1. Introduction
Lung cancer is the leading cause of death from cancer worldwide [
1]. Low-dose chest computed tomography (CT) for surveying lung cancer is increasingly used worldwide, and many small lung nodules are detected early nowadays [
2,
3]. Most of these small pulmonary nodules are suggested to be observed and regularly followed up at outpatient departments; however, some patients may be anxious about malignant tendencies and request to undergo surgery to remove these lesions. These tiny pure ground-glass nodules (GGN) in the lungs are difficult to detect manually for resection during surgery. CT-guided lesion localization is helpful for resection because the precise location of a lesion is determined using a dye or a hookwire [
4,
15], which guides the surgeon to be sure of the exact location of a target lesion and makes the operation smooth and safe. Traditionally, the two-stage CT-guided localization (TSCT), also known as pre-operative CT-guided localization, is used to localize tiny pure GGN or deep GGN in the lungs. Recently, the emergence of the hybrid operation room (HOR) makes one-stage CT-guided (OSCT) localization, also known as intraoperative CT-guided (IOCT) localization, more convenient and easier. The advent of the HOR has allowed the simultaneous localization and removal of small lung nodules. Some studies have demonstrated more benefits of OSCT localization in terms of safety and efficacy than TSCT localization [
5]. This report on a single-center experience aims to compare the efficacy and safety of TSCT to those of OSCT.
2. Materials and Methods
2.1. Study patients
This retrospective study aimed to compare the efficacy and safety of TSCT to those of OSCT. In this study, we collected data from patients with ipsilateral pulmonary nodules in whom localization was performed before surgical removal between October 2017 and January 2022 at Veteran General Hospital Kaohsiung (VHGKS). Our participants were divided into two groups; i.e., the OSCT and TSCT groups. Indications include the fact that the instance of solid nodules from the visceral pleura to the target lesion is deeper than 10 mm and the tiny solid nodules measured less than 10 mm [
6]. Any subsolid nodules, regardless of size or depth, are considered to be localized.
2.2. Two-stage computed tomography-guided localization
Two-stage computed tomography-guided localization was performed by an experienced radiologist in the CT suite. Most of the patients were positioned in the supine position because of the limitation of space in the CT scanner. The target lesion was scanned and the location was shown on the monitor. The puncture site was sterilized carefully, and the radiologist pierced into the lung near the target lesion with a 10-cm long, 20-gauge cannula needle housing a hookwire [
6]. Once the cannula needle was inserted into the target site, the hookwire was placed. After localization was completed, the patient was transferred to the original ward to wait for information about surgery. Then, the patient would be transferred to the operation room to undergo wedge resection for the removal of pulmonary lesions. Once the lesion was removed, the specimen was sent for a frozen section to check the tissue components. If the frozen section results reveal invasive adenocarcinoma, a lobectomy would be performed. If the frozen section results reveal atypical adenomatous hyperplasia, adenocarcinoma in situ (AIS), or minimally invasive adenocarcinoma (MIA), a lobectomy would not need to be performed [
6].
2.3. One-stage computed tomography-guided localization
One-stage computed tomography-guided localization was performed in the HOR. The localization procedure and surgical resection are both performed in the same place. The localization procedure was conducted by the thoracic surgeon. First of all, the patient was sent to the HOR. Once the induction of anesthesia was completed, the patient was placed in the appropriate position for CT-guided localization. Once the C-arm scanned the patient, the image of the target lesion showed up on the monitor. We planned the puncture side and the route of the cannula needle housing a hookwire [
7]. The skin of the puncture site was sterilized, and one 10-cm long, 20-gauge cannula needle housing a hookwire was inserted through it, along the required route, into the target site of the lung. Once the cannula needle approached the target site, the hookwire was placed to act as a marker of the ensuing resection. Then, the patient stayed in the same place, and the thoracic surgery team performed video-assisted thoracic surgery, usually wedge resection, for the removal of the lesion. After wedge resection was performed, the lesion would be sent for histopathological frozen section reporting. If the frozen section report revealed malignancy, a lobectomy would be performed.
2.4. Statistics Analysis
All study data were analyzed using IBM SPSS version 26. All continuous variables are summarized as means and standard deviations and compared with Student’s t-test. All categorical variables are summarized as counts and percentages and compared using Fisher’s exact test.
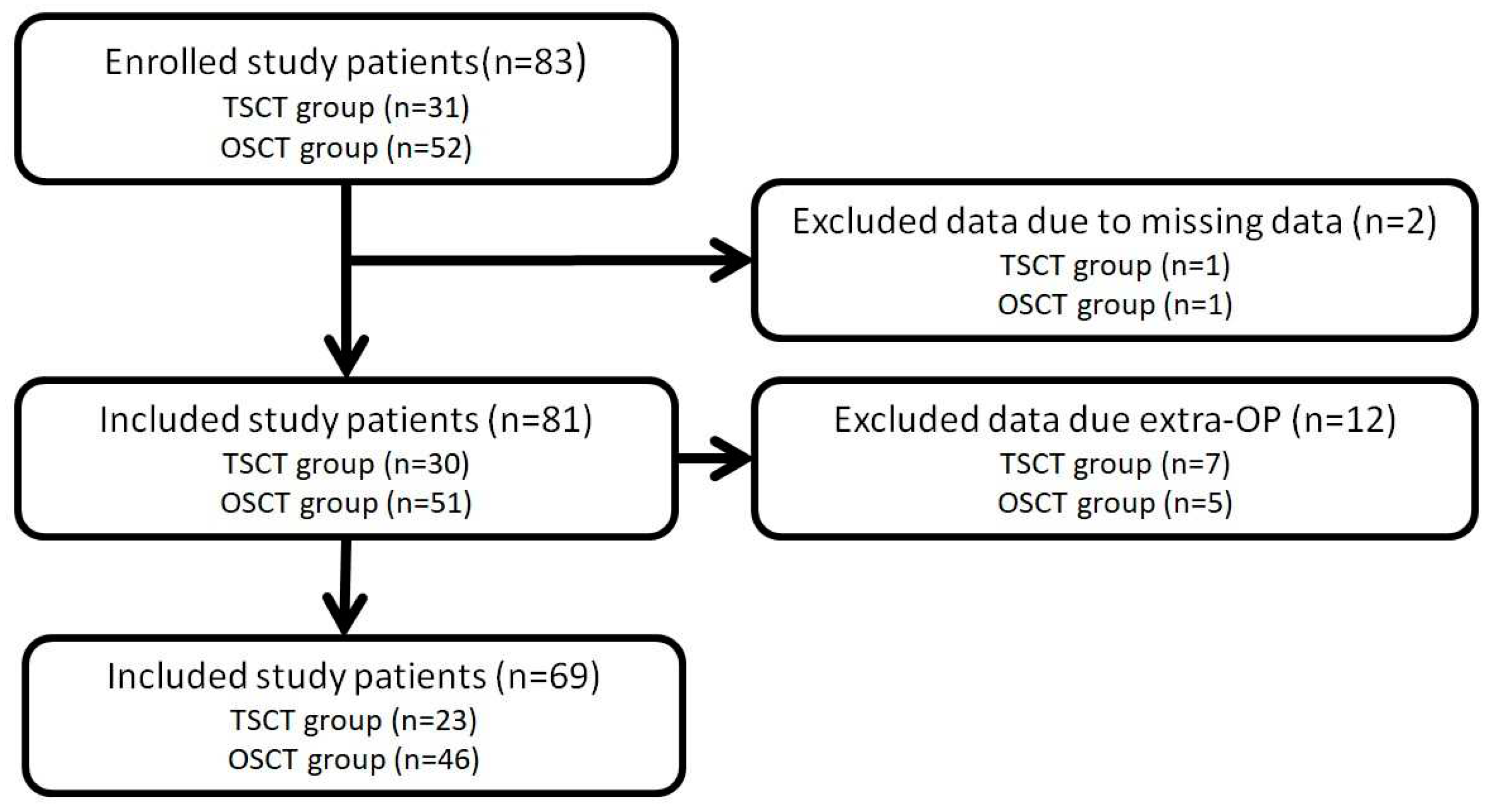
Figure 1.
Flowchart of the patient selection process.
Figure 1.
Flowchart of the patient selection process.
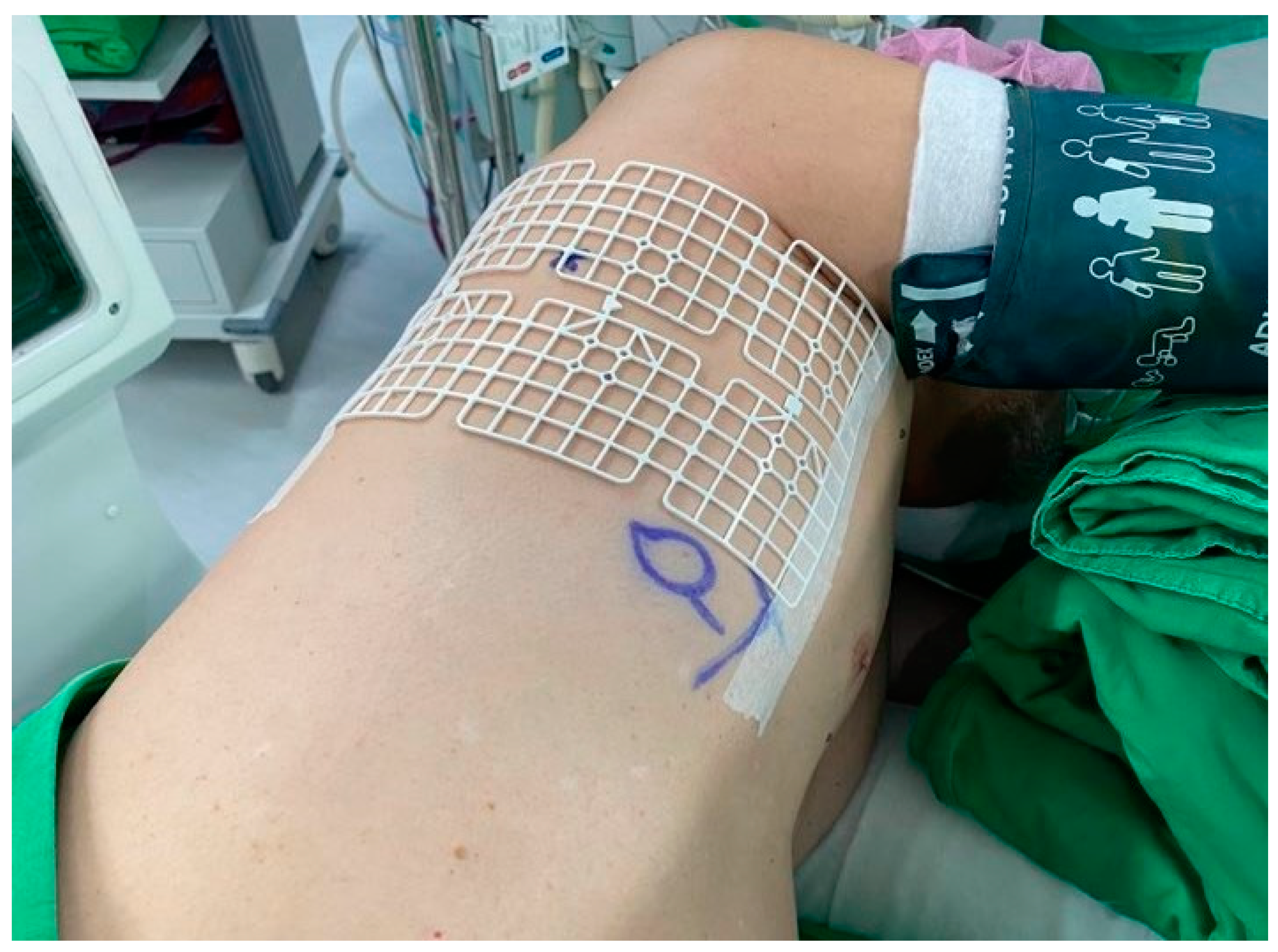
Figure 2.
Grid placed on the chest wall for localization.
Figure 2.
Grid placed on the chest wall for localization.
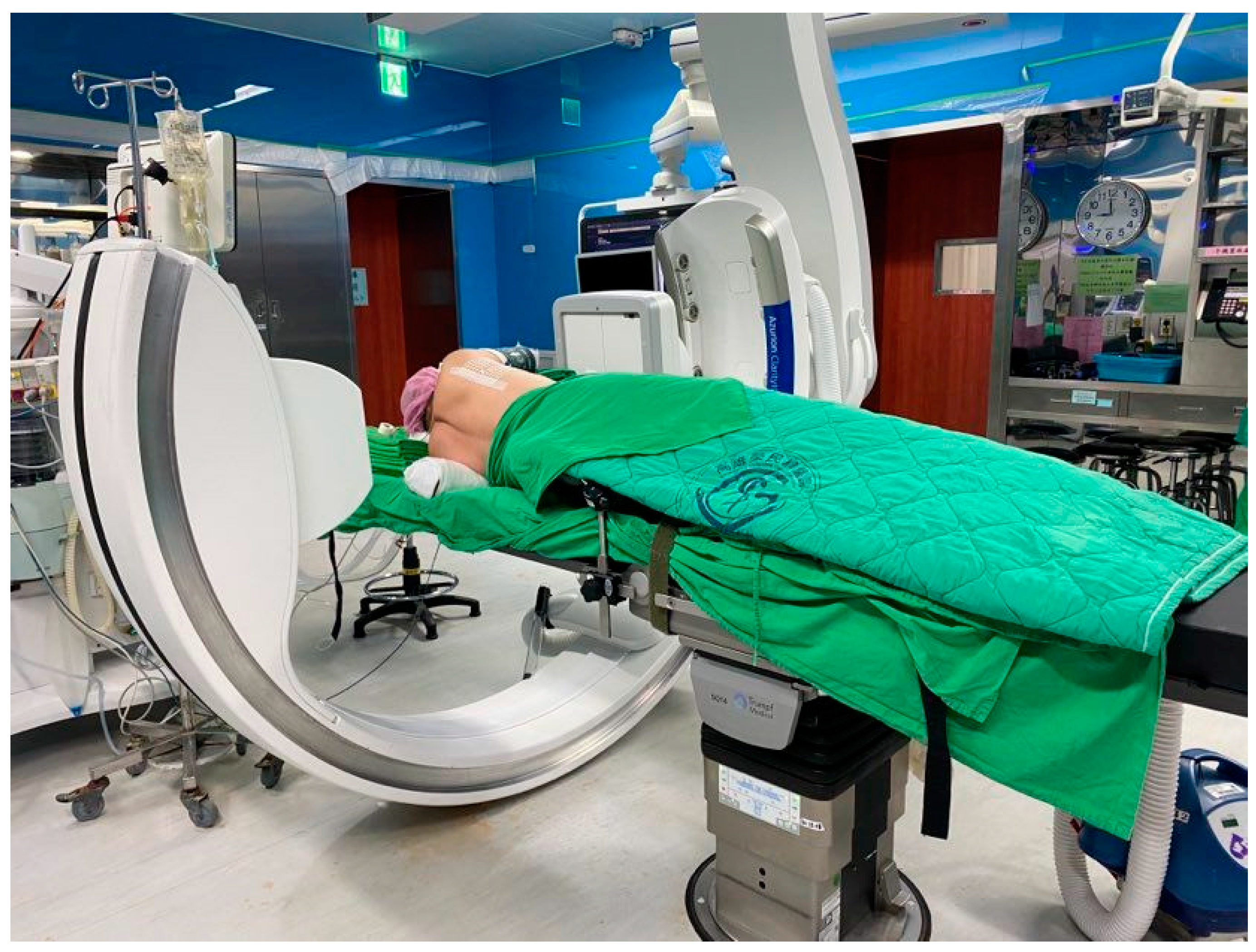
Figure 3.
C-arm scanning patients.
Figure 3.
C-arm scanning patients.
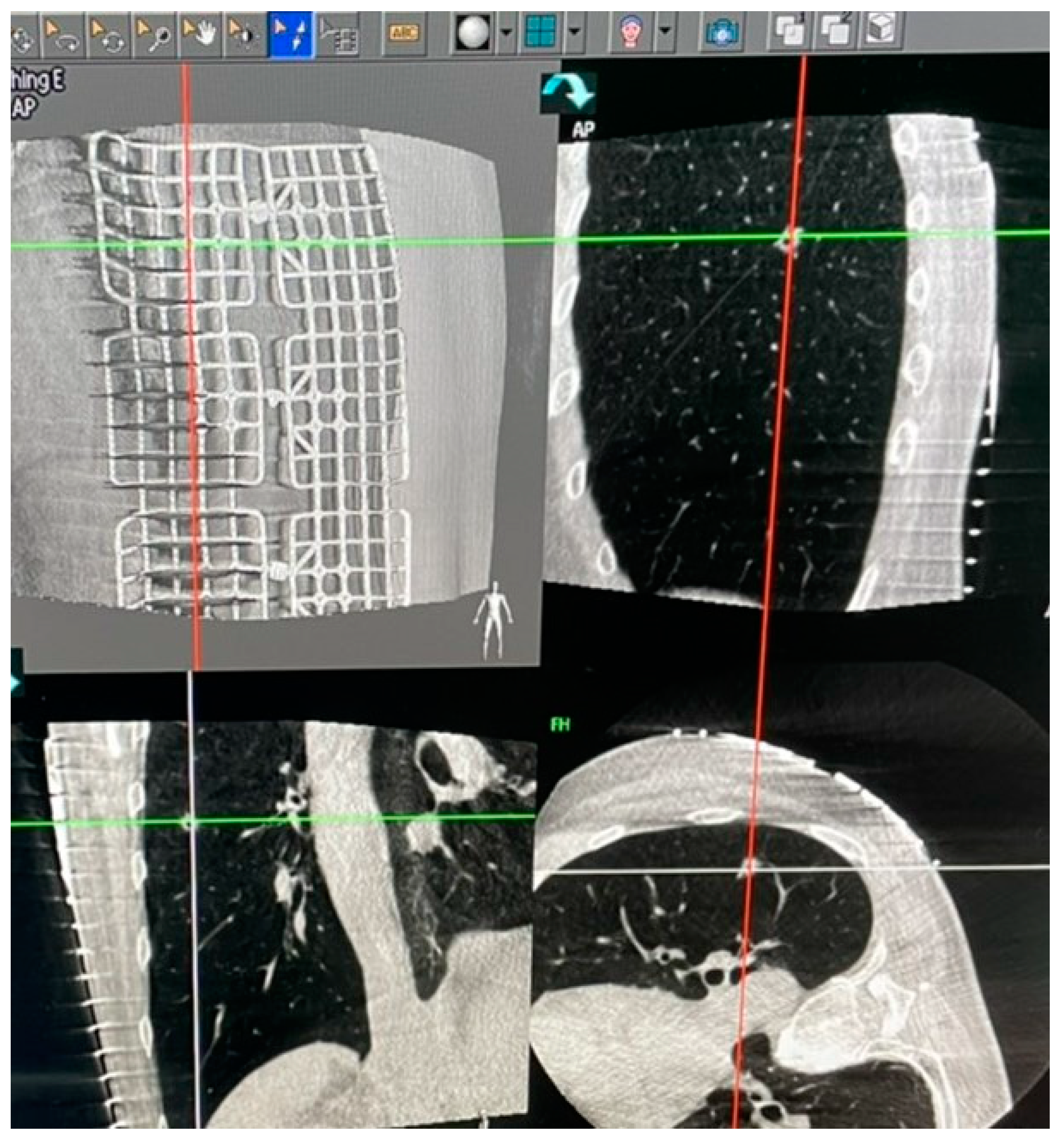
Figure 4.
Image calculated by the program showing site for localization needle penetration.
Figure 4.
Image calculated by the program showing site for localization needle penetration.
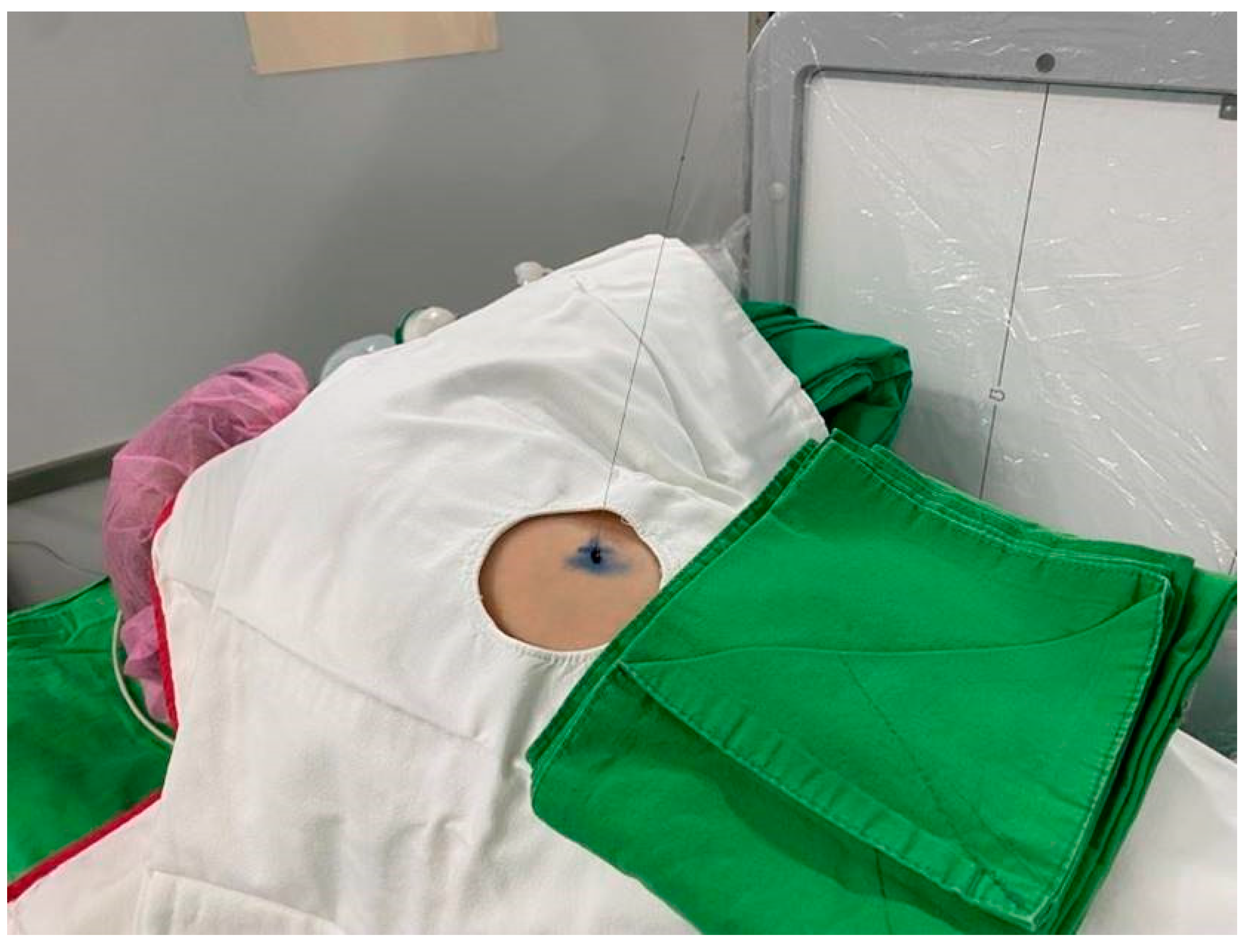
Figure 5.
Hookwire placed into the patient’s chest wall
Figure 5.
Hookwire placed into the patient’s chest wall
3. Results
During the study period, 81 study patients were collected; 30 in the TSCT group and 51 in the OSCT group. However, six patients concurrently undergoing a lobectomy and one patient concurrently undergoing a segmentectomy in the TSCT group were excluded. In the other group, we also excluded three patients and two patients concurrently undergoing lobectomies and mediastinal tumor resections, respectively, in the OSCT group. Finally, 23 and 46 patients were included in the TSCT and OSCT groups, respectively.
Table 1 shows the basic characteristics of the study patients. There were significant differences in age and lesion site. The mean age of our study participants was 56.83 years. The average age of the participants in the OSCT group was 54.82 and the average age in the TSCT group is 60.23 (p < 0.05). There were 23 male patients (33.3%) and 46 female patients (66.7%). In the OSCT group, 13 patients (28.3%) were males and 33 patients (71.7%) were females. In the TSCT group, 10 patients (43.5%) were males and 13 patients (56.5%) were females. There were 65 patients in ASA I-II (94.3%) and four patients in ASA III (5.7%). In the OSCT group, 43 patients (93.5%) were classified as ASA I-II and three patients (6.5%) were classified as ASA III. In the TSCT group, 22 patients were classified as ASA I–II and one patient was classified as ASA III. The proportions of right-sided lesions in the OSCT and TSCT groups were 56.5% and 78.2%, respectively (p < 0.05). According to previous CT findings, 64 lesions (92.8%) showed subsolid nodules (SSN) and five lesions (7.2%) showed solid nodules (SN). In the OSCT group, four lesions (8.7%) were SN and 42 lesions (91.3%) were SSN. In the TSCT group, one lesion (4.3%) was SN and 22 lesions (95.7%) were SSN. The mean pulmonary lesion size on CT image finding was 7.81 mm. In the OSCT group, the mean size of pulmonary lesions was 7.86 mm. In the TSCT group, the mean size of pulmonary lesions was 7.74 mm.
Table 2 summarizes the results of intraoperative and perioperative variables. The total number of perioperative complications of pneumothorax was 3 (4.3%). Only two patients (4.3%) experienced complications of pneumothorax in the OSCT group and one patient (4.3%) in the TSCT group. There was no significant difference in the incidence of perioperative complications of pneumothorax between the OSCT group and TSCT groups (p = 0.879). In the OSCT group, 28 patients (60.9%) were placed in the lateral decubitus position during localization and 18 patients (39.1%) were placed in the supine or prone position. In the TSCT group, 18 patients (78.2%) were placed in a supine or prone position and five patients (21.8%) were placed in the lateral decubitus position (p < 0.05). The mean blood loss was 6.93 ml in the OSCT group and 10.86 ml in the TSCT group (p < 0.05). After the procedure, most of the final pathological diagnoses were AIS and MIA, accounting for 45 (97.8%) and 22 (95.6%) in the OSCT and TSCT groups, respectively. The localization success rates in the OSCT and TSCT groups were 97.8% and 91.3%, respectively (p = 0.216).
Table 3 summarizes the efficacy index variables of time. The duration of the localization procedure was significantly shorter in the OSCT group than in the TSCT group (mean difference: -15.53 min). The time at risk was also significantly lower in the OSCT group than in the TSCT group (mean difference: -145.91 min). Because the patients in the OSCT group underwent the localization procedure and surgery in the same place, the time of localization and the time at risk were much shorter. Also, there was no significant difference in the surgery duration between the OSCT group and the TSCT group (168.94 min vs. 196.10 min, p > 0.05). However, the time spent under general anesthesia was much longer in the OSCT group than in the TSCT group (mean difference: +64.28 min). The total time of the procedure was significantly lower in the OSCT group than in the TSCT group (mean difference: -194.93 min).
4. Discussion
In our study, we evaluated the safety and efficacy of the OSCT and TSCT approaches to small pulmonary lesion resection. OSCT localization for small pulmonary lesions in the HOR is feasible, effective, and safe. The OSCT approach also significantly reduces the procedural time and time at risk. Therefore, patients in the OSCT group suffered from less fear and nervousness than those in the TSCT group. In our study, the mean age of the patients in the OSCT group was lower than that of patients in the TSCT group. As the use of LDCT increases, tiny pulmonary lesions are identified in more and more young patients. Therefore, this phenomenon explains why the mean age of the patients in the OSCT group is lower than that of patients in the TSCT group. The mean size of pulmonary lesions on CT images is approximately 7–8 mm, irrespective of the group in which they are found. According to NCCN guidelines, 6–8-mm pulmonary lesions in low-risk patients are preferably monitored every six months or yearly. Surgical resection is not the treatment of choice for these small lesions. In Taiwan, surgeons also explain this protocol to the patients who come to our outpatient department. However, the patients are usually nervous about the lesions in their lungs; so, they often opt for surgical resection of these lesions. If these patients prefer surgery, we would advise them to go for the localization of the lesions for accurate surgical resection.
In our study, the incidence of perioperative complications (such as pneumothorax) after localization and the rate of successful localization did not differ significantly between the OSCT group and the TSCT group. It indicates that localizations performed by thoracic surgeons are not inferior to those performed by an experienced radiologist. One case of pneumothorax in the OSCT group was a case of a pure ground-glass nodule over the right upper lobe. We performed localization in the HOR, and the whole procedure was smooth. However, after C-arm screening, the image showed that the localization needle was through-through from the right lower lobe to the right upper lobe with mild pneumothorax. Though the localization failed this time, we resected the target lesion successfully by tracing the pinhole on the lung’s surface to guess the location of the target lesion. One case of pneumothorax in the TSCT group was a result of hookwire dislodgement caused by severe agitation. However, we also successfully resected the target lesion by tracing the pinhole on the lung’s surface. Thus, localization performed by a thoracic surgeon might be better than that performed by a radiologist because the surgeon could resolve the complication in the operation room immediately if any acute situation arises.
Most patients in the OSCT group were placed in the lateral decubitus position during localization. This indicates that thoracic surgeons prefer placing patients in the lateral decubitus position because it is convenient for the surgical team to place the patient in this position to facilitate any operation that might be performed after localization. Also, most patients in the TSCT group were placed in the supine or prone position for localization. This might indicate that radiologists prefer placing patients in the supine or prone position due to the limited space available on the CT suite table. Because the CT in our hospital was not designed for intervention, it was not advisable for a radiologist to place patients in the lateral decubitus positions. The shortest possible trajectory of the localization needle was ideal for patients, and this trajectory determined the position of the patient for localization; however, the surgeon or radiologist performing the procedure could prefer specific positions for convenience.
The estimated intraoperative blood loss in the TSCT group was higher than that in the OSCT group. This indicated that the patient’s agitation after localization might cause a slight migration of the localization needle within the patient’s lung, which could cause bleeding. Thus, more blood loss was noticed in the TSCT group. Therefore, the OSCT localization method seems safer and more effective for patients undergoing localization.
In our study, the time of localization, time at risk, and total duration were all significantly shorter in the OSCT group than in the TSCT group. Because the patients underwent localization and surgery at the same place in the OSCT group, it saved much time that would have been used for transportation from the ward to the CT room. However, the time the patient spent under general anesthesia was significantly longer in the OSCT group than in the TSCT group, which might have increased the number of adverse events associated with anesthetics throughout the procedure. It may also have increased the cost of anesthetics, leading to an increase in the economic burden levied on patients.
In the study by Huang et al. [
8], they reported a successful and safe CT-guided localization method using microcoils to localize solitary pulmonary nodules. In another study by Fang et al. [
9], the technical developments were reviewed and different localization techniques were compared. The study mentioned the advantages and disadvantages of different localization techniques. An optimal method of localization for pulmonary nodules is yet to be established [
10]. Although there was a mild risk of pneumothorax and pulmonary hemorrhage due to dislodgement or migration, this technique was easier to perform and more widely used than other methods. The safety, effectiveness, and convenience of hookwire use for localization were acceptable [
11,
12]. Therefore, we use the hookwire as the tool to localize the target lesion, be it during OSCT or TSCT.
In the study by Chao et al. [
13], for those patients with multiple ipsilateral pulmonary nodules, OSCT localization performed in the HOR is associated with a shorter procedural time compared to the TSCT approach. In our study, we selected patients with only one pulmonary lesion undergoing wedge resection to eliminate certain confounding factors. Another radiation-free intraoperative localization approach using ultrasonography was also reported if the lung was completely deflated and could be extracted. Kondo et al. [
14] reported that intraoperative ultrasonography was also a safe and effective approach to the localization of pulmonary GGN. However, we did not compare this intraoperative localization method to the OSCT method. More studies are needed to investigate different intraoperative localization methods.
Our study has several limitations. First, this was a retrospective study including a relatively small number of cases. Our findings should be verified by conducting a prospective study with more cases. Second, this is a single-center study. Third, we did not collect data on radiation exposure because these data were missing, and we did not calculate the cost of anesthetics. Fourth, our study did not compare other methods of localizing pulmonary lesions. Non-percutaneous localization approaches such as electromagnetic navigation bronchoscopy [
16,
17] were not compared to the percutaneous localization method in our study. Finally, no oncological benefit could be observed in the OSCT group compared to the TSCT group because there was no significant difference in clinical outcomes between the two groups of patients with early lung cancer. Significant reductions in the procedural time and the time at risk could not be further translated to clinically significant differences between the two groups.
5. Conclusions
Compared to the TSCT, the OSCT-guided approach to pulmonary nodule localization in HOR significantly decreases the procedural time and risky time. The rate of successful localization and incidence of pneumothorax were similar in both groups.
Author Contributions
Conceptualization: K.-Y. H. and Y.-C. T; Methodology: K.-Y. H. and Y.-C. T; Resources: K.-Y. H.; Writing–original draft: K.-Y. H.; Writing–review & editing: Y.-C. T. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no external funding for this study.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to our data were retrospectively collected from the data base of Veterans General Hospital Kaohsiung.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, Lammers JJ, Weenink C, Yousaf-Khan U, Horeweg N, van 't Westeinde S, Prokop M, Mali WP, Mohamed Hoesein FAA, van Ooijen PMA, Aerts JGJV, den Bakker MA, Thunnissen E, Verschakelen J, Vliegenthart R, Walter JE, Ten Haaf K, Groen HJM, Oudkerk M. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020 Feb 6;382(6):503-513.
- Duffy SW, Field JK. Mortality Reduction with Low-Dose CT Screening for Lung Cancer. N Engl J Med. 2020 Feb 6;382(6):572-573. [CrossRef]
- Lee WY, Chen PH, Chen KC, Hsu HH, Chen JS. Computed Tomography-Guided Localization and Extended Segmentectomy for Non-Small Cell Lung Cancer. Diagnostics (Basel). 2022 Aug 24;12(9):2043. [CrossRef]
- Klinkenberg TJ, Dinjens L, Wolf RFE, van der Wekken AJ, van de Wauwer C, de Bock GH, Timens W, Mariani MA, Groen HJM. CT-guided Percutaneous Hookwire Localization Increases the Efficacy and Safety of VATS for Pulmonary Nodules. J Surg Oncol. 2017 Jun;115(7):898-904. [CrossRef]
- Fang HY, Chen KA, Wen YW, Wen CT, Pan KT, Chiu CH, Hsieh MJ, Chao YK. Efficacy and Safety of Preoperative vs. Intraoperative Computed Tomography-Guided Lung Tumor Localization: A Randomized Controlled Trial. Front Surg. 2022 Jan 7;8:809908. [CrossRef]
- Chao YK, Fang HY, Wen YW, Hsieh MJ, Wen CT. Intraoperative Computed Tomography-Guided Pulmonary Tumour Localization: A Thoracic Surgeon’s Learning Curve. Eur J Cardiothorac Surg. 2019 Mar 1;55(3):421-426. [CrossRef]
- Zhang H, Li Y, Yimin N, He Z, Chen X. CT-Guided Hook-Wire Localization of Malignant Pulmonary Nodules for Video Assisted Thoracoscopic Surgery. J Cardiothorac Surg. 2020 Oct 9;15(1):307. [CrossRef]
- Huang ZG, Wang CL, Sun HL, Li CD, Gao BX, Chen H, Yang MX. CT-Guided Microcoil Localization of Small Peripheral Pulmonary Nodules to Direct Video-Assisted Thoracoscopic Resection without the Aid of Intraoperative Fluoroscopy. Korean J Radiol. 2021 Jul;22(7):1124-1131. [CrossRef]
- Fang HY, Chang KW, Chao YK. Hybrid Operating Room for the Intraoperative CT-guided Localization of Pulmonary Nodules. Ann Transl Med. 2019 Jan;7(2):34. [CrossRef]
- Park CH, Han K, Hur J, Lee SM, Lee JW, Hwang SH, Seo JS, Lee KH, Kwon W, Kim TH, Choi BW. Comparative Effectiveness and Safety of Preoperative Lung Localization for Pulmonary Nodules: A Systematic Review and Meta-analysis. Chest. 2017 Feb;151(2):316-328.
- Ichinose J, Kohno T, Fujimori S, Harano T, Suzuki S. Efficacy and Complications of Computed Tomography-Guided Hook Wire Localization. Ann Thorac Surg. 2013 Oct;96(4):1203-1208. [CrossRef]
- Thistlethwaite PA, Gower JR, Hernandez M, Zhang Y, Picel AC, Roberts AC. Needle Localization of Small Pulmonary Nodules: Lessons learned. J Thorac Cardiovasc Surg. 2018 May;155(5):2140-2147.
- Chao YK, Fang HY, Pan KT, Wen CT, Hsieh MJ. Preoperative Versus Intraoperative Image-Guided Localization of Multiple Ipsilateral Lung Nodules. Eur J Cardiothorac Surg. 2020 Mar 1;57(3):488-495. [CrossRef]
- Kondo R, Yoshida K, Hamanaka K, Hashizume M, Ushiyama T, Hyogotani A, Kurai M, Kawakami S, Fukushima M, Amano J. Intraoperative Ultrasonographic Localization of Pulmonary Ground-Glass Opacities. J Thorac Cardiovasc Surg. 2009 Oct;138(4):837-42. [CrossRef]
- Ichinose J, Kohno T, Fujimori S, Harano T, Suzuki S. Efficacy and Complications of Computed Tomography-Guided Hook Wire Localization. Ann Thorac Surg. 2013 Oct;96(4):1203-1208. [CrossRef]
- Luo K, Lin Y, Lin X, Yu X, Wen J, Xi K, Lin P, Zhang L. Localization of Peripheral Pulmonary Lesions to Aid Surgical Resection: A Novel Approach for Electromagnetic Navigation Bronchoscopic Dye Marking. Eur J Cardiothorac Surg. 2017 Sep 1;52(3):516-521.
- Abbas A, Kadakia S, Ambur V, Muro K, Kaiser L. Intraoperative Electromagnetic Navigational Bronchoscopic Localization of Small, Deep, or Subsolid Pulmonary Nodules. J Thorac Cardiovasc Surg. 2017 Jun;153(6):1581-1590. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).