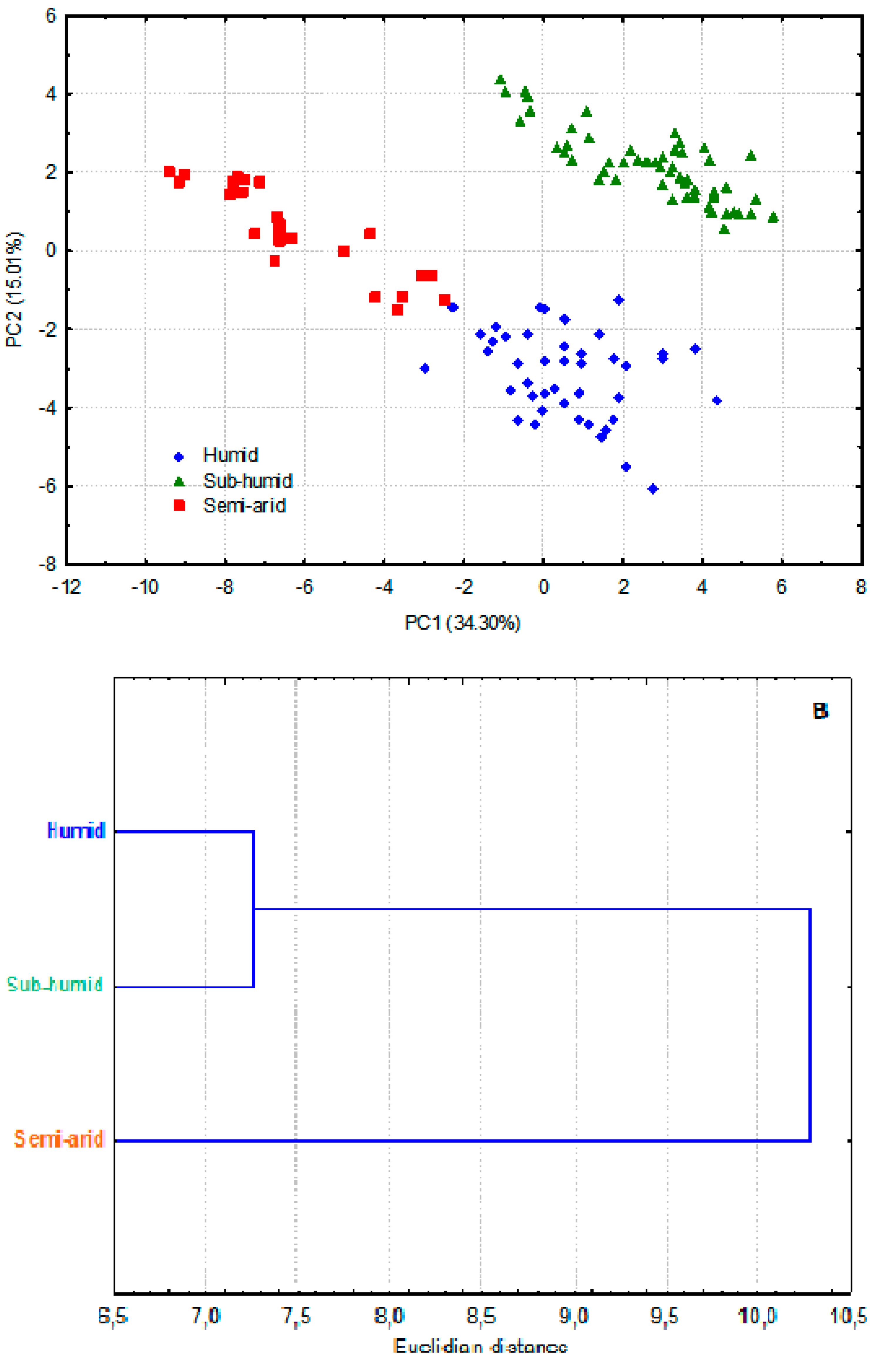
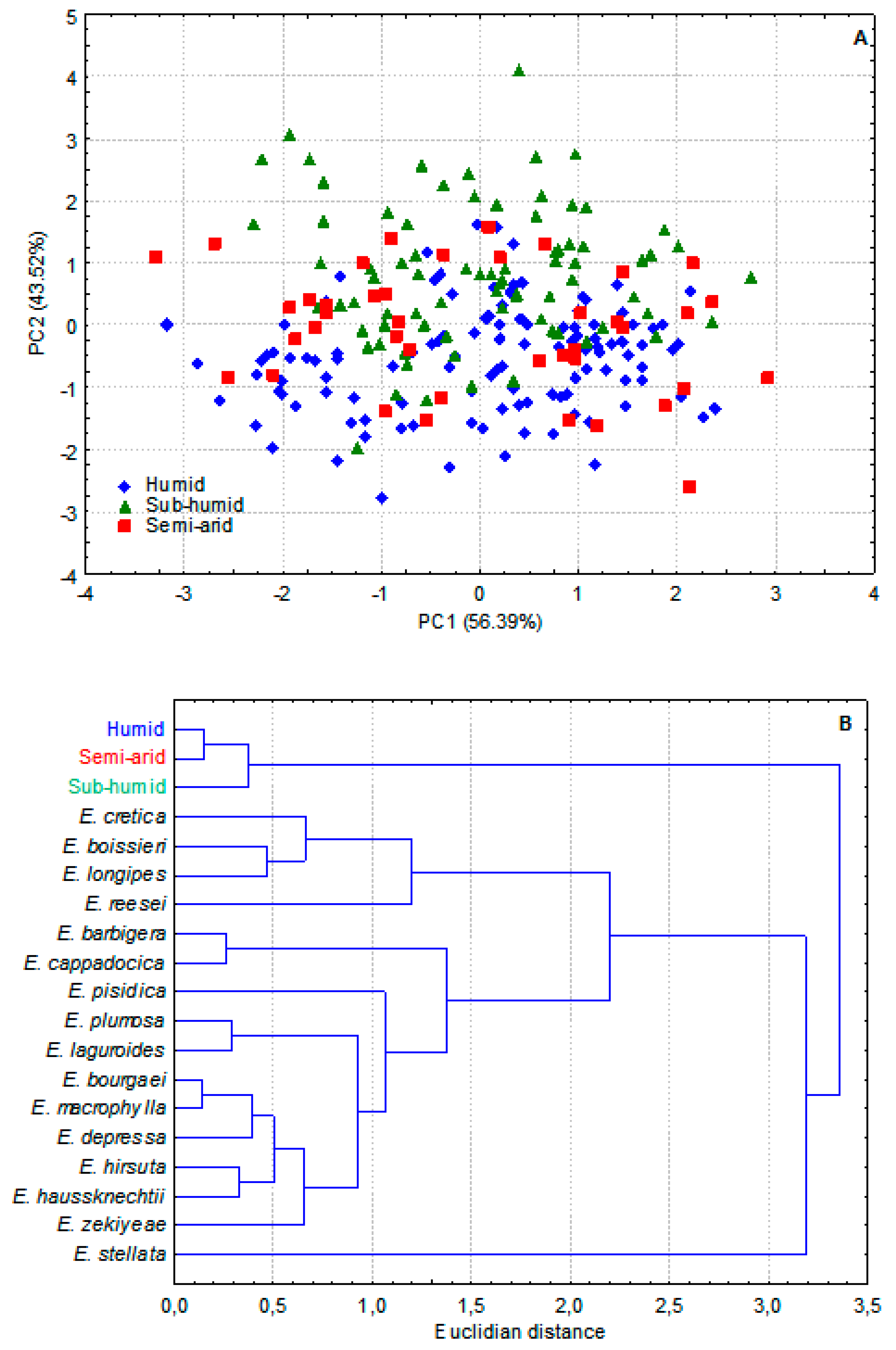
3.1. Whole plant morphology
3.2. Pollen grains size
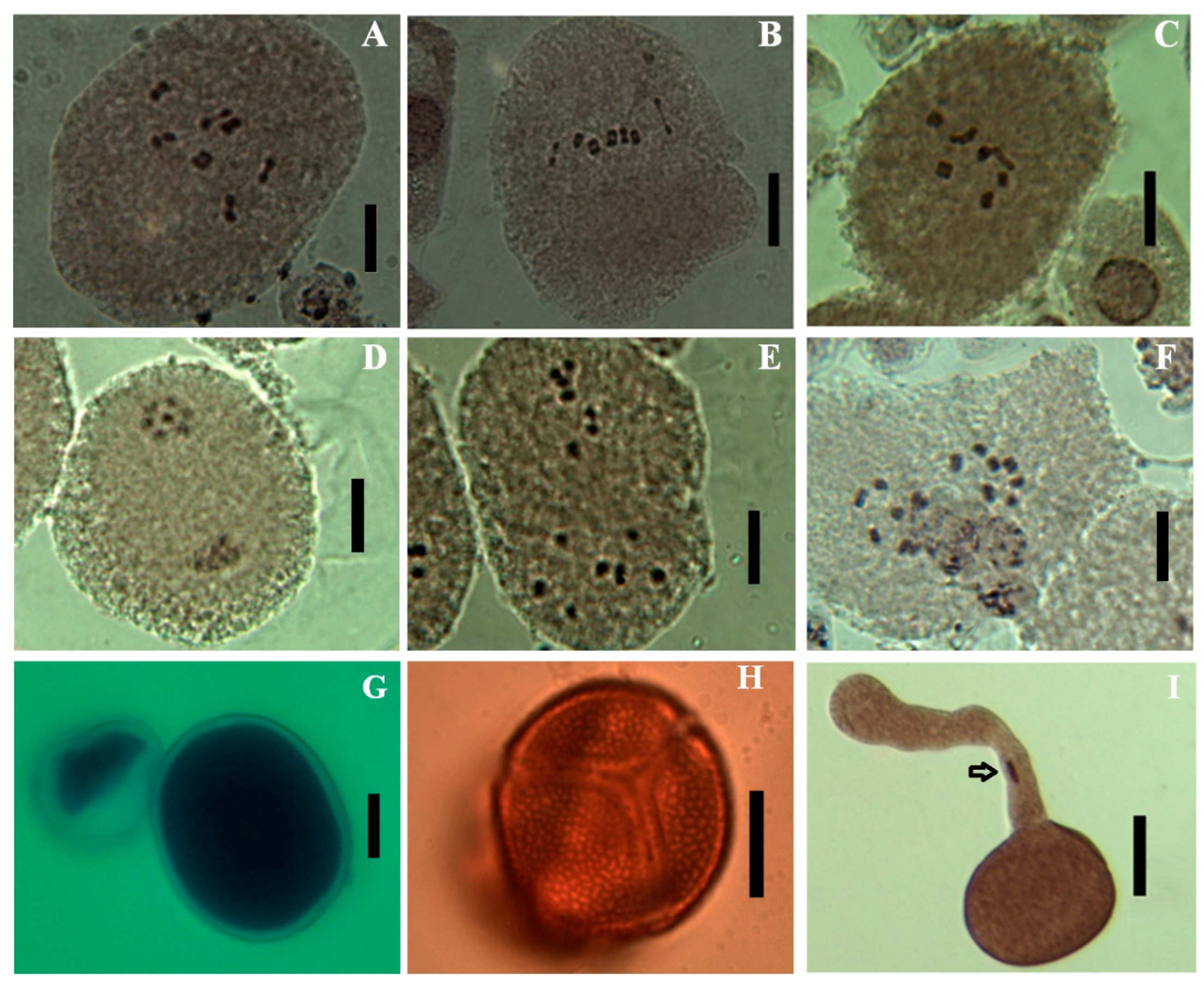
3.3. Chromosome numbers, meiotic abnormalities and pollen fertility
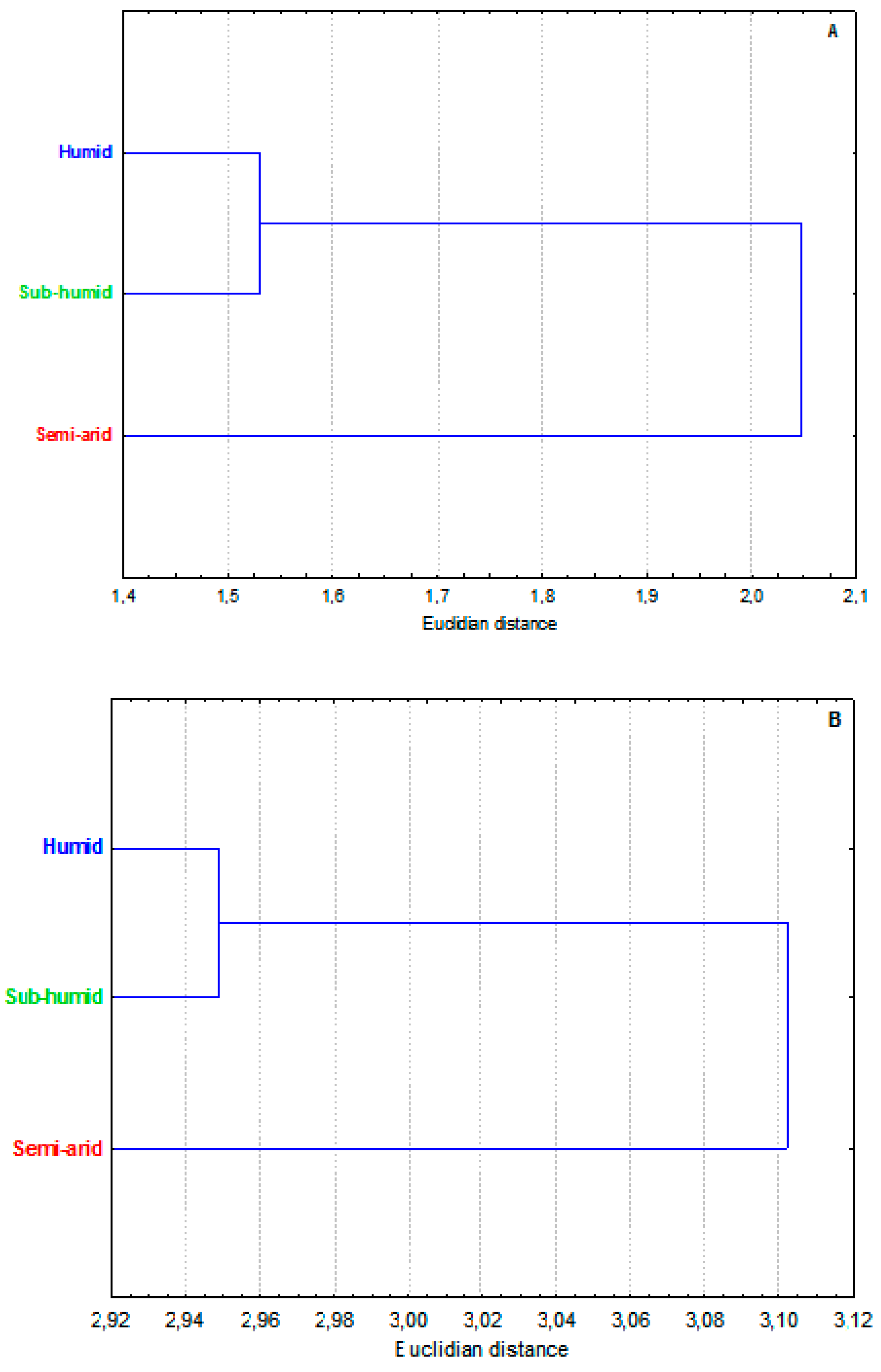
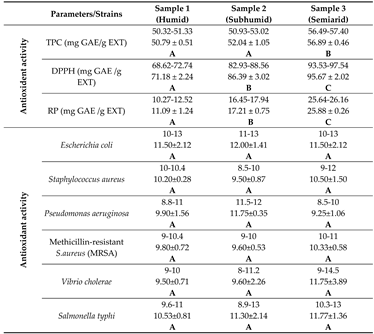
3.4. Antioxidant activity
3.5. Antibacterial activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
Appendix C
References
- Aytaç Z. (2000). The genus Ebenus L. (Leguminosae/Fabaceae) in Turkey Karaca Arboretum Magazine 5: 145–171.
- Aytaç Z., Yildirim H. (2018). Ebenus zekiyeae (Fabaceae), a new species from Turkey. Annales Botanici Fennici 55(4-6): 25–29. [CrossRef]
- Kaveh A. and Kazampour-Osaloo S. (2012). Cladistic analysis of the genus Ebenus (Fabaceae-Hedysareae) based on morphological data. Taxonomy and Biosystematics 4(11): 61–68. DOI: 20.1001.1.20088906.1391.4.11.7.8.
- Aytaç Z., Suludere Z., Pınar M. (2015). Examination of the leaflets hairs and stoma structures with the electron microscope of the genus Ebenus L. (Leguminosae) in Turkey. Biodicon 8(2): 32–50.
- Boissier E. (1872). Flora Orientalis: Save Enumeratio Plantarum In Oriente a Græcia et Ægypto ad Indfiæ Fines Hucusque Observatarum – vol. 2. APUD EUMDEM, 65, RUE DE LYON.
- Boulos L. (1999). Flora of Egypt. Vol. One (Azollaceae-Oxalidaceae). Cairo, Egypt: Al Hadara Publishing. 340 p.
- Jafri S.M.H. (1980). Fabaceae. In: Flora of Libya, V. 118, S.M.H. Jafri & A. El-Gadi (Eds.), Al Faateh University, FacuIty of Science, Department of Botany, Tripoli.
- Dobignard A., Chatelain C. (2012). Index synonymique de la flore d'Afrique du Nord. Vol.4, Dicotyledoneae : Fabaceae - Nymphaeaceae. Conservatoire et Jardin botaniques de la Ville de Genève, hors-série 11b.
- Quézel P., Santa S. (1962). Nouvelle flore d'Algérie et des régions désertiques méridionales - Tome 1. Ed CNRS. Paris.
- Huber-Morath, A. 1970: Ebenus L. In: Davis PH, Editor. Flora of Turkey and the East Aegean Islands, vol. 3. Edinburgh, UK: Edinburgh University Press, pp. 590–596.
- Çelebi A., Açik L., Aytaç Z. (2009). Biosystematic studies among Ebenus L. species based on morphological, RAPD-PCR and seed protein analyses in Turkey. Pak. J. Bot. 41(5): 2477–2486.
- Amirahmadi A., Kazampour-Osaloo S., Moein F., Kaveh A., Maassoumi A.A. (2014). Molecular systematics of the tribe Hedysareae (Fabaceae) based on nrDNA ITS and plastid trnL-F and matK sequences. Pl. Syst. Evol. 300:729–747. [CrossRef]
- Duan L., Wen J., Yang X., Liu P.L., Arslan E., Ertuğrul K., Chang Z.Y. (2015). Phylogeny of Hedysarum and tribe Hedysareae (Leguminosae: Papilionoideae) inferred from sequence data of ITS, matK, trnL-F and psbA-trnH. Taxon 64:49–64. [CrossRef]
- Kaveh A., Kazampour-Osaloo S. (2015). Estimation of Ebenus species divergence time based on nrDNA ITS and matK cpDNA sequences. 4th National Congress of Plants. 12, 13 May 2015, Tehran, Iran.
- Ghanavati F., Amirabadizadeh H. (2012 Medicinal). Pollen grain morphology in Iranian Hedysareae (Fabaceae). Crop Breeding Journal 2(1): 25–33. [CrossRef]
- Aytaç Z., Ünal F. & Pinar M.N. (2000). Morphological, palynological, and cytotaxonomical study of Ebenus longipes Boiss. & Bal. and E. argentea Siehe ex Bornm. (Leguminosae) from Turkey. Israel Journal of Plant Sciences 48: 321–326. [CrossRef]
- Pinar N.M., Vural C., Zytac Z. (2000). Pollen morphology of Ebenus L. (Leguminosae: subfamily Papilionoideae) in Turkey. Pak. J. Bot. 32(2): 303–310.
- Halbritter H., Auer W., Igersheim A. (2020). Ebenus cretica. In: PalDat - A palynological database. https://www.paldat.org/pub/Ebenus_cretica/305777;jsessionid=B9B8A714C47DE9C0CB28F0595C4D0398; accessed 2021-01-25.
- Molero J., Montserrat-Marti J. M. (1986). Números cromosomáticos de plantas marroquíes. Collectanea Botanica 16: 351–354.
- Davis P.H., Mill R.R., Kit Tan. (1988). Flora of Turkey and the East Aegean Islands, vol. 10 (supplement). Edinburgh, UK: Edinburgh University Press, pp. 381-382.
- Aksoy H., Ünal F., Aytaç Z. (2001). Karyological study on four endemic Ebenus L. taxa (Leguminosae) in Turkey. Caryologia, 54(4): 307–311. [CrossRef]
- Rice et al. 2015. The Chromosome Counts Database (CCDB) – a community resource of plant chromosome numbers. New Phytol. 206(1): 19–26.
- Gadoum N., Hamma A. (2016). Etude cytogénétique de l’espèce Ebenus pinnata Aiton (Fabaceae) du Golfe de Béjaïa et de la Vallée de la Soummam. Mémoire de Master en Sciences Biologiques. Université Abderrahmane Mira de Bejaia, Algérie. 58 p. http://univ-bejaia.dz/dspace/123456789/10254.
- Hadawat A.K., Madani S. (2022). Etude cytogénétique de quelques populations d’Ebenus pinnata Ait. (Fabacées) de la région de Béjaïa. Mémoire de Master en Sciences de l’Environnement. Université Abderrahmane Mira de Bejaia, Algérie. 49 p. http://univ-bejaia.dz/dspace/123456789/21618.
- Parra R., Valdés B., Gordillo I., Venanzi R. (1999). In: Kamari, G., Felber, F. and Garbari, F. (ed.): Meditenanean chromosome number reports – 9 – Fl. Medit. 9: 323–387.
- Uyar Z., Böke N., Türkay E., Koz Ö., Yas A.I., Kırmızıgül S. (2006). Flavonoid glycosides and methylinositol from Ebenus haussknechtii. Nat. Prod. Res. 20(11): 999–1007. [CrossRef]
- Özdemir E., Alpınar K. (2015). An ethnobotanical survey of medicinal plants in western part of central Taurus Mountains: Aladaglar (Nigde–Turkey). Journal of Ethnopharmacology166: 53–65. [CrossRef]
- Azcan N., Saricoban S., Demirci B., Aytaç Z., Baser K.H.C. (2001). Seed oils of fifteen Ebenus taxa growing in Turkey. Chemistry of Natural Compounds 37(3): 253–255. [CrossRef]
- Kültür S., Gürdal B., Sari A., Melikoğlu G., 2021. Traditional herbal remedies used in kidney diseases in Turkey: an overview. Turkish Journal of Botany 45: 269–287. [CrossRef]
- Ceylan R., Katani J., Zengina G., Mati S., Aktumsek A., Boroja T., Stanic S., Vladimir Mihailovic V., Gokalp Ozmen Guler G.O., Boga M., Yılmaz M.A. (2016). Chemical and biological fingerprints of two Fabaceae species (Cytisopsis dorycniifolia and Ebenus hirsuta): Are they novel sources of natural agents for pharmaceutical and food formulations? Industrial Crops and Products 84: 254–262. [CrossRef]
- Bektas E., Kaltalioglu K., Sahin H., Turkmen Z., Kandemir A., (2018). Analysis of phenolic compounds, antioxidant and antimicrobial properties of some endemic medicinal plants. International Journal of Secondary Metabolite 5(2): 75–86. [CrossRef]
- İmir N., Aydemir E., Şimşek E., Göktürk R., Yesilada E., Fişkin K. (2016). Cytotoxic and immunomodulatory effects of Ebenus boissieri Barbey on breast cancer cells. Genetics and Molecular Research 15(1). [CrossRef]
- Simsek E., Imir N., Aydemir E.R., Gokturk R.S., Yesilada E., Fiskin K. (2017). Caspase-mediated apoptotic effects of Ebenus boissieri Barbey extracts on human cervical cancer Cell Line HeLa. Pharmacognosy Magazine 13(50): 254–259. [CrossRef]
- Bhuwan J.C., Vijay J., Archana S.N., Piyush V., Minky M. (2022). Review on documented medicinal plants used for the treatment of cancer; Current Traditional Medicine 8(2): e111021197159. [CrossRef]
- Aydemir E.A., Simsek E., Imir N., Göktürk R.S., Yesilada E., Fiskin K. (2015). Cytotoxic and apoptotic effects of Ebenus boissieri Barbey on human lung cancer cell line A549. Pharmacognosy Magazine, 11(Suppl 1), S37. [CrossRef]
- Ingham J.L., 1978. Flavonoid and isoflavonoid compounds from leaves of sainfoin (Onobrychis viciifolia). Z. Naturforsch. 33: 146–148. [CrossRef]
- Kounadi S., Aligiannis N., Pongratz I., Lelovas P., Ismini D. and Skaltsounis A. (2011). Estrogenic activity of the methanolic extract of Ebenus cretica L. Planta Medica 77 - PM168. [CrossRef]
- Dontas I.., Kounadi S., Aligiannis N., Galanos A., Skaltsounis A. and Lelovas P., (2019). Plant extract administration and mild daily exercise increase bone density of ovariectomized rats. In Abstracts of 14th FELASA congress 2019, PC41, p. 157. Laboratory Animals 53(1): 28–203.
- Mitrocotsa D., Skaltsounis A.-L., S., Harvala C., Tillequin F. (1999). Flavonoid and terpene glycosides from European Ebenus species. Biochemical Systematics and Ecology 27: 305–307. [CrossRef]
- Mandokhail A., Samiullah, Khan K., Tareen A.H., Attiq-Ur-Rehman Kakar A.U.-R, Tariq S., Kakar N., (2020). Determination of antioxidants by four gifferent methods in medicinally jmportant plant Ebenus Stellata of Balochistan. Al-Nahrain Journal of Science.23(4): 13–18. [CrossRef]
- Abidin S.Z.U., Munem A., Khan R., Batiha G.E.-S., Amhad M., Zafar M., Khalil A.A.K., Hetta H.F., Mahmoud M.H., Sami A., Bhatti M.Z. (2021). Ethnoveterinary botanical survey of medicinal plants used in Pashto, Punjabi and Saraiki Communities of Southwest Pakistan. Veterinary Medicine and Science 7(5): 2068–2085. [CrossRef]
- Khodaparast A., Sayyah M., Sardari S. (2012). Anticonvulsant activity of hydroalcoholic extract and aqueous fraction of Ebenus stellata in mice. Iranian Journal of Basic Medical Sciences 15(3):. 811–819.
- Zameer S., Ali S., Gulmeena, Tareen R.B. (2022). Identification of volatile constituents and antimicrobial activity of Ebenus stellata. GU. J. Phytosci. 2(4): 214–222.
- Kiazai I., Samiullah, Khan N., Attiq-Ur-Rehman, Abdul Ghaffar, Abdul Baqi. (2019). Determination of heavy metals concentration in Astragalus anisacanthus and Ebenus stellata of Balochistan, Pakistan. Pure and Applied Biology 8(3): 2028–2035. [CrossRef]
- Yousif F., Hifnawy M.S., Soliman G., Boulos L., Labib T., Mahmoud S., Ramzy F., Yousif M., Hassan I., Mahmoud M., El-Hallouty S.M., El-Gendy M., Gohar L., El-Manawaty M., Fayyad W., El-Menshawi B.S. (2007). Large-scale in vitro. screening of Egyptian native and cultivated plants for schistosomicidal activity, Pharmaceutical Biology 45(6): 501–510. [CrossRef]
- Braham H., Ben Jannet H., Castedo L., Mighri Z. (2004). Isolation, for the first time, of a flavonoid glycoside and the (±)-catechin from the aerial parts of Ebenus pinnata. Journal de la Société Chimique de Tunisie 6: 153–160.
- Abreu P.M., Braham H., Ben Jannet H., Mighri Z., Matthew S. (2007). Antioxidant compounds from Ebenus pinnata. Fitoterapia 78: 32–34. [CrossRef]
- Nouioua W., Gaamoune S. (2018). Antioxidant, antimicrobial and anti-inflammatory activities development of methanol extracts of some species growing in the massif of Boutaleb, Setif, Algeria. International Journal of Pharmacy and Natural Medicines 6(1): 15–20.
- Mebarki A. (2005). Hydrologie des bassins de l’Est algérien : Ressources en eau, aménagement et environnement. Thèse de doctorat d’état (Géographie et Aménagement du Territoire, option : Hydrologie), Université Mentouri de Constantine, Algérie. 360 p.
- Dyer A.F. (1963). The use of lacto-propionic orcein in rapid squash. Methods for chromosome preparations. Stain Technol. 38: 85–90. [CrossRef]
- Mertens T.R., Hamnersmith R.L. (1998). Genetic laboratory investigations. Eleventh ed. Prentice Hall inc., Upper Saddle River, New Jersey.
- Singleton V.L., Orthofer, R., Lamuela-Raventos R.M. (1999). Analysis of total phenols and other oxidation substrates and sntioxidants by means of Folin-Ciocalteu RGAEent. Methods in Enzymology 299: 152–178. [CrossRef]
- Blois M.S. (1958). Antioxidant determinations by the use of a stable free radical. Nature 181(4617): 1199–1200. [CrossRef]
- Oyaizu M. (1986). Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Japanese journal of nutrition and dietetics 44(6): 307–315. [CrossRef]
- NCCLS. (1993). Performance standards for antimicrobial disc suspectibility tests. Approved Standard NCCLS Publication M2-A5, Villanova, PA, USA.
- Statsof, Inc. (2007). Statistica (data analysis software system). version 8.0. www. Statsoft.com.
- Fazlioğlu F. (2018). Phenotypic plasticity of ecotypes across habitats. Akademik Ziraat Dergisi 7(2): 253–258. [CrossRef]
- Hu X., Lan S., Song X.,Yang F., Zhang Z., Peng D., Ren M. (2021). Genetic divergence between two sympatric ecotypes of Phalaenopsis pulcherrima on Hainan Island. Diversity 13: 446. [CrossRef]
- Stronen A.V., Norman A.J., Wal E.V., Paquet P.C. (2021). The relevance of genetic structure in ecotype designation and conservation management. Evolutionary Applications 15(2): 185–202. [CrossRef]
- Knight C.A., Clancy R.B., Götzenberger L., Dann L., Beaulieu J.M. (2010). On the relationship between pollen size and genome size. Journal of Botany 2010, Article ID 612017, 7 pages. [CrossRef]
- Siddiqui S., Alrumman S.A. (2022). Methomyl, imbraclaobrid and clethodim induced cytomixis and syncytes behaviors in PMCs of Pisum sativum L: Causes and outcomes. Saudi J. Biol. Sci. 29(9): 103390. [CrossRef]
- Zhang Y., Wang Z. (2009). Phenolic composition and antioxidant activities of two Phlomis species: A correlation study. CR Biologies 332: 816–826. [CrossRef]
- Kumar S., Sandhir R., Ojha S. (2014). Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Research Notes 7, 560. [CrossRef]
- Ahuja I., De Vos R.C., Bones A.M., Hall R.D. (2010). Plant molecular stress responses face climate change. Trends in Plant Science 15(12): 664–674. [CrossRef]
- Rabeta M.S., Nur Faraniza R. (2013). Total phenolic content and ferric reducing antioxidant power of the leaves and fruits of Garcinia atrovirdis and Cynometra cauliflora. International Food Research Journal 20: 1691–1696.
- Kabtni S., Sdouga D., Bettaib Rebey I., Save M., Trifi-Farah N., Fauconnier M.L., Marghali S. (2020). Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations. Scientific reports 10(1): 8293. [CrossRef]
- De Abreu I.N., Mazzafera P. (2005). Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiology and Biochemistry 43(3): 241–248. [CrossRef]
- Al-Huqail A., El-Dakak R.M., Sanad M.N., Badr R.H., Ibrahim M.M., Soliman D. & Khan F. (2020). Effects of climate temperature and water stress on plant growth and accumulation of antioxidant compounds in sweet basil (Ocimum basilicum L.) leafy vegetable. Scientifica (Cairo). 2020 Feb 27; 2020: 3808909. [CrossRef]
| Climate stage | Humid | Sub-humid | Semi-arid |
| Locality name | Kherrata | Semaoun | Boudjelil |
| Population Code | H | SH | SA |
| GPS Localization | 36° 31' 33.30"N 5° 16' 49.73" |
36° 37' 27.16"N 4° 49' 7.20"E |
36° 22' 11"N 4° 26' 48.30"E |
|
Altitude/ Exposure |
612m/East | 195m/West | 270m/East |
| Soil | Red clay | Brown clay | White ground |
| Plant formation | Road bank | Garrigue, Grassland | Grassland, Sparse garrigue |
| Rainfall1 (mm) | 800-1000 | 600-800 | 600-800 |
| Number of plants | 44 | 55 | 27 |
|
Sampling date/ Plant codes |
08/06/2015/ H01-H20 03/06/2015/ H21-H35 15/07/2015/ H236-H44 |
20/05/2015/ SH01-SH15 09/06/2015/ SH16-SH25 09/06/2015/ SH26- SH34 20/05/2015/ SH35-SH47 28/06/2016 SH48-SH55 |
13/05/2015/ SA01-SA13 07/06/2015/ SA14-SA27 |
| N° | Coding | Character name | Type | Unit |
|---|---|---|---|---|
| 1 | DBP | Diameter at the base of the plant | C | cm |
| 2 | LLS | Length of the longest stem | C | cm |
| 3 | NS | Number of stems | D | Stems |
| 4 | DLS | Diameter of the longest stem | C | mm |
| 5 | LIN | Length of the third internode | C | cm |
| 6 | NI | Number of inflorescences (racemes) | D | Raceme |
| 7 | LS1 | Length of stipules | C | mm |
| 8 | WS | Width of stipules | C | mm |
| 9 | LL | Length of the leaf (3rd node) | C | cm |
| 10 | WL | Width of the leaf (3rd node) | C | cm |
| 11 | LP | Length of the petiole | C | cm |
| 12 | NPL | Number of pairs of leaflets (3rd node) | D | Pairs |
| 13 | LNLP | Lowest number of leaflets pairs | D | Pairs |
| 14 | HNLP | Highest number of leaflets pairs | D | Pairs |
| 15 | LLB | Length of the leaflet blade | C | cm |
| 16 | WLB | Width of the leaflet blade | C | mm |
| 17 | LIP | Length of the inflorescence peduncle (3rd node) | C | cm |
| 18 | HI | Height of the inflorescence | C | cm |
| 19 | DI | Diameter of the inflorescence | C | cm |
| 20 | LNF | Lowest number of flowers | D | Flower |
| 21 | HNF | Highest number of flowers | D | Flower |
| 22 | LFB | Length of the flower bract | C | mm |
| 23 | WFB | Width of the flower bract | C | mm |
| 24 | LC1 | Length of the flower calyx | C | mm |
| 25 | LC2 | Length of the corolla | C | mm |
| 26 | TLW | Total length of the wing | C | mm |
| 27 | WWB | Width of the wing blade | C | mm |
| 28 | WBW | Width at the base of the wing | C | mm |
| 29 | WMW | Width at the middle of the wing | C | mm |
| 30 | TWK | Total width of the keel | C | mm |
| 31 | LWPK | Length of the widest part of the keel | C | mm |
| 32 | TLK | Total length of the keel | C | mm |
| 33 | TLS | Total length of the standard | C | mm |
| 34 | LSB | Length of the standard blade | C | mm |
| 35 | WSB | Width of the standard blade | C | mm |
| 36 | LSP | Length of the standard ‘petiolule’ | C | mm |
| 37 | LCM | Length of the calyx at maturity | C | mm |
| 38 | LCT | Length of the calyx tube at maturity | C | mm |
| 39 | LHCT | Length of hairs at the base of the calyx teeth | C | mm |
| 40 | LPM | Length of the pod at maturity | C | mm |
| 41 | WPM | Width of the pod at maturity | C | mm |
| 42 | LS2 | Length of the seed | C | mm |
| 43 | WS | Width of the seed | C | mm |
| 44 | LR | Length of the radicle | C | mm |
| Extra variables (Not included in multivariate analyses) | ||||
| 45 | P | Polar diameter of pollen grains | C | µm |
| 46 | E | Equatorial diameter of pollen grains | C | µm |
| 47 | P/E | Rate of P and E diameters of pollen grains | C | |
| 48 | PxE | Product of P *E. | C | µm2 |
| 49 | PF | Pollen fertility rate | C | % |
| N° | Trait Code |
FL1 | FL2 | Humid | Sub-humid | Semi-arid |
|---|---|---|---|---|---|---|
| 1 | DBP | 0.49 | -0.24 | 0.73±0.19 B | 0.73±0.33 B | 0.55±0.25 A |
| 2 | LLS | 0.80 | 0.10 | 68.22±18.38 B | 89.28±28.93 C | 44.41±19.79 A |
| 3 | NS | 0.23 | -0.60 | 9.55±6.52 B | 4.65±2.47 A | 3.11±2.41 A |
| 4 | DLS | 0.47 | -0.41 | 4.19±1.12 B | 3.75±1.01 AB | 3.16±1.11 A |
| 5 | LIN | 0.67 | 0.16 | 5.27±1.04 B | 6.53±1.57 C | 4.36±1.58 A |
| 6 | NI | 0.52 | -0.39 | 57.57±28.25 B | 47.89±32.23 B | 17.89±23.80 A |
| 7 | LS1 | 0.66 | 0.17 | 10.98±1.67 A | 13.60±2.74 B | 10.10±2.71 A |
| 8 | WS | 0.56 | -0.13 | 4.22±0.75 B | 4.41±0.74 B | 3.64±1.00 A |
| 9 | LL | 0.77 | -0.05 | 10.01±2.06 B | 10.85±2.60 B | 6.91±1.93 A |
| 10 | WL | 0.67 | -0.06 | 5.19±0.80 B | 5.55±0.88 B | 4.64±0.66 A |
| 11 | LP | 0.73 | 0.03 | 4.56±1.21 B | 5.23±1.61 B | 2.96±0.98 A |
| 12 | NPL | 0.43 | -0.07 | 4.73±0.50 B | 4.78±0.50 B | 4.11±0.97 A |
| 13 | LNLP | -0.18 | -0.29 | 2.80±0.55 B | 2.45±0.50 A | 2.81±0.68 B |
| 14 | HNLP | 0.43 | -0.23 | 4.98±0.15 B | 4.91±0.35 B | 4.52±0.89 A |
| 15 | LLB | 0.70 | 0.03 | 2.45±0.33 B | 2.66±0.35 C | 2.15±0.36 A |
| 16 | WLB | 0.22 | -0.63 | 7.77±1.51 B | 6.14±1.21 A | 6.18±1.46 A |
| 17 | LIP | 0.56 | -0.31 | 22.00±3.17 B | 21.29±3.56 B | 18.20±3.94 A |
| 18 | HI | 0.43 | -0.49 | 5.21±1.41 B | 4.40±0.75 A | 3.88±1.61 A |
| 19 | DI | 0.33 | -0.16 | 2.44±0.31 A | 2.40±0.25 A | 2.30±0.31 A |
| 20 | LNF | 0.24 | 0.24 | 11.84±8.05 A | 16.64±7.17 B | 13.56±9.89 AB |
| 21 | HNF | 0.66 | -0.41 | 56.43±11.83 C | 49.44±9.47 B | 33.93±17.42 A |
| 22 | LFB | 0.08 | 0.29 | 8.15±0.62 A | 8.60±0.89 B | 8.46±1.04 AB |
| 23 | WFB | 0.06 | -0.12 | 3.29±0.32 A | 3.25±0.18 A | 3.19±0.43 A |
| 24 | LC1 | -0.05 | 0.25 | 12.72±0.94 A | 13.21±0.90 B | 13.38±1.06 B |
| 25 | LC2 | 0.13 | -0.25 | 8.21±0.44 A | 8.05±0.24 A | 7.98±0.70 A |
| 26 | TLW | 0.40 | 0.87 | 1.79±0.08 A | 3.54±0.13 C | 2.40±0.07 B |
| 27 | WWB | 0.45 | 0.85 | 1.37±0.09 A | 2.45±0.07 C | 1.67±0.05 B |
| 28 | WBW | -0.67 | 0.23 | 1.10±0.10 A | 1.14±0.06 A | 1.38±0.04 B |
| 29 | WMW | -0.02 | 0.89 | 0.43±0.05 A | 0.89±0.07 C | 0.83±0.07 B |
| 30 | TWK | -0.86 | -0.34 | 4.21±0.23 B | 3.62±0.10 A | 4.99±0.09 C |
| 31 | LWPK | -0.86 | -0.25 | 3.47±0.26 B | 3.12±0.08 A | 4.14±0.08 C |
| 32 | TLK | -0.81 | -0.29 | 6.36±0.36 B | 5.94±0.11 A | 6.99±0.08 C |
| 33 | TLS | -0.89 | -0.02 | 6.65±0.45 B | 6.30±0.23 A | 8.04±0.08 C |
| 34 | LSB | -0.86 | -0.29 | 5.13±0.47 B | 4.49±0.12 A | 6.06±0.05 C |
| 35 | WSB | -0.87 | -0.39 | 4.67±0.36 B | 3.36±0.16 A | 6.04±0.10 C |
| 36 | LSP | -0.31 | 0.38 | 1.72±0.47 A | 1.81±0.16 A | 2.02±0.10 B |
| 37 | LCM | -0.42 | 0.67 | 13.19±0.48 A | 14.50±0.89 B | 15.26±0.18 C |
| 38 | LCT | 0.04 | 0.81 | 2.80±0.14 A | 3.29±0.12 C | 3.19±0.12 B |
| 39 | LHCT | -0.15 | 0.22 | 3.27±0.05 A | 3.28±0.07 A | 3.29±0.03 A |
| 40 | LPM | -0.85 | 0.14 | 5.09±0.09 A | 5.08±0.11 A | 5.99±0.10 B |
| 41 | WPM | -0.71 | 0.40 | 3.04±0.10 A | 3.15±0.13 B | 3.46±0.07 C |
| 42 | LS2 | -0.87 | -0.06 | 2.35±0.07 B | 2.19±0.10 A | 3.00±0.08 C |
| 43 | WS | -0.85 | 0.06 | 2.05±0.05 A | 2.02±0.05 A | 2.34±0.05 B |
| 44 | LR | -0.77 | -0.34 | 2.14±0.05 B | 2.02±0.07 A | 2.25±0.05 C |
| 45 | P | NA | NA | 17.95±1.10 Ab | 19.47±1.27 Bb | 18.43±1.08 Ab |
| 46 | E | NA | NA | 12.80±1.18 Aa | 13.03±0.99 Aa | 12.84±1.37 Aa |
| 47 | P/E | NA | NA | 1.41±0.10 A | 1.50±0.11 B | 1.45±0.15 AB |
| 48 | PxE | NA | NA | 230.56±31.60 A | 254.31±30.81 B | 237.16±32.74 A |
| 49 | PF | NA | NA | 94.04±2.64 A | 95.01±2.02 A | 94.97±2.11 A |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





