1. Introduction
The COVID-19 pandemic has had significant global health implications, affecting millions of individuals worldwide. While the acute respiratory symptoms of COVID-19 have been extensively studied, emerging evidence suggests that the disease can have systemic effects, including disturbances in phosphocalcium metabolism [
1]. This manuscript review aims to summarize and critically analyze the current research on phosphocalcium metabolism disorders post-COVID, providing insights into the potential mechanisms and clinical implications [
2,
3,
4].
Phosphocalcium metabolism plays a crucial role in maintaining the balance of phosphorus and calcium in the body, which is essential for various physiological processes, including bone health, muscle function, nerve transmission, and cellular signaling [
5]. Any disturbances in this delicate equilibrium can lead to a spectrum of metabolic disorders, with significant implications for an individual’s overall well-being.
This manuscript review aims to provide a comprehensive analysis of the current state of research on phosphocalcium metabolism disorders post-COVID. It will delve into the existing literature to explore the impact of COVID-19 on phosphorus, calcium, and parathyroid hormone levels, along with the prevalence and clinical manifestations of these disturbances in recovering individuals [
6].
Understanding the consequences of COVID-19 on phosphocalcium metabolism is of paramount importance for healthcare professionals managing post-COVID patients. By identifying and addressing these metabolic disturbances, healthcare providers can potentially mitigate long-term complications and improve patient outcomes. Furthermore, this review will highlight potential avenues for future research to enhance our understanding of the pathophysiological mechanisms involved and develop effective strategies for managing phosphocalcium metabolism disorders in the aftermath of COVID-19.
In the following sections, this manuscript review will present a comprehensive overview of the research findings, providing valuable insights into the clinical implications and potential interventions for individuals experiencing phosphocalcium metabolism disorders after recovering from COVID-19.
2. Physiology
2.1. Phosphocalcium metabolism
The minerals calcium and phosphorus are essential components in the process of bone mineralization. Both minerals are found in bones and can be dissolved in serum or found intracellularly in soft tissues. Calcium and phosphorus are stored within the bone structure in the form of a crystalline compound known as hydroxyapatite. This compound not only acts as a storage site for these minerals but also serves as the fundamental building block of the bone, providing the necessary strength and support to bear the weight of the body. Calcium and phosphorus in serum exist in three forms: ionized, bound to albumin, or present in other ion complexes [
2]. Calcium is involved in various physiological processes, including muscle contraction, hormone, and neurotransmitter release, as well as enzyme activation and coagulation pathways [
3]. The presence of soluble calcium and phosphorus in serum is crucial for maintaining homeostasis and facilitating the proper functioning of various systems, including the nervous and muscular systems. Consequently, these levels are tightly regulated by hormones through processes such as intestinal absorption, bone resorption, renal excretion, and reabsorption [
7].
The regulation and monitoring of calcium levels in the bloodstream are closely managed through the utilization of calcium-sensing receptors (CaSR) [
8]. The calcium-sensing receptors (CaSRs) are a type of G-protein coupled receptors that are predominantly located in the kidneys and parathyroid glands. When calcium levels are high, an excessive amount of calcium binds to calcium-sensing receptors (CaSRs), leading to a decrease in the synthesis and secretion of parathyroid hormone. Additionally, it causes a reduction in the reabsorption of calcium by the kidneys [
8].
Phosphorus serves as a structural constituent of bone in the form of hydroxyapatite. Phosphorus primarily serves the crucial role of facilitating cellular energy production and supporting metabolic processes by acting as a constituent of adenosine triphosphate (ATP). The maintenance of serum phosphorus homeostasis is achieved through the ongoing processes of bone mineralization and resorption, which are regulated by a delicate equilibrium between osteoblasts, the cells responsible for bone formation, and osteoclasts, the cells involved in bone reabsorption [
2,
4].
The regulation of calcium and phosphorus in the body is primarily controlled by three hormones: vitamin D 25(OH), parathyroid hormone (PTH), and fibroblast growth factor 23 (FGF23) [
3].
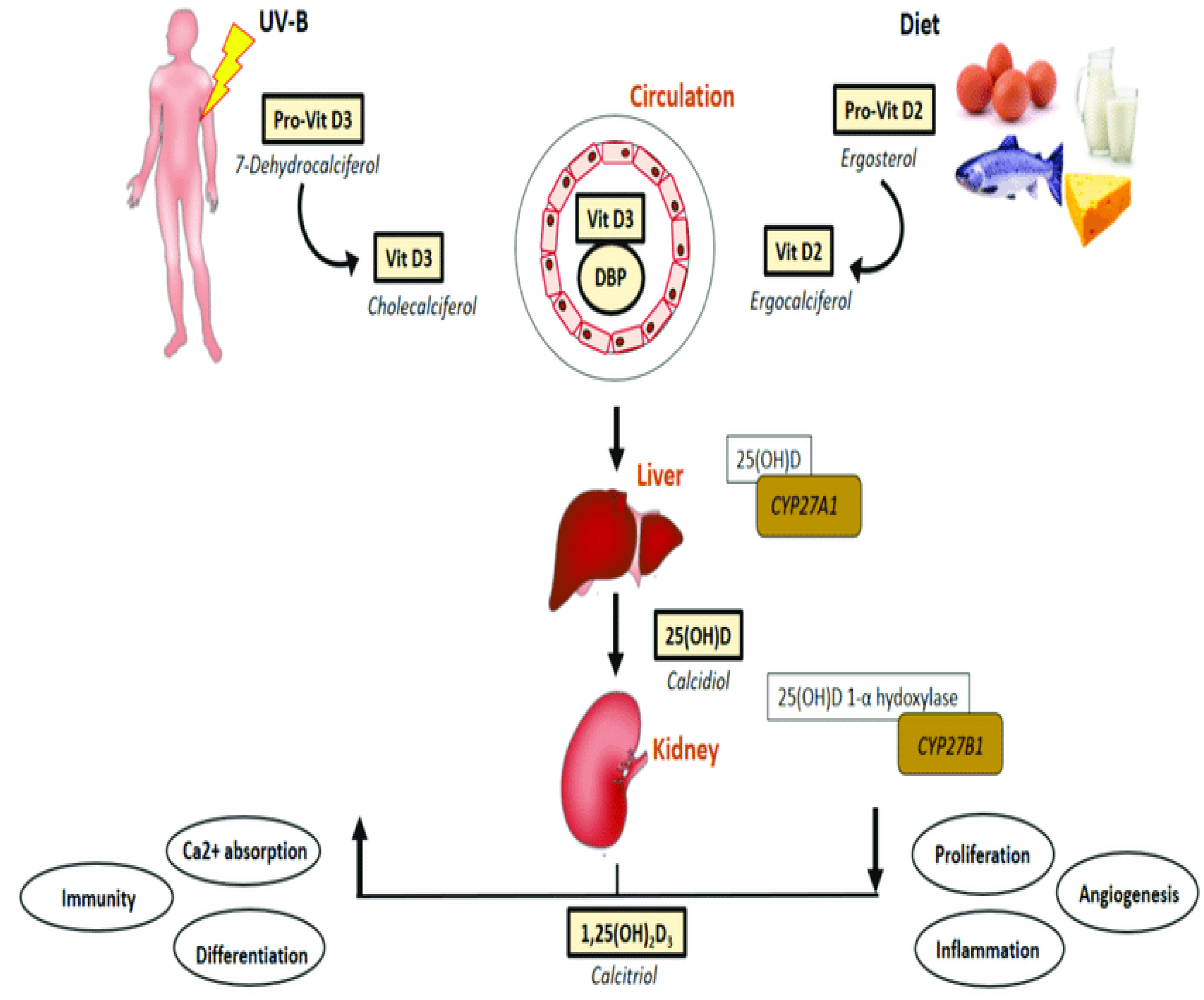
2.2. Regulation by Vitamin D
Various forms of vitamin D suffer biochemical conversion to an active form known as vitamin D1,25(OH)2 or calcitriol (
Figure 1). The synthesis of vitamin D3 (cholecalciferol) in the skin of animals begins with the conversion of 7-dehydrocholesterol through exposure to UVB radiation and heat. Vitamin D2, also known as ergocalciferol, is derived from plant and fungal sources [
2]. Additional sources of vitamin D include fish liver, oily fish, and its supplementation in milk and orange juice. Within the hepatic system, the hydroxylation process takes place, wherein D2 and D3 undergo conversion into calcidiol, also known as vitamin D25(OH) [
2]. The primary role of this intermediate metabolite is to serve as the reservoir for vitamin D. Within the renal system, calcidiol undergoes additional hydroxylation through the enzymatic action of 1α-hydroxylase, leading to the production of calcitriol, an active form of vitamin D. This process ultimately enhances the absorption of calcium and phosphorus in the intestines [
1,
2,
4].
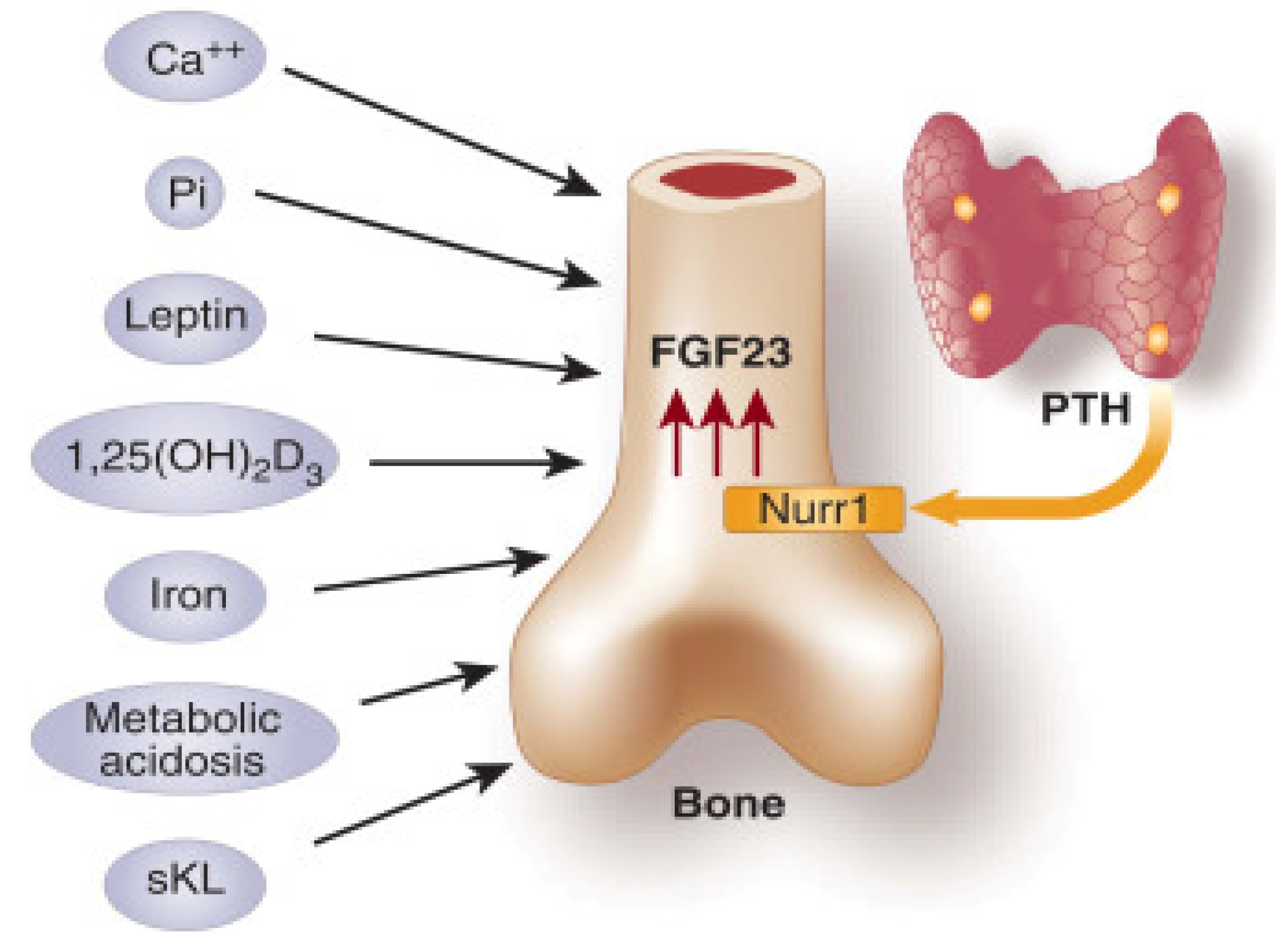
2.3. Regulation by PTH
Parathyroid hormone (PTH), which is secreted by the four parathyroid glands, plays a crucial role in regulating calcium and phosphorus levels in bone, the gastrointestinal tract, and the kidneys. The provided information is represented in
Figure 2. Within the renal tubules, parathyroid hormone (PTH) has the effect of augmenting the reabsorption of calcium while simultaneously enhancing the excretion of phosphorus [
2]. Within the gastrointestinal tract, parathyroid hormone (PTH) facilitates the process of reabsorption of calcium and phosphorus. This is achieved indirectly through the stimulation of 1α-hydroxylase, as supported by previous studies [
3]. Within the skeletal system, parathyroid hormone (PTH) serves as a stimulant for both osteoblasts and osteoclasts, leading to an elevation in bone turnover and ultimately resulting in bone resorption. The process of bone resorption leads to elevated levels of phosphorus and calcium. However, it is important to note that parathyroid hormone (PTH) also enhances the excretion of phosphorus in the kidneys [
9]. Consequently, the overall outcome is an elevation in serum calcium levels and a reduction in serum phosphorus levels [
2,
3]. The impact of parathyroid hormone (PTH) on renal function is characterized by a rapid onset, manifesting within minutes.
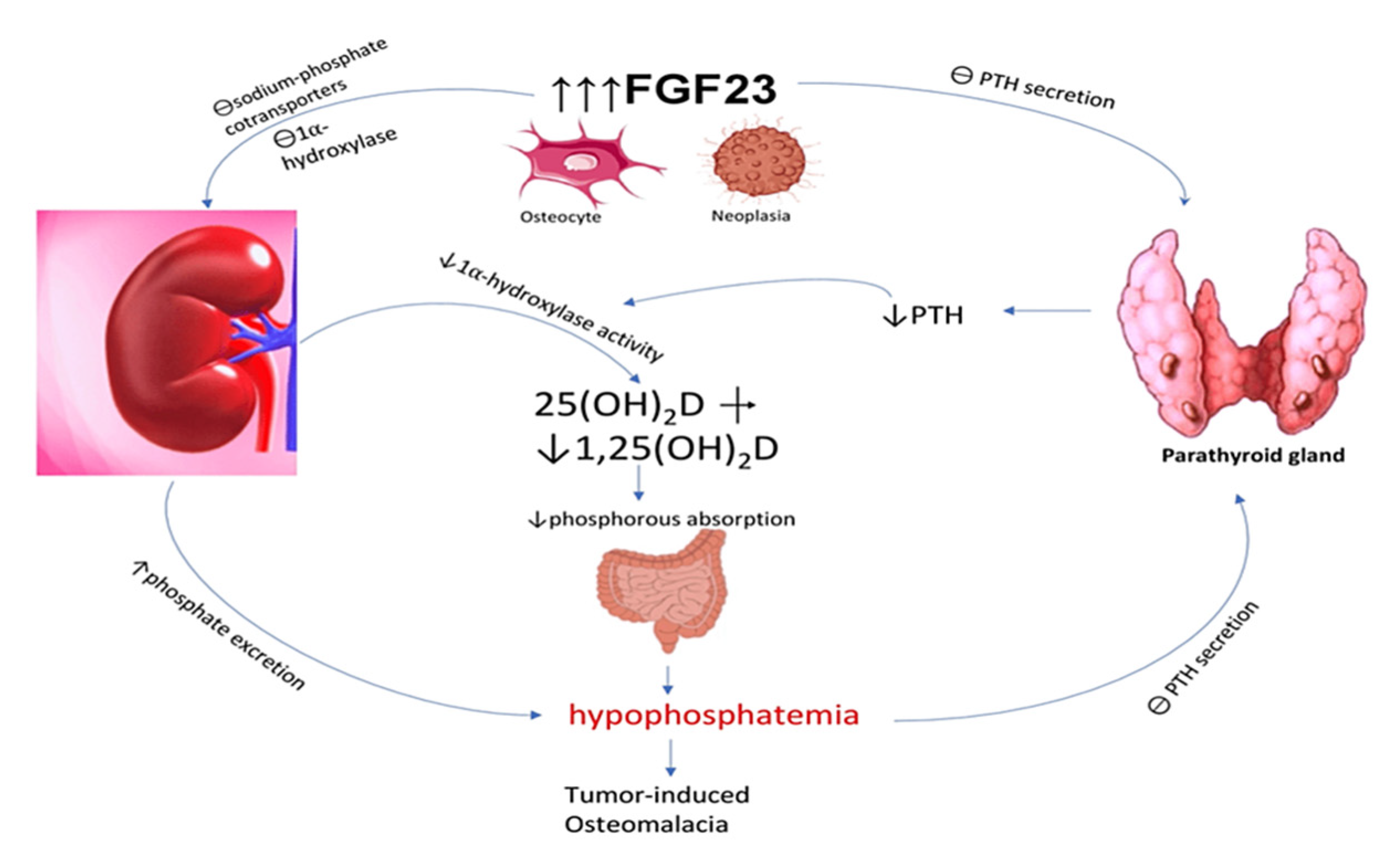
2.4. Regulation by FGF23
FGF23 is a significant hormone involved in the regulation of phosphorus levels. It functions as a phosphatonin, playing a crucial role in promoting the excretion of phosphorus in urine [
3] (
Figure 3). The hormone in question is presumed to activate its effects on the proximal renal tubule by binding to its receptor FGFR1, in conjunction with the co-receptor klotho [
3]. FGF23 functions as an inhibitor of 1α-hydroxylase and reduces the concentrations of calcitriol (vitamin D1,25(OH)2) in association with this receptor [
3,
5]. High levels of phosphate in the bloodstream trigger the skeletal system to produce fibroblast growth factor 23 (FGF23), which subsequently inhibits the reabsorption of phosphate in the renal tubules and the synthesis of calcitriol. FGF23 also promotes the production of 24α-hydroxylase, an enzyme responsible for the conversion of vitamin D25(OH) to vitamin D24,25(OH)2, which is an inactive metabolite.
3. Materials and methods
3.1. Literature Search Strategy:
A comprehensive search strategy was developed to identify relevant articles from an electronic database such as PubMed which were searched using keywords including “calcium metabolism disorders in COVID-19,” “phosphocalcium metabolism,” “hypophosphatemia in COVID-19,” “hypoparathyroidism in COVID-19, “ “hypocalcemia in COVID-19” and “post-COVID complications”. The search was limited to articles from the last 4 years to ensure the inclusion of recent research.
3.2. Inclusion and Exclusion Criteria:
Inclusion criteria:
Research articles investigating phosphocalcium metabolism disorders in patients who have recovered from COVID-19.
Studies with a clear study design and defined outcome measures related to phosphocalcium parameters.
Exclusion criteria:
Articles focusing solely on acute COVID-19 cases or pre-existing phosphocalcium metabolism disorders.
Review articles, editorials, and conference abstracts.
3.3. Data Extraction:
Two independent reviewers conducted the initial screening of the retrieved articles based on titles and abstracts.
Full-text screening was performed for potentially eligible articles, and discrepancies were resolved through discussion.
Data were extracted from the selected articles, including study characteristics (e.g., study design, sample size), patient demographics, COVID-19 severity, phosphocalcium-related parameters (calcium, phosphorus, PTH), and key findings related to phosphocalcium metabolism disorders.
3.4. Quality Assessment:
Studies with low methodological quality were carefully considered during data synthesis.
3.5. Data Synthesis:
The extracted data were synthesized to provide a descriptive overview of the selected studies’ characteristics, findings, and implications.
3.6. Limitations:
Potential limitations of this article review were acknowledged, such as the possibility of publication bias and the exclusion of non-English language articles.
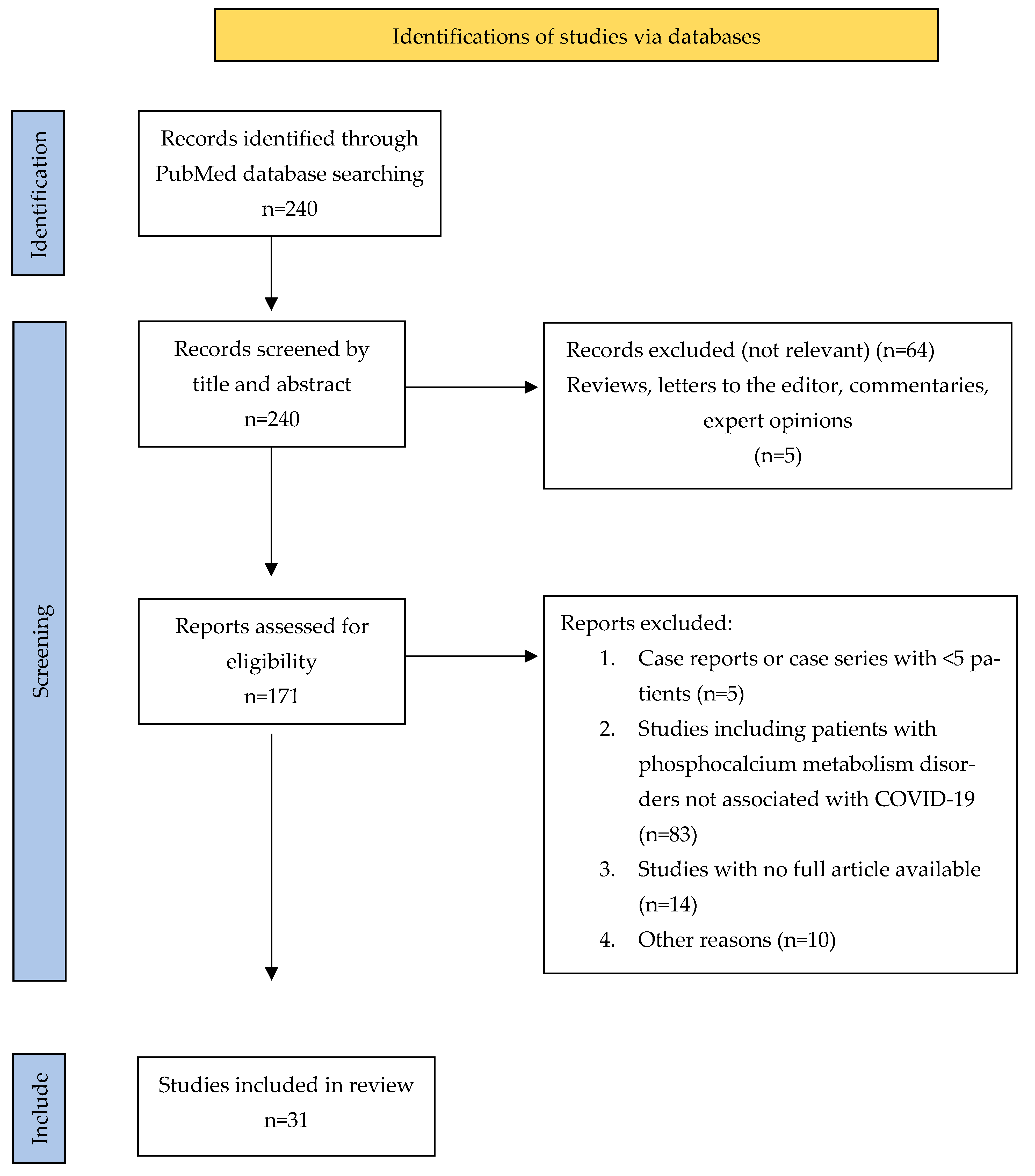
Figure 4.
PRISMA Flowchart of published studies related to COVID-19-associated phosphocalcium metabolism disorders.
Figure 4.
PRISMA Flowchart of published studies related to COVID-19-associated phosphocalcium metabolism disorders.
4. Results
4.1. Hypocalcemia
Low calcium levels were a common biochemical result during the first pandemic outbreak in Europe. Beginning with the seminal observation of a case of severe acute hypocalcemia in a patient previously thyroidectomized with infection with SARS-CoV-2 [
10], several studies taken worldwide to evaluate the possible causal or causal relationship between COVID-19 and hypocalcemia revealed an unexpectedly high prevalence of low calcium levels, ranging from 62.6 to 87.2% of patients, depending on the hypocalcemia definition used [
5,
11,
12].
In a recent study, researchers examined the relationship between calcium levels and various parameters related to inflammation, coagulopathy, and organ injury in individuals with COVID-19 [
13]. The findings revealed a negative association between calcium levels and these parameters, suggesting that higher calcium levels were linked to better outcomes. Additionally, the study identified hypocalcemia, or low calcium levels, as an independent risk factor for adverse effects in COVID-19 patients [
14]. This highlights the importance of calcium as a reliable and easily measurable biomarker for assessing disease severity in COVID-19. The results of this study provide valuable insights into the potential role of calcium in predicting and managing COVID-19 outcomes [
15]. The findings of this research were supported by subsequent systematic reviews and meta-analyses, which also concluded that there is a significant correlation between hypocalcemia and various indicators of disease severity (
Table 1) [
16,
17]. These indicators include hospitalization rates, length of hospital stay, admission to the intensive care unit, and the risk of mortality [
18,
19].
In their study, Cappellini et al. conducted a comprehensive analysis of calcium levels in a sample of 420 patients diagnosed with COVID-19, comparing them to a control group of 165 individuals without COVID-19. The researchers made a noteworthy discovery, as they observed a significant decrease in both serum total calcium and whole blood actual ionized calcium among the COVID-19 patients when compared to those without the virus. This finding shed light on the potential impact of COVID-19 on calcium homeostasis and highlights the importance of further investigation in this area [
1].
4.2. Hypophosphatemia
There is a suggested association between disturbances in phosphate metabolism and the impact of COVID-19 on the homeostasis of vitamin D, calcium, and phosphorus. The prevalence of vitamin D deficiency is high among individuals with severe COVID-19 infection [
20]. Povaliaeva et al. recently conducted a study that established a correlation between abnormal kidney function, abnormal vitamin D metabolism, and hypophosphatemia (
Table 2) [
21]. According to the authors, individuals with severe COVID-19 manifested atypical vitamin D metabolism, increased serum creatinine levels, and decreased serum phosphate levels [
21].
Figure 5.
Correlation between serum phosphate levels and Vitamin D, PTH, and calcium.
Figure 5.
Correlation between serum phosphate levels and Vitamin D, PTH, and calcium.
4.3. Hypovitaminosis D
The hypothesis of a strong correlation between VD and COVID-19 emerged during the initial stages of the pandemic, as VD has been widely recognized for its role in modulating both innate and adaptive immune responses [
22]. Vitamin D (VD) has been recognized for its antimicrobial properties and ability to inhibit viral activity. It also plays a role in regulating the adaptive immune response by promoting a transition from a pro-inflammatory state to a tolerogenic state. This results in the downregulation of immune responses mediated by T-helper-1 lymphocytes, the inhibition of pro-inflammatory cytokine production, and the promotion of regulatory T-cell maturation [
23].
Vitamin D (VD) has been recognized for its significant involvement in various metabolic pathways related to musculoskeletal health [
21]. Previous research has demonstrated that supplementation with VD has been shown to confer advantages in muscle recovery following periods of intense physical activity and tissue damage [
24,
25]. The studies found that VD levels were able to predict and exert an influence on the duration of illness and the time it took for recovery following an episode of acute severe pneumonia (
Table 3). To this day, the role of vitamin D in the occurrence of Long-COVID has only been examined in a limited number of small-scale studies [
26,
27]. In a recent pilot study involving elderly patients recovering from acute COVID-19, the effectiveness of a six-week therapy with 2,000 IU/day of cholecalciferol compared to placebo was examined. The study found that cholecalciferol therapy resulted in a reduction in creatinine kinase values and demonstrated a positive trend in improving overall health and physical well-being [
28,
29,
30,
31].
4.4. Hypoparathyroidism
The COVID-19 disease has been documented as a potential trigger for the decompensation of pre-existing primary hypoparathyroidism, which was previously well-tolerated by affected individuals. In their study, Bossoni et al. [
10] documented a clinical case involving a 72-year-old female patient who had previously undergone thyroidectomy [
32]. The patient exhibited a mild case of COVID-19 infection and presented with symptoms of acute perioral paresthesia and dysarthria. The laboratory analyses indicated a decrease in the concentration of calcium in the blood serum, an elevation in the concentration of phosphorus in the blood serum, and a decrease in the concentration of parathyroid hormone (PTH) in the blood serum [
33,
34,
35]. These findings suggest that the infection caused by the SARS-CoV-2 virus led to a significant decrease in calcium levels, particularly in the presence of underlying, asymptomatic hypoparathyroidism after surgery [
10].
4.5. Skeletal complications and vertebral fractures
Morphometric vertebral fractures (VFs) are considered significant clinical manifestations of osteoporosis and skeletal fragility. Recent reports have indicated a high prevalence of these fractures in patients with COVID-19 [
36,
37,
38]. VFs problems are associated with reduced survival rates, decreased respiratory function, and compromised quality of life in the general population [
36].
Hospitalized patients with COVID-19 may experience an elevated risk of fractures due to various concurrent factors [
39,
40]. These factors involve advanced age and comorbidities like diabetes, cardiovascular diseases, and hypertension.
Also, it has been previously reported that individuals who are hospitalized due to COVID-19 regularly show hypovitaminosis D, a condition that is known to be linked to decreased bone mineral density (BMD) and an elevated risk of fractures [
22,
41].
Vertebral fractures (VFs) and reduced bone mineral density (BMD) have been identified as factors that elevate the likelihood of developing pneumonia and hinder respiratory function, resulting in restrictive pulmonary dysfunction within the general population [
42]. VFs have been observed to have an impact on the respiratory function of individuals who have survived COVID-19 in the medium term. This influence, in turn, can have a substantial effect on their overall recovery process and may contribute to the occurrence of Long-COVID.
5. Incidence of phosphocalcium metabolism disorders related to COVID-19
The literature search revealed 240 studies, of which, ultimately, 31 studies were included in this review: 7 articles about hypocalcemia, 12 articles about hypophosphatemia and 12 articles about hypovitaminosis D.
Table 1.
Summary of included articles with hypocalcemia.
Table 1.
Summary of included articles with hypocalcemia.
| Study |
Study design |
Country |
Patients(n) |
Affected
Organ/ System |
| Yiquin W et al. [43] |
|
|
|
|
| Retrospective |
China |
125 |
Lungs |
| |
|
|
|
| Bálint D et al. [44] |
Multicenter Study |
Hungary |
451 |
Liver |
| Wessam O et al. [25] |
Prospective observational |
Oman |
445 |
Lungs |
| Meera M et al. [14] |
Retrospective observational |
England |
506 |
Lungs |
| Berta T et al. [45] |
Retrospective observational |
Spain |
316 |
Lungs |
| Jyot A et al. [46] |
Prospective observational |
India |
170 |
Heart |
| Jingmei L et al. [12] |
Prospective observational |
China |
69 |
Heart |
Table 2.
Summary of included articles with hypophosphatemia.
Table 2.
Summary of included articles with hypophosphatemia.
| Study |
Study design |
Country |
Patients(n) |
Affected Organ/ System |
| Rourang W et al. [4] |
|
|
|
|
| Case series |
China |
435 |
Kidney |
| |
|
|
|
| Zijin C et al. [47] |
Retrospective |
Hungary |
823 |
Kidney |
| Maram H et al. [48] |
Case series |
UAE |
128 |
Adrenal gland |
| Hannah W et al. [49] |
Cross-sectional |
Italy |
1226 |
Lungs |
| Marina V et al. [50] |
Prospective observational |
Switzerland |
104 |
Stomach |
| Baroncelli G et al. [51] |
Retrospective |
Italy |
26 |
Kidney |
| Hadavi M et al. [52] |
Retrospective |
Iran |
1346 |
Stomach |
| Kormann R et al. [53] |
Retrospective |
France |
42 |
Kidney |
| Benson Y et al. [54] |
Prospective observational |
USA |
88 |
Brain |
| Garcia F et al. [55] |
Case series |
Spain |
45 |
Kidney |
| Charles L et al. [56] |
Retrospective observational |
Singapore |
83 |
Kidney |
| Mnaff A et al. [57] |
Case series |
Kuwait |
78 |
Lungs |
Table 3.
Summary of included articles with hypovitaminosis D.
Table 3.
Summary of included articles with hypovitaminosis D.
| Study |
Study design |
Country |
Patients(n) |
Affected
Organ/ System |
| Saponaro F et al. [58] |
|
|
|
|
| Case series |
Italy |
93 |
Lungs |
| |
|
|
|
| Luigi di F et al. [5] |
Case series |
Italy |
78 |
Parathyroid |
| Singh S et al. [59] |
Retrospective |
India |
360 |
Lungs |
| Mohammed A et al. [60] |
Prospective observational |
Egypt |
124 |
Lungs |
| Manojlovic M et al. [61] |
Cross-sectional |
Serbia |
74 |
Heart |
| Mazziotti G et al. [62] |
Case series |
Italy |
348 |
Parathyroid |
| D’Alessandro A et al. [63] |
Multicenter Study |
Italy |
163 |
Lungs |
| Rimesh P et al. [16] |
Retrospective case |
India |
72 |
Bones |
| Rizaldy P et al. [64] |
Case series |
Indonesia |
10 |
Heart |
| Carpagnano G et al. [65] |
Retrospective |
Italy |
42 |
Lungs |
| Thiago J et al. [66] |
Cross-sectional |
Brazil |
176 |
Heart |
| Nascimento R et al. [67] |
Cross-sectional |
Brazil |
1478 |
Heart |
Table 4.
Baseline characteristics of included patients (SD= standard deviation, COPD= chronic obstructive pulmonary disease).
Table 4.
Baseline characteristics of included patients (SD= standard deviation, COPD= chronic obstructive pulmonary disease).
| Study |
Study design |
Patients(n) |
Age (Mean ± SD) |
COVID-19 Severity |
Duration of Hospitalization (Days) |
Phosphocalcium Findings |
Other Complications |
Comorbidity |
| Yiquin W et al. [43] |
|
|
|
|
|
|
|
|
| Retrospective |
125 |
55 ± 8.9 years |
Mild |
13 ± 3.9 |
Hypocalcemia: 64% |
Pneumonia |
Diabetes type 2
Hypertension
Coronary heart disease |
| |
|
|
|
|
|
|
|
Bálint D
et al. [44] |
Multicenter study |
451 |
58.2 ± 10.3 years |
Severe |
14 ± 3.9 |
Hypocalcemia: 45% |
Severe respiratory failures |
Cirrhosis |
| Wessam O et al. [25] |
Prospective |
445 |
50 ± 12.1 years |
Mild |
7.5 ± 2.1 |
Hypocalcemia: 75%
Hypovitaminosis D: 5% |
Chronic respiratory diseases |
Diabetes
Hypertension |
| Meera M et al. [14] |
Retrospective |
506 |
65 ± 7.2 years |
Severe |
25.6 ± 4.7 |
Hypocalcemia: 53% |
Lymphopenia
Hypoxia |
Obesity
Hypertension
Cancer |
| Berta T et al. [45] |
Retrospective |
316 |
65 ± 9.5 years |
Severe |
19.3 ± 3.5 |
Hypocalcemia: 63% |
ICU admission |
Cardiopathy
Dyslipidemia
Diabetes |
| Jyot A et al. [46] |
Prospective |
170 |
62 ± 11.7 years |
Critical |
23.7 ± 5.2 |
Hypocalcemia: 80% |
Acute respiratory distress syndrome |
Hypertension
Diabetes mellitus type 2 |
| Jingmei L et al. [12] |
Prospective |
|
68 ± 6.8 years |
Severe |
14.1 ± 1.9 |
Hypocalcemia: 62% |
Pneumonia |
Coronary heart disease
Hypertension |
| Rourang W et al. [4] |
Case series |
435 |
57 ± 10.5 years |
Severe |
10 ± 4 |
Hypophosphatemia: 7.6% |
Chronic liver disease |
COPD
Hypertension
Diabetes mellitus |
| Zijin C et al. [47] |
Retrospective |
823 |
60.9 ± 14.9 years |
Severe |
14.8 ± 6.3 |
Hypophosphatemia: 10% |
Acute liver injury |
COPD
Diabetes mellitus |
| Hannah W et al. [49] |
Cross sectional |
1226 |
61.2 ± 8.3 years |
Severe |
13.5 ± 2.8 |
Hypophosphatemia: 26% |
Pneumonia |
Cardiovascular disease
Septic shock |
| Marina V et al. [50] |
Prospective |
104 |
59 ± 14 years |
Critical |
20.3 ± 9 |
Hypophosphatemia : 33% |
Gastrointestinal problems |
Diabetes
Obesity |
| Hadavi M et al. [52] |
Retrospective |
1346 |
65.9 ± 1.1 years |
Severe |
14 ± 6 |
Hypophosphatemia : 10% |
Respiratory failure |
Cardiovascular disease
Hypertension
Autoimmune disease |
| Kormann R et al. [53] |
Retrospective |
42 |
63.4 ± 8 years |
Severe |
19 ± 12.2 |
Hypophosphatemia : 29% |
Kidney disease |
Hypertension
Dyslipidemia
COPD |
| Charles L et al. [56] |
Retrospective |
83 |
58 ± 12.7 years |
Severe |
14 ± 3.1 |
Hypophosphatemia : 18% |
Gastrointestinal problems |
Hypertension
Diabetes
Cancer |
The results from the reviewed studies indicate a high prevalence of phosphocalcium metabolism disorders among individuals with COVID-19, and there is often a correlation between the severity of these disorders and the clinical manifestation of the disease. Hypocalcemia and hypophosphatemia are frequently observed disturbances, particularly in patients with severe cases of COVID-19.
Multiple factors contribute to the occurrence of phosphocalcium abnormalities, encompassing the length and intensity of hospitalization, coexisting medical conditions, and the occurrence of additional complications like acute respiratory distress syndrome and acute kidney injury (refer to
Table 4). There is evidence to suggest that individuals diagnosed with chronic kidney disease or chronic heart disease may be more susceptible to the development of phosphocalcium disorders in the context of COVID-19.
The duration of hospital stay, and the severity of the disease are important variables that have a significant impact on phosphocalcium abnormalities in individuals diagnosed with COVID-19. Extended durations of hospitalization, particularly in cases of critical illness, have the potential to disrupt the balance of minerals in the body. This can be attributed to various factors such as reduced ability to move, changes in dietary consumption, and the presence of systemic inflammation. Consequently, it is imperative to closely monitor phosphocalcium levels during the entire duration of hospitalization in order to promptly intervene and mitigate complications associated with imbalances in these vital minerals.
The correlation between phosphocalcium disorders and specific comorbidities, such as chronic kidney disease and chronic heart disease, presents significant clinical implications. Individuals who have pre-existing medical conditions may exhibit increased susceptibility to disturbances in phosphocalcium metabolism after being infected with COVID-19. The management of phosphocalcium levels in these individuals may necessitate customized strategies, considering their pre-existing conditions and prescribed medication protocols.
6. Discussion
The COVID-19 pandemic has not only impacted the respiratory system, but it has demonstrated systemic implications, such as modifications in phosphocalcium metabolism. The objective of this article review was to provide a comprehensive summary and critical analysis of existing research about disorders in phosphocalcium metabolism following COVID-19 [
2,
16,
26]. The review aimed to offer valuable insights into the potential underlying mechanisms and clinical implications associated with these disorders.
The results derived from the examined studies highlight the frequency of phosphocalcium metabolism disorders among individuals in the process of recuperating from COVID-19. Various research studies have documented a variety of disruptions, such as hypophosphatemia, hypocalcemia, and secondary hypoparathyroidism [
19,
24,
33]. The results of this study suggest that COVID-19 has the potential to disturb the intricate equilibrium of phosphorus, calcium, and parathyroid hormone concentrations within the human body.
One possible mechanism that may contribute to the development of phosphocalcium metabolism disorders following COVID-19 is the direct impact of the virus on organs responsible for maintaining phosphocalcium balance. Multiple research studies have provided evidence regarding the existence of viral particles and the manifestation of viral receptors within the renal and parathyroid tissues [
33,
34]. This observation implies that SARS-CoV-2 has the potential to directly impact these organs, resulting in changes to phosphocalcium metabolism.
The COVID-19 infection has the potential to induce systemic inflammation and immune dysregulation, which in turn can have an impact on the regulation of phosphocalcium metabolism. The imbalances of phosphorus, calcium, and parathyroid hormone levels can occur because of the activation of the immune system and the release of inflammatory cytokines [
2,
3]. In addition, the administration of medications in the context of COVID-19 therapy, such as corticosteroids, has the potential to induce metabolic disruptions.
The clinical ramifications of disorders related to phosphocalcium metabolism following a COVID-19 infection are of considerable importance. The presence of hypophosphatemia and hypocalcemia may result in various clinical manifestations, such as muscle weakness, fatigue, bone pain, muscle cramps, tingling sensations, and potential cardiac arrhythmias [
3,
4,
16,
24].
The effective management of phosphocalcium metabolism disorders in individuals undergoing recovery from COVID-19 necessitates diligent monitoring and the implementation of suitable interventions. Systematic monitoring of phosphorus, calcium, and parathyroid hormone concentrations can facilitate the detection and management of any deviations from the optimal levels. The restoration and maintenance of optimal levels may require the utilization of nutritional supplementation, such as vitamin D and calcium. Furthermore, the restoration of phosphocalcium homeostasis could potentially be facilitated by the management of systemic inflammation and immune dysregulation.
Recognizing the constraints of the examined studies holds significance. Numerous studies exhibit limited sample sizes and heterogeneity concerning patient characteristics and methodologies. Additional investigation utilizing larger groups, standardized methodologies, and extended monitoring periods is imperative to enhance our comprehension of the frequency, underlying mechanisms, and medical consequences associated with disturbances in phosphocalcium metabolism post-COVID-19.
7. Conclusions
In conclusion, this review has explained the profound influence of COVID-19 on phosphocalcium metabolism, resulting in a range of metabolic dysfunctions. The results obtained from the examined studies highlight the high occurrence of disruptions in phosphocalcium metabolism among individuals in the process of recovering from COVID-19. These disruptions include hypophosphatemia, hypocalcemia, and secondary hypoparathyroidism.
The perturbations in phosphocalcium metabolism following COVID-19 are presumably influenced by a variety of factors, encompassing the direct impact of the virus on the kidneys and parathyroid glands, systemic inflammation, immune dysregulation, and the administration of medications during COVID-19 treatment. These mechanisms have the potential to disturb the intricate equilibrium of phosphorus, calcium, and parathyroid hormone, leading to imbalances and subsequent clinical manifestations.
The management of disorders related to phosphocalcium metabolism following a COVID-19 infection is of major significance to enhance patient outcomes. The regular monitoring of phosphorus, calcium, and parathyroid hormone levels is imperative for the timely identification and implementation of appropriate measures. The restoration and maintenance of optimal levels may require the utilization of nutritional supplementation, such as vitamin D and calcium. The restoration of phosphocalcium homeostasis can be facilitated by addressing systemic inflammation and immune dysregulation.
Nevertheless, it is crucial to recognize the constraints inherent in the examined studies, including limited sample sizes, variability in participant characteristics, and the necessity for additional investigations employing standardized methodologies and extended periods of observation. Future research endeavors should strive to enhance our comprehension regarding the frequency, underlying mechanisms, and medical ramifications of phosphocalcium metabolism disorders after the COVID-19 infection.
In summary, this manuscript review highlights the importance of phosphocalcium metabolism disorders in the population undergoing recovery from COVID-19. The results underscore the significance of surveillance, interventions, and additional investigation in this domain. Healthcare professionals have the potential to enhance the comprehensive care and outcomes of individuals after COVID by addressing these metabolic disturbances.
8. Future Perspectives
The review on phosphocalcium metabolism disorders post-COVID-19 provides valuable insights into the impact of SARS-CoV-2 infection on calcium, phosphorus, and vitamin D homeostasis in patients. Building on the existing knowledge, several future perspectives can guide further research and clinical practice in this area:
8.1. Long-term monitoring
Conducting longitudinal studies with extended follow-up periods is essential to understand the long-term consequences of phosphocalcium disorders post-COVID-19. Tracking patients beyond the acute phase will help assess the persistence of abnormalities and potential late-onset complications, such as osteoporosis and cardiovascular events.
8.2. Impact on bone health
Investigating the effects of phosphocalcium disorders on bone health is crucial. Long-term studies assessing bone mineral density, bone turnover markers, and fracture risk in post-COVID-19 patients can provide valuable insights into bone-related complications and guide preventive measures.
8.3. Optimal vitamin D supplementation
Conducting randomized controlled trials to determine the most effective and safe dosage of vitamin D supplementation in post-COVID-19 patients is essential. Understanding the optimal timing, duration, and formulation of supplementation can improve patient outcomes and reduce complications.
8.4. Immune system dysregulation
Exploring the immunological mechanisms underlying phosphocalcium metabolism disorders post-COVID-19 is critical. Investigating the role of immune dysregulation, cytokine storm, and chronic inflammation in disrupting calcium and phosphorus homeostasis can provide potential therapeutic targets.
8.5. Multidisciplinary care teams
Establishing multidisciplinary care teams involving endocrinologists, nephrologists, infectious disease specialists, and rehabilitation experts can provide comprehensive management for post-COVID-19 patients with phosphocalcium disorders. Collaboration among specialties can address the complexity of these conditions.
8.6. Global collaboration and data sharing
Encouraging international collaboration and data sharing among researchers and institutions can enhance the collective understanding of phosphocalcium disorders post-COVID-19. Large-scale, multinational studies can yield robust findings and facilitate more comprehensive guidelines.
8.7. Preparing for future outbreaks
Applying the knowledge gained from studying phosphocalcium disorders post-COVID-19 can help healthcare systems prepare for future infectious disease outbreaks. Lessons learned from managing these disorders can inform strategies for preventing and managing similar complications in future pandemics.
In conclusion, the future perspectives outlined above offer a roadmap for furthering our understanding of phosphocalcium metabolism disorders in the context of COVID-19 recovery. By addressing these perspectives through dedicated research and collaboration, we can optimize patient care, mitigate long-term consequences, and enhance public health measures to improve the overall well-being of post-COVID-19 patients.
Author Contributions
Conceptualization, L.A., L.B. and C.L.M.; methodology, C.B., L.N.; software, L.A., A.N.; validation, L.A., C.B., and C.M.; formal analysis, C.M., L.N., and C.L.M.; investigation, L.A., and L.B.; resources, L.A., B.I.S. and P.Z.; data curation, C.B., and C.L.M.; writing—original draft preparation, L.A., C.M., and L.B.; writing—review and editing, A.L.T.; visualization, L.A., and L.N.; supervision, A.L.T., B.I.S, and P.Z.; project administration, C.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cappellini, F.; Brivio, R.; Casati, M. Low Levels of Total and Ionized Calcium in Blood of COVID-19 Patients. Clin. Chem. Lab. Med. CCLM 2020, 58, e171–e173. [Google Scholar] [CrossRef] [PubMed]
- Gattineni, J. Inherited Disorders of Calcium and Phosphate Metabolism. Curr. Opin. Pediatr. 2014, 26, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Underland, L.; Markowitz, M. Calcium and Phosphate Hormones: Vitamin D, Parathyroid Hormone, and Fibroblast Growth Factor 23. Pediatr. Rev. 2020, 41, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; He, M. Hypophosphatemia at Admission Is Associated with Increased Mortality in COVID-19 Patients. Int. J. Gen. Med. 2021, Volume 14, 5313–5322. [Google Scholar] [CrossRef]
- Di Filippo, L.; Allora, A. Hypocalcemia in COVID-19 Is Associated with Low Vitamin D Levels and Impaired Compensatory PTH Response. Endocrine 2021, 74, 219–225. [Google Scholar] [CrossRef]
- Gittoes, N.J.; Criseno, S.; Appelman-Dijkstra, N.M.; Bollerslev, J. ENDOCRINOLOGY IN THE TIME OF COVID-19: Management of Calcium Metabolic Disorders and Osteoporosis. Eur. J. Endocrinol. 2020, 183, G57–G65. [Google Scholar] [CrossRef] [PubMed]
- Tatu, A.L.; Anghel, L. A Working Hypothesis on Vesicular Lesions Related to COVID-19 Infection, Koebner Phenomena Type V, and a Short Review of Related Data. Clin. Cosmet. Investig. Dermatol. 2021, Volume 14, 419–423. [Google Scholar] [CrossRef]
- Kos, C.H.; Karaplis, A.C. The Calcium-Sensing Receptor Is Required for Normal Calcium Homeostasis Independent of Parathyroid Hormone. J. Clin. Invest. 2003, 111, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Baroiu, L.; Anghel, L. The Role of D-Dimers in the Initial Evaluation of COVID-19. Ther. Clin. Risk Manag. 2022, Volume 18, 323–335. [Google Scholar] [CrossRef]
- Bossoni, S.; Chiesa, L. Severe Hypocalcemia in a Thyroidectomized Woman with Covid-19 Infection. Endocrine 2020, 68, 253–254. [Google Scholar] [CrossRef]
- Di Filippo, L.; Formenti, A.M. Hypocalcemia Is Highly Prevalent and Predicts Hospitalization in Patients with COVID-19. Endocrine 2020, 68, 475–478. [Google Scholar] [CrossRef]
- Liu, J.; Han, P. Prevalence and Predictive Value of Hypocalcemia in Severe COVID-19 Patients. J. Infect. Public Health 2020, 13, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Raesi, A.; Saedi Dezaki, E. Hypocalcemia in Covid-19: A Prognostic Marker for Severe Disease. Iran. J. Pathol. 2020, 16, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Ghani, H. Retrospective Case–Control Study to Evaluate Hypocalcaemia as a Distinguishing Feature of COVID-19 Compared with Other Infective Pneumonias and Its Association with Disease Severity. BMJ Open 2021, 11, e053810. [Google Scholar] [CrossRef]
- Minasi, A.; Andreadi, A. Hypocalcemia Is Associated with Adverse Outcomes in Patients Hospitalized with COVID-19. Endocrine 2022, 79, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Ram, S. High Prevalence of Hypocalcemia in Non-Severe COVID-19 Patients: A Retrospective Case-Control Study. Front. Med. 2021, 7, 590805. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.; Formenti, A.M. Hypocalcemia: The Quest for the Cause of a Major Biochemical Feature of COVID-19. Endocrine 2020, 70, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, E.; Alemzadeh, E. The Effect of Low Serum Calcium Level on the Severity and Mortality of Covid Patients. Immun. Inflamm. Dis. 2021, 9, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Martha, J.W.; Wibowo, A. Hypocalcemia Is Associated with Severe COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 337–342. [Google Scholar] [CrossRef]
- Mercola, J.; Grant, W.B. Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients 2020, 12, 3361. [Google Scholar] [CrossRef] [PubMed]
- Povaliaeva, A.; Malysheva, N. Impaired Vitamin D Metabolism in Hospitalized COVID-19 Patients. Pharmaceuticals 2022, 15, 906. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A. Hypovitaminosis D and the Endocrine Phenotype of COVID-19. Endocrine 2021, 72, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Bikle, D. MECHANISMS IN ENDOCRINOLOGY: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef] [PubMed]
- Hashemipour, S.; Kiani, S. Hypocalcemia in Hospitalized Patients with COVID-19: Roles of Hypovitaminosis D and Functional Hypoparathyroidism. J. Bone Miner. Metab. 2022, 40, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Osman, W.; Al Fahdi, F. Serum Calcium and Vitamin D Levels: Correlation with Severity of COVID-19 in Hospitalized Patients in Royal Hospital, Oman. Int. J. Infect. Dis. 2021, 107, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Bennouar, S.; Cherif, A.B. Vitamin D Deficiency and Low Serum Calcium as Predictors of Poor Prognosis in Patients with Severe COVID-19. J. Am. Coll. Nutr. 2021, 40, 104–110. [Google Scholar] [CrossRef]
- Dissanayake, H.A.; De Silva, N.L. Prognostic and Therapeutic Role of Vitamin D in COVID-19: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2022, 107, 1484–1502. [Google Scholar] [CrossRef]
- Caballero-García, A.; Pérez-Valdecantos, D. Effect of Vitamin D Supplementation on Muscle Status in Old Patients Recovering from COVID-19 Infection. Medicina (Mex.) 2021, 57, 1079. [Google Scholar] [CrossRef] [PubMed]
- Hopefl, R.; Ben-Eltriki, M. Association Between Vitamin D Levels and Inflammatory Markers in COVID-19 Patients: A Meta-Analysis of Observational Studies. J. Pharm. Pharm. Sci. 2022, 25, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, I.; Gatti, D. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front. Public Health 2021, 9, 736665. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.R.; Wibowo, A. Low Serum 25-Hydroxyvitamin D (Vitamin D) Level Is Associated With Susceptibility to COVID-19, Severity, and Mortality: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 660420. [Google Scholar] [CrossRef] [PubMed]
- Scappaticcio, L.; Pitoia, F. Impact of COVID-19 on the Thyroid Gland: An Update. Rev. Endocr. Metab. Disord. 2021, 22, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Tecilazich, F.; Formenti, A.M. Treatment of Hypoparathyroidism. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, J.-B.; Berchoux, E. Decompensated Primary Hypoparathyroidism in a Patient with COVID-19. Ann. Endocrinol. 2021, 82, 123–124. [Google Scholar] [CrossRef]
- Elkattawy, S.; Alyacoub, R. A Novel Case of Hypoparathyroidism Secondary to SARS-CoV-2 Infection. Cureus 2020. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.; Formenti, A.M. Radiological Thoracic Vertebral Fractures Are Highly Prevalent in COVID-19 and Predict Disease Outcomes. J. Clin. Endocrinol. Metab. 2021, 106, e602–e614. [Google Scholar] [CrossRef]
- Blanch-Rubió, J.; Soldevila-Domenech, N. Influence of Anti-Osteoporosis Treatments on the Incidence of COVID-19 in Patients with Non-Inflammatory Rheumatic Conditions. Aging 2020, 12, 19923–19937. [Google Scholar] [CrossRef]
- Formenti, A.M.; Pedone, E. Are Women with Osteoporosis Treated with Denosumab at Risk of Severe COVID-19? Endocrine 2020, 70, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Elderkin, A.L. Managing Fragility Fractures during the COVID-19 Pandemic. Nat. Rev. Endocrinol. 2020, 16, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Boussaid, S.; Makhlouf, Y. Association of SARS-COV2 and Lumbar Spine Fractures: Causal or Coincidental? J. Clin. Densitom. 2022, 25, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Yu, E.W. Vaccination for Coronavirus Disease 2019 and Relationship to Osteoporosis Care: Current Evidence and Suggested Approaches. J. Bone Miner. Res. 2021, 36, 1042–1047. [Google Scholar] [CrossRef]
- Di Filippo, L.; Compagnone, N. Vertebral Fractures at Hospitalization Predict Impaired Respiratory Function during Follow-up of COVID-19 Survivors. Endocrine 2022, 77, 392–400. [Google Scholar] [CrossRef]
- Wu, Y.; Hou, B. Risk Factors Associated With Long-Term Hospitalization in Patients With COVID-19: A Single-Centered, Retrospective Study. Front. Med. 2020, 7, 315. [Google Scholar] [CrossRef]
- Drácz, B.; Müller, V. Hypocalcemia on Admission Is a Predictor of Disease Progression in COVID-19 Patients with Cirrhosis: A Multicenter Study in Hungary. Biomedicines 2023, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Torres, B.; Alcubilla, P. Impact of Low Serum Calcium at Hospital Admission on SARS-CoV-2 Infection Outcome. Int. J. Infect. Dis. 2021, 104, 164–168. [Google Scholar] [CrossRef]
- Amrita, J. Role of Arterial Blood Gas (ABG) as a Valuable Assessment Tool of Disease Severity in SARS-CoV-2 Patients. J. Med. Biochem. 2022, 41, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gao, C. Hypophosphatemia Is an Independent Risk Factor for AKI among Hospitalized Patients with COVID-19 Infection. Ren. Fail. 2021, 43, 1329–1337. [Google Scholar] [CrossRef]
- Hashim, M.; Athar, S. New Onset Adrenal Insufficiency in a Patient with COVID-19. BMJ Case Rep. 2021, 14, e237690. [Google Scholar] [CrossRef]
- Wozniak, H.; Dos Santos Rocha, A. Hypophosphatemia on ICU Admission Is Associated with an Increased Length of Stay in the ICU and Time under Mechanical Ventilation. J. Clin. Med. 2022, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.V.; Pantet, O. Specific Nutrition and Metabolic Characteristics of Critically Ill Patients with Persistent COVID-19. J. Parenter. Enter. Nutr. 2022, 46, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Baroncelli, G.I.; Bertelloni, S. Management of Patients with X-Linked Hypophosphatemic Rickets during Covid-19 Pandemic Lockdown. J. Pediatr. Endocrinol. Metab. 2021, 34, 905–910. [Google Scholar] [CrossRef]
- Hadavi, M.; Taghinezhad, F. Hypo- and Hyperphosphatemia at Admission as Independent Factors of Mortality of COVID-19 Patients: Findings from a Retrospective Cohort Study. Int. J. Endocrinol. Metab. 2022, 20. [Google Scholar] [CrossRef]
- Kormann, R.; Jacquot, A. Coronavirus Disease 2019: Acute Fanconi Syndrome Precedes Acute Kidney Injury. Clin. Kidney J. 2020, sfaa109. [Google Scholar] [CrossRef] [PubMed]
- Yeh, B.; Bell, C. Seizures, Vitamin D Deficiency, and Severe Hypophosphatemia: The Unique Presentation of a SARS-CoV-2 Case. Cureus 2023. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, S.; Fernández-Morán, E. Tubulointerstitial Nephritis and Uveitis Syndrome and SARS-CoV-2 Infection in an Adolescent: Just a Coincidence in Time? Pediatr. Nephrol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Lew, C.C.H.; Ng, P.S. Nutrition Support Practices for Critically Ill Patients with Severe Acute Respiratory Syndrome Coronavirus-2: A Multicentre Observational Study in Singapore. Ann. Acad. Med. Singapore 2022, 51, 329–340. [Google Scholar] [CrossRef]
- Sabti, M.A.; Shamsaldeen, Y.A. Correcting Hypophosphataemia in a Paediatric Patient with Sanjad–Sakati Syndrome through a Single Oral Dose of Potassium Phosphate Intravenous Solution. SAGE Open Med. Case Rep. 2021, 9, 2050313X2098841. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, F.; Franzini, M. Is There a Crucial Link Between Vitamin D Status and Inflammatory Response in Patients With COVID-19? Front. Immunol. 2022, 12, 745713. [Google Scholar] [CrossRef]
- Singh, S.; Nimavat, N. An Epidemiological Investigation to Evaluate the Link between Hypovitaminosis D and COVID-19. J. Fam. Med. Prim. Care 2022, 11, 2630. [Google Scholar] [CrossRef]
- Teama, M.A.E.M.; Abdelhakam, D.A. Vitamin D Deficiency as a Predictor of Severity in Patients with COVID-19 Infection. Sci. Prog. 2021, 104, 003685042110368. [Google Scholar] [CrossRef] [PubMed]
- Manojlovic, M.; Ilincic, B.; Naglic, D.T.; Cabarkapa, V.; Djuric, A.P.; Kolarski, I.; Bojovic, M.; Urosevic, I.; Stokic, E.; Isenovic, E.R. Association between Vitamin D Hypovitaminosis and Severe Forms of COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5318–5326. [Google Scholar] [CrossRef] [PubMed]
- Mazziotti, G.; Lavezzi, E. Vitamin D Deficiency, Secondary Hyperparathyroidism and Respiratory Insufficiency in Hospitalized Patients with COVID-19. J. Endocrinol. Invest. 2021, 44, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Ciavardelli, D. Contribution of Vitamin D3 and Thiols Status to the Outcome of COVID-19 Disease in Italian Pediatric and Adult Patients. Sci. Rep. 2023, 2504. [Google Scholar] [CrossRef] [PubMed]
- Pinzon, R.T. ; Angela Vitamin D Deficiency among Patients with COVID-19: Case Series and Recent Literature Review. Trop. Med. Health 2020, 48, 102. [Google Scholar] [CrossRef]
- Carpagnano, G.E.; Di Lecce, V. Vitamin D Deficiency as a Predictor of Poor Prognosis in Patients with Acute Respiratory Failure Due to COVID-19. J. Endocrinol. Invest. 2021, 44, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.J.M.; Gonçalves, S.E.A.B. Prevalence of Obesity and Hypovitaminosis D in Elderly with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Nutr. ESPEN 2020, 40, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.A.; Fajardo, V.C. Work Hours as a Risk Factor for SARS-CoV-2 Infections: Cardiometabolic and Sleep Characteristics in Rotating Shift Workers. Sleep Sci. 2022, 15, 380–387. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).