Submitted:
19 July 2023
Posted:
21 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Novel Proposed EV Protein Biomarkers
3. Metabolism Related Proteins
4. Inflammation and Fibrosis
5. NOTCH Pathway
6. Wnt/β-Catenin Pathway
7. Plasma/Serum Secreted Proteins in NAFLD
8. Inter-Tissue Crosstalk
9. Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Loomba, R., S.L. Friedman, and G.I. Shulman, Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell, 2021. 184(10): p. 2537-2564. [CrossRef]
- Masoodi, M., et al., Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat Rev Gastroenterol Hepatol, 2021. 18(12): p. 835-856. [CrossRef]
- Kechagias, S., et al., Non-invasive diagnosis and staging of non-alcoholic fatty liver disease. Hormones (Athens), 2022. 21(3): p. 349-368. [CrossRef]
- Anstee, Q.M., L. Castera, and R. Loomba, Impact of non-invasive biomarkers on hepatology practice: Past, present and future. J Hepatol, 2022. 76(6): p. 1362-1378. [CrossRef]
- Hernandez Roman, J. and M.S. Siddiqui, The role of noninvasive biomarkers in diagnosis and risk stratification in nonalcoholic fatty liver disease. Endocrinol Diabetes Metab, 2020. 3(4): p. e00127. [CrossRef]
- Byrne, C.D. and G. Targher, NAFLD: a multisystem disease. J Hepatol, 2015. 62(1 Suppl): p. S47-64. [CrossRef]
- Park, H. , et al., Reappraisal of fibrosis-4 index and non-alcoholic fatty liver disease fibrosis score for advanced fibrosis in average-risk population. Front Med (Lausanne), 2022. 9: p. 1024836. [CrossRef]
- Sun, W. , et al., Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: A meta-analysis study. Hepatol Res, 2016. 46(9): p. 862-70. [CrossRef]
- van Niel, G. , et al., Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol, 2022. 23(5): p. 369-382. [CrossRef]
- Wu, D., H. Zhu, and H. Wang, Extracellular Vesicles in Non-alcoholic Fatty Liver Disease and Alcoholic Liver Disease. Front Physiol, 2021. 12: p. 707429. [CrossRef]
- Newman, L.A., K. Muller, and A. Rowland, Circulating cell-specific extracellular vesicles as biomarkers for the diagnosis and monitoring of chronic liver diseases. Cell Mol Life Sci, 2022. 79(5): p. 232. [CrossRef]
- Garcia-Martinez, I. , et al., Insights Into Extracellular Vesicles as Biomarker of NAFLD Pathogenesis. Front Med (Lausanne), 2020. 7: p. 395. [CrossRef]
- Baek, R. and M.M. Jorgensen, Multiplexed Phenotyping of Small Extracellular Vesicles Using Protein Microarray (EV Array). Methods Mol Biol, 2017. 1545: p. 117-127. [CrossRef]
- Breitwieser, K. , et al., Detailed Characterization of Small Extracellular Vesicles from Different Cell Types Based on Tetraspanin Composition by ExoView R100 Platform. Int J Mol Sci, 2022. 23(15). [CrossRef]
- Botha, J., H. R. Pugsley, and A. Handberg, Conventional, High-Resolution and Imaging Flow Cytometry: Benchmarking Performance in Characterisation of Extracellular Vesicles. Biomedicines, 2021. 9(2). [CrossRef]
- Nielsen, M.H. , et al., Acute Exercise Increases Plasma Levels of Muscle-Derived Microvesicles Carrying Fatty Acid Transport Proteins. J Clin Endocrinol Metab, 2019. 104(10): p. 4804-4814. [CrossRef]
- Motomura, W. , et al., Analysis of vanin-1 upregulation and lipid accumulation in hepatocytes in response to a high-fat diet and free fatty acids. J Clin Biochem Nutr, 2012. 51(3): p. 163-9. [CrossRef]
- Povero, D. , et al., Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal, 2013. 6(296): p. ra88. [CrossRef]
- Hendrikx, T. , et al., Soluble TREM2 levels reflect the recruitment and expansion of TREM2(+) macrophages that localize to fibrotic areas and limit NASH. J Hepatol, 2022. 77(5): p. 1373-1385. [CrossRef]
- Hou, J. , et al., TREM2 sustains macrophage-hepatocyte metabolic coordination in nonalcoholic fatty liver disease and sepsis. J Clin Invest, 2021. 131(4). [CrossRef]
- Mallach, A. , et al., The influence of the R47H triggering receptor expressed on myeloid cells 2 variant on microglial exosome profiles. Brain Commun, 2021. 3(2): p. fcab009. [CrossRef]
- Corey, K.E. , et al., ADAMTSL2 protein and a soluble biomarker signature identify at-risk non-alcoholic steatohepatitis and fibrosis in adults with NAFLD. J Hepatol, 2022. 76(1): p. 25-33. [CrossRef]
- Povero, D. , et al., Characterization and Proteome of Circulating Extracellular Vesicles as Potential Biomarkers for NASH. Hepatol Commun, 2020. 4(9): p. 1263-1278. [CrossRef]
- Garcia, N.A. , et al., Circulating exosomes deliver free fatty acids from the bloodstream to cardiac cells: Possible role of CD36. PLoS One, 2019. 14(5): p. e0217546. [CrossRef]
- Rada, P. , et al., Understanding lipotoxicity in NAFLD pathogenesis: is CD36 a key driver? Cell Death Dis, 2020. 11(9): p. 802. [CrossRef]
- Jung, J.W. , et al., Liver-originated small extracellular vesicles with TM4SF5 target brown adipose tissue for homeostatic glucose clearance. J Extracell Vesicles, 2022. 11(9): p. e12262. [CrossRef]
- Kim, E. , et al., TM4SF5-dependent crosstalk between hepatocytes and macrophages to reprogram the inflammatory environment. Cell Rep, 2021. 37(7): p. 110018. [CrossRef]
- Park, D. , et al., Tetraspanin TM4SF5 in hepatocytes negatively modulates SLC27A transporters during acute fatty acid supply. Arch Biochem Biophys, 2021. 710: p. 109004. [CrossRef]
- Luo, F., F. Oldoni, and A. Das, TM6SF2: A Novel Genetic Player in Nonalcoholic Fatty Liver and Cardiovascular Disease. Hepatol Commun, 2022. 6(3): p. 448-460. [CrossRef]
- Mann, J.P. , et al., Insights into genetic variants associated with NASH-fibrosis from metabolite profiling. Hum Mol Genet, 2020. 29(20): p. 3451-3463. [CrossRef]
- Li, Z. , et al., Liver sphingomyelin synthase 1 deficiency causes steatosis, steatohepatitis, fibrosis, and tumorigenesis: An effect of glucosylceramide accumulation. iScience, 2021. 24(12): p. 103449. [CrossRef]
- Granja, S.C. , et al., Non-Alcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma: Immunohistochemical Assessment of Markers of Cancer Cell Metabolism. Pathobiology, 2022. 89(3): p. 157-165. [CrossRef]
- Wan, L. , et al., Exosomes from activated hepatic stellate cells contain GLUT1 and PKM2: a role for exosomes in metabolic switch of liver nonparenchymal cells. FASEB J, 2019. 33(7): p. 8530-8542. [CrossRef]
- Shearer, A.M. , et al., PAR2 promotes impaired glucose uptake and insulin resistance in NAFLD through GLUT2 and Akt interference. Hepatology, 2022. 76(6): p. 1778-1793. [CrossRef]
- Roncal-Jimenez, C.A. , et al., Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism, 2011. 60(9): p. 1259-70. [CrossRef]
- Garcia, N.A. , et al., Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res, 2016. 109(3): p. 397-408. [CrossRef]
- Karim, S. , et al., Dysregulated hepatic expression of glucose transporters in chronic disease: contribution of semicarbazide-sensitive amine oxidase to hepatic glucose uptake. Am J Physiol Gastrointest Liver Physiol, 2014. 307(12): p. G1180-90. [CrossRef]
- de Gracia Hahn, D., A. Duret, and J.P. Mann, An AGTR1 Variant Worsens Nonalcoholic Fatty Liver Disease and the Metabolic Syndrome. Am J Gastroenterol, 2019. 114(4): p. 556-559. [CrossRef]
- Eshraghian, A., S. Iravani, and P. Azimzadeh, The Association between Angiotensin II Type 1 Receptor Gene A1166C Polymorphism and Non-alcoholic Fatty Liver Disease and Its Severity. Middle East J Dig Dis, 2018. 10(2): p. 96-104. [CrossRef]
- van der Graaff, D. , et al., Vasoconstrictor antagonism improves functional and structural vascular alterations and liver damage in rats with early NAFLD. JHEP Rep, 2022. 4(2): p. 100412. [CrossRef]
- Han, M. , et al., Hepatocyte caveolin-1 modulates metabolic gene profiles and functions in non-alcoholic fatty liver disease. Cell Death Dis, 2020. 11(2): p. 104. [CrossRef]
- Logozzi, M. , et al., High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One, 2009. 4(4): p. e5219. [CrossRef]
- Ibrahim, S.H. , et al., Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology, 2016. 63(3): p. 731-44. [CrossRef]
- Javeed, N. , et al., Pro-inflammatory beta cell small extracellular vesicles induce beta cell failure through activation of the CXCL10/CXCR3 axis in diabetes. Cell Rep, 2021. 36(8): p. 109613. [CrossRef]
- Nair, B. and L.R. Nath, Inevitable role of TGF-beta1 in progression of nonalcoholic fatty liver disease. J Recept Signal Transduct Res, 2020. 40(3): p. 195-200. [CrossRef]
- Shelke, G.V. , et al., Endosomal signalling via exosome surface TGFbeta-1. J Extracell Vesicles, 2019. 8(1): p. 1650458. [CrossRef]
- Rodrigues-Junior, D.M. , et al., Extracellular Vesicles and Transforming Growth Factor beta Signaling in Cancer. Front Cell Dev Biol, 2022. 10: p. 849938. [CrossRef]
- Wallace, S.J. , et al., Understanding the cellular interactome of non-alcoholic fatty liver disease. JHEP Rep, 2022. 4(8): p. 100524. [CrossRef]
- Yang, L. , et al., Transforming growth factor beta signaling in hepatocytes participates in steatohepatitis through regulation of cell death and lipid metabolism in mice. Hepatology, 2014. 59(2): p. 483-95. [CrossRef]
- Albadawy, R. , et al., Clinical Significance of HSPD1/MMP14/ITGB1/miR-6881-5P/Lnc-SPARCL1-1:2 RNA Panel in NAFLD/NASH Diagnosis: Egyptian Pilot Study. Biomedicines, 2021. 9(9). [CrossRef]
- Guo, Q. , et al., Integrin beta(1)-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol, 2019. 71(6): p. 1193-1205. [CrossRef]
- Miura, K. , et al., Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology, 2013. 57(2): p. 577-89. [CrossRef]
- Sharifnia, T. , et al., Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest Liver Physiol, 2015. 309(4): p. G270-8. [CrossRef]
- Sun, B.L. , et al., Involvement of eNAMPT/TLR4 inflammatory signaling in progression of non-alcoholic fatty liver disease, steatohepatitis, and fibrosis. FASEB J, 2023. 37(3): p. e22825. [CrossRef]
- Zhang, Y. , et al., Extracellular Vesicles with Exosome-like Features Transfer TLRs between Dendritic Cells. Immunohorizons, 2019. 3(6): p. 186-193. [CrossRef]
- Rossato, M. , et al., The P2 × 7 Receptor and NLRP3 Axis in Non-Alcoholic Fatty Liver Disease: A Brief Review. Cells, 2020. 9(4). [CrossRef]
- Mederacke, I. , et al., The purinergic P2Y14 receptor links hepatocyte death to hepatic stellate cell activation and fibrogenesis in the liver. Sci Transl Med, 2022. 14(639): p. eabe5795. [CrossRef]
- Boujedidi, H. , et al., CXCR4 dysfunction in non-alcoholic steatohepatitis in mice and patients. Clin Sci (Lond), 2015. 128(4): p. 257-67. [CrossRef]
- Li, M. , et al., Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene, 2018. 676: p. 101-109. [CrossRef]
- Wang, S. , et al., Emerging Importance of Chemokine Receptor CXCR4 and Its Ligand in Liver Disease. Front Cell Dev Biol, 2021. 9: p. 716842. [CrossRef]
- Lambrecht, J. , et al., A PDGFRbeta-based score predicts significant liver fibrosis in patients with chronic alcohol abuse, NAFLD and viral liver disease. EBioMedicine, 2019. 43: p. 501-512. [CrossRef]
- Geng, T. , et al., SphK1 mediates hepatic inflammation in a mouse model of NASH induced by high saturated fat feeding and initiates proinflammatory signaling in hepatocytes. J Lipid Res, 2015. 56(12): p. 2359-71. [CrossRef]
- Rigogliuso, S. , et al., An active form of sphingosine kinase-1 is released in the extracellular medium as component of membrane vesicles shed by two human tumor cell lines. J Oncol, 2010. 2010: p. 509329. [CrossRef]
- Williams, A.S. , et al., Integrin alpha1-null mice exhibit improved fatty liver when fed a high fat diet despite severe hepatic insulin resistance. J Biol Chem, 2015. 290(10): p. 6546-57. [CrossRef]
- Guo, X. , et al., Endothelial ACKR1 is induced by neutrophil contact and down-regulated by secretion in extracellular vesicles. Front Immunol, 2023. 14: p. 1181016. [CrossRef]
- Nasiri-Ansari, N. , et al., Endothelial Cell Dysfunction and Nonalcoholic Fatty Liver Disease (NAFLD): A Concise Review. Cells, 2022. 11(16). [CrossRef]
- Ramachandran, P. , et al., Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature, 2019. 575(7783): p. 512-518. [CrossRef]
- Katayama, A. , et al., Beneficial impact of Gpnmb and its significance as a biomarker in nonalcoholic steatohepatitis. Sci Rep, 2015. 5: p. 16920. [CrossRef]
- Angeloni, N.L. , et al., Pathways for Modulating Exosome Lipids Identified By High-Density Lipoprotein-Like Nanoparticle Binding to Scavenger Receptor Type B-1. Sci Rep, 2016. 6: p. 22915. [CrossRef]
- Ding, B.S. , et al., Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature, 2014. 505(7481): p. 97-102. [CrossRef]
- Hirsova, P. , et al., TRAIL Deletion Prevents Liver, but Not Adipose Tissue, Inflammation during Murine Diet-Induced Obesity. Hepatol Commun, 2017. 1(7): p. 648-662. [CrossRef]
- Bacil, G.P. , et al., Unraveling Hepatic Metabolomic Profiles and Morphological Outcomes in a Hybrid Model of NASH in Different Mouse Strains. Antioxidants (Basel), 2023. 12(2). [CrossRef]
- Osada-Oka, M. , et al., Macrophage-derived exosomes induce inflammatory factors in endothelial cells under hypertensive conditions. Hypertens Res, 2017. 40(4): p. 353-360. [CrossRef]
- Gonzalez-King, H. , et al., Hypoxia Inducible Factor-1alpha Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells, 2017. 35(7): p. 1747-1759. [CrossRef]
- Gonzalez-King, H. , et al., Non-classical Notch signaling by MDA-MB-231 breast cancer cell-derived small extracellular vesicles promotes malignancy in poorly invasive MCF-7 cells. Cancer Gene Ther, 2022. 29(7): p. 1056-1069. [CrossRef]
- Gridley, T. , Human Genetics. Notch, stroke and dementia. Nature, 1996. 383(6602): p. 673. [CrossRef]
- Olsauskas-Kuprys, R., A. Zlobin, and C. Osipo, Gamma secretase inhibitors of Notch signaling. Onco Targets Ther, 2013. 6: p. 943-55. [CrossRef]
- Sassoli, C. , et al., Mesenchymal stromal cells affect cardiomyocyte growth through juxtacrine Notch-1/Jagged-1 signaling and paracrine mechanisms: clues for cardiac regeneration. J Mol Cell Cardiol, 2011. 51(3): p. 399-408. [CrossRef]
- Xu, H. and L. Wang, The Role of Notch Signaling Pathway in Non-Alcoholic Fatty Liver Disease. Front Mol Biosci, 2021. 8: p. 792667. [CrossRef]
- Zhu, L. , et al., Upregulation of non-canonical Wnt ligands and oxidative glucose metabolism in NASH induced by methionine-choline deficient diet. Trends Cell Mol Biol, 2018. 13: p. 47-56.
- HM, A.E. , et al., Multi-omics characterization of a diet-induced obese model of non-alcoholic steatohepatitis. Sci Rep, 2020. 10(1): p. 1148. [CrossRef]
- Xiong, X. , et al., Landscape of Intercellular Crosstalk in Healthy and NASH Liver Revealed by Single-Cell Secretome Gene Analysis. Mol Cell, 2019. 75(3): p. 644-660 e5. [CrossRef]
- Scavo, M.P. , et al., Exosomal FZD-7 Expression Is Modulated by Different Lifestyle Interventions in Patients with NAFLD. Nutrients, 2022. 14(6). [CrossRef]
- Saponara, E. , et al., Loss of Hepatic Leucine-Rich Repeat-Containing G-Protein Coupled Receptors 4 and 5 Promotes Nonalcoholic Fatty Liver Disease. Am J Pathol, 2023. 193(2): p. 161-181. [CrossRef]
- Shree Harini, K. and D. Ezhilarasan, Wnt/beta-catenin signaling and its modulators in nonalcoholic fatty liver diseases. Hepatobiliary Pancreat Dis Int, 2023. 22(4): p. 333-345. [CrossRef]
- Chairoungdua, A. , et al., Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol, 2010. 190(6): p. 1079-91. [CrossRef]
- Dovrat, S. , et al., 14-3-3 and beta-catenin are secreted on extracellular vesicles to activate the oncogenic Wnt pathway. Mol Oncol, 2014. 8(5): p. 894-911. [CrossRef]
- Kalra, H. , et al., Extracellular vesicles containing oncogenic mutant beta-catenin activate Wnt signalling pathway in the recipient cells. J Extracell Vesicles, 2019. 8(1): p. 1690217. [CrossRef]
- Niu, L. , et al., Plasma proteome profiling discovers novel proteins associated with non-alcoholic fatty liver disease. Mol Syst Biol, 2019. 15(3): p. e8793. [CrossRef]
- Pitkanen, N. , et al., Afamin predicts the prevalence and incidence of nonalcoholic fatty liver disease. Clin Chem Lab Med, 2022. 60(2): p. 243-251. [CrossRef]
- Liu, Y., Y. Hu, and L. Deng, The Underlying Roles of Exosome-Associated PIGR in Fatty Acid Metabolism and Immune Signaling in Colorectal Cancer. J Oncol, 2022. 2022: p. 4675683. [CrossRef]
- Tey, S.K. , et al., Patient pIgR-enriched extracellular vesicles drive cancer stemness, tumorigenesis and metastasis in hepatocellular carcinoma. J Hepatol, 2022. 76(4): p. 883-895. [CrossRef]
- Veyel, D. , et al., Biomarker discovery for chronic liver diseases by multi-omics - a preclinical case study. Sci Rep, 2020. 10(1): p. 1314. [CrossRef]
- Aarts, S. , et al., Depletion of CD40 on CD11c(+) cells worsens the metabolic syndrome and ameliorates hepatic inflammation during NASH. Sci Rep, 2019. 9(1): p. 14702. [CrossRef]
- Yuan, M. , et al., CD40L/CD40 Regulates Adipokines and Cytokines by H3K4me3 Modification in Epicardial Adipocytes. J Cardiovasc Pharmacol, 2021. 78(2): p. 228-234. [CrossRef]
- He, S. , et al., LRG1 is an adipokine that mediates obesity-induced hepatosteatosis and insulin resistance. J Clin Invest, 2021. 131(24). [CrossRef]
- Zhong, M.E. , et al., Serum extracellular vesicles contain SPARC and LRG1 as biomarkers of colon cancer and differ by tumour primary location. EBioMedicine, 2019. 50: p. 211-223. [CrossRef]
- Chi, C. , et al., Exerkine fibronectin type-III domain-containing protein 5/irisin-enriched extracellular vesicles delay vascular ageing by increasing SIRT6 stability. Eur Heart J, 2022. 43(43): p. 4579-4595. [CrossRef]
- Wang, Y.D. , et al., New insight of obesity-associated NAFLD: Dysregulated "crosstalk" between multi-organ and the liver? Genes Dis, 2023. 10(3): p. 799-812. [CrossRef]
- Gu, H. , et al., ER stress-induced adipocytes secrete-aldo-keto reductase 1B7-containing exosomes that cause nonalcoholic steatohepatitis in mice. Free Radic Biol Med, 2021. 163: p. 220-233. [CrossRef]
- O'Farrell, M. , et al., FASN inhibition targets multiple drivers of NASH by reducing steatosis, inflammation and fibrosis in preclinical models. Sci Rep, 2022. 12(1): p. 15661. [CrossRef]
- Sano, S. , et al., Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun, 2014. 445(2): p. 327-33. [CrossRef]
- Gustafson, C.M. , et al., Age- and sex-specific differences in blood-borne microvesicles from apparently healthy humans. Biol Sex Differ, 2015. 6: p. 10. [CrossRef]
- Moreno-Vedia, J. , et al., Unveiling the Role of the Fatty Acid Binding Protein 4 in the Metabolic-Associated Fatty Liver Disease. Biomedicines, 2022. 10(1). [CrossRef]
- Phoonsawat, W. , et al., Adiponectin is partially associated with exosomes in mouse serum. Biochem Biophys Res Commun, 2014. 448(3): p. 261-6. [CrossRef]
- Botha, J. , et al., Bariatric surgery reduces CD36-bearing microvesicles of endothelial and monocyte origin. Nutr Metab (Lond), 2018. 15: p. 76. [CrossRef]
- Bansal, S. , et al., SARS-CoV-2 infection in lung transplant recipients induces circulating exosomes with SARS-CoV-2 spike protein S2. Clin Transl Med, 2021. 11(11): p. e576. [CrossRef]
- Peiseler, M. , et al., Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol, 2022. 77(4): p. 1136-1160. [CrossRef]
- Lee, Y.A. and S.L. Friedman, Inflammatory and fibrotic mechanisms in NAFLD-Implications for new treatment strategies. J Intern Med, 2022. 291(1): p. 11-31. [CrossRef]
- Liu, J. , et al., Wnt/beta-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther, 2022. 7(1): p. 3. [CrossRef]
- Goel, C., S. P. Monga, and K. Nejak-Bowen, Role and Regulation of Wnt/beta-Catenin in Hepatic Perivenous Zonation and Physiological Homeostasis. Am J Pathol, 2022. 192(1): p. 4-17. [CrossRef]
- Ma, R. , et al., Metabolic and non-metabolic liver zonation is established non-synchronously and requires sinusoidal Wnts. Elife, 2020. 9. [CrossRef]
- Gross, J.C. and L.C. Zelarayan, The Mingle-Mangle of Wnt Signaling and Extracellular Vesicles: Functional Implications for Heart Research. Front Cardiovasc Med, 2018. 5: p. 10. [CrossRef]
- Lee, E., H. Korf, and A. Vidal-Puig, An adipocentric perspective on the development and progression of non-alcoholic fatty liver disease. J Hepatol, 2023. 78(5): p. 1048-1062. [CrossRef]
- Chakravarthy, M.V. , et al., Harnessing Muscle-Liver Crosstalk to Treat Nonalcoholic Steatohepatitis. Front Endocrinol (Lausanne), 2020. 11: p. 592373. [CrossRef]
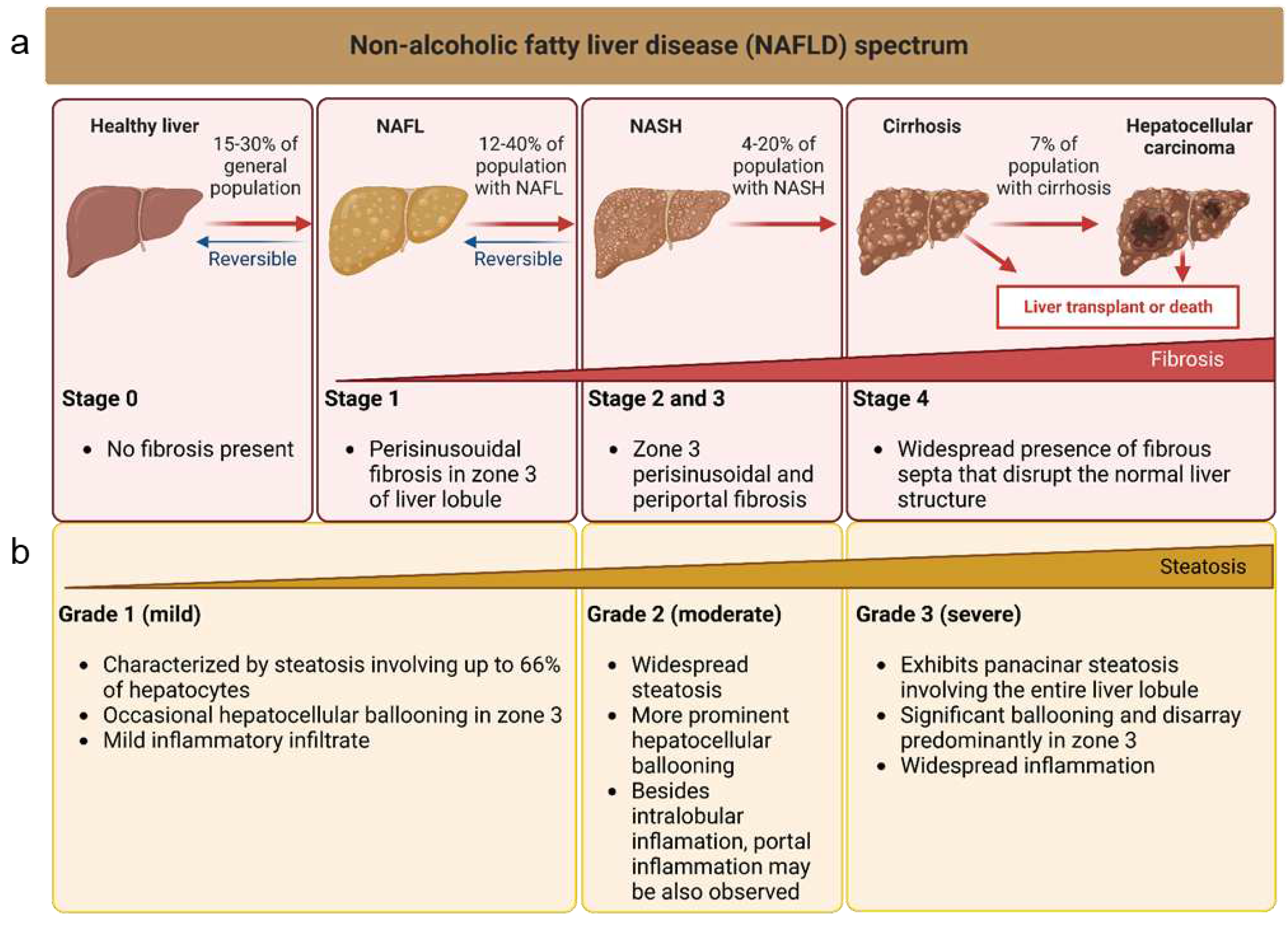
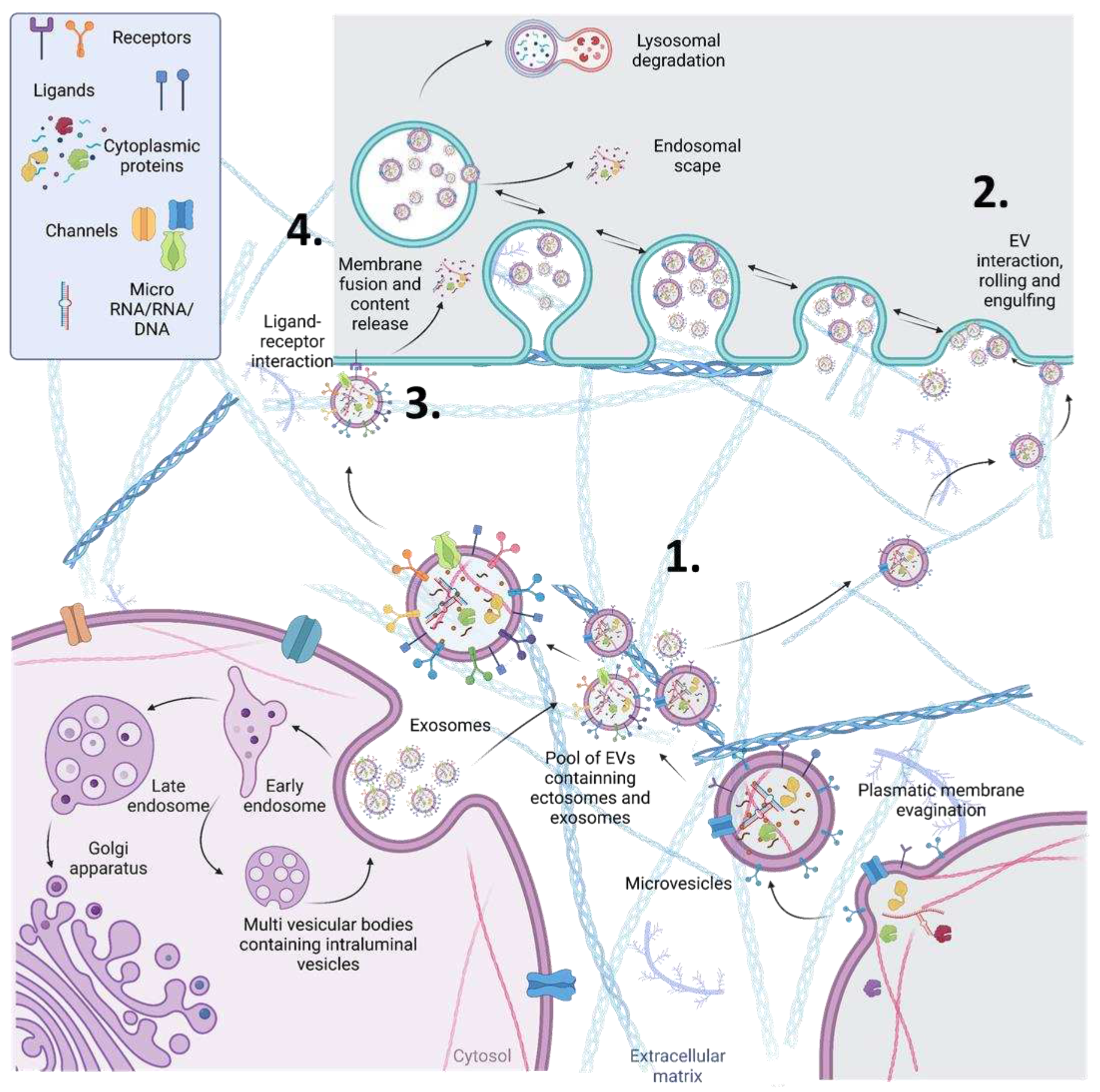

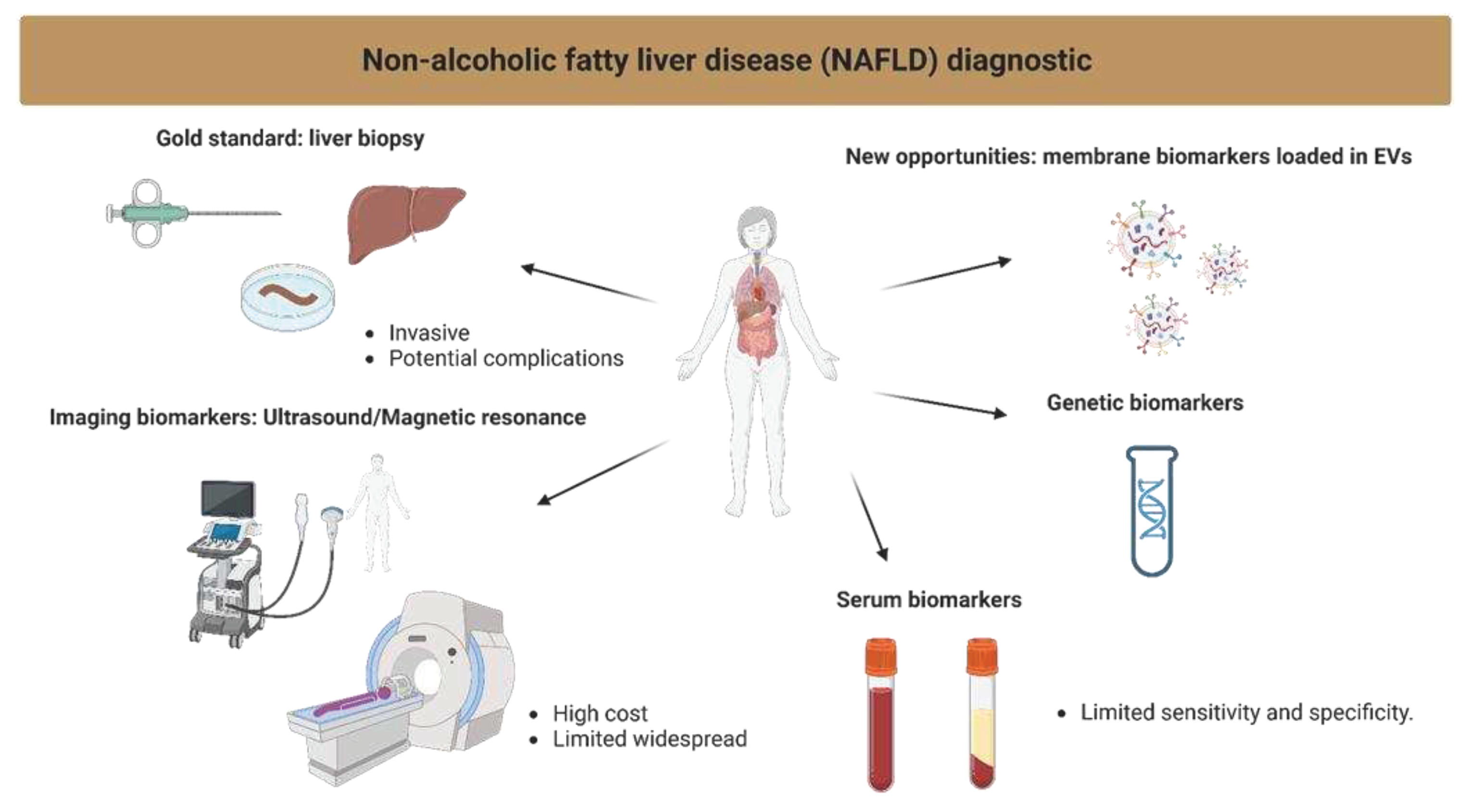
| Mechanism | Name | Description | References |
|---|---|---|---|
| Novel proposed biomarkers | VANIN-1 | Released on EVs surface from lipotoxic hepatocytes | [17,18] |
| TREM2 | Soluble TREM2 levels correlates with NAFLD to NASH progression | [19,20,21] | |
| ADAMTS2 | Soluble ADAMTS2 levels correlates with NAFLD to NASH progression | [22] | |
| IL13RA1 | upregulated levels in circulating EVs in NASH | [23] | |
| IL27RA | upregulated levels in circulating EVs in NASH | [23] | |
| ICAM2 | upregulated levels in circulating EVs in NASH | [23] | |
| STK16 | upregulated levels in circulating EVs in NASH | [23] | |
| Metabolism related proteins | CD36 | circulating levels of a soluble form of CD36 are abnormally elevated in NAFLD patients | [24,25] |
| TM4SF5 | liver derived EVs with TM4SF5 target brown adipose tissue for glucose clearance | [26,27,28] | |
| TM6SF2 | TM6SF2 variants were related to hepatic triglyceride in NAFLD and NASH | [29,30] | |
| SLC27A5 | upregulated levels in circulating EVs in NASH | [23,28] | |
| SGMS1 | NASH patients had higher liver GluCer synthase and higher plasma GluCer levels | [31] | |
| GLUT1 | increased liver GLUT1 levels correlates with higher degree of steatosis in NASH | [32,33] | |
| GLUT2 | Decreased liver levels in NAFLD | [34] | |
| GLUT5 | Increased liver levels in NAFLD induced by high fructose intake in rats | [35,36,37] | |
| GLUT4 | Altered liver levels in patients with chronic liver disease | [36,37] | |
| AGTR1 | gene variants of AGTR1 have been related with predisposition to develop NAFLD | [38,39,40] | |
| CAV1 | hepatocytes CAV1 modulates metabolic gene profiles and function in NAFLD | [41,42] | |
| Inflamation/fibrosis | CXCL10 | hepatocyte lipotoxicity induces the release of CXCL10-bearing vesicles | [43,44] |
| TGFB1 | promotes HSC activation and extracellular matrix production in NAFLD | [45,46] | |
| TGFB2 | drives multiple types of fibrosis during NAFLD to NASH progression | [47,48,49] | |
| TGFBR2 | drives multiple types of fibrosis during NAFLD to NASH progression | [47,48,49] | |
| TGFBR3 | drives multiple types of fibrosis during NAFLD to NASH progression | [47,48,49] | |
| TGFBR1 | drives multiple types of fibrosis during NAFLD to NASH progression | [47,48,49] | |
| ITGB1 | ITGB1 is released in EVs from hepatocytes under lipotoxic stress | [50,51] | |
| TLR2 | activate inflammasome in Kupffer cells/macrophages in NASH development | [52] | |
| TLR4 | contributes to NAFLD severity and NASH/hepatic fibrosis | [53,54,55] | |
| P2 × 7R | related to NAFLD and its inflammatory and fibrotic evolution | [56] | |
| P2Y14R | links hepatocyte death to hepatic stellate cell activation and fibrogenesis | [57] | |
| CXCR4 | functionally and mechanistically involved in the progression of liver fibrosis | [58,59,60] | |
| PDGFRA | increased liver expression levels in NASH patients | [31,61] | |
| PDGFRB | circulating levels of PDGFRB are progressively increased with increasing fibrosis stage | [31,61] | |
| SPHK1 | mediates hepatic inflammation in mice | [62,63] | |
| ITGA1 | facilitates hepatic insulin action while promoting lipid accumulation in mice | [64] | |
| ACKR1 | related to leucocyte recruitment by cirrhotic endothelial cells | [65,66,67] | |
| GPNMB | increased serum levels in NASH | [68] | |
| SCARB1 | SCARB1 deficiency increased inflammatory dyslipidaemia and adipocytes hypertrophy | [69] | |
| FGFR1 | central player in the response to liver injury and fibrosis | [70] | |
| TNFSF10 | increased liver expression levels in NASH | [71] | |
| CD68 | Increased liver infiltration with CD68+ macrophages are related to liver fibrosis | [72,73] | |
| NOTCH | NOTCH1 | directly involved in NAFLD development | [74,75,76,77,78,79] |
| NOTCH2 | directly involved in NAFLD development | [74,75,76,77,78,79] | |
| DLL1 | directly involved in NAFLD development | [74,75,76,77,78,79] | |
| DLL3 | directly involved in NAFLD development | [74,75,76,77,78,79] | |
| DLL4 | directly involved in NAFLD development | [74,75,76,77,78,79] | |
| JAG1 | directly involved in NAFLD development | [74,75,76,77,78,79] | |
| JAG2 | directly involved in NAFLD development | [74,75,76,77,78,79] | |
| WNT/β-catenin | WNT1 | related to hepatic glucose oxidation in NASH | [80] |
| WNT3a | related to hepatic glucose oxidation in NASH | [80] | |
| WNT5a | related to hepatic glucose oxidation in NASH | [80] | |
| WNT11 | related to hepatic glucose oxidation in NASH | [80] | |
| WNT2 | increased expression levels in liver pericentral endothelial cells in NASH | [81] | |
| RSPO3 | increased expression levels in liver pericentral endothelial cells in NASH | [81] | |
| WNT9b | altered liver expression levels in NASH | [82] | |
| WNT4 | altered liver expression levels in NASH | [82] | |
| FZD7 | modulated levels by lifestyle intervention in NAFLD patients | [83] | |
| LGR4/5 | its activity promotes NAFLD | [84] | |
| LRP6 | Mutations in LRP6 are one of the major causes of NAFLD induction | [85] | |
| DKK1 | related to hyperlipidaemia in NAFLD | [85] | |
| β-catenin | efector of the pathway. It has been found in EVs | [86,87,88] | |
| Plasma/serum secreted proteins in NAFLD | AFM | desregulated plasma levels in NAFLD and NASH patientis | [89,90] |
| PIGR | desregulated plasma levels in NAFLD and NASH patientis | [89,91,92,93] | |
| FTCD | proposed indicative biomarker for NAFLD to NASH progression | [22] | |
| Inter-tissue crosstalk | CD40 | CD40 expressing CD11c+ dendritic cells contribute to liver inflammation in NASH | [94,95] |
| LRG1 | adipokine that mediates obesity-induced hepatosteatosis and insulin resistance | [96,97] | |
| FNDC5 | serum irisin levels are reduced in patients with obesity-related NAFLD | [98,99] | |
| AKR1B7 | AKR1B7 in EVs derived from metabolic stressed adipocytes induce NASH in mice | [100] | |
| FASN | drives de novo lipogenesis, inflammation and fibrogenic signalling in NAFLD | [101,102] | |
| FABP4 | High levels of circulating FABP4 have been described in NAFLD patients | [103,104,105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





