Submitted:
20 July 2023
Posted:
21 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
The past
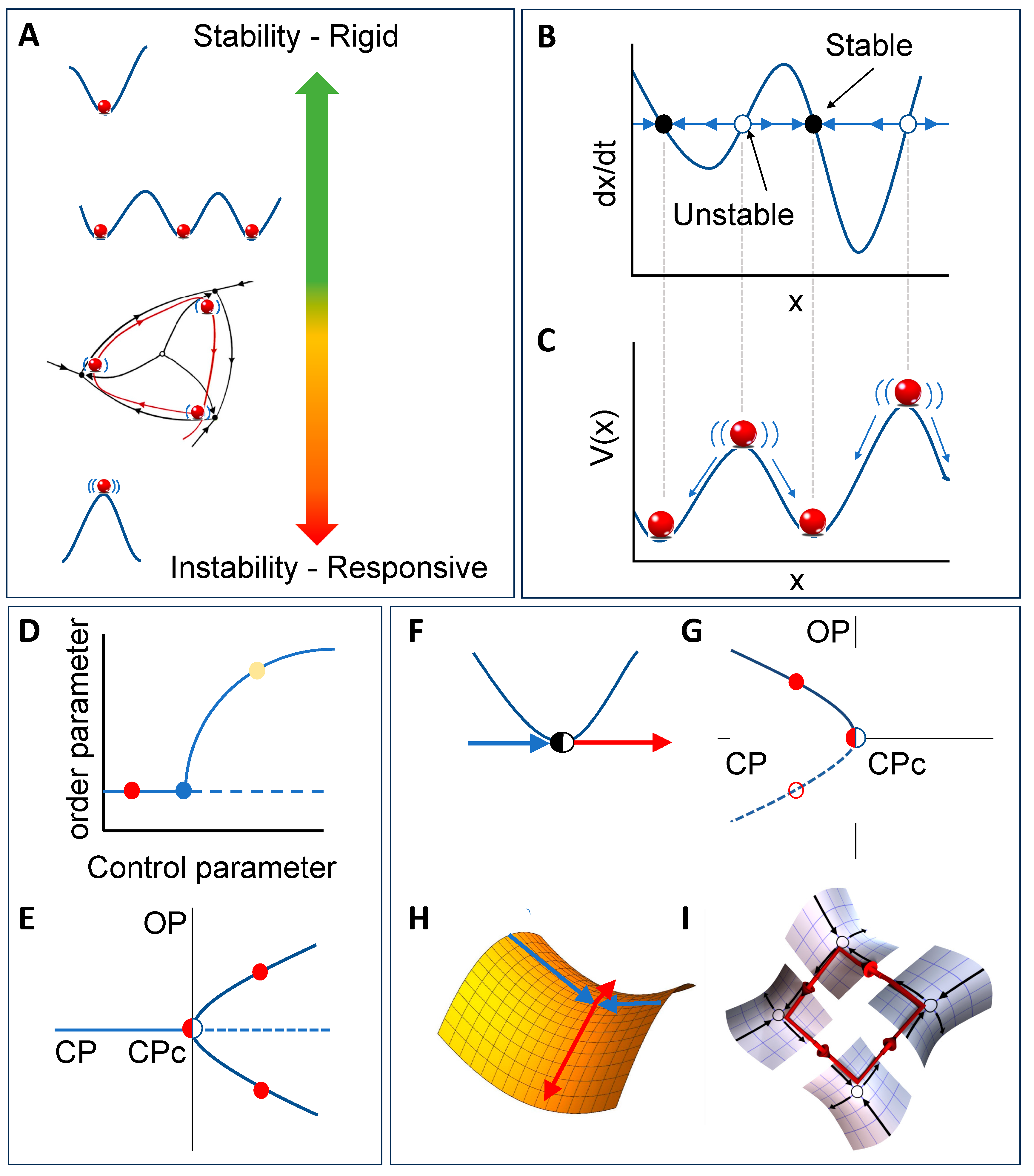
A phenomenological account of metastability
A brief history of metastability
The present
Practical signatures of metastability
Entropy of the spectral density
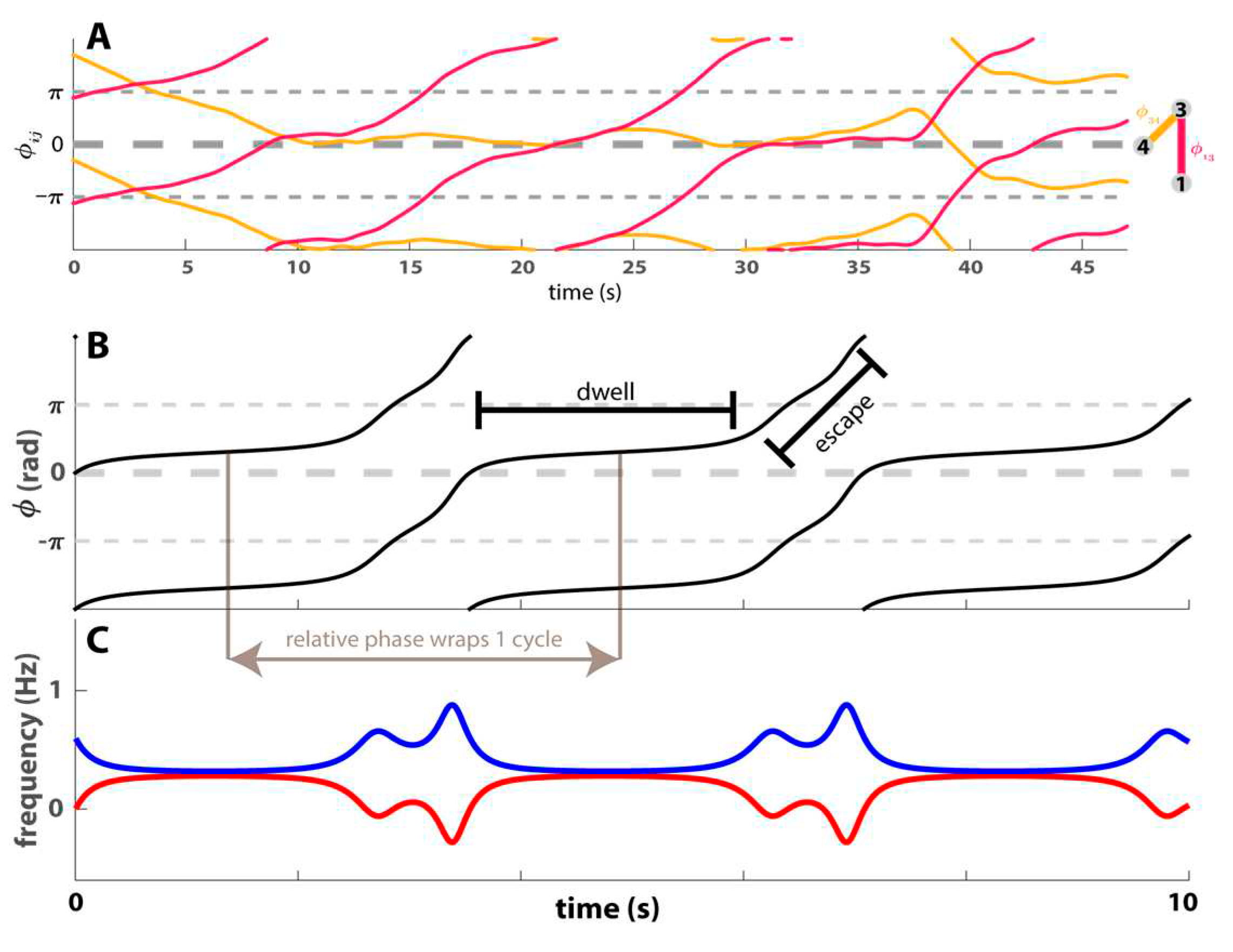
Ratio between dwell and escape time
Variance or standard deviation of the Kuramoto order parameter
Standard deviation of the average spatial coherence
Variance of functional connectivity
Fluctuation of relative frequency
Variance of the Leading Eigenvectors
Variance of the phase difference differential
Metastability in computational neuroscience
Metastability in Coordination Dynamics
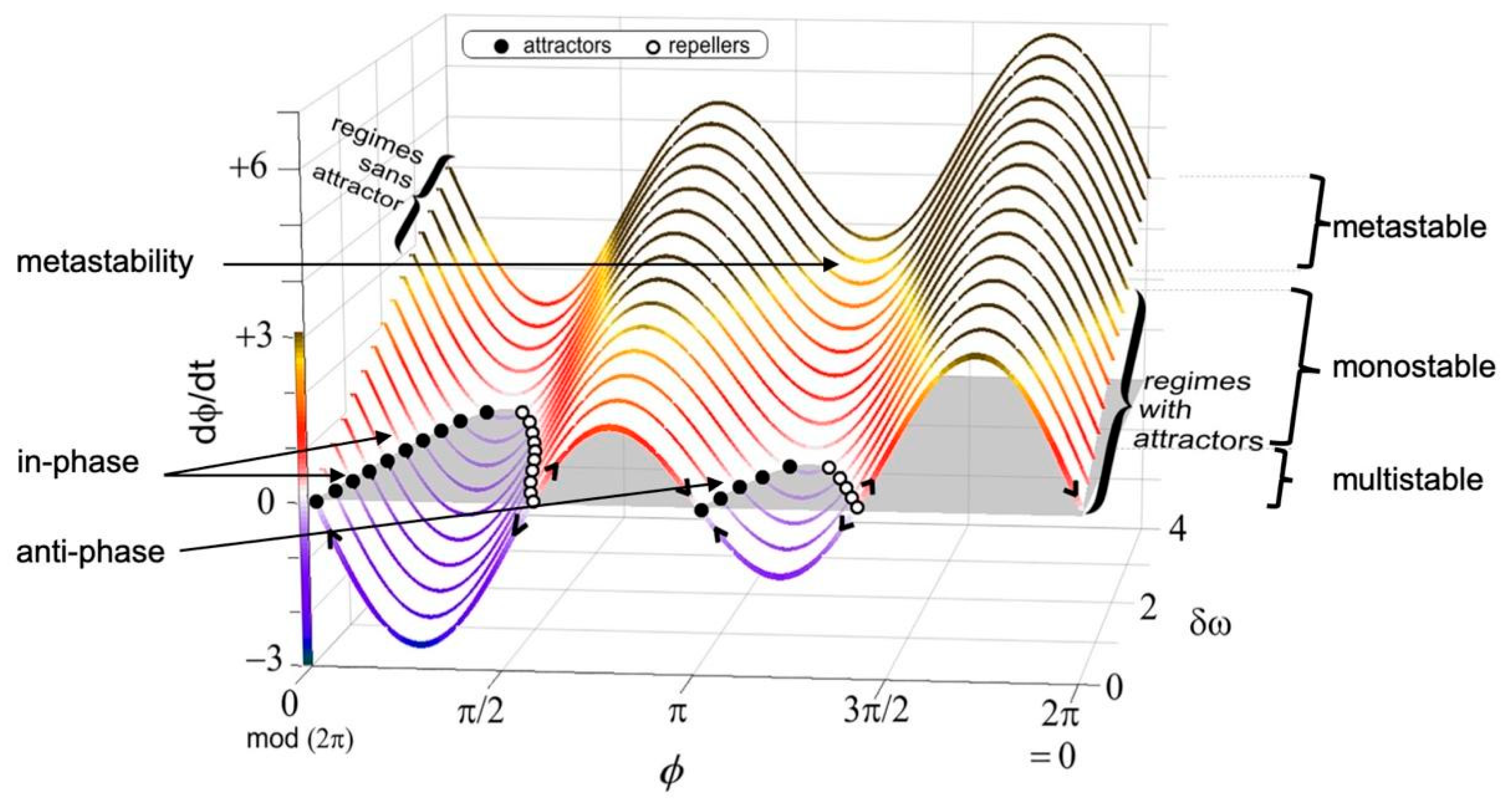
Noise-driven metastability with time delays
Hidden faces of metastability
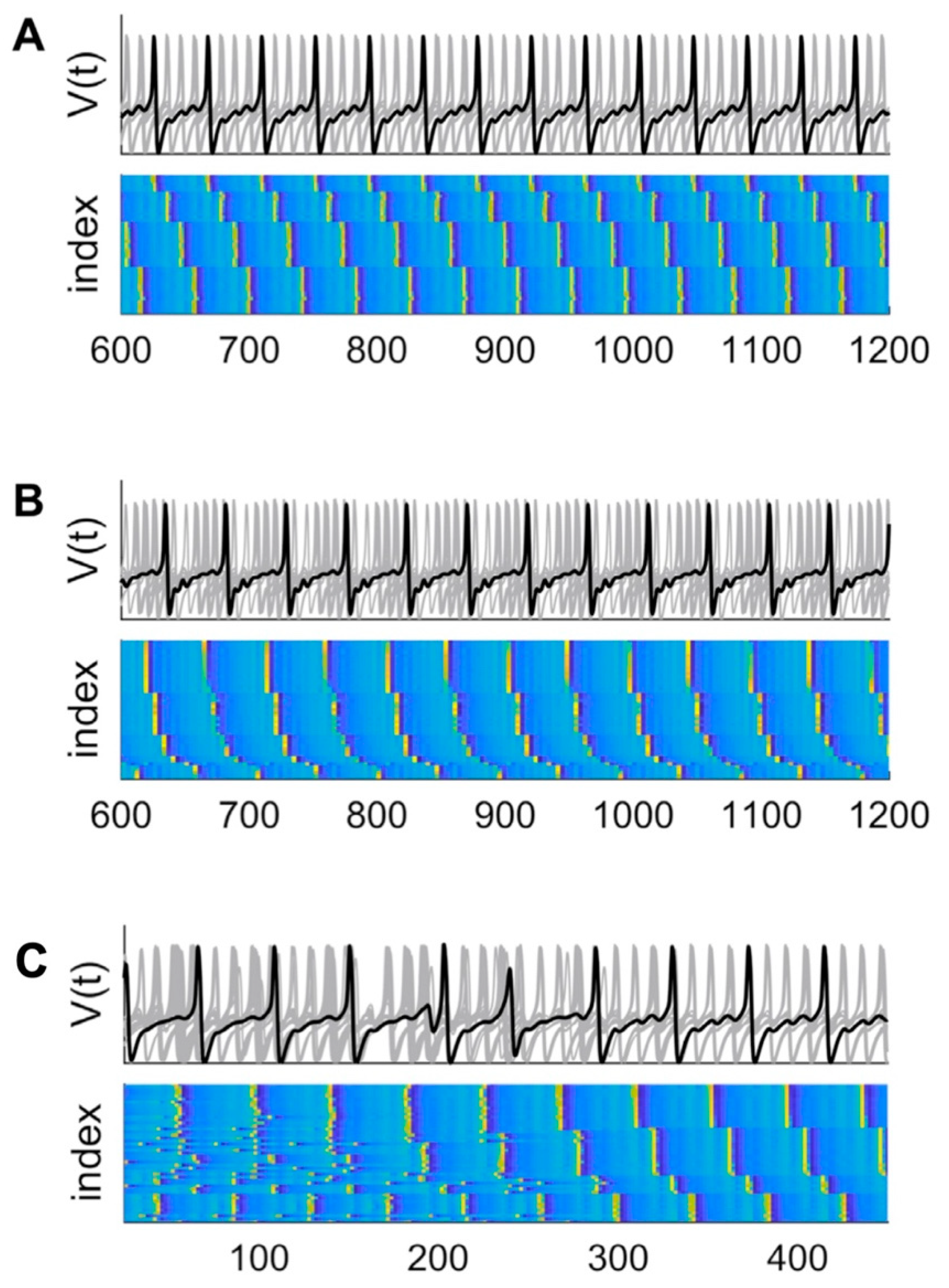
Metastability in neural mass models
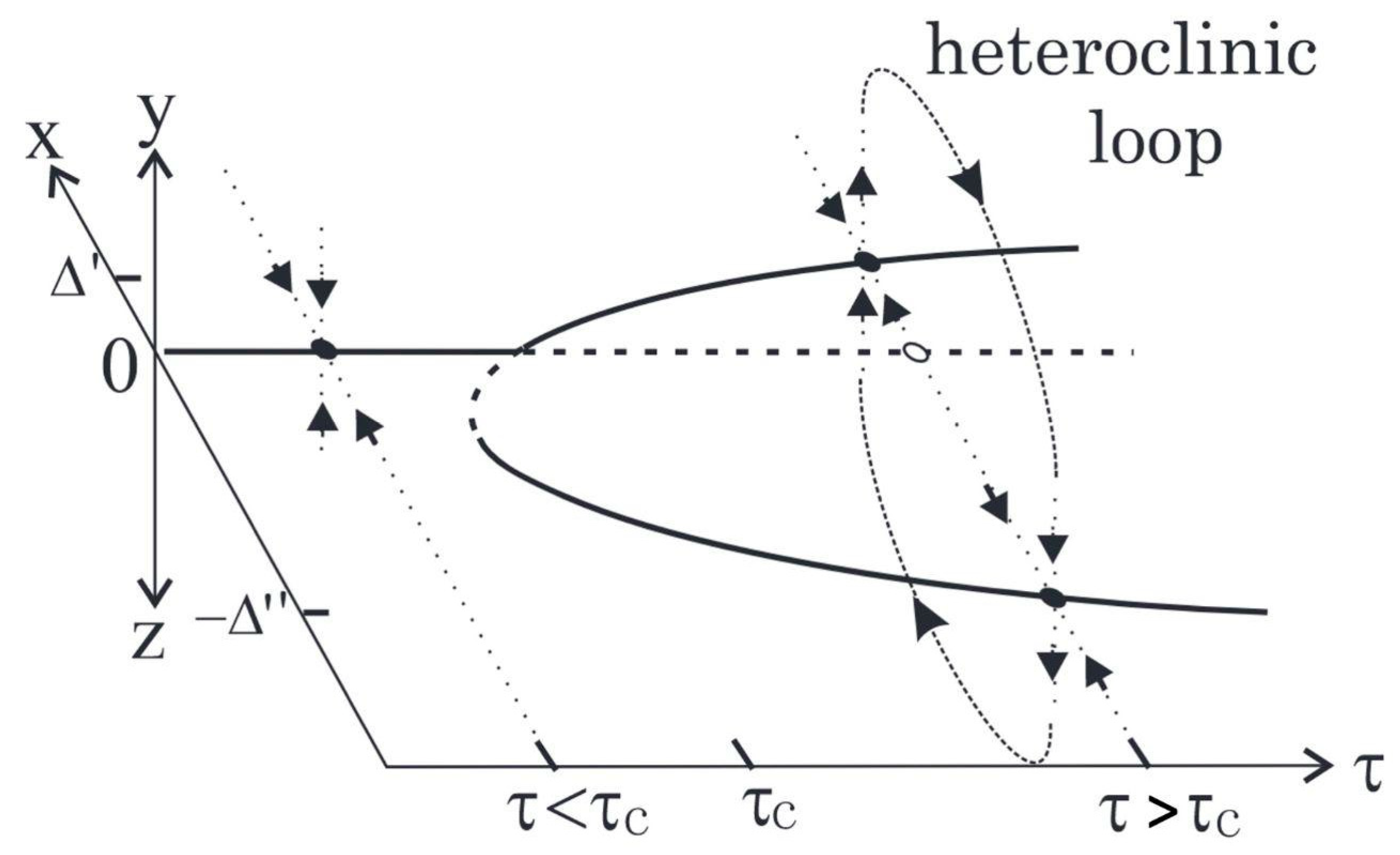
Metastability with saddles — Winnerless Competition
Metastability in models of coupled oscillators
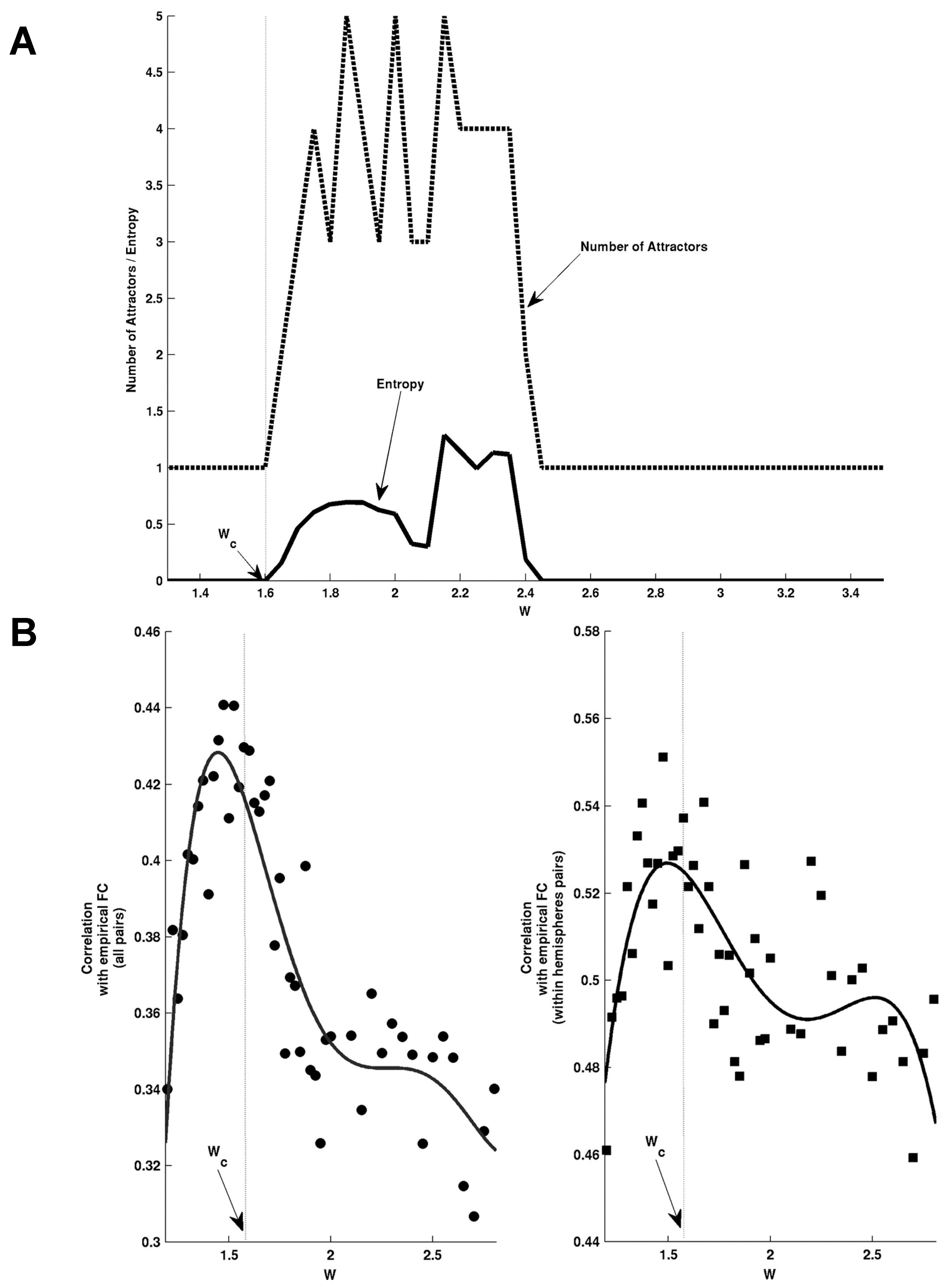
Multistable ghost attractors
Role of antiphase synchronisation
Identifying the determinants and moderators of metastability
The future
Beyond metastability — Turbulence
 |
“Big whorls have little whorls Which feed on their velocity, And little whorls have lesser whorls And so on to viscosity.” [117] |
Beyond first order coupling in the Kuramoto model
Kuramoto model with 2 Fourier modes
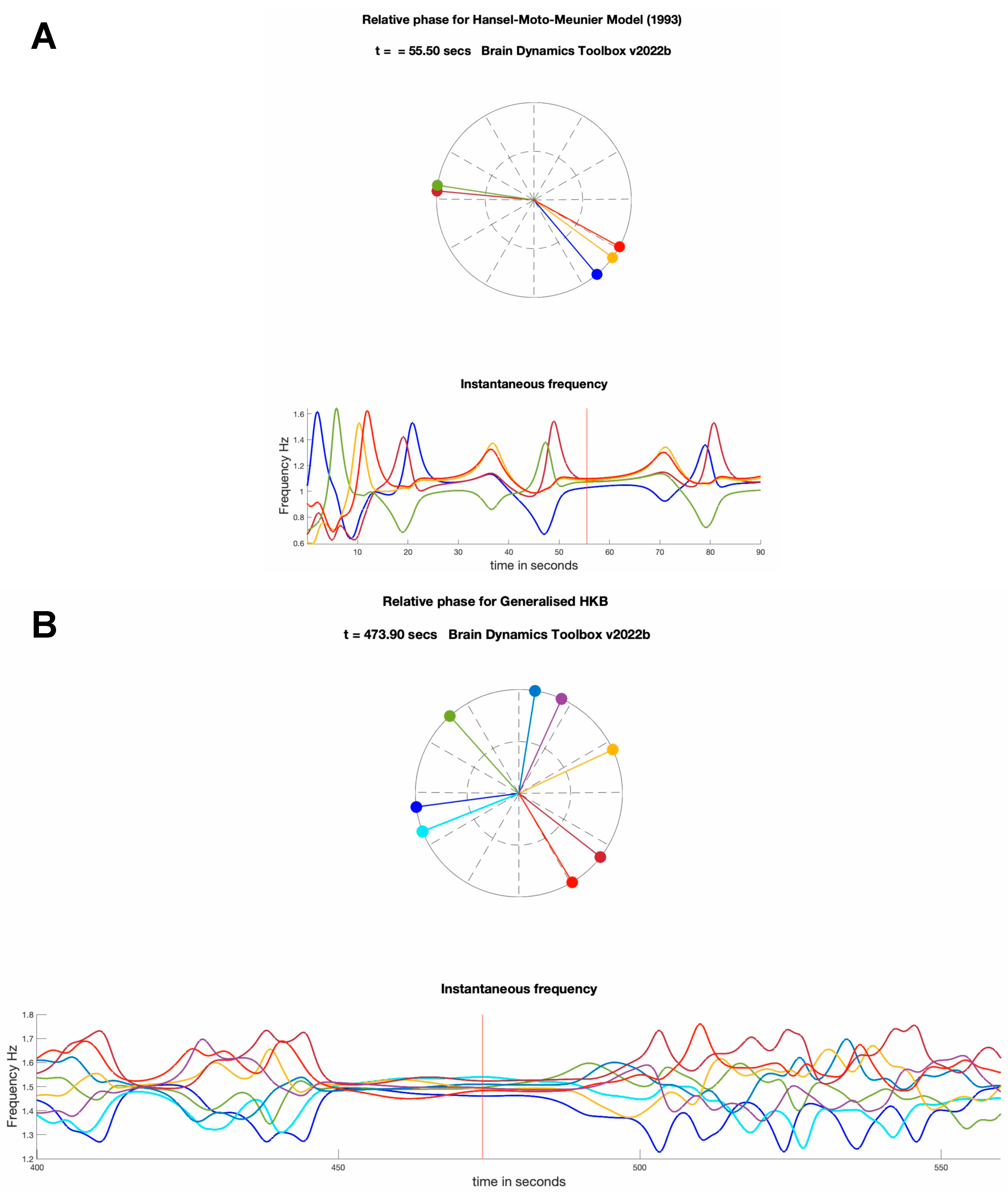
Generalised Haken-Kelso-Bunz model
Avenues for future empirical work
Concluding remarks
Supplementary Materials
Glossary
References
- Kelso, J.A.S. Dynamic patterns: The self-organization of brain and behavior; Dynamic patterns: The self-organization of brain and behavior; The MIT Press: Cambridge, MA, US, 1995; pp. xvii, 334; ISBN 978-0-262-11200-0. [Google Scholar]
- Kelso, J.A.S.; Tognoli, E. Toward a Complementary Neuroscience: Metastable Coordination Dynamics of the Brain. In Neurodynamics of Cognition and Consciousness; Perlovsky, L.I., Kozma, R., Eds.; Understanding Complex Systems; Springer: Berlin, Heidelberg, 2007; pp. 39–59. ISBN 978-3-540-73267-9. [Google Scholar]
- Tononi, G.; Sporns, O.; Edelman, G.M. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc. Natl. Acad. Sci. 1994, 91, 5033–5037. [Google Scholar] [CrossRef]
- Cocchi, L.; Gollo, L.L.; Zalesky, A.; Breakspear, M. Criticality in the brain: A synthesis of neurobiology, models and cognition. Prog. Neurobiol. 2017, 158, 132–152. [Google Scholar] [CrossRef]
- Kelso, J.A.S. Coordination Dynamics of Human Brain and Behavior. In Proceedings of the Evolution of Dynamical Structures in Complex Systems; Friedrich, R., Wunderlin, A., Eds.; Springer: Berlin, Heidelberg, 1992; pp. 223–234. [Google Scholar]
- Fuchs, A.; Kelso, J.A.S.; Haken, H. Phase transitions in the human brain: spatial mode dynamics. Int. J. Bifurc. Chaos 1992, 02, 917–939. [Google Scholar] [CrossRef]
- Kryukov, V. The metastable and unstable states in the brain. Neural Netw. 1988, 1, 264. [Google Scholar] [CrossRef]
- Breakspear, M. Dynamic models of large-scale brain activity. Nat. Neurosci. 2017, 20, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. Transients, Metastability, and Neuronal Dynamics. NeuroImage 1997, 5, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, M.I.; Huerta, R.; Varona, P.; Afraimovich, V.S. Transient Cognitive Dynamics, Metastability, and Decision Making. PLOS Comput. Biol. 2008, 4, e1000072. [Google Scholar] [CrossRef]
- Tsuda, I. Toward an interpretation of dynamic neural activity in terms of chaotic dynamical systems. Behav. Brain Sci. 2001, 24, 793–810. [Google Scholar] [CrossRef]
- Ashwin, P.; Postlethwaite, C. On designing heteroclinic networks from graphs. Phys. Nonlinear Phenom. 2013, 265, 26–39. [Google Scholar] [CrossRef]
- Tsuda, I. Chaotic itinerancy and its roles in cognitive neurodynamics. Curr. Opin. Neurobiol. 2015, 31, 67–71. [Google Scholar] [CrossRef]
- Rosas, F.E.; Mediano, P.A.M.; Luppi, A.I.; Varley, T.F.; Lizier, J.T.; Stramaglia, S.; Jensen, H.J.; Marinazzo, D. Disentangling high-order mechanisms and high-order behaviours in complex systems. Nat. Phys. 2022, 18, 476–477. [Google Scholar] [CrossRef]
- Haken, H. Cooperative phenomena in systems far from thermal equilibrium and in nonphysical systems. Rev. Mod. Phys. 1975, 47, 67–121. [Google Scholar] [CrossRef]
- Kelso, J.A.S.; Bressler, S.L.; Buchanan, S.; Ding, M.; Fuchs, A.; Holroyd, T. Cooperative and critical phenomena in the human brain revealed by multiple SQUIDS. In Measuring Chaos in The Human Brain; Duke, D.W., Pritchard, W.S., Eds.; WORLD SCIENTIFIC, 1991; pp. 1–266 ISBN 978-981-02-0701-4.
- Miets, H.A.; Chevalier, J. On the crystallization of sodium nitrate. Mineral. Mag. J. Mineral. Soc. 1906, 14, 123–133. [Google Scholar] [CrossRef]
- Shlosman, S. Metastable States. In Encyclopedia of Mathematical Physics; Françoise, J.-P., Naber, G.L., Tsun, T.S., Eds.; Academic Press: Oxford, 2006; pp. 417–420. ISBN 978-0-12-512666-3. [Google Scholar]
- Brinkman, B.A.W.; Yan, H.; Maffei, A.; Park, I.M.; Fontanini, A.; Wang, J.; La Camera, G. Metastable dynamics of neural circuits and networks. Appl. Phys. Rev. 2022, 9, 011313. [Google Scholar] [CrossRef] [PubMed]
- Holst, E.R.M. von The behavioural physiology of animals and man; : The collected papers of Erich von Holst; University of Miami Press: Coral Gables, Fla, 1973; ISBN 978-0-87024-261-8. [Google Scholar]
- DeGuzman; Kelso, J.A.S. The Flexible Dynamics of Biologial Coordination: Living in the Niche between Order and Disorder. In Principles Of Organization In Organisms; Mittenthal, J.E., Ed.; Addison-Wesley, 1992 ISBN 978-0-429-97218-8.
- Kelso, J.A.S. Phase transitions and critical behavior in human bimanual coordination. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1984, 246, R1000–R1004. [Google Scholar] [CrossRef]
- Kelso, J.A.S.; Del Colle, J.D.; Schöner, G. Action-perception as a pattern formation process. In Attention and performance 13: Motor representation and control; Lawrence Erlbaum Associates, Inc: Hillsdale, NJ, US, 1990; pp. 139–169. ISBN 978-0-8058-0565-9. [Google Scholar]
- Kelso, J.A.S.; Bressler, S.L.; Buchanan, S.; DeGuzman, G.C.; Ding, M.; Fuchs, A.; Holroyd, T. A phase transition in human brain and behavior. Phys. Lett. A 1992, 169, 134–144. [Google Scholar] [CrossRef]
- Pomeau, Y.; Manneville, P. Intermittent transition to turbulence in dissipative dynamical systems. Commun. Math. Phys. 1980, 74, 189–197. [Google Scholar] [CrossRef]
- Holden, A.V.; Kryukov, V.I. Neural Networks: theory and architecture. Manchester University Press; 1990.
- Friston, K.J.; Tononi, G.; Sporns, O.; Edelman, G.M. Characterising the complexity of neuronal interactions. Hum. Brain Mapp. 1995, 3, 302–314. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Aranibar, A. Evaluation of event-related desynchronization (ERD) preceding and following voluntary self-paced movement. Electroencephalogr. Clin. Neurophysiol. 1979, 46, 138–146. [Google Scholar] [CrossRef]
- Niebur, E.; Schuster, H.G.; Kammen, D.M. Collective frequencies and metastability in networks of limit-cycle oscillators with time delay. Phys. Rev. Lett. 1991, 67, 2753–2756. [Google Scholar] [CrossRef]
- Niebur, E.; Schuster, H.G.; Kammen, D.M. Systems of Relaxation Oscillators with Time-Delayed Coupling. In Neural Network Dynamics; Taylor, J.G., Caianiello, E.R., Cotterill, R.M.J., Clark, J.W., Eds.; Perspectives in Neural Computing; Springer London: London, 1992; pp. 226–233. ISBN 978-3-540-19771-3. [Google Scholar]
- Kelso, J.A.S. An Essay on Understanding the Mind. Ecol. Psychol. Publ. Int. Soc. Ecol. Psychol. 2008, 20, 180–208. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, Y. Mutual Entrainment. In Chemical Oscillations, Waves, and Turbulence; Springer Series in Synergetics; Springer Berlin Heidelberg: Berlin, Heidelberg, 1984; Vol. 19, pp. 60–88. ISBN 978-3-642-69691-6. [Google Scholar]
- Shanahan, M. Metastable chimera states in community-structured oscillator networks. Chaos Interdiscip. J. Nonlinear Sci. 2010, 20, 013108. [Google Scholar] [CrossRef] [PubMed]
- Abeysuriya, R.G.; Hadida, J.; Sotiropoulos, S.N.; Jbabdi, S.; Becker, R.; Hunt, B.A.E.; Brookes, M.J.; Woolrich, M.W. A biophysical model of dynamic balancing of excitation and inhibition in fast oscillatory large-scale networks. PLOS Comput. Biol. 2018, 14, e1006007. [Google Scholar] [CrossRef] [PubMed]
- Alderson, T.H.; Bokde, A.L.W.; Kelso, J.A.S.; Maguire, L.; Coyle, D. Metastable neural dynamics in Alzheimer’s disease are disrupted by lesions to the structural connectome. NeuroImage 2018, 183, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Deco, G.; Kringelbach, M.L.; Jirsa, V.K.; Ritter, P. The dynamics of resting fluctuations in the brain: metastability and its dynamical cortical core. Sci. Rep. 2017, 7, 3095. [Google Scholar] [CrossRef]
- Hancock, F.; Cabral, J.; Luppi, A.I.; Rosas, F.E.; Mediano, P.A.M.; Dipasquale, O.; Turkheimer, F.E. Metastability, fractal scaling, and synergistic information processing: What phase relationships reveal about intrinsic brain activity. NeuroImage 2022, 259, 119433. [Google Scholar] [CrossRef]
- Hellyer, P.J.; Scott, G.; Shanahan, M.; Sharp, D.J.; Leech, R. Cognitive Flexibility through Metastable Neural Dynamics Is Disrupted by Damage to the Structural Connectome. J. Neurosci. 2015, 35, 9050–9063. [Google Scholar] [CrossRef]
- Jobst, B.M.; Hindriks, R.; Laufs, H.; Tagliazucchi, E.; Hahn, G.; Ponce-Alvarez, A.; Stevner, A.B.A.; Kringelbach, M.L.; Deco, G. Increased Stability and Breakdown of Brain Effective Connectivity During Slow-Wave Sleep: Mechanistic Insights from Whole-Brain Computational Modelling. Sci. Rep. 2017, 7, 4634. [Google Scholar] [CrossRef]
- Lee, W.H.; Doucet, G.E.; Leibu, E.; Frangou, S. Resting-state network connectivity and metastability predict clinical symptoms in schizophrenia. Schizophr. Res. 2018, 201, 208–216. [Google Scholar] [CrossRef]
- Lee, W.H.; Frangou, S. Emergence of metastable dynamics in functional brain organization via spontaneous fMRI signal and whole-brain computational modeling. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 2017; pp. 4471–4474. [Google Scholar]
- Lord, L.-D.; Expert, P.; Atasoy, S.; Roseman, L.; Rapuano, K.; Lambiotte, R.; Nutt, D.J.; Deco, G.; Carhart-Harris, R.L.; Kringelbach, M.L.; et al. Dynamical exploration of the repertoire of brain networks at rest is modulated by psilocybin. NeuroImage 2019, 199, 127–142. [Google Scholar] [CrossRef]
- Mediano, P.A.M.; Rosas, F.E.; Farah, J.C.; Shanahan, M.; Bor, D.; Barrett, A.B. Integrated information as a common signature of dynamical and information-processing complexity. Chaos Interdiscip. J. Nonlinear Sci. 2022, 32, 013115. [Google Scholar] [CrossRef] [PubMed]
- Váša, F.; Shanahan, M.; Hellyer, P.J.; Scott, G.; Cabral, J.; Leech, R. Effects of lesions on synchrony and metastability in cortical networks. NeuroImage 2015, 118, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Glover, G.H. Time–frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage 2010, 50, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Hellyer, P.J.; Shanahan, M.; Scott, G.; Wise, R.J.S.; Sharp, D.J.; Leech, R. The Control of Global Brain Dynamics: Opposing Actions of Frontoparietal Control and Default Mode Networks on Attention. J. Neurosci. 2014, 34, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, E. The signatures of conscious access and its phenomenology are consistent with large-scale brain communication at criticality. Conscious. Cogn. 2017, 55, 136–147. [Google Scholar] [CrossRef]
- Alteriis, G. de; MacNicol, E.; Hancock, F.; Cash, D.; Expert, P.; Turkheimer, F.E. EiDA: A Lossless Approach for the Dynamic Analysis of Connectivity Patterns in Signals; Application to Resting State fMRI of a Model of Ageing. bioRxiv 2023. [Google Scholar] [CrossRef]
- Zhang, M.; Kelso, J.A.S.; Tognoli, E. Critical diversity: Divided or united states of social coordination. PLOS ONE 2018, 13, e0193843. [Google Scholar] [CrossRef]
- Zhang, M.; Beetle, C.; Kelso, J.A.S.; Tognoli, E. Connecting empirical phenomena and theoretical models of biological coordination across scales. J. R. Soc. Interface 2019, 16, 20190360. [Google Scholar] [CrossRef]
- Zhang, M.; Kalies, W.D.; Kelso, J.A.S.; Tognoli, E. Topological portraits of multiscale coordination dynamics. J. Neurosci. Methods 2020, 108672. [Google Scholar] [CrossRef]
- Alonso Martínez, S.; Deco, G.; Ter Horst, G.J.; Cabral, J. The Dynamics of Functional Brain Networks Associated With Depressive Symptoms in a Nonclinical Sample. Front. Neural Circuits 2020, 14, 570583. [Google Scholar] [CrossRef]
- Hancock, F.; Rosas, F.E.; McCutcheon, R.A.; Cabral, J.; Dipasquale, O.; Turkheimer, F.E. Metastability as a candidate neuromechanistic biomarker of schizophrenia pathology. PLOS ONE 2023, 18, e0282707. [Google Scholar] [CrossRef]
- Cabral, J.; Vidaurre, D.; Marques, P.; Magalhães, R.; Silva Moreira, P.; Miguel Soares, J.; Deco, G.; Sousa, N.; Kringelbach, M.L. Cognitive performance in healthy older adults relates to spontaneous switching between states of functional connectivity during rest. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vohryzek, J.; Deco, G.; Cessac, B.; Kringelbach, M.L.; Cabral, J. Ghost Attractors in Spontaneous Brain Activity: Recurrent Excursions Into Functionally-Relevant BOLD Phase-Locking States. Front. Syst. Neurosci. 2020, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Breakspear, M.; Williams, L.M.; Stam, C.J. A Novel Method for the Topographic Analysis of Neural Activity Reveals Formation and Dissolution of ‘Dynamic Cell Assemblies.’. J. Comput. Neurosci. 2004, 16, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Haken, H.; Kelso, J.A.S.; Bunz, H. A theoretical model of phase transitions in human hand movements. Biol. Cybern. 1985, 51, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Schöner, G.; Haken, H.; Kelso, J.A.S. A stochastic theory of phase transitions in human hand movement. Biol. Cybern. 1986, 53, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Tognoli, E.; Zhang, M.; Fuchs, A.; Beetle, C.; Kelso, J.A.S. Coordination Dynamics: A Foundation for Understanding Social Behavior. Front. Hum. Neurosci. 2020, 14, 317. [Google Scholar] [CrossRef]
- Coullet, P.; Tresser, C.; Arnéodo, A. Transition to stochasticity for a class of forced oscillators. Phys. Lett. A 1979, 72, 268–270. [Google Scholar] [CrossRef]
- Kelso, J.A.S.; Fuchs, A. Self-organizing dynamics of the human brain: Critical instabilities and Sil’nikov chaos. Chaos Woodbury N 1995, 5, 64–69. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500–544. [Google Scholar] [CrossRef]
- Hansel, D.; Mato, G.; Meunier, C. Clustering and slow switching in globally coupled phase oscillators. Phys. Rev. E 1993, 48, 3470–3477. [Google Scholar] [CrossRef] [PubMed]
- Kori, H.; Kuramoto, Y. Slow Switching in Globally Coupled Oscillators: Robustness and Occurrence through Delayed Coupling. Phys. Rev. E 2000, 63. [Google Scholar] [CrossRef] [PubMed]
- Hindmarsh, J.L.; Rose, R.M.; Huxley, A.F. A model of neuronal bursting using three coupled first order differential equations. Proc. R. Soc. Lond. B Biol. Sci. 1984, 221, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Ashwin, P.; Orosz, G.; Wordsworth, J.; Townley, S. Dynamics on Networks of Cluster States for Globally Coupled Phase Oscillators. SIAM J. Appl. Dyn. Syst. 2007, 6, 728–758. [Google Scholar] [CrossRef]
- Breakspear, M.; Terry, J.R.; Friston, K.J. Modulation of excitatory synaptic coupling facilitates synchronization and complex dynamics in a biophysical model of neuronal dynamics. Netw. Comput. Neural Syst. 2003, 14, 703–732. [Google Scholar] [CrossRef]
- Heitmann, S.; Breakspear, M. Putting the “dynamic” back into dynamic functional connectivity. Netw. Neurosci. 2018, 2, 150–174. [Google Scholar] [CrossRef]
- Morris, C.; Lecar, H. Voltage oscillations in the barnacle giant muscle fiber. Biophys. J. 1981, 35, 193–213. [Google Scholar] [CrossRef]
- Kaneko, K.; Tsuda, I. Networks of Chaotic Elements. In Complex Systems: Chaos and Beyond: A Constructive Approach with Applications in Life Sciences; Kaneko, K., Tsuda, I., Eds.; Springer: Berlin, Heidelberg, 2001; pp. 107–161. ISBN 978-3-642-56861-9. [Google Scholar]
- Stephan, K.E.; Kamper, L.; Bozkurt, A.; Burns, G.A.P.C.; Young, M.P.; Kötter, R. Advanced database methodology for the Collation of Connectivity data on the Macaque brain (CoCoMac). Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2001, 356, 1159–1186. [Google Scholar] [CrossRef]
- Sorrentino, F.; Pecora, L.M.; Hagerstrom, A.M.; Murphy, T.E.; Roy, R. Complete characterization of the stability of cluster synchronization in complex dynamical networks. Sci. Adv. 2016, 2, e1501737. [Google Scholar] [CrossRef]
- Hanggi, P. Escape from a metastable state. J. Stat. Phys. 1986, 42, 105–148. [Google Scholar] [CrossRef]
- Afraimovich, V.; Rabinovich, M.; Varona, P. Heteroclinic Contours in Neural Ensembles and the Winnerless Competition Principle. Int. J. Bifurc. Chaos 2003, 14. [Google Scholar] [CrossRef]
- Afraimovich, V.; Zhigulin, V.P.; Rabinovich, M.I. On the origin of reproducible sequential activity in neural circuits. Chaos Woodbury N 2004, 14, 1123–1129. [Google Scholar] [CrossRef]
- Afraimovich, V.; Muezzinoglu, M.K.; Rabinovich, M.I. Metastability and Transients in Brain Dynamics: Problems and Rigorous Results. In Long-range Interactions, Stochasticity and Fractional Dynamics: Dedicated to George M. Zaslavsky (1935–2008); Luo, A.C.J., Afraimovich, V., Eds.; Nonlinear Physical Science; Springer: Berlin, Heidelberg, 2010; pp. 133–175. ISBN 978-3-642-12343-6. [Google Scholar]
- Ashwin, P.; Karabacak, Ö.; Nowotny, T. Criteria for robustness of heteroclinic cycles in neural microcircuits. J. Math. Neurosci. 2011, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, Y.; Battogtokh, D. Coexistence of Coherence and Incoherence in Nonlocally Coupled Phase Oscillators. In Nonlinear Phenomena in Complex Systems; Haken, H., Ed.; 5; 2002; Vol. 4, pp. 380–385.
- Shanahan, M. Embodiment and the inner life: Cognition and Consciousness in the Space of Possible Minds; Oxford University Press: Oxford, New York, 2010; ISBN 978-0-19-922655-9. [Google Scholar]
- Baars, B.J. A Cognitive Theory of Consciousness; New York: Cambridge University Press, 1988. [Google Scholar]
- Dehaene, S.; Kerszberg, M.; Changeux, J.-P. A neuronal model of a global workspace in effortful cognitive tasks. Proc. Natl. Acad. Sci. 1998, 95, 14529–14534. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K. Clustering, coding, switching, hierarchical ordering, and control in a network of chaotic elements. Phys. Nonlinear Phenom. 1990, 41, 137–172. [Google Scholar] [CrossRef]
- Seth, A.K.; Izhikevich, E.; Reeke, G.N.; Edelman, G.M. Theories and measures of consciousness: An extended framework. Proc. Natl. Acad. Sci. 2006, 103, 10799–10804. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, M. Dynamical complexity in small-world networks of spiking neurons. Phys. Rev. E 2008, 78, 041924. [Google Scholar] [CrossRef]
- Freyer, F.; Roberts, J.A.; Becker, R.; Robinson, P.A.; Ritter, P.; Breakspear, M. Biophysical Mechanisms of Multistability in Resting-State Cortical Rhythms. J. Neurosci. 2011, 31, 6353–6361. [Google Scholar] [CrossRef]
- Ghosh, A.; Rho, Y.; McIntosh, A.R.; Kötter, R.; Jirsa, V.K. Noise during Rest Enables the Exploration of the Brain’s Dynamic Repertoire. PLOS Comput. Biol. 2008, 4, e1000196. [Google Scholar] [CrossRef]
- Deco, G.; Jirsa, V.K. Ongoing Cortical Activity at Rest: Criticality, Multistability, and Ghost Attractors. J. Neurosci. 2012, 32, 3366–3375. [Google Scholar] [CrossRef]
- Friston, K.J.; Mechelli, A.; Turner, R.; Price, C.J. Nonlinear Responses in fMRI: The Balloon Model, Volterra Kernels, and Other Hemodynamics. NeuroImage 2000, 12, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Deco, G.; Senden, M.; Jirsa, V. How anatomy shapes dynamics: a semi-analytical study of the brain at rest by a simple spin model. Front. Comput. Neurosci. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.; Kringelbach, M.L.; Deco, G. Functional connectivity dynamically evolves on multiple time-scales over a static structural connectome: Models and mechanisms. NeuroImage 2017, 160, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Kelso, J.A.S. Metastable Coordination Dynamics of Brain and Behavior. Brain Neural Netw. 2001, 8, 125–130. [Google Scholar] [CrossRef]
- Kelso, J.A.S. Multistability and metastability: understanding dynamic coordination in the brain. Philos. Trans. Biol. Sci. 2012, 367, 906–918. [Google Scholar] [CrossRef]
- Kelso, J.A.S.; DeGuzman, G.C.; Holroyd, T. Synergetic Dynamics of Biological Coordination with Special Reference to Phase Attraction and Intermittency. In Proceedings of the Rhythms in Physiological Systems; Haken, H., Koepchen, H.P., Eds.; Springer: Berlin, Heidelberg, 1991; pp. 195–213. [Google Scholar]
- Izhikevich, E.M. Dynamical systems in neuroscience: the geometry of excitability and bursting; Computational neuroscience; MIT Press: Cambridge, Mass, 2007; ISBN 978-0-262-09043-8. [Google Scholar]
- Deco, G.; Ponce-Alvarez, A.; Mantini, D.; Romani, G.L.; Hagmann, P.; Corbetta, M. Resting-State Functional Connectivity Emerges from Structurally and Dynamically Shaped Slow Linear Fluctuations. J. Neurosci. 2013, 33, 11239–11252. [Google Scholar] [CrossRef]
- Gollo, L.L.; Breakspear, M. The frustrated brain: from dynamics on motifs to communities and networks. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130532. [Google Scholar] [CrossRef]
- Roberts, J.A.; Gollo, L.L.; Abeysuriya, R.G.; Roberts, G.; Mitchell, P.B.; Woolrich, M.W.; Breakspear, M. Metastable brain waves. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Yang, H.; Shew, W.L.; Roy, R.; Plenz, D. Maximal Variability of Phase Synchrony in Cortical Networks with Neuronal Avalanches. J. Neurosci. 2012, 32, 1061–1072. [Google Scholar] [CrossRef]
- Kanamaru, T. Analysis of synchronization between two modules of pulse neural networks with excitatory and inhibitory connections. Neural Comput. 2006, 18, 1111–1131. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C. Organization of Anti-Phase Synchronization Pattern in Neural Networks: What are the Key Factors? Front. Syst. Neurosci. 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Neltner, L.; Hansel, D. On synchrony of weakly coupled neurons at low firing rate. Neural Comput. 2001, 13, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.; Hugues, E.; Sporns, O.; Deco, G. Role of local network oscillations in resting-state functional connectivity. NeuroImage 2011, 57, 130–139. [Google Scholar] [CrossRef]
- Pang, J.C.; Gollo, L.L.; Roberts, J.A. Stochastic synchronization of dynamics on the human connectome. NeuroImage 2021, 229, 117738. [Google Scholar] [CrossRef]
- Ponce-Alvarez, A.; Deco, G.; Hagmann, P.; Romani, G.L.; Mantini, D.; Corbetta, M. Resting-State Temporal Synchronization Networks Emerge from Connectivity Topology and Heterogeneity. PLOS Comput. Biol. 2015, 11, e1004100. [Google Scholar] [CrossRef] [PubMed]
- López-González, A.; Panda, R.; Ponce-Alvarez, A.; Zamora-López, G.; Escrichs, A.; Martial, C.; Thibaut, A.; Gosseries, O.; Kringelbach, M.L.; Annen, J.; et al. Loss of consciousness reduces the stability of brain hubs and the heterogeneity of brain dynamics. Commun. Biol. 2021, 4, 1–15. [Google Scholar] [CrossRef]
- Deco, G.; Jirsa, V.; McIntosh, A.R.; Sporns, O.; Kötter, R. Key role of coupling, delay, and noise in resting brain fluctuations. Proc. Natl. Acad. Sci. 2009, 106, 10302–10307. [Google Scholar] [CrossRef]
- Lynall, M.-E.; Bassett, D.S.; Kerwin, R.; McKenna, P.J.; Kitzbichler, M.; Muller, U.; Bullmore, E. Functional Connectivity and Brain Networks in Schizophrenia. J. Neurosci. 2010, 30, 9477–9487. [Google Scholar] [CrossRef]
- Córdova-Palomera, A.; Kaufmann, T.; Persson, K.; Alnæs, D.; Doan, N.T.; Moberget, T.; Lund, M.J.; Barca, M.L.; Engvig, A.; Brækhus, A.; et al. Disrupted global metastability and static and dynamic brain connectivity across individuals in the Alzheimer’s disease continuum. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Cabral, J.; Fernandes, H.M.; Van Hartevelt, T.J.; James, A.C.; Kringelbach, M.L.; Deco, G. Structural connectivity in schizophrenia and its impact on the dynamics of spontaneous functional networks. Chaos Woodbury N 2013, 23, 046111. [Google Scholar] [CrossRef]
- Kelly, S.; Jahanshad, N.; Zalesky, A.; Kochunov, P.; Agartz, I.; Alloza, C.; Andreassen, O.A.; Arango, C.; Banaj, N.; Bouix, S.; et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol. Psychiatry 2018, 23, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, M.P.; Fornito, A. Brain Networks in Schizophrenia. Neuropsychol. Rev. 2014, 24, 32–48. [Google Scholar] [CrossRef]
- Deco, G.; Tononi, G.; Boly, M.; Kringelbach, M.L. Rethinking segregation and integration: contributions of whole-brain modelling. Nat. Rev. Neurosci. 2015, 16, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Deco, G.; Cruzat, J.; Cabral, J.; Knudsen, G.M.; Carhart-Harris, R.L.; Whybrow, P.C.; Logothetis, N.K.; Kringelbach, M.L. Whole-Brain Multimodal Neuroimaging Model Using Serotonin Receptor Maps Explains Non-linear Functional Effects of LSD. Curr. Biol. 2018, 28, 3065-3074.e6. [CrossRef]
- Kringelbach, M.L.; Cruzat, J.; Cabral, J.; Knudsen, G.M.; Carhart-Harris, R.; Whybrow, P.C.; Logothetis, N.K.; Deco, G. Dynamic coupling of whole-brain neuronal and neurotransmitter systems. Proc. Natl. Acad. Sci. 2020, 201921475. [Google Scholar] [CrossRef]
- Luppi, A.I.; Carhart-Harris, R.L.; Roseman, L.; Pappas, I.; Menon, D.K.; Stamatakis, E.A. LSD alters dynamic integration and segregation in the human brain. NeuroImage 2021, 227, 117653. [Google Scholar] [CrossRef]
- Frisch, U. Turbulence: The Legacy of A.N. Kolmogorov; 1995; ISBN 978-0-521-45103-1.
- Richardson, L.F. Weather Prediction by Numerical Process | Numerical analysis; University Press, 1922.
- Deco, G.; Kemp, M.; Kringelbach, M.L. Leonardo da Vinci and the search for order in neuroscience. Curr. Biol. 2021, 31, R704–R709. [Google Scholar] [CrossRef] [PubMed]
- Deco, G.; Kringelbach, M.L. Turbulent-like Dynamics in the Human Brain. Cell Rep. 2020, 33, 108471. [Google Scholar] [CrossRef]
- Deco, G.; Sanz Perl, Y.; Vuust, P.; Tagliazucchi, E.; Kennedy, H.; Kringelbach, M.L. Rare long-range cortical connections enhance human information processing. Curr. Biol. 2021, 31, 4436–4448.e5. [Google Scholar] [CrossRef]
- Sheremet, A.; Qin, Y.; Kennedy, J.P.; Zhou, Y.; Maurer, A.P. Wave Turbulence and Energy Cascade in the Hippocampus. Front. Syst. Neurosci. 2019, 12. [Google Scholar] [CrossRef]
- Deco, G.; Liebana Garcia, S.; Sanz Perl, Y.; Sporns, O.; Kringelbach, M.L. The effect of turbulence in brain dynamics information transfer measured with magnetoencephalography. Commun. Phys. 2023, 6, 1–8. [Google Scholar] [CrossRef]
- Kawamura, Y.; Nakao, H.; Kuramoto, Y. Noise-induced turbulence in nonlocally coupled oscillators. Phys. Rev. E 2007, 75, 036209. [Google Scholar] [CrossRef] [PubMed]
- Shraiman, B.I.; Pumir, A.; van Saarloos, W.; Hohenberg, P.C.; Chaté, H.; Holen, M. Spatiotemporal chaos in the one-dimensional complex Ginzburg-Landau equation. Phys. Nonlinear Phenom. 1992, 57, 241–248. [Google Scholar] [CrossRef]
- Kelso, J.A.S. Unifying Large- and Small-Scale Theories of Coordination. Entropy 2021, 23, 537. [Google Scholar] [CrossRef] [PubMed]
- Hindmarsh, J.L.; Rose, R.M. A model of the nerve impulse using two first-order differential equations. Nature 1982, 296, 162–164. [Google Scholar] [CrossRef]
- Fuchs, A.; Jirsa, V.K.; Haken, H.; Kelso, J.A.S. Extending the HKB model of coordinated movement to oscillators with different eigenfrequencies. Biol. Cybern. 1996, 74, 21–30. [Google Scholar] [CrossRef]
- Kelso, J.A.S. The Haken–Kelso–Bunz (HKB) model: from matter to movement to mind. Biol. Cybern. 2021, 115, 305–322. [Google Scholar] [CrossRef]
- McKinley, J.; Zhang, M.; Wead, A.; Williams, C.; Tognoli, E.; Beetle, C. Third party stabilization of unstable coordination in systems of coupled oscillators. J. Phys. Conf. Ser. 2021, 2090. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Y.; Saggar, M. Cross-attractor repertoire provides new perspective on structure-function relationship in the brain. NeuroImage 2022, 259, 119401. [Google Scholar] [CrossRef]
- Markello, R.D.; Hansen, J.Y.; Liu, Z.-Q.; Bazinet, V.; Shafiei, G.; Suárez, L.E.; Blostein, N.; Seidlitz, J.; Baillet, S.; Satterthwaite, T.D.; et al. neuromaps: structural and functional interpretation of brain maps. Nat. Methods 2022, 19, 1472–1479. [Google Scholar] [CrossRef]
- Turkheimer, F.E.; Veronese, M.; Mondelli, V.; Cash, D.; Pariante, C.M. Sickness behaviour and depression: An updated model of peripheral-central immunity interactions. Brain. Behav. Immun. 2023, 111, 202–210. [Google Scholar] [CrossRef]
- Kutchko, K.M.; Fröhlich, F. Emergence of Metastable State Dynamics in Interconnected Cortical Networks with Propagation Delays. PLoS Comput. Biol. 2013, 9, e1003304. [Google Scholar] [CrossRef] [PubMed]
- Luppi, A.I.; Rosas, F.E.; Noonan, M.P.; Mediano, P.A.M.; Kringelbach, M.L.; Carhart-Harris, R.L.; Stamatakis, E.A.; Vernon, A.C.; Turkheimer, F.E. Oxygen and the Spark of Human Brain Evolution: Complex Interactions of Metabolism and Cortical Expansion across Development and Evolution. The Neuroscientist 2022, 10738584221138032. [Google Scholar] [CrossRef] [PubMed]
- Burt, J.B.; Demirtaş, M.; Eckner, W.J.; Navejar, N.M.; Ji, J.L.; Martin, W.J.; Bernacchia, A.; Anticevic, A.; Murray, J.D. Hierarchy of transcriptomic specialization across human cortex captured by structural neuroimaging topography. Nat. Neurosci. 2018, 21, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Vanes, L.D.; Mouchlianitis, E.; Barry, E.; Patel, K.; Wong, K.; Shergill, S.S. Cognitive correlates of abnormal myelination in psychosis. Sci. Rep. 2019, 9, 5162. [Google Scholar] [CrossRef]
- Valdés-Tovar, M.; Rodríguez-Ramírez, A.M.; Rodríguez-Cárdenas, L.; Sotelo-Ramírez, C.E.; Camarena, B.; Sanabrais-Jiménez, M.A.; Solís-Chagoyán, H.; Argueta, J.; López-Riquelme, G.O. Insights into myelin dysfunction in schizophrenia and bipolar disorder. World J. Psychiatry 2022, 12, 264–285. [Google Scholar] [CrossRef]
- Kaller, M.S.; Lazari, A.; Blanco-Duque, C.; Sampaio-Baptista, C.; Johansen-Berg, H. Myelin plasticity and behaviour—connecting the dots. Curr. Opin. Neurobiol. 2017, 47, 86–92. [Google Scholar] [CrossRef]
- Uhlhaas, P.J. High-Frequency Oscillations in Schizophrenia. Clin. EEG Neurosci. 2011, 42, 77–82. [Google Scholar] [CrossRef]
- Uhlhaas, P.J. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr. Opin. Neurobiol. 2013, 23, 283–290. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010, 11, 100. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Singer, W. High-frequency oscillations and the neurobiology of schizophrenia. Dialogues Clin. Neurosci. 2013, 15, 301–313. [Google Scholar] [CrossRef]
- Sumiyoshi, A.; Suzuki, H.; Ogawa, T.; Riera, J.J.; Shimokawa, H.; Kawashima, R. Coupling between gamma oscillation and fMRI signal in the rat somatosensory cortex: its dependence on systemic physiological parameters. NeuroImage 2012, 60, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Haider, B.; Duque, A.; Hasenstaub, A.R.; McCormick, D.A. Neocortical Network Activity In Vivo Is Generated through a Dynamic Balance of Excitation and Inhibition. J. Neurosci. 2006, 26, 4535–4545. [Google Scholar] [CrossRef] [PubMed]
- Gandal, M.J.; Sisti, J.; Klook, K.; Ortinski, P.I.; Leitman, V.; Liang, Y.; Thieu, T.; Anderson, R.; Pierce, R.C.; Jonak, G.; et al. GABA B -mediated rescue of altered excitatory–inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl. Psychiatry 2012, 2, e142–e142. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Curley, A.A.; Glausier, J.R.; Volk, D.W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012, 35, 57–67. [Google Scholar] [CrossRef]
- Mikanmaa, E.; Grent-’t-Jong, T.; Hua, L.; Recasens, M.; Thune, H.; Uhlhaas, P.J. Towards a neurodynamical understanding of the prodrome in schizophrenia. NeuroImage 2019, 190, 144–153. [Google Scholar] [CrossRef]
- Tatti, R.; Haley, M.S.; Swanson, O.K.; Tselha, T.; Maffei, A. Neurophysiology and Regulation of the Balance Between Excitation and Inhibition in Neocortical Circuits. Biol. Psychiatry 2017, 81, 821–831. [Google Scholar] [CrossRef]
- Thune, H.E.A. Auditory neural oscillations and excitation/inhibition balance in emerging psychosis. PhD, University of Glasgow, 2019.
- Hellyer, P.J.; Jachs, B.; Clopath, C.; Leech, R. Local inhibitory plasticity tunes macroscopic brain dynamics and allows the emergence of functional brain networks. NeuroImage 2016, 124, 85–95. [Google Scholar] [CrossRef]
- Turkheimer, F.E.; Leech, R.; Expert, P.; Lord, L.-D.; Vernon, A.C. The brain’s code and its canonical computational motifs. From sensory cortex to the default mode network: A multi-scale model of brain function in health and disease. Neurosci. Biobehav. Rev. 2015, 55, 211–222. [Google Scholar] [CrossRef]
- Xue, M.; Atallah, B.V.; Scanziani, M. Equalizing excitation–inhibition ratios across visual cortical neurons. Nature 2014, 511, 596–600. [Google Scholar] [CrossRef]
- Enrico, P.; Delvecchio, G.; Turtulici, N.; Pigoni, A.; Villa, F.M.; Perlini, C.; Rossetti, M.G.; Bellani, M.; Lasalvia, A.; Bonetto, C.; et al. Classification of Psychoses Based on Immunological Features: A Machine Learning Study in a Large Cohort of First-Episode and Chronic Patients. Schizophr. Bull. 2021, 47, 1141–1155. [Google Scholar] [CrossRef]
- Fraguas, D.; Díaz-Caneja, C.M.; Ayora, M.; Hernández-Álvarez, F.; Rodríguez-Quiroga, A.; Recio, S.; Leza, J.C.; Arango, C. Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-analysis. Schizophr. Bull. 2019, 45, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Molina, V.; Gispert, J.D.; Reig, S.; Sanz, J.; Pascau, J.; Santos, A.; Desco, M.; Palomo, T. Cerebral metabolic changes induced by clozapine in schizophrenia and related to clinical improvement. Psychopharmacology (Berl.) 2005, 178, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Tognoli, E.; Kelso, J.A.S. The Metastable Brain. Neuron 2014, 81, 35–48. [Google Scholar] [CrossRef]
- Alexander, M.L.; Alagapan, S.; Lugo, C.E.; Mellin, J.M.; Lustenberger, C.; Rubinow, D.R.; Fröhlich, F. Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD). Transl. Psychiatry 2019, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Avery, D.H.; Holtzheimer, P.E.; Fawaz, W.; Russo, J.; Neumaier, J.; Dunner, D.L.; Haynor, D.R.; Claypoole, K.H.; Wajdik, C.; Roy-Byrne, P. A Controlled Study of Repetitive Transcranial Magnetic Stimulation in Medication-Resistant Major Depression. Biol. Psychiatry 2006, 59, 187–194. [Google Scholar] [CrossRef]
- O’Reardon, J.P.; Solvason, H.B.; Janicak, P.G.; Sampson, S.; Isenberg, K.E.; Nahas, Z.; McDonald, W.M.; Avery, D.; Fitzgerald, P.B.; Loo, C.; et al. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol. Psychiatry 2007, 62, 1208–1216. [Google Scholar] [CrossRef]
- Zhang, M.; Force, R.B.; Walker, C.; Ahn, S.; Jarskog, L.F.; Frohlich, F. Alpha transcranial alternating current stimulation reduces depressive symptoms in people with schizophrenia and auditory hallucinations: a double-blind, randomized pilot clinical trial. Schizophrenia 2022, 8, 1–17. [Google Scholar] [CrossRef]
- Expert, P.; Lambiotte, R.; Chialvo, D.R.; Christensen, K.; Jensen, H.J.; Sharp, D.J.; Turkheimer, F. Self-similar correlation function in brain resting-state functional magnetic resonance imaging. J. R. Soc. Interface 2011, 8, 472–479. [Google Scholar] [CrossRef]
- Plenz, D.; Ribeiro, T.L.; Miller, S.R.; Kells, P.A.; Vakili, A.; Capek, E.L. Self-Organized Criticality in the Brain. Front. Phys. 2021, 9. [Google Scholar] [CrossRef]
| 1 | In preparing this paper, the authors found over 300 published neuroscience-related articles since 1988 that contained the word ‘metastability’. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




